Abstract
In this study, we tested the hypothesis that the documented transformation of 17β-estradiol (E2) from a counterinflammatory hormone in nondiabetic (ND) rats to a proinflammatory agent in rats with diabetes mellitus (DM) is due to an enhanced contribution from the receptor for advanced glycation end products (RAGE). Rhodamine 6G-labeled leukocytes were observed through a closed cranial window in rats. In vivo pial venular leukocyte adherence and infiltration were measured over 10 h reperfusion after transient forebrain ischemia in DM (streptozotocin) versus ND intact, ovariectomized (OVX), and E2-replaced (for 7–10 days) OVX (OVE) females. The role of RAGE was examined in two ways: 1) RAGE knockdown via topical application of RAGE antisense versus missense oligodeoxynucleotide or 2) intracerebroventricular injection of the RAGE decoy inhibitor, soluble RAGE. Among diabetic rats, the lowest levels of cortical RAGE mRNA and immunoreactivity of the RAGE ligand, AGE, were seen in OVX females, with significantly higher levels exhibited in intact and OVE females. However, results from the analysis of cortical RAGE protein only partially tracked those findings. When comparing ND to DM rats, cortical AGE immunoreactivity was significantly lower in OVE and intact females but similar in OVX rats. In DM rats, the level of postischemic leukocyte adhesion and infiltration (highest to lowest) was OVE > intact >> untreated OVX. In NDs, adhesion was highest in the untreated OVX group. Leukocyte extravasation was observed at >6 h postischemia but only in diabetic OVE and intact females and in ND OVX (untreated) rats. Pretreatment with RAGE antisense-oligodeoxynucleotide or soluble RAGE attenuated postischemic leukocyte adhesion and prevented infiltration but only in the diabetic OVE and intact groups. These results indicate that the exacerbation of postischemic leukocyte adhesion by chronic E2 replacement therapy in diabetic OVX females involves a RAGE-related mechanism. Targeting RAGE may restore the neuroprotective effect of E2 replacement therapy in diabetic females.
Keywords: transient forebrain ischemia, neutrophil, inflammation, diabetes mellitus
neuroprotective benefits of estrogen replacement therapy (ERT) have been established in multiple experimental ischemia models. However, the results of recent large-scale clinical studies have not supported the animal findings and even hinted that hormone replacement therapy may be detrimental (19). Indeed, we recently identified one circumstance where chronic ERT in ovariectomized (OVX) rats exacerbates ischemic brain damage-diabetes/chronic hyperglycemia (23). In nondiabetic rats, we have shown that one of the neuroprotective mechanisms of estrogen is to limit postischemic cerebral inflammation, as reflected by the extent of leukocyte adhesion/infiltration (21). On the other hand and in accord with neuropathological findings (23), in diabetic rats ERT is associated with a greater level of postischemic leukocyte activity in the brain, compared with that seen in untreated OVX females (32).
In the present study, we hypothesized that ERT-related exacerbation of postischemic inflammation in diabetic rats is linked to an increase in the presence of advanced glycation end products (AGE) and an enhanced function of the AGE receptor (RAGE). This derives from reports documenting chronic hyperglycemia-associated elevations in AGE (26), coupled with evidence that 17β-estradiol (E2) is capable of increasing RAGE transcription and protein expression (27). This may create an enhanced proinflammatory environment, which in the presence of an additional proinflammatory “hit” like ischemia-reperfusion, may lead to an amplification of inflammatory responses, such as increased neutrophil adhesion and infiltration. We therefore hypothesized that the blockade of RAGE would prevent the increased postischemic leukocyte activity, associated with ERT in diabetic OVX females, previously reported by us (32, 34). To that end, we examined the effects of RAGE knockdown, via antisense oligodeoxynucleotide (ODN) application, or RAGE blockade, using a RAGE decoy inhibitor, on postischemic leukocyte adhesion/infiltration in diabetic (streptozotocin injected) versus nondiabetic intact and OVX female rats with or without chronic ERT.
METHODS
Animals.
The study protocol was approved by the Institutional Animal Care and Use Committee. Three groups each of diabetic or nondiabetic female Sprague-Dawley rats (200–250 g at arrival) were used: intact, OVX, and E2-treated (0.1 mg·kg−1·day−1 ip for 7 days) OVX female rats (OVE). Ovariectomies were performed by the vendor (Charles River) 1 wk before shipment. Previous work from our laboratory demonstrated that the 0.1 mg/kg daily dose of E2 results in an average daily plasma E2 concentration that falls between the peak and nadir levels observed over the normal rat estrous cycle (31, 32). Streptozotocin (60 mg/kg iv) or vehicle was given at ∼2 wk following ovariectomy (see Ref. 34), and the rats were studied ∼6–8 wk later.
Surgical preparation and treatments.
In some rats, closed cranial windows were placed ∼48 h before the study. The procedure for “chronic” placement of cranial windows in experiments using topical applications of ODNs was described in a previous article (33). Aseptic techniques and hygienic postsurgical care were used to minimize the risk of infection resulting from chronic cranial window placement. Bupivacaine was applied locally at the wound site to provide analgesia during the early recovery period. At 48 h preceding the onset of ischemia, 300 μl of an artificial cerebrospinal fluid (aCSF) solution containing either 5 μM RAGE antisense (5′-AGCTACTGTCCCCGTTGG-3′) or 5 μM missense (5′-TCCATAGGCCTCTGTCGG-3′) ODN were injected into the space under the cranial window. That injection was repeated 24 h later. Six bases (3 at the 5′-end and 3 at the 3′-end) were phosphorothioated so as to minimize nuclease-mediated ODN breakdown. The specificity of the above rat RAGE antisense ODN sequence, which is identical to an ODN sequence previously reported to be selective and effective in knocking down RAGE expression (12), was confirmed by Basic Local Alignment Search Tool (BLAST) analysis. In experiments involving RAGE decoy inhibitor administration, 5 μl of a soluble RAGE (sRAGE) solution (84 μg/ml) were injected in anesthetized rats, via the intracerebroventricular route, through a burr hole (1.2 mm caudal to the bregma and 1.8 mm lateral to the sagittal suture at a depth of 4.0 mm) 24 h preceding ischemic onset. The controls received intracerebroventricular vehicle (aCSF) only. The cranial windows were prepared 24 h later. On the day of the study, the rats were anesthetized with isoflurane, followed by tracheotomy and mechanical ventilation. Paralysis was then induced with curare. During surgery, anesthesia was maintained with 1.2% isoflurane in 70% N2O-30% O2. The femoral arteries and veins were cannulated for blood sampling, arterial pressure monitoring, and drug infusions. Rectal temperature was servo-controlled at 37°C with a heating pad. The right common carotid artery was isolated, followed by insertion of a right subclavian venous catheter. In rats previously given intracerebroventricular injections of sRAGE or vehicle, the cranial windows were placed at this time. In the ODN-treated rats, the previously implanted cranial windows were reexposed. After the completion of surgery and window preparation, the isoflurane was discontinued and the rat was maintained on 70% N2O-30% O2-fentanyl (10 μg/kg initially; and 25 μg·kg−1·h−1 iv thereafter). The space under the window was suffused with 37°C aCSF that was equilibrated with 10% O2-5% CO2 with a balance of N2 (22).
Preparation of sRAGE.
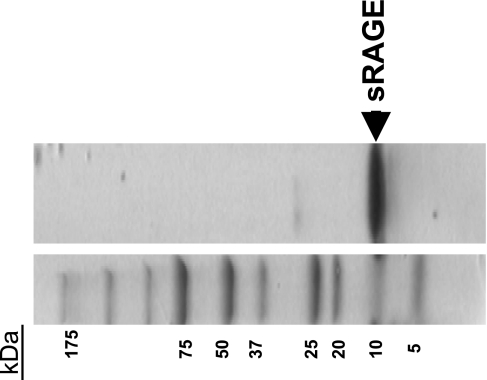
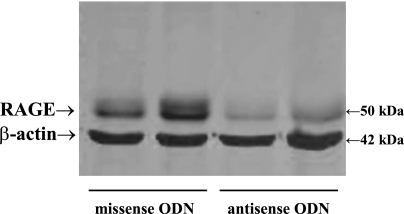
The sRAGE represents an amino acid sequence from the NH2-terminal variable domain, which contains the ligand-binding sites (5). The cDNA coding for the RAGE NH2-terminal AGE-binding domain (117–392 bp) was RT-PCR amplified from rat lung total RNA with a pair of primers: RAGE1 (5′-ATGGATCCAGCCCGGATCGAGAGGCCAC-3′) and RAGE2 (5′-GATTCGAATCTGGTAGACTCGGAGTC-3′). A 275-bp DNA, as predicted, was obtained and subsequently cloned into an Escherichia coli expression vector, pRSET B (Invitrogen), using BamHI and EcoRI cleavage sites, which were incorporated in the PCR primers. The sequence of pRSET-RAGE was confirmed by DNA sequencing (Research Resource Center, DNA facilities, UIC). The pRSET-RAGE DNA was transformed into Escherichia coli expression strain BL21-DE3, and the expression was induced by adding 0.2 mM isopropyl-β-d-thiogalactopyranoside. Large-scale expression was performed after the expression conditions were optimized. Endotoxin was removed using Detoxy-gel (Pierce, Rockford, IL). The complete sequence of the recombinant sRAGE used in the experiments (amino acids 28 through 106 of the full-length rat protein) was NH2-ARIGEPLMLSCKGAPKKPTQKLEWKLNTGRTEAWKVLSPQGDPWDSVARILPNGSLLLPAIGIVDEGTFRCRATNRLGKEVKSNYRVRVYQI-COOH, yielding a peptide with a molecular mass of ≅10 kDa. This is consistent with the literature (5) and was confirmed by Western blot analysis (Fig. 1). To that end, recombinant sRAGE was separated by 7.5–20% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The blot was blocked with 0.5× Odyssey blocking buffer for 1 h and then hybridized with anti-mouse/rat RAGE antibody (diluted 1 to 3,000 in the blocking buffer; R&D Systems, Minneapolis, MN) at 4°C overnight. After being washed several times with PBS plus 0.5% Tween 20 (PBST), the blot was hybridized with an anti-rat IgG antibody conjugated with infrared fluorescent dye (donkey anti-rat IgG IRDye 800 from Rockland Immunochemicals, Gilbertsville, PA, at 1 to 4,000 dilution in the blocking buffer). After the hybridization, the blot was washed three times with PBST and scanned at 800 nm on an Odyssey Infrared Image System from LI-COR Biotechnology (Lincoln, NE).
Fig. 1.
The soluble receptor for advanced glycation end products (RAGE) decoy inhibitor, sRAGE, synthesized in the present study using an Escherichia coli expression system, appears as a single ∼10-kDa band, representing the RAGE NH2-terminal variable (V) domain (Ref. 5). The V domain (sRAGE) protein contains the advanced glycation end product (AGE)-binding site.
Ischemia and leukocyte activity monitoring.
A 0.8-mm-diameter laser-Doppler flow probe (Perimed, Jarfälla, Sweden) was secured to the cranial window above the right parietal cortex, and baseline measurements (in perfusion units) were recorded. Right forebrain ischemia was produced by clamping the right common carotid artery, combined with blood withdrawal from the subclavian vein, to decrease cortical cerebral blood flow to 20% of baseline, as measured by laser-Doppler flowmetry. Reperfusion was established after 20 min in the diabetic and 30 min in the nondiabetic rats. The shorter ischemic duration used in the diabetic group arises from earlier observations of a greater postischemic lethality in diabetic versus nondiabetic animals when exposed to 30 min of transient forebrain ischemia (23, 31, 32). The 20-min ischemic duration in the diabetic rats is associated with a lethality rate in females (∼15%) that is similar to the rate seen following 30 min of ischemia in nondiabetic females, based on the average for intact, OVX, and OVE rats. Leukocytes were labeled with rhodamine 6G (200 μg/ml in 0.9% saline) given initially as an intravenous bolus (l ml) and followed by a continuous infusion at a rate of l ml/h (14, 20). The leukocyte activity of pial venules was monitored using a rhodamine-filtered Nikon microscope equipped with an epi-illumination darkfield system (33, 34). Images were captured using a digital video camera (CoolSNAP, Photometrics, Tucson, AZ) and saved for later analysis. In most cases, as done in previous studies from our laboratory (34), leukocyte adhesion was quantitated as the percentage of the viewed venular area occupied by adherent (nonmobile) intravascular rhodamine 6G-labeled leukocytes. In some animals, leukocyte infiltration (i.e., the presence of extravascular leukocytes) was observed. In those cases, the total area of intra- and extravascular leukocyte presence was expressed in relation to the viewed venular area. The difference between these values (i.e., total leukocyte presence) and the adherent (intravascular) leukocyte percentages at equivalent time points in each animal was then calculated. This provided us with an estimation of leukocyte extravasation/infiltration (see Ref. 34). In sham-operated ischemic, diabetic, and OVE females, we previously reported only negligible leukocyte adhesion (and no extravasation) during a 10–12-h observation period (35).
mRNA and protein analyses.
Samples of cortex from the three major diabetic study groups were obtained for analysis of RAGE mRNA and, as a control, β-actin mRNA, using real-time RT-PCR. We employed procedures similar to those described in earlier publications from our laboratory (6, 7). Relative mRNA concentrations were calculated from the takeoff points of the PCR reactions, using the comparative 2−ΔΔCT method (15). For RAGE, the forward primer sequence was 5′-TGGCACTTGGATGGGAAACCT-3′, and the reverse sequence was 5′-CCTTGGGCTGGGGTCACT-3′, with an expected product length of 128 bp. For β-actin, the forward primer was 5′-CCTGAAGTACCCCATTGAACA-3′, and the reverse sequence was 5′-CACACGCAGCTCATTGTAGAA-3′, which yielded a 92-bp product. Correct product synthesis was confirmed by agarose gel electrophoresis and melting curve analysis.
To complement the mRNA findings, cortical tissue was harvested for Western immunoblot analysis of RAGE abundance. We compared cerebral cortical RAGE protein expression in age-matched diabetic versus nondiabetic intact, OVX, and OVE female rats. Brain cortices of PBS-perfused rats were sonicated in ice in a lysis buffer with the following composition: 20 mM Tris·HCl, 0.4% SDS, 1 mM EDTA, 1 mM PMSF, 2% protease cocktail inhibitor (Sigma-Aldrich, St. Louis, MO), and 1 mM sodium orthovanadate. The homogenate was centrifuged at 100,000 g for 1 h, and the supernatant was collected for protein determination (via the Bradford method). Twenty micrograms of protein were loaded in each lane of a 10% SDS-PAGE gel. After transfer to a polyvinylidene difluoride membrane, the blots were blocked with 0.5× Odyssey blocking buffer for 40 min and incubated with anti-β-actin (0.5 μg/ml, mouse monoclonal from Sigma-Aldrich) and then hybridized with a donkey anti-mouse IgG secondary antibody conjugated with infrared fluorescent dye (IRDye 700 from LI-COR, at 1:10,000 dilution in the blocking buffer) and subsequently scanned at 700 nm using the Odyssey Imager. The blots were hybridized with anti-mouse/rat RAGE antibody (R&D, 1:500) overnight at 4°C and then 60 min with donkey anti-rat secondary infrared fluorescent antibody (800 nm, 1:5,000, Rockland) and scanned at 800 nm. After incubation with either primary or secondary antibodies, the membranes were washed four times for 5 min with PBST. Fluorescence was analyzed using the Odyssey software. The software settings relative to gain and background correction as well as antibody incubation times were kept identical to ensure comparability between different membranes. Immunoblots using α-tubulin as a housekeeping protein were also performed and produced results (data not shown) identical to those obtained when RAGE was expressed relative to β-actin. In a few instances, at the end of the experiments involving ODN treatments of OVE diabetic females, superficial cortical tissue samples were acquired from the exposed brain surface under the cranial windows (33), frozen, and stored at −80°C for later Western immunoblot analysis of RAGE expression (see above).
Finally, in diabetic and nondiabetic intact, OVX, and OVE females, brains were perfusion fixed (in buffered 4% paraformaldehyde) and paraffin embedded according to procedures described in an earlier article (31). Coronal sections (7 μm) were prepared for immunohistochemical analysis of carboxymethyllysine (CML), the most prevalent AGE (CML-AGE). A monoclonal anti-CML antibody (working concentration 2 μg/ml, clone 6D12, Research Diagnostics) was used. The second antibody was a donkey anti-mouse conjugated to Cy3 (from Jackson ImmunoResearch). Sections were viewed through a fluorescence microscope (Nikon), and a 0.35-mm2 region of the sensorimotor cortex (at ∼0–1 mm caudal to the bregma) was captured using a Spot 2 digital camera and the MetaMorph program. In some instances, higher magnification views were obtained. The images were captured under identical conditions of light exposure. The images were subsequently processed and analyzed semiquantitatively using the ImagePro Plus program (Media Cybernetics, Silver Spring, MD). Thus the captured images were converted to eight-bit gray scale to permit threshold binarization. For all images, the intensity threshold for subsequent counting was 200 (from a scale of 0–256), and only objects > 0.0015% of the viewed area were counted. The percentage of the viewed cortical area displaying CML-AGE immunoreactivity was then estimated.
Statistics.
For comparisons within a given experiment (total vs. intravascular leukocyte presence, blood gasses, pH, and mean arterial blood pressure), statistical analyses were performed using a two-way ANOVA with a post hoc Student-Newman-Keuls test for multiple comparison procedures. For statistical comparisons between intact, OVX, and OVE rats within a given subgroup (and a given treatment), a one-way ANOVA was used, with a post hoc Tukey analysis applied to compare responses at identical time points. That same analysis was used for comparisons of RAGE-to-β-actin ratios in Western immunoblots and between missense and antisense treatments or sRAGE and vehicle treatments at each time point within a specific rat group. SigmaStat (version 3.5; Richmond, CA) was used. A level of P < 0.05 was considered significant in all statistical tests. Values are presented as means ± SE. All drugs/chemicals were obtained from Sigma-Aldrich unless otherwise stated.
RESULTS
Arterial Po2 was maintained above 100 mmHg during the entire study in all animals. When comparing initial and end-reperfusion values, some modest reductions in mean arterial blood pressure were seen in most groups (mean reduction = 17%). The end-reperfusion arterial pH values were moderately lower than the preischemic value in most experimental groups (average decline = 0.03 units). With few exceptions, arterial Pco2 levels remained relatively constant when comparing preischemic to end-reperfusion values. Average body weights were similar in the diabetic and nondiabetic rats (∼280 g) with a trend toward higher body weights in untreated OVX females. In diabetic rats, the overall plasma glucose levels ranged from 22 to 35 mM with no differences among groups, whereas nondiabetic values were in the range of 6 to 10 mM.
CML-AGE expression.
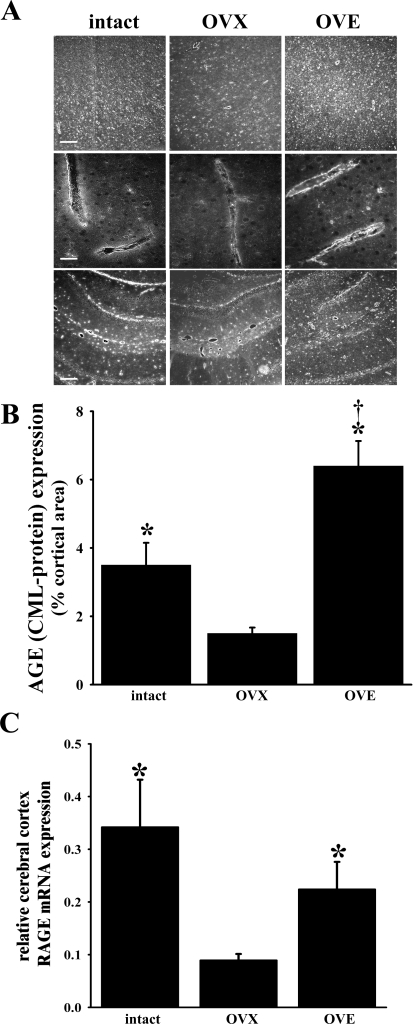
Regional cerebral expression of the RAGE ligand, AGE, was evaluated via immunohistochemistry, using an anti-CML-AGE antibody. In the present study, CML-AGE immunoreactivity in most brain regions was observed in all three diabetic groups. In Fig. 2A, representative photomicrographs of cortex at low (top) and high magnifications (middle) and hippocampus (bottom) are provided. It should be noted that CML-AGE expression was especially concentrated in and around blood vessels with an immunoreactivity pattern that appeared to be most intense in the intact and OVE groups, compared with the OVX group, and seemed to favor the endothelium, although we cannot exclude a perivascular astrocytic expression. The vascular expression is particularly evident in higher magnification views of cortical parenchymal microvessels (Fig. 2A, middle row). We performed a semiquantitative analysis of cortical CML-AGE immunoreactivity in the three diabetic groups (n = 6 to 7 for each), as well as the three nondiabetic groups (n = 5 to 7 for each). The results for diabetic rats are summarized in Fig. 2B. The percentage of the viewed cortical area displaying CML-AGE immunoreactivity in the diabetic animals was highest in the OVE group (6.4 ± 0.7%), intermediate in the intact females (3.5 ± 0.7%), and lowest in the non-ERT OVX group (1.5 ± 0.2%). Significant differences were observed between the OVE and the intact or OVX group. The difference between the intact and OVX groups was also significant. The same relative pattern of CML-AGE immunoreactivity among groups appeared to be present when viewing sections at the level of the hippocampus (Fig. 2A, bottom row). Modest CML-AGE immunoreactivity in age-matched nondiabetic female cortices was observed as 91.1 ± 0.1%, 1.1 ± 0.1%, and 1.0 ± 0.1% in intact, OVX, and OVE females, respectively (data not shown). However, when compared with their diabetic counterparts, significant differences were detected in OVE and intact, but not OVX, females. An omission of the primary antibody was accompanied by an absence of immunoreactivity in the above groups (not shown), indicating the lack of nonspecific fluorescence in the CML-AGE immunohistochemical analysis.
Fig. 2.
A: immunoreactivity of the AGE, carboxymethyllysine (CML) in the cortex (top and middle) and hippocampus (bottom) of intact, ovariectomized (OVX), and E2-treated OVX (OVE) diabetic females. Scale bars (representative of the entire row) = 100 μm (top and bottom) and 25 μm (middle). B: analysis of the relative expression of CML in the cerebral cortex of intact (n = 7), OVX (n = 6), and OVE (n = 6) diabetic females (represented by A, top). Results are presented as the percentage of the viewed cortical area expressing immunoreactivity. C: RAGE mRNA expression in brain tissue samples harvested from the cerebral cortical surface of intact, OVX, and OVE diabetic females (n = 3 in each group). Relative mRNA expression values were obtained via real-time PCR, using β-actin as an internal control. In each sample analyzed, the takeoff points (CT) were obtained for RAGE and β-actin. Relative mRNA expression was calculated using the 2−ΔΔCT method (15). For B and C, values are means ± SE. *P < 0.05 vs. OVX; †P < 0.05 vs. intact.
RAGE mRNA expression.
Real-time RT-PCR analysis of RAGE mRNA in brain tissue samples harvested from the cortex of diabetic rats revealed a level of RAGE expression in the OVX group that was significantly lower compared with the intact and OVE females (the latter 2 groups exhibiting roughly equivalent levels of expression; Fig. 2C).
RAGE protein expression.
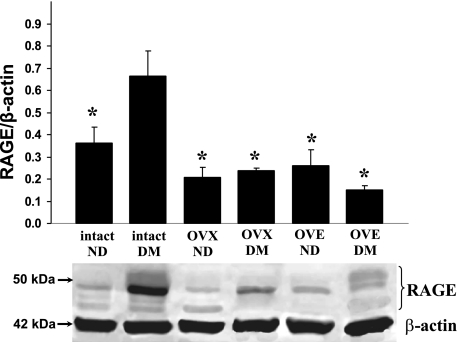
In cortical surface tissue harvested from diabetic females, Western immunoblot analyses of RAGE protein expression, relative to the expression of β-actin, in intact, untreated OVX, and OVE females revealed a relative pattern of abundance that had some similarities, but also differences, compared with the mRNA findings. Similar to the mRNA data, relative RAGE expression (Fig. 3) in the intact diabetic females was significantly higher (by nearly 3-fold) compared with untreated diabetic OVX rats. However, unlike the mRNA results, RAGE protein expression in diabetic intact females was significantly higher than that in the OVE group (by ∼4-fold). In addition, also in contrast to mRNA findings, relative RAGE expression in the diabetic OVE females was lower compared with the OVX females, although not significantly so. A significantly higher relative expression of RAGE (∼2-fold) was seen when comparing diabetic versus nondiabetic intact females (Fig. 3). Immunoreactivity of the loading control, β-actin, showed no appreciable variations among samples.
Fig. 3.
Representative Western immunoblots depicting RAGE (∼50-kDa bands) and β-actin (∼42-kDa bands) expression (bottom) along with RAGE band intensities expressed relative to β-actin (top) in samples obtained from the superficial cerebral cortex are shown. The groups are nondiabetic (ND) and diabetic mellitus (DM) intact female rats (n = 6 for both), ND and DM OVX female rats (n = 4 and 6, respectively), and ND and DM OVE rats (n = 5 and 6, respectively). For RAGE measurements, all bands between 45 and 55 kDa were included in the analysis. Values are means ± SE. *P < 0.05 vs. intact DM.
Postischemic leukocyte behavior: effects of RAGE antisense ODN.
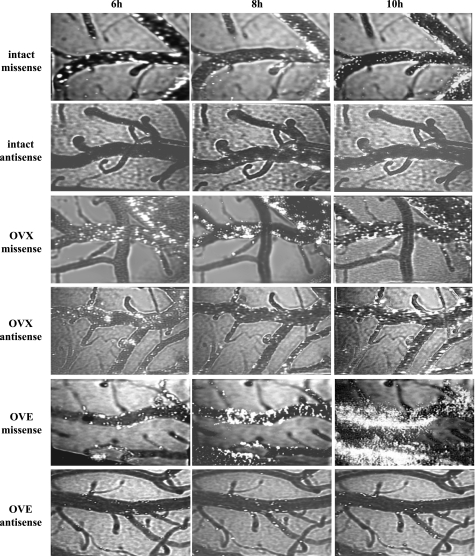
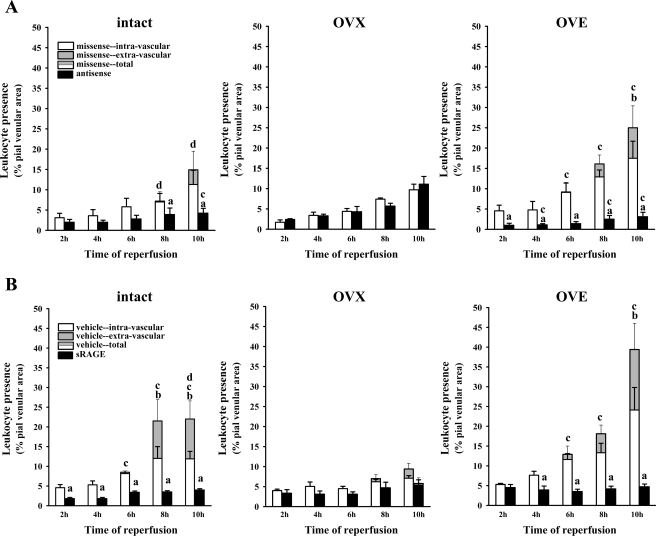
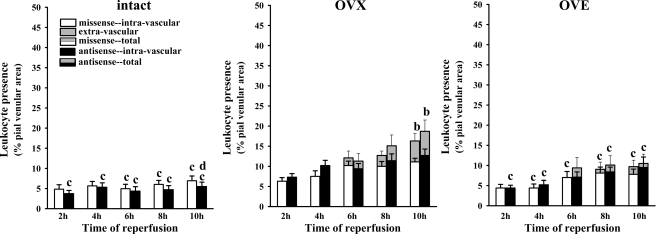
In presenting these data, as we have done in previous studies (34), both adherent intravascular and extravasated leukocytes are considered. In all cases, the percentages of adherent leukocytes in pial venules at the 2-, 4-, 6-, 8-, and 10-h reperfusion time points were measured (see methods). However, within the 6–10-h time interval, leukocyte extravasation was sometimes observed. Extravasated leukocytes were defined as fluorescent cells lying outside of the intravascular space. Evidence of leukocyte extravasation in diabetic females, when present, was detected only at time points ≥ 6 h and only in the missense-exposed, OVE, and, to a lesser extent, intact female rats. This is illustrated in representative frames captured from video recordings in the diabetic rat groups (Fig. 4). To provide a quantitation of leukocyte extravasation (when present), extravascular leukocyte presence was calculated by subtracting the intravascular leukocyte area (expressed as a percentage of the viewed venular area) from the total leukocyte area (also expressed as a percentage of the viewed venular area) at specific time points. No extravasation was seen in any of the rats treated with RAGE antisense ODN (Fig. 5A). Furthermore, RAGE knockdown was accompanied by marked reductions (70–90%, relative to the missense ODN-treated group) in postischemic leukocyte adhesion in the OVE females, but no reduction was seen in the OVX females without E2 supplementation. On the other hand, in the intact female group, the level of postischemic leukocyte adhesion was 50–60% lower at most time points in the antisense ODN-treated versus the missense ODN-treated group, although only at 8 and 10 h were the differences statistically significant. Western immunoblot analyses of superficial cortex tissue samples (largely composed of pial vessels, glia limitans, and some layer I cells) harvested from RAGE antisense and missense ODN-treated rats confirmed that the antisense ODN treatment was effective in reducing RAGE expression (Fig. 6). In nondiabetic females (Fig. 7), the only significant signs of extravasation were observed in OVX females at 8 and 10 h reperfusion. However, unlike the intact and OVE diabetic rats, no differential effect of antisense versus missense ODN treatments was observed in the nondiabetic OVX females, irrespective of whether extravasation was present. A more direct comparison of the influence of RAGE knockdown in diabetic versus nondiabetic intact, OVX, and OVE females is provided in Table 1. Here the data are expressed as the total leukocyte presence at 10 h reperfusion in antisense- relative to missense-treated rats. The only rats where significant reductions in total leukocyte presence were observed were the diabetic intact (70% reduction) and OVE (86% reduction) females. Thus the absence of any significant difference between the antisense versus the missense ODN treatments in diabetic OVX and all nondiabetic female groups would indicate an absence of RAGE influence on postischemic leukocyte behavior in these groups.
Fig. 4.
Representative images of postischemic leukocyte behavior at 6, 8, and 10 h reperfusion in and around pial venules in missense oligodeoxynucleotide (ODN) vs. antisense ODN-treated diabetic intact, OVX, and OVE rats. Note the markedly greater leukocyte presence and the increasing appearance of extravasated leukocytes in the missense-exposed rats given E2 replacement.
Fig. 5.
A: postischemic leukocyte behavior (over 2–10 h reperfusion), following 48 h exposure to topically applied RAGE antisense or missense ODN, in diabetic intact [n = 5 (antisense), and n = 4 (missense)], OVX [n = 6 (antisense), and n = 7 (missense)], and OVE [n = 7 (antisense), and n = 7 (missense)] females. The data are expressed as the percentage of the viewed venular area occupied by adherent (i.e., “nonrolling”) leukocytes present within the intravascular compartment [missense (white bars) or antisense (black bars)] or as the area percentage of leukocytes found extravascularly (gray-shaded portion of bars only, seen solely in missense-treated rats). Total leukocyte presence (intravascular + extravascular leukocytes) is represented by the combined height of the gray/white bars. The SE bars arising from the tops of the gray portions of the data bars relate to total leukocyte presence. The SE bars originating from the tops of the white data bar portions relate to intravascular leukocytes. Statistical comparisons (P < 0.05) at each time point: aintravascular adhesion-antisense vs. missense, btotal vs. intravascular leukocyte presence, cintact or OVE total missense or antisense vs. OVX, and dintact vs. OVE total leukocyte presence. B: postischemic leukocyte behavior at 24 h following intracerebroventricular injection of sRAGE or artificial cerebrospinal fluid vehicle in diabetic intact, OVX, and OVE females (n = 4 in all cases, except OVE + sRAGE, where n = 6). The data are expressed as the percentage of the viewed venular area occupied by adherent leukocytes present within the intravascular compartment [vehicle (white bars) or sRAGE (black bars)] or as the area percentage of leukocytes found extravascularly (gray-shaded portion of bars only, seen solely in vehicle-treated rats). Substituting “vehicle” for “missense” and “sRAGE” for “antisense,” SE bar descriptions and statistical symbol definitions are the same as in A.
Fig. 6.
Western immunoblot analysis of RAGE expression in pial tissue harvested from OVE diabetic female rats exposed to either missense ODN (lanes 1 and 2) or RAGE antisense ODN (lanes 3 and 4) for 48 h.
Fig. 7.
Postischemic leukocyte behavior (over 2–10 h reperfusion), following 48 h exposure to topically applied RAGE antisense or missense ODN in nondiabetic intact [n = 4 (antisense), and n = 4 (missense)], OVX [n = 5 (antisense), and n = 4 (missense)], and OVE [n = 6 (antisense), and n = 5 (missense)] females. The data are expressed as the percentage of the viewed venular area occupied by adherent leukocytes present within the intravascular compartment [missense (white bars) or antisense (black bars)] or as the area percentage of leukocytes found extravascularly (gray-shaded portion of bars). Total leukocyte presence (intravascular + extravascular leukocytes) is represented by the combined height of the gray/white or gray/black bars. For definition of symbols, see Fig. 5 legend. It should be noted that in all female groups, no significant differences in leukocyte behavior were observed when comparing antisense to missense ODN treatments at each time point. This is strongly suggestive of an absence of postischemic RAGE influence in nondiabetic rats, irrespective of hormone status.
Table 1.
Effect of RAGE knockdown or decoy inhibition via sRAGE on total (intravascular plus extravascular) leukocyte presence at 10 h reperfusion
| RAGE Knockdown (AS-ODN Relative to MS-ODN) | RAGE Decoy Inhibition (sRAGE Relative to Vehicle) | |
|---|---|---|
| Diabetic | ||
| Intact | ↓70%* | ↓81%* |
| OVX | ↑11%† | ↓38%† |
| OVE | ↓86%* | ↓88%* |
| Nondiabetic | ||
| Intact | ↓19%† | not tested |
| OVX | ↑14%† | not tested |
| OVE | ↑11%† | not tested |
RAGE, receptor for advanced glycation end products; sRAGE, soluble RAGE; AS, antisense, MS, missense; ODN oligodeoxynucleotide; OVX, ovariectomized; OVE, 17β-estradiol-replaced OVX.
P < 0.05 AS-ODN vs. MS-ODN or sRAGE vs. vehicle (diabetic and nondiabetic data derived from results summarized in Figs. 5 and 7, respectively).
Not significant.
Postischemic leukocyte behavior: effects of sRAGE.
Intracerebroventricular treatment of diabetic rats with the RAGE decoy inhibitor, sRAGE, compared with vehicle, yielded findings (Fig. 5B, and Table 1) similar to those seen in diabetic animals treated with antisense versus missense ODNs; that is, sRAGE versus vehicle administration was associated with a significantly lower postischemic leukocyte presence in both intact and OVE females and a complete prevention of leukocyte infiltration. Furthermore, similar to the experiments involving antisense ODN applications, no significant changes in leukocyte presence were observed in diabetic sRAGE- versus vehicle-treated OVX females (see Table 1). This too can be taken as evidence of a lack of RAGE influence in this group.
DISCUSSION
We previously reported that, in nondiabetic OVX female rats provided with chronic ERT, the level of pial venular leukocyte adhesion and infiltration following transient forebrain ischemia was much lower than that observed in the absence of ERT (21). However, in the diabetic rat, ERT had the opposite effect (32). The results of the present study suggested that this “transformation” in the diabetic rat was related to an increased contribution from RAGE. This was based on several pieces of evidence. First, a greater cerebral expression of a principal RAGE ligand, CML-AGE, favoring vascular/perivascular tissue, was seen in diabetic intact and OVE versus untreated OVX females. Second, this finding of elevated presence of ligand in diabetic intact or OVE females (relative to their untreated OVX counterparts) was paralleled by cerebral cortical RAGE mRNA expression data. However, that expression pattern was not mirrored by RAGE protein findings in the diabetic cerebral cortex, where, in relation to untreated OVX females, RAGE protein levels were only elevated in intact females but surprisingly not in OVE females. Third, RAGE knockdown, via antisense ODN applications, and RAGE blockade (using the decoy inhibitor, sRAGE) were accompanied by substantial reductions in postischemic leukocyte adhesion/infiltration in the OVE as well as in the intact diabetic group. In contrast, antisense ODN and sRAGE exposure were associated with no changes in postischemic leukocyte behavior in untreated OVX females. Finally, consistent with a lack of RAGE influence, the levels of postischemic leukocyte adhesion/infiltration in nondiabetic females were unaffected by RAGE antisense ODN applications, irrespective of hormone status.
Evidence from non-central nervous system tissues exposed to proinflammatory conditions indicates a link between RAGE activation and enhanced leukocyte adhesion and transendothelial migration (e.g., Ref. 4). In the present investigation, RAGE functional enhancement can be inferred under circumstances where RAGE knockdown and/or sRAGE treatment resulted in significant reductions in postischemic leukocyte activity. Accordingly, only intact and OVE diabetic females, but not diabetic OVX or any of the nondiabetic rats, were likely to have experienced enhanced RAGE function. This suggests an estrogen-related elevated postischemic RAGE activity in diabetic female brains.
How does one account for this apparent RAGE-potentiating effect of estrogen in the diabetic rat? One possibility would be that chronic hyperglycemia-linked factors [e.g., increased AGE levels (8, 13)] are coupled with an estrogen-associated enhanced expression of RAGE. The latter has some support in the literature, where it has been reported that prolonged exposure to E2, via a NF-κB-independent but Sp-1-dependent transcriptional mechanism, can elicit an increase in expression of RAGE message and protein in cultured vascular endothelial cells (17, 27). However, in the present study, although we did observe RAGE mRNA expression patterns in diabetic rats that appeared to correlate with the presence of estrogen (Fig. 2), RAGE protein levels only partially tracked these findings. Thus cortical RAGE protein expression in the diabetic intact females was roughly threefold greater than in their OVX counterparts, similar to mRNA findings. In contrast, OVE diabetic females did not display increased RAGE expression compared with untreated OVX females. Nevertheless, it should be noted that protein expression and function changing in opposite directions are not unprecedented in the diabetic brain. For example, in the diabetic rat cerebral vasculature, endothelial nitric oxide synthase expression is upregulated, although endothelial nitric oxide synthase-dependent vasodilating function is substantially impaired (29).
With respect to RAGE, there is a scenario whereby the RAGE protein expression one measures may not track RAGE function. Thus, whereas only the full-length RAGE is actively coupled to proinflammatory gene transcription, endogenous generation of sRAGE fragments, containing the external ligand-binding domain, can be generated and act as decoy blockers of RAGE function. The soluble forms, such as the proteolytically cleaved sRAGE or alternatively spliced endogenous-secreted form of RAGE (18), lack the short transmembrane and cytoplasmic domains and, as such, may differ in molecular mass with the full-length form by only a small margin. This results in the presence of multiple bands around 50 kDa. Since the RAGE immunoblot analysis did not consistently within a given group yield the same number of bands, we took the more conservative approach of including all of the (2–4) bands observed at the level of the 50-kDa marker, expressed relative to β-actin, for our RAGE protein analyses. Thus, in the presence of higher levels of endogenous sRAGE decoy inhibitors relative to full-length RAGE, one might anticipate reduced RAGE function. In contrast, a lesser relative presence of sRAGEs could be associated with a greater RAGE function (e.g., Ref. 1), possibly arising from elevated availability of the RAGE ligand, AGE. Consistent with its AGE binding, the decoy function, an inverse relationship between sRAGE and CML-AGE, has been reported in diabetic subjects (9). Perhaps the seemingly greater abundance of brain CML-AGE that we observed in estrogen-exposed (especially OVE) females reflects a lower endogenous sRAGE presence. However, because of a lack of definitive information regarding endogenous decoy inhibitor availability, issues related to endogenous inhibitory activity must remain unresolved.
In the present study, AGE and RAGE expression analyses were performed only in preischemic brains. The possibility exists that postischemic analyses may yield better correlations for a number of reasons. For example, increased cerebral RAGE expression has been reported in association with nondiabetic rodent ischemia models (10, 16, 35). Also, diabetic, as opposed to nondiabetic, rats are very likely to be exposed to higher levels of RAGE ligand (AGE), and an increased presence of ligand has been linked to an enhanced RAGE expression (e.g., Refs. 8 and 11). Thus, in the diabetic and estrogen-exposed preischemic brain, a neuropathological potential for RAGE may exist. This could act to prime the brain to respond to ischemia-reperfusion with a potentiation of RAGE activation and an exaggerated inflammatory response: the so-called “two hit” phenomenon described by Schmidt and coworkers (24). In the absence of a second hit, the brain may not experience dysfunction despite elevated levels of AGE and RAGE (see, for example, Ref. 28). The need for a double hit to produce RAGE-related brain damage could also explain why only the diabetic intact and OVE groups responded to RAGE-targeted interventions in the present study; that is, the absence of a treatment effect in the remaining groups (i.e., diabetic OVX and all nondiabetic animals) may relate to the fact that these rats only experienced the second-hit ischemia-reperfusion.
As one additional consideration, it should be noted that RAGE is a multiligand receptor that interacts with a number of non-AGE ligands, including S100 proteins (calgranulins), high-mobility group B1 protein (amphoterin), and leukocyte β2-integrins (2, 3). These ligands may have some relevance in the present study where leukocyte behavior is used as an indicator of RAGE activity; that is, RAGE has been proposed as an endothelial counterreceptor for the leukocyte β2-integrin Mac-1, high-mobility group B1 protein has been linked to enhanced neutrophil survival (30), and S100 proteins are constitutively expressed in leukocytes (25).
In conclusion, in accord with recent reports from our laboratory, E2 supplementation of diabetic OVX females is associated with an exacerbation of leukocyte/inflammatory activity in the postischemic brain. In the absence of any intervention, the magnitude of postischemic leukocyte adhesion/infiltration in diabetic females was OVE > intact >> OVX. In the presence of RAGE knockdown or exogenous sRAGE, both the intact and the E2-treated groups showed substantial reductions in adhesion and a complete absence of infiltration, whereas OVX rats were virtually unaffected by RAGE-targeted interventions. Application of RAGE antisense ODN had no effect on postischemic leukocyte behavior in nondiabetic females, irrespective of estrogen status. However, additional questions remain. This includes the divergent findings when comparing RAGE protein abundance and sensitivity to RAGE-related interventions in intact versus OVE diabetic females. To address this in future studies, one might consider examining whether the preischemic RAGE expression relationships we found carry over to the postischemic state, as well as exploring the possible influence of progesterone (which is present in the intact but lacking in the E2-treated animals) and other modulators of RAGE activity. Nevertheless, at a minimum, the current findings suggest that the RAGE effect on postischemic inflammation that we observed was unique to the diabetic state and strongly influenced by the presence of estrogen. Moreover, recent findings from our laboratory indicated that the specific blockade of postischemic blood to brain neutrophil extravasation in OVE diabetic females resulted in significant neuroprotection (34). Thus, since RAGE blockade prevents postischemic neutrophil extravasation in diabetic, estrogen-exposed females, we anticipate that RAGE blockade would also provide significant ischemic neuroprotection in these animals.
GRANTS
This study was supported by National Institutes of Health Grants DK-65629 and HL-52594.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Susan Anderson and Dennis Riley for expert technical assistance.
REFERENCES
- 1.Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis 196: 9–21, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med 83: 876–886, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Chavakis T, Bierhaus A, Al Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med 198: 1507–1515, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavakis T, Bierhaus A, Nawroth PP. RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect 6: 1219–1225, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Dattilo BM, Fritz G, Leclerc E, Kooi CW, Heizmann CW, Chazin WJ. The extracellular region of the receptor for advanced glycation end products is composed of two independent structural units. Biochemistry 46: 6957–6970, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dello Russo C, Gavrilyuk V, Weinberg G, Almeida A, Bolanos JP, Palmer J, Pelligrino D, Galea E, Feinstein DL. Peroxisome proliferator-activated receptor gamma thiazolidinedione agonists increase glucose metabolism in astrocytes. J Biol Chem 278: 5828–5836, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Gavrilyuk V, Dello Russo C, Heneka MT, Pelligrino D, Weinberg G, Feinstein DL. Norepinephrine increases I kappa B alpha expression in astrocytes. J Biol Chem 277: 29662–29668, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114: 597–605, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Grossin N, Wautier MP, Meas T, Guillausseau PJ, Massin P, Wautier JL. Severity of diabetic microvascular complications is associated with a low soluble RAGE level. Diabetes Metab 34: 392–395, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Hassid BG, Nair MN, Ducruet AF, Otten ML, Komotar RJ, Pinsky DJ, Schmidt AM, Yan SF, Connolly ES. Neuronal RAGE expression modulates severity of injury following transient focal cerebral ischemia. J Clin Neurosci 16: 302–306, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Herold K, Moser B, Chen Y, Zeng S, Yan SF, Ramasamy R, Emond J, Clynes R, Schmidt AM. Receptor for advanced glycation end products (RAGE) in a dash to the rescue: inflammatory signals gone awry in the primal response to stress. J Leukoc Biol 82: 204–212, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J Cell Biochem 81: 102–113, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Kaneko M, Bucciarelli L, Hwang YC, Lee L, Yan SF, Schmidt AM, Ramasamy R. Aldose reductase and AGE-RAGE pathways: key players in myocardial ischemic injury. Ann NY Acad Sci 1043: 702–709, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Lindauer U, Dreier J, Angstwurm K, Rubin I, Villringer A, Einhaupl KM, Dirnagl U. Role of nitric oxide synthase inhibition in leukocyte-endothelium interaction in the rat pial microvasculature. J Cereb Blood Flow Metab 16: 1143–1152, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Ma L, Carter RJ, Morton AJ, Nicholson LF. RAGE is expressed in pyramidal cells of the hippocampus following moderate hypoxic-ischemic brain injury in rats. Brain Res 966: 167–174, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee TK, Reynolds PR, Hoidal JR. Differential effect of estrogen receptor alpha and beta agonists on the receptor for advanced glycation end product expression in human microvascular endothelial cells. Biochim Biophys Acta 1745: 300–309, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J 22: 3716–3727, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 288: 321–333, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Santizo R, Pelligrino DA. Estrogen reduces leukocyte adhesion in the cerebral circulation of female rats. J Cereb Blood Flow Metab 19: 1061–1065, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Santizo RA, Koenig HM, Pelligrino DA. Estrogen and leukocyte adhesion following transient forebrain ischemia in rats. Stroke 31: 2231–2235, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Santizo RA, Xu HL, Galea E, Muyskens S, Baughman VL, Pelligrino DA. Combined endothelial nitric oxide synthase upregulation and caveolin-1 downregulation decrease leukocyte adhesion in pial venules of ovariectomized female rats. Stroke 33: 613–616, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Santizo RA, Xu HL, Ye S, Baughman VL, Pelligrino DA. Loss of benefit from estrogen replacement therapy in diabetic ovariectomized female rats subjected to transient forebrain ischemia. Brain Res 956: 86–95, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 108: 949–955, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepherd CE, Goyette J, Utter V, Rahimi F, Yang Z, Geczy CL, Halliday GM. Inflammatory S100A9 and S100A12 proteins in Alzheimer's disease. Neurobiol Aging 27: 1554–1563, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev 1: 1–15, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem 275: 25781–25790, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Thangthaeng N, Sumien N, Forster MJ. Dissociation of functional status from accrual of CML and RAGE in the aged mouse brain. Exp Gerontol 43: 1077–1085, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trauernicht AK, Sun H, Patel KP, Mayhan WG. Enalapril prevents impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in diabetic rats. Stroke 34: 2698–2703, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev 17: 189–201, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Santizo R, Baughman VL, Pelligrino DA. Estrogen provides neuroprotection in transient forebrain ischemia through perfusion-independent mechanisms in rats. Stroke 30: 630–637, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Xu HL, Baughman VL, Pelligrino DA. Estrogen replacement treatment in diabetic ovariectomized female rats potentiates postischemic leukocyte adhesion in cerebral venules. Stroke 35: 1974–1978, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Xu HL, Galea E, Santizo RA, Baughman VL, Pelligrino DA. The key role of caveolin-1 in estrogen-mediated regulation of endothelial nitric oxide synthase function in cerebral arterioles in vivo. J Cereb Blood Flow Metab 21: 907–913, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Xu HL, Salter-Cid L, Linnik MD, Wang EY, Paisansathan C, Pelligrino DA. Vascular adhesion protein-1 plays an important role in postischemic inflammation and neuropathology in diabetic, estrogen-treated ovariectomized female rats subjected to transient forebrain ischemia. J Pharmacol Exp Ther 317: 19–29, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Zhai DX, Kong QF, Xu WS, Bai SS, Peng HS, Zhao K, Li GZ, Wang DD, Sun B, Wang JH, Wang GY, Li HL. RAGE expression is up-regulated in human cerebral ischemia and pMCAO rats. Neurosci Lett 445: 117–121, 2008 [DOI] [PubMed] [Google Scholar]