Abstract
Platelet-activating factor (PAF) acetylhydrolase plays a crucial role inactivating the potent inflammatory mediator, PAF. PAF is implicated in the initiation and propagation of acute lung injury. Although PAF acetylhydrolase is a constitutively active plasma protein, increased PAF production during inflammatory events may necessitate an increase in PAF acetylhydrolase activity in the local environment. A series of experiments were conducted to determine whether the systemic administration of LPS to Sprague-Dawley rats resulted in enhanced expression of PAF acetylhydrolase in lung tissue. Ribonuclease protection assays revealed a dramatic increase in PAF acetylhydrolase mRNA, which peaked at 24 h following in vivo LPS administration. The increase in PAF acetylhydrolase mRNA was dose dependent and was detected when as little as 10 μg/kg of LPS was administered. Western blot analyses of lung tissue homogenates confirmed an increased production of PAF acetylhydrolase protein in response to LPS. In addition, Western blot analyses revealed the rat PAF acetylhydrolase protein exhibited heterogeneous molecular weights with predominant species migrating at 63 and 67 kDa. Some of the molecular weight heterogeneity likely resulted from extensive glycosylation of the secreted protein. Immunohistochemical analyses of lung tissue sections and colocalization experiments revealed a heterogenous population of cells that express the plasma-type PAF acetylhydrolase. Lung interstitial macrophages were PAF acetylhydrolase positive, but surprisingly, alveolar macrophages did not increase expression of PAF acetylhydrolase in response to systemic LPS administration. In addition, rat granulocytes consisting primarily of neutrophils were strongly positive for PAF acetylhydrolase in the LPS-exposed lung tissue. The absence of immunoreactive PAF acetylhydrolase in alveolar macrophages obtained from bronchial alveolar lavage confirmed that systemic LPS administration resulted in enhanced PAF acetylhydrolase expression only in a subset of lung macrophages.
Keywords: lipid mediator, inflammation, lipoprotein-associated phospholipase A2, acute lung injury, lipopolysaccharide, endotoxin
the effects of the inflammatory mediator platelet-activating factor (PAF) on lung physiology and pathophysiology have been well documented. PAF administration induces bronchoconstriction, increases bronchial hyperreactivity, increases vascular permeability, and stimulates bronchial mucus secretion and polymorphonuclear leukocyte infiltration of the airways (14). PAF is produced by inflammatory cells and cells within the lung tissue and the progression and outcome of inflammation depends, in part, on the synthesis and disposition of this lipid mediator. Potential mechanisms of PAF-induced inflammation and vascular lung injury include the stimulation of oxidant production by inflammatory cells, the enhanced adhesion of neutrophils to pulmonary endothelium, and the direct vasoactive effects on the pulmonary microvasculature (12). Transgenic mice that overexpress PAF receptors demonstrated an increased bronchial hyperreactivity to methacholine and an increased mortality to endotoxin exposure (24). Disruption of the PAF receptor gene in mice demonstrated an essential role of the PAF receptor in allergen-induced airway obstruction and bronchoconstriction (23). Endotoxemia also produces a complex pulmonary response, which includes alterations in both airway and vascular function. Endotoxin exposure can result in acute changes in pulmonary hemodynamics, lung vascular permeability, and lung mechanics (7). The endotoxin-induced changes meet the criteria generally used for the diagnosis of Adult Respiratory Distress Syndrome (ARDS) in humans (2). Inflammatory reactions in lung tissue involve the participation of numerous cellular and humoral mechanisms and engage the participation of macrophages, neutrophils, platelets, and the coagulation system. It is clear that a network of complex regulatory molecules and their receptors participate in the instigation and perpetuation of acute lung injury. Chang et al. (11) documented marked increases in PAF concentrations in blood and lung homogenates from rats after endotoxin injection. Further, these authors demonstrated a significant attenuation of endotoxin-induced accumulation of extravascular lung water and albumin and loss of hypoxic vasoconstriction by pretreatment with PAF receptor antagonists. One endogenous mechanism to inactivate PAF is the secreted and intracellular PAF acetylhydrolases.
Plasma PAF acetylhydrolase (lipoprotein-associated phospholipase A2. Lp-PLA2) is a distinct Ca2+-independent enzyme that inactivates the potent phospholipid mediator PAF and other structurally similar bioactive lipids produced in response to oxidative damage (37). This circulating enzyme has received a tremendous amount of attention from the recent discovery that elevated levels of PAF acetylhydrolase are an independent risk factor for future coronary events (4, 5, 26, 35, 36). Two independent laboratories initially cloned and characterized the extracellular human plasma PAF acetylhydrolase. Tjoelker et al. (47) isolated a 44-kDa protein from human plasma and cloned the corresponding cDNA from a macrophage library. Tew et al. (45) also purified the human plasma PAF acetylhydrolase and demonstrated that the protein ranged in size from a 43-kDa nonglycosylated protein to a 65- to 67-kDa glycosylated form. Asano et al. (3) demonstrated that plasma PAF acetylhydrolase activity originates from hematopoietic lineage cells, such as macrophages. Normal lung tissue contains the highest PAF acetylhydrolase activity of any organ with the exception of the kidney (41). However, it is uncertain to what extent the extracellular and/or the intracellular isoform of PAF acetylhydrolase contributes to this activity.
The effect of LPS on plasma PAF acetylhydrolase expression and activity remains controversial. We previously demonstrated marked up-regulation of PAF acetylhydrolase specifically in liver Kupffer cells of LPS-treated rats (21, 22). However, Gomes et al. (19) reported acute decreases in plasma PAF-AH activity in mice subjected to cecal ligation and puncture or challenged with LPS, and in human patients with sepsis. In many cell culture models, LPS has been documented to decrease PAF acetylhydrolase expression. In a myelocytic leukemic cell line (HL-60), which produced and secreted PAF acetylhydrolase after the cells differentiated into macrophages, LPS inhibited the secretion of PAF acetylhydrolase in a dose-dependent manner (33). Likewise, I have observed a 50% decrease in PAF acetylhydrolase mRNA in cultured Kupffer cells incubated with LPS (21). IFN-γ and LPS decreased the human PAF acetylhydrolase promoter activity by 35% and 50%, respectively, in monocyte-derived macrophages and various established macrophage cell lines (9). However, the same laboratory subsequently demonstrated that LPS administration to murine RAW264.7 and human THP-1 macrophages resulted in transcriptional upregulation of PAF acetylhydrolase via a p38 mitogen-activated protein kinase (p38 MAPK)-dependent pathway (50). Changes in the in vivo activity of plasma PAF acetylhydrolase have been documented in conjunction with asthma (31), systemic lupus erythematosus (44), hypertension (40), chronic cholestasis (29), and necrotizing entercolitis (10, 17). These in vivo reports document both increased and decreased PAF acetylhydrolase activity in response to pathophysiological conditions, but increased PAF acetylhydrolase levels directly correlate with the risk of future coronary events (4, 5, 26, 35, 36). Because of the profound role of PAF in contributing to lung pathophysiology, my laboratory investigated the expression and localization of plasma PAF acetylhydrolase in rat lung tissue following endotoxin challenge.
MATERIALS AND METHODS
Reagents.
Escherichia coli lipopolysaccharide (055:B5), collagenase (Type IV from Clostridium histolyticum), protease E (type XIV from Streptomyces griseus), and bovine serum albumin (BSA, fraction V, essentially fatty acid free) were purchased from Sigma (St. Louis, MO, USA). N-Glycosidase F was purchased from Boehringer Mannheim (Mannheim, Germany). Metrizamide [2-(3-acetamido-5-N-methylacetamido-2,4,6-triiodobenz-amido)-2-deoxy-d-glucose] was obtained from Nyegaard (Oslo, Norway). The mouse anti-rat ED1 and mouse anti-rat HIS48 antibodies were purchased from Serotec (Raleigh, NC). The rat cDNA homologue of the human plasma-type PAF acetylhydrolase was kindly provided by ICOS (Bothell, WA). Unless specifically stated otherwise, all references to PAF acetylhydrolase refer to the plasma-type PAF acetylhydrolase. All other chemicals were of the highest grade possible.
Endotoxin exposure.
Male Sprague-Dawley rats weighing between 300 and 350 g were fed a standard laboratory chow ad libitum. To effect a systemic endotoxin exposure model, LPS (E. coli serotype 055:B5; 3 mg/kg; 1 × 106 endotoxin units) dissolved in a sterile solution of 0.1% BSA in saline was infused slowly through a 27-gauge needle into the tail vein of conscious restrained rats. The physical restraint of the rodents was performed with an approved restraining cage specifically designed to avoid any pain, injury, and discomfort to the animals and the injections were performed as quickly as possible. In control animals, a solution of sterile 0.1% BSA in saline without LPS was infused. At the indicated times, rats were administered a lethal dose of pentobarbital sodium (ip), the thorax was opened, and the trachea was separated from the thymus and esophagus. The trachea was cut below the larynx, and the trachea and lungs were removed from the animal. For RNA analyses, lung tissue was harvested and freeze-clamped immediately in liquid nitrogen and stored at −80°C. Saline- and LPS-treated rats (n ≥ 3) were used for the collection of lung tissue. For the collection of bronchial lavage fluid (BAL), rats receiving LPS or saline were given a lethal injection of pentobarbital sodium at the indicated times after exposure. The lungs were removed, as described above. BAL was collected by inserting a small metal cannula into the trachea and alternatively instilling and aspirating four successive 5-ml volumes of PBS. All animals receiving LPS exhibited symptoms of illness, including ruffled fur, lethargy, and diarrhea. All animal experiments conformed to National Institutes of Health guidelines (publication no. 86–23, revised 1985) for the humane use and care of laboratory animals and were approved by the University of Texas Health Science Center at San Antonio institutional animal care and use committee (protocol # 0012-34-05-C).
RNA isolation and ribonuclease protection assays.
All RNA isolation procedures were based on the method of Chomczynski and Sacchi (13). Briefly, 1 g of frozen lung tissue was pulverized in liquid nitrogen and homogenized in 5 ml of TRIzol (GIBCO BRL, Gaithersburg, MD). After the addition of 1 ml of chloroform and phase separation, the RNA was precipitated with 2.5 ml of isopropyl alcohol. Ribonuclease protection assays (RPA) employed a truncated rat plasma PAF acetylhydrolase cDNA, as previously described (21). 32P-labeled antisense RNA complimentary to rat PAF acetylhydrolase and rat GAPDH (triGAPDH; Ambion, Austin, TX, USA) was synthesized in vitro (MaxiScript; Ambion), and hybridized in solution with 80 μg of lung total RNA. The specific activity of the GAPDH antisense RNA was reduced by including 0.5 mM unlabeled UTP in the in vitro transcription reaction. RPA experiments were performed using the RPAII reagents (Ambion). After RNase digestion, the protected probe fragments were separated on a 6% polyacrylamide/urea gel and the gel was exposed to a Phosphorimager screen. Differences in the specific activity of the two probes can be visualized in the control lane, which contains the undigested RNA probes. Visualization and quantitation of the amount of protected PAF acetylhydrolase and GAPDH RNA probes were performed using a Storm PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Yeast tRNA was included as a negative control.
Western blot analyses.
Flash-frozen lung tissue was homogenized in 5-ml RIPA buffer (PBS containing 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS plus 10 mg/ml PMSF and 30 μl/ml aprotinin). Cell lysate protein concentrations were quantitated using the bicinchoninic acid protein assay (Pierce ThermoFisher Scientific, Rockford, IL). The resulting cell lysate (20 μg) was mixed with 2× SDS sample buffer (125 mM Tris-HCl pH 6.8, 200 mM DTT, 4% SDS 0.2% bromophenol blue and 20% gycerol) and subjected to SDS-PAGE (10% gel) using the buffer system of Laemmli (27). The separated proteins were transferred electrophoretically to PVDF membranes (Millipore, Bedford, MA), using a semi-dry transfer blot system. Immediately following the transfer, total membrane-bound proteins were visualized by staining with 0.2% Ponceau S to demonstrate equal protein loading and ensure proper electrophoresis and transfer of samples occurred prior to Western blotting. Following destaining, the membranes were subsequently blocked in Tris-buffered saline (TBS, pH 7.4) containing 5% nonfat dried milk powder for 2 h at room temperature. Visualization of the PAF acetylhydrolase protein was performed using a rabbit anti-rat PAF acetylhydrolase antibody as previously described (22). For the indicated experiment, the rat lungs were perfused for 10 min with PBS prior to collection and freezing. Kupffer cell lysates from primary cultures of Kupffer cells (resident liver macrophages) isolated from rat livers using a modification of the centrifugal elutriation procedure of Knook and Sleyster (25), as described previously (18), and rat serum were used as PAF acetylhydrolase-positive controls.
Glycosidase treatment.
Aliquots of lung tissue homogenates (20 μg), cultured Kupffer cell lysates (20 μg) or rat serum (10 μl of 1/100 dilution in PBS), were heat denatured in the presence of 1% SDS and incubated with 0.3 units of N-glycosidase F (Boehringer Mannheim) in a final reaction mixture containing 20 mM potassium phosphate, 0.1% SDS, and 0.5% NP40 and allowed to proceed at 37°C overnight. Control samples not receiving N-glycosidase F were incubated for an identical period of time to assay for protease activity.
Immunohistochemical localization analyses.
Rats received LPS or saline via the tail vein 24 h prior to lung tissue isolation. Briefly, after pentobarbital sodium overdose, the lungs, while still connected to the heart, were removed, and a small metal cannula was inserted into the trachea and a 1:1 solution of 2 M sucrose/Tissue Tek OCT (Milles Scientific Laboratories, Naperville, IL) was slowly instilled to inflate the lungs. The lung lobes were flash frozen in liquid nitrogen and stored at −80°C. Smaller pieces of lung tissue were removed by a scalpel, embedded in OCT compound, and snap frozen in isopentane in a liquid nitrogen bath. Sectioning and mounting of the lung tissue were performed by the Institutional Pathology Core facility. Immunohistochemistry was performed on 5-μm slices previously fixed for 10 min in −20°C acetone/methanol (1:1), as previously described (22). The tissue sections were rinsed extensively in PBS and blocked in 5% normal goat serum for 1 h at room temperature in a humidified chamber. The sections were then incubated overnight at 4°C in a 1/200 dilution of affinity-purified rabbit anti-PAF acetylhydrolase antibody and a 1/700 dilution of mouse anti-rat ED1 or a 1/200 dilution of mouse anti-rat HIS48 (Serotec, Raleigh, NC). After washing extensively in PBS, the lung sections were incubated with a Cy3-conjugated goat anti-rabbit secondary antibody (1/800; Jackson Laboratories, Bar Harbor, ME) and a FITC-conjugated goat anti-mouse secondary antibody (1/200; Jackson Laboratories) for 2 h at room temperature. Both secondary antibodies were previously absorbed against rat serum proteins by the manufacturer. The sections were washed extensively in PBS before the immunofluorescence was visualized using an Olympus BX60 (Olympus America, Center Valley, PA) microscope equipped with Cy3 and FITC filter sets. Control sections included incubation without primary antibody and with preimmune serum at appropriate dilutions. No specific localization of PAF acetylhydrolase was detected on control tissue sections. After the tissue sections were surveyed, representative photographs were obtained using a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI). The numbers of positive cells were quantified by examining four independent images (captured with a 10× objective) per treatment group. For ease of cell counting, each image was divided into four equal quadrants, and the number of positive cells was determined by three laboratory personnel blinded to the treatment. Cell numbers are reported as the means ± SD. Independent t-tests were used to assess statistical difference between saline and LPS treatment groups. For the immunocytochemical staining of cytocentrifuged BAL cells, a sensitive immunoperoxidase method was employed and used according to the manufacturer's directions. (Vectastain ABC kit; Vector Laboratories, Burlingame, CA). Endogenous peroxidase activity of the BAL cells was quenched by treatment with 0.3% hydrogen peroxide in methanol for 30 min prior to incubation with the rabbit anti-PAF acetylhydrolase antibody.
RESULTS
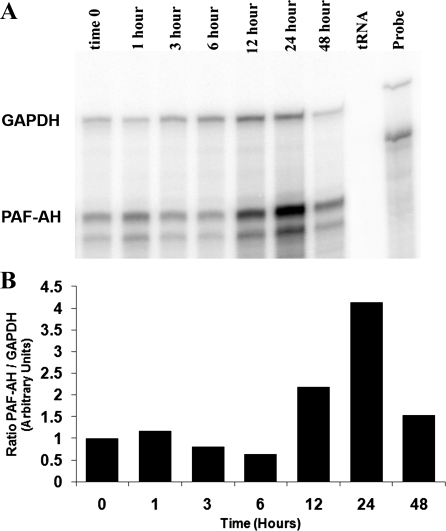
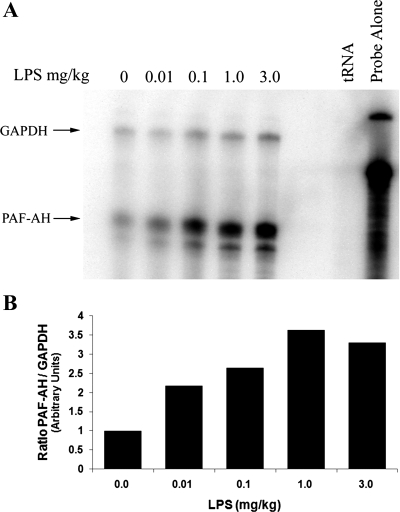
Previously, my laboratory demonstrated that the in vivo administration of LPS to rats resulted in substantial induction of PAF acetylhydrolase mRNA in numerous tissues with the largest increase detected in lung tissue (22). Basal levels of PAF acetylhydrolase mRNA were barely detectable in most normal tissues, but we detected significantly more basal expression of PAF acetylhydrolase in lung and spleen tissue (22). Nonetheless, pronounced endotoxin-mediated upregulation of the PAF acetylhydrolase mRNA was evident in all the tissues examined (22). Because of the crucial role PAF plays in lung pathophysiology, as well as the large induction in PAF acetylhydrolase mRNA observed in LPS-treated lung tissue, our goal was to specifically examine the expression and localization of PAF acetylhydrolase in LPS-exposed lung tissue. PAF acetylhydrolase mRNA levels were detected using a RPA of lung tissue RNA isolated from LPS-exposed animals. Rats were administered LPS (3 mg/kg, 0.1% BSA in PBS) via their tail vein and at 0, 1, 3, 6, 12, 24, and 48 h following administration the lung tissue was harvested. Total lung RNA was isolated and then analyzed for PAF acetylhydrolase mRNA levels. GAPDH levels were also detected and used as an internal control for RNA loading. Yeast tRNA was hybridized with both anti-sense RNA probes and served as a negative control. Three microliters of a 1/100 dilution of the undigested probes was run in parallel to illustrate the integrity and relative specific activity of the radiolabeled probes. The result of a representative time course experiment is depicted in Fig. 1A. A twofold increase in PAF acetylhydrolase mRNA was detected at 12 h, peaked at 24 h (four-fold), and by 48 h, was approaching previous baseline levels (Fig. 1B). In contrast to levels detected in most other tissues (22), basal levels of PAF acetylhydrolase mRNA were readily apparent in the lung tissue. Although two protected fragments were detected for PAF acetylhydrolase (Fig. 1A), this is likely a result of either an RNase A hypersensitive site or the result of a single nucleotide difference between the cloned cDNA and the rat PAF acetylhydrolase transcript and does not represent an alternatively spliced exon in this region of the rat PAF acetylhydrolase gene (K. M. Howard, unpublished observations). To investigate the sensitivity of the response to LPS, a dose-response experiment was performed. Rats received 0.01, 0.1, 1.0, or 3 mg/kg LPS, and lung tissue was harvested at 24 h. Control animals (not receiving LPS) received saline alone. Following RNA isolation, the PAF acetylhydrolase mRNA levels were detected by RPA (Fig. 2A) . A twofold increase in PAF acetylhydrolase mRNA levels was detected with the administration of as little as 0.01 mg/kg LPS. The PAF acetylhydrolase levels appeared to be fully induced with 1.0 mg/kg (3.5-fold), and the administration of a larger dose (3 mg/kg) did not result in any further increases.
Fig. 1.
Time course of platelet-activating factor (PAF) acetylhydrolase mRNA induction in lung tissue from LPS-exposed animals. Lung tissue was harvested from rats exposed to LPS (3 mg/kg) for 0, 1, 3, 6, 12, 24, and 48 h and immediately frozen in liquid nitrogen. Total RNA was prepared from the lung tissue, as detailed in the materials and methods. Aliquots of total RNA (80 μg) were analyzed by ribonuclease protection assays (RPA). The total RNA was hybridized in solution with 32P-labeled antisense RNA probes for PAF acetylhydrolase (245 bp) and GAPDH (355 bp). The RNA-RNA hybrids were then digested with RNase A/T1 and separated on a 6% polyacrylamide, 8-M urea gel and exposed to a phosphorimage screen (A). Probe, undigested full-length antisense probes; tRNA, RNase digested negative control. GAPDH and PAF acetylhydrolase (PAF-AH) labels are adjacent to the respective protected fragments. The RPA shown is representative of three independent experiments. B: densitometric quantitation of the representative RPA.
Fig. 2.
Induction of lung tissue PAF acetylhydrolase mRNA in response to varying doses of LPS. Twenty-four hours after infusion of 0, 0.01, 0.1, 1.0, and 3.0 mg/kg LPS, lung tissue was harvested, and total RNA was isolated as described in the materials and methods. A: aliquots of total RNA (80 μg) were analyzed by RPA. The total RNA was hybridized in solution with 32P-labeled antisense RNA probes for PAF acetylhydrolase (245 bp) and GAPDH (355 bp). The RNA-RNA hybrids were then digested with RNase A/T1 and separated on a 6% polyacrylamide, 8-M urea gel and exposed to a phosphorimage screen. Probe Alone, undigested full-length antisense probes; tRNA, RNase digested negative control. The arrows point to protected fragments for GAPDH and PAF acetylhydrolase (PAF-AH). The RPA shown is representative of three independent experiments. B: densitometric quantitation of the representative RPA.
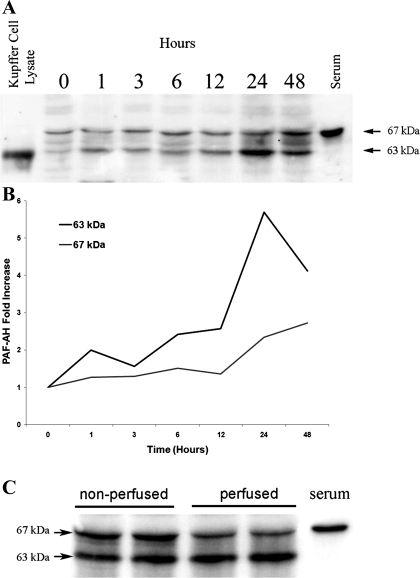
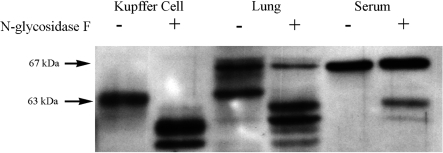
To investigate whether a concomitant increase of PAF acetylhydrolase protein was produced in response to in vivo LPS administration, Western blot analyses were performed using a rat-specific PAF acetylhydrolase antibody previously produced in my laboratory (22). Analyses of protein derived from cultured Kupffer cell lysates and from normal rat serum were used as positive controls. Surprisingly, Western blot results revealed that molecular weight heterogeneity of the PAF acetylhydrolase protein was present in the lung tissue lysates with two prominent protein bands detected at 67 and 63 kDa (Fig. 3A). The immunoreactive protein detected in Kupffer cell lysates migrates at an approximate molecular weight of 63 kDa, while that detected in diluted serum samples migrates at 67 kDa. The Western blot of LPS-exposed lung tissue samples demonstrated an increase with time of the immunoreactive PAF acetylhydrolase proteins (Fig. 3B). This increase was most apparent in the 63 kDa species detected and peaked at 24 h following the LPS administration (5.5-fold). These initial Western blot experiments were performed on lung tissue homogenates in which the lungs were removed immediately at the indicated times following LPS exposure. Because the 67-kDa species was similar in size to that detected in the serum sample, additional experiments perfused the lung vasculature with PBS prior to the excision of the lung and preparation of the lung tissue lysates. Figure 3C demonstrated that approximately one-half of the 67-kDa isoform detected in the nonperfused lung tissue homogenates was the result of the presence of serum PAF acetylhydrolase contained within the lung vasculature during isolation. Tew et al. (45) reported that the human serum PAF acetylhydrolase protein is extensively glycosylated. Therefore, it was likely that the molecular heterogeneity detected in the Western blots represents rat PAF acetylhydrolase isoforms that are the result of glycosylation of a single gene product. To explore the extent of glycosylation of the rat enzyme, protein lysates were subjected to enzymatic glycosidase digestion. Treatment of the Kupffer cell, serum, and lung tissue homogenates with PNGase F caused a shift in the migration of the immunoreactive proteins (Fig. 4). The 67-kDa isoform was more resistant to treatment with the glycosidase, and only partial digestion was achieved under numerous experimental digestion conditions. Although the plasma PAF acetylhydrolase contains ∼41% amino acid identity with the 40-kDa intracellular PAF acetylhydrolase, the antibody I used does not cross-react with this protein (data not shown).
Fig. 3.
Western blot analysis of PAF acetylhydrolase protein induction in lung tissue from LPS-exposed animals. Lung tissue from the indicated samples was homogenized in RIPA, and aliquots (20 μg) were separated by 10% SDS-PAGE and transferred to PVDF membranes. PAF acetylhydrolase protein was detected using a rabbit anti-rat PAF acetylhydrolase antibody (1/1,000) and a horseradish peroxidase labeled goat anti-rabbit secondary antibody (1/1,000). The peroxidase-labeled proteins were visualized using an enhanced chemiluminescence detection system. A: LPS (3 mg/kg) was administered and at 0, 1, 3, 6, 12, 24, and 48 h later the lung tissue was harvested and tissue homogenates were subjected to Western blot analysis. Rat serum (1/100) or cultured rat Kupffer cell lysates (20 μg) were used as a positive control. B: line graph of the quantitation of the 63 and 67 kDa PAF acetylhydrolase immunoreactive bands. C: Western blot of tissue homogenates prepared from lung tissue either removed directly after 24 h of LPS exposure (nonperfused) or after the lung vasculature was perfused for 10 min with PBS (perfused). Rat serum (1/100) was run in parallel and used as a positive control.
Fig. 4.
Digestion with N-glycosidase alters the mobility of PAF acetylhydrolase proteins. Samples of cultured Kupffer cell lysates, lung tissue homogenates, and rat serum were incubated with N-glycosidase F, as detailed in the materials and methods. Control digestions were incubated in the appropriate buffer lacking N-glycosidase F for an equal amount of time. Western blot analysis to detect PAF acetylhydrolase was performed as described previously. (-, no N-glycosidase F; +, N glycosidase F).
After demonstrating that the systemic administration of LPS resulted in both a time- and dose-dependent increase in PAF acetylhydrolase mRNA and protein, the next goal was identifying the specific cell-type(s) responsible for this increased PAF acetylhydrolase production in lung tissue. To identify the cells responsible, immunohistochemical colocalization experiments were performed on tissue sections prepared from lung tissue harvested 24 h after exposure to saline or LPS (Fig. 5). To determine whether lung tissue macrophages were responsible for the increase in PAF acetylhydrolase detected in the Western blots, these tissue sections were incubated with antibodies that react with rat PAF acetylhydrolase (22) and rat ED1. A majority of both rat tissue macrophages and free macrophages (alveolar and peritoneal) are strongly positive for a cytoplasmic rat antigen, ED1 (6, 16).
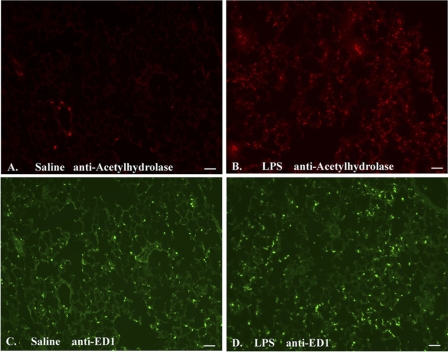
Fig. 5.
Immunohistochemical localization of lung PAF acetylhydrolase and rat ED1 in saline- and LPS-infused animals. Lung tissue sections (5 μm) from saline (A and C) and LPS (B and D) -treated animals 24 h after exposure were cryopreserved and fixed in a solution of methanol:acetone (1:1). The lung sections were incubated with affinity-purified rabbit anti rat PAF acetylhydrolase (1/200) and mouse anti-rat ED1 (1/700) antibodies. Localization of the anti-PAF acetylhydrolase antibody was detected using a Cy3-conjugated goat anti-rabbit secondary antibody (red). Localization of the anti-ED1 antibody was detected using a goat anti-mouse FITC-conjugated secondary antibody (green). Scale bar represents 50 μm: Original magnification: ×100. A and B: anti-PAF acetylhydrolase (red). C and D: anti-ED1 (green).
Lung tissue sections prepared from a saline-exposed animal demonstrated the presence of rare PAF acetylhydrolase-expressing cells (Fig. 5A, red). The scale bar shown in the figure represents 50 μm. Figure 5B illustrates the immunoreactive PAF acetylhydrolase cells in lung tissue 24 h following in vivo systemic LPS exposure. A dramatic increase in the number of PAF acetylhydrolase-positive cells that were dispersed throughout the lung was detected (saline 7.5 ± 7.5 positive cells vs. LPS 161 ± 32, P < 0.001). An increased background immunofluorescence revealed thickened alveolar walls and some punctate localization within the interstitium. Figure 5, C and D illustrated the localization of ED1-positive macrophages in both the saline and LPS-exposed tissue (green). ED1-positive macrophages are clearly evident in normal lung tissue, and a statistically significant increase in the number of ED1-positive cells in the LPS-exposed animal was detected (saline 109 ± 8; LPS 143 ± 16.5; P = 0.01).
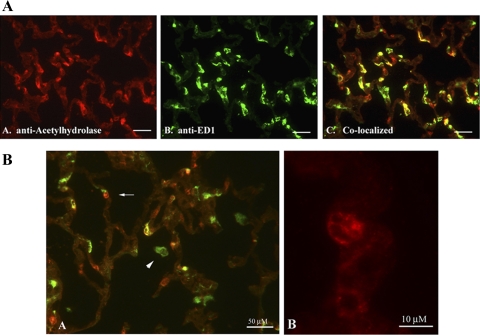
As hypothesized, PAF acetylhydrolase colocalized with many ED1-positive macrophages in the LPS-exposed lung tissue. An examination of these lung tissue sections at higher magnification is illustrated in Fig. 6A. Figure 6, A and B are photographs of the immunohistochemical localization of PAF acetylhydrolase (red) and ED1 (green), respectively. The merged image is depicted in Fig. 6A, subpanel C and illustrated the colocalization of PAF acetylhydrolase and ED1 (yellow). Throughout the lung section, a mixed population of cells was observed representing all combinations of phenotype (PAF acetylhydrolase and ED1 positive, yellow; PAF acetylhydrolase positive and ED1 negative, red; and PAF acetylhydrolase negative and ED1 positive, green). Surprisingly, the presence of a subpopulation of macrophages that were not positive for PAF acetylhydrolase was detected. Figure 6B better illustrates these cells at higher magnification. In subpanel A, the arrowhead points to a PAF acetylhydrolase-negative and ED1-positive cell floating within the alveolar space, most likely an alveolar macrophage. Unexpectedly, PAF acetylhydrolase-positive cells that were not positive for the macrophage antigen ED1 were also detected (arrow). At higher magnification (subpanel B), these ED1 negative cells appeared to exhibit a polymorphonuclear appearance.
Fig. 6.
Colocalization of PAF acetylhydrolase and ED1 in LPS-exposed lung tissue. Lung tissue sections isolated from animals exposed to LPS (3 mg/kg) for 24 h were cryopreserved and fixed in a solution of methanol:acetone (1:1). The lung sections were incubated with affinity-purified rabbit anti-rat PAF acetylhydrolase (1/200) and mouse anti-rat ED1 (1/700) antibodies. Localization of the anti-PAF acetylhydrolase antibody was detected using a Cy3-conjugated goat anti-rabbit secondary antibody (red). Localization of the anti-ED1 antibody was detected using a goat anti-mouse FITC-conjugated secondary antibody (green). A: original magnification: ×400. A, A: anti-PAF acetylhydrolase. A, B: anti-ED1. A, C: merged image. Scale bar shown represents 50 μm. B: merged images at higher magnification. Arrowhead points to an ED1-positive, PAF acetylhydrolase-negative cell. Arrow points to a PAF acetylhydrolase-positive, ED1-negative cell. Scale bar shown represents 50 μm (B, subpart A) and 10 μm (B, subpart B).
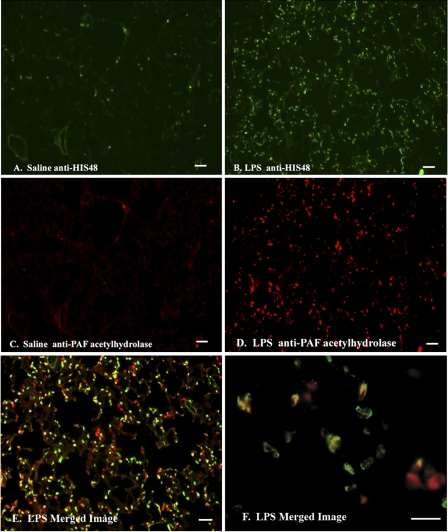
Additional immunohistochemical colocalization experiments were performed to identify the ED1-negative, PAF acetylhydrolase-positive cells. Lung tissue sections obtained 24 h after exposure to saline or LPS injections were incubated with antibodies that react with the rat PAF acetylhydrolase and HIS48, a rat granulocyte marker. Lung tissue sections prepared from the saline control animals demonstrated sparsely separated HIS48-positive cells (Fig. 7A, green). In vivo treatment with LPS caused massive infiltration of granulocytes into the lung tissue at 24 h (Fig. 7B; Saline 19.5 ± positive cells vs. LPS 184 ± 7.6; P < 0.001). Likewise, PAF acetylhydrolase-positive cells were barely detectable in the saline-exposed lungs (C). As was seen, in Fig. 6, LPS administration resulted in a dramatic increase in the number of PAF acetylhydrolase-positive cells (D; Saline 23.5 ± 7.2 positive cells vs. LPS 237 ± 42; P < 0.001 ). Merging of the PAF acetylhydrolase and HIS48 images taken of the LPS-exposed lung tissue confirmed that heterogeneous populations of cells are PAF acetylhydrolase positive (E and F). Notably, the PAF acetylhydrolase antibody colocalized with the HIS48 granulocyte marker (E and F, yellow).
Fig. 7.
Immunohistochemical analyses of PAF acetylhydrolase and HIS48 in saline- and LPS-exposed lung tissue. Lung tissue sections (5 μm) from saline (A and C)- and LPS (B and D–F)-treated animals 24 h after exposure were cryopreserved and fixed in a solution of methanol:acetone (1:1). The lung sections were incubated with affinity-purified rabbit anti-rat PAF acetylhydrolase (1/200) and mouse anti-rat HIS48 (1/200) antibodies. Localization of the anti-PAF acetylhydrolase antibody was detected using a Cy3-conjugated goat anti-rabbit secondary antibody (red). Localization of the anti-HIS48 antibody was detected using a goat anti-mouse FITC-conjugated secondary antibody (green). Scale bar represent 50 μm. A and B: anti-HIS48. C and D: anti-PAF acetylhydrolase. E and F: merged images.
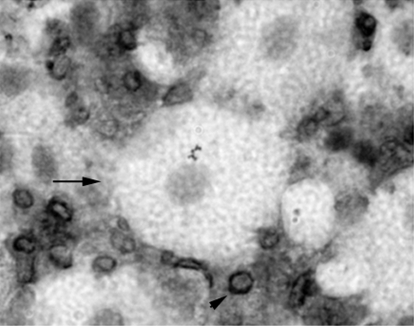
Because of the examination of LPS-exposed lung tissue sections suggested that alveolar macrophages did not respond with an increase in PAF acetylhydrolase protein production, additional immunohistochemistry on alveolar macrophages isolated from animals exposed to systemic LPS administration were performed. We obtained bronchial alveolar lavage (BAL) samples from LPS-exposed animals and performed immunocytochemistry for PAF acetylhydrolase on the cytocentrifuged samples. PAF acetylhydrolase immunoreactivity was not detected in the larger alveolar macrophages isolated from a 24-h exposed animal (Fig. 8, arrow); however, immunoreactivity was detected in smaller cells isolated from the lavage samples (black staining; arrowhead).
Fig. 8.
PAF acetylhydrolase cytochemistry on cells obtained from bronchial alveolar lavage (BAL) fluid isolated from LPS-exposed animals. Rats received LPS (3 mg/kg), and BAL was collected at 24 h after the administration. BAL was collected by instilling and collecting four successive 5-ml volumes of PBS. The lavage fluid was centrifuged, and the cell pellet was resuspended in 1 ml PBS. Immunocytochemistry was performed on cytospin-prepared slides. Arrow points to a large PAF acetylhydrolase-negative alveolar macrophage. Arrowhead points to small PAF acetylhydrolase-positive BAL cell.
DISCUSSION
The in vivo administration of a systemic dose of LPS resulted in substantial lung injury characterized by massive neutrophil infiltration, edema, and extravasation of protein and water into the alveolar space as reported previously (7, 8). We previously demonstrated that of the numerous tissues examined, lung tissue was most responsive with respect to increased levels of PAF acetylhydrolase mRNA in response to systemic LPS administration (22). The purpose of this research study was to further investigate the expression and cell-specific localization of PAF acetylhydrolase in lung tissue following in vivo administration of LPS.
Administration of LPS increased PAF acetylhydrolase RNA and protein in lung tissue (Figs. 1–3). This increase was most pronounced at 24 h following administration and identical to the time of peak PAF acetylhydrolase mRNA, protein, and activity production in response to LPS detected in the liver (21, 22). Plasma PAF acetylhydrolase performs an essential role in controlling the pathophysiological effects of PAF and PAF-like lipid mediators. The delayed induction of PAF acetylhydrolase expression could reflect the need to downregulate and minimize any subsequent actions of PAF and PAF-like phospholipids generated during the inflammatory sequela. For instance, the initial PAF-instigated inflammatory response is necessary and beneficial, but a mechanism to halt this signaling before tissue injury results is required. Therefore, increased production of PAF acetylhydrolase within the affected tissue likely functions to minimize subsequent injury and promote host recovery.
Alternatively, the increased expression and protein production of PAF acetylhydrolase in response to LPS could reflect the need to replenish the supply of active PAF acetylhydrolase. Several research groups demonstrated that PAF acetylhydrolase activity is susceptible to irreversible oxidative inactivation (1, 28, 32). This may explain why increased and decreased activity of PAF acetylhydrolase have been reported in numerous studies. A wide variation in circulating PAF acetylhydrolase activity has been documented in critically ill patients with sepsis. PAF acetylhydrolase activity of patients was below controls but markedly increased over time, and higher activities were seen in patients with severe sepsis or septic shock compared with those without organ failure (15). Likewise, Gomes et al. (19) found moderately decreased values for plasma PAF acetylhydrolase activity in patients with sepsis and septic shock. In contrast, PAF acetylhydrolase activity has been consistently shown to increase in BAL fluid in both critically ill human patients and in animal models of acute lung injury (20, 34, 39, 48).
Although a 3 mg/kg dose of LPS was routinely used in these experiments, increased PAF acetylhydrolase mRNA expression was detected in response to 0.01 mg/kg (Fig. 2). This relatively small dose of LPS is comparable to dosages used for desensitization experiments, in which small amounts of LPS are used to render the animal less sensitive to subsequent lethal doses of LPS (30). The increased expression of PAF acetylhydrolase in response to small doses of LPS could contribute to subsequent induced tolerance to LPS via the increased degradation of PAF and PAF-like molecules. Gomes et al. (19) demonstrated that the administration of recombinant PAF acetylhydrolase dramatically reduced mortality and inflammation in mice challenged with LPS or subjected to cecal ligation and puncture.
In addition to demonstrating an increase in PAF acetylhydrolase protein production following LPS administration, Western blot analyses revealed the presence of molecular weight heterogeneity of the PAF acetylhydrolase protein (Fig. 4). Rat plasma-type PAF acetylhydrolase circulates predominantly as a 67-kDA species, while the protein detected from cellular lysates of cultured macrophages migrates at 63 kDa. Sequence analysis of the rat PAF acetylhydrolase gene predicts a 50-kDa protein, which includes the ∼20-amino acid leader sequence. The variation in molecular weights detected likely arises as a result of glycosylation of the PAF acetylhydrolase protein, and the differences detected between the intracellular and extracellular proteins are a reflection of different stages of post-translational processing. Human plasma PAF acetylhydrolase was initially purified and characterized as a 43-kDa protein, although several truncated species with differing NH2-terminal sequences were identified (43, 46). Subsequently, both Tew et al. (45) and Tselepis et al. (49) demonstrated that human PAF acetylhydrolase contains 9–10 kDa of heterogeneous asparagine-conjugated sugar chains containing sialic acid but that it does not contain any serine/threonine-linked glycans. Tew et al. (45) reported the purified human plasma PAF acetylhydrolase protein ranged in size from a 43-kDA nonglycosylated protein to a 65- to 67-kDa glycosylated form. The report of molecular weight heterogeneity for the human PAF acetylhydrolase agrees with the multiple species of rat PAF acetylhydrolase I detected in the Western blots.
To understand the potential role of PAF acetylhydrolase in minimizing lung injury, a definitive identification of the cells responsible for PAF acetylhydrolase expression and secretion is necessary. This identification of the PAF acetylhydrolase-secreting cells can provide insight into understanding, in the local environment, where an increased level of PAF acetylhydrolase is needed to control PAF and PAF-like lipid molecules and contribute insight into the mode of action of this anti-inflammatory enzyme. Immunohistochemical colocalization experiments confirmed the hypothesis that lung macrophages are activated to express high levels of PAF acetylhydrolase following endotoxin exposure. Utilizing the presence of a naturally occurring missense mutation in the PAF acetylhydrolase gene in a small percentage of the Japanese population, Asano et al. (3) investigated PAF acetylhydrolase activity in patients undergoing allogenic bone marrow transplant. This study demonstrated that the recipient's PAF acetylhydrolase activity depended upon the donor's genotype and confirmed that PAF acetylhydrolase activity arises from cells of hematopoietic origin (3). It is generally accepted that macrophages are a significant source of plasma PAF acetylhydrolase. Monocytes do not express PAF acetylhydrolase until differentiated into macrophages in culture, and cultured macrophages are capable of secreting large amounts of PAF acetylhydrolase (33, 42). Immunohistochemistry on lung tissue isolated from the saline-infused controls revealed few PAF acetylhydrolase-positive cells, even though resident tissue macrophages (as evidenced by the presence of reactivity to the rat macrophage antigen ED1) were present. The lack of significant PAF acetylhydrolase expression in normal lung tissue is similar to the low levels of PAF acetylhydrolase expression detected in normal liver tissue (22). Following stimulation with LPS, the number of ED1-positive macrophages present in the lung slightly increased, but the number of PAF-AH-positive cells dramatically increased. Lung tissue contains at least 3 unique classes of resident macrophages, including alveolar macrophages, which line the alveoli; interstitial macrophages, which lie within the interstitium; and the pulmonary intravascular macrophages, which populate the airways. These resident macrophages contain unique functional differences depending upon the surrounding environment. Surprisingly, not all lung macrophages identified by positive reactivity to the rat macrophage antigen ED1 were induced to express PAF acetylhydrolase in our study. Some of these ED1-positive, PAF acetylhydrolase-negative cells could be circulating monocytes present in the vasculature, as monocytes do not express PAF acetylhydrolase (42). After the systemic administration of LPS, alveolar macrophages did not respond with an induction of PAF acetylhydrolase protein production (Figs. 6 and 8), although smaller leukocytes, most likely neutrophils in the BAL, showed positive PAF acetylhydrolase staining (see below). Numerous studies have documented increased PAF acetylhydrolase activity in BAL fluid in both critically ill patients and in animal models of acute lung injury (20, 34, 39). Grissom et al. (20) demonstrated that PAF acetylhydrolase activity is increased in BAL fluid from patients with ARDS and detected PAF acetylhydrolase mRNA in alveolar macrophages. However, this RT-PCR result was obtained from BAL cells that were characterized as having anywhere from 35 to 63% neutrophils present. In a porcine model of oleic acid-induced acute lung injury, Salluh et al. (39) demonstrated increased BAL fluid PAF acetylhydrolase activity and observed PAF acetylhydrolase immunostaining of macrophages and epithelial cells in the lung tissue. In their studies, increased PAF acetylhydrolase activity did not correlate with increased protein content in the lavage fluid, and the increase in PAF acetylhydrolase activity detected was several hours after the increase in protein content. The authors concluded that increases in PAF acetylhydrolase levels are, in part, a result of local production of PAF acetylhydrolase rather than the flooding of the alveolar compartment with plasma proteins. Our results demonstrating an increase in PAF acetylhydrolase in a subset of lung tissue macrophages supports the hypothesis that PAF acetylhydrolase is upregulated in response to inflammatory challenge and is produced locally to control and limit inflammation. The lack of upregulation of PAF acetylhydrolase expression in alveolar macrophages could result from the method of LPS exposure (i.e., systemic vs. intratracheal) or could result from inherent functional differences of these macrophages. The immunohistochemical colocalization experiments demonstrated significant immunoreactivity to the PAF acetylhydrolase antibody in cells that were not positive for the macrophage marker ED1. ED1 is a monoclonal antibody that recognizes the rat homologue of human CD68 and the antigen is expressed by the majority of tissue macrophages (6). Although we cannot entirely eliminate the possibility that some tissue macrophages may not be recognized by the ED1 antibody and therefore display only reactivity to the PAF acetylhydrolase antibody, additional colocalization experiments demonstrated that these PAF acetylhydrolase-positive cells also display reactivity to an antibody directed against HIS48, a rat granulocyte marker frequently used to identify rat neutrophils (38).
In conclusion, systemic administration of LPS results in the induction of PAF acetylhydrolase mRNA in lung tissue and differential PAF acetylhydrolase protein expression in a subset of lung tissue macrophages. Systemic LPS exposure also resulted in the appearance of PAF acetylhydrolase in some lung tissue granulocytes. The elevated levels of PAF acetylhydrolase may represent a means of controlling the proinflammatory actions of PAF and oxidized phospholipids in compartmentalized regions of the lung. The temporal expression of PAF acetylhydrolase with peak induction at 24 h following LPS challenge allows for initial PAF signaling and cellular responses but serves to limit further tissue injury from subsequent lipid mediator formation.
GRANTS
This research was supported by National Institutes of Health Grant HL66130.
DISCLOSURES
No conflict of interest are declared by the author.
ACKNOWLEDGMENTS
The author thanks Merle S. Olson, Dean of the Graduate School of Biomedical Science, University of Texas Health Science Center at San Antonio for donating his research equipment to my laboratory for the completion of these studies. In addition, my appreciation is extended to Susan O'Malley for critical reading of this manuscript.
Present address for K. M. Howard: Department of Biomedical Sciences, University of Nevada, Las Vegas School of Dental Medicine, Las Vegas, NV 89106.
REFERENCES
- 1.Ambrosio G, Oriente A, Napoli C, Palumbo G, Chiariello P, Marone G, Condorelli M, Chiariello M, Triggiani M. Oxygen radicals inhibit human plasma acetylhydrolase, the enzyme that catabolizes platelet-activating factor. J Clin Invest 93: 2408– 2416, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artigas A, Bernard GR, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, Lamy M, Marini JJ, Matthay MA, Pinsky MR, Spragg R, Suter PM. The American-European Consensus Conference on ARDS, part 2: Ventilatory, pharmacologic, supportive therapy, study design strategies, and issues related to recovery and remodeling. Acute respiratory distress syndrome. Am J Respir Crit Care Med 157: 1332– 1347, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Asano K, Okamoto S, Fukunaga K, Shiomi T, Mori T, Iwata M, Ikeda Y, Yamaguchi K. Cellular source(s) of platelet-activating-factor acetylhydrolase activity in plasma. Biochem Biophys Res Commun 261: 511– 514, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Chambless LE, Myerson M, Wu KK, Sharrett AR, Boerwinkle E. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study.[see comment]. Arch Intern Med 165: 2479– 2484, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study.[see comment]. Circulation 109: 837– 842, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Beelen RHJ, Estermans IL, Dopp EA, Dijkstra CD. Monoclonal antibodies ED1, ED2, and ED3 against rat macrophages: expression of recognized antigens in different stages of differentiation. Trans Proced XIX : 3166– 3170, 1987 [Google Scholar]
- 7.Brigham KL, Begley CJ, Bernard GR, Hutchison AA, Loyd JE, Lucht WD, Meyrick B, Newman JH, Niedermeyer ME, Ogletree ML. Septicemia and lung injury. Clin Lab Med 3: 719– 744, 1983 [PubMed] [Google Scholar]
- 8.Brigham KL, Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis 133: 913– 927, 1986 [PubMed] [Google Scholar]
- 9.Cao Y, Stafforini DM, Zimmerman GA, McIntyre TM, Prescott SM. Expression of plasma platelet-activating factor acetylhydrolase is transcriptionally regulated by mediators of inflammation. J Biol Chem 273: 4012– 4020, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Caplan MS, Sun XM, Hseuh W, Hageman JR. Role of platelet activating factor and tumor necrosis factor-alpha in neonatal necrotizing enterocolitis. J Pediatr 116: 960– 964, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Chang SW, Feddersen CO, Henson PM, Voelkel NF. Platelet-activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest 79: 1498– 1509, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CR, Voelkel NF, Chang SW. Platelet-activating factor potentiates protamine-induced lung edema. Role of eicosanoids. Am J Respir Crit Care Med 149: 34– 40, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156– 159, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Christman BW, Lefferts PL, King GA, Snapper JR. Role of circulating platelets and granulocytes in PAF-induced pulmonary dysfunction in awake sheep. J Appl Physiol 64: 2033– 2041, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Claus RA, Russwurm S, Dohrn B, Bauer M, Losche W. Plasma platelet-activating factor acetylhydrolase activity in critically ill patients. Crit Care Med 33: 1416– 1419, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in rat recognized by monoclonal antibodies ED1, ED2 and ED3. Adv Exp Med Biol 186: 409– 419, 1985 [DOI] [PubMed] [Google Scholar]
- 17.Furukawa M, Lee EL, Johnston JM. Platelet-activating factor-induced ischemic bowel necrosis: the effect of platelet-activating factor acetylhydrolase. Pediatr Res 34: 237– 241, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Gandhi CR, Stephenson K, Olson MS. A comparative study of endothelin- and platelet-activating-factor-mediated signal transduction and prostaglandin synthesis in rat Kupffer cells. Biochem J 281: 485– 492, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomes RN, Bozza FA, Amancio RT, Japiassu AM, Vianna RCS, Larangeira AP, Gouvea JM, Bastos MS, Zimmerman GA, Stafforini DM, Prescott SM, Bozza PT, Castro-Faria-Neto HC. Exogenous platelet-activating factor acetylhydrolase reduces mortality in mice with systemic inflammatory response syndrome and sepsis. Shock 26: 41– 49, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Grissom CK, Orme JF, Richer LD, McIntyre TM, Zimmerman GA, Elstad MR. Platelet-activating factor acetylhydrolase is increased in lung lavage fluid from patients with acute respiratory distress syndrome. Crit Care Med 31: 770– 775, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Howard KM, Miller JE, Miwa M, Olson MS. Cell-specific regulation of expression of plasma-type platelet-activating factor acetylhydrolase in the liver. J Biol Chem 272: 27543– 27548, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Howard KM, Olson MS. The expression and localization of plasma platelet-activating factor acetylhydrolase in endotoxemic rats. J Biol Chem 275: 19891– 19896, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, Cao W, Kume K, Fukuchi Y, Ikuta K, Miyazaki J, Kumada M, Shimizu T. Impaired anaphylatic respinses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med 187: 1779– 1788, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishii S, Nagase T, Tashiro F, Ikuta K, Sato S, Waga I, Kume K, Miyazaki J, Shimizu T. Bronchial hyperreactivity, increased endotoxin lethality and melanocytic tumorigenesis in transgenic mice overexpressing platelet-activating factor receptor. EMBO J 16: 133– 142, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knook DL, Sleyster EC. Separation of Kupffer and endothelial cells of the rat liver by centrifugal elutriation. Exp Cell Res 99: 444– 449, 1976 [DOI] [PubMed] [Google Scholar]
- 26.Koenig W, Twardella D, Brenner H, Rothenbacher D. Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol 26: 1586– 1593, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680– 685, 1970 [DOI] [PubMed] [Google Scholar]
- 28.MacRitchie AN, Gardner AA, Prescott SM, Stafforini DM. Molecular basis for susceptibility of plasma platelet-activating factor acetylhydrolase to oxidative inactivation. FASEB J 21: 1164– 1176, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Meade CJ, Birke F, Metcalfe S, Watson C, Jamieson N, Neild G. Serum PAF-acetylhydrolase in severe renal or hepatic disease in man: relationship to circulating levels of PAF and effects of nephrectomy or transplantation. J Lipid Mediat Cell Signal 9: 205– 215, 1994 [PubMed] [Google Scholar]
- 30.Mengozzi M, Ghezzi P. Cytokine down-regulation in endotoxin tolerance. Eur Cytokine Netw 4: 89– 98, 1993 [PubMed] [Google Scholar]
- 31.Miwa M, Miyake T, Yamanaka T, Sugatani J, Suzuki Y, Sakata S, Araki Y, Matsumoto M. Characterization of serum platelet-activating factor (PAF) acetylhydrolase. Correlation between deficiency of serum PAF acetylhydrolase and respiratory symptoms in asthmatic children. J Clin Invest 82: 1983– 1991, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyaura S, Eguchi H, Johnston JM. Effect of a cigarette smoke extract on the metabolism of the proinflammatory autacoid, platelet-activating factor. Circ Res 70: 341– 347, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Nakamura M, Honda Z, Izumi T, Sakanaka C, Mutoh H, Minami M, Bito H, Seyama Y, Matsumoto T, Noma M. Molecular cloning and expression of platelet-activating factor receptor from human leukocytes. J Biol Chem 266: 20400– 20405, 1991 [PubMed] [Google Scholar]
- 34.Nakos G, Pneumatikos J, Tsangaris I, Tellis C, Lekka M. Proteins and phospholipids in BAL from patients with hydrostatic pulmonary edema. Am J Respir Crit Care Med 155: 945– 951, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Oei HHS, van der Meer IM, Hofman A, Koudstaal PJ, Stijnen T, Breteler MMB, Witteman JCM. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation 111: 570– 575, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Packard CJ, O'Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GD. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group[see comment]. N Engl J Med 343: 1148– 1155, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu Rev Biochem 69: 419– 445, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Reckless J, Tatalick LM, Grainger DJ. The pan-chemokine inhibitor NR58–3.143 abolishes tumour necrosis factor-alpha accumulation and leucocyte recruitment induced by lipopolysaccharide in vivo. Immunology 103: 244– 254, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salluh JI, Pino AV, Silva AR, Gomes RN, Souza HS, Lapa e Silva JR, Jandre FC, Giannella-Neto A, Zimmerman GA, Stafforini DM, Prescott SM, Castro-Faria-Neto HC, Bozza PT, Bozza FA. Lung production of platelet-activating factor acetylhydrolase in oleic acid-induced acute lung injury. Prostaglandins Leukot Essent Fatty Acids 77: 1– 8, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Satoh K, Imaizumi T, Kawamura Y, Yoshida H, Takamatsu S, Takamatsu M. Increased activity of the platelet-activating factor acetylhydrolase in plasma low density lipoprotein from patients with essential hypertension. Prostaglandins 37: 673– 682, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Snyder F, Blank ML, Lee TC. Metabolism of platelet activating factor in lung. Annals NY Acad Sci 629: 168– 175, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Stafforini DM, Elstad MR, McIntyre TM, Zimmerman GA, Prescott SM. Human macrophages secret platelet-activating factor acetylhydrolase. J Biol Chem 265: 9682– 9687, 1990 [PubMed] [Google Scholar]
- 43.Stafforini DM, Prescott SM, McIntyre TM. Human plasma platelet-activating factor acetylhydrolase. Purif Prop J Biol Chem 262: 4223– 4230, 1987 [PubMed] [Google Scholar]
- 44.Tetta C, Bussolino F, Modena V, Montrucchio G, Segoloni G, Pescarmona G, Camussi G. Release of platelet-activating factor in systemic lupus erythematosus. Int Arch Allergy Appl Immunol 91: 244– 256, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Tew DG, Southan C, Rice SQ, Lawrence MP, Li H, Boyd HF, Moores K, Gloger IS, Macphee CH. Purification, properties, sequencing, and cloning of a lipoprotein-associated, serine-dependent phospholipase involved in the oxidative modification of low-density lipoproteins. Arterioscler Thromb Vasc Biol 16: 591– 599, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Tjoelker LW, Eberhardt C, Unger J, Trong HL, Zimmerman GA, McIntyre TM, Stafforini DM, Prescott SM, Gray PW. Plasma platelet-activating factor acetylhydrolase is a secreted phospholipase A2 with a catalytic triad. J Biol Chem 270: 25481– 25487, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Tjoelker LW, Wilder C, Eberhardt C, Stafforini DM, Dietsch G, Schimpf B, Hooper S, Le Trong H, Cousens LS, Zimmerman GA. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase [see comments]. Nature 374: 549– 553, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Triggiani M, De Marino V, Sofia M, Faraone S, Ambrosio G, Carratu L, Marone G. Characterization of platelet-activating factor acetylhydrolase in human bronchoalveolar lavage. Am J Respir Crit Care Med 156: 94– 100, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Tselepis AD, Karabina SAP, Stengel D, Piedagnel R, Chapman MJ, Ninio E. N-linked glycosylation of macrophage-derived PAF-AH is a major determinant of enzyme association with plasma HDL. J Lipid Res 42: 1645– 1654, 2001 [PubMed] [Google Scholar]
- 50.Wu X, Zimmerman GA, Prescott SM, Stafforini DM. The p38 MAPK pathway mediates transcriptional activation of the plasma platelet-activating factor acetylhydrolase gene in macrophages stimulated with lipopolysaccharide. J Biol Chem 279: 36158– 36165, 2004 [DOI] [PubMed] [Google Scholar]