Abstract
Maintenance of functional vascular networks requires structural adaptation of vessel diameters in response to hemodynamic and metabolic conditions. The mechanisms by which diameters respond to the metabolic state are not known, but may involve the release of vasoactive substances in response to low oxygen by tissue (“tissue signaling”, e.g., CO2, adenosine), by vessel walls (“wall signaling”, e.g., prostaglandins, adenosine), and/or by red blood cells (RBCs) (“RBC signaling”, e.g., ATP and nitric oxide). Here, the goal was to test the potential of each of these locations of oxygen-dependent signaling to control steady-state vascular diameters and tissue oxygenation. A previously developed theoretical model of structural diameter adaptation based on experimental data on microvascular network morphology and hemodynamics was used. Resulting network characteristics were analyzed with regard to tissue oxygenation (Oxdef; percentage of tissue volume with Po2 < 1 Torr) and the difference between estimated blood flow velocities and corresponding experimental data [velocity error (Verr); root mean square deviation of estimated vs. measured velocity]. Wall signaling led to Oxdef < 1% and to the closest hemodynamic similarity (Verr: 0.60). Tissue signaling also resulted in a low oxygen deficit, but a higher Verr (0.73) and systematic diameter deviations. RBC signaling led to widespread hypoxia (Oxdef: 4.7%), unrealistic velocity distributions (Verr: 0.81), and shrinkage of small vessels. The results suggest that wall signaling plays a central role in structural control of vessel diameters in microvascular networks of given angioarchitecture. Tissue-derived and RBC-derived signaling of oxygen levels may be more relevant for the regulation of angiogenesis and/or smooth muscle tone.
Keywords: oxygen sensing, vascular diameter, angioadaptation
structural adaptation in response to both hemodynamic and metabolic conditions is necessary for the maintenance of functionally adequate vessel networks (59, 70). If it is assumed that vessel diameters respond only to hemodynamic stimuli, i.e., wall shear stress and intravascular pressure, theoretical arguments show that complex microvascular networks degrade to single arteriovenous shunts (36, 32, 55). Therefore, responses to metabolic signals are needed to generate and preserve multiple parallel flow pathways, which are essential for meeting the metabolic needs of the tissue because of the limited diffusion distance of metabolites, especially oxygen. As a crucial requirement for cell energy metabolism that is contained only in small quantities in tissue, oxygen is of particular importance as a metabolic signal for the adjustment of vessel diameters.
The goal of the present work is to examine the potential ability of several mechanisms of oxygen responsiveness to provide adequate structural control of microvessel diameters. Relatively little direct experimental evidence is available on metabolic control of structural diameter adaptation. Therefore, one may start from considering oxygen response mechanisms involved in the acute control of blood flow, by variation of smooth muscle tone in arterioles and small arteries. These mechanisms may also be involved in structural adaptation, since sustained changes in tone have been shown to lead to corresponding changes of structural vessel diameter (3, 44). However, it should be noted that structural diameter adaptation does not necessarily require preceding changes in smooth muscle tone. As an example, vascular meshes generated by vasculogenesis during embryonic development do not possess proper smooth muscle layers and thus smooth muscle tone. Nevertheless, they remodel to give well-organized microvascular networks exhibiting systematic vessel diameter variation along arteriovenous flow pathways. Similarly, adult capillaries are subject to structural diameter adjustment (23, 41).
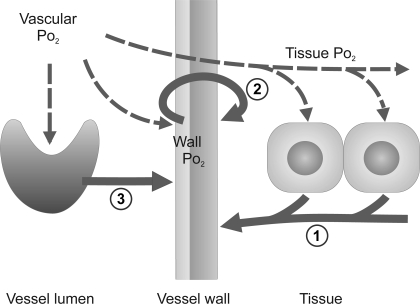
A number of physiological models have been proposed for the mechanism by which vascular diameters respond to local oxygen levels via changes in smooth muscle tone. These models generally assume that vasoactive substances are released at a rate that increases with decreasing local oxygen availability. Three main potential sources for such vasoactive substances have been identified: the parenchymal tissue, the red blood cells (RBCs), and the vessel wall (Fig. 1), as discussed in detail below.
Fig. 1.
Schematic drawing of metabolic signaling mechanisms identified in vivo. Vascular oxygen level affects hemoglobin saturation in red blood cells (RBC) and diffuses to vessel wall and tissue cells (dashed arrows). Resulting local oxygen levels evoke release of metabolic signaling substances from these structures (solid arrows), which, on reaching the vessel wall, cause diameter changes. The corresponding signaling modes investigated in this study are “tissue signaling” (1), “wall signaling” (2), and “RBC signaling” (3).
Several substances are known to be released by tissue cells in an oxygen-dependent manner, evoking vasodilation after diffusion to nearby vessel segments. Among these substances are adenosine, a degradation product of adenosine triphosphate (ATP), that accumulates when oxygen supply and demand are mismatched (6), and carbon dioxide that is produced in proportion to the rate of oxygen consumption and probably acts on vascular smooth muscle cells via a decrease in local pH (8).
Based on findings of oxygen-dependent release of various vasodilators, RBCs have also been suggested to be involved in vascular tone regulation. RBCs were shown to deliver ATP from intracellular pools in association with offloading of oxygen from the hemoglobin (5, 20, 21, 28, 29). Hypoxia was also shown to cause the release of nitric oxide (NO) from RBC hemoglobin via induction of allosteric transition from the relaxed to the tense state (40, 48, 64). A further mechanism involves the deoxyhemoglobin-mediated reduction of nitrite to NO (12, 24, 46).
As a third possibility, the vessel wall itself may generate vasoactive substances in response to changes in local oxygen tension. Various potent vasodilators are produced by endothelial cells in direct response to low Po2, including NO, prostaglandins, “endothelium-derived hyperpolarizing factor”, and adenosine (9, 14, 19, 31, 43, 45, 50). Alternatively, low oxygen levels may lead to changes in smooth muscle cell function, causing muscle relaxation (for review, see Ref. 65).
The experimental evidence cited above shows the implication of these three different modes of metabolic signaling, referred to in the following as “tissue signaling,” “wall signaling,” and “RBC signaling” for the regulation of vascular tone. Here, we analyze the capability of each of these metabolic signaling modes to control the structural adaptation of vessel diameters in microvascular networks in response to local oxygen levels. A previously developed mathematical model of structural diameter adaptation in terminal vascular beds (55) is used. Such mathematical simulations have the advantage of allowing explicit control of the biological mechanisms represented in the model.
In the model used here, structural adaptation of vessel diameters in microvascular networks with given topologies is simulated by considering the responses of each segment to local hemodynamic and metabolic stimuli, including the contribution of information transfer along the vessel wall. All vessel segments (arterioles, capillaries, and venules) are treated equivalently with respect to their remodeling responses to adaptive stimuli. The model is based on experimental data of complete microvascular networks from the rat mesentery. In the experimental setup used, no significant spontaneous smooth muscle tone was detected in the microvessels analyzed. Thus the measured vessel diameters represent the eventual result of structural vascular adaptation to hemodynamic and metabolic conditions. Correspondingly, only steady-state conditions resulting from long-term vessel diameter adjustment to a maintained metabolic and hemodynamic state are considered.
In the present study, the three metabolic signaling modes (tissue signaling, wall signaling, and RBC signaling) are tested separately. For each mode, simulation results are then compared with experimental data to determine which mode is most consistent with observations and, therefore, most likely to represent an actual mechanism for structural diameter adaptation in vivo.
MATERIAL AND METHODS
Experimental data.
After approval by the University and State authorities for animal welfare, all surgical and experimental procedures were carried out in accordance with the German Animal Protection Act. Microvascular networks in fat-free and lymph vessel-free portions of the mesenteries of two male Wistar rats (∼300 g body wt) from the animal facilities of the Charité Berlin were observed by intravital video microscopy in a scanning procedure lasting ∼20 min, as described previously (54). Heart rate, arterial pressure, anesthetic level, and fluid balance were monitored. In the experimental setup used, no significant spontaneous smooth muscle tone was detected in the microvessels analyzed. The vasodilator papaverine (10−4 M) was continuously applied to prevent possible development of vascular tone during the scanning procedure. Vessels in the thin mesenteric membrane, as studied here, are known to exhibit only low smooth muscle tone compared with other terminal vascular beds, e.g., in skeletal muscle. The existence of a finite vessel tone before the experiment with the organ still in situ cannot be excluded. However, since there is no indication for any significant tone in situ, the observed (structural) diameters and flow velocities are assumed to constitute the best obtainable data set representing the result of long-term adjustment of vessel diameters to hemodynamic and metabolic conditions.
Diameter, length, hematocrit, and blood flow velocity were measured offline in all segments between branch points using a digital image analysis system. Velocity measurements are based on the principle of spatial correlation analysis, which allows reliable measurements of velocities up to ∼40 mm/s (51, 52). Perfusion rates of all boundary segments were calculated from measured vessel diameters and blood flow velocities. Results are presented for a microvascular network containing 576 vessel segments. The network was supplied with oxygenated blood by a main feeding arteriole (diameter 28 μm, blood flow 410 nl/min) and drained by a main venule (diameter 59 μm), as primary boundaries, and included secondary boundaries: 30 inputs and 4 outputs, with vessel diameters of 10 and 12 μm (median), and blood flow of 3 and 8 nl/min (median), respectively. For comparison, a second mesenteric network with 389 vessel segments was examined.
Reconstruction and discretization.
Arrangement of vessels in the tissue was reconstructed from video recordings offline using network reconstruction software developed in house. Network data were generated by interactively tracing vessel segments as polygonal curves from numerous fields of view that were video recorded during intravital microscopy. The resulting database describes the topological (vessel connection) and the morphological (segmental length, diameter, and location) network structure and was completed with measured vessel parameters (e.g., hematocrit, blood flow velocity).
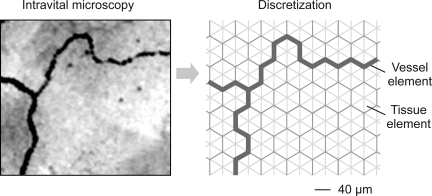
For simulation of diffusive exchange, a discretization approach was used. All vessel segments of the almost planar microvascular network were mapped onto a regular hexagonal (“honeycomb”) grid (Fig. 2). Hexagonal grids were chosen because three grid lines meet at any junction point, corresponding to segments in vascular bifurcations, at a uniform angle of 120° between grid lines. Each vessel segment linking two adjacent bifurcations was converted into a connected series of vessel elements corresponding to the edges of the hexagonal grid. The (three-dimensional) crossing of two segments cannot be directly projected to a two-dimensional grid. Therefore, crossings were represented using the two nodes adjacent to the crossing, with the segments involved crossing in the course of the connecting hexagonal edge between these nodes. On this hexagonal edge, two vessel elements were generated in the model, one for each vessel segment of the crossing, sharing localization in the tissue. The main network studied contained 18 vessel crossings.
Fig. 2.
Approach for definition of vessel elements (grid lines) and tissue elements (triangles) from videomicroscopic recordings by discretization. Locations and connections of vessels visible on videorecordings (left) are used to map the microvascular network to a dense hexagonal grid, with any hexagon being composed of six triangles (right).
A hexagonal edge length of 40 μm, equivalent to about one-tenth of average segment length measured in vivo, was chosen as a compromise between precision of vascular network representation and computational load in simulations. Discretization of curved vessel segments to a series of straight elements usually leads to a summed length of elements that deviates from the measured segment length. To correct for this, a factor was determined for each segment by dividing the measured segment length by the summed length of its vessel elements obtained by discretization. The length of all hexagonal edges of the segment is then multiplied by this factor. The resulting adjusted hexagonal edge length is used for the calculation of diffusive exchange area.
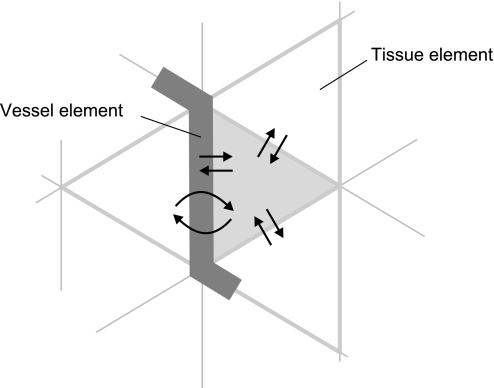
The area of each hexagon was divided into six equilateral triangles, defining tissue elements. Tissue elements were assigned a thickness of 20 μm, according to literature data for the rat mesentery (4). Each tissue element has three neighboring tissue elements (elements with one common edge) and, possibly, one neighboring vessel element (on the hexagonal edge). Each vessel element has neighboring tissue elements at opposing sides.
Discretization of the main mesenteric network, which contains 576 vessel segments, resulted in 4,740 vessel elements and 31,379 tissue elements. Network and tissue parameters were visualized using in-house computer software based on the graphic libraries of OpenGL for graphical presentation of vessel networks and tissue elements.
Model simulation.
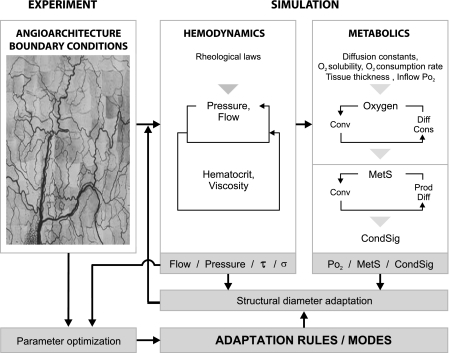
The overall approach used in the theoretical simulations has been described previously (55, 59) and is summarized in Fig. 3. The input to the model consists of experimental data on microvascular network structure and conditions in boundary segments. Based on these data, vascular network hemodynamic parameters are calculated, which serve as the input for the determination of oxygen distribution in vascular network and tissue. Local oxygen content is assumed to stimulate the production of a metabolic signal substance, according to the different modes tested (see below). Starting from experimentally measured diameters for all segments, hemodynamic and metabolic stimuli evoke vessel diameter adjustment in each vessel segment, according to the assumed set of adaptation rules (see below, Adaptation model). The quantitative impact of individual stimuli on segment diameter change is determined by parameters in the adaptation rules, which were optimized via comparison of simulated to experimental blood flow velocities. Physiological constants used in this model are given in Table 1. Adjustment of vessel diameters in the mesenteric vascular network in response to the local oxygenation was simulated, assuming one of the three modes: tissue signaling, wall signaling, or RBC signaling.
Fig. 3.
Simulation of structural diameter adaptation. Experimental data on network structure and rheological laws are used to simulate blood flow, transmural pressure, hematocrit, and viscosity for each vessel segment. Oxygen distribution in the vascular network and the tissue is estimated via an iterative loop, alternately calculating oxygen convection (Conv) in the network and oxygen diffusion (Diff) and consumption (Cons) in the tissue. The adaptation model is shown with shaded background. A metabolic signaling substance (MetS) is produced (Prod), dependent on the calculated local oxygen level, according to the selected mode (tissue signaling, wall signaling, RBC signaling) and undergoes diffusion and convection. Vascular concentration of MetS leads to the generation of a signal that is conducted upstream (CondSig) along flow pathways. Stimuli derived from hemodynamic conditions (flow, pressure, wall shear stress τ, and wall stress σ) and metabolic conditions determine diameter adaptation for each vessel segment, according to an assumed set of adaptation rules. Comparison of simulated with experimental network data is performed to optimize parameters used in the adaptation.
Table 1.
Standard values of model parameters for oxygen transport and consumption
| Description | Parameter | Value | Unit | Reference No. |
|---|---|---|---|---|
| Blood parameters | ||||
| Oxygen binding capacity of RBC | CRBC | 0.52 | ml O2/ml | 10 |
| Po2 at half-maximal hemoglobin saturation | Po2 50 | 38 | Torr | 30 |
| Hill equation exponent | n | 3 | 30 | |
| Solubility of oxygen in RBC | αRBC | 3.38×10−5 | cm3 O2·cm−3·Torr−1 | 1 |
| Solubility of oxygen in plasma | αpl | 3×10−5 | cm3 O2·cm−3·Torr−1 | 33 |
| Krogh diffusion coefficient in plasma | Kpl | 8.3×10−10 | cm3 O2·cm−1·s−1·Torr−1 | 33 |
| Tissue parameters | ||||
| Oxygen diffusion coefficient | Dt | 1.04×10−5 | cm2/s | 69 |
| Solubility of oxygen | αt | 3×10−5 | cm3 O2·cm−3·Torr−1 | 69 |
| Krogh diffusion coefficient | Kt = Dt ·αt | 3.12×10−10 | cm3 O2·cm−1·s−1·Torr−1 | |
| Oxygen demand | M0 | 0.01 | cm3 O2·cm−3·min−1 | 27, 66 |
| Po2 at half-maximal oxygen consumption | P0 | 1 | Torr | 13, 62 |
| Thickness of the mesentery | 20 | μm | 4 |
RBC, red blood cells.
Network hemodynamics.
Input data for the calculation of vascular network hemodynamics comprise morphological data (diameter and length) for each segment and data on network topology (segment connection matrix). Boundary conditions comprise flow or pressure for all segments entering or leaving the network, as well as the discharge hematocrit in all inflow segments. Details of the hemodynamic model (Fig. 3, top middle panel) have been described earlier (61). The flow resistance of each vessel segment is estimated, taking into account the experimentally determined variation of apparent viscosity of blood with vessel diameter and hematocrit, Fahraeus-Lindqvist effect (61), and the phase separation of RBCs and plasma in diverging bifurcations (60). Blood flow for each segment is determined by alternately calculating rheological parameters (segment hematocrit, viscosity) and hemodynamic parameters (segment blood flow, nodal pressure) until stable values are achieved. Values of wall shear stress in each segment are calculated from the pressure, diameter, and length values.
Oxygen distribution.
The input to the determination of oxygen distribution consists of topological data (tissue and vessel element arrangement) and morphological data (tissue element edge length and height, vessel element diameter and length), and of vessel network convective transport characteristics (blood flow and discharge hematocrit for each segment). Steady-state oxygen distribution in the vascular network and the surrounding tissue is determined by iteratively calculating oxygen convection in the vascular network, oxygen diffusion and oxygen consumption by the tissue (Fig. 3, top part of top right panel), until convergence is achieved (average change of tissue Po2 < 10−3 Torr). The model obeys mass conservation, and the difference between total oxygen inflow and outflow equals the summed oxygen consumption by the tissue, assuming zero diffusive flux at the outer boundaries of the tissue region considered.
A finite difference model of oxygen transport was developed using the vessel elements (subscript “ves”) and tissue elements (subscript “tis”) generated as described above. Each element is characterized by a single Po2. In the model, a linear Po2 gradient between centers of neighboring elements is assumed. This gradient is estimated as ΔPo2/Δs, where Δs is the distance between these centers. Temporal discretization to time intervals Δt was performed using an explicit single-step procedure in which new parameter values are derived from values at the previous step.
The convective oxygen flux (JO2conv) of a vessel element is determined, considering both hemoglobin-bound oxygen and oxygen dissolved in RBCs and plasma, according to
| (1) |
| (2) |
and Q is blood flow, cves is oxygen volume concentration of the blood, HD is discharge hematocrit; CRBC is oxygen-carrying capacity of erythrocytes, Po2ves is Po2 of the vessel element, and αRBC and αpl are oxygen solubility in RBCs and plasma, respectively. Differences between plasma and intraerythrocyte Po2 are neglected (25). Erythrocyte oxygen saturation (So2) as a function of Po2, So2 (Po2ves), is determined according to the Hill equation, So2 (Po2ves) = Po2 vesn/(Po2 vesn+ Po2 50n), assuming values typical for rat blood for the Hill coefficient (n) and the half-saturation oxygen pressure (Po2 50) (Table 1).
Oxygen diffusion is assumed between tissue elements and between tissue elements and all types of vessels (arterioles, capillaries, venules). Within the tissue, oxygen consumption, diffusivity, and solubility are assumed to be uniform. Oxygen diffusion rate (JO2diff) is described according to Fick's law of diffusion by
| (3) |
where K is the Krogh diffusion coefficient of tissue or blood plasma, i.e., the product of oxygen solubility and oxygen diffusion coefficient. For diffusion between two tissue elements, the area for diffusive exchange (A) corresponds to hexagonal edge length, multiplied by the thickness of the tissue, reduced by the vessel element diameter in the presence of a vessel element. For diffusion between a tissue and a vessel element, width and height of the diffusion area are the adjusted length of the vessel element and one-half of the vessel element perimeter, respectively. If the diameter of the vessel element exceeded the assumed thickness of the tissue of 20 μm, the thickness of adjacent tissue elements was increased to equal the vessel diameter (4). For each diffusion time step and each element, diffusive oxygen exchange rate JO2diff with all neighboring elements is calculated (Fig. 4).
Fig. 4.
Simulation of oxygen diffusion in the finite difference model of oxygen transport. Any finite element is assumed to exchange oxygen by diffusion with all neighboring elements, resulting in a net oxygen loss or gain for each element. For a selected tissue element (shaded triangle), simulated exchange processes with its neighboring vessel and tissue elements are indicated by arrows.
Rate of total diffusive oxygen loss (JO2 totdiff) for tissue and vessel elements is determined from all individual exchange processes with neighboring elements according to
| (4) |
where i enumerates all neighboring vessel and tissue elements of the element under consideration, Po2 is the Po2 of this element. For neighboring element i, Po2i is the Po2, Ai is the area for diffusive exchange with the element under consideration, and Δsi is the distance of its center to the center of the element under consideration.
The oxygen consumption rate (M) of the tissue is assumed to depend on Po2, according to Michaelis-Menten kinetics with
| (5) |
where M0 is oxygen demand, i.e., consumption when oxygen supply is not limiting, Po2tis is the Po2 of the tissue element, and P0 is the Po2 at which oxygen consumption equals 50% of demand (Table 1). Experimental data on the oxygen demand in the rat mesentery differ significantly. Reported values include, e.g., 60 nl O2·cm−3·s−1 (∼0.004 cm3 O2·cm−3·min−1) (27) and 2.4 × 10−4 ml O2·cm−3·s−1 (∼0.014 cm3 O2·cm−3·min−1) (66). In the model, M0 was set to 0.01 cm3 O2·cm−3·min−1 as a compromise between such experimental data. Based on literature data on intra- and extravascular Po2 values (16, 17, 68) and corresponding to studies by Pittman and coworkers (49, 67), the vessel wall was considered as part of the tissue.
In each step (k) of the iterative scheme, oxygen volume concentration (c) and Po2 in tissue and vessel elements of the preceding time step (k − 1) are used to calculate oxygen diffusion, consumption, and convection, resulting in updated values of c and Po2. First, for all finite elements (vessel elements, tissue elements), the oxygen diffusion exchange with all directly neighboring elements is calculated using Po2 values of the previous time step for all elements, and the rate of total diffusive oxygen loss, JO2 totdiff (k), is determined according to Eq. 4.
Next, oxygen content (Vo2) and oxygen volume concentration in each element are updated. In tissue elements, Vo2 of the actual time step is determined by subtracting (adding) the total diffusive oxygen loss (gain) and the amount of oxygen consumed per time interval from Vo2 of the previous time step according to
| (6) |
where Vtis is the volume of the tissue element. Vo2 is used to calculate the oxygen volume concentration according to ctis(k) = Vo2 tis(k)/Vtis.
In vessel elements, updating Vo2 content combines the consideration of diffusive oxygen loss to the tissue and of convective oxygen transport through the network. Starting with the known oxygen inflow in input boundaries, JO2 in,vesconv of all vessel elements is updated along flow pathways using the oxygen outflow (JO2 out,vesconv) their respective feeding vessel elements. JO2 out,vesconv is calculated from the flow rate and the oxygen volume concentration of the outflowing blood (cout,ves). Vo2,ves(k) is then determined on the basis of oxygen mass balance in the vessel, considering the intravascular V̇o2 and oxygen transport by convection and diffusion as
| (7) |
where Vves is the volume of the element and cves(k) = (cin,ves + cout,ves(k))/ 2 is the mean oxygen volume concentration in the vessel element. The mean oxygen volume concentration in the vessel element is determined by cves(k) = [cin,ves(k)+ cout,ves(k)]/2 and is used to calculate the new Vo2 according to Vo2 ves(k) = cves(k) Vves.
Finally, Po2 is updated in each element using c(k). In tissue elements, Po2tis is calculated according to Po2 tis(k) = αtc tis(k), where αt is the solubility of oxygen in the tissue. In vessel elements, Po2ves and So2 are determined by an iterative solution of Eq. 2. Calculations are repeated until the average change of tissue Po2 between two consecutive time steps is smaller than the set criterion (average change of Po2tis < 10−3 Torr).
For validation of the O2 transport model simulation, a theoretical test system was created. The test system consisted of one vessel segment and a large, adjacent tissue region, which was completely avascular to avoid interactions with other segments. The simulated Po2 profile along a line perpendicular to the vessel wall meets theoretical expectations of an exponential oxygen decline within the tissue and lies within the brackets of different in vivo findings for the rat mesentery using the phosphorescence quenching method (27, 66). An alternative approach to validate the present model is the analysis of overall oxygen extraction in the vascular network. For the network studied, the model predicts an arteriovenous Po2 decline of 8.4–19.5 Torr in dependence on the Po2 assumed for the main arteriole of 70–95 Torr. This corresponds to experimental findings in the rat mesentery of 12.8 Torr (26).
Adaptation model.
The adaptation model (Fig. 3, bottom, gray background) is based on the hypothesis that the steady-state vessel diameter of each vessel segment is determined by responses to several stimuli derived from local hemodynamic and metabolic conditions/signals. A logarithmic relationship between signal and evoked adaptive stimulus was assumed in accordance with the Weber-Fechner law. Diameter changes triggered by these stimuli are assumed to be governed by the same rules in all segments of the network. The diameter adaptation process is inherently unbounded, and the final (steady-state) diameter of a given vessel is only determined by the adaptive stimuli, derived from the surrounding conditions, and the assumed adaptation rules.
For each vessel segment, the sum of hemodynamic and metabolic stimuli gives the net adaptive stimulus Stot, which determines the change (ΔD) of the vessel diameter (D) per time step (Δt) according to
| (8) |
with
| (9) |
Hemodynamic stimuli (Sτ, Sp) are derived from local wall shear stress (τ) and transmural pressure (p), respectively. Metabolic stimuli (Sm, Sc) correspond to the local concentration of a metabolic signal substance at the vessel wall and to a conducted signal, respectively (see below). The constants kh and km determine the impact of overall hemodynamic and of metabolic stimuli for diameter adjustment, respectively. Because the net adaptive stimulus Stot is zero in the equilibrium state, only the relative strength of hemodynamic and metabolic stimuli is relevant, and kh was set to 1. Thus the relative impact of metabolic stimuli compared with hemodynamic stimuli is determined by km. The constant kp is used to weigh the relative impact of pressure effects to shear stress effects. The constant kc relates the effects of conducted stimuli to the effects of stimuli derived from local metabolite concentration.
The parameter ks was introduced into the model to balance the effect of adaptive stimuli for vessel diameter adjustment derived from wall shear stress and the metabolic situation, which are assumed to evoke diameter increase (see below). Thus ks can be interpreted mathematically as a construct to allow the achievement of equilibrium. On the other hand, ks can be interpreted biologically to represent a “shrinking tendency” of vessels in the absence of positive growth stimuli. This is in accordance with results in experimental studies, showing atrophy or degradation in the absence of positive trophic stimuli, e.g., in unloaded or denervated muscles, and in cell cultures if no growth/survival factors are added to the culture medium.
Vessel diameter adaptation is simulated as an iterative process, alternately updating surrounding conditions (hemodynamic, metabolic) and vessel diameters. Since no information on dynamics of adaptive response of vessel diameters is included in the present approach, the time step duration Δt is an arbitrary parameter.
Increased wall shear stress is assumed to lead to an increase in vessel diameter, and the signal from wall shear stress was calculated as Sτ = log [(τ + τo)/ε], where τo [τo = 0.103 dyn/cm2 (56)] is a small constant included to ensure that Sτ remains bounded for very low shear stress values. The dimensional constants ε (numerical value of 1) here and in the following equations are introduced to ensure correct dimensions.
The stimulus caused by transmural pressure is assumed to decrease vessel diameter and is calculated as Sp = −log [τe(p)/ε], assuming that the transmural pressure (p) controls the set point of the vascular feedback control to shear stress τe(p). According to experimental data, this set point level τe(p) increases sigmoidally with p as τe(p) = [100 − 86 exp(−5,000 {log [log (p/ε)]}5.4)]ε (58).
The metabolic signals are assumed to correspond to the local Po2 or So2. According to the aim of the present study, three metabolic signaling modes were implemented, with rules for release of the metabolic signaling substance exhibiting the same general structure. The signal substance is assumed to be released at a rate Jm (measured in arbitrary units), depending on the following: 1) the oxygen-dependent hypothetical vasoactive metabolic signal substance is generated if Po2 (So2) in the respective oxygen-sensing structure falls below a reference level (Po2ref, So2ref), assuming a linear increase of production rate with decreasing V̇o2. The oxygen-dependent signal is set to zero, in case the present value exceeds the reference value; 2) the size of the substance-releasing structure; and 3) a scaling constant βX introduced to guarantee that, under “baseline” conditions, i.e., assuming experimental segment diameters, the total amount of metabolite released per time is the same for all tested modes. Metabolite release resulting from tissue signaling was arbitrarily chosen as a reference, setting the respective scaling constant, βT, to 1. The scaling constants for wall signaling, βW, and RBC signaling, βRBC, were adjusted accordingly.
In tissue signaling, signal substance is released from each tissue element according to
| (10) |
where the size of the substance-releasing structure is represented by the volume of the tissue element (Vtis).
In wall signaling, substance release was assumed to occur in proportion to segment length (L), with substance flux added to the blood calculated as
| (11) |
with βW = 1.38. For a given Po2, the evoked relative diameter change (ΔD/D) was assumed to be identical in vessels of equal length and flow rate, which requires the generation of identical metabolic stimuli (Sm) in these vessels. As Sm is assumed to depend on the metabolite concentration in the flowing blood (see below, Eq. 14), respective metabolite release rates from the vessel wall have also to be identical, although these vessels may exhibit differing inner surface areas due to unequal diameters. Consequently, metabolite release was assumed to be independent of vessel diameters.
In RBC signaling, corresponding to experimental findings on ATP release from erythrocytes (39), signal substance is released in response to the local value of So2 and in proportion to the local (tube) hematocrit (HT) according to
| (12) |
(βRBC = 0.03), where So2seg and So2ref are So2 for the average Po2 of the segment and for the reference oxygen partial pressure, respectively; Vseg is the intravascular volume.
In tissue signaling, the metabolic signal substance reaches wall and lumen of vessel segments only after diffusion. A diffusion coefficient of Dmet = 4 × 10−6 cm2/s, as determined for nucleotides (18, 35), was used. In contrast, the metabolic signaling substance is delivered into the flowing blood directly in wall signaling and RBC signaling. The metabolic signal substance entering the segment lumen locally in each vessel segment (Jm,seg) is added to the amount of metabolic signal substance convected from upstream regions (Jm,segup), resulting in substance flux at the downstream end of the segment (Jm,segdown), according to
| (13) |
At vascular branch points, the metabolic signal substance is distributed in proportion to blood flow. No decay of the signal substance was assumed. Metabolic signal substance transport is determined according to the same approach as is used for oxygen, described above.
The local concentration of metabolic signal substance in the blood (cm) is used to calculate the metabolic stimulus (Sm) according to
| (14) |
If the signal substance is transported exclusively by intravascular convection, cm corresponds to the mean substance concentration in the vessel segment (cm,seg). Intravascular concentration is determined according to cm,seg = Jm,seg/(Q˙ + Q˙ref), where Q˙ is segment blood flow. Q˙ref is a small constant included to keep concentration values bounded for very low segment blood flow. In case of additional diffusive transport across the tissue, cm is determined from both the segment concentration and the average concentration in all tissue elements directly adjacent to the segment (cm,tis), according to cm = (cm,seg + cm,tis)/2.
In accordance with experimental and theoretical findings, showing the importance of information transfer along flow pathways for coordinating diameter adjustment in vascular networks (63) and for preventing arteriovenous shunts (56), metabolic information is assumed to be conducted upstream along vessel walls throughout the entire network (11), in addition to the downstream convection. A local conducted signal (Jc, dimensionless) is generated in each vessel in proportion to the local metabolic stimulus and to segment length according to
| (15) |
Jc is added to the conducted signal reaching the segment from downstream parts of the vessel network (Jcdown). At converging bifurcations, the signal is equally distributed to the (upstream) feeding segments, independent of their blood flow, in diverging bifurcations signals of the (downstream) daughter segments sum up. The conducted signal declines along vessel segments with exponential length constant Lc, leading to a conducted signal on the upstream end of the segment (Jcup), according to
| (16) |
assuming that the metabolic signal entering the segment from downstream segments decays over the total segment length, whereas the metabolic signal added locally decays over a one-half segment length only. The conducted stimulus (Sc) for diameter adaptation based on the midsegment conducted signal is defined as
| (17) |
where Jref is a reference conducted signal.
Experimentally recorded microvascular networks exhibit secondary input and output boundaries, in addition to the main feeding and draining vessels. For secondary boundaries, values must be assigned for So2 and flux of metabolic signal substance (inputs) and conducted signals (outputs). To minimize possible artifactual effects of these assigned values on simulation results, the respective values were estimated relative to calculated values of pressure and flow for main feeding and draining segments.
For all simulation runs, the following boundary conditions were set as close as possible to the in vivo state while testing different adaptation characteristics: Po2 in the main feeding arteriole (value: 95 Torr, estimated); discharge hematocrit in input boundary segments (values: individually measured for each segment); transmural pressure in the main draining venule [value: 13.8 Torr, according to measurements (61)]; and flow rates in all other segments entering or leaving the network (values: calculated from measured flow velocities and vessel diameters). Alternatively, it would have been feasible to assign pressure values (instead of flow rates) to all boundary segments. However, flow rates were chosen, with the exception of the main venule, because they could be based on actual experimental values.
Diameter adaptation was simulated until convergence was achieved (segment average diameter change < 10−5 μm). Usually ∼2,000 adaptation runs corresponding to 10- to 20-min calculation time on a conventional personal computer (2.5 GHz) were needed.
Variation of model parameters.
For each metabolic signaling mode, the combination of adaptation parameters leading to vascular network structures with highest similarity to in vivo structures was determined. The most sensitive indicator for functional similarity is given by the blood flow velocity error (Verr), indicating the difference between blood flow velocities observed in vivo and those resulting from simulated adaptation. Verr is calculated as root mean square relative deviation (RMS) of predicted from measured vessel segment blood flow velocities. Velocity differences are especially sensitive, since even small changes of diameters elicit large changes in flow velocity. In addition, the relative measurement errors of velocities are smaller than those of vessel diameters (59).
The deviation of velocities predicted by the flow model for experimentally measured diameters (without simulated adaptation) from corresponding measured velocities resulted in a Verr of 0.61 (RMS) with a SE of the mean of about ±0.01 (57). The value of 0.61 is close to the achievable minimum, given the experimental and theoretical constraints (61). Increases of the Verr above this reference are used to evaluate hemodynamic similarity to the in vivo situation reached by different metabolic signaling modes, corresponding to findings of earlier related studies (61, 57). Such increases proved to react sensitively and consistently to modifications of adaptation modes or parameters (55). The robustness of the Verr depends on the size of the network analyzed and is thus supported here by the relatively high number of vessel segments studied (576 segments).
In the present study, a downhill simplex procedure (47) for the optimization of the model parameters (km, kc, kp, ks, Po2ref, Q˙ref, Jref, Lc) (59) was used to minimize Verr for each metabolic signaling mode. The total vascular volume results from the simulated adaptation process. For the network state at minimal Verr, tissue oxygen deficit was determined as the percentage of tissue elements exhibiting a Po2 < 1 Torr. In addition, diameter error (Diaerr) and hematocrit error were calculated as RMS deviation of predicted from measured vessel diameter or vessel hematocrit, respectively.
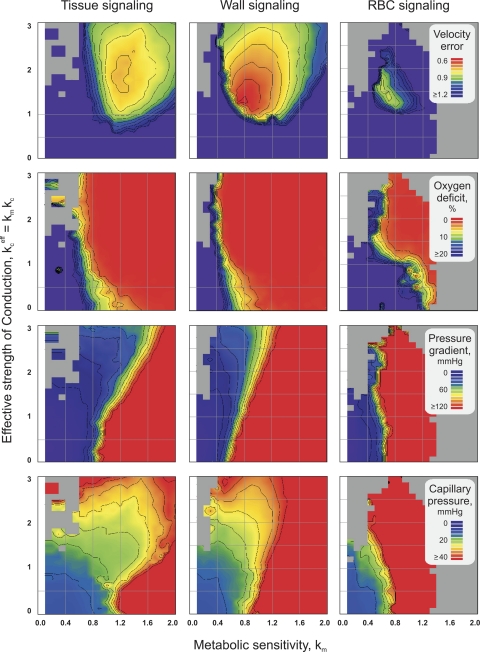
In addition, a systematic variation of the two most relevant parameters of the simulated adaptation, the relative strength of metabolic vs. hemodynamic stimuli (km) and the effective strength of conducted signaling of the metabolic information (kceff = km kc), was used to investigate overall effects of individual metabolic signaling modes on functional network properties. The constant kp was not included in the scans because of the low impact on the respective results (55). The model constant km was varied from 0.1 to 2.0 in steps of 0.1, and kceff was varied from 0.0 to 3.0 in steps of 0.125. For each combination of km and kceff in the two-dimensional scans, the Verr, the tissue oxygen deficit, the pressure difference between the feeding arteriole and the draining venule, and the mean capillary pressure were determined. During the scans, ks was used to fix the total intravascular volume of the network to the experimentally determined value to counteract changes in the effective strength of stimuli derived from a given situation accompanying the variation of km and kceff.
RESULTS
Detailed results are given for the network with 576 vessel segments and an oxygen level in the main feeding arteriole of 95 Torr. Similar results were obtained for this network, assuming a feeding Po2 of 70 Torr (26), and for the second mesenterial network with 389 vessel segments (data not shown).
For each metabolic signaling mode, the network characteristics at minimal Verr and the corresponding optimized model parameters (km, kc, kp, ks, Po2ref, Q˙ref, Jref) are shown in Table 2. Optimizations robustly yielded values for Lc substantially higher than the maximal flow path length through the network (∼9 mm). Therefore, Lc was set to infinity in the model for all metabolic signaling modes, corresponding to an unattenuated spread of the conducted signal. Such a nondecaying propagation of signals along the vessel wall was also found experimentally (22).
Table 2.
Characteristic network parameters obtained by model simulations of hemodynamics and oxygen distribution using experimental diameters and diameters after simulated adaptation with different metabolic signaling modes
| Experimental Diameters | Simulated Diameter Adaptation |
|||
|---|---|---|---|---|
| Tissue Signaling | Wall Signaling | RBC Signaling | ||
| Network characteristics | ||||
| Verr | 0.61 | 0.73 | 0.60 | 0.81 |
| Diaerr | 0 | 0.50 | 0.38 | 0.68 |
| Hcerr | 0.51 | 0.65 | 0.58 | 0.92 |
| OxDef, % | 0.7 | 0.2 | 0.2 | 4.7 |
| Vol, nl | 44 | 43 | 42 | 35 |
| Optimized adaptation parameters | ||||
| km | 1.30 | 0.80 | 0.64 | |
| kc | 1.52 | 1.60 | 2.17 | |
| kp | 0.90 | 0.35 | 0.65 | |
| ks | 3.24 | 2.51 | 1.89 | |
| Pref, mmHg | 115 | 95 | 92 | |
| Q˙ref, nl/min | 0.007 | 0.012 | 0.012 | |
| Jref | 4,822 | 4,925 | 5,537 | |
See text for definitions of acronyms. For simulated adaptation, optimized values of adaptation parameters are given.
The lowest value of Verr, 0.60, was achieved, assuming wall signaling. Other signaling modes (tissue signaling, RBC signaling) resulted in significantly higher Verr values. The largest value, obtained for RBC signaling, indicates unrealistic network structures and function. Diaerr and hematocrit error also exhibited minimal values, assuming wall signaling. The oxygen deficit was <1% in wall signaling and tissue signaling, while RBC signaling resulted in an oxygen deficit of 4.7%.
For experimental diameters, the estimated oxygen deficit was 0.7%, whereas it is reasonable to assume that, in vivo, no oxygen deficit is present. Various causes may underlie this discrepancy, including simplifying assumptions made in the model (e.g., with respect to the assumed oxygen diffusivity, tissue thickness, homogeneity of oxygen consumption, and absence of oxygen exchange with the surrounding peritoneal fluid). However, due to their effect on flow resistance, measurement errors in vessel diameters may play a dominant role. Accordingly, simulated adaptation, which eliminates possible measurement errors in vessel diameters, led to lower oxygen deficits for wall signaling and tissue signaling.
Functional network properties resulting from systematic variation of the metabolic sensitivity (km) and strength of conductive transfer of metabolic information upstream (kceff = km kc) are shown in Fig. 5. Lower values of Verr and oxygen deficit of the tissue (rows 1 and 2) indicate higher consistency with data obtained for the in vivo network. For the bulk pressure drop across the network and for the mean capillary pressure (rows 3 and 4), closest to consistency with experimental data is achieved within defined pressure ranges (50–90 and 23–27 Torr, respectively).
Fig. 5.
Effects of varying the relative impact of metabolic stimuli compared with hemodynamic stimuli (km) and the effective strength of reactions to metabolic signals conducted upstream (kceff) on network properties. In all plots, light gray regions correspond to numeric instabilities in the calculation of hemodynamics. Row 1: matching between simulated and experimental blood flow velocities in the network (velocity error Verr). Row 2: tissue oxygen deficit (percentage of tissue area with Po2 < 1 Torr). Row 3: bulk pressure drop across vascular network. Row 4: mean capillary pressure.
The Verr (Fig. 5, first row) reaches a single minimum in each mode, with values given in Table 2. However, individual minimal values of Verr markedly differ for the different signaling modes, with the lowest Verr reached by wall signaling. For all modes, tissue oxygen deficit (Fig. 5, second row) occurs if km values are lower than a critical level. The pressure difference between the feeding arteriole and the draining venule (Fig. 5, third row) and the mean capillary pressure (Fig. 5, fourth row) both rise with increasing km in all three metabolic signaling modes.
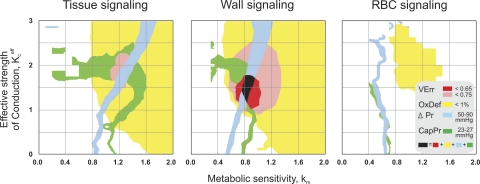
For the comparison of the different metabolic signaling modes tested, it is relevant to identify parameter domains in which all critical functional network properties exhibit realistic values compared with experimental data (Fig. 6). For all modes, tissue oxygen deficit is absent over wide ranges of model constants, whereas physiological values for the pressure difference between the feeding arteriole and the draining venule and the capillary pressure are obtained only for narrow bands of model parameter combinations. In tissue signaling and wall signaling, parameter combinations exist that yield a coincidence of the lowest respective Verr, minimal oxygen deficit, and physiological pressure values. However, regions of low Verr are larger, and the minimal Verr is markedly lower in wall signaling than in tissue signaling. In RBC signaling, no overlap of ranges for model parameter combinations leading to adequate tissue oxygenation and pressure distributions is observed. Also, low levels of Verr are not obtained for any parameter combination.
Fig. 6.
Synopsis of combinations of km and kceff leading to low Verr, absence of oxygen deficit (OxDef), and realistic values for the pressure drop across vascular network (ΔPr) and mean capillary pressure (CapPr). Assuming both tissue signaling and wall signaling, network structures at minimal Verr coincide with physiological values of all other parameters (OxDef, ΔPr, CapPr). However, minimal Verr is markedly lower for wall signaling (<0.65) than for tissue signaling (0.65 < Verr < 0.75). In RBC signaling, minimal Verr exceeds 0.75, and model constant combinations leading to physiological oxygen and pressure distributions do not overlap.
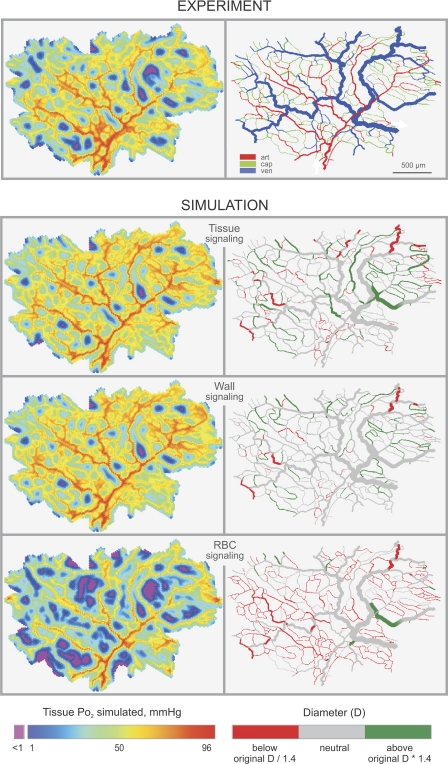
Figure 7 shows distributions of tissue oxygen levels and deviations between predicted and observed diameters for the three metabolic signaling modes, analyzed at the respective minimal levels of Verr. Both tissue signaling and wall signaling led to oxygen distributions within the tissue, similar to those estimated for in vivo network structures. However, tissue signaling caused unrealistic diameter increase in some feeding arterioles and shrinkage of some large venules. In the case of wall signaling, significant diameter deviations were fewest and exhibited a seemingly random distribution. The most pronounced diameter deviations were seen as a result of RBC signaling with excessive shrinking of small vessels.
Fig. 7.
Predicted tissue oxygen levels (left) and vessel diameters (right) in a mesenteric microvascular network. Experiment, results with measured diameters; vessels color-coded for segment type derived from adjacent bifurcations (diverging, converging). art, Arteriole; cap, capillary; ven, venule. White arrows indicate flow direction in the feeding arteriole and the draining venule. Simulation, results for three assumed signaling modes. Vessels are color-coded for deviation of predicted from measured diameters. Tissue signaling gives good tissue oxygenation, but some distal arterioles are unrealistically large, and some venules are too small. Wall signaling gives good tissue oxygenation and no systematic diameter deviations. RBC signaling results in large undersupplied tissue regions and shrinking of small vessels.
DISCUSSION
In the present study, the ability of different modes of metabolic signaling in response to the local oxygenation to achieve adequate oxygen supply to the tissue and realistic vascular network hemodynamics by long-term structural adjustment of vessel diameters in microvascular networks was studied. Of the three modes of metabolic signaling considered (Fig. 1), wall signaling gives functionally most adequate results with low oxygen deficit in the tissue, in combination with a high similarity to measured data, as evidenced by low blood flow velocity deviations (Verr). Tissue signaling also provides realistic oxygen distributions, but leads to a somewhat higher systematic deviation of flow velocities and vessel diameters from those measured in vivo. RBC signaling results in poor oxygen supply and diameter distributions, which strongly differ from experimental data.
This study is based on experimental data from the rat mesentery (60). The mesentery has a relatively low metabolic demand (27, 66), some of which might be met by diffusion from the surrounding peritoneal fluid. Two extreme cases can be considered. 1) The surrounding peritoneal fluid is well stirred, leading to a homogenous Po2 at all parts of the mesenteric surface. The mesenteric membrane would thus be homogenously supplied with oxygen. Microvascular networks in the mesentery would not be needed for oxygen supply. 2) The peritoneal fluid is unstirred. Because the peritoneal fluid exhibits low oxygen solubility, each tissue region would control the Po2 of the adjacent peritoneal fluid, necessitating a microvasculature for adequate oxygen supply.
In the mesenteries studied, networks exhibited relatively homogenous distributions of microvessels, supporting assumption 2. Also, measured network morphologies and flows resulted in adequate oxygen supply to the tissue, when oxygen consumption typical for connective tissue was assumed. This suggests that vessel diameters observed in vivo represent the result of the adjustment to the metabolic demand of the surrounding mesenterial tissue (58). In the oxygen transport model used here, the mesentery was consequently assumed to be supplied with oxygen only by its vasculature. A limited additional supply with oxygen by the peritoneal fluid would tend to homogenize oxygenation, but would not change the conclusions of the present study. The conclusions of the present study regarding metabolic control of structural adaptation reflect general principles of the mechanisms considered, as discussed below, which should not depend critically on the specific angioarchitecture and design of a given microvascular bed or on the exact level of metabolic demand.
For a given oxygenation, the amount of metabolic signaling substance released depends on characteristics of the signaling structures. In RBC signaling, the metabolic substance is delivered in proportion to the number of RBCs in the vessel, which is determined by vessel volume and hematocrit. Thus the mismatch between oxygen supply and demand, assuming RBC signaling, can be explained as follows. Microvessels in underperfused tissue regions are characterized by low RBC flux and/or content. The amount of signaling substance released in a given vessel of the underperfused region at a given RBC So2 may, therefore, also be low, evoking a weak metabolic stimulus, allowing further decrease in vascular diameter and blood flow, in a positive feedback mechanism. This cannot be overcome by the fact that the pronounced decrease in So2 in underperfused tissue regions naturally increases the amount of metabolic signaling substance released by each RBC (39). Consequently, both vessel volume and hematocrit may further decline, corresponding to a further reduction of the number of signaling RBCs, again leading to a diameter decline.
In additional simulations, phase separation effects (53, 54) were eliminated, leading to a homogenous hematocrit throughout the network. The absence of a decrease in Verr (Verr = 0.81) indicates that resulting amounts of metabolic signal substance released were still insufficient to guarantee adequate capillary diameters.
These considerations are supported by supplementary simulations (data not shown) on RBC signaling, assuming release of the metabolic signal substance proportional to the decline of hemoglobin So2 between entrance and exit of a segment, according to studies on the liberation of NO from S-nitrosohemoglobin by RBCs (40). In this case, the metabolite is released from each RBC in a finite amount during the arteriovenous oxygen desaturation cycle of hemoglobin. Thus the amount of signaling substance depends not only on hematocrit, but additionally on blood volume flow, which are both reduced in underperfused tissue regions. Accordingly, simulated adaptation with this type of RBC metabolite release led to even stronger hypoxia and even higher deviations from experimentally determined network characteristics.
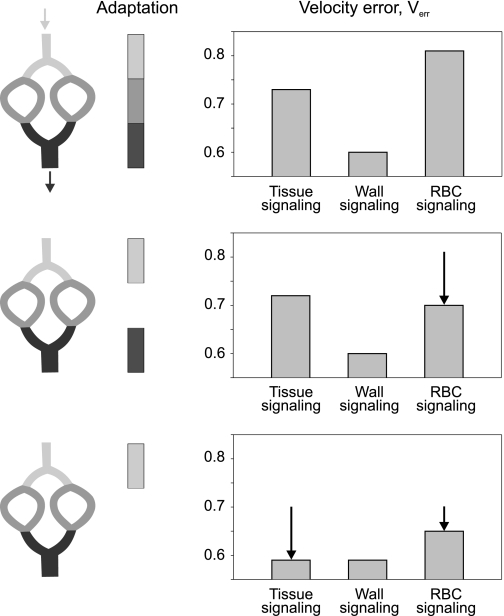
Although RBC signaling seems inadequate for structural control of blood flow distribution in microvascular networks, this mechanism may well play a role in adjusting total network blood flow to acute changes in tissue metabolic demand (2). Provided that the distribution of structural vessel diameters is adjusted by other metabolic signaling modes, the So2 in collecting venules reflects the overall match of oxygen supply to demand in the network. In case of relative underperfusion, a large RBC population experiences low So2, leading to a strong RBC-derived metabolic signal in draining venules. This signal could be transferred to feeding arterioles by upstream conduction along the vessel wall (11) and/or diffusion of a metabolic signaling substance (34), especially for parallel arteriole-venule arrangements, evoking arteriolar diameter increase and augmenting total network blood flow. A role for RBC signaling in the control of arteriolar vessel diameters is supported by findings of additional calculations, in which adaptation was allowed only in arterioles and venules or only in arterioles, respectively, and adaptation parameters were optimized as described before (see Fig. 8). When only arterioles were allowed to adjust their diameters, Verr for RBC signaling was markedly reduced (Fig. 8, bottom). However, the reference value of Verr determined for experimentally measured diameters (Verr = 0.61) was not reached.
Fig. 8.
Effects of applying simulated diameter adaptation to all segment types (top row), only to arterioles and venules (middle row), and only to arterioles (bottom row) on minimal Verr. Arrows (diagrams, right) correspond to the difference of Verr from values in the row above. For wall signaling, low levels of Verr are obtained, irrespective of the extent of simulated adaptation. For tissue signaling, Verr is reduced to low levels, if venular diameters are not adapted. In RBC signaling, Verr is markedly reduced, if adaptation is restricted to arterioles.
The described feedback problems do not occur for wall signaling or tissue signaling. In these modes, the number of cells generating the signaling substance is independent of local hemodynamics. Tissue cells might seem to be the most probable origin of metabolic signals for vascular diameter adaptation, since the primary task of microvascular networks is the adequate supply of the tissue with substrates. Surprisingly, the present study suggests that wall signaling is better suited to control vessel diameters than tissue signaling. Both tissue signaling and wall signaling are capable of preventing oxygen deficit in tissue, but wall signaling leads to a lower Verr and Diaerr.
This apparent paradox may be explained by considering the factors influencing oxygen levels at the oxygen sensor in both signaling modes. The oxygenation of tissue cells is determined both by their distance to the nearest vessels (diffusion-limited transport) and by the perfusion rate in these vessels (flow-limited transport). The perfusion rate, in turn, depends on vascular diameter. The diffusion distance can be modified only by changes in vessel density regulated by angiogenesis or vessel pruning. Consequently, control of vascular diameters by metabolic signals derived from the tissue cannot guarantee adequate tissue oxygenation, if vessel density is too low. In this situation, tissue cells would continue to produce increased levels of the metabolic signaling substance, despite excessive increase of vessel lumen in the respective regions, compromising the feedback between the metabolic signal production and tissue hypoxia. In Fig. 7, this is reflected by the increased number of arterioles, with diameters enlarged relative to the experimental measurements.
In contrast to tissue hypoxia, hypoxia of the vessel wall occurs only when perfusion is insufficient relative to oxygen extraction. This can be addressed by increased vessel diameter, leading to reduced resistance and increased flow rate. Thus a signal derived from the vessel wall is better suited for controlling vascular diameters. Signals delivered by tissue cells, on the other hand, would be better suited to regulate vessel density by inducing angiogenesis (or pruning). In the present study, no hypoxia was observed in the rat mesenteries serving as a reference when oxygen demand typical for connective tissue was assumed in the oxygen transport model. Thus there were probably no strong angiogenetic signals present.
The difference between tissue signaling and wall signaling with respect to the achieved degree of similarity to real network characteristics (Verr, Diaerr) seems to be small. However, this difference is robust and reproducible and is maintained for a number of modifications of the adaptation scheme including the following: 1) not allowing capillaries to adjust their diameters (tissue signaling: Verr = 0.72, wall signaling: Verr = 0.61; Fig. 8, middle); 2) keeping the discharge hematocrit constant at a value of 0.4 throughout the network by abolishing phase separation (tissue signaling: Verr = 0.73, wall signaling: Verr = 0.60); and 3) using a different network angioarchitecture, i.e., the second, smaller mesenterial network (tissue signaling: Diaerr = 0.40, wall signaling: Diaerr = 0.28; flow velocities were not measured for this network).
For tissue signaling, a reduction of Verr to the reference value (Verr = 0.61, which corresponds to the values obtained from wall signaling); however, was observed when venular diameters were fixed, in addition to fixing capillary diameters (Fig. 8, bottom). Therefore, the signals generated in this mode seem to be inadequate to regulate venular adaptation. A possible explanation may be that the caliber of venules should relate more to the oxygen extraction in the drained capillary bed in contrast to the oxygenation of the tissue in which they are embedded (which is influenced by oxygen exchange with neighboring vessels).
In the modeling approach used, only one metabolic signaling mode was assumed to be active at a time. By this means, consequences of the signaling mode under consideration for network properties are revealed more clearly, and the ability of each mode to play a leading role can be evaluated. However, different metabolic signaling modes may cooperate in vivo. Corresponding experimental data are difficult to obtain because of methodological difficulties in guaranteeing stable conditions and also in blocking all but one metabolic signaling mode over periods of time needed for structural adaptation.
Both in vitro and in vivo approaches have been used experimentally. Isolated vessels perfused with RBC-free solutions with different Po2 exhibited an oxygen reactivity that depended on the integrity of the endothelial cell layer (9, 14, 31, 38, 45, 50), suggesting wall signaling to have an important impact on vessel diameter adaptation. It was also shown that, in isolated rat cerebral arterioles (passive maximum diameter 76.4 ± 4.5 μm), low extraluminal oxygen tension resulted in vasodilation, only if the vessels were perfused with RBCs (15), suggesting the importance of RBC signaling. In an extended in vitro approach, larger septal artery segments from rat hearts, with and without a layer of surrounding cardiac tissue, were used (42). In this setup, hypoxic relaxation was augmented by the presence of parenchyma, indicating tissue signaling to play a role in tone regulation upon impaired oxygen supply.
Experiments directly addressing the relative impact of tissue and vessel-derived, oxygen-dependent signals in diameter adaptation in vivo are rare. In acute experiments in the hamster cheek pouch, Jackson and colleagues (7, 37, 38) used a technique that allows arterioles to be separated from the surrounding parenchyma. In these studies, global changes in superfusate Po2 evoked significant, reproducible changes in arteriolar tone and diameter (37). However, this was not the case for local Po2 changes at the vessel wall, produced by microapplication of fluid to the vessel surface or by cannulation and perfusion of the arteriole. In addition, observed vascular diameter changes were not influenced by the presence of RBCs in the vessel lumen (7, 37). Jackson (37) hypothesized that oxygen sensors are located either in the parenchyma adjacent to the vessel segment studied or in vessels downstream. The latter alternative would require conduction of signals derived from the local metabolic situation upstream along vessel walls.
Thus experimental findings on the localization of the oxygen sensor for acute diameter adaptation are still not congruent. For structural adaptation, corresponding experimental data are not even available. Also, the molecular mechanisms of oxygen sensing in terminal vascular beds are not well understood. Identification of mechanisms for oxygen sensing by vessel walls, under conditions in which Po2 is relatively high and metabolism is not limited by oxygen availability, is an important unresolved question.
In conclusion, the present study indicates that vessel wall signaling is centrally involved in the long-term, steady-state adjustment of vessel diameters in microvascular networks in response to metabolic signals. Tissue signaling, which seems to be of minor importance for this type of diameter adaptation, may have a crucial role in stimulating the generation of new vessels by sprouting angiogenesis in hypoxic tissue regions. RBC signaling, although incapable of guaranteeing adequate structural diameter adaptation within the network, may play a supporting role for the adjustment of network total blood flow to metabolic demand, via changes in vessel tone.
GRANTS
This study was supported by Deutsche Forschungsgemeinschaft Grant FOR 341/TP1 and by National Heart, Lung, and Blood Institute Grant HL034555.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
Inspiring discussions with F. Reglin are gratefully acknowledged.
REFERENCES
- 1.Altman PL, Dittmer DS. In: Respiration and Circulation, Bethesda, MD: Fed. Am. Soc. Exp. Biol., 1971, p. 139 [Google Scholar]
- 2.Arciero JC, Carlson BE, Secomb TW. Theoretical model of metabolic blood flow regulation: roles of ATP release by red blood cells and conducted responses. Am J Physiol Heart Circ Physiol 295: H1562–H1571, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakker EN, Der Meulen ET, Spaan JA, VanBavel E. Organoid culture of cannulated rat resistance arteries: effect of serum factors on vasoactivity and remodeling. Am J Physiol Heart Circ Physiol 278: H1233–H1240, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Barber BJ, Oppenheimer J, Zawieja DC, Zimmermann HA. Variations in rat mesenteric tissue thickness due to microvasculature. Am J Physiol Gastrointest Liver Physiol 253: G549–G556, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26: 40–47, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol 204: 317–322, 1963 [DOI] [PubMed] [Google Scholar]
- 7.Brekke JF, Jackson WF, Segal SS. Arteriolar smooth muscle Ca2+ dynamics during blood flow control in hamster cheek pouch. J Appl Physiol 101: 307–315, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Broten TP, Romson JL, Fullerton DA, Van Winkle DM, Feigl EO. Synergistic action of myocardial oxygen and carbon dioxide in controlling coronary blood flow. Circ Res 68: 531–542, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Busse R, Pohl U, Kellner C, Klemm U. Endothelial cells are involved in the vasodilatory response to hypoxia. Pflügers Arch 397: 78–80, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Clark A, Jr, Federspiel WJ, Clark PA, Cokelet GR. Oxygen delivery from red cells. Biophys J 47: 171–181, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res 56: 43–53, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Costa LE, Mendez G, Boveris A. Oxygen dependence of mitochondrial function measured by high-resolution respirometry in long-term hypoxic rats. Am J Physiol Cell Physiol 273: C852–C858, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am J Physiol Heart Circ Physiol 292: H3072–H3078, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG., Jr Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol 278: H1294–H1298, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension. Circ Res 27: 669–678, 1970 [DOI] [PubMed] [Google Scholar]
- 17.Duling BR, Kuschinsky W, Wahl M. Measurements of the perivascular Po2 in the vicinity of the pial vessels of the cat. Pflügers Arch 383: 29–34, 1979 [DOI] [PubMed] [Google Scholar]
- 18.Dworkin M, Keller KH. Solubility and diffusion coefficient of adenosine 3′:5′-monophosphate. J Biol Chem 252: 864–865, 1977 [PubMed] [Google Scholar]
- 19.Earley S, Pastuszyn A, Walker BR. Cytochrome p-450 epoxygenase products contribute to attenuated vasoconstriction after chronic hypoxia. Am J Physiol Heart Circ Physiol 285: H127–H136, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc 36: 35–41, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal 11: 251–266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujino H, Kohzuki H, Takeda I, Kiyooka T, Miyasaka T, Mohri S, Shimizu J, Kajiya F. Regression of capillary network in atrophied soleus muscle induced by hindlimb unweighting. J Appl Physiol 98: 1407–1413, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med 36: 707–717, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Goldman D, Popel AS. A computational study of the effect of capillary network anastomoses and tortuosity on oxygen transport. J Theor Biol 206: 181–194, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Golub AS, Barker MC, Pittman RN. Microvascular oxygen tension in the rat mesentery. Am J Physiol Heart Circ Physiol 294: H21–H28, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Golub AS, Barker MC, Pittman RN. Po2 profiles near arterioles and tissue oxygen consumption in rat mesentery. Am J Physiol Heart Circ Physiol 293: H1097–H1106, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol 572: 295–305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Gray LH, Steadman JM. Determination of the oxyhemoglobin dissociation curves for mouse and rat blood. J Physiol 175: 161–171, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grser T, Rubanyi GM. Different mechanisms of hypoxic relaxation in canine coronary arteries and rat abdominal aortas. J Cardiovasc Pharmacol 20, Suppl 12: S117–S119, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Hacking WJG, VanBavel E, Spaan JAE. Shear stress is not sufficient to control growth of vascular networks: a model study. Am J Physiol Heart Circ Physiol 270: H364–H375, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Hellums JD, Nair PK, Huang NS, Ohshima N. Simulation of intraluminal gas transport processes in the microcirculation. Ann Biomed Eng 24: 1–24, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Hester RL, Hammer LW. Venular-arteriolar communication in the regulation of blood flow. Am J Physiol Regul Integr Comp Physiol 282: R1280–R1285, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Hubley MJ, Rosanske RC, Moerland TS. Diffusion coefficients of ATP and creatine phosphate in isolated muscle: pulsed gradient 31P NMR of small biological samples. NMR Biomed 8: 72–78, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Hudetz AG, Kiani MF. The role of wall shear stress in microvascular network adaptation. Adv Exp Med Biol 316: 31–39, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Jackson WF. Arteriolar oxygen reactivity: where is the sensor? Am J Physiol Heart Circ Physiol 253: H1120–H1126, 1987 [DOI] [PubMed] [Google Scholar]
- 38.Jackson WF, Duling BR. The oxygen sensitivity of hamster cheek pouch arterioles. In vitro and in situ studies. Circ Res 53: 515–525, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380: 221–226, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Kano Y, Shimegi S, Takahashi H, Masuda K, Katsuta S. Changes in capillary luminal diameter in rat soleus muscle after hind-limb suspension. Acta Physiol Scand 169: 271–276, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Kerkhof CJ, van der Linden PJ, Sipkema P. Role of myocardium and endothelium in coronary vascular smooth muscle responses to hypoxia. Am J Physiol Heart Circ Physiol 282: H1296–H1303, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Marshall JM. Adenosine and muscle vasodilatation in acute systemic hypoxia. Acta Physiol Scand 168: 561–573, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Lemus LA, Hill MA, Bolz SS, Pohl U, Meininger GA. Acute mechanoadaptation of vascular smooth muscle cells in response to continuous arteriolar vasoconstriction: implications for functional remodeling. FASEB J 18: 708–710, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Messina EJ, Sun D, Koller A, Wolin MS, Kaley G. Increases in oxygen tension evoke arteriolar constriction by inhibiting endothelial prostaglandin synthesis. Microvasc Res 48: 151–160, 1994 [DOI] [PubMed] [Google Scholar]
- 46.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem 278: 46349–46356, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Nelder JA, Mead R. A simplex method for function minimization. Comput J 7: 308–313, 1965 [Google Scholar]
- 48.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature 409: 622–626, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Pittman RN, Golub AS, Schleicher WF. Rate of decrease of Po2 from an arteriole with arrested flow. Adv Exp Med Biol 566: 257–262, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Pohl U, Busse R. Hypoxia stimulates release of endothelium-derived relaxant factor. Am J Physiol Heart Circ Physiol 256: H1595–H1600, 1989 [DOI] [PubMed] [Google Scholar]
- 51.Pries AR. A versatile video image analysis system for microcirculatory research. Int J Microcirc Clin Exp 7: 327–345, 1988 [PubMed] [Google Scholar]
- 52.Pries AR, Kanzow G, Gaehtgens P. Microphotometric determination of hematocrit in small vessels. Am J Physiol Heart Circ Physiol 245: H167–H177, 1983 [DOI] [PubMed] [Google Scholar]
- 53.Pries AR, Ley K, Claassen M, Gaehtgens P. Red cell distribution at microvascular bifurcations. Microvasc Res 38: 81–101, 1989 [DOI] [PubMed] [Google Scholar]
- 54.Pries AR, Ley K, Gaehtgens P. Generalization of the Fahraeus principle for microvessel networks. Am J Physiol Heart Circ Physiol 251: H1324–H1332, 1986 [DOI] [PubMed] [Google Scholar]
- 55.Pries AR, Reglin B, Secomb TW. Structural adaptation of microvascular networks: functional roles of adaptive responses. Am J Physiol Heart Circ Physiol 281: H1015–H1025, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Pries AR, Reglin B, Secomb TW. Structural response of microcirculatory networks to changes in demand: information transfer by shear stress. Am J Physiol Heart Circ Physiol 284: H2204–H2212, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Pries AR, Secomb TW. Microvascular blood viscosity in vivo and the endothelial surface layer. Am J Physiol Heart Circ Physiol 289: H2657–H2664, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Pries AR, Secomb TW, Gaehtgens P. Design principles of vascular beds. Circ Res 77: 1017–1023, 1995 [DOI] [PubMed] [Google Scholar]
- 59.Pries AR, Secomb TW, Gaehtgens P. Structural adaptation and stability of microvascular networks: theory and simulations. Am J Physiol Heart Circ Physiol 275: H349–H360, 1998 [DOI] [PubMed] [Google Scholar]
- 60.Pries AR, Secomb TW, Gaehtgens P, Gross JF. Blood flow in microvascular networks: experiments and simulation. Circ Res 67: 826–834, 1990 [DOI] [PubMed] [Google Scholar]
- 61.Pries AR, Secomb TW, Gessner T, Sperandio MB, Gross JF, Gaehtgens P. Resistance to blood flow in microvessels in vivo. Circ Res 75: 904–915, 1994 [DOI] [PubMed] [Google Scholar]
- 62.Richmond KN, Shonat RD, Lynch RM, Johnson PC. Critical Po2 of skeletal muscle in vivo. Am J Physiol Heart Circ Physiol 277: H1831–H1840, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science 234: 868–870, 1986 [DOI] [PubMed] [Google Scholar]
- 64.Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276: 2034–2037, 1997 [DOI] [PubMed] [Google Scholar]
- 65.Taggart MJ, Wray S. Hypoxia and smooth muscle function: key regulatory events during metabolic stress. J Physiol 509: 315–325, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsai AG, Friesenecker B, Mazzoni MC, Kerger H, Buerk DG, Johnson PC, Intaglietta M. Microvascular and tissue oxygen gradients in the rat mesentery. Proc Natl Acad Sci USA 95: 6590–6595, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vadapalli A, Pittman RN, Popel AS. Estimating oxygen transport resistance of the microvascular wall. Am J Physiol Heart Circ Physiol 279: H657–H671, 2000 [DOI] [PubMed] [Google Scholar]
- 68.Wilson DF, Lee WM, Makonnen S, Finikova O, Apreleva S, Vinogradov SA. Oxygen pressures in the interstitial space and their relationship to those in the blood plasma in resting skeletal muscle. J Appl Physiol 101: 1648–1656, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Yaegashi K, Itoh T, Kosaka T, Fukushima H, Morimoto T. Diffusivity of oxygen in microvascular beds as determined from Po2 distribution maps. Am J Physiol Heart Circ Physiol 270: H1390–H1397, 1996 [DOI] [PubMed] [Google Scholar]
- 70.Zakrzewicz A, Secomb TW, Pries AR. Angioadaptation: keeping the vascular system in shape. News Physiol Sci 17: 197–201, 2002 [DOI] [PubMed] [Google Scholar]