Abstract
In Drosophila, genetic techniques relying on stochastic chromosomal rearrangements involve the generation and screening of a large number of fly stocks to isolate a few lines of interest. Here, we describe a PCR-based method allowing non-lethal molecular characterization of single flies. Using this procedure, individual candidate recombinant animals can be genotyped and selected one generation earlier than with extant methodology and, importantly, before stocks are established. This advance should significantly accelerate and facilitate several of the most fundamental and routine techniques in Drosophila genetics.
Introduction
Chromosomal recombination (CR) and mobilization of transposable elements (MTE) are two fundamental techniques in Drosophila genetics. CR is commonly used to associate or dissociate two alleles on different loci of a chromosome. MTE generates two classes of excisions: 1) precise, or revertants, where the transposon is completely removed leaving a wild-type chromosome and 2) imprecise, where rearrangements occur to either the transposon or the surrounding sequence (1). These methods have two major limitations. First, they rely on largely unpredictable chromosomal rearrangements. Hence, reliable characterization of the resulting DNA structure requires molecular genotyping, usually by polymerase chain reaction (PCR) and DNA sequencing. Second, these methods are generally inefficient. Since recombination frequency is proportional to the distance between the two loci of interest, achieving CR between two close loci requires screening a large number of candidate animals. Similarly, for MTE, isolating an imprecise excision causing a deletion in a given gene may require screening hundreds of lines, particularly when dealing with a large locus. These genetic experiments therefore typically involve generating single animals bearing unique chromosomal rearrangements, from which dozens or even hundreds of individual stocks are derived and molecularly screened to identify a small number of strains to be kept for phenotypic study (1). A procedure allowing non-lethal genotyping of single animals would allow direct molecular screening of first-generation candidate recombinants, i.e. at least one generation earlier than with classic methodology, with stocks being established only from the few interesting individuals. Such a method should contribute to making CR and MTE efforts substantially faster, cheaper and less burdensome. Here, we describe a PCR-based protocol for genotyping single Drosophila fruit flies using small body parts. Our technique allows DNA purification and amplification from wings of a single, live fly with no significant impairment of robustness or reproductive ability.
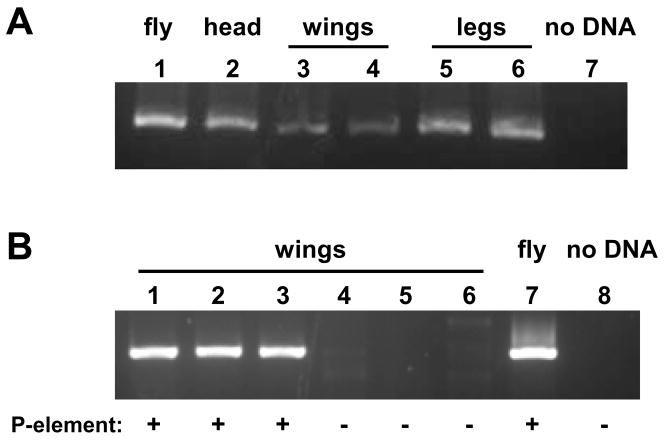
DNA can be purified from a single fly (2). This method is also effective when using a single head or thorax, but not smaller body parts (Fig. 1A and data not shown), and therefore requires the genotyped animal to be sacrificed. We started by developing a protocol allowing DNA purification from a pair of fly legs. The two forelegs of an anesthetized male were sectioned at the proximal femur, placed in a 0.2 mL PCR tube and covered with 10 μL of 400 μg/mL protease K (1:50 dilution of a 20 mg/ml stock diluted in buffer A: 10 mM Tris-Cl at pH 8.2, 1 mM EDTA and 25 mM NaCl). Optimal results were obtained when the biological sample was entirely submerged in the buffer. Importantly, homogenization was not necessary in these conditions and sufficient DNA was obtained by virtue of the protease digestion. The tubes were incubated at 37 °C for 1 h and then at 95 °C for 2 min to inactivate the protease. A 540-bp fragment of the Drosophila actin (Act42A) gene was amplified using the following PCR conditions: 1× iProof buffer (Bio-rad), 0.2 mM dNTPs, 0.5 μM primers (Forward: 5′-GGTCGCGATTTAACCGACTACCTGAT; Reverse: 5′-AGCAGATGTGGATCTCGAAGCAAGAG), 3 μL fly leg DNA template, 0.4 U iProof High-fidelity DNA polymerase (Bio-rad) and water to a total volume of 20 μL. Thermocycler conditions were: 1 cycle of initial denaturation (98 °C 30 sec); 35 cycles of denaturation (98 °C 10 sec), annealing (63 °C 15 sec) and extension (72 °C 20 sec); and 1 cycle of final extension (72 °C 10 min). Running 5 μL of each reaction on an agarose gel revealed a single band of the expected size, similar in intensity to the product obtained from head DNA (Fig. 1A, lanes 5 and 6).
Fig. 1.
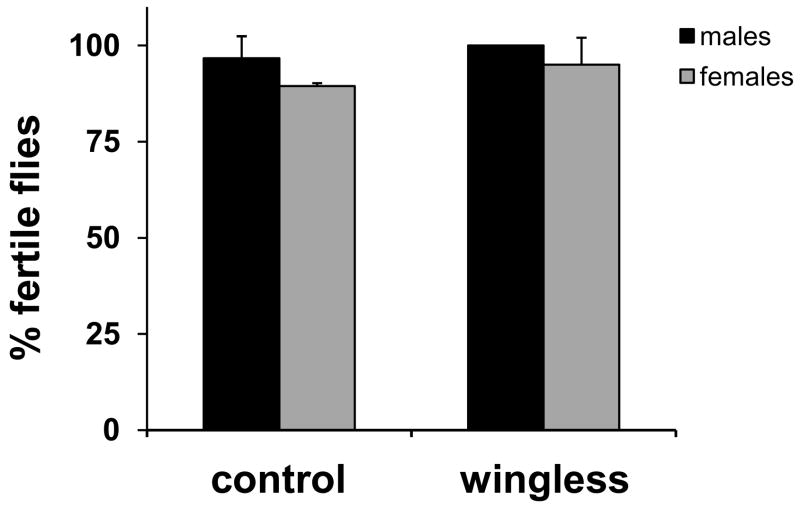
PCR amplification of DNA isolated from discrete body parts of a single fly. (A) A 540-bp fragment of the Act42A gene was PCR-amplified from DNA isolated by either the lethal purification method (ref. 2) – Lanes 1–2 – or our non-lethal protocol – Lanes 3–7. Biological samples used were: a whole fly (lane 1), a single head (lane 2), a pair of wings (lanes 3–4) or a pair of legs (lanes 5–6). Lane 7 shows a control reaction in which no template was added. (B) DNA purified from wings of flies bearing the UAS-dSOD2 P-element insertion (lanes 1–3), but not control flies (lanes 4–6), allows PCR amplification of a product specific to the P-element. Lane 7, PCR from DNA extracted from a whole fly bearing the P-element. Lane 8, control reaction with no template added.
Although our method isolated DNA suitable for PCR from a small body part, leg amputation is an invasive procedure likely to affect fitness. Since the goal of non-lethal genotyping is to select animals with which to establish a stock, flies must not only survive genotyping but also remain robust and fertile. We therefore asked if fly wings can be used as the biological sample. Our protocol successfully isolated enough DNA from a pair of fly wings (sectioned immediately distal to the wing base) to yield a visible band after PCR (Fig. 1A, lanes 3 and 4). Increasing the concentration of protease was not beneficial, and shortening the incubation time reduced extraction efficiency (data not shown). As seen previously with leg-derived DNA, the results were most consistent when the wings were fully covered by the protease solution. Overall, using two wings for the extraction gave optimal reproducibility.
To test the specificity of the amplification from wing DNA, we PCR-amplified a sequence specific to the UAS-dSOD2 transgenic insertion (3) (the primers used were Forward: 5′-AGTACTGTCCTCCGAGCGGA and Reverse: 5′-TAGGGCAGCTTCGGTAGGGT). A PCR product was obtained from DNA extracted from wings of UAS-dSOD2 flies, but not from controls lacking the transgene (Fig. 1B).
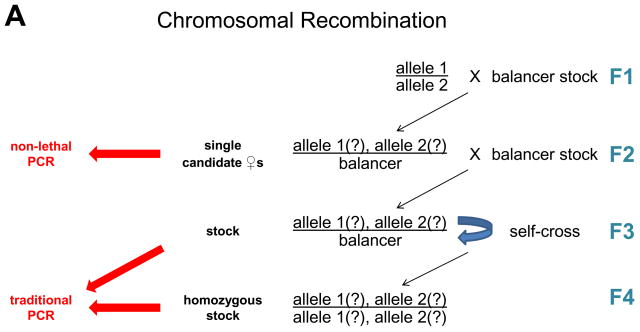
We next asked if double wing ablation affects reproductive ability. The courtship ritual of Drosophila males includes unilateral wing vibrations thought to influence female receptivity (4). We tested the ability of wingless flies to generate viable progeny when housed with a winged cohort of the opposite gender. The performance of wingless animals of both genders was indistinguishable from winged controls (Fig. 2). This result demonstrates that double wing ablation does not affect long-term reproductive ability and constitutes a convenient non-lethal genotyping method.
Fig. 2.
Wing ablation does not affect the fertility of males or females. Wingless or control flies were housed with one animal of the opposite gender in culture vials for 7 days and scored for viable progeny. Results represent the average ± SD of 2 (females) or 3 (males) trials. N = 10 animals per trial.
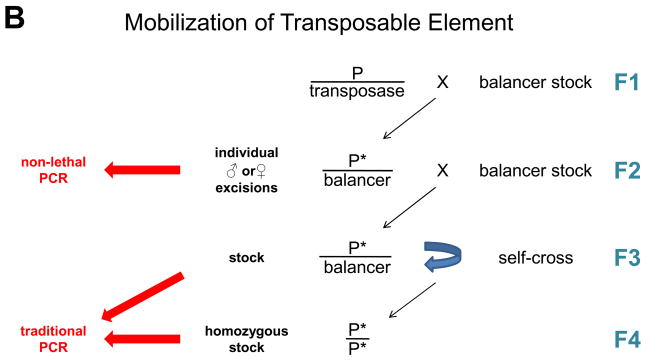
A method for non-lethal genotyping has been described in the honeybee (5). The advent of a protocol designed specifically for non-lethal PCR in Drosophila should significantly facilitate molecular genetics in this well-established genetic model organism. Excisions isolated through a visible marker such as eye color and candidate recombinants can sometimes be screened phenotypically. In numerous cases, however, because the phenotype is too burdensome to screen with or unknown (such as in the case of reverse genetics), screening relies on molecular techniques. In these instances, non-lethal PCR can replace current molecular methods with a significant saving in time and cost, since it allows genotyping at least one generation earlier, i.e. before stocks are established (Fig. 3). Candidate animals can be screened molecularly one by one or in small batches until a chromosomal event of interest is isolated, virtually eliminating unnecessary labor. As with current methodology, the number of animals screened before interesting stocks are isolated varies from experiment to experiment. In practice, since a recombinant between two alleles located on different chromosomal arms occurs, in average, 50% of the time, it often suffices to genotype two or three candidates to isolate such a line, with this number rising as the distance between the alleles diminishes. As for MTE, the frequency of a particular excision event is a function of the properties of each transposon and its insertion site and ranges from very frequent – e.g. precise excisions of Piggybac transposons, typically requiring only one or two candidates to be genotyped – to very infrequent – e.g. an imprecise excision resulting in a null allele of a large locus, which may require several hundred candidates to be screened.
Fig. 3.
Since first-generation recombinants bear the putative rearrangement—a recombination in CR and an excision in MTE—over a balancer chromosome, the PCR product should be unique to the chromosome of interest. Specifically, our method is useful in CR experiments to confirm the recombination of insertions of known sequence (by either using two primers complementary to the insertion or one on the insertion and another on the flanking genomic region) and deletions (using flanking primers). For MTE, useful primer sets include those with a primer on one end of the transposon and another on the neighboring genomic region, as well as primers flanking the insert, useful in identifying imprecise excisions where the size of the amplicon is reduced upon deletion of part of the endogenous chromosome.
Finally, our protocol is particularly useful in local transposition, a specific case of MTE that takes advantage of the propensity of transposable elements to hop locally. The goal of local transposition is to mobilize an insert into a specific nearby gene of interest (6). Candidate animals can be effectively screened using a primer on one end of the insertion coupled with a set of primers in the target gene.
Supplementary Material
Acknowledgments
We thank B. Zid for comments on the manuscript. This work was supported by funding from a Lawrence L. and Audrey W. Ferguson Fellowship (to G.B.C.), the National Institutes of Health (K99AG030493, to W. W. J.; AG16630, AG24366 and DK070154, to S. B.) and the National Science Foundation (MCB-0418479, to S.B.).
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
Note added in proof: It has been brought to the attention of the authors that a similar method has been previously described in a non–peer-reviewed, non–indexed publication by Gleason et al. [Gleason, J.M., K.A. Cropp, and R.S. Dewoody. 2004. DNA preparations from fly wings for molecular marker assisted crosses. Drosophila Info. Serv. 87:107-108.]
References
- 1.Ashburner M, Golic KG, Hawley RS. Drosophila, a laboratory handbook. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1989. [Google Scholar]
- 2.Gloor GB, Preston CR, Johnson-Schlitz DM, Nassif NA, Phillis RW, Benz WK, Robertson HM, Engels WR. Type I repressors of P element mobility. Genetics. 1993;135:81–95. doi: 10.1093/genetics/135.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Missirlis F, Hu J, Kirby K, Hilliker AJ, Rouault TA, Phillips JP. Compartment-specific protection of iron-sulfur proteins by superoxide dismutase. J Biol Chem. 2003;278:47365–9. doi: 10.1074/jbc.M307700200. [DOI] [PubMed] [Google Scholar]
- 4.Kyriacou CP, Hall JC. Interspecific genetic control of courtship song production and reception in Drosophila. Science. 1986;232:494–497. doi: 10.1126/science.3083506. [DOI] [PubMed] [Google Scholar]
- 5.Châline N, Ratnieks FLW, Raine NE, Badcock NS, Burke T. Apidologie. 2004;35:311–318. [Google Scholar]
- 6.Adams MD, Sekelsky JJ. From sequence to phenotype: reverse genetics in Drosophila melanogaster. Nat Rev Genet. 2002;3:189–98. doi: 10.1038/nrg752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.