Abstract
Mitochondrial reactive oxygen species (ROS) are potentially important in vascular oxygen-sensing mechanisms because hypoxia appears to be a stimulus for mitochondrial ROS generation; however, scavenging of endogenous ROS does not alter relaxation of endothelium-denuded bovine coronary arteries (BCA) to hypoxia. The purpose of this study was to investigate the influence of increasing mitochondrial ROS on the relaxation of BCA to hypoxia. Increasing mitochondrial superoxide with inhibitors of electron transport (10 μM rotenone and antimycin) and by opening mitochondrial ATP-dependent K+ channels with 100 μM diazoxide were observed in this study to attenuate relaxation of BCA precontracted with 30 mM KCl to hypoxia by 68–76% and 38%, respectively. This effect of rotenone is not prevented by inhibiting NADPH oxidase (Nox) activation or scavenging superoxide with Peg-SOD; however, it is reversed 85% and 26% by increasing the consumption of intracellular peroxide by 0.1 mM ebselen and 32.5 U/ml Peg-catalase. Because inhibition of extracellular signal-regulated kinase (ERK) mitogen-activated protein (MAP) kinase (10 μM PD-98059), but not src kinase or rho kinase, also reverses the effects of rotenone by 69%, the peroxide-elicited force-enhancing effects of ERK appear to be attenuating the response to hypoxia. Rotenone increased the phosphorylation of ERK (by 163%). Activation of ERK in BCA with 0.1 mM peroxide or endogenous peroxide generated by stimulating Nox2 with a stretch treatment or contraction with 100 nM U-46619 also attenuated relaxation to hypoxia. Thus coronary arterial relaxation to hypoxia may be attenuated by pathophysiological conditions associated with increased peroxide generation by mitochondria or other sources that stimulate ERK.
Keywords: oxygen sensing, redox signaling, superoxide
multiple mechanisms potentially influence the effects of hypoxia on the coronary circulation and other systemic vascular segments (20, 21, 25). Coronary arterial smooth muscle has relaxing mechanisms that are directly activated by hypoxia (5, 24). Although the influence of hypoxia on reactive oxygen species (ROS) generated by mitochondria (13, 17, 27) and NADPH oxidases (Nox) (28) have been observed to participate in oxygen-sensing mechanisms detected in the vascular smooth muscle of systemic and pulmonary arteries, ROS-independent mechanisms have been identified that seem to be major factors in the relaxation of isolated coronary arteries to hypoxia (5, 24). The endothelium-denuded bovine coronary arteries (BCA) examined in the current study show a relaxation to hypoxia that appears to originate from a metabolic stress that promotes the oxidation of cytosolic NADPH, a potential coordinator of multiple processes that lower intracellular calcium (5, 6). Hypoxia and inhibiting complex I or complex III in the proximal part of the electron transport chain (ETC) can increase mitochondrial ROS generation in BCA (4) and in rat renal arteries (13). However, hypoxia decreases superoxide in mitochondria when BCA are contracted, under the conditions where relaxation to hypoxia is observed (4). In addition, our previous studies have shown that proximal mitochondrial ETC inhibitors that increase superoxide do not activate the relaxation caused by hypoxia (4, 6), and the distal ETC inhibitor cyanide did not inhibit BCA relaxation to hypoxia (5) under conditions where it does not appear to alter mitochondrial ROS generation (2, 13). Thus the influence of mitochondrial-derived ROS on responses to hypoxia is potentially an important factor that needs to be better defined.
Recent studies are providing evidence that many conditions associated with aging-related vascular disease processes and stressed physiological conditions result in increased mitochondrial oxidant production in vascular smooth muscle (1, 11, 12). Thus interactions between mitochondrial-derived ROS and vascular oxygen-sensing mechanisms could be important factors in vascular disease processes. The purpose of this study was to investigate mechanisms involved in our initial observation that inhibitors of ETC that increase superoxide in BCA attenuate relaxation to hypoxia. Because scavenging intracellular peroxide was found to reverse the actions of mitochondrial ETC inhibitors, we focused on examining the role of peroxide-activated force-enhancing mechanisms previously identified (7, 18) in BCA associated with extracellular signal-regulated kinase (ERK), src, and rho kinases.
MATERIALS AND METHODS
Measurement of force generation.
Isolated endothelium-denuded artery rings were prepared from left anterior descending or circumflex of BCA of slaughterhouse-derived calf hearts and subsequently studied by previously described methods (4, 7, 15, 18). Arterial rings were mounted on wire hooks attached to Grass force displacement transducers for measurement of changes in isometric force on a Grass Instruments polygraph. Arteries were incubated in Krebs buffer for 1 h, gassed with 21% O2, 5% CO2, and 74% N2 at an optimal passive tension of 5 g. Next, the vessels were depolarized with Krebs buffer containing 130 mM KCl in place of NaCl to enhance the reproducibility of subsequent contractions. After being washed with Krebs buffer, the vessels were reequilibrated with Krebs buffer for another hour before conducting the experiments. The rings were contracted with 30 mM KCl under 21% O2, 5% CO2, and 74% N2 to obtain a control contractile response in the absence of any treatments. After a 15-min reequilibration in Krebs buffer, treatments to modulate the response to hypoxia described in the results were then conducted over a 30-min period. The agents used in this study were obtained from Sigma Chemical or sources previously described (4, 7, 18). Arteries were then contracted to 30 mM KCl or 100 nM U-46619 in the presence of the probes examined, and, after reaching a plateau of steady-state force, BCA were exposed to hypoxia (95% N2, 5% CO2, Po2 ≈ 8–10 torr). Data were analyzed as the percent relaxation of force generated by 30 mM KCl or 100 nM U-46619, and the effects of drugs on the contraction to 30 mM KCl were evaluated as the percent of the force generation by the control contraction to 30 mM KCl. The majority of the study was conducted in BCA precontracted with 30 mM KCl to minimize the influence of the contractile agent on ROS mediation of responses, because, as previously reported (7, 18), contraction with 30 mM KCl does not increase superoxide, and force generation by this contractile agent was only altered by treatments with the stretch protocol and rho kinase inhibitor under the conditions examined in this study.
Detection of changes in superoxide and H2O2 by chemiluminescence.
Relative changes in the levels of superoxide in BCA were detected by lucigenin-enhanced chemiluminescence, as previously described (4, 15). Endothelium-removed BCA segments (n = 8) were initially incubated in Krebs buffer for 1 h, gassed with 21% O2, 5% CO2 and 74% N2, and then the BCA segments continued to incubate for another 30 min in the absence and presence of mitochondrial inhibitors and/or superoxide scavengers. By the end of the incubation, the arterial segments were placed in plastic scintillation minivials containing 5 μM lucigenin in the absence or presence of agents potentially modulating superoxide, in a final volume of 1 ml of air-equilibrated Krebs solution buffered with 10 mM HEPES-NaOH (pH 7.4). Baseline chemiluminescence was recorded before adding the BCA segments. The chemiluminescence was measured in a liquid scintillation counter (Beckman LS-6000IC); all manipulations were performed in the darkroom. Scintillation counting was performed two to three times after adding the BCA rings to obtain a stable reading. Baseline was then subtracted from the scintillation counting produced under each condition before adjusting the data for the amount of tissue present. For H2O2 measurements, 10 μM luminol and 1 nM horseradish peroxidase were substituted for lucigenin. Data are expressed as counts per minutes per gram tissue weight.
Western blot analysis of changes in ERK phosphorylation.
Western blot analysis was performed on BCA rings frozen in liquid nitrogen 20 min after hypoxic treatment under the experimental conditions described in results using methods described previously (18). Briefly, BCA pulverized in liquid nitrogen were added to lysis buffer [50 mM Tris·HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, protease cocktail (Sigma Chemical, St. Louis, MO)], followed by centrifugation to obtain the supernatant that was used for both Western analysis of protein (20 μg) on 10% SDS-PAGE and measurement of protein content. Total and phosphorylated forms of ERK antibodies were from Sigma/RBI, and secondary anti-rabbit and anti-mouse antibodies were from Sigma/RBI. After transfer to nitrocellulose membranes and exposure to the primary and secondary antibodies, the intensities of bands were detected using an Amersham ECL kit and standard X-ray film (Kodak X-Omat, Rochester, NY). Protein levels were measured by densitometric analysis using the Kodak one-dimensional software (Kodak Rochester). Data were analyzed as the percent of control BCA of the ratio of phospho-p44-ERK to total ERK.
Statistical analysis.
Data are shown as means ± SE. Statistical analyses were performed by Student's t-test or by ANOVA followed by the Tukey-Kramer Multiple-Comparison test for comparisons between two groups or multiple groups, respectively. P < 0.05 was considered statistically significant.
RESULTS
Influence of mitochondrial electron transport inhibitors on the detection of superoxide by lucigenin and relaxation to hypoxia in BCA.
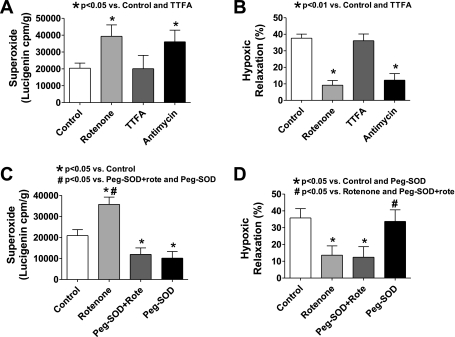
Previous work from our laboratory (4) documented evidence that the complex I and III inhibitors rotenone and antimycin increased mitochondrial superoxide generation that was detected by 5 μM lucigenin in BCA under conditions where rotenone was shown to increase mitochondrial superoxide, without altering the detection of extramitochondrial superoxide. The lucigenin measurements from this previous study documenting the increase in superoxide elicited by 10 μM rotenone and 10 μM antimycin are included in Fig. 1A for comparisons made in Fig. 1 with additional BCA superoxide measurements and vascular relaxation to hypoxia data obtained from the same groups of animals that were initially studied. As shown in Fig. 1A, the mitochondrial complex II inhibitor thenoyltrifluoroacetone (TTFA) did not influence BCA superoxide generation that was detected by lucigenin-enhanced chemiluminescence under conditions where it was increased by rotenone and antimycin. It was previously reported that treatment of BCA with rotenone or antimycin did not have an effect on contraction to 30 mM KCl (4, 6). As shown by the data in Fig. 1B, the presence of these mitochondrial electron transport inhibitors greatly impaired relaxation to hypoxia by 76% and 68% in BCA precontracted with 30 mM KCl that were treated with rotenone or antimycin, respectively. In contrast, pretreatment with concentrations of the complex II inhibitor TTFA previously observed to inhibit vascular superoxide generation (1) did not have a detectible effect on relaxation to hypoxia (Fig. 1B) under conditions where it did not influence the detection of superoxide (Fig. 1A).
Fig. 1.
Effects of mitochondrial electron transport inhibitors on relaxation of bovine coronary arteries (BCA) precontracted with 30 mM KCl to hypoxia and the role of changes in superoxide detected by lucigenin chemiluminescence. A: effects of rotenone, antimycin, and TTFA on superoxide generation detected by 5 μM lucigenin chemiluminescence (n = 8–14 experiments). B: effects of these mitochondrial electron transport inhibitors on relaxation of BCA precontracted with 30 mM KCl to hypoxia (n = 8–17). Note that rotenone and antimycin, but not TTFA, increased superoxide and inhibit relaxation to hypoxia. C: scavenging superoxide with the tissue-permeable superoxide scavenger polyethylene glycol (Peg)-superoxide dismutase (SOD) (30 U/ml) decreases the detection of superoxide by lucigenin in both rotenone-treated and nontreated BCA (n = 8). D: absence of an effect of Peg-SOD on both relaxation of BCA precontracted with 30 mM KCl to hypoxia and the inhibitory effect of rotenone on this response (n = 7).
Effects of scavenging superoxide with polyethylene glycol-superoxide dismutase on BCA relaxation to hypoxia and superoxide detection in the absence and presence of rotenone.
The membrane-permeable form of superoxide dismutase polyethylene glycol (Peg)-superoxide dismutase (SOD) (30 U/ml) decreased superoxide production detected by lucigenin chemiluminescence in both control and rotenone-treated BCA to a similar level (Fig. 1C), documenting that it is an effective scavenger of the source of superoxide observed to be increased by rotenone. Peg-SOD pretreatment did not alter relaxation of BCA precontracted with 30 mM KCl to hypoxia, and it did not reverse the inhibitory effect of rotenone on hypoxic vasorelaxation (Fig. 1D).
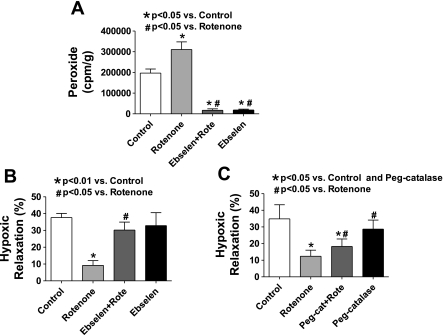
Effects of scavenging peroxide with ebselen on BCA relaxation to hypoxia and peroxide detection in the absence and presence of rotenone.
The release of H2O2 detectible as the increase in horseradish peroxidase-catalyzed luminol chemiluminescence in response to rotenone was examined. As shown in Fig. 2A, rotenone increased the detection of H2O2 release by 60% compared with untreated control BCA. Scavenging peroxide with ebselen (0.1 mM), a glutathione peroxidase mimetic that can decrease intracellular peroxide, decreased the detection of H2O2 release by ∼90% from BCA in the absence or presence of rotenone when compared with arteries not treated with ebselen (Fig. 2A). Although ebselen can also efficiently scavenge peroxynitrite, the relaxation to hypoxia is not influenced (15) by inhibition of nitric oxide biosynthesis in the endothelium-denuded BCA employed in this study. In confirmation of previously reported observations (16), ebselen did not alter the contraction to 30 mM KCl (data not shown) or have a detectible effect on the subsequent relaxation elicited by hypoxia (Fig. 2B). However, the presence of ebselen reversed the inhibition of hypoxic vasorelaxation caused by rotenone pretreatment. Another membrane-permeable peroxide scavenger, Peg-catalase, was also used to examine the possible involvement of H2O2 in the inhibitory effect rotenone has on relaxation of BCA to hypoxia. Preincubation with Peg-catalase did not alter the relaxation of 30 mM KCl-contracted BCA to hypoxia, but, coincubation of Peg-catalase and rotenone partially reversed the inhibitory effect of rotenone (see Fig. 2C). Although the origin of the greater efficiency of ebselen in reversing the effects of rotenone compared with catalase is not known, increased permeability of ebselen in subcellular regions is theorized to be a factor.
Fig. 2.
Effect of peroxide scavengers on rotenone-elicited increases in the detection of peroxide and its inhibition of relaxation to hypoxia in BCA precontracted with 30 mM KCl. A: levels of peroxide detected by luminol chemiluminescence in the presence of horseradish peroxidase are increased by rotenone in BCA when compared with control arteries. The glutathione peroxidase mimetic ebselen (0.1 mM) essentially eliminates the detection of BCA peroxide in the absence or presence of rotenone (n = 7–17). B: ebselen reversed the inhibitory effect of rotenone on hypoxic relaxation in BCA precontracted with 30 mM KCl (n = 5–7). C: Peg-catalase partially reversed the inhibitory effect of rotenone on hypoxic relaxation in BCA precontracted with 30 mM KCl (n = 7).
Effect of a mitochondrial ATP-dependent K+ channel opener, exogenously added H2O2, and an inhibitor of Nox oxidase activation on hypoxic vasorelaxation in BCA.
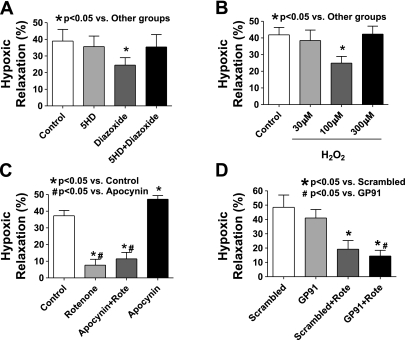
Because peroxide derived from superoxide generated by the mitochondrial ETC appeared to be inhibiting hypoxic vasorelaxation, the effects of increasing mitochondrial superoxide generation were examined with a mitochondrial potassium channel (mito KATP) opener diazoxide, which has been shown to increase in superoxide in a manner reversed by the mitoKATP channel blocker 5-hydroxydecanoate (5-HD) (29). As shown in Fig. 3A, the presence of diazoxide (100 μM) inhibited relaxation of BCA to hypoxia by 38%, and 5-HD (200 μM) reversed the inhibitory effect of diazoxide. These results further support that mitochondrial-derived ROS impaired hypoxia vasorelaxation in BCA.
Fig. 3.
Increasing mitochondrial reactive oxygen species (ROS) generation by opening mitochondrial potassium (mito KATP) channels with diazoxide inhibits relaxation to hypoxia, whereas inhibiting NADPH oxidase (Nox) activation with apocynin or gp91dstat does not reverse the attenuation of hypoxic vasorelaxation by rotenone in BCA precontracted with 30 mM KCl. A: diazoxide (100 μM) impairs hypoxia-induced relaxation in BCA in a manner that is prevented by 5-hydroxydecanoate (5-HD, 200 μM), an inhibitor of mito KATP channels (n = 6–7). B: exogenously added 100 μM H2O2 impairs relaxation to hypoxia (n = 7). Apocynin (100 μM; C) and 50 μM gp91dstat (GP91; D) did not reverse the inhibitory effect of rotenone on relaxation to hypoxia in BCA.
Exogenously added H2O2 induced different effects on the response to hypoxia in BCA depending on the dose used. In the presence of 100 μM H2O2, the relaxation to hypoxia was decreased by 41%, whereas 30 and 300 μM concentrations of peroxide did not have detectible effects (see Fig. 3B).
Apocynin, an inhibitor of Nox oxidase activation and basal superoxide in BCA detected by lucigenin chemiluminescence (8, 18), did not reverse the inhibitory effect of rotenone on the relaxation of BCA to hypoxia (see Fig. 3C). However, apo cynin itself caused an increase in relaxation to hypoxia that did not appear to be due to the generation of peroxide by Nox oxidase because scavenging peroxide with ebselen did not enhance relaxation to hypoxia. Inhibition of Nox oxidase activation and basal superoxide in BCA detected by lucigenin chemiluminescence by the more specific peptide agent 50 μM gp91dstat (7) did not increase the relaxation of BCA to hypoxia or reverse the inhibitory effect of rotenone (see Fig. 3D), suggesting that Nox oxidase was not influencing the relaxation to hypoxia or participating in the inhibitory actions of rotenone on this response.
Inhibition of ERK mitogen-activated protein kinase, but not src or rho kinases, prevents detection of the effects of rotenone on impairing relaxation of BCA to hypoxia.
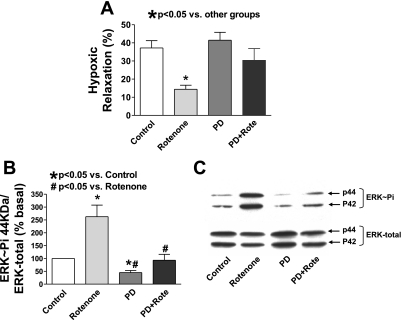
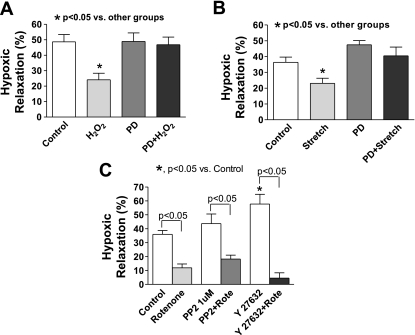
To further study the mechanisms underlying the effect of mitochondrial ROS on hypoxic relaxation in BCA, we examined several force-enhancing pathways that had been shown to be regulated by ROS signaling (10, 23). Previous study from our laboratory (18) found that activation of ERK by a stretch treatment stimulating increasing peroxide production derived from the p47phox activation of Nox-2 elicited enhanced force generation in BCA. The data in Fig. 4A show that inhibition of ERK activation by the mitogen/extracellular signal-regulated kinase inhibitor PD-98059 reversed the impairment of hypoxic relaxation in BCA by rotenone without directly influencing the hypoxic response (Fig. 4A). Western analysis of rotenone-treated coronary arteries (Fig. 4, B and C) demonstrated that rotenone caused a 2.6-fold increase in the levels of the phosphorylated forms of ERK (263 ± 45%, n = 15). PD-98059 alone decreased ERK phosphorylation by 55%. In the presence of PD-98059, rotenone did not increase the phosphorylated form of ERK. PD-98059 also reversed impaired hypoxic relaxation induced by exogenously added peroxide (100 μM) (Fig. 5A). Exposure of BCA to the stretch treatment protocol that stimulates ERK phosphorylation by endogenous peroxide production (18) on the relaxation to hypoxia was also examined. The data in Fig. 5B show that a 20-min stretch pretreatment of BCA impaired hypoxic relaxation in BCA by ∼37%, and the presence of PD-98059 during exposure to hypoxia reversed detection of this inhibition. These results are all consistent with elevated peroxide levels impairing relaxation of BCA to hypoxia through activation of the ERK mitogen-activated protein (MAP) kinase pathway.
Fig. 4.
Rotenone activates extracellular signal-regulated kinase (ERK) mitogen-activated protein (MAP) kinase, and inhibition of ERK activation reverses the inhibitory effect of rotenone on relaxation to hypoxia in BCA precontracted with 30 mM KCl. A: inhibition of ERK activation with PD-98059 (PD, 10 μM) reverses the impairment of relaxation of BCA to hypoxia by rotenone (n = 12). B and C: representative Western blot analysis and summary data showing that rotenone enhanced ERK phosphorylation (ERK∼Pi) in a manner inhibited by PD-98059 (n = 15).
Fig. 5.
Inhibition of ERK MAP kinase reverses the attenuation of relaxation to hypoxia by exogenous peroxide and stimulation of endogenous peroxide derived from Nox oxidase by stretch (18) in BCA precontracted with 30 mM KCl. However, the peroxide-mediated effects of rotenone on this response are not prevented by agents that suppress the activities of src or rho kinases. A: inhibition of ERK activation by PD-98059 (10 μM, PD) reverses the inhibitory effect of 100 μM peroxide on hypoxic relaxation in BCA (n = 5–8). B: stretch significantly impairs hypoxia-induced relaxation in BCA, and PD-98059 reverses the inhibitory effect of stretch (n = 8). C: inhibition of src or rho kinase by 4-amino-5-(4-cholophenyl)-7-(t-butyl)pyrazolo-[3,4-d]pyrimidine (PP2, 1 μM) or Y-27632 (1 μM), respectively, did not reverse the inhibitory effect of rotenone on hypoxic relaxation in BCA (n = 4–10).
The potential involvement of an src kinase-Rho kinase pathway in the inhibitory action of rotenone in impairing hypoxic vasorelaxation was also examined, since both of these pathways have been observed to participate in a peroxide-regulated contractile mechanism in BCA (7) and vascular smooth muscle hypoxic signaling (22, 26). As shown in Fig. 5C, treatment of BCA with the 4-amino-5-(4-cholophenyl)-7-(t-butyl)pyrazolo-[3,4-d]pyrimidine (PP2) inhibitor of src kinase and the Y-27632 inhibitor of Rho kinase did not significantly impair relaxation to hypoxia employing concentrations that were previously observed to modulate contractile responses in BCA associated with activating these kinase systems (7).
Influence of peroxide generated by contraction with U-46619 in the absence and presence of rotenone on the relaxation of BCA to hypoxia.
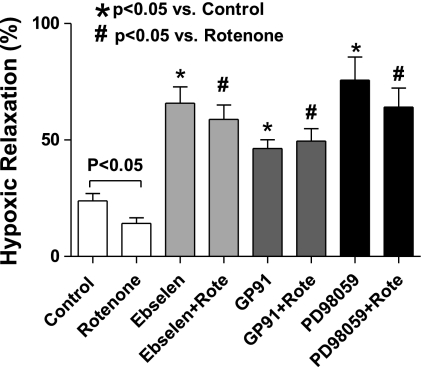
The contraction of BCA to the prostaglandin H2/thromboxane A2 receptor agonist U-46619 was previously shown to be partially mediated through peroxide generated by a protein kinase C-mediated activation of Nox2 (7). Experiments in the present study used to examine the effects of hypoxia confirmed the previously reported (7) attenuation of contraction to 100 nM U-46619 in the presence of ebselen and gp91dstat (data not shown). As shown in Fig. 6, rotenone was observed to inhibit the relaxation to hypoxia in the presence of U-46619. The inhibitory effects of rotenone on the relaxation to hypoxia were not observed in the presence of ebselen or PD-98059, suggesting that rotenone was inhibiting this response through peroxide-activating ERK. Relaxation to hypoxia was also enhanced by treatment with gp91dstat; however, the presence of rotenone did not alter this enhancement caused by inhibition of Nox2 activation. Rotenone did not significantly (data not shown) alter force generation to 100 nM U-46619 under any of the conditions examined in Fig. 6. Interestingly, PD-98059 depressed the contraction to U-46619 by 40% and enhanced the relaxation to hypoxia. Thus the depression of contraction to U-46619 and enhancement of relaxation to hypoxia in the presence of gp91dstat, ebselen, and PD-98059 suggest that U-46619 was stimulating contraction through a Nox oxidase-derived peroxide-mediated stimulation of ERK, and this process was functioning to decrease the relaxation to hypoxia that was observed.
Fig. 6.
Effects of 100 μM ebselen, 50 μM gp91dstat (GP91), and 10 μM PD-98059 in the absence and presence of rotenone on the relaxation to hypoxia in BCA precontracted with 100 nM U-46619 (n = 6–12).
DISCUSSION
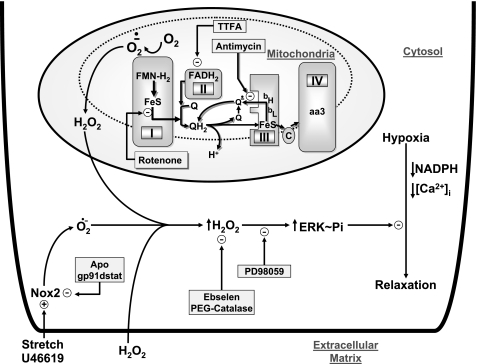
The data in this study provide further evidence supporting previous observations (4, 5, 15, 16) for the absence of a role for ROS in mediating the relaxation of endothelium-denuded BCA to hypoxia, since scavenging superoxide with Peg-SOD or scavenging peroxide with Peg-catalase and ebselen did not attenuate this response. However, as illustrated in the model in Fig. 7, when the generation of mitochondrial ROS is increased by inhibiting electron transport, mitochondria appear to release peroxide in amounts that attenuate the response to hypoxia through stimulating ERK MAP kinase. In addition, activating ERK through exposure of BCA to extracellular peroxide or stimulating its production by endogenous Nox oxidase activity also inhibits relaxation to hypoxia (see Fig. 7).
Fig. 7.
Model showing how the relaxation of BCA to hypoxia is attenuated by inhibition of mitochondrial electron transport sites in Complex I and Complex III through an increase in superoxide generation from Complex I, which results in peroxide release from mitochondria in amounts that can stimulate the force-enhancing effects of ERK. The model includes alternative pathways for attenuating relaxation to hypoxia by ERK stimulation as a result of exposure to extracellular peroxide or stimulation of Nox2 by U-46619 or stretch. Potential sites where probes used in this study are thought to influence the response to hypoxia are also shown. Arrows in the regions of complexes I-IV show pathways of election transfer between flavins (FMN-H2/FADH2), iron sulfur centers (Fe-S), the oxidized (Q), one electron-reduced semiquinone (Q·−), and the two electron-reduced QH2 hydroquinone form of coenzyme Q. Cytochromes (bH, bL, c, aa3) and molecular oxygen (O2) are associated with the generation of superoxide (O2·−). APO, apocynin.
Relaxation of BCA to hypoxia is attenuated by increasing mitochondrial peroxide.
Stimulation of an increase in superoxide generation from complex I by inhibitors of mitochondrial ETC appears to inhibit the relaxation of BCA when they are subsequently exposed to hypoxia through the generation of peroxide. Our previous observations (4) that rotenone increased the detection of superoxide generation by BCA with lucigenin suggested complex I was a source of mitochondrial superoxide generation, because the other well-characterized site of superoxide generation in the region of complex III should have been inhibited by rotenone. Although antimycin could theoretically increase superoxide generation from complex III, it will also increase its generation from complex I as well because it also increases electron density needed for superoxide generation in this portion of the ETC. The complex II inhibitor TTFA did not alter the detection of superoxide or the relaxation to hypoxia, suggesting that endogenous substrates fueling electron transport through complex II were not influencing superoxide generation or the response to hypoxia under the conditions examined. Our previous observations (4) that rotenone increased mitochondrial superoxide detected by mitosox but not extramitochondrial superoxide detected by dihydroethidine suggest that the release of mitochondrial superoxide in cytosolic regions could not be detected under the conditions examined. Because Peg-SOD was observed to inhibit the detection of rotenone-stimulated increases in superoxide, it appears that Peg-SOD can gain access to the mitochondrial source of superoxide detected by lucigenin. Data in the present study with horseradish peroxidase and luminol showing detection of an increase in the release of peroxide from BCA treated with rotenone suggest that mitochondria were releasing peroxide in amounts that could be detected in the extracellular region of BCA. Because the scavenging of intracellular peroxide by Peg-catalase and ebselen, but not intracellular superoxide by Peg-SOD, reversed the rotenone-elicited inhibition of relaxation to hypoxia, these observations suggest increased mitochondrial peroxide release can function to inhibit processes mediating the relaxation to hypoxia.
Peroxide attenuates relaxation to hypoxia by stimulating ERK.
Based on our previous studies on the role of peroxide in enhancing force generation in BCA (7, 18), the participation of ERK, src, and rho kinases in the inhibitory effects of rotenone on the relaxation to hypoxia were examined. Observations of an increase in ERK phosphorylation by rotenone and an attenuation of rotenone's effects on relaxation to hypoxia by inhibition of ERK activation with PD-90859 suggested that the stimulation of ERK by mitochondrial-derived peroxide was functioning to prevent detection of relaxation to hypoxia. In our study, pretreatment with rotenone did not induce force generation or effect on the subsequent contraction to 30 mM KCl or 100 nM U-46619 in the absence or presence of PD-90859, suggesting that peroxide-mediated activation of ERK elicited by rotenone under these conditions may be functioning as a process that selectively interferes with the hypoxia-elicited relaxing mechanisms in BCA. Observations that opening mitochondrial KATP channels with diazoxide inhibited relaxation to hypoxia in a manner prevented by 5-HD suggest that an alternative mechanism of promoting the generation of mitochondrial-derived ROS could also be functioning through stimulating ERK. Our observations in BCA confirm a previous report (11) that these mitochondrial KATP channel-modulating agents can promote a ROS-mediated activation of ERK in cultured vascular smooth muscle cells and in vivo. Based on the actions of the src and Rho kinase inhibitors PP2 and Y-27632, respectively, a role for these other force-enhancing mechanisms in the actions of rotenone on hypoxia could not be detected. The increase in relaxation to hypoxia in the presence of Y-27632 could either be due to rho kinase causing a basal inhibition of the relaxation to hypoxia or due to relaxation appearing to be enhanced by the lower level of force generated by 30 mM KCl in the presence of this agent. Because our previous studies detected a role for ERK in force-enhancing responses elicited by exposure of BCA to peroxide and to a stretch protocol that activates Nox oxidase by a p47phox-associated process (18), these approaches for increasing peroxide were investigated for their effects on the relaxation to hypoxia. Both a dose of 0.1 mM peroxide and treatment with stretch attenuated BCA relaxation to hypoxia in a manner that was prevented by treatment with the inhibitor of ERK activation PD-98059. The form of Nox oxidase in BCA that can be activated by binding p47phox appears to be Nox2 (8). Based on the absence of detection of an effect of blocking this mechanism of oxidase activation by apocynin and gp91dstat on the effects of rotenone in BCA contracted with 30 mM KCl, Nox2 did appear to participate in the attenuation of relaxation to hypoxia elicited by this complex I-associated inhibitor of mitochondrial electron transport. Our previous studies (18) suggest that the absence of an effect of 30 μM peroxide on relaxation to hypoxia is probably because this dose appears to be below the threshold for noticeable activation of force-enhancing effects of ERK. The absence of an effect of the 300 μM dose of peroxide on relaxation to hypoxia could be related to additional relaxing mechanisms activated under these conditions which appear to function to antagonize the force-enhancing effects of simultaneously activating ERK (18). For example, metabolism of this higher dose of peroxide may enhance cytosolic NADPH oxidation, a process thought to be associated with expression of relaxation to hypoxia (5). Although a src kinase-epidermal growth factor receptor pathway of activating ERK has been identified, there also appears to be a thiol oxidation-elicited mechanism of activating ERK that is not inhibited by PP2 (18, 19), suggesting that mitochondrial-derived peroxide can activate ERK by this alternative mechanism. Thus the activation of ERK by peroxide derived from mitochondria, Nox2 activation, or extracellular sources appears to be a common pathway in functioning as an inhibitor of BCA relaxation to hypoxia.
The mechanisms reported in this study could have roles in altered responses to hypoxia observed (3, 9, 14) in aging-related vascular diseases such as diabetes and hypertension, which are associated with increased mitochondrial and/or Nox oxidase-derived ROS generation. Although hypoxic regulation of microcirculatory blood flow appears to involve multiple mechanisms (25), including vasodilation elicited by tissue-derived hydrogen peroxide (21), the ERK-mediated attenuation of vascular smooth muscle relaxation to hypoxia reported in the present study could be a factor responsible for altered responses seen in vascular disease.
GRANTS
This study was support by National Heart, Lung, and Blood Institute Grants HL-31069, HL-43023, and HL-66331. A greater part of experiments reported in this study contributed toward a partial fulfillment of the requirements for a PhD degree in Physiology for Q. Gao.
REFERENCES
- 1.Bagi Z, Toth E, Koller A, Kaley G. Microvascular dysfunction after transient high glucose is caused by superoxide-dependent reduction in the bioavailability of NO and BH4. Am J Physiol Heart Circ Physiol 287: H626–H633, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Cadenas E, Boveris A. Enhancement of hydrogen peroxide formation by protophores and ionophores in antimycin-supplemented mitochondria. Biochem J 188: 31–37, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ely SW, Sun CW, Knabb RM, Gidday JM, Rubio R, Berne RM. Adenosine and metabolic regulation of coronary blood flow in dogs with renal hypertension. Hypertension 5: 943–950, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Gao Q, Wolin MS. Effects of hypoxia on relationships between cytosolic and mitochondrial NAD(P)H redox and superoxide generation in coronary arterial smooth muscle. Am J Physiol Heart Circ Physiol 295: H978–H989, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupte SA, Wolin MS. Hypoxia promotes relaxation of bovine coronary arteries through lowering cytosolic NADPH. Am J Physiol Heart Circ Physiol 290: H2228–H2238, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Gupte SA, Arshad M, Viola S, Kaminski PM, Ungvari Z, Rabbani G, Koller A, Wolin MS. Pentose phosphate pathway coordinates multiple redox-controlled relaxing mechanisms in bovine coronary arteries. Am J Physiol Heart Circ Physiol 285: H2316–H2326, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Gupte SA, Kaminski PM, George S, Kouznestova L, Olson SC, Mathew R, Hintze TH, Wolin MS. Peroxide generation by p47phox-Src activation of Nox2 has a key role in protein kinase C-induced arterial smooth muscle contraction. Am J Physiol Heart Circ Physiol 296: H1048–H1057, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupte SA, Kaminski PM, Floyd B, Agarwal R, Ali N, Ahmad M, Edwards J, Wolin MS. Cytosolic NADPH may regulate differences in basal Nox oxidase-derived superoxide generation in bovine coronary and pulmonary arteries. Am J Physiol Heart Circ Physiol 288: H13–H21, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Gupte SA, Wolin MS. Oxidant and redox signaling in vascular oxygen sensing: implications for systemic and pulmonary hypertension. Antioxid Redox Signal 10: 1137–1152, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol 287: H1495–H1500, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension 45: 438–444, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Labinskyy N, Mukhopadhyay P, Toth J, Szalai G, Veres M, Losonczy G, Pinto JT, Pacher P, Ballabh P, Podlutsky A, Austad SN, Csiszar A, Ungvari Z. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol 296: H946–H956, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res 90: 1307–1315, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Miura H, Wachtel RE, Loberiza FR, Jr, Saito T, Miura M, Nicolosi AC, Gutterman DD. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res 92: 151–158, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Mohazzab- HKM, Kaminski PM, Fayngersh RP, Wolin MS. Oxygen-elicited responses in calf coronary arteries: role of H2O2 production via NADH-derived superoxide. Am J Physiol Heart Circ Physiol 270: H1044–H1053, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Mohazzab- HKM, Agarwal R, Wolin MS. Influence of glutathione peroxidase on coronary artery responses to alterations in Po2 and H2O2. Am J Physiol Heart Circ Physiol 276: H235–H241, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol 98: 390–403, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res 92: 23–31, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Oeckler RA, Arcuino E, Ahmad M, Olson SC, Wolin MS. Cytosolic NADH redox and thiol oxidation regulate pulmonary arterial force through ERK MAP kinase. Am J Physiol Lung Cell Mol Physiol 288: L1017–L1025, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Park KH, Rubin LE, Gross SS, Levi R. Nitric oxide is a mediator of hypoxic coronary vasodilatation. Relation to adenosine and cyclooxygenase-derived metabolites. Circ Res 71: 992–1001, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol 26: 2614–2621, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Sato H, Sato M, Kanai H, Uchiyama T, Iso T, Ohyama Y, Sakamoto H, Tamura J, Nagai R, Kurabayashi M. Mitochondrial reactive oxygen species and c-Src play a critical role in hypoxic response in vascular smooth muscle cells. Cardiovasc Res 67: 714–722, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Thakali K, Davenport L, Fink GD, Watts SW. Cyclooxygenase, p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase MAPK, Rho kinase, and Src mediate hydrogen peroxide-induced contraction of rat thoracic aorta and vena cava. J Pharmacol Exp Ther 320: 236–243, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Thorne GD, Ishida Y, Paul RJ. Hypoxic vasorelaxation: Ca2+-dependent, Ca2+-independent mechanisms. Cell Calcium 36: 201–208, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol 97: 404–415, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Wardle RL, Gu M, Ishida Y, Paul RJ. Rho kinase is an effector underlying Ca2+-desensitizing hypoxic relaxation in porcine coronary artery. Am J Physiol Heart Circ Physiol 293: H23–H29, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Waypa GB, Schumacker PT. Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. J Appl Physiol 98: 404–414, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Wolin MS, Ahmad M, Gupte SA. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol 289: L159–L173, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res 97: 354–362, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]