Abstract
Class IA (p85/p110) PI 3-kinases play a major role in regulating cell growth, survival, and motility. Activating mutations in the p110α isoform of the class IA catalytic subunit (PIK3CA) are commonly found in human cancers. These mutations lead to increased proliferation and transformation in cultured cells, but their effects on cell motility and tumor metastasis have not been evaluated. We used lentiviral-mediated gene transfer and knockdown to produce stable MDA-MB-231 cells in which the endogenous human p110α is replaced with either wild type bovine p110α, or the two most common activating p110α mutants: the helical domain mutant E545K and the kinase domain mutant H1047R. The PI3K/Akt pathway was hyperactivated in cells expressing physiological levels of helical or kinase domain mutants. Cells expressing either mutant showed increased motility in vitro, but only cells expressing the helical domain mutant showed increased directionality in a chemotaxis assay. In SCID mice, xenograft tumors expressing either mutant showed increased rates of tumor growth as compared to tumors expressing wild type p110α. However, tumors expressing the p110α helical domain mutant showed a marked increase in both tumor cell intravasation into the blood, and tumor cell extravasation into the lung after tail vein injection, as compared to tumors expressing wild type p110α or the kinase domain mutant. Our observations suggest that when compared to kinase domain mutations in a genetically identical background, expression of helical domain mutants of p110α produce a more severe metastatic phenotype.
Introduction
Phosphoinositide 3-kinases (PI 3-kinases) signal to multiple downstream pathway by the specific phosphorylation of the D3 position of the inositol headgroup. The class IA isoforms contain distinct regulatory (p85α, p85β, p55α and p50γ) and catalytic (p110α, p110β and p110δ) subunits. The p85α and p110α isoforms are mutated in human cancers, and the p110α mutants are oncogenic in vitro and in vivo (1).
The bulk of p110α mutations occur at two hotspots: an acidic cluster in the helical domain (E542, E545 and E546) and a residue in the kinase domain (H1047). Vogt and coworkers have shown that the E545K and H1047R mutants synergistically induce transformation in chick fibroblasts (2), suggesting that these mutations activate PI 3-kinase in mechanistically distinct manners. The helical domain mutations disrupt an inhibitory interface with the nSH2 domain of the p85, mimicking the effect of phosphotyrosine protein binding to the nSH2 domain (3). Consistent with this model, helical domain mutants are not activated by tyrosyl phosphopeptides but are activated by oncogenic Ras, which binds to the Ras-binding domain (RBD) of p110α (2, 4). In contrast, the p110α H1047R mutant is still inhibited by p85 (J.M. Backer unpublished), and p85/p110 dimers containing the H1047R mutant are activated by phosphopeptides (5). However, p85/p110α-H1047R mutants are not activated by oncogenic Ras, suggesting that the H1047R mutation mimics the effects of Ras binding to the RBD of p110α (2, 4).
These different mechanisms of activation could lead to different localization of PI 3-kinase activity in the cell. Both mutants bind to p85, and would be recruited to sites of receptor or docking protein tyrosine phosphorylation in growth factor stimulated cells. However, recruitment of a helical domain mutant to a tyrosine phosphorylated receptor would not lead to a gradient of PI 3-kinase activity, since these mutants are not additionally activated by SH2 domain occupancy (3). In contrast, kinase domain mutants are activated by SH2 domain occupancy, and would be more active at the site of recruitment than in the cytosol (5). Additional differences in the activity of membrane targeted versus cytosolic PI 3-kinase would be caused by binding to GTP-Ras; helical domain mutants would show increased activity upon targeting to a Ras-rich membrane domain, whereas kinase domain mutants would not (2, 4). Given recent studies showing activation of Ras isoforms in distinct membrane domains (6), this could also lead to different gradients of cytosolic versus membrane targeted activity for the two types of mutant.
While overexpression of either helical versus kinase domain mutants of p110α causes increased cell growth and transformation (7–10), studies using different methods to introduce mutant p110α have yielded discordant results as to whether their phenotypes differ in vivo (11, 12). However, Parsons and colleagues defined a gene expression signature indicative of a loss of PTEN-mediated inhibition of PI 3-kinase signaling (13); of tumors showing both the PTEN loss signature and mutation of p110α, 67% of the tumors contained kinase domain mutants, and only 19% contained helical domain or C2 domain mutants. These data strongly suggest that helical and kinase domain mutants have distinct physiological phenotypes in human cancers.
This study specifically examines the contribution of helical domain versus kinase mutations in p110α to the metastatic properties of human breast cancer cells. To address this question, we used the human cell line MDA-MB-231, which is capable of producing tumors in SCID mice, but is normal for both PI 3-kinase and PTEN. Using a lentiviral strategy, we stably replaced endogenous human p110α with physiological levels of wild type or mutant bovine p110α. Both helical domain and kinase domain mutants cause similar increases in tumor growth in vivo, as compared to cells expressing wild type p110α. However, cells expressing helical domain mutants are more chemotactic in vitro, and show markedly increased rates of intravasation and extravasation in vivo. These data suggest that helical domain mutants of p110α confer an increased metastatic potential, which could have important implications for the prognosis of patients whose tumors contain p110α mutations.
Materials and Methods
Antibodies
Affinity-purified rabbit antibodies against p110α and p85α have been previously described (14). Mouse anti-Myc antibodies were produced in-house. Anti-p-Akt and anti-Akt antibodies were purchased from Cell Signaling Technology (Danvers, MA).
Lentiviral construct and lentivirus generation
Bovine p110α bearing a C-terminal myc tag was subcloned into a modified lentiviral vector with a blasticidin selection marker (15). The oncogenic mutations E545K and H1047R, and kinase-disabling mutation R916P, were introduced into p110α using Quick Change site-directed mutagenesis kit (Stratagene, La Jolla, CA) and confirmed by sequencing. Human p110α shRNAs in pLKO.1-puro vector were purchased from Sigma (St. Louis, MI). To package the lentivirus, HEK293T cells were transfected with lentiviral vectors encoding no insert, wild type or mutant bovine p110α, or shRNA against human p110α, along with the packaging vectors pVSVG and pCMVdR. Recombinant lentivirus was collected from the tissue culture supernatant 48h after transfection.
Cell culture and stable cell lines
The human breast cancer cell line MDA-MB-231 was obtained from American Type Culture Collection (Manassas, VA) and maintained in monolayer cultures in DMEM supplemented with 10% fetal bovine serum (FBS). Cells were infected at various MOI with lentivirus expressing wild type or mutant c-terminally myc-tagged bovine p110α and selected with blasticidin. Resistant populations were blotted with anti-myc antibody to identify lines in which myc-tagged bovine p110α expression was at a physiological level. The stable lines were then subjected to a second infection with lentivirus expressing shRNA against human p110α and then selected with puromycin. Stable knockdown of human p110α was determined by real time PCR, and by Western blot analysis of parallel cultures of cells not expressing bovine p110α. This procedure resulted in four cells lines that had been subjected to sequential stable lentiviral bovine p110α transfection and human p110α knockdown. As a control, cell lines in which stable knockdown was followed by expression of helical or kinase domain mutants of p110α were also evaluated in vitro for p110 expression, Akt phosphorylation, protrusion, motility, and wound healing, and showed similar results to cell lines obtained using the replacement strategy (Supplemental Figures 1,2).
Immunoprecipitation and Western Blots
Stable cells were rinsed in cold PBS, lysed in a buffer containing 120 mM NaCl, 20 mM Tris (pH 7.5), 1 mM MgCl2, 1 mM CaCl2, 10% glycerol, 1 % NP-40, 1mM DTT, and protease inhibitor cocktail (Roche). Proteins were immunoprecipitated with anti-Myc, anti-p85, anti-p110α, anti-p110β (all produced in house) or anti-p110δ (Epitomics) antibodies. Blots were probed with anti-p85, anti-p110α (produced in house) or anti-p110δ (Epitomics) antibodies. For Akt immunoblots, stable MDA-MB-231 lines were starved for 4h in starvation medium (DMEM/0.5% FBS/0.8% BSA), stimulated without or with 5nm EGF for 3min, and immediately lysed in hot sample buffer. Proteins were separated by 10% SDS-PAGE, and anti-Akt and anti-pAkt (Cell Signaling Technologies) blots were visualized using ECL (Amersham).
Anti-PIP3 staining
Cells were fixed and stained as previously described using anti PIP3 antibodies (Echelon) (16).
Protrusion assays
Stable MDA-MB-231 cells were seeded in 35mm dish coated with matrigel. After 12h of adhesion, cells were incubated in starvation medium for 4h, and stimulated with 2.5nm EGF (Invitrogen). Phase contrast time-lapse images of the cells were collected every 20s and digitized using a Scion frame-grabber. Cell surface area changes were analyzed using NIH ImageJ software.
Boyden Chamber Assay
Transwell chambers (6.5mm-diameter, 8 um pore size; Costar) were coated with collagen I (BD Bioscience) overnight and then rinsed with medium plus 0.8 % BSA before use. MDA-MB-231 cells expressing wild type or mutant p110α (5 ×104 cells per well) were applied to the upper chamber in starvation medium. EGF (0 or 2.5nM) was added to the lower chamber as a chemoattractant. After 4h migration at 37°C, cells in the lower surface were fixed, stained with DAPI and counted.
Wound healing assay
Cells were grown to confluency on culture plates and a wound was made in the monolayer with a sterile P200 pipette tip (~0.5 mm in width). After wounding, the cells were washed to remove debris and new media was added. Phase-contrast images of the wounded area were taken at 0h, 4 h, and 20h after wounding. Wound widths were measured at a minimum of ten different points for each wound, and the average rate of wound closure during the first 4h of wound healing was calculated.
Dunn chamber chemotaxis assay
Cells (2 × 105/dish) were seeded onto the matrigel-coated coverslips and allowed to attach overnight. The next day, cells were starved for 4h in starvation medium, and the coverslips with attached cells were inverted and mounted onto a Dunn chemotaxis chamber (Hawksley Technology, Lancing, United Kingdom) as described (17). The inner well of the chamber was filled with starvation medium only, while the outer well was filled with starvation medium containing 5 or 0.5nm EGF as a chemoattractant as indicated. Time lapse images of moving cells were recorded every 2min over a 3h period. Cells movements were tracked manually, and analyses of migration and chemotaxis was performed using Mathematica notebooks written and provided by Professors GA Dunn and GE Jones, King’s College London (18).
Animal Models
Xenograft tumors were produced by injection of 1 × 106 MDA-MB-231 cells expressing wild-type or mutant bovine p110α into the mammary glands of 5–7 week old female SCID mice (19). Tumors size was measured weekly. The mice were sacrificed when tumors reached 1.2–1.3cm in diameter.
Tumor Cell blood burden
The blood burden of tumor cells were measured as described (20). SCID mice bearing xenograft tumors were anesthetized with isoflurane and blood was drawn from the right ventricle of the heart using a heparin-coated 25-gauge needle and a 1 ml syringe. Blood was placed in a tissue culture dish containing DMEM with 10% FBS and incubated overnight. The dishes were rinsed with PBS twice on the following day and the DMEM medium was replaced every three days thereafter. Puromycin was added to the medium on the 5th day and colonies were counted on the 14th day. Each colony was scored as representing one tumor cell from the blood, and the numbers were normalized to the blood volume taken from the heart.
Lung Metastases
1×106 MDA-MB-231 cells stably expressing wild-type or mutant bovine p110α were injected intravenously into the tail vein of SCID mice. After 4 weeks (5 animals per groups) or 7 weeks (at least 3 animals per group), the mice were sacrificed. Lungs were collected, fixed in 10% neutral buffered formalin and embedded in paraffin followed by serial sectioning. Five sections (100 μm apart) from each lung were stained with Haematoxylin and Eosin (H&E) and photographed. The tumor nodules were quantified by using Image J software.
Statistics
Quantitative data are expressed as the mean ± SEM. Statistical analysis was performed using ANOVA, unpaired Student’s t-test, or Mann-Whitney U test. A p value less than 0.05 was considered statistically significant.
Results
Replacement of endogenous p110α with wild type or mutant bovine p110α in MDA-MB-231 cells
To test the effect of distinct oncogenic p110α mutations on motility and metastasis, we established stable MDA-MB-231 cell lines in which endogenous human p110α was replaced with wild type or mutant bovine p110α. We used an approach similar to a knockdown/rescue strategy, except that we first expressed wild type or mutant bovine p110α, then knocked down the endogenous human p110α. This avoids possible adaptive responses to survival in the absence of p110α during the knockdown phase. The MDA-MB-231 line was chosen because it is capable of forming tumors in SCID mice, but has normal alleles for both p110α and PTEN (http://www.sanger.ac.uk/perl/genetics/CGP/cosmic?action=sample&id=905960). The mutants tested are the two most commonly mutated sites in p110α, E545K in the helical domain and H1047R in the kinase domain. The replacement strategy was accomplished using lentiviral mediated gene transfer as described in the Methods section. Cells infected with empty viruses were used as controls.
Infection of control MDA-MB-231 with lentivirus expressing p110α shRNA caused an over 90% knockdown at the protein level, as shown by immunoprecipitation with anti-p110α antibody and blots with anti-p110α antibody (Fig. 1A); knockdown was 70% efficient at the mRNA level, as detected by real-time PCR (data not shown). In a traditional knockdown/rescue strategy, exogenous bovine p110α would be expressed at a level comparable to that of endogenous human p110α in control cells, as detected by blotting with anti-p110α antibody (Fig. 1A). Similarly, in the p110α replacement cell lines used in this study, knockdown of human p110α was 80% efficient by real time PCR (data not shown), and total levels of bovine p110α were similar to that of human p110α in control cells (Fig. 1B). Levels of total p85, p110β and p110δ were also similar to that seen in control cells (Fig. 1B). Anti-myc immunoprecipitates from cell lines in which human p110α was replaced with wild type or mutant bovine p110α also showed similar levels of bovine myc-p110α expression and myc-p110α-associated p85 (Fig. 1C). Thus, the stable cells lines expressed bovine p110α at physiological levels.
Figure 1. p110α expression, Akt phosphorylation and PIP3 production in cell lines expressing mutant bovine p110α in place of wild type.
A. Anti-p110α immunoprecipitates were prepared from control MDA-MB-231 cells, cells infected with a lentiviral shRNA construct targeting human p110α, or cells in which human p110α was rescued with wild type bovine p110α. The immunoprecipitates were immunoblotted with anti-p110α (top panel) or anti-myc (lower panel) antibody. B. Control cells or stable MDA-MB-231 cells in which human p110α was replaced with wild type or mutant bovine p110α (helical domain: E545K; kinase domain: H1047R; kinase dead: KD) were immunoprecipitated with anti-p110α,β or δ antibodies. Immunoprecipitates or whole cell lysates were blotted for p110α or δ or p85 as indicated. C. Control cells or stable MDA-MB-231 cells in which human p110α was replaced with wild type or mutant bovine p110α were immunoprecipitated with anti-myc antibody, and blotted with anti-p110α (top panel) or anti-p85α (middle panel) antibodies. Whole cell lysates from these cell lines were blotted with anti β-actin (lower panel) as a loading control. D. Control MDA-MB-231 cells or cells in which human p110α was replaced with bovine wild type or mutant p110α were starved in starvation medium for 4h, and stimulated without or with 5nm EGF for 3min. Equal amount of cell lysate was separated by 10% SDS-PAGE and blotted for P-S473-Akt or total Akt. E. Control MDA-MB-231 cells or cells in which human p110α was replaced with bovine wild type or mutant p110α were serum starved overnight, fixed and stained with anti-PIP3 antibody as described. Phase contrast and immunofluorescence images for each cell line are shown.
Consistent with the p110α expression data, EGF-stimulated phosphorylation of Akt was significantly decreased in cells in which p110α was replaced with a kinase-dead mutant, whereas cells expressing wild type bovine p110α showed a level of Akt phosphorylation similar to that seen in control cells (Fig. 1D). In contrast, MDA-MB-231 cells expressing either helical domain or kinase domain p110α mutants showed elevated basal levels of Akt phosphorylation as well as enhanced EGF-stimulated Akt phosphorylation. We also measured production of PIP3 by immunofluorescence analysis of fixed cells using a previously characterized antibody specific for PIP3 (16). In quiescent cells, PIP3 was clearly increased in cells expressing helical and kinase domain mutants of p110α (Fig. 1E). These results show that PI 3-kinase signaling is increased in cells expressing the p110α E545K or H1047R mutants at physiological levels.
p110α oncogenic mutants cause enhanced EGF-stimulated protrusion and cell motility
Actin-mediated protrusion at the leading edge of moving cells is an early step in carcinoma cell motility (21). The EGF-stimulated protrusion of MD-MBA-231 lines was measured by time-lapse video microscopy. Cells in which human p110α was replaced with wild type bovine p110α showed a rate and extent of protrusion that was similar to control MDA-MB-231 cells. Cells expressing a kinase dead p110α mutant (KD) showed minimal protrusion after EGF stimulation. This result is similar to our previous finding that the p110α isoform is required for EGF stimulated protrusion in carcinoma cells (14). Cells expressing either helical domain (E545K) or kinase domain (H1047R) mutants exhibited significantly greater protrusion in response to EGF compared to control cells (Fig. 2A). These results indicate that oncogenic p110α mutants promote cell protrusion in carcinoma cells.
Figure 2. Expression of p110α mutants increases protrusion and migration.
A: Stable MDA-MB-231 cells expressing wild type or mutant (helical domain: E545K; kinase domain: H1047R) bovine p110α were seeded in 35mm dish coated with matrigel. After 12h to allow adhesion, the cells were starved for 4h, and stimulated with 2.5nm EGF. Time-lapse images were collected every 20s. The surface area of each cell was measured using ImageJ, and normalized to the initial cell area. The data are the mean ± SEM from 15–20 cells. B. Cells were plated collagen-coated transwell chambers, and incubated for 4h without or with EGF in the lower chamber. The cells were fixed and stained with DAPI, and the number of cells on the lower filter surface was counted. The data are the mean ± SEM from 3 experiments. C: Monolayer cultures of stable MDA-MB-231 cells expressing wild type or mutant bovine p110α were wounded with a P200 pipette tip. Phase-contrast images of the wound area were taken at the 0h and 20h. Images from the 0h and 20h incubations are shown. D: The data are the Mean ± SEM, and statistical significance were determined using a two-tailed students t-test. *: p<0.05; **: p<0.01 compared with cell expressing wild type p110α.
To test the effects of mutant p110α on cell motility, we evaluated MDA-MB-231 cells expressing wild type p110α or oncogenic p110α mutants in a Boyden Chamber transwell assay. Cells expressing either helical domain or kinase domain mutations showed an increase in migration relative to cells expressing wild type p110α, both in the absence or presence of EGF (Fig. 2B). Similarly, in a wound-healing assay, cells expressing wild type p110α exhibited a similar rate of wound closure as control MDA-MB-231 cells. However, significantly faster wound closure was observed in cells expressing helical or kinase domain mutants of p110α cells, with over 60% of the open area covered by the cells in a 20h period (Fig. 2C, top). These differences were not due to differential proliferation, as rates of cell growth for cells expressing wild type or mutant p110α were identical in both normal and reduced serum (data not shown). Quantification of wounding-induced migration during the first 4h of wound healing showed that the migration rate was increased 1.5–2 fold in cells expressing mutant p110α, as compared to cells expressing wild type p110α (Fig. 2C, bottom). These data show that oncogenic mutations in p110α confer enhanced cell motility in vitro.
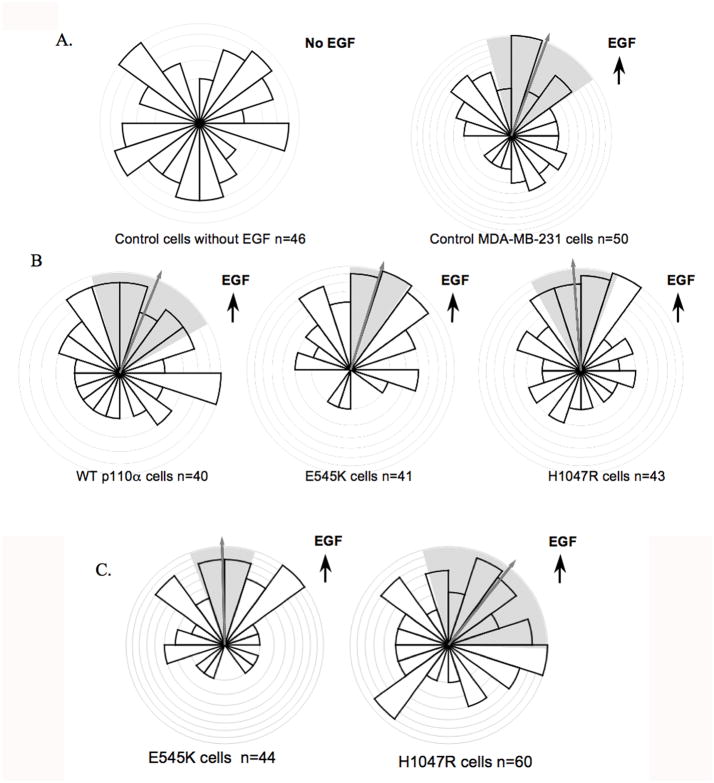
Helical domain mutation leads to increased directionality in MDA-MB-231 cells
To determine whether p110α helical and kinase domain mutations have an effect on chemotaxis, we used the Dunn chamber assay, which uses time-lapse video microscopy to monitor the cell movement under the influence of a linear gradient of diffusing chemoattractant (17, 22). As expected, MDA-MB-231 cells moved in a non-directional manner in the absence of EGF, but showed clear directional movement in the presence of a 0 to 5nm EGF gradient (Fig. 3A). Interestingly, directional movement of cells expressing the kinase domain mutant was similar to that of cell expressing wild-type p110α (Fig 3B). However, cells expressing the helical domain mutant exhibited significantly greater directionality than either the kinase domain mutant or wild type cells (Fig. 3B) (p< 0.05 using the Moore test (23)). The comparison between cells expressing helical and kinase domain mutants was repeated at a lower dose of EGF (0 to 0.5nm), and yielded an even more obvious enhancement of chemotaxis in the helical domain cells (p < 0.001) (Fig. 3C). Cells expressing the helical domain mutant also showed greater persistence (0.24 ±0.03 versus 0.17 ± 0.02, p < .05), although the speed was not significantly different (data not shown). Taken together, these data show that while expression of either oncogenic mutant increases cell motility, expression of the helical domain mutant enhances directional sensing in a chemoattractant gradient. This prompted us to evaluate the potential differential effects of the two p110α mutations on intravasation and extravasation, two important components of metastasis in vivo.
Figure 3. Expression of the helical domain mutant increases chemotaxis.
Stable cells were placed in Dunn chambers in the presence or absence of EGF. Cells migration was recorded by time-lapse video microscopy, and analyses of migration and chemotaxis was performed using Mathematica notebooks written and provided by Professors GA Dunn and GE Jones, King’s College London (18). The bar height represents the proportion of cells moving in a particular direction, and the arrow and the shading indicate the mean direction of cell migration and its 95% confidence interval, respectively. A. Chemotaxis of control MDA-MB-231 cells in the absence or presence of a 0 to 5nm EGF gradient. B. Chemotaxis of cells expressing wild type or mutant bovine p110α in a 0–5nm EGF gradient. 40–50 cells were counted for each group. p < 0.005 helical domain mutant (E545K) vs. wild type; p < 0.05 helical domain mutant (E545K) vs. kinase domain mutant (H1047R) in the Moore test. C. Chemotaxis of helical domain and kinase domain mutants in a 0 to 0.5nm EGF gradient. p < .001 in the Moore test.
Helical domain mutations causes enhanced intravasation as compared to kinase domain mutations in vivo
Cells expressing wild type or mutant p110α were injected into the mammary glands of SCID mice. Although no significant differences were observed in the proliferation rates of these cell lines in vitro, even under low serum conditions (data not shown), xenograft tumor expressing helical or kinase domain mutants of p110α grew much faster than those expressing wild type p110α (Fig. 4A). Tumors expressing the helical domain mutants showed a statistically significant enhancement in growth rate relative to tumors expressing the kinase domain mutants, although the difference was small when compared to the difference between mutant and wild type p110α tumors. The rapid growth of tumors expressing kinase and helical domain presumably involves interactions with stromal factors not seen in the in vitro proliferation experiments.
Figure 4. The helical domain mutation enhances tumor growth and in vivo intravasation.
Cell lines expressing wild type or mutant p110α were injected into the right mammary fat pads of SCID mice. A. Spontaneous tumor size was recorded every week. **: p <0.01 helical domain (E545K) vs. kinase domain (H1047R). B. Blood burden (intravasation) experiments were performed once the tumor volumes reached 1.2–1.3cm. Adherent tumor cells were counted 14 days after plating of the blood samples. Data are normalized for the blood volume for each sample. n=6–11 mice per mutant type. **: p<0.01 E545K vs. wild type; *: p<0.05 H1047R vs. wild type; ***: p<0.01 E545K vs. H1047R in Mann-Whitney U test.
We assessed the ability of the tumor cells to intravasate into the blood by measuring the tumor cell blood burden as described (20). Tumor cell intravasation is affected by tumor size, and the blood tumor burdens were therefore measured when the xenograft tumors reached identical sizes (1.2–1.3cm). Because of the differential growth rates, measurements were performed after 7 weeks for tumors expressing helical domain mutants, 8 weeks for tumors expressing kinase domain mutants, and 13 weeks for tumors expressing wild type p110α, such that tumor size was similar for each cell type. Colony counts from mice carrying helical domain or kinase domain mutants were higher than those with wild type p110α (Fig. 4B). Interestingly, cells expressing the helical domain mutations exhibited a 3.4 fold higher rate of intravasation than cells expressing the kinase domain mutation, regardless of the fact that mice carrying helical domain tumors were sacrificed 1 week earlier than mice carrying kinase domain tumors. These results strongly suggest that p110α oncogenic mutations increase breast cancer metastasis by promoting mobilization of mammary tumor cells into the circulation, and that the helical domain mutation E545K promotes intravasation more robustly than the kinase domain mutation H1047R.
Helical domain mutations enhance tumor cell extravasation
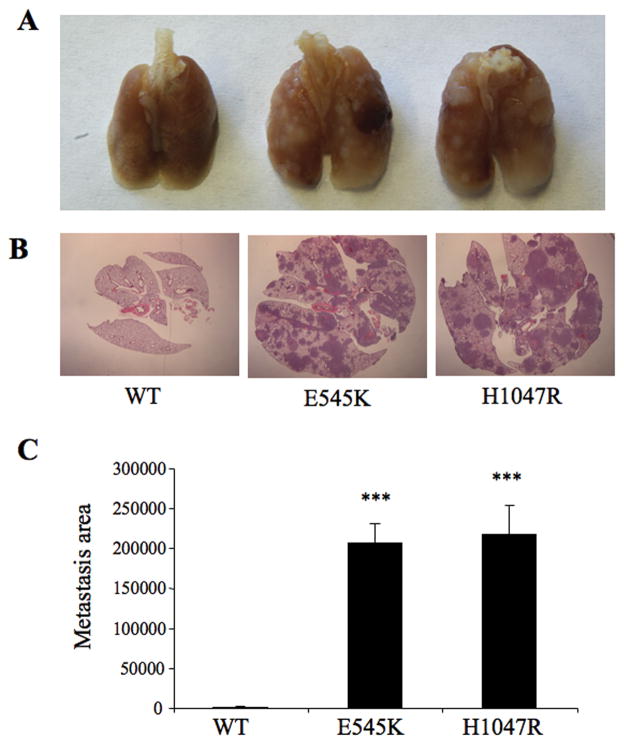
The ability of tumor cells expressing wild type or mutant p110α to extravasate into the lung was measured by injecting identical numbers of each cell line into the tail veins of SCID mice. The lungs were analyzed at 7 weeks, a time by which control MDA-MB-231 cells show measurable levels of lung metastasis (data not shown). As expected, metastatic foci were detectable in histological sections from mice injected with cells expressing wild type p110α (Fig. 5A, 5B). However, mice injected with cells expressing helical domain or kinase domain mutants of p110α E545K or H1047R cells developed extensive metastatic nodules evidenced both by gross and histological analysis (Fig. 5A, 5B). The florid metastases caused high mortality in both groups (data not shown), and made it difficult to assess differences between two mutations. We therefore analyzed a separate cohort of mice at 4 weeks after tail vein injection. By this time, cells expressing the helical domain mutant showed a significantly higher level of metastasis than cells expressing the kinase domain mutant, as evidenced by an increased number of metastatic foci and increased total metastatic area (Fig. 6). The dramatic differences in extravasation are unlikely to be explained by the slightly higher growth rate of helical domain versus kinase domain tumors, and suggest tumor cells expressing helical domain mutants of p110α show a much higher rate of migration from the vasculature into the lung.
Figure 5. Tail vein injection of cells expressing mutant p110α lead to increased lung metastases.
Stable cells expressing wild type or mutant p110α (helical domain: E545K; kinase domain: H1047R) were injected into the lateral tail vein of SCID mice. Mice were sacrificed 7 weeks after tail vein injection. A: Lungs from control and mutant p110α mice. B: Lung metastases were visualized by H&E staining. C: The area of lung metastases were determined by Image J. ***, p<0.001, relative to wild type.
Figure 6. Increased lung metastasis in cells expressing helical domain versus kinase domain mutants of p110α.
Stable cell lines were injected into the lateral tail vein of SCID mice as in Figure 5. Mice were sacrificed 4 weeks after tail vein injection. A: Lung metastases were visualized by H&E staining. B: The area of lung metastases were determined by Image J. C: The number of metastatic foci were determined by Image J. ***: p<0.001, relative to E545K.
Discussion
The bulk of oncogenic mutations of p110α cluster in two hot spots: in an acid cluster in the helical domain (E542, E545 and Q546) and in the C-terminus of the kinase domain (H1047). Both helical domain and kinase domain mutants of p110α cause increased lipid kinase activity in cells, but by different mechanisms (2–5). The distinct mechanisms by which the helical domain and kinase domain mutants activate p85/p110 dimers might lead to distinct patterns of PI[3,4,5]P3 production in cells.
When we directly compared the effect of p110α helical domain versus kinase domain mutations in cell lines with otherwise identical genetic backgrounds, expression of the helical domain mutation lead to increased chemotaxis in vitro, and increased activity during in vivo metastasis assays. Cells expressing the helical domain mutant showed a small but significant increase in tumor growth rate as compared to cells expressing the kinase domain mutant; both mutant cell lines produced tumors much faster than cells expressing wild type p110α. However, the presence of tumor cells in the blood of animals with helical domain mutants was nearly 3-fold higher than in animals with kinase domain tumors. Similarly, metastasis to the lung was much faster upon tail vein injection of helical domain opposed to kinase domain cells. These differences did not correspond to marked differences in Akt activation in the two cell lines, consistent with the idea that site specific PI 3-kinase activity might be important in defining the phenotype of these mutants in vivo.
Several previous studies have compared signaling by overexpressed helical domain and kinase domain mutants of p110α. Overexpression of either N-terminally tagged mutant in NIH 3T3 cells or mammary epithelial cells led to increased Akt activation, growth in soft agar, disruption of mammary acinar morphogenesis in 3D culture, and tumor formation in a xenograft model(7–9). Similar results were seen in Ba/F3 mouse pro-B cells (10). In these studies, the phenotype produced by overexpression of either the helical domain or kinase domain mutants were similar. A concern in some of these studies is the use of N-terminal epitope tags, which stabilize p110α independently of binding to p85α and might obscure differences between the mutants (24). The C-terminal tag used in this study does not stabilize p110α, although it is possible that it could still have some unforeseen effect on signaling in vivo. Alternatively, Samuels et al. used a genetic strategy to silence the expression of helical domain or kinase domain mutants in human cell lines expressing a single copy of the mutant allele (DLD1 and HCT116 cells, respectively) (12). The helical and kinase domain mutant lines both led to increased levels of tumor formation and metastasis in a xenograft model, as compared to the cells in which the mutant allele was ablated. However, a direct comparison of the in vivo phenotypes of the helical versus kinase domain mutants was difficult, given that different cells lines were used. In a more recent study, knock-in of helical versus kinase domain mutations led to the activation of a similar range of downstream activators in MCF-10A cells (25). We also failed to see differences between the helical domain and kinase domain mutants in responses such as protrusion or motility in the Boyden chamber, but we did see differences in complex behaviors such as in vitro chemotaxis in an EGF gradient and metastatic behavior in vivo.
In contrast, clear differences between the helical domain and kinase domain mutants were seen in studies using retroviral expression of untagged p110α mutants in chicken fibroblasts; this method depends on endogenous p85 for stabilization of p110α, and should not lead to overexpression. Although both mutations lead to increased PI 3-kinase activity, expression of the kinase domain mutation led to more robust Akt activation and foci formation in chicken fibroblasts, and tumor production in the chicken embryo chorioallantoic membrane assay (11, 26). These studies are not consistent with our data, which show similar rates of tumor growth but increased metastatic behavior for the helical domain relative to the kinase domain mutants. However, multiple differences in the systems used (stromal factors, interactions with macrophages and other inflammatory cells, cell type and species-specific differences) could explain the different results.
Our data would suggest that in human breast cancer cells identical in other respects, the presence of helical domain mutants of p110α would predict a more aggressive metastatic phenotype. The clinical evidence in support of this hypothesis is mixed. A number of studies have suggested that mutations in p110α correlate with more severe disease in breast, colon, and endometrial cancer, but these studies did not separately compare helical domain versus kinase domain mutants (27–32). Helical domain mutants were found to predominate, relative to kinase domain mutations, in aggressive lobular breast carcinoma (33), and were found to be independently associated with poor prognosis in these tumors (34). Other studies found an association of kinase domain mutants, but not helical domains, with poor prognosis in breast cancer and endometrial cancer (35–38). Finally, as mentioned earlier, kinase domain mutants were found at a higher rate than helical or C2 domain mutants (67% versus 19%) in tumors with a PTEN loss gene signature, which correlates with poor prognosis in a number of tumor types (13).
The relative effect of different p110α mutations on patient prognosis is likely to be complex, and will undoubtedly also be influenced by the presence of other oncogenic mutations in a given tumor. For example, recent data from Vasudevan et al. suggest that in some breast cancer lines expressing helical domain mutants of p110α, adaptations have occurred such that activation of Akt is minimal, and anchorage independent growth relies on activation of PDK1 and SGK3 (39). In MDA-MB-231 cells, the Ras/Erk pathway is activated by mutations in both K-Ras and B-Raf (http://www.sanger.ac.uk/perl/genetics/CGP/cosmic?action=sample&id=905960). K-Ras associates with distinct non-raft regions of the plasma membrane (6). Given that helical domain mutants of p110α show increased activity in the presence of oncogenic Ras, whereas kinase domain mutants do not (2), the presence of constitutively active K-Ras in MDA-MB-231 cells could lead to a localized activation of helical domain that would not occur in cells expressing kinase domain mutants of p85/p110α. It will be important to determine how such differential targeting regulates chemotaxis and metastatic behavior in breast cancer lines expressing mutant p110α.
Supplementary Material
MDA-MB-231 cells expressing helical or kinase domain mutants of p110α were produced using a classical knockdown-rescue strategy as described. A. Stable lines were immunoprecipitated with anti-p110α antibody and blotted for p110α or myc. B. Control cells, replacement cells expressing wild type or kinase dead bovine p110α, and knockdown/rescue cells expressing helical or kinase domain mutants of p110α, were incubated without or with EGF and blotted for total and pS473-Akt.
A. Control cells, replacement cells expressing wild type or kinase dead bovine p110α, and knockdown/rescue cells expressing helical or kinase domain mutants of p110α, were stimulated with EGF and images were recorded by time-lapse video microscopy. Cell areas were measured with NIH ImageJ and expressed relative to initial area. B. Cell lines described above were plated collagen-coated transwell chambers, and incubated for 4h without or with EGF in the lower chamber. The cells were fixed and stained with DAPI, and the number of cells on the lower filter surface was counted. C. Monolayer cultures of cells described above were wounded with a P200 pipette tip. Phase-contrast images of the wound area were taken at time 0, 4 and 20h. The average rate of wound closure during the first 4h of wound healing was calculated.
Acknowledgments
We would like to thank Dr. Gareth Jones, King’s College London, for his generous assistance with the Dunn Chamber data analysis. This work is funded by NIH GM55692 (J.M.B.) CA113395 (J.S.C.), NIH PO1 CA 100324 (J.M.B. J.E.S., J.S.C.), the Albert Einstein Cancer Center (P30 CA013330), and an award from the Janey Fund (J.M.B.). Sumanta Goswami is the recipient of the Young Investigator Award from Breast Cancer Alliance Inc.
References
- 1.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 2.Zhao L, Vogt PK. Helical domain and kinase domain mutations in p110alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc Natl Acad Sci U S A. 2008;105:2652–7. doi: 10.1073/pnas.0712169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miled N, Yan Y, Hon WC, et al. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science. 2007;317:239–42. doi: 10.1126/science.1135394. [DOI] [PubMed] [Google Scholar]
- 4.Chaussade C, Cho K, Mawson C, Rewcastle GW, Shepherd PR. Functional differences between two classes of oncogenic mutation in the PIK3CA gene. Biochem Biophys Res Commun. 2009;381:577–81. doi: 10.1016/j.bbrc.2009.02.081. [DOI] [PubMed] [Google Scholar]
- 5.Carson JD, Van Aller G, Lehr R, et al. Effects of oncogenic p110alpha subunit mutations on the lipid kinase activity of phosphoinositide 3-kinase. Biochem J. 2008;409:519–24. doi: 10.1042/BJ20070681. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg S, Henis YI. Interactions of Ras proteins with the plasma membrane and their roles in signaling. Cell Signal. 2008;20:31–9. doi: 10.1016/j.cellsig.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Ikenoue T, Kanai F, Hikiba Y, et al. Functional analysis of PIK3CA gene mutations in human colorectal cancer. Cancer Res. 2005;65:4562–7. doi: 10.1158/0008-5472.CAN-04-4114. [DOI] [PubMed] [Google Scholar]
- 8.Zhao JJ, Liu Z, Wang L, Shin E, Loda MF, Roberts TM. The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci U S A. 2005;102:18443–8. doi: 10.1073/pnas.0508988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isakoff SJ, Engelman JA, Irie HY, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–1000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 10.Horn S, Bergholz U, Jucker M, et al. Mutations in the catalytic subunit of class IA PI3K confer leukemogenic potential to hematopoietic cells. Oncogene. 2008;27:4096–106. doi: 10.1038/onc.2008.40. [DOI] [PubMed] [Google Scholar]
- 11.Bader AG, Kang S, Vogt PK. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci U S A. 2006;103:1475–9. doi: 10.1073/pnas.0510857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–73. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Saal LH, Johansson P, Holm K, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007;104:7564–9. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill K, Welti S, Yu JH, et al. Specific requirement for the p85-p110α phosphatidylinositol 3-kinase during epidermal growth factor-stimulated actin nucleation in breast cancer cells. JBiolChem. 2000;275:3741–4. doi: 10.1074/jbc.275.6.3741. [DOI] [PubMed] [Google Scholar]
- 15.Dull T, Zufferey R, Kelly M, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–71. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yip SC, Eddy RJ, Branch AM, et al. Quantification of PtdIns(3,4,5)P(3) dynamics in EGF-stimulated carcinoma cells: a comparison of PH-domain-mediated methods with immunological methods. Biochem J. 2008;411:441–8. doi: 10.1042/BJ20071179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones GE, Ridley AJ, Zicha D. Rho GTPases and cell migration: measurement of macrophage chemotaxis. Methods Enzymol. 2000;325:449–62. doi: 10.1016/s0076-6879(00)25465-7. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed T, Shea K, Masters JR, Jones GE, Wells CM. A PAK4-LIMK1 pathway drives prostate cancer cell migration downstream of HGF. Cell Signal. 2008;20:1320–8. doi: 10.1016/j.cellsig.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Wyckoff J, Wang W, Lin EY, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 20.Wyckoff JB, Jones JG, Condeelis JS, Segall JE. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 2000;60:2504–11. [PubMed] [Google Scholar]
- 21.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–52. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zicha D, Dunn G, Jones G. Analyzing chemotaxis using the Dunn direct-viewing chamber. Methods Mol Biol. 1997;75:449–57. doi: 10.1385/0-89603-441-0:449. [DOI] [PubMed] [Google Scholar]
- 23.Moore BR. A modificatoin of the Rayleigh test for vector data. Biometrika. 1980;67:175–80. [Google Scholar]
- 24.Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: Stabilization and inhibition of the p110-alpha catalytic subunit by the p85 regulatory subunit. MolCellBiol. 1998;18:1379–87. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gustin JP, Karakas B, Weiss MB, et al. Knockin of mutant PIK3CA activates multiple oncogenic pathways. Proc Natl Acad Sci U S A. 2009;106:2835–40. doi: 10.1073/pnas.0813351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A. 2005;102:802–7. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Li SY, Rong M, Grieu F, Iacopetta B. PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res Treat. 2006;96:91–5. doi: 10.1007/s10549-005-9048-0. [DOI] [PubMed] [Google Scholar]
- 29.Barault L, Veyrie N, Jooste V, et al. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer. 2008;122:2255–9. doi: 10.1002/ijc.23388. [DOI] [PubMed] [Google Scholar]
- 30.Kato S, Iida S, Higuchi T, et al. PIK3CA mutation is predictive of poor survival in patients with colorectal cancer. Int J Cancer. 2007;121:1771–8. doi: 10.1002/ijc.22890. [DOI] [PubMed] [Google Scholar]
- 31.Ogino S, Nosho K, Kirkner GJ, et al. PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol. 2009;27:1477–84. doi: 10.1200/JCO.2008.18.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvesen HB, Carter SL, Mannelqvist M, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci U S A. 2009;106:4834–9. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buttitta F, Felicioni L, Barassi F, et al. PIK3CA mutation and histological type in breast carcinoma: high frequency of mutations in lobular carcinoma. J Pathol. 2006;208:350–5. doi: 10.1002/path.1908. [DOI] [PubMed] [Google Scholar]
- 34.Barbareschi M, Buttitta F, Felicioni L, et al. Different prognostic roles of mutations in the helical and kinase domains of the PIK3CA gene in breast carcinomas. Clin Cancer Res. 2007;13:6064–9. doi: 10.1158/1078-0432.CCR-07-0266. [DOI] [PubMed] [Google Scholar]
- 35.Lai YL, Mau BL, Cheng WH, Chen HM, Chiu HH, Tzen CY. PIK3CA exon 20 mutation is independently associated with a poor prognosis in breast cancer patients. Ann Surg Oncol. 2008;15:1064–9. doi: 10.1245/s10434-007-9751-7. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15:2472–8. doi: 10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]
- 37.Catasus L, Gallardo A, Cuatrecasas M, Prat J. PIK3CA mutations in the kinase domain (exon 20) of uterine endometrial adenocarcinomas are associated with adverse prognostic parameters. Mod Pathol. 2008;21:131–9. doi: 10.1038/modpathol.3800992. [DOI] [PubMed] [Google Scholar]
- 38.Catasus L, Gallardo A, Cuatrecasas M, Prat J. Concomitant PI3K-AKT and p53 alterations in endometrial carcinomas are associated with poor prognosis. Mod Pathol. 2009;22:522–9. doi: 10.1038/modpathol.2009.5. [DOI] [PubMed] [Google Scholar]
- 39.Vasudevan K, Barbie D, Davies M, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009 doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MDA-MB-231 cells expressing helical or kinase domain mutants of p110α were produced using a classical knockdown-rescue strategy as described. A. Stable lines were immunoprecipitated with anti-p110α antibody and blotted for p110α or myc. B. Control cells, replacement cells expressing wild type or kinase dead bovine p110α, and knockdown/rescue cells expressing helical or kinase domain mutants of p110α, were incubated without or with EGF and blotted for total and pS473-Akt.
A. Control cells, replacement cells expressing wild type or kinase dead bovine p110α, and knockdown/rescue cells expressing helical or kinase domain mutants of p110α, were stimulated with EGF and images were recorded by time-lapse video microscopy. Cell areas were measured with NIH ImageJ and expressed relative to initial area. B. Cell lines described above were plated collagen-coated transwell chambers, and incubated for 4h without or with EGF in the lower chamber. The cells were fixed and stained with DAPI, and the number of cells on the lower filter surface was counted. C. Monolayer cultures of cells described above were wounded with a P200 pipette tip. Phase-contrast images of the wound area were taken at time 0, 4 and 20h. The average rate of wound closure during the first 4h of wound healing was calculated.