Abstract
Exposure of preterm infants to hyperoxia impairs vascular growth, contributing to the development of bronchopulmonary dysplasia and retinopathy of prematurity. Disruption of vascular endothelial growth factor (VEGF)-nitric oxide (NO) signaling impairs vascular growth. Endothelial progenitor cells (EPCs) may play an important role in vascular growth. Endothelial colony-forming cells (ECFCs), a type of EPC, from human preterm cord blood are more susceptible to hyperoxia-induced growth impairment than term ECFCs. Therefore, we hypothesized that hyperoxia disrupts VEGF-NO signaling and impairs growth in preterm ECFCs and that exogenous VEGF or NO preserves growth in hyperoxia. Growth kinetics of preterm cord blood-derived ECFCs (gestational ages, 27–34 wk) were assessed in room air (RA) and hyperoxia (40–50% oxygen) with or without VEGF, NO, or Nω-nitro-l-arginine. VEGF, VEGF receptor-2 (VEGFR-2), and endothelial NO synthase (eNOS) protein expression and NO production were compared. Compared with RA controls, hyperoxia significantly decreased growth, VEGFR-2 and eNOS expression, and NO production. VEGF treatment restored growth in hyperoxia to values measured in RA controls and significantly increased eNOS expression in hyperoxia. NO treatment also increased growth in hyperoxia. Nω-nitro-l-arginine treatment inhibited VEGF-augmented growth in RA and hyperoxia. We conclude that hyperoxia decreases growth and disrupts VEGF-NO signaling in human preterm ECFCs. VEGF treatment restores growth in hyperoxia by increasing NO production. NO treatment also increases growth during hyperoxia. Exogenous VEGF or NO may protect preterm ECFCs from the adverse effects of hyperoxia and preservation of ECFC function may improve outcomes of preterm infants.
Keywords: endothelial progenitor cells, umbilical cord blood, bronchopulmonary dysplasia, retinopathy of prematurity, nitric oxide synthase
despite improvements in perinatal care, chronic lung disease after premature birth, known as bronchopulmonary dysplasia (BPD), remains a major clinical problem, with an estimated 10–15 thousand affected infants in the United States each year (8, 57). Patients with BPD have persistent abnormalities in gas exchange, exercise intolerance, a high risk for pulmonary hypertension, and recurrent respiratory infections (1, 19, 40, 57). BPD is primarily characterized by impaired lung vascular and alveolar growth, including alveolar simplification, decreased lung surface area, and reduced growth of the capillary bed (15, 30, 38). Several mechanisms contribute to the pathogenesis of BPD, including exposure to hyperoxia. Experimental and clinical studies (14, 52) have shown that hyperoxia impairs lung vascular and alveolar growth in preterm infants.
In addition to the high risk for BPD, hyperoxia also contributes to abnormal vascular growth in the developing retina, causing retinopathy of prematurity (ROP; Ref. 56). Laboratory studies (22, 56) have demonstrated that high oxygen tension disrupts normal vascularization of the immature retina, leading to marked injury followed by impaired recovery that is characterized by excessive vascular growth.
Altered vascular endothelial growth factor (VEGF) signaling contributes to hyperoxia-induced vascular disease in both BPD and ROP. In human studies of BPD, VEGF was decreased in tracheal fluid samples from at risk premature neonates who subsequently developed BPD, and lung VEGF and VEGF receptor-1 (VEGFR-1) expression was decreased in infants who died with BPD (9, 43). In animal studies, hyperoxia decreases alveolar VEGF expression (48), and selective VEGF receptor inhibition reduces lung vascular growth and alveolarization (35, 44). These results suggest that endothelial-epithelial cross-talk, especially via VEGF signaling, is critical for normal lung growth following birth and that disruption of VEGF signaling impairs lung vascular growth and alveolarization. In addition to models of neonatal lung disease, impaired VEGF signaling has also been demonstrated in experimental models of ROP, with hyperoxia causing an early decrease in VEGF expression during early retinal injury, which is followed by late elevation of VEGF that mediates the excessive retinal vascularization of progressive disease (56).
In the developing lung and elsewhere, nitric oxide (NO) mediates the angiogenic effects of VEGF via activation of VEGFR-2 and stimulation of downstream signaling pathways (7, 47, 50, 54). Previous work from our laboratory (41) showed that VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats and that inhaled NO improves lung growth in infant rats after either hyperoxia or treatment with a VEGF receptor inhibitor (46, 58). However, mechanisms through which VEGF or NO improve lung growth after hyperoxia are not fully understood.
In addition to angiogenic mechanisms, vascular growth during development also occurs through vasculogenesis (16, 27). Vasculogenesis is the process of blood vessel formation via differentiation of endothelial progenitor cells (EPCs) into endothelial cells (39). Several studies (4, 12, 18, 53, 61) of adult vascular diseases have demonstrated that EPCs may play an important role in the pathogenesis or treatment of vascular injury. However, the roles of EPCs during normal vascular development or in response to injury, especially in the setting of prematurity, remain unknown.
Several distinct populations of cells have been reported as EPCs (2, 26, 45). In the majority of studies to date, EPCs were identified and enumerated by two methodologies: flow cytometry or in vitro cell culture. By flow cytometry, EPCs have typically been identified as cells expressing CD34, CD133, and VEGFR-2 (51). In cell culture, there is a distinction between early outgrowth EPCs (also known as colony-forming unit endothelial cells or CFU-ECs) and late outgrowth EPCs [known as endothelial colony-forming cells (ECFCs); Ref. 31, 51]. CFU-ECs are not highly proliferative and have characteristics of both angiogenic macrophages and hematopoietic cells (62). ECFCs are characterized by a cobblestone-like morphology in culture, express endothelial cell surface markers, have a high proliferative potential and the capacity for self-renewal, are able to develop a vascular network in vivo, and do not ingest bacteria (31, 33, 51).
In studies of CFU-ECs, VEGF is one of several factors involved in EPC mobilization from bone marrow (3, 24, 60, 63), recruitment and homing to sites of neovascularization (24, 34, 63), and development (60, 63). VEGF increases mobilization through PI3K/Akt-dependent activation of endothelial NO synthase (eNOS; Ref. 60). eNOS is required at the site of vessel formation by EPCs (17) and regulates proliferation, apoptosis, and differentiation of EPCs (49). Jiang et al. (36, 37) demonstrated that hypoxia-inducible factor-1α (HIF-1α) regulates VEGF, VEGFR-2, and eNOS expression. In addition, previous work from our laboratory (6) demonstrated that hyperoxia reduces lung VEGF, VEGFR-2, and eNOS expression, and CD45−1/Sca-1+/CD133+/VEGFR-2+ cell levels in the blood, bone marrow, and lungs in a mouse model of BPD.
With regard to studies of ECFCs, Ingram et al. (32) showed that oxidant treatment decreases the clonogenic capacity and increases apoptosis of ECFCs. We (5) recently reported that umbilical cord blood from preterm infants contains more ECFCs than term infants; that preterm ECFCs have greater growth potential but are more susceptible to hyperoxia-induced oxidative stress; and that the adverse effects of hyperoxia on preterm ECFC growth are prevented by treatment with the antioxidants, SOD, and catalase. These studies suggest that hyperoxia alters preterm ECFC function, but mechanisms underlying these effects are unclear. Although impaired VEGF and NO signaling have been linked with vascular injury in the developing newborn (9, 43, 50), the direct effects of hyperoxia on VEGF-NO signaling in human ECFCs are not known. Therefore, we hypothesized that hyperoxia decreases preterm ECFC growth through disruption of VEGF-NO signaling and that treatment with VEGF or NO would restore or enhance preterm ECFC growth.
MATERIALS AND METHODS
Cord Blood Samples and ECFC Isolation
The Colorado Multiple Institutional Review Board approved all protocols included in this study. Preterm cord blood samples (gestational age; 27–34 wk) were obtained at birth from deliveries at the University of Colorado Anschutz Inpatient Pavilion. After informed consent was obtained, cord blood was collected in a heparinized 60-ml syringe directly from the umbilical vein. Samples were transferred to heparinized tubes and transported to the research laboratory at room temperature. All cord blood samples were processed within 24 h of collection. ECFCs were isolated and characterized by light microscopy, flow cytometry, and immunohistochemistry, as previously described (5, 45).
Cord blood samples were processed as follows. Fifteen milliliters of blood were diluted in 20 ml PBS (Invitrogen, Carlsbad, CA) and placed in 50-ml conical vials. Blood was underlaid with 15 ml of Ficoll-Paque PLUS (Amersham Biosciences, Piscataway, NJ) using a steel pipetting needle (Popper and Sons, Lincoln, RI) and then centrifuged at 740 g for 30 min. The mononuclear cell buffy coat was then collected and washed three times with complete EGM-2 media (EBM-2 media with SingleQuot Bullet kit; hydrocortisone, human epidermal growth factor, human fibroblast growth factor-basic, insulin-like growth factor-1, VEGF, ascorbic acid, heparin, and gentamycin; Lonza, Mapleton, IL), 10% FBS (Hyclone; Logan, UT), and 2% antibiotic/antimycotic (penicillin, streptomycin, and amphotericin B; Invitrogen). Mononuclear cells were plated on cell culture plates coated with type 1 rat-tail collagen (BD Biosciences, San Jose, CA) at a density of 5 × 106 cells/cm2. Cells were incubated in room air (37°C, 5% CO2) for 14 days. Complete EGM-2 media were changed daily for 7 days and then three times per week. ECFCs were quantified on day 14. Single colonies were then passaged using sterile cloning disks (Labcor Products, San Diego, CA) and expanded by standard techniques. Low passage cells (p3 to p5) were used for each of the studies.
Characterization of Preterm ECFCs
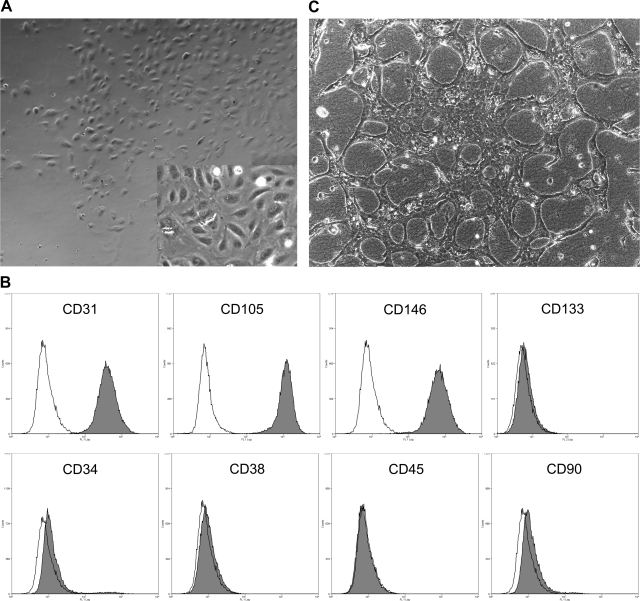
Isolated preterm ECFCs developed a cobblestone-like morphology (Fig. 1A). By flow cytometry, these cells were positive for endothelial cell makers (CD31, CD105, and CD146), weakly positive for CD34, and negative for CD133, a marker of cell immaturity (Fig. 1B). Additionally, these cells were negative for hematopoietic (CD38 and CD45) and fibroblast (CD90) markers (Fig. 1B). By immunohistochemistry, VE-cadherin and vWF were strongly positive (data not shown). Preterm ECFCs spontaneously formed vascular-like structures in a three-dimensional tube formation assay with collagen gel (Fig. 1C).
Fig. 1.
Endothelial colony forming cells (ECFCs) develop a cobblestone-like morphology in culture and express endothelial cell surface markers. A: image of typical preterm ECFCs [magnification: low-power, ×40; high-power (inset), ×100]. B: flow cytometry of preterm ECFCs. Each single-stain result (gray) was compared with the appropriate isotype control (white). Preterm ECFCs were strongly positive for endothelial cell lineage makers, CD31, CD105, and CD146, weakly positive for CD34 and negative for CD133, a marker of cell immaturity. Cells were negative for the hematopoietic makers CD38 and CD45 and the fibroblast marker CD90. C: preterm ECFCs in an in vitro tube formation assay.
Flow cytometry analysis.
We analyzed cultured preterm ECFCs using fluorescence activated cell sorting. We resuspended 0.5–1.0 × 106 cells in 80 μl of DNase staining buffer (PBS with 2% FBS, 80 U/ml dornase alfa; Sigma, St. Louis, MO) to prevent cell clumping and 20 μl mouse serum to prevent nonspecific binding of antibodies. Cells were then washed and stained with one or more of following: CD45-PE-cyanine 7 (PCy7; Beckman Coulter IM3548U; Fullerton, CA), CD133-PE (Miltenyi Biotec 130-080-80; Auburn, CA), CD31-FITC (BD 557508), CD34-allophycocyanin (BD 555824), CD38-FITC (BD 335790), CD90-FITC (BD 555595), CD105-FITC (BioLegend 312403; San Diego, CA), CD146-FITC (R&D Systems FAB932F; Minneapolis, MN), the appropriate FITC-mouse IgG1κ (BD 555748), or PE-mouse IgG1κ (BD 559320) isotype controls. Immediately before analysis, propidium iodide was applied to the samples to identify dead cells. Cells were analyzed using a Beckman Coulter FC500 flow cytometer.
Immunohistochemistry.
Preterm ECFCs were plated on type-1 collagen-coated 4-chamber tissue culture slides (BD Falcon) at a density of 2,500 cells per well. Cells were incubated in room air (37°C, 5% CO2) for 3–4 days until 90% confluent. They were washed with PBS, fixed with 4% paraformaldehyde (15 min), and then permeabilized with methanol (15 min) and 0.1% Triton-X (5 min). Then, 2% horse serum and 0.1% BSA were used as blocking agents to prevent nonspecific binding. Cells were washed with 0.1% BSA in PBS after blocking and between primary and secondary antibodies. Primary antibodies included the following endothelial markers: eNOS (BD 610297), CD31 (Dako M0823; Denmark), vWF-8 (Dako A0082), VE-cadherin (Cayman 160840; Ann Arbor, MI), and VEGFR-2 (Santa Cruz sc-504; Santa Cruz, CA). Cells were then incubated with appropriate FITC-conjugated goat-anti-mouse (Invitrogen A21202) and PE-Texas red-conjugated goat-anti-rabbit (Invitrogen A21207) secondary antibodies. Finally, nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Vector; Burlingame, CA). Images were obtained using an Olympus IX71 fluorescence microscope (Olympus; Center Valley, PA). Three filters were used to acquire images: DAPI (blue); eNOS (green); and vWF, VE-cadherin, and VEGFR-2 (red).
Tube formation assay.
The ability of ECFCs to form vascular structures in vitro was assayed by mixing 1 × 105 ECFCs in 7.5% Cultrex type I bovine collagen (R&D Systems 344210001) and endothelial basal medium-2 (EBM-2; Lonza; no supplements). This cell/collagen/media mixture (0.5 ml) was then added to each well of a 12-well plate and incubated for 60 min. After incubation, 0.5 ml of complete EGM-2 media (1% FBS) was added to each well. The media were changed daily and the center of each well was imaged at 48 h using an Olympus IX71 fluorescence microscope (Olympus).
ECFC growth assay.
Preterm ECFCs were plated in complete EGM-2 media with 10% FBS on collagen-coated 6-well tissue culture plates at a density of 2.5 × 104 cells/well and incubated overnight in room air. Cells were grown in EGM-2 media with 2.5% FBS in room air or hyperoxia, at 40–50% oxygen, for 6 days.
In preliminary experiments, ECFCs from human term cord blood were exposed to higher concentrations of oxygen than used in this study (60% and 80% oxygen). We found that with extreme hyperoxia, ECFC growth was not only inhibited, but cell detachment and death occurred as demonstrated by decreasing cell counts. When exposed to 40% oxygen, the growth of term ECFCs was not significantly different from that of room air controls. However, preterm ECFC cell number was significantly decreased after exposure to 40% oxygen. Based on these results, we chose to use 40–50% oxygen in these studies. In addition, this level may better reflect the physiological levels that human newborns are exposed to after premature birth.
In preliminary serum-titration studies, we further found that ECFCs proliferate equally in 10% FBS and 2.5% FBS. To minimize the effects of FBS, we used the lower serum concentration for all growth assays. Moreover, because supplementation of EGM-2 media with VEGF might mask the effects of exogenous VEGF in our study protocol, VEGF was not added to the control media.
Three types of growth assays were performed with preterm ECFCs from four cord blood samples (n = 4) in each study. The conditions for the three assays were as follows: 1) room air with and without recombinant human VEGF (rhVEGF; 25 ng/ml; R&D Systems 293-VE); hyperoxia with and without rhVEGF; 2) room air with and without NO gas (10 ppm) and hyperoxia with and without NO gas; and 3) room air and hyperoxia with the combination of rhVEGF with or without Nω-nitro-l-arginine (LNA; 2 mmol/l; Sigma N5501) to inhibit NO production. Concentrations of rhVEGF, NO gas, and LNA were established by preliminary in vitro studies and earlier publications (20, 21, 55, 59). Media were changed every day and cells were counted in triplicate on days 0, 1, 3, 4, and 6. For counting, cells were removed from wells by enzymatic digestion with 0.25% trypsin/2.21 mmol/l EDTA (Mediatech; Manassas, VA). Cell counts were performed using a hemocytometer and the results were averaged for each time point.
Western blot analysis for VEGF, VEGFR-2, and eNOS.
On days 3 and 6, cells from the room air and hyperoxic control and hyperoxia with rhVEGF groups of the growth assays were washed with ice-cold PBS and lysed in RIPA buffer (50 mM Tris·HCl, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, and 0.1% SDS) containing a protease inhibitor cocktail (Sigma). Cell lysates were scraped off dishes and centrifuged at 10,000 g for 20 min at 4°C. Protein content in the supernatant was determined by the Bradford method, using BSA as the standard (11). Then, 15 μg of protein sample per lane were resolved by SDS-polyacrylamide gel electrophoresis, and proteins from the gel were transferred to polyvinylidene difluoride membranes. Blots were blocked for 30 min in ECL Advance blocking agent (Amersham Pharmacia Biotech, Buckinghamshire, UK) in PBS or Tris-buffered saline with 0.05% Tween 20 (Acros Organics; Belgium). These blots were incubated at 4°C overnight with a rabbit polyclonal antibody against human VEGF (Santa Cruz sc-507; Santa Cruz, CA; 1: 500) or VEGF receptor 2 (VEGFR-2; Santa Cruz sc-504; 1: 500) or a purified mouse anti-eNOS/NOS type III antibody (BD 610297; 1: 500). Antibodies were diluted in ECL Advance blocking agent in PBS or Tris-buffered saline with 0.05% Tween 20. Blots were then incubated for 1 h at room temperature with either a goat anti-rabbit IgG-horseradish peroxidase secondary antibody (Santa Cruz sc-2054; 1: 5,000–40,000) or a goat anti-mouse IgG-horseradish peroxidase antibody (Chemicon, Millipore, AP124P, Billerica, MA; 1: 20,000). After being washed, bands were visualized by enhanced chemiluminescence (ECL Advance kit; Amersham Pharmacia Biotech). Recombinant human VEGF was used as a control. Blots were also incubated with a mouse monoclonal anti-β-actin antibody (Sigma A-2228; 1:10,000) for normalization.
Densitometry was performed using NIH Image (version 1.61). Changes in VEGF, VEGFR-2, and eNOS protein expression were analyzed after normalizing for β-actin expression.
ELISA.
VEGF concentration in the ECFC-conditioned media from the room air and hyperoxic control groups on days 3 and 6 of the growth assays was measured with a Quantikine ELISA kit (R&D Systems).
NO production assay.
Preterm ECFCs were plated in complete EGM-2 media with 10% FBS on collagen-coated 6-well tissue culture plates at a density of 2.5 × 105 cells/well and incubated overnight in room air. Cells were pretreated for 24 h in room air or in hyperoxia (50% oxygen) in EGM-2 media with 2.5% FBS without VEGF supplementation. ECFCs were incubated for 30 min at 37°C in HBSS (Mediatech) containing 0.5 μmol/l of 3-amino,4-aminomethyl-2′,7′-difluorofluorescein diacetate, cell-permeable NO fluorescent indicator (DAF-FM DA; Calbiochem 251520; San Diego, CA) for loading and were washed with PBS. ECFCs were then incubated for 30 min at 37°C in EBM-2 in the presence or absence of rhVEGF (25 ng/ml; R&D Systems) and harvested by enzymatic digestion with 0.25% trypsin/2.21 mmol/l EDTA. Fluorescence intensity was measured by flow cytometry in the system for FITC. Mean fluorescence intensity was compared using Summit software (version 4.3; Dako-Cytomation).
Statistical Analysis
Data are means ± SE. Statistical analysis was performed with the Prism software package (version 4, GraphPad Software, San Diego, CA). Paired t-test was used to compare densitometry units for VEGF, VEGFR2, and eNOS protein expression by Western blot analysis. Growth kinetics and NO production were compared by repeated measures two-way ANOVA with Bonferroni posttest analysis. P values <0.05 were considered significant.
RESULTS
Hyperoxia Impairs Preterm ECFC Growth and VEGF Treatment Increases Cell Growth in Room Air and Hyperoxia
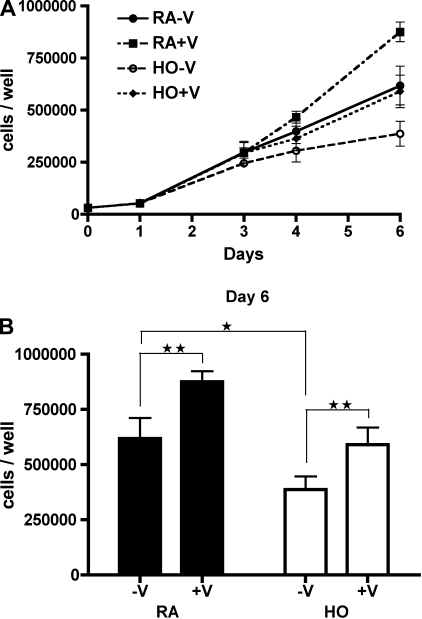
Preterm ECFC growth kinetics were studied under the following conditions: room air without rhVEGF (RA-V); room air with rhVEGF (25 ng/ml, RA+V); hyperoxia (40–50% O2) without rhVEGF (HO-V); and hyperoxia with rhVEGF (HO+V; Fig. 2A). After 6 days, preterm ECFC number from the RA-V group was significantly greater than from the HO-V group (6.2 ± 0.9 × 105 vs. 3.9 ± 0.6 × 105 cells/well; P < 0.05; Fig. 2B). The number of ECFCs in the RA+V group was significantly greater than that of the RA-V group (8.8 ± 0.5 × 105 vs. 6.2 ± 0.9 × 105 cells/well; P < 0.01; Fig. 2B). Additionally, the number of ECFCs in the HO+V group was significantly greater than that of the HO-V group (5.9 ± 0.8 × 105 vs. 3.9 ± 0.6 × 105 cells/well; P < 0.01; Fig. 2B) and similar to that of the RA-V control group (5.9 ± 0.8 × 105 vs. 6.2 ± 0.9 × 105 cells/well; P = NS; Fig. 2B).
Fig. 2.
Hyperoxia impairs preterm ECFC growth and VEGF treatment increases growth in room air and hyperoxia. A: Growth curves of preterm ECFCs in room air or hyperoxia (40–50% oxygen) with or without recombinant human (rh)VEGF (25 ng/ml). Cells were grown in the following conditions for 6 days: room air without VEGF (RA-V; •); room air with VEGF (RA+V; ▪); hyperoxia without VEGF (HO-V; ○); and hyperoxia with VEGF (HO+V; ⧫). B: ECFC numbers on day 6. Hyperoxia reduced preterm ECFC growth, and VEGF treatment significantly increased ECFC growth in both room air and hyperoxia. Error bars represent SE. *P < 0.05, **P < 0.01. n = 4.
Hyperoxia Decreases Protein Expression of VEGFR-2 and eNOS but not VEGF in Preterm ECFCs
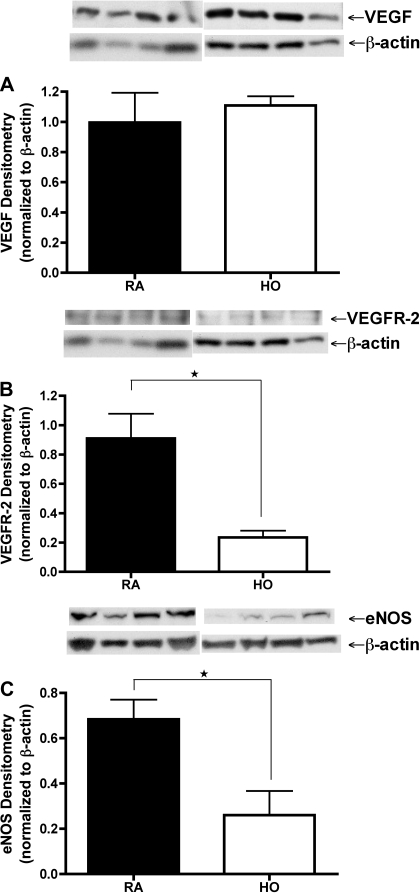
To determine if hyperoxia decreases VEGF-NO signaling in preterm ECFCs, we collected cell lysates and conditioned media on days 3 and 6 from RA-V and HO-V groups of growth assays. We measured VEGF protein content by Western blot analysis using cell lysates and by ELISA using conditioned media. Additionally, we measured VEGFR-2 and eNOS protein contents by Western blotting. There was no difference in VEGF protein content between RA-V and HO-V groups on day 6 (Fig. 3A). In addition, VEGF concentrations from conditioned media on day 6 as measured by ELISA were below the detectable range in both groups (data not shown). However, VEGFR-2 and eNOS protein contents were markedly reduced in the HO-V group compared with RA-V group on day 6 by 74.3 and 61.9%, respectively (Fig. 3, B and C). Results on day 3 ECFCs were the same as those studied on day 6.
Fig. 3.
Hyperoxia decreases expression of VEGFR-2 and eNOS but not VEGF protein in preterm ECFCs. Western blot analyses of cell lysates exposed to hyperoxia (50% oxygen) on day 6 of growth assay (HO, open bars) are compared with room air controls (RA, filled bars). A: VEGF protein in preterm ECFCs is unchanged by hyperoxia exposure. VEGFR-2 (B) and endothelial nitric oxide synthase (eNOS; C) protein in preterm ECFCs are markedly reduced in hyperoxia compared with room air controls. Error bars represent SE. *P < 0.05. n = 4.
Hyperoxia Decreases Basal NO Production and Inhibits the Ability to Produce NO in Response to VEGF in Preterm ECFCs
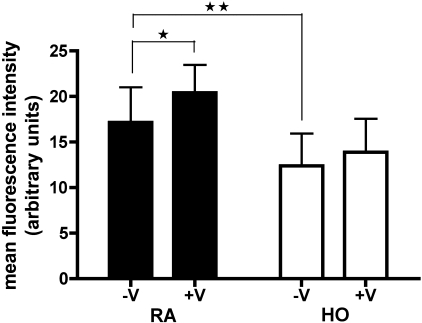
To determine whether hyperoxia decreases NO production by preterm ECFCs and whether exogenous VEGF increases NO production by preterm ECFCs in hyperoxia, we measured NO production with the cell-permeable NO fluorescent indicator DAF-FM diacetate by flow cytometry. Cells were pretreated in RA or HO (50% oxygen) for 24 h and then stimulated with or without 30-min exposures to rhVEGF (±V, 25 ng/ml). NO production in the HO-V group was significantly decreased compared with the RA-V group (P < 0.01; Fig. 4). Exogenous VEGF significantly increased NO production in room air (RA-V vs. RA+V; P < 0.05; Fig. 4). In contrast to RA ECFCs, VEGF treatment did not stimulate NO production in hyperoxia, suggesting that hyperoxia decreases basal NO production by preterm ECFCs and that ECFCs lose their ability to produce NO in response to acute VEGF treatment after 24 h of hyperoxia.
Fig. 4.
Hyperoxia decreases basal NO production and the ability to produce NO in response to VEGF in preterm ECFCs, as measured with the cell-permeable NO fluorescent indicator 3-amino,4-aminomethyl-2′,7′-difluorofluorescein (DAF-FM) diacetate, by flow cytometry. Cells were pretreated in room air (filled bars) or hyperoxia (50% oxygen, open bars) for 24 h and then stimulated with or without 30-min exposures to rhVEGF (±V, 25 ng/ml, right and left, respectively, in each pretreatment). NO production after hyperoxia was decreased compared with the room air group. VEGF acutely stimulated NO production by the room air cells but had no effect on cells exposed to hyperoxia. Error bars represent SE. *P < 0.05, **P < 0.01; n = 4.
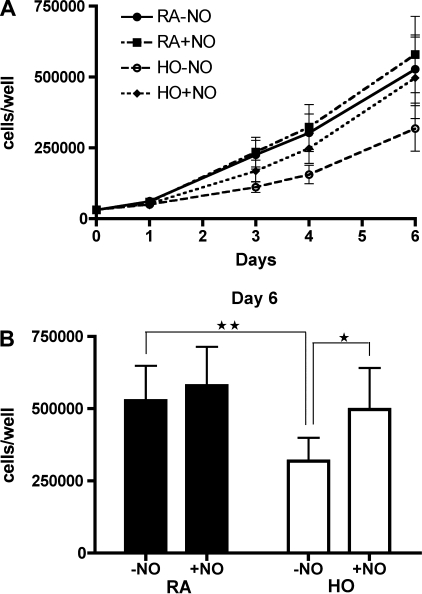
NO Treatment Increases Preterm ECFC Growth in Hyperoxia
Preterm ECFC growth kinetics were studied under the following conditions: room air without NO gas (RA-NO); room air with NO gas (10 ppm, RA+NO); hyperoxia (50% O2) without NO gas (HO-NO); and hyperoxia with NO gas (HO+NO; Fig. 5A). The number of preterm ECFCs on day 6 in the HO+NO group was significantly greater than that of the HO-NO group (5.0 ± 1.4 × 105 vs. 3.1 ± 0.8 × 105 cells/well; P < 0.05; Fig. 5B) and similar to that of the RA-NO group (5.0 ± 1.4 × 105 vs. 5.3 ± 1.2 × 105 cells/well; P = NS; Fig. 5B). However, NO treatment did not alter preterm ECFC growth in room air (RA-NO vs. RA+NO; 5.3 ± 1.2 × 105 vs. 5.8 ± 1.3 × 105 cells/well; P = NS; Fig. 5B).
Fig. 5.
NO treatment increases preterm ECFC growth in hyperoxia. A: growth curves of preterm ECFCs during exposure to room air or hyperoxia (50% oxygen), with or without NO gas (10 ppm) for 6 days: room air without NO (RA-NO; •); room air with NO (RA+NO, ▪); hyperoxia without NO (HO-NO; ○); and hyperoxia with NO (HO+NO; ⧫). B: ECFC numbers on day 6. NO treatment did not alter preterm ECFC growth from room air cells but restored growth of ECFCs exposed to hyperoxia to room air values after 6 days. Error bars represent SE. *P < 0.05, **P < 0.01; n = 4.
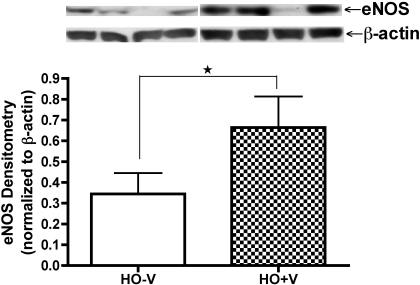
VEGF Treatment Increases eNOS Protein Expression in Preterm ECFCs in Hyperoxia
To determine the effect of VEGF treatment on eNOS protein expression in preterm ECFCs in hyperoxia, we collected cell lysates on days 3 and 6 from HO-V and HO+V groups of growth assays. We measured eNOS protein contents by Western blotting. There was no difference in eNOS protein content between HO-V and HO+V groups on day 3 (data not shown). However, eNOS protein contents were significantly increased in the HO+V group compared with HO-V group on day 6 by 193% (P < 0.05; Fig. 6).
Fig. 6.
VEGF treatment increases eNOS protein expression in preterm ECFCs in hyperoxia. Western blot analysis for eNOS from cell lysates exposed to hyperoxia (50% oxygen) with or without rhVEGF (±V; 25 ng/ml) on day 6 of growth assay (HO-V, open bar; HO+V, hatched bar). Error bars represent SE. *P < 0.05; n = 4.
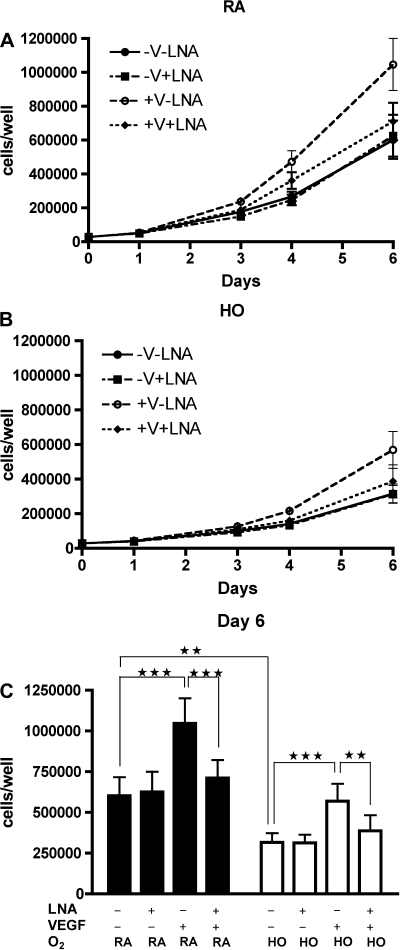
NOS Inhibition Reduces the Effects of VEGF Treatment on Preterm ECFC Growth
To determine whether the effect of VEGF treatment on preterm ECFC growth is mediated by NO, we studied the effects of NOS inhibition on the growth of preterm ECFCs treated with VEGF in room air and hyperoxia. Growth kinetics were studied under the following conditions: room air with or without rhVEGF (25 ng/ml) with or without LNA (2 mmol/l; RA+V+L, RA-V+L, RA+V-L, and RA-V-L; Fig. 7A); hyperoxia (50% O2) with or without rhVEGF with or without LNA (HO+V+L, HO-V+L, HO+V-L, and HO-V-L; Fig. 7B). In both room air and hyperoxia, NOS inhibition prevented VEGF-augmented preterm ECFC growth (RA+V-L vs. RA+V+L; 10.5 ± 1.5 × 105 vs. 7.1 ± 1.1 × 105 cells/well; P < 0.001: HO+V-L vs. HO + V+L; 5.7 ± 1.1 × 105 vs. 3.9 ± 1.0 × 105 cells/well; P < 0.01; Fig. 7C), but NOS inhibition did not affect preterm ECFC growth under basal conditions (RA-V-L vs. RA-V+L; 6.0 ± 1.1 × 105 vs. 6.3 ± 1.2 × 105 cells/well; P = NS: HO-V-L vs. HO-V+L; 3.2 ± 0.6 × 105 vs. 3.1 ± 0.5 × 105 cells/well; P = NS; Fig. 7C).
Fig. 7.
Effect of VEGF treatment on preterm ECFC growth is mediated by NO. Growth curves of preterm ECFCs in room air (A) and in hyperoxia (B; 50% oxygen) with or without rhVEGF (25 ng/ml) or Nω-nitro-l-arginine (LNA; 2 mmol/l). Study conditions included without VEGF and without LNA (-V-L; •); without VEGF and with LNA (-V+L; ▪); with VEGF and without LNA (+V-L; ○); and with both VEGF and LNA (+V+L, ⧫). C: ECFC numbers on day 6 in room air and hyperoxia. NOS inhibition prevented VEGF-augmented preterm ECFC growth but did not affect growth under basal conditions in both room air and hyperoxia. Error bars represent SE. **P < 0.01, ***P < 0.001; n = 4.
DISCUSSION
In this study, we show that hyperoxia impairs preterm ECFC growth, decreases protein expression of VEGFR-2 and eNOS, and reduces NO production in preterm ECFCs. We found that VEGF and NO treatment preserve preterm ECFC growth in hyperoxia. Additionally, we demonstrate that the effect of VEGF treatment on preterm ECFC growth is mediated by NO. These results suggest that hyperoxia reduces preterm ECFC function by downregulation of VEGF-NO signaling and that exogenous VEGF and NO can protect preterm ECFCs from the inhibitory effects of hyperoxia on cell proliferation.
These findings are interesting because this is the first report that demonstrates the effect of hyperoxia on VEGF-NO signaling in human preterm ECFCs and the effects of exogenous VEGF and NO on preterm ECFC growth. Insights into EPC biology during development and in pathophysiological conditions may provide new concepts for understanding the disease process and developing therapeutic options for vascular diseases, such as BPD and ROP. These findings suggest that hyperoxia-induced vascular diseases in preterm infants may potentially be related to impaired EPC proliferation and growth and that VEGF and NO therapies may augment EPC function. Whether inhaled NO can augment EPC function in premature infants is unknown. As noted in studies of EPCs in adult vascular diseases (4, 12, 18, 53, 61), cord blood-derived ECFCs from preterm infants may potentially serve as a biomarker to predict disease severity or as a therapeutic option in neonatology in the future. Recently, Borghesi et al. (10) reported that extremely preterm infants who display lower ECFC numbers at birth have an increased risk of developing BPD.
Previous studies (42) have shown that VEGF elicits mitogenic, antiapoptotic, and proangiogenic effects in endothelial cells, mainly through activation of VEGFR-2 and downstream pathways, including eNOS expression and activation, and NO production. Jiang et al. (36, 37) demonstrated that knockdown and overexpression of HIF-1α decreases and increases VEGF, VEGFR-2, and eNOS expression in CFU-ECs, respectively. Thus we speculate that HIF-1α downregulation by hyperoxia may be a mechanism through which VEGFR-2 and eNOS are decreased by hyperoxia in preterm ECFCs. Failure to respond to VEGF may be attributed to decreased VEGFR-2 and eNOS protein expression; however, we cannot exclude the possibility that decreased NO production reflects decreased NO bioavailability by reactive oxygen species due to hyperoxia (13).
Our results suggest that disruption of VEGF-NO signaling during hyperoxia reduces preterm ECFC growth. Hyperoxia decreased VEGFR-2 and eNOS levels and prevented NO production after VEGF stimulation. However, there were no differences in VEGF expression between room air- and hyperoxia-exposed ECFCs. This suggests that hyperoxia disrupts the VEGF-NO signaling pathway, not by altering VEGF levels or production, but rather by impairing the cellular ability to acutely respond to VEGF. Nevertheless, VEGF supplementation during hyperoxia protected ECFCs from hyperoxia-induced growth impairment. Moreover, NOS inhibition had no effect on preterm ECFC growth in the basal condition. These results suggest that the basal NO level in our culture system is too low to affect preterm ECFC growth and that decreased VEGF signaling through VEGFR-2 but not decreasing NO production is involved in the mechanisms through which hyperoxia reduces preterm ECFC growth.
Additionally, Ingram et al. (32) demonstrated that oxidant treatment decreases the clonogenic capacity of ECFCs and increases apoptosis. Baker et al. (5) previously showed that preterm ECFCs are susceptible to hyperoxia-induced oxidative stress, a process that can be prevented by treatment with the antioxidants. Therefore, although hyperoxia decreases VEGFR-2 and eNOS expression, preterm ECFC growth may also be decreased by another mechanism such as increased oxidative stress, which may lead to increased apoptosis.
Results from this study suggest that the effects of VEGF on preterm ECFC growth are at least partly mediated by NO. The ability of exogenous VEGF to stimulate NO production decreases after exposure to hyperoxia suggesting that hyperoxia disrupts this process. However, in hyperoxia VEGF treatment partially restores eNOS protein expression and NO treatment restores the growth of ECFCs to baseline (room air) levels. We speculate that exogenous VEGF increases preterm ECFC growth partially by protecting ECFCs from decrease in eNOS expression and by stimulating NO production in hyperoxia.
The effects of NO on mature endothelial cell growth have been previously studied by other laboratories (25, 64), but the effects of NO on endothelial cell or EPC growth under hyperoxic conditions have not been fully studied. Hoetzer et al. (28) demonstrated that eNOS inhibition does not alter EPC colony-forming capacity. However, the cells used in their study were peripheral blood-derived, adult CFU-ECs, not cord blood-derived, preterm ECFCs. Additionally, eNOS expression and capacity of NO production are different among adult CFU-ECs, ECFCs, and mature endothelial cells. Basal eNOS protein expression in ECFCs is lower than mature endothelial cells but higher than CFU-ECs (23). The capacity of NO production by VEGF stimulation of ECFCs is higher than that of CFU-ECs (29).
Decreased eNOS expression, impaired eNOS activation or activity, and accelerated NO degradation by ROS such as peroxynitrite (formed when superoxide combines with NO) all cause a decline in NO bioavailability (13). In the present study, NOS inhibition had no effect on preterm ECFC growth in basal conditions and VEGF-induced endogenous NO increased growth in both room air and hyperoxia. However, pharmacological doses of exogenous NO only increased growth in hyperoxia. We cannot fully explain these results but speculate that the basal level of endogenous NO in our culture system may be too low to affect preterm ECFC growth. Additionally, the main mechanisms through which exogenous NO and VEGF-induced endogenous NO affect preterm ECFC growth may be different due to different administration routes, production sites, or NO amounts.
Pharmacological levels of exogenous NO may increase growth mainly through a hyperoxia-specific effect. For example, increased scavenging of superoxide could result in optimization of the balance between ROS and NO or an overcoming of the decreased NO bioavailability during oxidative stress. Endogenous NO may increase growth mainly through hyperoxia-nonspecific effect on ECFCs, for example, activating soluble guanylate cyclase and increasing cGMP.
We conclude that hyperoxia decreases growth and disrupts VEGF-NO signaling in preterm ECFCs, that VEGF treatment restores preterm ECFC growth in hyperoxia by increasing NO production and that NO treatment increases preterm ECFC growth during hyperoxia. Finally, we speculate that exogenous VEGF or NO may partially protect preterm ECFCs from the effects of hyperoxia and that protection of preterm ECFCs from the adverse effects of hyperoxia may improve outcomes in preterm infants at risk for BPD and ROP.
GRANTS
This work was supported by the Thrasher Grant Program (PN200601-194 to S. H. Abman), National Heart, Lung, and Blood Institute Grants RO1-HL-085703 and RO1-HL-068702 (to S. H. Abman) and K12- HL-090147-01 (to C. D. Baker), and the American Thoracic Society (ATS 06-060 to V. Balasubramaniam; Career Development Award to C. D. Baker).
REFERENCES
- 1.Abman SH. Pulmonary hypertension in chronic lung disease of infancy: pathogenesis, pathophysiology, and treatment. In: Chronic Lung Disease in Early Infancy, edited by Bland RD, Coalson JJ. New York, NY: M. Dekker, 1999, p. 619– 668 [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964– 966, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18: 3964– 3972, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assumus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation 106: 3009– 3017, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Baker CD, Ryan SL, Ingram DA, Seedorf GJ, Abman SH, Balasubramaniam V. Endothelial colony forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med 180: 454– 461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L1073– L1084, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase-deficient mouse. Am J Physiol Lung Cell Mol Physiol 284: L964– L971, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 357: 1946– 1955, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and Tie-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 164: 1971– 1980, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Borghesi A, Massa M, Campanelli R, Bollani L, Tzialla C, Figar TA, Ferrari G, Bonetti E, Chiesa G, de Silvestri A, Spinillo A, Rosti V, Stronati M. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med 180: 540– 546, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248– 254, 1976 [DOI] [PubMed] [Google Scholar]
- 12.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med 172: 854– 860, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular disease: the role of oxidant stress. Circ Res 87: 840– 844, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Chess PR, D'Angio CT, Pryhuber GS, Maniscalco WM. Pathogenesis of bronchopulmonary dysplasia. Semin Perinatol 30: 171– 180, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Coalson JJ. Pathology of chronic lung disease of early infancy. In: Chronic Lung Disease in Early Infancy, edited by Bland RD, Coalson JJ. New York, NY: M. Dekker, 1999, p. 85– 124 [Google Scholar]
- 16.DeMello DE, Reid LM. Embryonic and early fetal development of human lung vasculature and its functional implications. Pediatr Dev Pathol 3: 439– 449, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Duda DG, Fukumura D, Jain RK. Role of eNOS in neovascularization: NO for endothelial progenitor cells. Trends Mol Med 10: 143– 145, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Fadini GP, Schiavon M, Cantini M, Baesso I, Facco M, Miorin M, Tassinato M, de Kreutzenberg SV, Avogaro A, Agostini C. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells 24: 1806– 1813, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Filippone M, Sartor M, Zacchello F, Baraldi E. Flow limitation in infants with bronchopulmonary dysplasia and respiratory function at school age. Lancet 361: 753– 754, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Gensch C, Clever YP, Werner C, Hanhoun M, Bohm M, Laufs U. The PPAR-γ agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis 192: 67– 74, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Gien J, Seedorf GJ, Balasubramaniam V, Markham NE, Abman SH. Intrauterine pulmonary hypertension impairs angiogenesis in vitro: role of vascular endothelial growth factor-nitric oxide signaling. Am J Respir Crit Care Med 176: 1146– 1153, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu X, El-Remessy AB, Brooks SE, Al-Shabrawey M, Tsai NT, Caldwell RB. Hyperoxia induces retinal vascular endothelial cell apoptosis through formation of peroxynitrite. Am J Physiol Cell Physiol 285: C546– C554, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Gulati RG, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res 93: 1023– 1025, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MAS, Rafii S. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cell. J Exp Med 193: 1005– 1014, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellaer R, Polack T, Grabner R, Till U. Nitric oxide inhibits proliferation of human endothelial cells via a mechanism independent of cGMP. Atherosclerosis 144: 49– 57, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348: 593– 600, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Hislop AA. Airway and blood vessel interaction during lung development. J Anat 201: 325– 334, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoetzer GL, Irmiger HM, Keith RS, Westbrook KM, DeSouza CA. Endothelial nitric oxide synthase inhibition does not alter endothelial progenitor cell colony forming capacity or migratory activity. J Cardiovasc Pharmacol 46: 387– 389, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24: 288– 293, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 29: 710– 717, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood 106: 1525– 1531, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Ingram DA, Krier TR, Mead LE, McGuire C, Prater DN, Bhavsar J, Saadatzadeh MR, Bijangi-Vishehsaraei K, Li F, Yoder MC, Haneline LS. Clonogenic endothelial progenitor cells are sensitive to oxidative stress. Stem Cells 25: 297– 304, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104: 2752– 2760, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masude H, Hayashi S, Silver M, Isner JM, Asahara T. Endothelial progenitor cell vascular endothelial growth factor transfer for vascular regeneration. Circulation 105: 732– 738, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 279: L600– L607, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Jiang M, Wang B, He B, Fan H, Guo TB, Shao Q, Gao L, Liu Y. Angiogenesis by transplantation of HIF-1 alpha modified EPCs into ischemic limbs. J Cell Biochem 103: 321– 334, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Jiang M, Wang B, He B, Fan H, Guo TB, Shao Q, Gao L, Liu Y. Inhibition of hypoxia-inducible factor-1alpha and endothelial progenitor cell differentiation by adenoviral transfer of small interfering RNA in vitro. J Vasc Res 43: 511– 521, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723– 1729, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Kassmeyer S, Plendl J, Custoidis P, Bahramsoltani M. New insights in vascular development: vasculogenesis and endothelial progenitor cells. Anat Histol Embryol 38: 1– 11, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet 367: 1421– 1431, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol 289: L529– L535, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Lahm T, Crisostomo PR, Markel TA, Wang M, Lillemore KD, Meldrum DR. The critical role of vascular endothelial growth factor in pulmonary vascular remodeling after lung injury. Shock 28: 4– 14, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Lassus P, Turanlahti M, Heikkila P, Anderson LC, Nupponen I, Sarnesto A, Anderson S. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 164: 1981– 1987, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 283: L555– L562, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 105: 71– 77, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res 58: 22– 29, 2005 [DOI] [PubMed] [Google Scholar]
- 47.MacRitchie AN, Albertine KH, Sun J, Lei PS, Jensen SC, Freestone AA, Clair PM, Dahl MJ, Godfrey EA, Carlton DP, Bland RD. Reduced endothelial nitric oxide synthase in lungs of chronically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol 281: L1011– L1020, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Maniscalco WM, Watkins RH, D'Angio CT, Ryan RM. Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am J Respir Cell Mol Biol 16: 557– 567, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Ozuyaman B, Ebner P, Niesler U, Ziemann J, Kleinbongard P, Jax T, Godecke A, Kelm M, Kalka C. Nitric oxide differentially regulates proliferation and mobilization of endothelial progenitor cells but not of hematopoietic stem cells. Thromb Haemost 94: 770– 772, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 100: 3131– 3139, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia 21: 1141– 1149, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Roberts RJ, Weesner KM, Bucher JR. Oxygen-induced alterations in lung vascular development in the newborn rat. Pediatr Res 17: 368– 375, 1983 [DOI] [PubMed] [Google Scholar]
- 53.Schmidt LC, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation 111: 2981– 2987, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Shen BQ, Lee DY, Zioncheck TF. Vascular endothelial growth factor governs endothelial nitric-oxide synthase expression via a KDR/Flk-1 receptor and a protein kinase C signaling pathway. J Biol Chem 274: 33057– 33063, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Smadja DM, Bieche I, Helley D, Laurendeau I, Simonin G, Muller L, Aiach M, Gaussem P. Increased VEGFR2 expression during human late endothelial progenitor cells expansion enhances in vitro angiogenesis with up-regulation of integrin α6. J Cell Mol Med 11: 1149– 1161, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith LEH. Through the eyes of a child: understanding retinopathy through ROP. The Friedenwald lecture. Invest Ophthalmol Vis Sci 49: 5177– 5182, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol 67: 623– 661, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Tang JR, Markham NE, Lin YJ, McMurtry IF, Maxey A, Kinsella JP, Abman SH. Inhaled Nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol 287: L344– L351, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impaires endothelial progenitor cell mobilization and function in diabetes. Diabetes 56: 666– 674, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95: 343– 353, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Wang XX, Zhang FR, Shang YP, Zhu JZ, Xie XD, Tao QM, Zhu JH, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary artery hypertension: a pilot randomized controlled trial. J Am Coll Cardiol 49: 1566– 1571, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109: 1801– 1809, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res 78: 413– 421, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Zheng J, Wen Y, Austin JL, Chen DB. Exogenous nitric oxide stimulates cell proliferation via activation of a mitogen-activated protein kinase pathway in ovine fetoplacental artery endothelial cells. Biol Reprod 74: 375– 382, 2006 [DOI] [PubMed] [Google Scholar]