Abstract
Pseudomonas aeruginosa is a leading cause of hospital-acquired pneumonia and an important pathogen in patients with chronic lung disease, such as cystic fibrosis and bronchiectasis. The contribution of Toll-like receptor 5 (TLR5) to the innate immune response to this organism is incompletely understood. We exposed wild-type and TLR5-deficient (Tlr5−/−) mice to aerosolized P. aeruginosa at low and high inocula and assessed bacterial clearance, lung inflammation, and cytokine production 4 and 24 h after infection. Bacterial clearance was impaired in Tlr5−/− mice after low-inoculum, but not high-inoculum, infection. Early bronchoalveolar accumulation of neutrophils was reduced in Tlr5−/− mice after low- and high-dose infection. Cytokine responses, including markedly impaired monocyte chemoattractant protein-1 production 4 h after low- and high-inoculum challenge, were selectively altered in Tlr5−/− mice. In contrast, there was no impairment of bacterial clearance, neutrophil recruitment, or monocyte chemoattractant protein-1 production in Tlr5−/− mice after infection with a nonflagellated isotypic strain of P. aeruginosa. Thus TLR5-mediated recognition of flagellin is involved in activating pulmonary defenses against P. aeruginosa and contributes to antibacterial resistance in a manner that is partially inoculum dependent. These data are the first to demonstrate a unique role for TLR5 in the innate immune response to P. aeruginosa lung infection.
Keywords: lung infection, host defense, Pseudomonas, Toll-like receptors
pseudomonas aeruginosa is a leading cause of hospital-acquired pneumonia (14, 15) and an important pathogen in patients with chronic lung disease, such as cystic fibrosis and bronchiectasis, who are often colonized with highly resistant strains (23, 33). Host resistance to acute P. aeruginosa pneumonia requires activation of an innate immune response that recruits neutrophils to the lungs (41, 50). We previously demonstrated that successful initiation of the acute inflammatory response to and clearance of P. aeruginosa lung infection requires myeloid differentiation factor 88 (MyD88). This adapter molecule is essential for signaling by members of the IL-1 receptor superfamily, including most Toll-like receptors (TLRs) (45). TLRs are pattern recognition receptors that trigger a signaling cascade resulting in nuclear translocation of NF-κB and transcription of proinflammatory cytokine genes (2). More than 10 TLRs have been characterized, and each of these TLRs recognizes specific pathogen-associated molecular patterns (3, 41). The P. aeruginosa bacterium contains a variety of pathogen-associated molecular patterns that allow its recognition by several TLRs, including TLR2 (peptidoglycan and lipopeptides), TLR4 (LPS), TLR5 (flagellin), and TLR9 (cytosine-phosphate-guanosine DNA).
Whereas MyD88 is essential for successful resistance to P. aeruginosa pneumonia (45), TLR2, TLR4, and TLR9 are not required for the clearance of this pathogen from the lungs (47). TLR2-deficient (Tlr2−/−) mice have a selective defect in cytokine response to this organism and no impairment in neutrophil recruitment; TLR4-deficient (Tlr4−/−) mice have a broader impairment in cytokine and inflammatory response to infection, but Tlr2−/− and Tlr4−/− mice are able to resolve the infection. We previously examined the role of TLR5 in this model indirectly by infecting Tlr2−/−, Tlr4−/−, or Tlr2/4−/− mice with a flagellin-deficient strain of P. aeruginosa. Cytokine responses and neutrophil recruitment were more blunted in Tlr4−/− and Tlr2/4−/− mice infected with flagellin-deficient than wild-type (WT) P. aeruginosa (47). These observations suggest a role for TLR5 in the MyD88-dependent response to P. aeruginosa that warrants further study.
Several observations support a role for TLR5 in the host response to lung infection. TLR5 is highly expressed in human and murine lung tissue (43, 51) by airway epithelial cells, neutrophils, and alveolar macrophages (6, 24, 28, 31, 35). TLR5 recognizes a conserved monomeric protein component of flagellin (48) and signals through the MyD88 pathway to upregulate expression of proinflammatory cytokines (2). In airway epithelial cells, flagellin stimulation also upregulates TLR5 expression and mobilization from the basal to the apical cell surface (1). Neutrophil influx and the production of some cytokines in response to purified flagellin instilled in the murine lung is dependent on TLR5 (11). We previously reported that TLR5-deficient (Tlr5−1/−) mice exhibit dysregulated inflammatory responses to pulmonary infection with Legionella pneumophila (17) and that humans with a common stop codon polymorphism in the ligand-binding region of TLR5 are hypersusceptible to pneumonic legionellosis (18).
Prior research also predicts that the recognition of flagellin by TLR5 is likely to play a role in the host response to P. aeruginosa lung infection. TLR5 appears to be important for the recognition of P. aeruginosa by airway epithelial cells (1, 8, 19, 52) and for neutrophil responses to this organism (24). Animal models have demonstrated that the acute inflammatory response to purified flagellin is dependent on TLR5 (11), but the specific role of TLR5 in regulating the host response to live P. aeruginosa has not been elucidated. Mice infected with the nonflagellated ΔfliC mutant do not have a reduced inflammatory response compared with mice infected with a WT strain, but strains of P. aeruginosa that produce excess flagellin induce a greater inflammatory response in the lung than WT strains (7, 47). The only published study of P. aeruginosa lung infection in Tlr5−/− mice found no significant difference in mortality compared with WT animals but suggested a cooperative relationship between TLR4 and TLR5 in activating host resistance (11).
In this study, we sought to clarify the role of TLR5 in the innate immune responses to acute P. aeruginosa pneumonia. We used a well-defined murine model to compare the responses of Tlr5−/− and WT mice to acute lower respiratory tract infection with P. aeruginosa. We found that TLR5 contributes to the innate immune response to P. aeruginosa in a manner that is most evident at a low level of infection.
MATERIALS AND METHODS
Mice.
Male and female WT C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Tlr5−/− mice were provided by Dr. S. Akira (University of Osaka, Osaka, Japan) (5) and were backcrossed to C57BL/6 mice eight times. Genotypes were confirmed by PCR analysis of genomic DNA obtained from tail biopsies. Mice were housed under specific pathogen-free conditions with unlimited access to sterile food and water, and all animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Washington. Mice were 10–14 wk of age at the time of experimentation.
Bacteria.
WT P. aeruginosa strain PAK was a gift from Dr. S. Lory (Harvard University); the ΔfliC mutant of the PAK strain was generously provided by Dr. A. Prince (Columbia University) (10). Bacteria were prepared as described elsewhere (46). Frozen bacteria were thawed, inoculated into Luria-Bertani (LB) broth (Invitrogen, Carlsbad, CA), and incubated for 6 h at 37°C on a rotating platform. This broth was diluted 1:1,000 into each of four flasks containing 250 ml of fresh LB broth and incubated again for 16–18 h at 37°C on the rotating platform. Bacteria then were pelleted, washed twice with PBS containing 1 mM MgCl2, and resuspended in 18 ml of PBS with MgCl2. Bacterial concentrations were estimated by optical density and quantitated by culture of serial dilutions on LB agar. We performed two experiments, each with “low” and “high” concentrations of the PAK strain of P. aeruginosa, to achieve mean depositions of 6.4 × 104 colony-forming units (CFU)/lung (“low inoculum”) and 7.9 × 105 CFU/lung (“high inoculum”). A single low-inoculum experiment with the ΔfliC mutant achieved a mean deposition of 1.1 × 105 CFU/lung.
Mouse infection and tissue processing.
Mice were infected with P. aeruginosa as previously described (45–47). In each experiment with the PAK strain of P. aeruginosa, 8–10 WT mice and 8–10 Tlr5−/− mice, as well as 3 or 4 additional WT mice used to confirm bacterial deposition, were exposed to aerosolized bacteria simultaneously. A total of four experiments were performed with the PAK strain: two at low inoculum and two at high inoculum. An additional single experiment was performed with the ΔfliC mutant of the PAK strain. Mice were placed in wire mesh cages enclosed within a 55-liter Plexiglas cylinder connected via 10-cm ducting to a 16-liter aerosol chamber. The 18-ml bacterial slurry was divided into two nebulizers (Salter, Arvin, CA) and aerosolized into the chamber for 30 min via forced air at 15–18 psi. Airflow through the chamber was maintained at 20 l/min by negative pressure. After infection, the mice were returned to their cages, with the exception of the three or four mice used to determine bacterial deposition in the lungs. These animals were euthanized by intraperitoneal injection of pentobarbital sodium and exsanguinated by external cardiac puncture. Left lungs were homogenized in 1 ml of PBS, and the homogenate was serially diluted and quantitatively cultured on LB agar. Colonies were counted after 48 h of incubation at 37°C. This procedure was repeated at 4 h in the ΔfliC experiment and at 4 and 24 h in the remaining mice, with four or five mice of each genotype used at each time point. At 24 h, spleens also were harvested, homogenized in 1 ml of PBS, and quantitatively cultured.
Bronchoalveolar lavage.
At 4 and 24 h, the trachea of each mouse was cannulated, and the right lung was lavaged with 0.6 ml of warm 0.9% NaCl-0.6 mM EDTA solution. This procedure was repeated with 0.5-ml volumes for a total of four washes. The pooled lavage fluid was centrifuged at 330 g, and the supernatants were removed for storage at −80°C. The cell pellets were resuspended in RPMI 1640 medium (HyClone Laboratories, Logan, UT) with 10% heat-inactivated FBS, and cells were counted in a hemocytometer. Differentials were determined from examination of cytocentrifuge slides (Thermo Shandon, Pittsburgh, PA) that were stained with a modified Wright-Giemsa technique (Diff-Quik, Dade Behring, Dudingen, Switzerland).
Histopathology.
After bronchoalveolar lavage (BAL), the right lungs of each animal were inflated to 15-cm pressure with 4% paraformaldehyde and immersed in the same fixative for ≥48 h, then they were transferred to a 70% ethanol solution. Fixed lung tissue was embedded in paraffin, sectioned, and stained with hematoxylin-eosin. Tissue sections were examined by a veterinary pathologist who was unaware of bacterial inoculum, animal genotype, or time point. Three scores were assigned, with each individual component graded on a scale of 0–4, as described elsewhere (44). The bronchiolar injury score combines peribronchiolar inflammation and bronchiolar necrosis, the vascular injury score combines perivascular inflammation and vasculitis, and the alveolar injury score combines alveolar wall injury and number of alveolar inflammatory cells per four random high-power fields. Cell counts were converted to a scale of 1–4 as follows: 1 for 20–150 cells, 2 for 151–300 cells, 3 for 301–450 cells, 4 for ≥451 cells.
Measurement of cytokines.
After removal of 100 μl of homogenized left lung tissue for quantitative culture, the remainder of the lung homogenate was diluted 1:1 in lysis buffer containing protease inhibitor (Roche Diagnostics, Mannheim, Germany). After a 30-min incubation on ice, samples were centrifuged at 1,500 g, and supernatants were stored at −80°C until assays were performed. IFN-γ, IL-1β, macrophage inflammatory protein-2 (MIP-2), KC, monocyte chemoattractant protein-1 (MCP-1), IL-6, granulocyte-macrophage colony-stimulating factor, and TNF-α were measured in BAL fluid and lung homogenates using a microbead array and Luminex 100 analyzer (Austin, TX). In two experiments, one low-inoculum and one high-inoculum, IL-6 was measured by an ELISA kit (Duoset, R & D Systems, Minneapolis, MN) because of a nationwide shortage of IL-6 Luminex beads.
Data analysis.
In vivo challenge experiments were performed twice at each inoculum. Bacterial counts, cell counts, and cytokine levels are expressed as means ± SE. Results for continuous variables were compared using Student's t-test. Histopathology scores were compared by Mann-Whitney U-test. P ≤ 0.05 was considered statistically significant. Prism software (GraphPad, Irvine, CA) was used for statistical analysis and graph preparation.
RESULTS
Bacterial clearance is impaired in Tlr5−/− mice after low-inoculum P. aeruginosa infection.
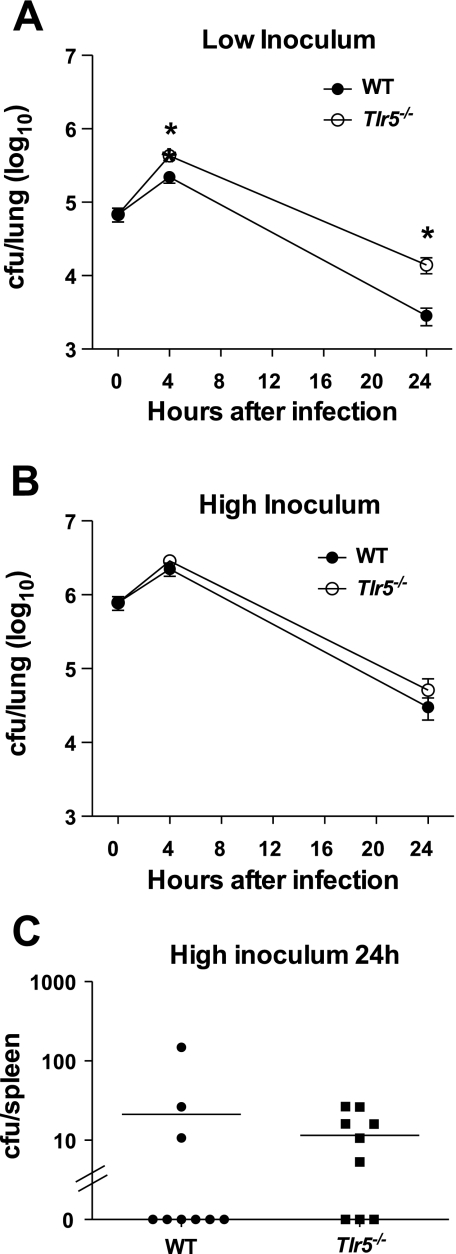
To characterize the role of TLR5 in the inflammatory response to and bacterial clearance of P. aeruginosa lung infection, we exposed WT and Tlr5−/− mice to aerosolized P. aeruginosa strain PAK at a low inoculum of 105 CFU/lung. At 4 h after infection, Tlr5−/− mice had more than twice as many bacteria in their lungs as WT mice (P < 0.05). This difference persisted and, in fact, increased at 24 h, when Tlr5−/− mice harbored >10-fold more bacteria in their lungs than WT mice (Fig. 1A). Although Tlr5−/− mice were able to effect a net reduction in bacterial burden, clearance was notably delayed compared with WT mice. To determine whether the role of TLR5 in bacterial clearance varied by severity of infection, we repeated the experiments with a 10-fold-higher inoculum. At this higher infection dose, no significant difference in lung bacterial counts was observed between WT and Tlr5−/− mice at 4 or 24 h (Fig. 1B). In contrast to the low-inoculum experiments, small numbers of bacteria were cultured from the spleen 24 h after high-inoculum infection only. Spleen bacterial counts were not different between WT and Tlr5−/− mice (Fig. 1C). These findings demonstrate that TLR5 plays a role in the clearance of P. aeruginosa from the lungs that is inoculum-dependent.
Fig. 1.
A: impaired bacterial clearance in Toll-like receptor (TLR) 5 (TLR5)-deficient (Tlr5−/−) mice at 4 and 24 h after low-inoculum Pseudomonas aeruginosa infection. CFU, colony-forming units; WT, wild-type. B: no difference in bacterial clearance between WT and Tlr5−/− mice after high-inoculum infection. C: positive splenic cultures 24 h after high-inoculum infection only, with no difference in bacterial load between WT and Tlr5−/− mice. Values in A–C represent combined results of 2 independent experiments; data are individual values; lines represent means ± SE in A and B (n = 9–10 mice at each time point). *P < 0.05 (by 2-tailed Student's t-test).
Early inflammatory responses are impaired in Tlr5−/− mice.
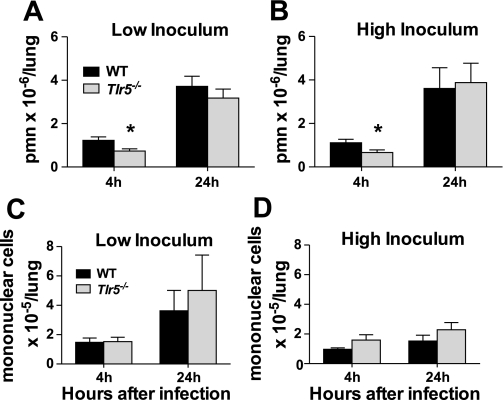
After low- and high-inoculum infection, the number of neutrophils in 4-h BAL samples was significantly diminished in Tlr5−/− compared with WT mice. By 24 h after infection, the number of neutrophils was similar in the lungs of low- and high-inoculum-infected Tlr5−/− mice (Fig. 2, A and B). Mononuclear cell counts did not differ significantly between WT and Tlr5−/− animals at either inoculum or at either time point (Fig. 2C). Therefore, TLR5-dependent signaling contributes to the early influx of neutrophils to the bronchoalveolar air spaces after P. aeruginosa infection.
Fig. 2.
A: bronchoalveolar lavage (BAL) neutrophil count is reduced in Tlr5−/− mice 4 h after low-inoculum P. aeruginosa infection but similar to WT mice at 24 h. PMN, polymorphonuclear leukocytes. B: similar findings at high inoculum. C and D: no difference in BAL mononuclear cell count between WT and Tlr5−/− mice at 4 or 24 h in low- or high-inoculum experiments. Each graph represents combined results of 2 independent experiments. Values are means ± SE (n = 9–10 mice per group at each time point). *P < 0.05 (by 2-tailed Student's t-test).
Histopathology is altered in Tlr5−/− mice.
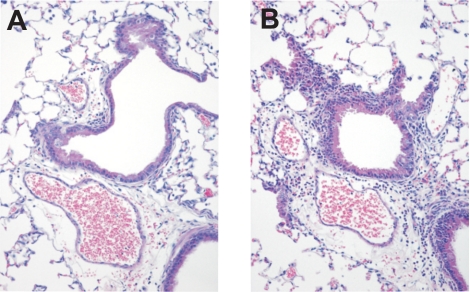
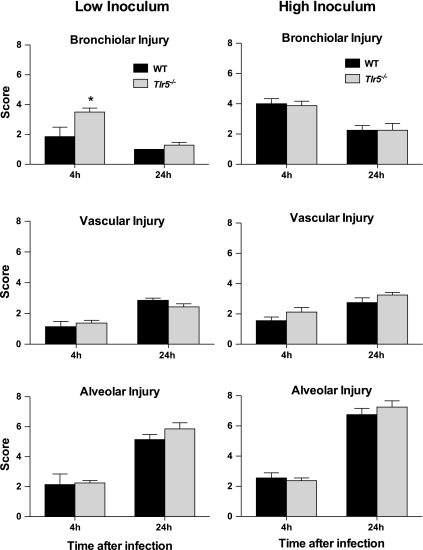
Neutrophilic peribronchial inflammation and occasional focal bronchiolar cell necrosis are the most prominent histopathological changes early after P. aeruginosa lung infection. At 4 h after low-inoculum infection, Tlr5−/− mice exhibited significantly greater peribronchiolar inflammation (Fig. 3) and had a higher bronchiolar injury score than WT mice (Fig. 4A). There was no significant difference in vascular or alveolar injury between groups at 4 h, and there were no histological differences between Tlr5−/− and WT lungs after high-inoculum infection (Fig. 4, B and C).
Fig. 3.
Representative images of hematoxylin-eosin-stained lung sections from low-inoculum experiment at 4 h. Note greater degree of predominantly neutrophilic peribronchiolar inflammation in Tlr5−/− (B) than WT (A) mice. Neutrophils are minimally present within alveolar spaces but are evident within edema fluid-widened perivascular spaces of both strains. Magnification ×20.
Fig. 4.
Histopathological findings. Hematoxylin-eosin-stained fixed lung tissue sections were graded on a scale of 0–8 in the categories of bronchial, vascular, and alveolar injury. Pathological findings were nearly identical between WT and Tlr5−/− mice, with 1 exception: significantly greater bronchial injury at 4 h in the low-inoculum experiments in Tlr5−/− mice. Values are means ± SE (n = 7–8 mice per group at each time point). *P < 0.05 (by Mann-Whitney U-test).
MCP-1 and TNF-α responses are impaired in Tlr5−/− mice.
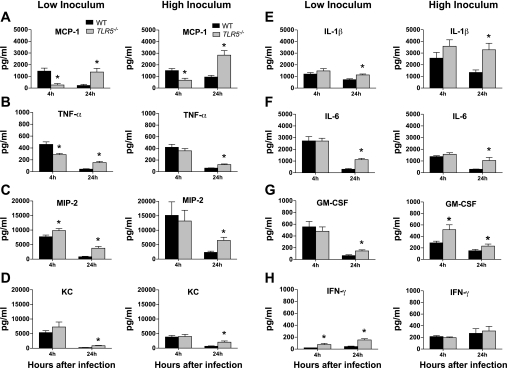
Intrapulmonary cytokine responses were selectively altered in Tlr5−/− mice. After low-inoculum infection, intrapulmonary levels of MCP-1 and TNF-α were significantly reduced in Tlr5−/− mice compared with WT animals at the 4-h time point (Fig. 5, A and B). In contrast, MIP-2 levels were augmented by 25% in Tlr5−/− mice compared with WT controls (Fig. 5C). IFN-γ concentrations also were higher in Tlr5−/− mice, but levels of this cytokine were extremely low in both groups. After high-inoculum infection, MCP-1 levels also were significantly lower in Tlr5−/− than WT mice (Fig. 5A). Other cytokine levels were similar in the two groups of mice after high-inoculum infection, with the exception of increased levels of granulocyte-macrophage colony-stimulating factor in Tlr5−/− animals, although absolute values of this cytokine were very low (Fig. 5G). By 24 h in low- and high-inoculum experiments, levels of all cytokines were greater in Tlr5−/− than WT mice, with the exception of similar levels of IFN-γ in high-inoculum experiments (Fig. 5H).
Fig. 5.
Cytokine levels in lung homogenates. Left: low-inoculum infection. At 4 h, levels of monocyte chemoattractant protein 1 (MCP-1) were markedly reduced in Tlr5−/− mice (A), and TNF-α levels were less strikingly reduced (B); production of macrophage inflammatory protein-2 (MIP-2) was augmented (C), there were no differences in KC, IL-1β, IL-6, or granulocyte-macrophage colony-stimulating factor (GM-CSF) (D–G), and IFN-γ production was augmented, but levels of this cytokine were extremely low (H). At 24 h, levels of nearly all cytokines were higher in Tlr5−/− mice. Right: high-inoculum infection. At 4 h, MCP-1 levels remained reduced in Tlr5−/− mice (A), and GM-CSF levels were augmented, but levels of this cytokine were also very low (G). No other differences were seen between groups. At 24 h, higher levels of most cytokines were identified in Tlr5−/− mice. Values are means ± SE (n = 9–10 mice per group at each time point). *P < 0.05 (by 2-tailed Student's t-test).
Tlr5−/− mice have no impairment in bacterial clearance or immune response to nonflagellated P. aeruginosa.
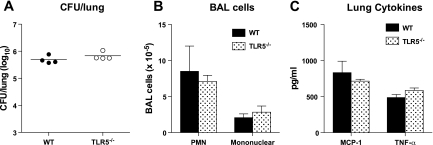
To confirm that the impairment in the response of Tlr5−/− mice to P. aeruginosa is indeed attributable to the TLR5-flagellin interaction, we exposed WT and Tlr5−/− mice to the aerosolized ΔfliC mutant of the PAK strain of P. aeruginosa, which differs from the PAK strain only in the absence of flagellin. We used an inoculum of 1.1 × 105 CFU/lung, which is similar to the low-inoculum experiments with the PAK strain, in which we identified impaired responses in Tlr5−/− mice. In response to ΔfliC, the number of bacteria was similar in lungs of Tlr5−/− and WT mice, and we found no difference in neutrophil recruitment or other cell counts or in production of MIP-2 or TNF-α (Fig. 6).
Fig. 6.
A: no impairment in bacterial clearance 4 h after low-inoculum P. aeruginosa strain ΔfliC infection in Tlr5−/− mice. Data are individual data points from 1 experiment (n = 4 mice per genotype); lines represent means. B and C: no impairment in neutrophil recruitment to the lung or production of MCP-1 or TNF-α in Tlr5−/− mice. Values are means ± SE.
DISCUSSION
Our key findings are that Tlr5−/− mice exhibit an inoculum-dependent defect in bacterial clearance after infection with P. aeruginosa, in association with dysregulated early cytokine responses and delayed accumulation of bronchoalveolar neutrophils. None of these findings are present in Tlr5−/− mice infected with a nonflagellated form of the bacteria, suggesting that the flagellin-TLR5 axis plays a unique role in the early innate immune response to P. aeruginosa in the lungs.
Previously, we reported a near-complete lack of inflammatory response and overwhelming mortality in P. aeruginosa-infected mice deficient in the adaptor molecule MyD88 (45). Tlr2−/− mice have minimal impairment in their response to this organism. Mice with dominant-negative (9, 36) or targeted deletions (37, 47) of TLR4 have a diminished inflammatory response to aerosolized P. aeruginosa, but neither of these proteins alone is required for resolution of acute infection. TLR5 has therefore generated interest as another MyD88-dependent TLR that may be an important component of the innate immune response to P. aeruginosa in the lung. The innate immune response to flagellin in the murine lung, as measured by cytokine production and neutrophil recruitment, is dependent on TLR5 (11). However, it also has been reported that absence of the flagellin-TLR5 axis alone does not impair bacterial clearance of P. aeruginosa (7, 38). This has been investigated using flagellin-deficient organisms (7, 38), a model confounded by the decreased motility of this strain.
Our experiments represent the first demonstration of a unique role for TLR5 in early containment of P. aeruginosa infection. This role is most striking at a low inoculum and is diminished at a higher inoculum, illustrating the concept of innate immunity as a complex system in which cooperative relationships between receptors can compensate for the absence of a single protein. Previously published work using Tlr5−/− mice to study P. aeruginosa pulmonary infection utilized a nasal instillation method and focused exclusively on survival (11). These experiments identified no difference in survival between Tlr5−/− and WT mice, although mortality was increased or earlier in animals deficient in both TLR4 and TLR5. Our studies have allowed us to discern more subtle differences in the responses of Tlr5−/− and WT mice to infection and paint a picture of the more consequential role of TLR5 at lower levels of infection.
We have found that absence of TLR5 impairs neutrophil influx to the bronchoalveolar air spaces of the lungs early after P. aeruginosa infection at low and high bacterial inocula. We identified a similar impairment in Tlr5−/− mice with L. pneumophila pneumonia (17). TLR5 signaling also has recently been reported to regulate neutrophil accumulation in murine colon tumor xenografts (40). Other investigators found that neutrophil-depleted mice, or mice in which neutrophil recruitment is impeded by administration of a blocking antibody against chemokine receptor CXCR2, are unable to clear P. aeruginosa lung infection and have increased mortality (32, 50). Therefore, blunted neutrophil recruitment likely contributes to the impaired bacterial clearance we observed in Tlr5−/− mice. However, Tlr4−/− mice also have reduced neutrophil counts after P. aeruginosa infection, yet they are able to clear the bacteria as quickly as WT mice.
We infer that TLR5 signaling plays a key role in neutrophil recruitment to the lungs after P. aeruginosa infection and that this contributes to, but is not the sole explanation for, the impaired bacterial clearance observed in our low-inoculum experiments. Neutrophil function also may be negatively affected by the absence of TLR5, inasmuch as TLR5 activation has been demonstrated to enhance neutrophil cytokine secretion and phagocytic function in the lungs (24). The lower levels of TNF-α we identified in Tlr5−/− animals in our low-inoculum experiments may also negatively affect neutrophil function. This cytokine has recently been found to play a role in key elements of murine neutrophil function, including adhesion and priming of the oxidative burst (26, 34).
The mechanism of delayed neutrophil recruitment into the airways of Tlr5−/− mice is not clear. We did not observe a defect in whole lung CXC chemokine responses in these animals; indeed, early MIP-2 production in response to low-inoculum infection was augmented in the absence of TLR5. Similarly, we previously also found an unimpaired CXC chemokine response to inhalation of L. pneumophila in Tlr5−/− mice (17). However, TLR5 deficiency may result in localized, cell-specific defects in the response to P. aeruginosa infection that are not evident when whole lung responses are measured. Our pathological finding of greater peribronchiolar inflammation in Tlr5−/− mice 4 h after low-inoculum infection suggests that there may be an airway-specific effect of TLR5 deficiency on neutrophil trafficking across the epithelium. Bone marrow chimera studies that demonstrated that the response to flagellin in the lung is dependent on non-bone marrow-derived cells (11), such as airway epithelial cells, further support a cell-specific role for TLR5. Our own chimera studies showed that the early lung inflammatory response to P. aeruginosa is dependent on NF-κB activation in non-bone marrow-derived cells (16), and other investigators similarly reported that neutrophilic inflammation in response to P. aeruginosa is reduced when NF-κB activity is inhibited in airway epithelial cells (42). Further studies examining cell-specific patterns of TLR5-dependent activation are necessary to further characterize the role of this receptor in regulating pulmonary innate immune responses.
One of the most striking findings in these experiments was the impaired early secretion of MCP-1 in Tlr5−/− mice infected by low- and high-inoculum P. aeruginosa. MCP-1 is best known as a monocyte chemoattractant, but reduced levels did not result in fewer mononuclear cells in the BAL fluid of Tlr5−/− mice. This finding is consistent with published work demonstrating that MCP-1 is important for alveolar macrophage function but does not affect the overall number of BAL macrophages in response to P. aeruginosa lung infection (4, 22). MCP-1 also plays a role in resolution of inflammation (4, 25), and it can induce migration of bone marrow neutrophils and circulating neutrophils in models of intra-abdominal sepsis or vasculitis (20, 21, 49). Several authors identified a connection between MCP-1 levels and neutrophil infiltration in models of lung and intra-abdominal inflammation (12, 13, 27, 29, 30, 39). In addition, MCP-1 neutralization led to impaired bacterial clearance and increased mortality following a cecal ligation-and-puncture model of peritonitis (29).
Therefore, the impaired bacterial clearance we identified in Tlr5−/− mice may be due to a combination of reduced neutrophil number and impaired neutrophil and macrophage functional response to infection, mediated, at least in part, by diminished levels of TNF-α and MCP-1. Finally, reduced levels of MCP-1 provide additional reason to focus on the cell-specific nature of the TLR5 response to P. aeruginosa. Although a variety of cell types secrete this cytokine, production of MCP-1 by alveolar epithelial cells appears to be critically important for alveolar macrophage function in P. aeruginosa pneumonia (4, 22).
Early secretion of most cytokines other than MCP-1 and TNF-α was not impaired in Tlr5−/− mice, which is somewhat surprising, given diminished IL-6 and KC secretion noted by other authors in Tlr5−/− mice exposed to intranasal flagellin (11) or WT mice exposed to flagellin-deficient bacteria (38). The higher inocula, different method of infection, and use of a less virulent organism in the latter experiment may contribute to this difference. We also identified higher levels of most cytokines in Tlr5−/− than WT mice 24 h after infection. This finding may reflect the higher bacterial count in the lungs of Tlr5−/− mice at the later time point. This was most prominent in the low-inoculum experiment, where these counts were statistically significantly different, but even in the high-inoculum experiments a trend toward more bacteria was observed in the lungs of Tlr5−/− animals.
In summary, we have demonstrated that TLR5 plays an inoculum-dependent role in the early innate immune response to P. aeruginosa. Reduced neutrophil influx into the airways and likely altered immune cell function, perhaps mediated in part by impaired MCP-1 and TNF-α production, contribute to reduced bacterial clearance. The combined effect is best seen at low levels of infection, whereas in more severe infections other intact components of the innate immune system are able to compensate for the absence of TLR5 to normalize bacterial clearance, even though measurable impairments in the inflammatory response persist. Further investigation into the role of TLR5-dependent immune cell function, as distinguished from neutrophil or monocyte influx into the lungs, in the clearance of this bacteria is needed. In addition, future studies should explore the resident cell type-specific role of TLR5, particularly non-bone marrow-derived cells, such as airway epithelial cells, in mediating the response to P. aeruginosa. More knowledge in these areas will be necessary as we aim to better understand the contribution of this protein to the innate immune response to acute P. aeruginosa infection.
GRANTS
This work was supported in part by National Institutes of Health Grants AI-061464, HL-54972, and HL-073996.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and Toll-like receptor 2 as well as Toll-like receptor 5. Am J Respir Cell Mol Biol 30: 627– 634, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Akira S. Toll-like receptor signaling. J Biol Chem 278: 38105– 38108, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 4: 499– 511, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Amano H, Morimoto K, Senba M, Wang H, Ishida Y, Kumatori A, Yoshimine H, Oishi K, Mukaida N, Nagatake T. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J Immunol 172: 398– 409, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Andersen-Nissen E, Hawn TR, Smith KD, Nachman A, Lampano AE, Uematsu S, Akira S, Aderem A. Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J Immunol 178: 4717– 4720, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Applequist SE, Wallin RP, Ljunggren HG. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int Immunol 14: 1065– 1074, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Balloy V, Verma A, Kuravi S, Si-Tahar M, Chignard M, Ramphal R. The role of flagellin versus motility in acute lung disease caused by Pseudomonas aeruginosa. J Infect Dis 196: 289– 296, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cobb LM, Mychaleckyj JC, Wozniak DJ, Lopez-Boado YS. Pseudomonas aeruginosa flagellin and alginate elicit very distinct gene expression patterns in airway epithelial cells: implications for cystic fibrosis disease. J Immunol 173: 5659– 5670, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Faure K, Sawa T, Ajayi T, Fujimoto J, Moriyama K, Shime N, Wiener-Kronish JP. TLR4 signaling is essential for survival in acute lung injury induced by virulent Pseudomonas aeruginosa secreting type III secretory toxins. Respir Res 5: 1, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, Prince A. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun 66: 43– 51, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galan JE, Flavell RA, Alexopoulou L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci USA 103: 12487– 12492, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frink M, Lu A, Thobe BM, Hsieh YC, Choudhry MA, Schwacha MG, Kunkel SL, Chaudry IH. Monocyte chemoattractant protein-1 influences trauma-hemorrhage-induced distal organ damage via regulation of keratinocyte-derived chemokine production. Am J Physiol Regul Integr Comp Physiol 292: R1110– R1116, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Asano M, Wang H, Matsushima K, Takeya M, Kuziel WA, Mukaida N, Yokoyama H. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol 14: 2503– 2515, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Garau J, Gomez L. Pseudomonas aeruginosa pneumonia. Curr Opin Infect Dis 16: 135– 143, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 41: 848– 854, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hajjar AM, Harowicz H, Liggitt HD, Fink PJ, Wilson CB, Skerrett SJ. An essential role for non-bone marrow-derived cells in control of Pseudomonas aeruginosa pneumonia. Am J Respir Cell Mol Biol 33: 470– 475, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawn TR, Berrington WR, Smith IA, Uematsu S, Akira S, Aderem A, Smith KD, Skerrett SJ. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J Immunol 179: 6981– 6987, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Hawn TR, Verbon A, Lettinga KD, Zhao LP, Li SS, Laws RJ, Skerrett SJ, Beutler B, Schroeder L, Nachman A, Ozinsky A, Smith KD, Aderem A. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J Exp Med 198: 1563– 1572, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hybiske K, Ichikawa JK, Huang V, Lory SJ, Machen TE. Cystic fibrosis airway epithelial cell polarity and bacterial flagellin determine host response to Pseudomonas aeruginosa. Cell Microbiol 6: 49– 63, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Iida S, Kohro T, Kodama T, Nagata S, Fukunaga R. Identification of CCR2, flotillin, and gp49B genes as new G-CSF targets during neutrophilic differentiation. J Leukoc Biol 78: 481– 490, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Johnston B, Burns AR, Suematsu M, Issekutz TB, Woodman RC, Kubes P. Chronic inflammation upregulates chemokine receptors and induces neutrophil migration to monocyte chemoattractant protein-1. J Clin Invest 103: 1269– 1276, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannan S, Huang H, Seeger D, Audet A, Chen Y, Huang C, Gao H, Li S, Wu M. Alveolar epithelial type II cells activate alveolar macrophages and mitigate P. aeruginosa infection. PLoS ONE 4: e4891, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Microbiologic follow-up study in adult bronchiectasis. Respir Med 101: 1633– 1638, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Koller B, Kappler M, Latzin P, Gaggar A, Schreiner M, Takyar S, Kormann M, Kabesch M, Roos D, Griese M, Hartl D. TLR expression on neutrophils at the pulmonary site of infection: TLR1/TLR2-mediated up-regulation of TLR5 expression in cystic fibrosis lung disease. J Immunol 181: 2753– 2763, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA 94: 12053– 12058, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauterbach M, O'Donnell P, Asano K, Mayadas TN. Role of TNF priming and adhesion molecules in neutrophil recruitment to intravascular immune complexes. J Leukoc Biol 83: 1423– 1430, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Li P, Garcia GE, Xia Y, Wu W, Gersch C, Park PW, Truong L, Wilson CB, Johnson R, Feng L. Blocking of monocyte chemoattractant protein-1 during tubulointerstitial nephritis resulted in delayed neutrophil clearance. Am J Pathol 167: 637– 649, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, Chen W, Cross AS, Gendler SJ, Gewirtz AT, Kim KC. Enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol 176: 3890– 3894, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Strieter RM, Kunkel SL. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J Immunol 163: 6148– 6154, 1999 [PubMed] [Google Scholar]
- 30.Maus U, von Grote K, Kuziel WA, Mack M, Miller EJ, Cihak J, Stangassinger M, Maus R, Schlondorff D, Seeger W, Lohmeyer J. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med 166: 268– 273, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol 170: 5165– 5175, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol 14: 123– 132, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Oliver A, Canton R, Campo P, Baquero F, Blazquez J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288: 1251– 1254, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Onnheim K, Bylund J, Boulay F, Dahlgren C, Forsman H. Tumour necrosis factor (TNF)-α primes murine neutrophils when triggered via formyl peptide receptor-related sequence 2, the murine orthologue of human formyl peptide receptor-like 1, through a process involving the type I TNF receptor and subcellular granule mobilization. Immunology 125: 591– 600, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oshikawa K, Sugiyama Y. Gene expression of Toll-like receptors and associated molecules induced by inflammatory stimuli in the primary alveolar macrophage. Biochem Biophys Res Commun 305: 649– 655, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Power MR, Peng Y, Maydanski E, Marshall JS, Lin TJ. The development of early host response to Pseudomonas aeruginosa lung infection is critically dependent on myeloid differentiation factor 88 in mice. J Biol Chem 279: 49315– 49322, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Ramphal R, Balloy V, Huerre M, Si-Tahar M, Chignard M. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J Immunol 175: 3927– 3934, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Ramphal R, Balloy V, Jyot J, Verma A, Si-Tahar M, Chignard M. Control of Pseudomonas aeruginosa in the lung requires the recognition of either lipopolysaccharide or flagellin. J Immunol 181: 586– 592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reichel CA, Khandoga A, Anders HJ, Schlondorff D, Luckow B, Krombach F. Chemokine receptors Ccr1, Ccr2, and Ccr5 mediate neutrophil migration to postischemic tissue. J Leukoc Biol 79: 114– 122, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Rhee SH, Im E, Pothoulakis C. Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology 135: 518– 528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 171: 1209– 1223, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadikot RT, Zeng H, Joo M, Everhart MB, Sherrill TP, Li B, Cheng DS, Yull FE, Christman JW, Blackwell TS. Targeted immunomodulation of the NF-κB pathway in airway epithelium impacts host defense against Pseudomonas aeruginosa. J Immunol 176: 4923– 4930, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Sebastiani G, Leveque G, Lariviere L, Laroche L, Skamene E, Gros P, Malo D. Cloning and characterization of the murine Toll-like receptor 5 (Tlr5) gene: sequence and mRNA expression studies in Salmonella-susceptible MOLF/Ei mice. Genomics 64: 230– 240, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol 287: L143– L152, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol 172: 3377– 3381, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Skerrett SJ, Martin TR, Chi EY, Peschon JJ, Mohler KM, Wilson CB. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 276: L715– L727, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 292: L312– L322, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol 4: 1247– 1253, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Speyer CL, Gao H, Rancilio NJ, Neff TA, Huffnagle GB, Sarma JV, Ward PA. Novel chemokine responsiveness and mobilization of neutrophils during sepsis. Am J Pathol 165: 2187– 2196, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai WC, Strieter RM, Mehrad B, Newstead MW, Zeng X, Standiford TJ. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun 68: 4289– 4296, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol 168: 554– 561, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun 73: 7151– 7160, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]