Abstract
Vasovagal syncope may be due to a transient cerebral hypoperfusion that accompanies frequency entrainment between arterial pressure (AP) and cerebral blood flow velocity (CBFV). We hypothesized that cerebral autoregulation fails during fainting; a phase synchronization index (PhSI) between AP and CBFV was used as a nonlinear, nonstationary, time-dependent measurement of cerebral autoregulation. Twelve healthy control subjects and twelve subjects with a history of vasovagal syncope underwent 10-min tilt table testing with the continuous measurement of AP, CBFV, heart rate (HR), end-tidal CO2 (ETco2), and respiratory frequency. Time intervals were defined to compare physiologically equivalent periods in fainters and control subjects. A PhSI value of 0 corresponds to an absence of phase synchronization and efficient cerebral autoregulation, whereas a PhSI value of 1 corresponds to complete phase synchronization and inefficient cerebral autoregulation. During supine baseline conditions, both control and syncope groups demonstrated similar oscillatory changes in phase, with mean PhSI values of 0.58 ± 0.04 and 0.54 ± 0.02, respectively. Throughout tilt, control subjects demonstrated similar PhSI values compared with supine conditions. Approximately 2 min before fainting, syncopal subjects demonstrated a sharp decrease in PhSI (0.23 ± 0.06), representing efficient cerebral autoregulation. Immediately after this period, PhSI increased sharply, suggesting inefficient cerebral autoregulation, and remained elevated at the time of faint (0.92 ± 0.02) and during the early recovery period (0.79 ± 0.04) immediately after the return to the supine position. Our data demonstrate rapid, biphasic changes in cerebral autoregulation, which are temporally related to vasovagal syncope. Thus, a sudden period of highly efficient cerebral autoregulation precedes the virtual loss of autoregulation, which continued during and after the faint.
Keywords: syncope, cerebral blood flow, nonlinear, cerebral blood flow velocity
the 2004 task force on syncope (6) defined fainting as “a transient, self-limited loss of consciousness, usually leading to falling. The onset is relatively rapid, and subsequent recovery is spontaneous, complete, and usually prompt. The underlying mechanism is transient global cerebral hypoperfusion.” As the most common form of fainting, vasovagal syncope is considered to be mediated by systemic hypotension causing global cerebral hypoperfusion and resulting from peripheral vasodilation due to sympathetic withdrawal and vagal-induced bradycardia (15).
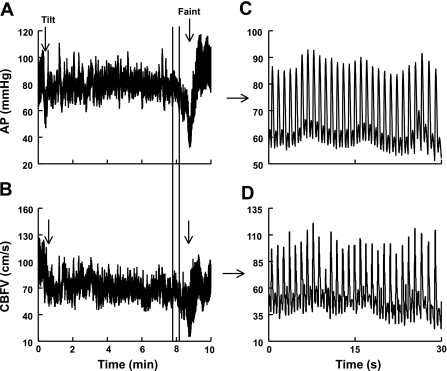
Previously, we (51) showed that hyperpnea and a decrease in end-tidal CO2 (ETco2) occur before vasovagal syncope and may play a role in the sympathoinhibition that accompanies syncope. Additionally, using multiresolution wavelet analysis, we (30) demonstrated a gradual decrease in systolic blood pressure (SBP), decrease in heart rate (HR), and increase in total peripheral resistance (TPR) that occurred well in advance of the faint (30). These changes may coincide with the increase in respiratory tidal volume and decrease in ETco2 that we measured. Thereafter, at the time of faint, there was a precipitous fall in HR, TPR, arterial blood pressure (AP), and cerebral blood flow (CBF). The decrease in CBF near faint is closely linked to the decrease in AP. Immediately before faint, oscillations in CBF appear to vary in synchrony (or “in phase”) with oscillations in AP, as shown in Fig. 1. Hence, while decreased ETco2 contributes to cerebral hypoperfusion at the time of fainting, the effective synchronization of AP and CBF means that a large decrease in blood pressure could result in an exaggerated decrease in CBF. This strong linkage between AP and CBF suggests a loss of autoregulation and is curious because it occurs before blood pressure reaches the reported lower threshold for autoregulatory failure (11, 17, 24). Similar decreases in CBF are typically absent in quadrupedal mammals during comparable hypotensive stress (9, 54).
Fig. 1.
Arterial pressure (AP) and cerebral blood flow velocity (CBFV) from a representative fainter. A: AP during tilt. B: CBFV during tilt. C: magnified AP between the marker lines in A before faint. D: magnified CBFV between the marker lines in B before faint. Immediately before faint, AP and CBFV become synchronized, and oscillations in AP are transferred to oscillations in CBFV.
The idea that cerebral autoregulation might fail during vasovagal syncope is not new, although it remains controversial in part because prior investigations have primarily used linear approaches, assuming stationary pressure and flow signals. Linear, steady-state relationships between AP and CBF velocity (CBFV) are often quantified by cross-correlation analyses in the time domain (10) or by equivalent transfer function analyses in the frequency domain (10, 58). Using transcranial Doppler ultrasound to monitor CBFV, a “paradoxic vasoconstrictive-type pattern” (20) was found in the cerebral vasculature before decreases in AP during syncope in adults and children (20, 50). This suggested that cerebral autoregulation was disrupted at the time of faint. However, much of this steady-state reduction in CBF has since been attributed to hypocapnia-induced vasoconstriction (7, 8, 56). Taking this into consideration, Schondorf et al. (41) used linear, stationary, time-independent transfer function analysis to quantify changes in cerebral autoregulation during syncope and concluded that autoregulation remained intact. On the other hand, Carey et al. (8) used the dynamic autoregulatory index and concluded that cerebral autoregulation deteriorates before and at the time of faint.
One concern with these analyses is that the changes in AP, HR, TPR, and CBF during orthostasis in fainters are nonlinear, time dependent, and nonstationary and, therefore, poorly amenable to linear, time-independent analyses (24). Giller and Mueller (17), who studied linear and nonlinear methods in healthy volunteers, concluded that CBF and CBFV were particularly nonlinear, highly nonstationary, and required alternate methods for quantification. Similarly, Panerai et al. (32) found that a nonlinear model of cerebral autoregulation was superior to linear models even during resting supine conditions in healthy subjects.
One particularly simple nonparametric technique of nonlinear analysis uses phase synchronization methods, rigorously defined as “the operation of oscillatory systems in unison such that their phases and frequencies closely relate” (37). When that occurs, the systems are said to be “phase locked” or “frequency entrained.” Phase synchronization techniques have been previously used by Latka et al. (24) to describe cerebral autoregulation at rest. Methods of phase synchronization are particularly appropriate to the study of AP and CBFV in vasovagal faint, where decreased synchronization corresponds to a gain in cerebral autoregulation (independence of CBFV and AP), whereas increased synchronization corresponds to a loss of autoregulation. On visual inspection, the time course of AP and CBFV are closely linked in fainting humans; therefore, we hypothesized that cerebral autoregulation fails during fainting, with the relationship between AP and CBFV demonstrating increased time-dependent phase synchronization.
METHODS
Subjects
To test our hypothesis, we studied 12 subjects with a history of vasovagal syncope (mean age: 17 ± 1 yr, 7 females and 5 males) and 12 healthy control subjects (mean age: 18 ± 1 yr, 6 females and 6 males). The age range of all subjects was 15–21 yr old. The study was approved by the Institutional Review Board of New York Medical College. Signed informed consent was obtained from all subjects. Syncopal subjects were referred to our center after at least three episodes of fainting during the last 6 mo. Syncopal subjects had previously undergone clinical evaluation, including a detailed history, physical examination, electrocardiography, and echocardiography, which ruled out other medical causes for their syncope. Control subjects were healthy volunteers who responded to advertisements or were selected from a database of volunteers and had no previous history of orthostatic intolerance.
Any subject with any systemic illness, including cardiac disease or other forms of orthostatic intolerance, competitive athletic training, recent pregnancy in the last 3 mo, or recent prolonged periods of bed rest were excluded from this study. The use of nicotine was also exclusionary. No subjects were on medications. All subjects refrained from caffeine or xanthine-containing substances for 72 h before the test.
Because prolonged upright tilt may invoke a fainting response in young healthy subjects, we limited the head-up tilt (HUT) testing to 10 min. We (30, 51) have previously demonstrated that this is a sufficient tilt time for the comparison of orthostatic changes between control and syncopal subjects. All subjects in the syncope group fainted during the 10-min HUT without any provocation, whereas no subjects in the control group fainted or experienced presyncopal symptoms.
Protocol
Testing began at 9:30 AM in a climate-controlled room at 70°F after a 12-h overnight fast. Subjects were familiarized with the procedure and instrumented for ECG, Finometer for continuous blood pressure monitoring, transcranial Doppler ultrasound sonography of the left middle cerebral artery (MCA), respiratory plethysmography, and capnography.
After a 30-min acclimation period, supine baseline measurements were recorded continuously for at least 5 min. After the supine measurements, subjects were tilted upright to 70° upright for a maximum of 10 min with continuous measurements. Upon fainting, subjects were lowered to the supine position. Vasovagal syncope was defined as a loss of consciousness and postural tone while upright with both a decrease in HR and a decrease in SBP to < 60 mmHg. Most faints immediately followed presyncopal symptoms, such as lightheadedness, nausea, or diaphoresis.
Detail of Methods
Time intervals.
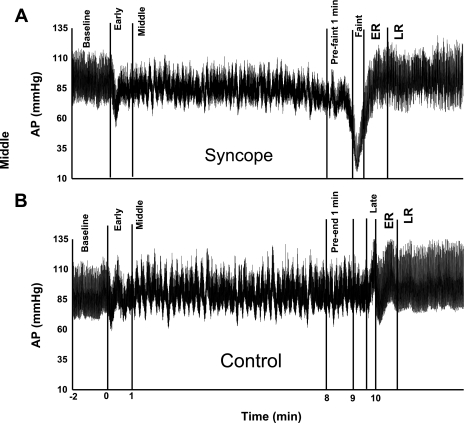
Because fainters do not exhibit the same temporal response to tilt, we (30, 51) have previous used time intervals to describe equivalent physiological events before fainting. Therefore, to allow for equivalent comparisons, we defined seven time intervals. The first interval was termed “baseline” and describes the time period before tilt when all subjects were under supine resting conditions. The second interval was termed “early” and describes the period between subjects being tilted upright until 1 min after tilt. Transient initial orthostatic hypotension often occurs in even healthy young volunteers during this period and has its own distinct physiology (57). Thus, this period is transient and unrelated to syncope and was not measured (52). The third interval was termed “middle” and describes the period of subjects being upright 1 min after tilt until 1 min before faint. Because subjects fainted at different times, the exact length of this interval varied with each subject. The fourth interval was termed “prefaint 1 min” and describes the period 1 min before faint until the onset of fainting. The fifth interval was termed “faint” and describes the period during the fainting episode (on average, this was 16 s). The sixth interval was entitled “early recovery” (ER) and describes a 1-min period after the faint after the tilt table being returned to the supine position. The seventh interval was termed “late recovery” (LR) and describes the 2-min period after ER. Figure 2A shows these time intervals for a representative syncopal subject.
Fig. 2.
Time intervals for 10-min head-up tilt (HUT) table testing. Note that time 0 is the beginning of HUT. A: set of time intervals for a representative fainting subject. Time intervals for the syncope group were as follows: baseline (supine conditions before being tilted upright), early (from the time of tilt until 1 min later), middle (from the previous point until 1 min before the faint), prefaint 1 min (1 min before the faint), faint [the time of faint until the return to the supine position (mean time of 16 s)], early recovery (ER; from the return to the supine position until 1 min later), and late recovery (LR; from the previous point until 2 min later). B: set of time intervals for a representative control subject. For the control group, the baseline, early, and middle time intervals were the same as in the syncope group. Preend 1 min, 1 min before returning to the supine position; late, the final 16 s of tilt until the return to the supine position. ER and LR time intervals were the same as in the syncope group.
Similar points were defined for control subjects based on the average time for each time interval in the syncope group. The first two intervals were identical for volunteers and syncopal subjects. Similarly, we excluded the “early” time interval from analysis, as stated above. The third interval (“middle”) was defined as the period of subjects being upright 1 min after tilt until minute 9 of the tilt. The fourth interval (“pre-end 1 min”) was defined as minutes 9–10 of the tilt. The fifth interval was called “late” and was defined as the last 16 s after minute 10 of tilt, corresponding to the “faint” time interval in the syncope group. The last two intervals were identical to those for syncopal subjects. Figure 2B shows the time intervals for a representative control subject.
HR and blood pressure monitoring.
A single lead ECG continuously monitored HR. AP was continuously monitored by a Finometer (Finometer, FMS, Amsterdam, The Netherlands) device, which used photoplethysmography of the right index or middle fingers calibrated to the brachial artery pressure. The Finometer has a height sensor and software that corrects for finger-to-heart distance. Pulse pressure (PP) was calculated by finding the difference between SBP and diastolic blood pressure (DBP). Mean AP (MAP) was calculated by the following equation: MAP = (1/3 × SBP) + (2/3 × DBP). MAP at the level of the head (MAPh) was estimated as MAP − 15 mmHg (11).
Respiratory and ETco2 monitoring.
Respiratory inductance plethysmography (Respitrace, NIMS Scientific, Miami Beach, FL) measured continuous respiratory frequency. ETco2 was measured continuously via a nasal cannula attached to a capnograph (Smith Medical PM, Waukesha, WI).
Transcranial Doppler and CBFV monitoring.
We monitored flow velocity of the left MCA continuously using a 2-MHz transcranial Doppler ultrasound system (Multigon, Yonkers, NY). The probe was placed over each subject's temporal window, the optimal signal and depth were acquired, and the probe was fixed at a constant angle by means of a custom headband. Pulse CBFV was calculated by finding the difference between systolic CBFV and diastolic CBFV. The cerebrovascular resistance index (CVRi) was estimated from MAPh and mean CBFV as follows: CVRi = MAPh/mean CBFV.
CO2 reactivity was calculated at the percent change in mean CBFV per change in ETco2. This calculation was based on the average expired value of ETco2 during each time interval and the percent change in mean CBFV from baseline during that interval.
Phase Synchronization Index Methods
Phase synchronization, also known as phase locking, is a well-established concept in nonlinear dynamics: two or more oscillators, each with its own dominant frequency, become entrained or cosynchronous, causing the difference in phase between the oscillators to become nearly constant; the oscillator frequencies, therefore, become equal (37). The phase difference need not be zero to have phase synchronization. A nonzero constant difference in phase viewed in the time domain corresponds to a time lag between the oscillators. Phase synchronization requires small interactions between the independent oscillators (33). This is distinct from generalized synchronization, in which large interactions produce a scaled amplitude and a constant phase difference in both signals, which, therefore, operate identically {i.e., S1(t) = f[S2(t)], where S1(t) is one signal and f[S2(t)] is the function (f) of another signal} (37).
Phase synchronization can be analyzed by diverse methods and quantified by a number of indexes (26, 34). Analyses may be linearly based and time invariant, as exemplified by linear cross-correlation or cross-covariance (coherence based) systems, or nonlinear. In general, linear, time-invariant indexes are less well suited to nonlinear and nonstationary signals, as occur during fainting. Nonlinear measures include information or entropy-based measures, nonlinear interdependencies, or methods that use estimates of instantaneous phase, as used here (34).
Scalar time-dependent quantities, such as AP and CBFV, have no intrinsic phase, although the appearance of well-defined oscillations in some signals leads one to infer a measure of phase. The concept of phase requires the concept of complex numbers and is not obvious in the study of real-valued signals. Thus, a complex extension of the real-valued function needs to be constructed. One way that this can be accomplished is to use a complex filter approach such as continuous wavelets (24). An alternative approach, which we used, is discussed further in the appendix. If a (co)sinusoid oscillation is detected, it is taken to be the real part [Acos(ωt + ϕ)] of the complex function Acos(ωt + ϕ) + iAsin(ωt + ϕ), where A is the amplitude, ω is the angular frequency (equal to 2πf), t is time, ϕ is a real constant, and i is the imaginary number. This formula is known as the analytic extension of the cosine function and is differentiable at every point in the complex plane. The formula can also be expressed in polar form as the Euler relation Aei(ωt + ϕ), where (ωt + ϕ) is referred to as the phase and ei(ωt + ϕ) is 2π periodic. This can be generalized to represent any time-dependent complex function as A(t)eiΦ(t), where A(t) is now a time-dependent amplitude and Φ(t) is the time-dependent phase. The analytic function concept represents a way to generalize any signal to the complex plane, thus defining phase. Thus, given that a real signal can be expressed as f(t) = A(t)cosΦ(t), the imaginary part can be constructed as the “analytic extension” of f(t) using the Hilbert transform (see the appendix) (4, 10). Thus, real and imaginary parts are constructed, yielding a computation of analytic phase Φ(t). For this to make intuitive sense, the signal must oscillate around zero. Thus, very low-frequency contributions to the signal are detrended. We used a Butterworth bandpass filter. Each filtered time domain signal was examined and oscillated around zero. We imposed a preprocessing step by bandpass filtering the CBF and AP signals from 0.01 to 0.5 Hz so that phase trajectories encircle the origin and oscillations derived from the cardiac cycle are excluded. Included, then, is only a relatively narrow range of low-frequency oscillations contained in blood pressure and CBF signals while excluding periodic HR changes and 0 Hz. Signals were then resampled at 2 Hz.
Hilbert transform and preprocessing steps were imposed on both AP and CBFV to yield Φ(t)AP and Φ(t)CBF. Instantaneous frequencies can be obtained from the derivatives of the phases. However, during periods of interest during which the slopes of Φ(t)AP and Φ(t)CBF were nearly constant, we found it most practical to obtain frequencies in a least-squared sense. Phase differences {ΔΦ(t) = [Φ(t)AP − Φ(t)CBFV]} are constructed as shown in Fig. 3A, in which each phase is unwrapped, allowing for phases greater than ±2π. This results, however, in identical values of eiΔΦ(t). Regions in the time domain, where phase differences are nearly constant (“flat”), correspond to phase locking with entrained frequencies, as shown in Fig. 3B. For completely uncoupled oscillators, the distribution of ΔΦ(t) is uniform [see Fig. 3C, where the distribution of the unwrapped ΔΦ(t) is shown as a histogram]. For more highly coupled oscillators, the distribution is sharply peaked, as shown in Fig. 3D. A more quantitative perspective for discrete time systems can be obtained by summing sequential values of eiΔΦ(t) over N time intervals, which creates a straight line in the complex plane if ΔΦ(t) is constant (completely synchronized) and, otherwise, a random walk in the complex plane if each ΔΦ(t) is entirely random (completely unsynchronized). This can be used to define a phase synchronization index (PhSI) of γ = <[eiΔΦ(t)]> = 1 for complete synchronization (and a single frequency peak) or γ = <[eiΔΦ(t)]> = 0 for complete lack of synchrony, where the vertical lines signify absolute value, and the symbols < > signify time average (34). The index can be more easily calculated using the following equivalent formula:
which is the specific synchronization index used throughout.
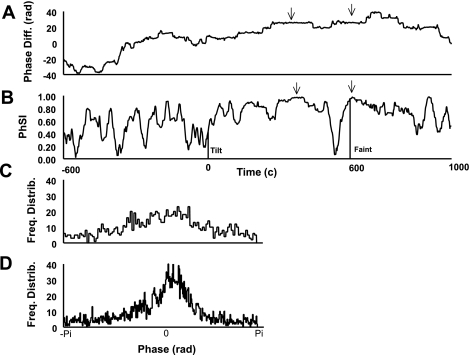
Fig. 3.
A: phase difference [Diff; in radians (Rad); ΔΦ] between the phases for AP and CBFV {ΔΦ(t) = [Φ(t)AP − Φ(t)CBFV]}. Note the flat regions (two examples denoted by arrows), which represent a constant phase difference (or phase locking) over a time period. Also, note the “bumps,” which represent oscillations of 2π due to noise and have no mathematical effect on phase difference. B: phase synchronization index (PhSI) for a representative syncopal subject during HUT. Note the areas of maximum phase (as denoted by the arrows), which are at equivalent time periods as the flat phase-locked regions in A. This demonstrates that the maximum PhSI corresponds to phase locking, whereas a lack of phase locking occurs at the minimum PhSI. Time 0 is the beginning of tilt. The vertical line next to “tilt” and “faint” indicate the time at which each event occurred. C: the representative frequency distribution (Freq Distrib) for phase shows a relatively uniform distribution, as seen in uncoupled oscillators. D: the representative frequency distribution for phase shows a sharply peaked distribution, as seen in highly coupled oscillators.
Data Analysis and Statistics
Data were sampled at 200 Hz, digitalized, stored in a computer, and analyzed offline. Two investigators, who were blind to the subject group, independently validated each time interval and reached similar conclusions. SPSS version 13 was used for analysis. Data are expressed as averages over each time interval. Independent, two-tailed Student's t-test compared demographic data between groups. ANOVA for repeated measures compared changes between and within each group during each time interval, with a post hoc Bonferroni comparison used if there was significance. All values are presented as means ± SE. Significance was set at P < 0.05.
RESULTS
Subject Demographics
There were no statistical differences in age (18 ± 1 vs. 19 ± 1 yr), height (163 ± 3 vs. 168 ± 2 cm), weight (63 ± 3 vs. 65 ± 2 kg), or sex between the syncope and control groups.
Time Intervals
Time intervals are shown for a representative syncopal and control subject in Fig. 2. The average duration of each time interval in the syncope and control groups did not differ; specifically, the baseline interval (syncope group: 306 ± 18 s vs. control group: 312 ± 24 s), early interval (syncope group: 60 ± 0.1 s vs. control group: 60 ± 0 s), middle interval (syncope group: 454 ± 69 s vs. control group: 468 ± 12 s), prefaint/pre-end 1 min interval (syncope group: 60 ± 0 s vs. control group: 60 ± 0 s), faint/late interval (syncope group: 15.8 ± 0.4 s vs. control group: 16.1 ± 0.1 s), ER interval (syncope group: 60 ± 0.1 s vs. control group: 60 ± 0 s), and LR interval (syncope group: 120 ± 0.1 s vs. control group: 115 ± 2.5 s) were not statistically different. The average time of tilt for the syncope group was 590 ± 24 s and that for the control group was 604 s ± 8 s. The average time to faint in the syncope group was 574 ± 26 s.
HR and Blood Pressure
Table 1 shows average values for SBP, DBP, MAP, PP, HR, and R-R interval (RRI). During baseline, there were no statistical differences between the syncope and control groups. During the middle interval, the syncope group had a higher HR than controls (P < 0.05). During the prefaint 1 min interval, the syncope group had a lower SBP (P < 0.01) and a lower MAP (P < 0.05). During the faint interval, the syncope group had a lower SBP (P < 0.001), DBP (P < 0.001), MAP (P < 0.001), PP (P < 0.001), HR (P < 0.01), and RRI (P < 0.01). During the ER interval, the syncope group had a lower SBP (P < 0.01), MAP (P < 0.05), and PP (P < 0.01). HR was similar in both groups during early recovery. During the LR interval, there were no statistical differences in AP or HR between groups.
Table 1.
Measurement responses during each time interval
| Baseline |
Middle |
Pre-End/Prefaint 1 Min |
Late/Faint |
ER |
LR |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurement | Control Group | Syncope Group | Control Group | Syncope Group | Control Group | Syncope Group | Control Group | Syncope Group | Control Group | Syncope Group | Control Group | Syncope Group |
| Systolic blood pressure, mmHg | 123±3 | 119±3 | 119±3 | 120±3 | 116±4 | 102±3† | 116±4 | 69±4‡ | 117±3 | 103±3† | 120±3 | 116±3 |
| Diastolic blood pressure, mmHg | 64±2 | 64±3 | 67±3 | 72±2 | 66±3 | 59±2 | 65±3 | 34±3‡ | 63±3 | 59±2 | 64±2 | 66±2 |
| Mean arterial pressure, mmHg | 84±2 | 82±3 | 84±3 | 88±2 | 82±3 | 74±2* | 82±3 | 46±3‡ | 81±3 | 74±2* | 83±2 | 82±2 |
| Pulse pressure, mmHg | 59±3 | 54±3 | 53±3 | 48±3 | 51±3 | 43±3 | 51±3 | 36±2‡ | 54±3 | 44±2† | 56±2 | 50±2 |
| Heart rate, beats/min | 65±3 | 70±3 | 86±4 | 101±5* | 89±4 | 101±8 | 88±4 | 64±7† | 70±3 | 74±4 | 62±2 | 65±4 |
| R-R interval, ms | 951±43 | 890±37 | 717±35 | 645±33 | 698±40 | 644±36 | 702±39 | 1093±121† | 895±38 | 862±32 | 993±39 | 956±44 |
| Respiratory frequency, breaths/min | 16±0.5 | 17±0.7 | 15±0.8 | 20±0.7‡ | 15±0.7 | 19±1† | 16±0.6 | 22±3* | 15±5 | 20±2* | 16±0.6 | 19±2 |
| End-tidal CO2, mmHg | 43±0.9 | 42±0.6 | 40±1 | 40±0.7 | 39±1 | 36±1 | 40±1 | 34±1† | 43±1 | 39±1† | 43±0.8 | 41±0.9* |
| Mean CBFV (cm/s) | 69±4 | 74±5 | 62±4 | 64±4 | 61±4 | 49±4* | 61±4 | 39±4‡ | 65±4 | 61±5 | 66±4 | 68±5 |
| Systolic CBFV (cm/s) | 108±6 | 111±7 | 97±5 | 98±7 | 95±5 | 86±6 | 95±6 | 94±11 | 106±5 | 110±10 | 107±5 | 103±7 |
| Diastolic CBFV (cm/s) | 47±3 | 50±4 | 44±3 | 45±3 | 42±3 | 31±2† | 43±3 | 17±2‡ | 43±3 | 37±3 | 44±3 | 47±5 |
| Pulse CBFV (cm/s) | 61±3 | 61±3 | 53±3 | 53±4 | 53±3 | 46±5 | 52±3 | 78±10* | 64±3 | 73±6 | 63±3 | 56±3 |
| Cerebrovascular resistance index, mmHg·cm−1·s−1 | 1.26±0.09 | 1.19±0.11 | 1.16±0.10 | 1.22±0.12 | 1.18±0.12 | 1.30±0.15* | 1.17±0.12 | 0.90±0.13 | 1.06±0.10 | 1.09±0.14 | 1.09±0.11 | 1.05±0.09 |
| CO2 reactivity, %/mmHg | N/A | N/A | 12±2 | 10±1 | 14±3 | 21±2* | 13±3 | 30±4‡ | 1±2 | 13±3† | 0.36±1 | 8±4 |
Values are means ± SE. While upright, fainters exhibited a decline in blood pressure, end-tidal CO2, and cerebral blood flow velocity (CBFV), whereas heart rate and respiratory frequency increased. ER, early recovery; LR, late recovery; N/A, not applicable.
P < 0.05,
P < 0.01, and
P < 0.001 compared with the control group.
Respiratory Frequency and ETco2
During baseline, there were no differences in respiratory frequency or ETco2 between groups. As shown in Table 1, the syncope group had a greater respiratory frequency than the control group at the middle interval (P < 0.001), prefaint 1 min interval (P < 0.01), faint interval (P < 0.05), and ER interval (P < 0.05).
Because baseline ETco2 values may vary between subjects, we report both group mean absolute values and group mean percent change from baseline. Group mean ETco2 values were lower for the syncope group than the control group during the faint interval (P < 0.01), during the ER interval (P < 0.01), and during the LR interval (P < 0.05). The syncope group demonstrated a greater percent change in ETco2 than the control group at the prefaint 1 min interval (syncope group: −15 ± 1% vs. control group: −9 ± 2%, P < 0.05), faint interval (syncope group: −20 ± 2% vs. control group: −8 ± 2%, P < 0.001), and ER interval (syncope group: −9 ± 2% vs. control group: −0.7 ± 1%, P < 0.01).
CBFV
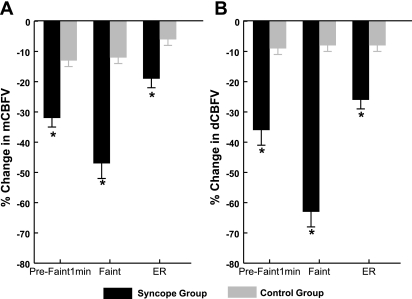
Because baseline CBFV values may vary between subjects, we report both group mean absolute values and group mean percent change from baseline. Mean values are shown in Table 1. Mean CBFV was significantly lower in the syncope group than the control group at the prefaint 1 min (P < 0.05) and faint (P < 0.001) intervals. As shown in Fig. 4A, the percent change in mean CBFV was significantly greater in the syncope group than the control group at the prefaint 1 min, faint, and ER intervals (P < 0.001). Absolute values for systolic CBFV were not different between groups, although the percent change was significant at the prefaint 1 min interval (syncope group: −21 ± 4% vs. control group: −11 ± 2%, P < 0.05). Diastolic CBFV was significantly reduced in the syncope group compared with the control group at the prefaint 1 min (P < 0.01) and faint (P < 0.001) intervals. As shown in Fig. 4B, the percent change in diastolic CBFV was different between the syncope and control groups at the prefaint 1 min, faint, and ER intervals (P < 0.001).
Fig. 4.
A: percent change in mean CBFV (mCBFV). During the prefaint 1 min time interval, the syncope group had a 32 ± 3% decrease in mCBFV compared with the 13 ± 2% decrease in the control group. At the faint time interval, the syncope group had a 47 ± 5% decrease in mCBFV compared with the 12 ± 2% decrease in the control group. At the ER time interval, the syncope group had a 19 ± 3% decrease in mCBFV compared with the 6 ± 2% decrease in the control group. B: percent change in diastolic CBFV (dCBFV). During the prefaint 1 min time interval, the syncope group had a 36 ± 5% decrease in dCBFV compared with the 9 ± 2% decrease in the control group. At the faint time interval, the syncope group had a 63 ± 5% decrease in dCBFV compared with the 8 ± 2% decrease in the control group. At the ER time interval, the syncope group had a 26 ± 3% decrease in dCBFV compared with the 8 ± 2% decrease in the control group. *P < 0.001 compared with the control group.
Absolute values for CVRi are shown in Table 1, and no baseline differences were found between groups. Because of the use of mean CBFV in the calculation, CVRi was converted to the percent change from baseline for analysis at each time interval. During the middle interval, syncopal subjects increased CVRi from baseline by 24 ± 4% versus the 8 ± 2% decrease in controls (P < 0.02). During the prefaint 1 min interval, syncopal subjects increased CVRi from baseline by 37 ± 9% versus the 6 ± 3% decrease in controls (P < 0.01). At the late interval of tilt, control subjects exhibited a decrease in CVRi of 7 ± 2%. At the faint interval, syncopal subjects exhibited two different types of responses. In one group (n = 4), CVRi increased from baseline by 31 ± 13% (P < 0.02 compared with controls), whereas in another group (n = 8), CVRi decreased by 27 ± 6% (P < 0.001 compared with controls). The change in CVRi was not different between either group during the ER and LR intervals.
While the CO2 reactivity could not account for the change in mean CBFV for the syncope group at the prefaint 1 min (predicted: 21 ± 2% decrease vs. actual: 32 ± 3% decrease, P < 0.01) and faint (predicted: 30 ± 4% decrease vs. actual: 47 ± 5% decrease, P < 0.01) intervals, the CO2 reactivity accounted for the change in mean CBFV for the control group at all intervals.
PhSI
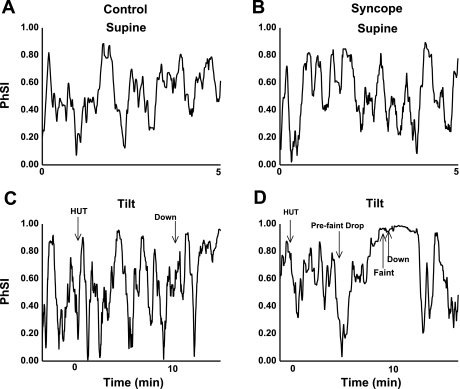
As shown in Fig. 5, A and B, phase synchronization oscillated between nearly zero (unsynchronized) and nearly 1 (synchronized) in both groups during supine resting conditions. This was an unexpected and novel finding. Table 2 shows mean group PhSI values. Mean PhSI values were the same for the syncope and control groups during the baseline period. At the prefaint/preend 1 min interval, the syncope group had a higher mean synchronization (P < 0.05) and a higher maximum synchronization (P < 0.001) compared with the control group. At the faint interval, the phase synchronization of the syncope group all approached maximum, whereas the control group maintained normal oscillations (P < 0.001). At the ER interval, the syncope group had a higher mean synchronization compared with the control group (syncope group: 0.79 ± 0.04 vs. control group: 0.64 ± 0.04, P < 0.01). The high phase synchronization stayed near maximum for the syncope group after the return to the supine position for an average of 60 ± 5 s (see Fig. 5D for a representative syncopal subject). Again, no such pattern was seen in control subjects (see Fig. 5C for a representative control subject).
Fig. 5.
PhSI. A: synchronization for a representative control subject during resting supine conditions. B: synchronization for a representative syncopal subject during resting supine conditions. C: synchronization for a representative control subject during 70° HUT. D: synchronization for a representative syncopal subject during 70° HUT. Note that in C and D, time 0 is defined as the beginning of HUT. During resting supine conditions, the control and syncope groups did not differ. During HUT, control subjects had oscillations in phase synchronization similar to baseline. In the syncope group, fainters demonstrated a prefaint drop in synchronization followed by a steady increase to maximum synchronization at faint (as noted by the arrows in D). This maximum synchronization was stable and maintained after fainters returned to the supine position. “Down” denotes the return to supine conditions.
Table 2.
Phase synchronization index during each time interval
| Baseline |
Middle |
Pre-End/Prefaint 1 Min |
Late/Faint |
ER |
LR |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Group | Syncope Group | Control Group | Syncope Group | Control Group | Syncope Group | Control Group | Syncope Group | Control Group | Syncope Group | Control Group | Syncope Group | |
| Mean | 0.58±0.04 | 0.54±0.02 | 0.63±0.02 | 0.58±0.02 | 0.58±0.03 | 0.73±0.04* | 0.55±0.06 | 0.92±0.02‡ | 0.64±0.04 | 0.79±0.04† | 0.65±0.05 | 0.64±0.04 |
| Minimum | 0.12±0.04 | 0.09±0.01 | 0.12±0.03 | 0.09±0.02 | 0.30±0.06 | 0.45±0.08 | 0.41±0.06 | 0.85±0.04‡ | 0.33±0.06 | 0.51±0.10 | 0.27±0.08 | 0.26±0.05 |
| Maximum | 0.91±0.03 | 0.90±0.02 | 0.95±0.01 | 0.94±0.01 | 0.84±0.02 | 0.95±0.02‡ | 0.69±0.05 | 0.97±0.01‡ | 0.91±0.03 | 0.96±0.01 | 0.92±0.02 | 0.92±0.02 |
Values are means ± SE. During the faint, arterial pressure and CBFV became highly synchronous in syncopal subjects, whereas control subjects maintained relatively stable variation in phase synchronization throughout the tilt.
P < 0.05,
P < 0.01, and
P < 0.001 compared with the control group.
Before faint and based on visual inspection, every subject in the syncope group demonstrated a sharp decrease in PhSI from 0.74 ± 0.07 to 0.17 ± 0.04. This occurred on average 119 ± 16 s before faint (as shown for a representative subject in Fig. 5D). Immediately after this sharp decrease, PhSI sharply increased and remained elevated at the time of faint in all syncopal subjects, similar to that shown in Fig. 5D. No such pattern was seen in control subjects, who maintained oscillations similar to baseline oscillations in PhSI throughout upright tilt, similar to that shown in Fig. 5C.
DISCUSSION
Main and Novel Findings
The presence of complete synchronization between AP and CBFV implies the absence of autoregulation. Our data demonstrate a rapid biphasic change of autoregulation, which is induced by and temporally related to vasovagal syncope. Approximately 2 min before fainting, syncopal subjects have a sudden rapid decrease in phase synchronization between AP and CBFV followed by a prolonged increase in phase synchronization linking AP and mean CBFV. The 2-min period corresponds to a period of increased cerebral autoregulation, which is followed by a virtual loss of autoregulation during the faint and into ER. During this period of autoregulatory failure, CBFV appears to be linked to blood pressure as if a linear passive resistance relationship existed between the two. This fulfills criteria for phase synchronization and may also fulfill criteria for generalized synchronization, as explained earlier. Cerebral autoregulation in fainters does not recover immediately upon becoming supine and roughly parallels the time course of clinical recovery, which can be prolonged after fainting.
Our data also show that phase synchronization is a dynamic quantity even under resting conditions. At rest, autoregulation is not a constant, as shown in Fig. 5; rather, it is intermittent and variable, as suggested by Giller and Mueller (17) and Latka et al. (24) We postulate that during the period of AP-CBF asynchrony, CBF is tightly controlled by neuronal activities and brain metabolism and unrelated to AP, whereas during the period of synchronization, CBF is determined by AP alone. Thus, synchronization unlinks CBF from cerebral metabolic control. We further propose that AP and CBF are two quasistable attractors of a noisy chaotic nonlinear system. These nonlinear interactions results in bistable conditions of synchrony and asynchrony, which can be affected by baroreceptor unloading, as occurs during orthostasis.
Strictly speaking, phase synchronization, as measured here, is related to dynamic autoregulation. Thus, AP and CBFV data are passed through a bandpass filter as part of a preprocessing step, which removes slow changes in AP and CBFV due to very-low-frequency and 0-Hz data points as well as HR dependency. However, when examined separately, mean and diastolic CBFV slowly fall during the tilt and at the faint (see Fig. 4), whereas systolic CBFV appears to be better sustained. This fall in mean CBFV and diastolic CBFV parallels a similar slow decrease in AP and implies a decrease in “static” autoregulation as well.
Does Hypocapnia Account for Reduced CBFV?
The change in ETco2 during tilt cannot account for all of the reduction in CBFV during the prefaint 1 min and faint intervals when synchronization is maximum, although the effects of CO2 are still important and may account for a portion. A 3.5% reduction in CBFV per mmHg change in ETco2 is expected (22, 23). For example, during the faint interval, mean CBFV decreased 47 ± 5% (see Fig. 4A), whereas the CO2 reactivity accounted for a 30 ± 4% decrease.
We measured CO2 reactivity based on the average change in expired ETco2 associated with the percent change in mean CBFV, and spontaneous breathing pattern changes and tilt-related respiratory mechanisms may influence the outcome (44). Furthermore, ETco2 may misrepresent arterial Pco2 by ∼3 mmHg (5) and lead to an overcalculation of our CO2 reactivity. Additionally, we speculate that decreasing ETco2 potentiates the declining AP and CBFV and increases CVRi, contributing to cerebral hypoperfusion and faint.
We speculate that CO2 itself may be able to alter synchronization, and this will be investigated in future experiments using controlled CO2. Our speculation is based on previous work by Aaslid et al. (1, 2), who found that hypocapnia augments cerebral autoregulation. Also, Ainslie et al. (3) concluded that hyperventilation may directly influence dynamic autoregulation. Furthermore, Mitsis et al. (27) stated that CO2 effects on cerebral autoregulation are highly nonlinear and need to be modeled as such.
CBF and Autoregulation During Syncope
The literature suggests that the decrease in CBFV appears to precede the decrease in AP in syncopal subjects (11). Supporting this, Hughson et al. (21) described a phase lead of CBFV over AP but concluded it to be due to the physiology of resistance changes in the cerebrovasculature. It would appear that cerebrovasoconstriction occurs in syncopal subjects before fainting. Assuming linear passive resistor pressure-flow relations, one could state that the CVRi steadily increases until fainting. Increased resistance decreases CBF and leads to cerebral hypoperfusion. Grubb et al. (20) first reported this “paradoxical response” in fainting adults, and Sung et al. (50) reported it in fainting children. They both suggested that a deregulation in cerebral autoregulation may occur. On the other hand, Diehl et al. (12) studied syncope and found increased cerebrovascular resistance but intact autoregulation before faint. Similarly, Serrador et al. (47) found decreased CBFV and increased cerebrovascular resistance in association with orthostatic intolerance during HUT after a parabolic flight. They concluded that cerebral hypoperfusion was not related to changes in autoregulation. Thus, it is controversial whether cerebral autoregulation is affected during syncope. Differences in methods and the use of linear measurements may account for these discrepancies.
In many studies (11, 46, 56, 58) about cerebral autoregulation, CBF changes often precede AP changes in both time and frequency domain analyses, suggesting a directional relationship of change transferring from CBF to AP. One explanation requires a change in the causal paradigm such that changes in CBF convey information to the brain that potentially alters AP. This new paradigm involves a “hemoneural hypothesis,” which suggests that CBF may directly influence neural activity and hemodynamic responses (28). Additional recent evidence supporting this idea comes from Sirotin and Das (49), who demonstrated the brain's ability to preemptively increase CBF before anticipated neural activity. Alternatively, the order reversal can be explained within the nonlinear framework by assuming bidirectional coupling, which enables the phenomenon of “anticipatory synchronization” (42, 43). An analysis of coupling direction is possible but is beyond the scope of the current work.
Our results are supported by Carey et al. (8), who used the dynamic autoregulatory index and concluded that cerebral autoregulation decreased immediately before and during the time of faint. On the other hand, our results contrast with those of Schondorf et al. (41), who used linear transfer function analysis to report that dynamic cerebral autoregulation was maintained during presyncope in adults. This may be closer to our own results than seems evident in that synchronization index analyses show that autoregulation is intact until shortly before fainting. However, excluding the time interval during which AP and CBFV decreases obscured appreciation for the loss of autoregulation. Also, during tilt, Schondorf et al. (41) showed a high coherence at a frequency of ∼0.1 Hz that decreased at presyncope. This decreased linear coherence may be analogous to the decrease in PhSI, which we measured. We (31) have previously related low coherence to efficient autoregulation and high coherence to less efficient autoregulation (31). However, low coherence can be caused by noise, and its use as a measure of autoregulation is often avoided on this basis. Synchronization indexes have no such limitations in that noise of sufficient amplitude shows up as “phase slips,” in which there is a multiple of 2π change in phase (33). These slips have absolutely no effect on our phase index because they depend on sine and cosine terms, which are unaffected by 2π changes.
Beyond the presyncope period, PhSI shows that autoregulation steadily drops off and syncope ensues. Transfer function analysis is not able to accurately reflect this behavior because fainting is nonlinear and strongly time dependent. Although the present study only included subjects who fainted within 10 min of tilt, we have applied the methods to a set of young syncopal patients (n = 4) who had longer tilts lasting up to 30 min until fainting was reached (data not shown). These subjects demonstrated similar patterns in phase synchronization before faint [baseline PhSI: 0.55 ± 0.03, prefaint PhSI: 0.71 ± 0.03, a sharp decrease in PhSI (0.18 ± 0.02) seen immediately before faint, and faint PhSI: 0.94 ± 0.02], as described above. Therefore, we believe the pattern described of strong autoregulation followed by near complete loss of autoregulation may be typical of young individuals developing vasovagal syncope as syncope approaches during longer durations of HUT.
Utility of the Phase Synchronization Index as an Index of Cerebral Autoregulation
There is no single unique way to introduce the complex number-based notion of phase into a real signal. While Hilbert transform approaches are common (26), continuous wavelet approaches have also been used. For example, Latka et al. (24) used the wavelet approach to describe phase dynamics as a way to model cerebral autoregulation. Our mean PhSI values for control subjects at rest were very similar to Latka et al.'s data, and, indeed, the two methods in general yielded similar results.
Why Phase Synchronization?
Phase synchronization may be described as the frequency entrainment of two weakly interacting oscillators, independent of their amplitudes (37). Typically, autonomous oscillators operate on a limit cycle, such that for any change in amplitude due to a discomposure, the amplitude returns to its prior original value (19). While it is difficult to alter the amplitude of this cycle, it is typically easy to change phase. Thus, in the physiological realm, oscillations in AP often directly affect CBF oscillations. This is the antithesis of cerebral autoregulation, where, by definition and within limits, CBF is maintained relatively constant regardless of AP. This shows up as the absence of a relationship between amplitudes and phases of CBFV and AP and is believed to be the result of independent flow control by myogenic, metabolic, and perhaps autonomic control, to a lesser degree.
If we consider AP and CBFV initially as independent oscillators operating on limit cycles, then large external forces (such as seen during forced changes in blood pressure during lower body negative pressure) will almost completely synchronize the phase and amplitude of AP and CBFV. This describes a state of generalized synchronization and only occurs with very strong coupling perturbing all limit cycle geometry in both amplitude and phase. Under autoregulatory conditions, coupling is weak, the intrinsic cycling geometry is unaffected, and the smaller coupling signal serves only to move one or both oscillators along the limit cycle in such a way that they become tuned to operate at some fixed phase difference and fixed frequency, such as occurs here (i.e., phase synchronization). Failure of phase synchronization between AP and CBFV is due to cerebral autoregulation (17, 24). When phase differences lock, i.e., become nearly constant, changes in AP and CBF are directly transferred and no effector (no cerebral autoregulation) is acting to modulate CBF.
Phase synchronization has been studied in other physiological systems. Examples include research on cardiac rhythms (55), circadian rhythms (18), cardiorespiratory interactions (36, 39, 40), and visual sensory neuroscience (14, 48). Neurological disease states, such as epilepsy (38), are related to oscillatory synchronization between different parts of the brain and were initially thought to be autonomous oscillators. Additionally, changes in intracranial pressure may synchronize with changes in AP and provide a new area for research (25). On the other hand, synchronization of neuron groupings may help to perform complex integrated actions (53), and its absence may lead to excessive oscillatory behavior. As a time-dependent measure of autoregulation, phase synchronization between AP and CBF may be a useful tool to help better understand the control mechanisms that influence autoregulation.
Limitations
Modeling of cerebral autoregulation using PhSI is only a representation of what may actually occur in the brain. We do not intend our index to be all encompassing. Rather, we developed it as a time-dependent, nonlinear, and nonstationary measure of changes between the oscillations in AP and CBFV that assumes autoregulation to be efficient when synchronization is low. We are also aware that it is not the only index of phase synchronization that may be used. Prior work has suggested that all such indexes yield similar results (24).
The use of time intervals enables a comparison of events with similar physiology rather than an identical time course. As best we could, we used time intervals with equivalent durations for both control and syncopal subjects. This should allow equal quanta of data to be analyzed and compared.
The HUT time was limited to 10 min because extended upright periods may cause healthy subjects to produce false positive faints (51). Although this may make our results most applicable to those who faint within 10 min of being upright, similar patterns may be seen in those who faint after more extended periods of tilt.
Trancranial Doppler was used to measure CBFV rather than CBF. Changes in CBFV correlate with changes in CBF as long as the diameter of the MCA does not change. Studies (13, 45) have found that the MCA diameter is stable during orthostasis and is not affected by changing levels of CO2. Also, the MCA may not be the most representative cerebral artery for the study of syncope. Practical considerations dictated its use, but the future development of new probes and probe-holding devices may permit the continuous interrogation of the basilar arteries and other cerebral arteries of interest.
The reported abrupt fall in the PhSI before faint was based on visual inspection of the signals rather than a more objective analysis. While this may induce bias, we believe that it did not, because all the syncope subjects displayed this unique pattern before faint. Thus, it appears that this pattern is unique to syncope phase synchronization.
Our calculations of CO2 reactivity were based on expired ETco2 values during tilt. Other influences, such as spontaneous breathing pattern changes or tilt-related mechanisms, may affect this calculation. We are limited by not having standardized the CO2 reactivity calculation by using inspired CO2. This may have lead to an overcalculation of CO2 reactivity (5).
We used a correction factor of 15 mmHg for the hydrostatic gradient from the heart to the brain based on Dan et al. (11). We failed to measure the distance between the heart and transcranial Doppler ultrasound probe in our subjects before testing and, therefore, could not use the standard correction factor of 0.7788 mmHg/cm. However, since height does not change within an individual when upright, this does not change the conclusions or temporal patterns.
AP was measured beat to beat using finger photoplethysmography. This method assumes that changes in finger pressure are representative of changes in the rest of the body.
Additionally, we implicitly assumed that intracranial pressure does not change during the course of the experiments. This is not accurate in that orthostasis changes cerebral spinal fluid and intracranial pressure.
We were unable to fully describe the effects of changing ETco2 on phase synchronization between AP and CBFV. As far as we are aware, no one has studied these effects, and further research is required.
Summary
Using phase synchronization as a nonlinear, time-dependent measure of cerebral autoregulation, we have demonstrated that in individuals who faint within 10 min of tilt, phase synchronization between AP and CBFV is decreased (implying increased autoregulation) 2 min before faint and then steadily increases (implying decreased autoregulation) at the faint. This high synchronization is maintained for about a minute after the return to the supine position. The data indicate a failure in cerebral autoregulation at faint.
APPENDIX
As stated previously, real scalar time-dependent quantities, such as AP and CBFV, have no intrinsic phase, which is a quantity that appropriately belongs to the complex plane. Clearly, phase can be introduced in any number of ways, and one of the best known ways is by filtering or transforming the signal. Thus, for example, Fourier transforms readily yield f = 0 symmetric complex transforms of real-valued functions in the frequency domain. Such methods can be extended to nonlinear time-dependent signals by using more versatile filtering with wavelets (24). However, a consistent way to introduce and define phase of any real-valued function of continuous time is to use the analytic signal concept of Gabor (16). The approach is based on the Hilbert transform. The analytic extension of the signal is denoted z(t) = y(t) + i>y˜(t), where the following equation is the Hilbert transform of the original real-valued function:
where z is the analytic extension of the signal in the complex plane, y is the original scaler signal in the real plane, y˜ is the Hilbert transformation of the signal, P is the Cauchy principle value, and τ is the time displacement. This transform is just the convolution of y(t) with 1/t; therefore, it emphasizes the local properties of the analyzed signal, or
where the symbol * denotes the conventional convolution integral.
Thus, by the convolution theorem, the Fourier transform of y˜(t) is Y˜ = −(i sgn f)Y, where Y is the Fourier transform of the original function, sgn = 1 for f > 0 and −1 for f < 0, and f is frequency.
When reassembled, the Fourier transform of the constructed analytic signal [Z(f) = (1 + sgn f)Y(f)], which is just zero for f < 0 and twice Y(f) for f > 0. Inverting the Fourier transform generates the analytic extension, which is a fast and easy way to generate the analytic signal corresponding to the initial real-valued signal. For example, if y(t) = Acos(ωt + ϕ), then z(t) computes as Acos(ωt + ϕ) + iAsin(ωt + ϕ), which corresponds exactly to our notion that the cosine is the real value of z.
Euler's formula, eix = cos(x) + isin(x), as demonstrated in the 18th century by the application of infinite series, can then be used to express z(t) as Aei(ωt + ϕ) for the simple example. In general, where A and x may be a function of time [say, x(t) = Φ(t) and A = A(t)], we obtain the following equation:
where A is the amplitude, Φ(t) is the phase, and eiΦ(t) is 2π periodic, which was calculated using the Euler formula and Hilbert transform in combination.
Hilbert transform and preprocessing steps were imposed on both AP and CBFV to yield Φ(t)AP and Φ(t)CBF. Instantaneous frequencies can be obtained from the derivatives of the phases. Phase differences {ΔΦ(t) = [Φ(t)AP − Φ(t)CBFV]} were constructed. Regions in the time domain, where phase differences are nearly constant (“flat”), correspond to phase locking with entrained frequencies (see Fig. 3, A and B).
Over the range of our bandpass filter, both CBFV and AP can behave as approximately nonlinearly coupled autonomous oscillating systems. Such systems typically follow a limit cycle, which is geometrically unaltered by relatively weak interactions. Thus, amplitudes are relatively unaffected, as would be the case of general synchronization, whereas phases are relatively easily changed, such that systems may advance or retard their timing over the limit cycle in synchrony, leading to phase synchronization. The specifics of the oscillatory phenomena need not be specified for phase synchronization to occur; the only requirements are nonlinearity, autonomous oscillatory properties, and relatively weak interactions. In general, such dynamic systems demonstrate n:m synchronization, in which [nΦ(t)1 − mΦ(t)2] are about constant (where n and m are positive integers, such that n oscillations of variable 1 occur during m oscillations of variable 2). Such phenomena occur in cardiopulmonary synchronization (29, 35, 36). Given the phasic nature of the cardiac cycle and its relation to both blood pressure and blood flow, 1:1 coupling is expected and assumed. Thus, for AP − CBFV interactions over the passband of interest, phase synchronization occurs when ΔΦ(t) = [Φ(t)AP − Φ(t)CBFV] is nearly a constant over, say, N time intervals, as shown in Fig. 3, A and B. Averaging eiΔΦ(t) over N time intervals yields a convenient index of synchronization because, by the Euler formula, eiΔΦ(t) represents a unit vector in the complex plane expressed in polar coordinates. If the angle ΔΦ(t) is nearly constant for each time interval (i.e., there is phase synchronization), then vector summation result in a total distance from the origin of approximately N × 1. This distance divided by the number of intervals (N) is ∼1, the state of complete synchronization. Otherwise, if individual ΔΦ(t) are randomly related (completely unsynchronized), a random walk is traced out in the complex plane.
Thus, we defined a PhSI of γ = <[eiΔΦ(t)]> = 1 for complete synchronization (and a single frequency peak) or γ = <[eiΔΦ(t)]> = 0 for complete lack of synchrony, where the vertical lines signify absolute value and the symbols < > signify time average (34). Using the Euler formula, eiΔΦ(t) = cos[ΔΦ(t)] + isin[ΔΦ(t)] and <[eiΔΦ(t)]> = <cos[ΔΦ(t)]> + i<sin[ΔΦ(t)]>. Taking the modulus or absolute value of the complex quantity results in the following more computationally convenient form:
which is the specific synchronization index used throughout.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-074873 and 1-R01-HL-087803, American Heart Association Grant 0735603T, and a grant from the Chronic Fatigue and Immune Deficiency Syndrome Association of America.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. Newman, Dr. Gewitz, and the members of the Division of Pediatric Cardiology for the continued support. The authors thank our mentors Dr. Hintze and Dr. Kaley for the frequent inspiration. The authors also thank Ryan Aguinaldo for the assistance regarding the literature.
REFERENCES
- 1.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke 20: 45–52, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Aaslid R, Newell DW, Stooss R, Sorteberg W, Lindegaard KF. Assessment of cerebral autoregulation dynamics from simultaneous arterial and venous transcranial Doppler recordings in humans. Stroke 22: 1148–1154, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Ainslie PN, Celi L, McGrattan K, Peebles K, Ogoh S. Dynamic cerebral autoregulation and baroreflex sensitivity during modest and severe step changes in arterial Pco2. Brain Res 1230: 115–124, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bendat JS, Piersol AG. Random Data Analysis and Measurement Procedures. New York: Wiley-Interscience, 1971 [Google Scholar]
- 5.Bjurstedt H, Hesser CM, Liljestrand G, Matell G. Effects of posture on alveolar-arterial CO2 and O2 differences and on alveolar dead space in man. Acta Physiol Scand 54: 65–82, 1962 [DOI] [PubMed] [Google Scholar]
- 6.Brignole M, Alboni P, Benditt DG, Bergfeldt L, Blanc JJ, Bloch Thomsen PE, van Dijk JG, Fitzpatrick A, Hohnloser S, Janousek J, Kapoor W, Kenny RA, Kulakowski P, Masotti G, Moya A, Raviele A, Sutton R, Theodorakis G, Ungar A, Wieling W. Guidelines on management (diagnosis and treatment) of syncope–update 2004. Europace 6: 467–537, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Carey BJ, Eames PJ, Panerai RB, Potter JF. Carbon dioxide, critical closing pressure and cerebral haemodynamics prior to vasovagal syncope in humans. Clin Sci (Lond) 101: 351–358, 2001 [PubMed] [Google Scholar]
- 8.Carey BJ, Manktelow BN, Panerai RB, Potter JF. Cerebral autoregulatory responses to head-up tilt in normal subjects and patients with recurrent vasovagal syncope. Circulation 104: 898–902, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chen RY, Fan FC, Schuessler GB, Simchon S, Kim S, Chien S. Regional cerebral blood flow and oxygen consumption of the canine brain during hemorrhagic hypotension. Stroke 15: 343–350, 1984 [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Hu K, Stanley HE, Novak V, Ivanov PC. Cross-correlation of instantaneous phase increments in pressure-flow fluctuations: applications to cerebral autoregulation. Phys Rev E Stat Nonlin Soft Matter Phys 73: 031915, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dan D, Hoag JB, Ellenbogen KA, Wood MA, Eckberg DL, Gilligan DM. Cerebral blood flow velocity declines before arterial pressure in patients with orthostatic vasovagal presyncope. J Am Coll Cardiol 39: 1039–1045, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Diehl RR, Linden D, Chalkiadaki A, Diehl A. Cerebrovascular mechanisms in neurocardiogenic syncope with and without postural tachycardia syndrome. J Auton Nerv Syst 76: 159–166, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Djurberg HG, Seed RF, Evans DA, Brohi FA, Pyper DL, Tjan GT, al Moutaery KR. Lack of effect of CO2 on cerebral arterial diameter in man. J Clin Anesth 10: 646–651, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Engel AK, Konig P, Gray CM, Singer W. Stimulus-dependent neuronal oscillations in cat visual cortex: inter-columnar interaction as determined by cross-correlation analysis. Eur J Neurosci 2: 588–606, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Fenton AM, Hammill SC, Rea RF, Low PA, Shen WK. Vasovagal syncope. Ann Intern Med 133: 714–725, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Gabor D. Theory of communication. J IEEE (Lond) 93: 429–457, 1946 [Google Scholar]
- 17.Giller CA, Mueller M. Linearity and non-linearity in cerebral hemodynamics. Med Eng Phys 25: 633–646, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Glass L, Mackey M. From Clocks to Chaos: the Rhythms of Life. Princeton, NJ: Princeton Univ. Press, 1988 [Google Scholar]
- 19.Glass L. Synchronization and rhythmic processes in physiology. Nature 410: 277–284, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Grubb BP, Gerard G, Roush K, Temesy-Armos P, Montford P, Elliott L, Hahn H, Brewster P. Cerebral vasoconstriction during head-upright tilt-induced vasovagal syncope. A paradoxic and unexpected response. Circulation 84: 1157–1164, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Hughson RL, Edwards MR, O'Leary DD, Shoemaker JK. Critical analysis of cerebrovascular autoregulation during repeated head-up tilt. Stroke 32: 2403–2408, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Ide K, Eliasziw M, Poulin MJ. Relationship between middle cerebral artery blood velocity and end-tidal Pco2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol 95: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Kastrup A, Thomas C, Hartmann C, Schabet M. Sex dependency of cerebrovascular CO2 reactivity in normal subjects. Stroke 28: 2353–2356, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Latka M, Turalska M, Glaubic-Latka M, Kolodziej W, Latka D, West BJ. Phase dynamics in cerebral autoregulation. Am J Physiol Heart Circ Physiol 289: H2272–H2279, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Lemaire JJ, Khalil T, Cervenansky F, Gindre G, Boire JY, Bazin JE, Irthum B, Chazal J. Slow pressure waves in the cranial enclosure. Acta Neurochir (Wien) 144: 243–254, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Lo MT, Hu K, Liu Y, Peng CK, Novak V. Multimodal pressure flow analysis: application of Hilbert Huang transform in cerebral blood flow regulation. EURASIP J Appl Signal Processing 2008: 785243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitsis GD, Poulin MJ, Robbins PA, Marmarelis VZ. Nonlinear modeling of the dynamic effects of arterial pressure and CO2 variations on cerebral blood flow in healthy humans. IEEE Trans Biomed Eng 51: 1932–1943, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Moore CI, Cao R. The hemo-neural hypothesis: on the role of blood flow in information processing. J Neurophysiol 99: 2035–2047, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mrowka R, Cimponeriu L, Patzak A, Rosenblum MG. Directionality of coupling of physiological subsystems: age-related changes of cardiorespiratory interaction during different sleep stages in babies. Am J Physiol Regul Integr Comp Physiol 285: R1395–R1401, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Nowak JA, Ocon A, Taneja I, Medow MS, Stewart JM. Multiresolution wavelet analysis of time-dependent physiological responses in syncopal youths. Am J Physiol Heart Circ Physiol 296: H171–H179, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ocon A, Medow MS, Taneja I, Clarke D, Stewart JM. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 297: H664–H673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panerai RB, Dawson SL, Potter JF. Linear and nonlinear analysis of human dynamic cerebral autoregulation. Am J Physiol Heart Circ Physiol 277: H1089–H1099, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Pikovsky A, Rosenblum M, Kurths J. Synchronization: a Universal Concept in Nonlinear Sciences. Cambridge: Cambridge Univ. Press, 2001 [Google Scholar]
- 34.Quian QR, Kraskov A, Kreuz T, Grassberger P. Performance of different synchronization measures in real data: a case study on electroencephalographic signals. Phys Rev E Stat Nonlin Soft Matter Phys 65: 041903, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Rosenblum MG, Cimponeriu L, Bezerianos A, Patzak A, Mrowka R. Identification of coupling direction: application to cardiorespiratory interaction. Phys Rev E Stat Nonlin Soft Matter Phys 65: 041909, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Rosenblum MG, Kurths J, Pikovsky A, Schafer C, Tass P, Abel HH. Synchronization in noisy systems and cardiorespiratory interaction. IEEE Eng Med Biol Mag 17: 46–53, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Rosenblum M, Pikovsky A, Kurths J, Schäfer C, Tass P. Phase synchronization: from theory to data analysis. In: Handbook of Biological Physics, edited by Moss F, Gielen S. Amsterdam: Elsevier Science, 2001, p. 279–321 [Google Scholar]
- 38.Sakkalis V, Doru Giurc Neanu C, Xanthopoulos P, Zervakis M, Tsiaras V, Yang Y, Karakonstantaki E, Michelyannis S. Assessment of linear and nonlinear synchroniztion measures for analyzing EEG in a mild epileptic paradigm. IEEE Trans Inf Technol Biomed 13: 433–441, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Schafer C, Rosenblum MG, Abel HH, Kurths J. Synchronization in the human cardiorespiratory system. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics 60: 857–870, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Schafer C, Rosenblum MG, Kurths J, Abel HH. Heartbeat synchronized with ventilation. Nature 392: 239–240, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Schondorf R, Stein R, Roberts R, Benoit J, Cupples W. Dynamic cerebral autoregulation is preserved in neurally mediated syncope. J Appl Physiol 91: 2493–2502, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Senthilkumar DV, Lakshmanan M. Transition from anticipatory to lag synchronization via complete synchronization in time-delay systems. Phys Rev E Stat Nonlin Soft Matter Phys 71: 016211, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Senthilkumar DV, Lakshmanan M, Kurths J. Phase synchronization in time-delay systems. Phys Rev E Stat Nonlin Soft Matter Phys 74: 035205, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Serrador JM, Hughson RL, Kowalchuk JM, Bondar RL, Gelb AW. Cerebral blood flow during orthostasis: role of arterial CO2. Am J Physiol Regul Integr Comp Physiol 290: R1087–R1093, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Serrador JM, Schlegel TT, Black FO, Wood SJ. Cerebral hypoperfusion precedes nausea during centrifugation. Aviat Space Environ Med 76: 91–96, 2005 [PMC free article] [PubMed] [Google Scholar]
- 47.Serrador JM, Shoemaker JK, Brown TE, Kassam MS, Bondar RL, Schlegel TT. Cerebral vasoconstriction precedes orthostatic intolerance after parabolic flight. Brain Res Bull 53: 113–120, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci 18: 555–586, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Sirotin YB, Das A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature 457: 475–479, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung RY, Du ZD, Yu CW, Yam MC, Fok TF. Cerebral blood flow during vasovagal syncope induced by active standing or head up tilt. Arch Dis Child 82: 154–158, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taneja I, Medow MS, Glover JL, Raghunath NK, Stewart JM. Increased vasoconstriction predisposes to hyperpnea and postural faint. Am J Physiol Heart Circ Physiol 295: H372–H381, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas KN, Cotter JD, Galvin SD, Williams MJ, Willie CK, Ainslie PN. Initial orthostatic hypotension is unrelated to orthostatic tolerance in healthy young subjects. J Appl Physiol 107: 506–517, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Timmermann L, Florin E, Reck C. Pathological cerebral oscillatory activity in Parkinson's disease: a critical review on methods, data and hypotheses. Expert Rev Med Devices 4: 651–661, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Tuor UI, Farrar JK. Pial vessel caliber and cerebral blood flow during hemorrhage and hypercapnia in the rabbit. Am J Physiol Heart Circ Physiol 247: H40–H51, 1984 [DOI] [PubMed] [Google Scholar]
- 55.van der Pol B, van der Mark J. The heartbeat considered as a relaxation oscillation, and an electrical model of the heart. London Edinburgh Dublin Philosoph Magazine J Sci Ser 76: 763–775, 1928 [Google Scholar]
- 56.van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol 94: 833–848, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Wieling W, Krediet CT, van DN, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 112: 157–165, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 274: H233–H241, 1998 [DOI] [PubMed] [Google Scholar]