Abstract
The goal of this work was to investigate the hemodynamic effects of simultaneous left ventricular (LV) pacing site (LVPS) and interventricular pacing delay (VVD) variation with biventricular pacing (BiVP) during acute LV failure. Simultaneously varying LVPS and VVD with BiVP has been shown to improve hemodynamics during acute right ventricular (RV) failure. However, effects during acute LV failure have not been reported. In six open-chest pigs, acute LV volume overload was induced by regurgitant flow via an aortic-LV conduit. Epicardial BiVP was implemented with right atrial and ventricular leads and a custom LV pacing array. Fifty-four LVPS-VVD combinations were tested in random order. Cardiac output was evaluated by aortic flow probe, ventricular systolic function by maximum rate of ventricular pressure change, and mechanical interventricular synchrony by normalized RV-LV pressure diagram area. Simultaneous LVPS-VVD variation improved all measures of cardiac function. The observed effect was different for each functional index, with evidence of LVPS-VVD interaction. Compared with effects of LVPS-VVD variation in a model of acute RV failure, hemodynamic changes were markedly different. However, in both models, maximum rate of ventricular pressure change of the failing ventricle was improved with synchronous interventricular contraction, suggesting that, in acute ventricular failure, BiVP can recruit the unstressed ventricle to support systolic function of the failing one. Thus simultaneously varying LVPS and VVD with BiVP during acute ventricular failure can improve cardiac function by “interventricular assist”, with hemodynamic effects dependent on the type of failure. This supports the potential utility of temporary BiVP for the treatment of acute ventricular failure commonly seen after cardiac surgery.
Keywords: heart failure, cardiac pacing, hemodynamics, cardiac mechanics, cardiac surgery
in 2005, approximately 699,000 patients underwent open-heart surgery in the United States (28). In the postoperative period, this group often faces acute ventricular failure and subsequent low cardiac output, requiring treatment with volume infusion, inotropic and vasoactive agents, diuretics, and mechanical assistance devices. While effective, these therapies have been associated with numerous complications, increasing mortality and morbidity, along with length of hospital stay and cost (16). Thus new strategies for treatment of postoperative ventricular failure have the potential to significantly improve patient outcomes and reduce costs. Biventricular pacing (BiVP) may be useful in this setting.
Permanent BiVP, or cardiac resynchronization therapy, is now commonly recommended as treatment for patients with congestive heart failure (15, 30). By reversing inter- and intraventricular conduction delay, BiVP improves ventricular systolic and diastolic function, benefiting long-term cardiac function and geometry (7). BiVP is also associated with acute improvements in cardiac function. Importantly, this is not associated with changes in myocardial energy demand (21), unlike most inotropic agents, which improve contractility at the expense of increased oxygen consumption and energy store depletion (29). Acute functional benefit without increased energy cost makes BiVP an excellent option for treatment of postoperative ventricular failure, and initial studies have shown its utility after cardiac surgery (14, 20, 23, 24, 33).
The efficacy of BiVP can be improved by varying atrioventricular (AVD) and interventricular pacing delay (VVD) and left ventricular (LV) pacing site (LVPS) (10). Developing techniques for postoperative BiVP optimization, as well as understanding the effects of these parameters on function, are important steps to the implementation of BiVP for postoperative care. We have been investigating optimization of temporary BiVP in patients after cardiac surgery and also in experimental animal models of acute ventricular failure. In a pig model of acute right ventricular (RV) pressure overload, our laboratory demonstrated that RV-first pacing with variation of VVD improved cardiac output with improved RV contractility (26). This was associated with increased interventricular synchrony, suggesting that RV systolic function was dependent on support from the unstressed LV. Most recently, our laboratory reported hemodynamic effects of simultaneous LVPS-VVD variation with BiVP in this model (4). However, effects of BiVP during acute LV failure have not been reported. Accordingly, we studied simultaneous variation of LVPS and VVD with BiVP in a pig model of acute LV volume overload. We anticipated that function would be improved by combinations of LVPS and VVD and that differences between effects in LV and RV failure would help clarify mechanisms of action and potential clinical value of temporary BiVP for the treatment of various forms of acute heart failure.
EXPERIMENTAL PROCEDURES
Studies were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the Columbia University Institutional Animal Care and Use Committee.
Animal preparation.
Experiments were carried out in six male Yorkshire pigs (40–50 kg), anesthetized intramuscularly with atropine (0.02 mg/kg), ketamine (20 mg/kg), and xylazine (0.5 mg/kg), followed by oral endotracheal intubation. Mechanical ventilation was performed with a mixture of oxygen (100%) and titrated isoflurane (2.0%). To maintain blood volume, 0.9% saline solution was administered intravenously at 10 ml·kg−1·h−1 for the first hour, and then 5 ml·kg−1·h−1 for the duration of the study. Anticoagulation was achieved with 100 U/kg hourly intravenous boluses of sodium heparin.
Standard limb leads were placed for surface electrocardiogram monitoring. A femoral arterial line was inserted to measure peripheral arterial pressure. The chest was opened by median sternotomy, and the pericardium was incised longitudinally with traction sutures placed about its free edges to expose and support the heart. A solid-state pressure transducer catheter (5 French, Millar Instruments, Houston, TX) was inserted into the RV via stab wound in the apex to measure instantaneous pressures. Pressure in the LV was monitored by a similar catheter, inserted across the aortic valve via the left carotid artery, which also allowed measurement of aortic pressure. An ultrasonic flow probe (24-mm diameter, Transonic Systems, Ithaca, NY) was placed around the ascending aorta to measure instantaneous aortic volume flow. Bipolar temporary epicardial pacing leads (Medtronic, Houston, TX) were clipped to the right atrial appendage and sewn onto the anterior surface of the RV. For implementation of BiVP with various LVPS, a custom multielectrode temporary pacing array composed of bipolar pacing leads (Capsure Epi 4968, Medtronic) sutured to a Gore-Tex patch (W. L. Gore & Associates, Newark, DE) was placed within the pericardial space posterior to the LV and secured in place by gently approximating the pericardium to the LV, as previously reported (4). This allowed rapid testing of three basal (obtuse margin, circumflex, and posterior descending artery) and two midventricular (inferolateral and inferomedial) LVPS. An additional pacing lead was sewn onto the surface of the LV at the apex.
A modified version of a preparation described by Welch et al. (34) was utilized for induction of acute LV volume overload. This involved creation of an ascending aorta-LV apex conduit from a DLP cannula (12-French, Medtronic) with the midsection replaced by a segment of thoracic aorta harvested from another animal and stored in lactated Ringer and formalin (2.0%) solution. The conduit was presoaked in 10 U/ml heparinized saline solution. It was inserted into the ascending aorta via a stab wound and purse-string suture and flushed with heparinized saline. The distal end was inserted in the LV apex via a stab wound and purse-string suture, flushed with heparinized saline, de-aired, and closed using surgical tubing clamps. To prevent clotting in the conduit, the sodium heparin dose was increased to 300 U/kg hourly intravenous boluses. To stabilize the preparation, a 0.008 U·kg−1·min−1 intravenous drip of vasopressin was initiated. An ultrasonic flow probe was placed around the tissue segment of the conduit (10-mm diameter, Transonic Systems) to measure regurgitant flow from the aorta to the LV.
BiVP protocol.
The pacing leads were connected to a custom temporary, external BiVP unit containing a shock-mounted permanent BiVP device (InSync III 8042, Medtronic). Dual-chamber (DDD) RV pacing was initiated with a heart rate of 100 beats/min and an AVD of 150 ms. A two-dimensional, short-axis LV echocardiogram was acquired (VingMed CFM 800, GE Medical Systems, Milwaukee, WI). Pacing was stopped, and complete atrioventricular block was established by atrioventricular node ablation with injection of 0.1-ml aliquots of 100% ethanol into the region of the bundle of His, which allowed complete control of myocardial activation sequence, as previously described (17). This was immediately followed by reinstitution of DDD-RV pacing. A second LV echocardiogram was acquired to confirm there was no adverse effect of ethanol injection on septal function. The surgical tubing clamps were gradually released until flow through the aortic-LV conduit (“retrograde” flow) was 30% of total LV output. The vasopressin dose was titrated to stabilize the preparation for the remainder of the experiment, which successfully maintained mean arterial pressure within 5 mmHg of the value measured before opening the conduit. After 1 h of overload, the pacing mode was converted to DDD-BiV, and the presence of atrioventricular block and proper sensing and pacing function of the leads and pacing array was confirmed. VVD was varied between +80 and −80 ms (positive VVD indicates RV-first pacing) in 20-ms increments, with six LVPS. Thus a total of 54 VVD-LVPS combinations were tested, in random order. Each combination was tested for 15 s, followed by 15 s of DDD-RV pacing. Animals were humanely killed at the conclusion of the experiment.
Data acquisition and analysis.
Hemodynamic signals were sampled by an analog-to-digital converter (ADInstruments, Milford, MA) and recorded on a personal computer (Apple Computer, Cupertino, CA). Offline analysis was performed using custom routines implemented in Matlab (The MathWorks, Natick, MA), as described previously (26). Briefly, end diastole in each ventricle was defined as the point immediately before the rate of ventricular pressure change exceeded 10% of maximum (dP/dtmax). Cardiac output was calculated by integrating the aortic flow signal over each cardiac cycle, which included all flow (effective forward flow plus retrograde flow), as the flow probe was placed proximal to the conduit. Ventricular systolic function was assessed by dP/dtmax. Mechanical interventricular synchrony was quantified with the area enclosed by the normalized RV-LV pressure diagram (APP) (31). This expresses synchrony based on pressure during the complete cardiac cycle, with a loop area of zero indicating complete synchrony and a maximum area of one indicating complete asynchrony. For our purposes, a counterclockwise loop was given positive values, indicating RV pressure preceding LV pressure. Variables were averaged over the second half of each testing interval, excluding any ectopic cardiac cycles. Results were displayed using response surfaces (27), linearly interpolated between measured values.
Statistical analysis.
For the comparison of hemodynamic data in control, immediately after induction of LV volume overload, and after 1 h of loading, a repeated-measures ANOVA was utilized. For the comparison of hemodynamic data across LVPS and VVD and between RV pressure overload and LV volume overload, a three-factor analysis of variance with repeated measures on two factors was utilized. Here the subjects were measured under all levels of both factors (LVPS and VVD), within a particular treatment group (RV pressure overload or LV volume overload). Three-way and two-way interaction terms were tested for and interpreted. If significant, post hoc analyses were conducted as suggested by Keppel (18). A P value < 0.05 was considered significant for all tests. Data are presented as means ± SE. All data were analyzed using SAS system software (SAS Institute, Cary, NC).
RESULTS
Acute LV volume overload.
Table 1 presents the immediate hemodynamic effects of inducing LV volume overload, as well as changes after 1 h of loading, averaged across the six pigs. The induction of volume overload immediately increased cardiac output (in this case, total flow, i.e., effective forward flow plus retrograde flow), LV end-diastolic pressure, and LV dP/dtmax, while it decreased diastolic aortic pressure. After 1 h, cardiac output and LV end-diastolic pressure had returned to their baseline values (meaning effective forward flow was reduced), LV and RV dP/dtmax and mean and systolic aortic pressure were decreased to below baseline, and diastolic aortic pressure was still reduced. RV end-diastolic pressure and mechanical interventricular synchrony (APP) were unaffected by volume loading.
Table 1.
Hemodynamic effects of left ventricular volume overload
| Functional Index | Control | LVVO (Acute) | LVVO (1 h) |
|---|---|---|---|
| CO, l/min | 2.2±0.2 | 2.9±0.1† | 2.3±0.3‡ |
| Systolic AoP, mmHg | 94±7 | 94±8 | 83±3†‡ |
| Diastolic AoP, mmHg | 73±6 | 67±5† | 59±2† |
| LV EDP, mmHg | 7.6±1.4 | 8.5±1.4† | 7.6±0.6† |
| LV dP/dtmax, mmHg/s | 703±57 | 743±59† | 635±54†‡ |
| RV EDP, mmHg | 3.9±0.6 | 4.3±1.0 | 2.4±0.6 |
| RV dP/dtmax, mmHg/s | 143±14 | 148±4 | 138±13†‡ |
| Interventricular synchrony (APP)* | 0.48±0.07 | 0.47±0.07 | 0.46±0.04 |
Values are mean ± SE. LVVO, left ventricular volume overload; CO, cardiac output (systemic plus retrograde flow); AoP, aortic pressure; LV, left ventricle; EDP, end-diastolic pressure; dP/dtmax, maximum rate of ventricular pressure increase; RV, right ventricle; APP, area of the normalized right-left ventricular pressure diagram.
Positive APP indicates RV preceding LV pressure.
P < 0.05 vs. control.
P < 0.05 vs. LVVO (Acute).
Simultaneous variation of LVPS and VVD with BiVP.
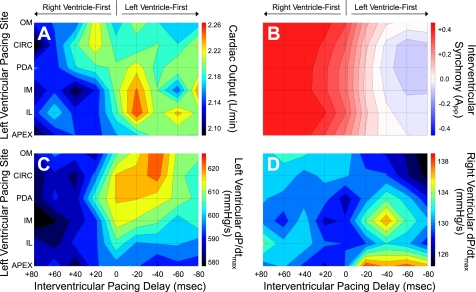
Figure 1 shows response surfaces of cardiac output, mechanical interventricular synchrony (APP), and LV and RV dP/dtmax from simultaneous LVPS-VVD variation with BiVP during acute LV volume overload averaged across the six pigs. Qualitatively, all functional indexes were improved with LV-first pacing (VVD < 0). However, the LVPS that maximized function differed for each index. Cardiac output was highest with BiVP at the midventricle (LVPS = inferolateral/inferomedial), APP was most synchronous and LV dP/dtmax the greatest at the anterior base (LVPS = obtuse margin/circumflex), and RV dP/dtmax was best at the apex. To check whether there was a dependence of the hemodynamic response on the order that the LVPS-VVD combinations were tested, in the one pig where hemodynamic stability permitted, all combinations were repeated with a new randomization. The response of each functional index was the same for the two protocols.
Fig. 1.
Hemodynamic response surfaces during simultaneous variation of left ventricular pacing site and interventricular pacing delay with biventricular pacing during acute left ventricular volume overload. Presented here are hemodynamic response surfaces from simultaneous changes in left ventricular pacing site and interventricular pacing delay with biventricular pacing during acute left ventricular volume overload averaged across 6 pigs. A: cardiac output. B: interventricular synchrony [area enclosed by the normalized RV-LV pressure diagram (APP)]. C: maximum rate of left ventricular pressure increase (dP/dtmax). D: right ventricular dP/dtmax. For surfaces of cardiac output and dP/dtmax, red represents the highest values. For surfaces of interventricular synchrony, red represents right preceding left ventricular pressure, blue represents left preceding right ventricular pressure, and white represents complete synchrony. Positive interventricular pacing delay indicates right ventricle-first pacing, as indicated by the arrows. CIRC, circumflex; IL, inferolateral; IM, inferomedial; OM, obtuse margin; PDA, posterior descending artery.
Table 2 gives details from statistical analysis of the response surfaces. There was a significant effect of varying LVPS on mechanical interventricular synchrony (APP) and LV and RV dP/dtmax, but not on cardiac output. On the other hand, changes in VVD affected all functional indexes. As a result, there was significant LVPS-VVD interaction for each of the indexes, except cardiac output.
Table 2.
Hemodynamic effects of simultaneous variation of left ventricular pacing site and interventricular pacing delay with biventricular pacing during acute left ventricular volume overload
| Best Settings |
|||||
|---|---|---|---|---|---|
| Functional Index | LVPS | VVD†, ms | Change From Worst Settings | Change From Default Settings‡ | LVPS-VVD Interaction |
| CO, l/min | No effect | −20 | 8±2% | 3±1% | P = 0.293 |
| Interventricular synchrony (APP)* | Apex | −80 | −0.45±0.03 | −0.31±0.02 | P < 0.001 |
| LV dP/dtmax, mmHg/s | OM | −40 | 9±3% | 1±1% | P = 0.012 |
| RV dP/dtmax, mmHg/s | Apex | −20 | 12±3% | 7±4% | P < 0.001 |
Values are mean ± SE. LVPS, left ventricular pacing site; VVD, interventricular pacing delay; No effect, lack of significant parameter effect; OM, obtuse margin.
Positive APP indicates RV preceding LV pressure.
Positive VVD indicates RV-first pacing.
Default settings (the most common clinically implemented biventricular pacing protocol): LVPS = circumflex; VVD = 0 ms.
DISCUSSION
The present study investigated the hemodynamic effects of simultaneous LVPS-VVD variation with BiVP in a model of acute LV failure. It was shown that optimizing LVPS and VVD by simultaneous variation with BiVP improved cardiac output, mechanical interventricular synchrony, and ventricular systolic function, with a different effect on each functional index.
Comparison to acute RV failure.
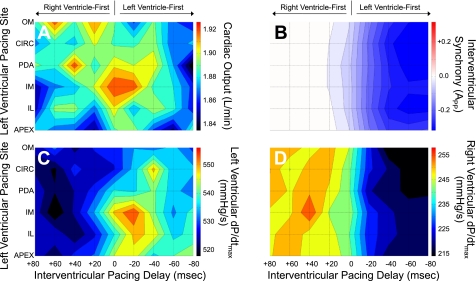
In an attempt to clarify underlying mechanisms, we compared the results to a similar experiment performed in a model of acute RV failure produced by RV pressure overload (4). Figure 2 shows hemodynamic response surfaces generated by applying the analysis described here to the data from that model. In contrast to that seen during acute LV volume overload, VVD that maximized function was qualitatively different for the various functional indexes. Cardiac output was highest with simultaneous pacing (VVD = 0), LV dP/dtmax was greatest with LV-first pacing (VVD < 0), and APP was most synchronous and RV dP/dtmax best with RV-first pacing (VVD > 0). In this case, cardiac output and LV dP/dtmax were both maximized with BiVP at the posterior midventricle (LVPS = inferomedial), but there was little effect of LVPS on APP or RV dP/dtmax. Table 3 gives further details from statistical analysis of the response surfaces. Additionally, when the hemodynamic effects of varying LVPS and VVD were directly compared between the two models, they were significantly different for all of the hemodynamic indexes (cardiac output, P < 0.001; LV dP/dtmax, P = 0.035; RV dP/dtmax, P < 0.001; APP, P < 0.001).
Fig. 2.
Hemodynamic response surfaces during simultaneous variation of left ventricular pacing site and interventricular pacing delay with biventricular pacing during acute right ventricular pressure overload. Presented here are hemodynamic response surfaces from simultaneous changes in left ventricular pacing site and interventricular pacing delay with biventricular pacing during acute right ventricular pressure overload averaged across 6 pigs. A: cardiac output. B: interventricular synchrony (APP). C: dP/dtmax. D: right ventricular dP/dtmax. For surfaces of cardiac output and dP/dtmax, red represents the highest values. For surfaces of interventricular synchrony, red represents right preceding left ventricular pressure, blue represents left preceding right ventricular pressure, and white represents complete synchrony. Positive interventricular pacing delay indicates right ventricle-first pacing, as indicated by the arrows.
Table 3.
Hemodynamic effects of simultaneous variation of left ventricular pacing site and interventricular pacing delay with biventricular pacing during acute right ventricular pressure overload
| Best Settings |
|||||
|---|---|---|---|---|---|
| Functional Index | LVPS | VVD†, ms | Change From Worst Settings | Change From Default Settings‡ | LVPS-VVD Interaction |
| CO, l/min | No effect | 0 | 5±2% | 2±2% | P = 0.980 |
| Interventricular synchrony (APP)* | IM | +20 | 0.32±0.03 | 0.04±0.02 | P = 0.047 |
| LV dP/dtmax, mmHg/s | No effect | No effect | P = 0.199 | ||
| RV dP/dtmax, mmHg/s | No effect | +40 | 20±5% | 6±2% | P = 0.814 |
Values are mean ± SE. IM, inferomedial.
Positive APP indicates RV preceding LV pressure.
Positive VVD indicates RV-first pacing.
Default settings (the most common clinically implemented biventricular pacing protocol): LVPS = circumflex; VVD = 0 ms.
“Interventricular assist” of the failing ventricle.
While the response to variations in LVPS and VVD was markedly different between the two acute failure models, there was one important similarity. In both, systolic function of the failing ventricle (dP/dtmax) was maximized when interventricular contraction was close to synchronous (APP = 0). Previously, in our model of acute RV pressure overload, we demonstrated that varying VVD changed RV systolic function, which was associated with changes in interventricular synchrony (26). It was shown that RV dP/dtmax was highest with synchronous interventricular contraction (APP = 0), suggesting that RV systolic function was dependent on support from the unstressed LV. The results presented here further support this hypothesis and extend this idea to both ventricles. It appears that, during acute ventricular failure, varying BiVP parameters can recruit the unstressed ventricle to support function of the failing one by “interventricular assist”. The ventricles contribute directly to each other's contraction via mechanical coupling, as continuity of the myocardium allows transmission of forces between the ventricles (9), and changes in interventricular synchrony may change the amount and timing of this contribution. This effect has been demonstrated in studies by Damiano et al. using an electrically isolated RV preparation (12, 13). By varying the pacing interval between the LV and RV (which is, in fact, the earliest example of VVD variation with BiVP), they showed that one component of ventricular pressure can be directly attributed to RV contraction, whereas the other component is directly related to contraction of the LV and septum. Therefore, with synchronous contraction, the unstressed ventricle may be able to contribute to the contraction of the failing one, thus improving its systolic function. Interestingly, results from the present study suggest that the effectiveness of this support appears dependent on LVPS, as all pacing sites produced nearly synchronous contraction at the correct VVD, but did not maximize systolic function of the failing ventricle. This has important implications for LVPS selection and warrants further study.
Pathology dependence of BiVP.
It is suggested that the efficacy and optimization of BiVP may depend on the specific cardiac pathology, accounting for the differences in hemodynamic response between patients. This idea is supported here by the different response to variations of LVPS and VVD between the two acute ventricular failure models. An analogous result was found in a study by Byrne et al. (11) that compared BiVP in failing canine hearts with pure right and left bundle branch block, showing that there was less improvement with BiVP in the right bundle branch block hearts, and that RV pacing alone provided the same degree of benefit. This has important implications for temporary BiVP implementation, as it supports the need for optimization in each patient, and suggests that this may be facilitated by understanding its relation to the underlying pathology.
Simultaneous parameter variation and parameter interaction.
A weakness of most BiVP studies varying more than one pacing parameter is that each is tested independently. Varying one parameter at a time does not account for their interdependence or a possible nonlinear, cumulative effect. Ideally, protocols should explore multiple variables simultaneously. This is supported by the LVPS-VVD interaction demonstrated in the present study, as well as other studies that have reported parameter interaction with simultaneous variation of AVD and VVD (22, 25, 35, 36). Parameter interaction may also help explain some disparities between results of various BiVP studies in the literature. For example, previous studies of VVD optimization during acute RV pressure overload (26) and LV volume overload (3) from our laboratory have shown different effects of VVD than the present study; however, in those studies, LVPS was fixed at the obtuse margin. In fact, if one considers only the data with LVPS at the obtuse margin from the present study, there is good agreement with the previous results. Similarly, in a patient study by Lane et al. (19), while they did not simultaneously vary LVPS and VVD, they showed that optimum VVD often differed when BiVP was implemented with a lateral compared with an inferior LVPS.
Clinical implications.
The present study provides an alternate mechanism for benefits of BiVP in acute ventricular failure, which might be helpful after cardiac surgery and other transient forms of myocardial depression. The traditional view of BiVP is that it reverses regional dyssynchrony within the failing ventricle. The present results demonstrate that BiVP can also recruit the healthy ventricle to assist its distressed counterpart.
In previous clinical studies (1, 2, 5), and currently as part of the National Institutes of Health sponsored Biventricular Pacing After Cardiac Surgery Trial (32), we have been performing LVPS and VVD optimization in patients following cardiac surgery. The advantage of that setting, like in the experimental models presented here, is that the open chest provides access to the entire surface of the LV, so that temporary epicardial pacing arrays, or multiple leads sewn to the surface of the heart, can be utilized to rapidly test pacing site, with VVD altered using a standard pacemaker programmer. Additionally, with aortic flow probes, cardiac output can be measured in real time, thus providing an index for optimization (alternative indexes can also be used, derived from pressure or echocardiography, for example). Once the chest is closed, we have successfully tested two LV sites, with leads sewn to the surface of the LV during surgery and using PulseCO to measure cardiac output (6, 8). However, in settings in which cardiac surgery is not involved, novel technologies will need to be developed to implement similar protocols.
Selection of an appropriate functional index for optimization also merits further discussion. Cardiac output is employed as the primary end-point in our clinical studies. This is clinically appropriate, as postoperative benefits of BiVP are dependent on organ perfusion. However, accurate real-time cardiac output measurement requires invasive techniques, and cardiac output requires time to equilibrate after changes in pacing protocols. Thus alternate parameters for optimization require consideration. In this respect, it is important to note that, in the present study, maximization of the failing ventricle's systolic function did not maximize cardiac output. The relationship of cardiac output to mechanical synchrony and systolic function illustrated here appears complex, so, while synchronous contraction may best support the failing ventricle, mechanical interventricular synchrony may not be the best target, if the goal is to maximize cardiac output. What indexes are best for BiVP optimization needs further evaluation.
Study limitations.
An important limitation of the present study was the lack of chronic heart failure and acute ischemia, which are present to some degree in most forms of surgical heart disease. Greater heterogeneity of electrical and mechanical myocardial properties induced by ischemia could alter the effects of varying LVPS and VVD. This study could also have greatly benefited from a measurement of intraventricular synchrony, as this is commonly viewed as the most important parameter in evaluating the mechanisms and efficacy of BiVP. This is currently being evaluated with tissue Doppler echocardiography in our models of acute ventricular failure. Additionally, our system is currently being updated to include conductance catheter measurement of ventricular volume for pressure-volume analysis, which allows measurement of indexes combining pressure and volume output, such as stroke work, which may be more sensitive parameters for optimization. Finally, as variability of measured cardiac output and dP/dtmax was inherently larger than of APP, more experiments could have improved the results from statistical analysis of the generated response surfaces.
Conclusions.
The work presented here shows that simultaneously varying LVPS and VVD with BiVP can improve cardiac function during acute ventricular failure. This may have important implications for temporary BiVP and its optimization and supports the potential utility of optimized temporary BiVP for the treatment of acute failure commonly seen after cardiac surgery. Clinical studies are warranted to explicitly determine the value of optimized temporary BiVP in the postoperative period, as well as investigate appropriate hemodynamic indexes and techniques for its clinical implementation.
GRANTS
This work was supported by a grant from the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01-HL080152 to H. M. Spotnitz) and by the Department of Surgery at Columbia University. H. M. Spotnitz is the George H. Humphreys, II, Professor of Surgery.
DISCLOSURES
There are no disclosures to report.
ACKNOWLEDGMENTS
We thank all of the hard working support staff at the Columbia University Medical Center who made this work possible.
This study was presented in part at the 36th Congress of the International Union of Physiological Sciences Sessions, Kyoto, Japan, 27 July-1 August 2009.
Present addresses: T. A. Quinn, Postdoctoral Research Fellow, Department of Physiology, Anatomy, and Genetics, University of Oxford, UK; J. W. Holmes, Associate Professor of Biomedical Engineering and Medicine, Robert M. Berne Cardiovascular Research Center, University of Virginia, Charlottesville, VA.
REFERENCES
- 1.Berberian G, Cabreriza SE, Quinn TA, Garofalo CA, Spotnitz HM. Left ventricular pacing site-timing optimization during biventricular pacing using a multi-electrode patch. Ann Thorac Surg 82: 2292–2294, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Berberian G, Kanter JP, Quinn TA, Spotnitz HM. Optimized perioperative biventricular pacing in setting of right heart failure. Europace 7: 385–387, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Berberian G, Quinn TA, Cabreriza SE, Garofalo CA, Barrios DM, Weinberg AD, Spotnitz HM. Load dependence of cardiac output in biventricular pacing: left ventricular volume overload in pigs. J Thorac Cardiovasc Surg 131: 666–670, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Berberian G, Quinn TA, Cabreriza SE, Kenny JE, Garofalo CA, Weinberg AD, Spotnitz HM. Left ventricular pacing site and timing optimization during biventricular pacing using a multielectrode patch in pigs. J Thorac Cardiovasc Surg 134: 574–578, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Berberian G, Quinn TA, Kanter JP, Curtis LJ, Cabreriza SE, Weinberg AD, Spotnitz HM. Optimized biventricular pacing in atrioventricular block after cardiac surgery. Ann Thorac Surg 80: 870–875, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Berberian G, Quinn TA, Vigilance DW, Park DY, Cabreriza SE, Curtis LJ, Spotnitz HM. Validation study of PulseCO system for continuous cardiac output measurement. ASAIO J 51: 37–40, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bilchick KC, Helm RH, Kass DA. Physiology of biventricular pacing. Curr Cardiol Rep 9: 358–365, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Booth JH, Quinn TA, Richmond ME, Cabreriza SE, Weinberg AD, Johnston T, Spotnitz HM. Cardiac output measurement by arterial pressure waveform analysis during optimization of biventricular pacing after cardiac surgery. ASAIO J 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bove AA, Santamore WP. Ventricular interdependence. Prog Cardiovasc Dis 23: 365–388, 1981 [DOI] [PubMed] [Google Scholar]
- 10.Burri H, Sunthorn H, Shah D, Lerch R. Optimization of device programming for cardiac resynchronization therapy. Pacing Clin Electrophysiol 29: 1416–1425, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Byrne MJ, Helm RH, Daya S, Osman NF, Halperin HR, Berger RD, Kass DA, Lardo AC. Diminished left ventricular dyssynchrony and impact of resynchronization in failing hearts with right versus left bundle branch block. J Am Coll Cardiol 50: 1484–1490, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Damiano RJ, Jr, Cox JL, Lowe JE, Santamore WP. Left ventricular pressure effects on right ventricular pressure and volume outflow. Cathet Cardiovasc Diagn 19: 269–278, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Damiano RJ, Jr, La Follette P, Jr, Cox JL, Lowe JE, Santamore WP. Significant left ventricular contribution to right ventricular systolic function. Am J Physiol Heart Circ Physiol 261: H1514–H1524, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Dzemali O, Bakhtiary F, Israel CW, Ackermann H, Moritz A, Kleine P. Impact of different pacing modes on left ventricular function following cardiopulmonary bypass. Thorac Cardiovasc Surg 56: 87–92, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Riegel B, Tarkington LG, Yancy CW. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 117: e350–e408, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Ferraris VA, Ferraris SP, Singh A. Operative outcome and hospital cost. J Thorac Cardiovasc Surg 115: 593–602, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Kenny JE, Berberian G, Rabkin DG, Cabreriza SE, Quinn TA, Curtis LJ, Spotnitz HM. Ethanol induction of complete heart block in swine. J Surg Res 132: 142–146, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Keppel G. Design and Analysis: A Researchers Handbook Upper Saddle River, NJ: Prentice Hall, 1991 [Google Scholar]
- 19.Lane RE, Chow AW, Mayet J, Francis DP, Peters NS, Schilling RJ, Davies DW. The interaction of interventricular pacing intervals and left ventricular lead position during temporary biventricular pacing evaluated by tissue Doppler imaging. Heart 93: 1426–1432, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muehlschlegel JD, Peng YG, Lobato EB, Hess PJ, Jr, Martin TD, Klodell CT., Jr Temporary biventricular pacing postcardiopulmonary bypass in patients with reduced ejection fraction. J Card Surg 23: 324–330, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Nelson GS, Berger RD, Fetics BJ, Talbot M, Spinelli JC, Hare JM, Kass DA. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation 102: 3053–3059, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Perego GB, Chianca R, Facchini M, Frattola A, Balla E, Zucchi S, Cavaglia S, Vicini I, Negretto M, Osculati G. Simultaneous vs. sequential biventricular pacing in dilated cardiomyopathy: an acute hemodynamic study. Eur J Heart Fail 5: 305–313, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Pham PP, Balaji S, Shen I, Ungerleider R, Li X, Sahn DJ. Impact of conventional versus biventricular pacing on hemodynamics and tissue Doppler imaging indexes of resynchronization postoperatively in children with congenital heart disease. J Am Coll Cardiol 46: 2284–2289, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Pichlmaier M, Bagaev E, Lichtenberg A, Teebken O, Klein G, Niehaus M, Haverich A. Four-chamber pacing in patients with poor ejection fraction but normal QRS durations undergoing open heart surgery. Pacing Clin Electrophysiol 31: 184–191, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Porciani MC, Dondina C, Macioce R, Demarchi G, Pieragnoli P, Musilli N, Colella A, Ricciardi G, Michelucci A, Padeletti L. Echocardiographic examination of atrioventricular and interventricular delay optimization in cardiac resynchronization therapy. Am J Cardiol 95: 1108–1110, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Quinn TA, Berberian G, Cabreriza SE, Maskin LJ, Weinberg AD, Holmes JW, Spotnitz HM. Effects of sequential biventricular pacing during acute right ventricular pressure overload. Am J Physiol Heart Circ Physiol 291: H2380–H2387, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Quinn TA, Rabkin DG, Cabreriza SE, Curtis LJ, Spotnitz HM. Visualization of the effect of atrial-ventricular and right-left delay on cardiac output during biventricular pacing (Abstract). J Am Coll Cardiol 43: 404A, 2004 [Google Scholar]
- 28.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25–e146, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Suga H, Hisano R, Goto Y, Yamada O, Igarashi Y. Effect of positive inotropic agents on the relation between oxygen consumption and systolic pressure volume area in canine left ventricle. Circ Res 53: 306–318, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H, Gasparini M, Linde C, Morgado FB, Oto A, Sutton R, Trusz-Gluza M. Guidelines for cardiac pacing and cardiac resynchronization therapy: The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology Developed in collaboration with the European Heart Rhythm Association. Eur Heart J 28: 2256–2295, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Verbeek XA, Vernooy K, Peschar M, Van Der Nagel T, Van Hunnik A, Prinzen FW. Quantification of interventricular asynchrony during LBBB and ventricular pacing. Am J Physiol Heart Circ Physiol 283: H1370–H1378, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Wang DY, Richmond ME, Quinn TA, Mirani AJ, Rusanov A, Yalamanchi V, Cabreriza SE, Weinberg AD, Spotnitz HM. Optimized temporary biventricular pacing acutely improves intraoperative cardiac output after weaning from cardiopulmonary bypass (Abstract). Circulation 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisse U, Isgro F, Werling C, Lehmann A, Saggau W. Impact of atrio-biventricular pacing to poor left-ventricular function after CABG. Thorac Cardiovasc Surg 50: 131–135, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Welch GH, Jr, Braunwald E, Sarnoff SJ. Hemodynamic effects of quantitatively varied experimental aortic regurgitation. Circ Res 5: 546–551, 1957 [DOI] [PubMed] [Google Scholar]
- 35.Whinnett ZI, Davies JE, Willson K, Manisty CH, Chow AW, Foale RA, Davies DW, Hughes AD, Mayet J, Francis DP. Haemodynamic effects of changes in atrioventricular and interventricular delay in cardiac resynchronisation therapy show a consistent pattern: analysis of shape, magnitude and relative importance of atrioventricular and interventricular delay. Heart 92: 1628–1634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuber M, Toggweiler S, Roos M, Kobza R, Jamshidi P, Erne P. Comparison of different approaches for optimization of atrioventricular and interventricular delay in biventricular pacing. Europace 10: 367–373, 2008 [DOI] [PubMed] [Google Scholar]