Abstract
Acute lung injury (ALI) is an inflammatory disorder associated with recruitment and activation of neutrophils in lungs. Rac2, a member of the Rho GTPase subfamily, is an essential regulator of neutrophil degranulation, superoxide release, and chemotaxis. Here, we hypothesized that Rac2 is important in mediating lung injury. Using a model of IgG immune complex-mediated ALI, we showed that injury was attenuated in rac2−/− mice compared with wild-type (WT) mice undergoing ALI, with significant decreases in alveolar leukocyte numbers, vascular leakage, and the inflammatory mediators, myeloperoxidase (MPO) and matrix metalloproteinases (MMPs). Reduced injury in rac2−/− mice was not associated with diminished cytokine and chemokine production, since bronchoalveolar lavage (BAL) levels of IL-17, TNF, CCL3, CXCL1, and CXCL2 were similarly increased in WT and rac2−/− mice with ALI compared with sham-treated mice (no ALI). BAL levels of MMP-2 and MMP-9 were significantly decreased in the airways of rac2−/− mice with ALI. Immunohistochemical analysis revealed that MMP-2 and MMP-9 expression was evident in alveolar macrophages and interstitial neutrophils in WT ALI. In contrast, MMP-positive cells were less prominent in rac2−/− mice with ALI. Chimeric mice showed that Rac2-mediated lung injury was dependent on hematopoietic cells derived from bone marrow. We propose that lung injury in response to immune complex deposition is dependent on Rac2 in alveolar macrophages and neutrophils.
Keywords: neutrophils, macrophages, bronchoalveolar lavage, cytokines, bone marrow chimeras, matrix metalloproteinases
acute lung injury (ALI) is a common clinical disorder involving acute respiratory failure caused by injury to alveolar epithelial and endothelial barriers in the lung (4, 42). Its severe form is acute respiratory distress syndrome (ARDS), which is accompanied by a high rate of morbidity and mortality (20–50%; Refs. 3, 36, 42). A current medical intervention for ALI is to maintain patients on a low tidal volume respiratory ventilation protocol (17). Several drug intervention strategies have been tested for ALI but have failed to reduce mortality associated with the disease (17, 50).
Neutrophil recruitment and activation are major causative factors in ALI and lead to tissue-damaging degranulation and superoxide release. Regulation of mediator release from neutrophils occurs through ligand-receptor stimulation of Rho GTPases. Of these, all three Rac proteins are expressed in neutrophils (Rac1, Rac2, and Rac3), whereas Rac1 and Rac2 have been investigated more extensively than Rac3 in these cells (9, 24, 30). Rac1 and Rac2 exhibit 92% amino acid identity and are functionally interchangeable in their ability to regulate superoxide generation and chemotaxis, although human neutrophils mainly express Rac2 (9). However, Rac1 and Rac2 serve distinct, nonredundant functions in neutrophils. Whereas Rac1 controls cell spreading, Rac2 is critical for migration as well as superoxide release and exocytosis of azurophilic granules (2, 22). Specifically, Rac2 is required for F-actin formation in neutrophils, which is essential for numerous cellular activities including chemotaxis, superoxide release, and degranulation (19, 41). Primary granule exocytosis in neutrophils is dependent on Rac2-dependent actin remodeling, demonstrated by the inability of Rac2−/− neutrophils to translocate primary granules to the cell membrane for their release (1, 2).
The function of Rac2 in a neutrophil-mediated inflammatory model has not been investigated. Our aim was to elucidate the role of Rac2 in experimental ALI induced by IgG immune complex deposition in the airways (reverse passive Arthus reaction), a model of lung inflammation and injury. This model of injury is specifically dependent on neutrophil recruitment to the airways and their activation based on experiments in rats (27). In this study, we examined the effects of gene deletion of Rac2 in immune complex-mediated ALI in mice (41).
MATERIALS AND METHODS
Mice.
Rac2-deficient mice (rac2−/−) were established by gene disruption (41). All gene-deleted mice were backcrossed against the C57BL/6 strain background for >11 generations. Animals were bred on-site and housed under specific virus antigen-free conditions on a 12:12-h light-dark cycle and fed autoclaved food and water as needed. All experiments used male mice at 8–12 wk of age. Wild-type (WT) animals were C57BL/6 mice housed and maintained in the same manner as for rac2−/− mice. Animal experiments were approved for ethics by the University of Alberta Animal Policy and Welfare Committee.
Immune complex-mediated ALI.
Isoflurane inhalation using a precision vaporizer instrument (Ohio Medical, Gurnee, IL), and intraperitoneal injections of ketamine and xylazine (1.5 mg and 150 μg per mouse, respectively) were used for anesthesia and analgesia. The reverse passive Arthus reaction was induced in the airways of the animal by intratracheal injection of 40 μl containing 16, 40, or 160 μg of rabbit anti-ovalbumin IgG (Valeant Pharmaceuticals International, Costa Mesa, CA) in water, followed by an intravenous tail vein injection of 100-μl low endotoxin (<0.1 IU/ml) 4 mg/ml ovalbumin (Sigma-Aldrich, Oakville, Ontario, Canada) dissolved in saline (5, 11, 35). Sham-treated mice were C57BL/6 WT and rac2−/− animals that were subjected to intratracheal injection of 40-μl saline followed by intravenous injection of 400-μg ovalbumin. Anti-ovalbumin was administered via tracheal injection in anesthetized animals following surgical dissection of the skin surrounding the throat and upper thoracic cavity and separation of the underlying musculature. The antibody was injected by needle and syringe through the second and third tracheal cartilage rings, and immediately following this, the animals were sutured and allowed to recover. Mice were killed 6 h after injections by an intraperitoneal overdose of ketamine and xylazine (15 and 1.5 mg per mouse, respectively). In agreement with previous findings, we determined that maximal lung injury was evident at 6 h past injection of anti-ovalbumin and ovalbumin (23), and significant albumin leakage into the alveoli was obtained at a dose of 160-μg anti-ovalbumin. These procedures were performed in a sterile environment. We chose 160-μg anti-ovalbumin as the optimal dose to detect lung inflammatory events that are dependent on Rac2 expression.
Bronchoalveolar lavage fluid.
Collection of bronchoalveolar lavage (BAL) fluid occurred 6 h following injection of intravenous ovalbumin (1-ml PBS × 6). After BAL fluid was centrifuged at 300 g for 5 min, the total and differential cell counts of the BAL fluid were determined from the cell pellet using Diff-Quik (Fisher Scientific, Nepean, Ontario, Canada) on cell cytospins. For analysis of markers in BAL supernatants, 1 ml of PBS was used for BAL collection to ensure an adequate concentration could be measured.
Marker assays for BAL supernatants.
Myeloperoxidase (MPO) was assayed using tetramethylbenzidine (TMB) as the substrate, as previously described (2, 32). Briefly, 150 μl of TMB substrate solution was added to 50 μl of BAL in a 96-well microplate and incubated at room temperature for 15 min before termination of the reaction with 50 μl of 1 M H2SO4. Microplates were read spectrophotometrically at 450 nm using a Power Wave XS plate reader (BioTek Instruments, Winooski, VT). Absorbance values for background (PBS only) were subtracted from sample MPO values. Lactoferrin was assayed in BAL and cell supernatants by ELISA measurement based on cross-reactivity of human anti-lactoferrin (Sigma-Aldrich) for murine lactoferrin (2). Murine albumin was determined by ELISA (Bethyl Laboratories, Montgomery, TX). Cytokines (IL-1β, IL-17, and TNF), chemokines (CCL3, CXCL1, and CXCL2), and matrix metalloproteinase (MMP-2 and MMP-9) were determined by a customized Pierce SearchLight multiplex ELISA assay system (Pierce, Rockford, IL).
Lung histology, immunohistochemistry, and confocal microscopy analysis.
For murine lung histology, control and treated C57BL/6 mice were euthanized without lavage. Following dissection of the thoracic cavity to expose the lungs, tracheae were cannulated, and 1.0–1.5 ml (based on individual weight) 10% buffered formalin in PBS (pH 7.2) was gently instilled by syringe through the cannula into the lungs. Lungs were inflated to a degree that they filled the original dimensions of the thoracic cavity. Care was taken to gradually infuse the buffered formalin to prevent overinflation and alveolar damage. After instillation of buffered formalin, tracheae were tied off with surgical suture below the cannulation point and dissected above the suture position, and lungs were removed in toto from the thorax for immersion in 10% buffered formaldehyde for 24 h before paraffin embedding and routine histological processing. In each case, the right lobe of the lung was used for histological analysis for consistency between animals. Lungs were sectioned at 4 μm followed by hematoxylin and eosin (H&E) staining. We confirmed that lungs were consistently inflated to a similar degree in each animal by examination of the histological appearance of alveolar spaces in stained lung sections from sham-treated and untreated animals, which usually showed intact, unbroken architecture and little evidence of collapsed tissue. In quantitative histology, hemorrhage and inflammation were assessed using the following scale: 1 for mild focal inflammation, 2 for mild diffuse inflammation, 3 for intense inflammation with focal hemorrhage and intraalveolar neutrophils, and 4 for diffuse, intense inflammation with widespread neutrophilic infiltration. The quality of lung histological sections, and quantification of inflammatory indices, was confirmed in a blinded manner by a licensed anatomic pathologist with fellowship training in lung pathology and cytopathology. Images were acquired at ambient temperature using a Nikon Eclipse model E600 microscope with ×20/×40 objectives [1.0 numerical aperture (NA)] configured for standard bright field microscopy, an 11-megapixel Nikon DXM 1200 digital camera attached to the microscope, and control software, Nikon ACT-1 (Nikon, Mississauga, Ontario, Canada). In immunofluorescence analysis, lungs were removed immediately after anesthesia overdose without fixation, frozen in liquid nitrogen, and sectioned on a cryostat before staining with monoclonal antibodies to marker Gr-1 conjugated to Alexa 488 (mouse Ly-6G, specific for murine neutrophils; eBioscience, San Diego, CA) and surfactant protein C conjugated to phycoerythrin (SP-C; Chemicon International, Temecula, CA). Cell nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). Slides were prepared with mounting media and visualized at ambient temperature. Images of immunofluorescence were obtained on an Olympus FV1000 confocal microscopy system using a Plan Apo ×63 objective (1.4 NA; Olympus, Mississauga, Ontario, Canada). In immunohistochemistry, paraffin-embedded lung samples were sectioned and subjected to antibody labeling following deparaffinization using the iVIEW DAB Detection System on the Ventana Discovery XT system (Ventana Medical Systems, Tucson, AZ). For antigen detection, 40 μg/ml mouse anti-MMP-2 (cross-reactive for mouse MMP-2; clone 42-5D11; Millipore, Billerica, MA) or 20 μg/ml rat anti-mouse MMP-9 (clone 116134; R&D Systems, Minneapolis, MN) was detected by biotinylated anti-mouse IgG that was cross-reactive for rat anti-MMP-9, following a mouse-on-mouse block step using the manufacturer's protocol (Vector M.O.M. Basic Kit; Vector Laboratories, Burlingame, CA). Mouse IgG1 (BD Biosciences Canada, Mississauga, Ontario, Canada) was used at 40 μg/ml in place of primary antibody for isotype controls.
Measurement of O2•− release from neutrophils.
Generation of extracellular O2•− from cells in suspension was measured as previously described (31). SOD-inhibitable absorbance was calculated using 2.11 × 104 M−1·cm−1 for reduced cytochrome c.
Chimera generation.
Mice were irradiated with a single dose of ∼10 Gy from a 137Cs source. Following irradiation (18–24 h), mice were reconstituted with freshly prepared bone marrow cells (1 × 106 cells per animal) from donor animals by intravenous injection using a previously reported technique (16). Bone marrow cells were prepared by flushing femurs and tibias by syringe and 26-gauge needles in a laminar flow hood and placing cells in sterile HBSS or using a mortar and pestle with femurs and tibias in fetal calf serum and HBSS. Bone marrow cells were counted and resuspended in fresh HBSS at 10 × 106 per milliliter. A volume of 100-μl bone marrow cell suspension was injected through the tail vein 18–24 h after irradiation. Mice were then housed in clean conventional housing for 6–8 wk to allow full bone marrow reconstitution before experimentation.
Statistical analysis.
All data in this paper were analyzed by 1-way ANOVA together with Tukey post hoc analysis. Analyses were conducted on a minimum of 3 animals and up to 17 animals in at least 3 separate experiments. Error bars indicate SE, and significance was set at P < 0.05.
RESULTS
Rac2 mediates airway neutrophil infiltration and lung injury.
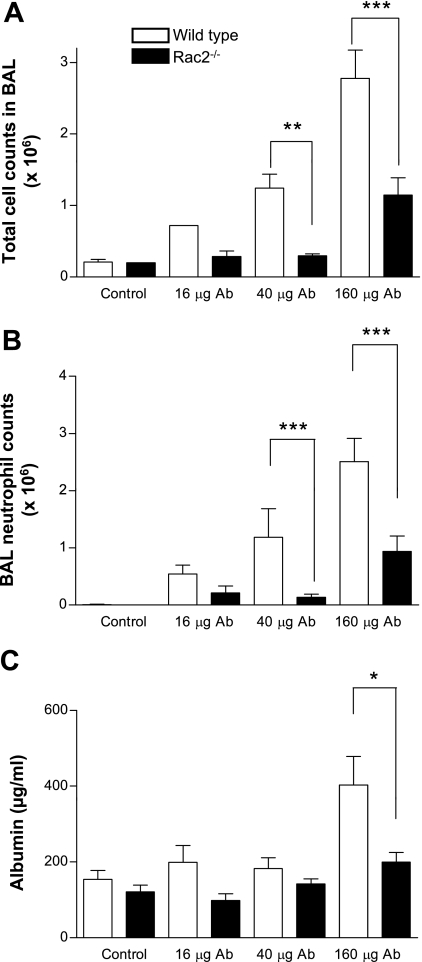
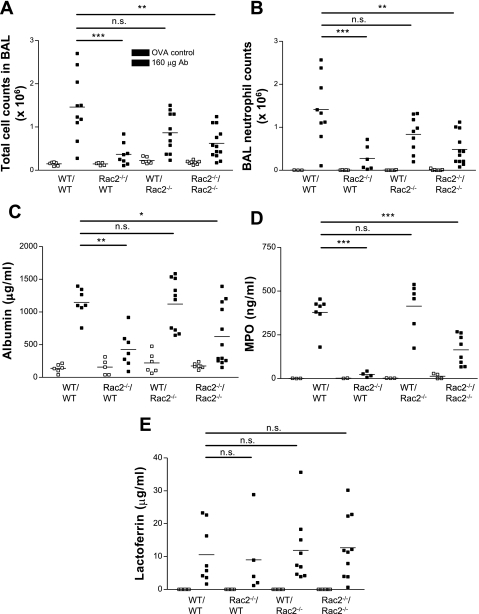
Lung injury was induced in alveolar spaces using the reverse passive Arthus reaction. Six hours after intratracheal administration of anti-ovalbumin IgG (16–160 μg) followed by intravenous tail vein injection of ovalbumin (400 μg), mice were euthanized and subjected to BAL. In WT mice, increased BAL leukocyte infiltration occurred, the majority of which were neutrophils (>90% at doses ≥40 μg of antibody; Fig. 1, A and B). In contrast, Rac2-deficient mice showed significantly less cellular inflammation and fewer alveolar neutrophils in response to increasing doses of antibody to ovalbumin. The decrease in total alveolar cell numbers in Rac2-deficient mice was entirely related to reduced neutrophils, as alveolar macrophage numbers did not vary between WT and Rac2-null animals in response to increasing antibody dosage. There were no significant increases in lymphocytes or dendritic cells in BAL samples in response to ALI. Animals did not show any mortality in response to these doses of antibody and ovalbumin. Increasing the doses of antibody and antigen led to a breakdown of lung tissue in WT animals, with widespread hemorrhage and injury. BAL samples from WT ALI contained a mixture of airway and systemic blood cells as well as structural cells. Thus multiple mechanisms unrelated to Rac2 may become activated when large doses are used, which would complicate our interpretations of the findings. Therefore, we chose to use the lower dose of 160 μg of anti-ovalbumin (with 400 μg of ovalbumin) in the following experiments to determine the specific role of Rac2 in lung inflammation and injury.
Fig. 1.
Rac2 is required for leukocyte infiltration and protein leakage in immune complex-mediated acute lung injury (ALI). Mice were treated with increasing doses of intratracheal anti-ovalbumin (40 μl) together with intravenous ovalbumin (400 μg). Control mice were subjected to sham surgery with intratracheally administered saline (40 μl) followed by intravenous ovalbumin (400 μg). Bronchoalveolar lavage (BAL) samples were analyzed from mice euthanized at 6 h postinjection for total cell counts (A), neutrophil counts (B), and albumin (C) in wild-type (WT) and rac2−/− mice (n = 11–17). *P < 0.05; **P < 0.01; ***P < 0.001. Ab, antibody.
Lung injury was measured by determining albumin concentrations in BAL fluid as an indication of plasma leakage. Albumin levels were increased in BAL from WT controls at the highest dose of anti-ovalbumin tested (160 μg; Fig. 1C). However, in Rac2-deficient animals, albumin did not increase over control (ovalbumin-injected) animals, indicating that injury was attenuated. These parameters remained similar at 24 h postinduction of ALI (data not shown).
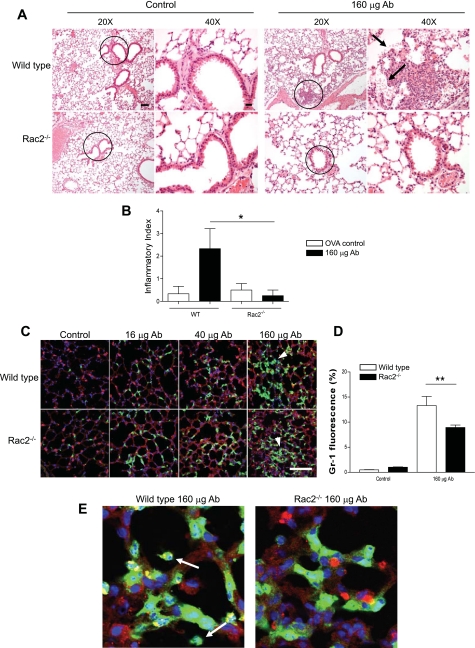
Lung parenchyma in WT and rac2−/− ALI was examined by histology (Fig. 2A). Quantitative histology on lung sections indicated that WT ALI consistently showed a patchy distribution of inflammatory foci, with focal hemorrhage, intraalveolar neutrophils, as well as neutrophilic inflammation in interstitial spaces, septal walls, and airway walls (Fig. 2B). These were significantly reduced in rac2−/− ALI, although some parenchymal neutrophilic accumulation was evident in these animals.
Fig. 2.
Neutrophil recruitment to lung parenchyma in WT and rac2−/− mice with ALI. A: lungs stained with hematoxylin and eosin (H&E) from WT and rac2−/− mice exhibited similar increases in leukocyte infiltration following administration of 160 μg of anti-ovalbumin, although alveolar hemorrhage was more prominent in WT lungs (arrows). The circles indicate the regions shown at higher magnification at right. Scale bars: low magnification, 50 μm; high magnification, 20 μm. B: quantitative histology of H&E-stained lung sections, carried out in blinded, unbiased analysis. Bar graph shows means ± SE of sections of lung measuring ≥200 mm2 (3–4 mice per condition). C: immunofluorescence of lung sections from mice treated with increasing doses of anti-ovalbumin. Sections were stained for the neutrophil marker Gr-1 (green) shown in combination with the epithelial cell marker surfactant protein C (SP-C; red) and counterstained with nuclear marker 4′,6′-diamidino-2-phenylindole (DAPI; blue). Scale bar, 100 μm. Arrowheads indicate clusters of interstitial neutrophils. D: percentage of Gr-1 fluorescence obtained from WT and rac2−/− mice in response to immune complexes. Average fluorescent percentages were obtained from 10 randomly selected fields from lung sections, and green fluorescence (Gr-1+ cells) was quantified using the Olympus FluoView confocal software program (n = 3 mice). *P < 0.05; **P < 0.01. E: higher magnification of images shown in B indicating extravasation of neutrophils (arrows) into alveolar spaces in WT but not rac2−/− lungs with ALI. OVA, ovalbumin.
Neutrophils are recruited to lung interstitium in Rac2-deficient mice.
Neutrophils from Rac2-deficient mice have been shown to have defective chemotaxis related to altered F-actin dynamics while expressing normal levels of adhesion molecules CD11a, CD11b, CD11c, CD18, and CD61 (19, 22, 41). To determine the extent of neutrophil recruitment to lungs in rac2−/− ALI, immunofluorescence of neutrophils (Gr-1+ cells) was determined in lung sections from mice with ALI. Neutrophil recruitment to the lung interstitium was observed in both WT and Rac2-deficient ALI (Fig. 2C). More neutrophils were found in the lung parenchyma of Rac2-deficient ALI than in control animals injected with ovalbumin only, as determined by the percentage of Gr-1 immunofluorescence averaged from 10 fields (at ×200 magnification) randomly chosen from midsagittal lung sections (Fig. 2D). At higher magnifications, Gr-1+ neutrophils in WT ALI were found in alveolar spaces; in contrast, in rac2−/− ALI, neutrophils were less evident in alveolar spaces (Fig. 2E).
Cytokines and chemokines in airways of Rac2-deficient mice are elevated during lung injury.
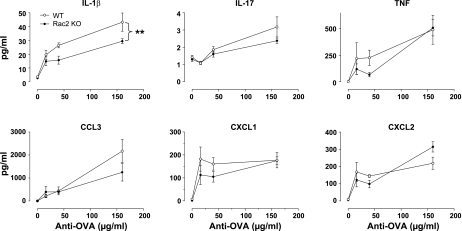
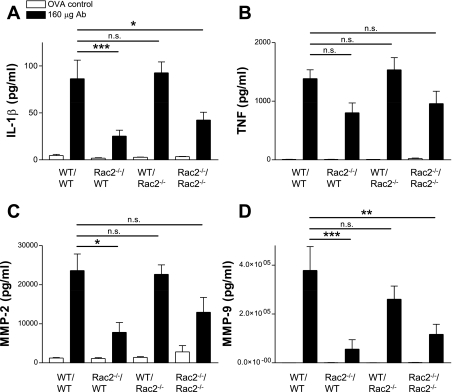
To investigate the possibility that reduction of neutrophil egression into the airways in Rac2-deficient mice may be related to reduced cytokine or chemokine release, we measured BAL levels of a selected group of immunomodulatory cytokines. IL-1β, TNF, and the murine homolog of human IL-8, CXCL1 (formerly keratinocyte-derived cytokine; KC) are critical for recruitment of neutrophils and initiating injury in this model (39). IL-17 released from T cells is a potent stimulus for stromal endothelial cells, leading to IL-8-dependent neutrophil recruitment (29). CXCL1 is required to establish a chemotactic gradient for neutrophils from damaged epithelium by binding to syndecan-1, generated by matrilysin-induced cleavage of this heparin sulphate proteoglycan bound to epithelial cells (33). In addition, CCL3 [formerly macrophage inflammatory protein-1α (MIP-1α)] and CXCL2 (formerly MIP-2) are associated with the onset of inflammation in immune complex-mediated ALI and are required for the recruitment of neutrophils (43, 44). As expected, all BAL cytokines measured in this study were significantly elevated over control values in WT mice with ALI, particularly at the highest dose of antibody used (160 μg; Fig. 3). However, there were no significant differences in TNF, IL-17, CCL3, CXCL1, or CXCL2 release between Rac2-deficient and WT ALI. One exception was IL-1β, which produced at lower levels in Rac2-deficient mice compared with WT ALI (P < 0.01 by 2-way ANOVA; n = 5), although it was still elevated above control values. These findings suggest that cytokine release in alveolar spaces, with the exception of IL-1β, was not significantly reduced in Rac2-deficient mice in this model of lung injury.
Fig. 3.
Cytokine and chemokines profiles in immune complex-mediated ALI from WT and rac2−/− mice. Values are shown for cytokines (IL-1β, IL-17, and TNF) and chemokines (CCL3, CXCL1, and CXCL2) in BAL samples from mice treated with increasing doses of anti-ovalbumin and 400-μg ovalbumin (n = 5). **P < 0.01. KO, knockout.
Rac2 is required for release of MPO and MMP-2 and MMP-9 in lung injury.
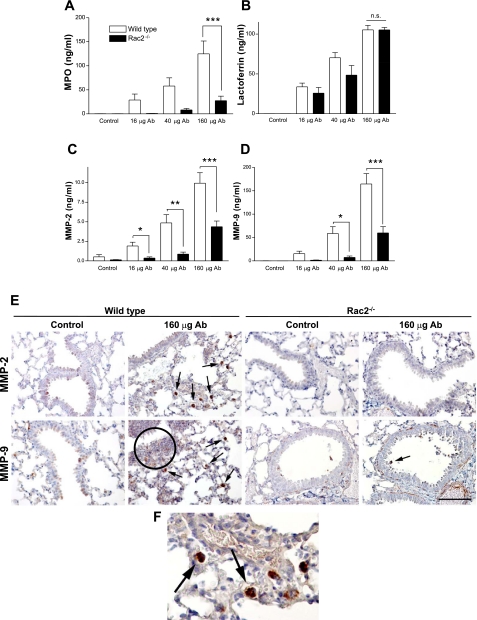
We next determined the levels of granule-derived mediators MPO, lactoferrin, and MMP-2/-9 in BAL samples obtained from WT and Rac2-deficient mice with ALI. In rac2−/− animals with ALI, MPO was reduced in BAL samples (Fig. 4A). In contrast, BAL levels of lactoferrin were elevated in both WT and Rac2-deficient mice during lung injury (Fig. 4B). These findings suggest that Rac2−/− neutrophils recruited to lung interstitial spaces release secondary granules, enriched in lactoferrin, into parenchymal tissues and alveolar spaces.
Fig. 4.
Proinflammatory mediator release in lung injury responses to immune complexes. WT and rac2−/− mice were treated with increasing doses of immune complexes, and levels of myeloperoxidase (MPO; A), lactoferrin (B), matrix metalloproteinase-2 (MMP-2; C), and MMP-9 (D) were assayed in BAL samples (n = 5). *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. E: immunohistochemistry of paraffin-embedded lung sections from WT and rac2−/− animals following control treatment (ovalbumin alone) and ALI, showing MMP-2 and MMP-9 immunoreactivity indicated by the red/brown diaminobenzidine (DAB) stain. Sections were counterstained by hematoxylin (blue) to show lung morphology. Arrows indicate alveolar macrophages that were positive for MMP-2 or MMP-9. F: magnified section of lung from WT ALI showing MMP-2 immunoreactivity in alveolar macrophages. Circle, region showing neutrophilic inflammation showing staining for MMP-9. Scale bar, 100 μm.
Interestingly, MMP-2 and MMP-9 were also significantly diminished in BAL from Rac2-deficient ALI (Fig. 4, C and D). Reduced activity of MMP-2/-9 in BAL samples from Rac2-deficient mice was confirmed by gelatin zymography (data not shown). In vitro experiments with Rac2−/− neutrophils demonstrated decreased MMP-2 and MMP-9 release on immune complex stimulation (P. Lacy, unpublished observations).
However, MMP-2 and MMP-9 expression was not specific to neutrophils, as determined by immunohistochemical analysis of lung sections (Fig. 4, E and F). Staining for MMP-2/-9 was not strongly evident in control WT or rac2−/− animals, although there was faint staining in bronchial epithelial cells. In WT ALI, elevated MMP-2 and MMP-9 expression was present in tissue inflammatory cells, predominantly in alveolar macrophages, with weaker staining associated with interstitial neutrophils (Fig. 4E, circle, and 4F, magnified inset). Although some staining was evident in airway epithelial cells in WT control and ALI lung sections, there was no marked increase in MMP-2 or MMP-9 in epithelial cells from ALI lungs. In contrast, lung sections from rac2−/− ALI showed greatly diminished levels of all cells positive for MMP-2 and MMP-9, particularly alveolar macrophages, in correlation with reduced numbers of MMP-2/-9-positive neutrophils.
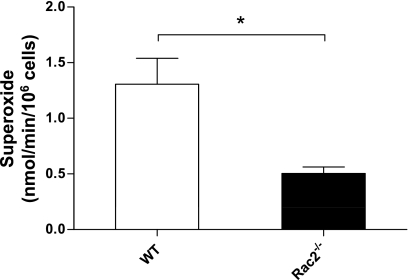
Since IgG-immune complex-mediated ALI has been shown to be dependent on neutrophil-derived oxidants (27), we tested the possibility that production of oxygen radicals may also be reduced in rac2−/− ALI. However, WT and rac2−/− ALI lung sections did not show increased antibody staining of the oxidant, nitrotyrosine, over control lungs, even at 160-μg antibody (data not shown). In addition, we tested for oxidative stress markers (using commercial assays from Hycult for nitrotyrosine and from OxisResearch for lipid peroxidation markers and oxidized glutathione) in BAL samples and found no increases in WT or rac2−/− ALI mice over control animals. Specifically, each of the nitrotyrosine, lipid peroxidation, and kinetic GSH/GSSG assays demonstrated robust standard curve responses, but none of the BAL samples from sham-treated and injured WT and rac2−/− mice showed significant increases above baseline absorbance values (data not shown). However, when bone marrow neutrophils were isolated from WT and rac2−/− mice and stimulated in vitro to generate superoxide by measurement of cytochrome c reduction, significant attenuation in superoxide production was detected in Rac2−/− neutrophils (Fig. 5). These data suggest that neutrophils derived from rac2−/− bone marrow were deficient in generating oxidants, although we did not find significant changes in the levels of products of oxidant-related damage in the airways.
Fig. 5.
Superoxide generation from bone marrow neutrophils obtained from WT and rac2−/− mice. Freshly isolated bone marrow neutrophils were stimulated with PMA (500 ng/ml), and O2•− production was measured using cytochrome c reduction by spectrophotometry. *P < 0.05 (n = 4).
Defect in transepithelial migration is associated with hematopoietic cells.
Transepithelial migration of neutrophils in Rac2-deficient mice may be related to a defect in Rac2 expression in hematopoietic and/or nonhematopoietic tissues. To investigate this, we utilized adoptive transfer of marrow cells from Rac2-deficient mice into lethally irradiated WT mice to generate chimeras. These were compared with irradiated rac2−/− mice reconstituted with CD45.1+ WT bone marrow. As controls, we reconstituted irradiated WT mice with CD45.1+ WT bone marrow and Rac2-deficient recipient mice with Rac2-deficient marrow. This chimeric model has been shown to reconstitute 99% of peripheral blood neutrophils (16). After bone marrow reconstitution (6–8 wk), chimeras were subjected to ALI using 160-μg anti-ovalbumin and 400-μg ovalbumin and compared with ovalbumin-only controls. This dose of antibody was chosen for its ability to increase vascular leakage in WT mice (Fig. 1C). To confirm marrow replacement with donor cells in the airways during ALI, we determined the percentage of CD45.1+/Gr-1+ cells in BAL fluid from CD45.1+/CD45.1− chimeras. We found that 82 ± 5% of Gr-1+ BAL neutrophils were from donor animals (CD45.1+/Gr-1+) in WT/WT chimeras with ALI (n = 8). This did not differ significantly from WT/Rac2−/−, which had 79 ± 8% CD45.1+/Gr-1+ neutrophils in their BAL fluid following ALI (n = 5). There were insignificant levels of CD45.1+/Gr-1+ neutrophils detected in BAL fluid from WT/WT or WT/Rac2−/− control animals or from chimeras that had received rac2−/− bone marrow transplants. These observations indicate that the majority of neutrophils in the airways of WT/WT and WT/Rac2−/− chimeras with ALI were derived from CD45.1+ donor bone marrow, confirming marrow engraftment along with neutrophil proliferation and recruitment to the airways during ALI. In addition, engraftment and recruitment of WT bone marrow in rac2−/− recipients appeared to be similar to WT/WT chimeras, indicating that there were no defects in WT donor neutrophil reconstitution or accumulation in the lungs of WT/Rac2−/− animals with ALI.
In bone marrow chimeras, WT mice reconstituted with WT bone marrow (WT/WT) exhibited significant increases in total cell numbers in BAL samples in ALI, most of which were neutrophils (Fig. 6, A and B). The numbers of alveolar macrophages did not vary between any of the control and ALI chimeras. This suggests that the numbers of macrophages recruited to the airways were unaffected by immune complex activation in bone marrow chimeras. In contrast, irradiated WT mice injected with Rac2-deficient marrow (Rac2−/−/WT) had reduced BAL total cell numbers compared with WT/WT ALI, which was largely the result of decreased neutrophil numbers, with no significant changes in alveolar macrophages, dendritic cells, or lymphocytes. Irradiated Rac2-deficient mice reconstituted with WT bone marrow (WT/Rac2−/−) did not significantly differ in BAL cell numbers from WT/WT ALI, suggesting that Rac2-dependent inflammation and injury in this model was specifically dependent on hematopoietically derived cells. Rac2-deficient mice reconstituted with Rac2-deficient marrow (Rac2−/−/Rac2−/−) exhibited reduced inflammation similar to that found in WT mice given Rac2-deficient bone marrow (Rac2−/−/WT).
Fig. 6.
Immune complex-mediated ALI in chimeras of WT and rac2−/− mice. Total cell counts (A) and neutrophil counts (B) in BAL samples from irradiated WT mice reconstituted with WT marrow (WT/WT) or rac2−/− marrow (Rac2−/−/WT) were compared with irradiated rac2−/− mice injected with WT marrow (WT/Rac2−/−) or rac2−/− marrow (Rac2−/−/Rac2−/−). Levels of albumin (C), MPO (D), and lactoferrin (E) are also shown from the same samples. *P < 0.05; **P < 0.01; ***P < 0.001.
Similar trends of vascular leakage (albumin) and MPO release were observed in WT/WT compared with WT/Rac2−/− chimeras, which were significantly reduced in Rac2−/−/WT and Rac2−/−/Rac2−/− chimeras (Fig. 6, C–E). Lactoferrin levels were similarly elevated in all chimeras with ALI, suggesting neutrophil activation. An apparent increase in alveolar MPO was observed in Rac2−/−/Rac2−/− chimeras with ALI compared with Rac2−/−/WT chimeras with ALI; however, this was not significant based on ANOVA and post hoc analysis.
Levels of IL-1β in BAL samples were increased in WT/WT ALI over Rac2−/−/WT ALI, although TNF secretion was similar across all chimeras (Fig. 7, A and B). MMP-2/-9 levels were also reconstituted in WT/WT mice compared with Rac2−/−/WT ALI (Fig. 7, C and D). These findings suggest that alveolar neutrophil recruitment and subsequent lung injury in response to immune complex-mediated ALI and release of MPO, IL-1β, and MMP-2/-9 are dependent on Rac2-expressing hematopoietic cells.
Fig. 7.
Release of cytokines and metalloproteases in BAL samples from chimeras. IL-1β (A), TNF (B), MMP-2 (C), and MMP-9 (D) release was determined in BAL samples obtained from WT and rac2−/− chimeras (n = 6–9). *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
In this study, we show that the Rho GTPase, Rac2, has an essential role in the pathogenesis of ALI. Disruption of the gene encoding Rac2 significantly attenuated lung inflammation and injury induced by immune complex deposition. Neutrophils were observed to accumulate in interstitial lung tissues during ALI in rac2−/− animals, but few transmigrated into alveolar spaces. The levels of alveolar MPO and MMP-2/-9 were attenuated in rac2−/− mice with ALI. Alveolar macrophages expressing MMP-2/-9 were prominent in WT ALI but were not evident in lungs from rac2−/− mice. These observations indicate that Rac2 may be involved in mediating MMP production from alveolar macrophages and neutrophils as well as neutrophil migration into alveolar spaces, resulting in lung injury. Although disruption of the gene encoding Rac2 attenuated immune complex-mediated lung injury, it did not abolish the pathology. At higher doses of antibody in rac2−/− mice with ALI, increased alveolar neutrophil counts were observed, suggesting that other pathways may compensate for Rac2-mediated cell transmigration in this model, such as Rac1, Rac3, Cdc42, and Rho.
ALI and ARDS are characterized by interstitial lung inflammation and injury. Inflammation in these disorders is associated with infiltration of leukocytes including monocytes/macrophages and neutrophils and involves endothelial and epithelial barrier dysfunction. Recruited leukocytes generate proinflammatory mediators, leading to edema and alveolar hemorrhage, a process associated with clinical findings of ALI defined by rapid-onset bilateral pulmonary infiltrates and hypoxemia (4, 36). Immune complex-mediated ALI is an established model that most closely resembles transfusion-related ALI (TRALI), which is dependent on Fcγ receptor activation by IgG complexed to antigen or cellular receptors (34, 45). Lung injury in this model has previously been shown to be dependent on activated neutrophils releasing reactive oxygen species and granule products (7, 23, 26–28).
In murine models of IgG immune complex-mediated ALI, the inflammatory cascade begins with cross-linking of FcγRIII receptors (CD16) on macrophages by immune complexes along with immune complex-mediated production of complement C5a (25, 46). Alveolar macrophages are activated and quickly release proinflammatory cytokines TNF and IL-1β (23), upregulating selectins and ICAM-1 on vascular endothelial cells to facilitate adhesion of neutrophils and their transmigration to sites of immune complex deposition (47). In addition, activated alveolar macrophages generate a range of chemokines to attract neutrophils, including CCL3, CXCL1, and CXCL2 (6, 18, 23). Neutrophils are recruited from the bloodstream and are activated to release reactive oxygen species and toxic granule-derived mediators including MPO and lactoferrin (7, 23, 26–28). Other mediators released from a variety of lung cells that contribute to injury include MMP-2 and MMP-9 (21).
Lung injury induced by immune complexes is dependent on MMP-2/-9, released by many cells, including macrophages, fibroblasts, smooth muscle cells, and neutrophils (14, 21). Macrophages are the main cell types that express MMP-2/-9 during immune complex-mediated ALI (21, 38). We found elevated levels of MMP-2/-9-positive cells in lungs from WT ALI, with the majority of these cells being morphologically similar to alveolar macrophages and interstitial neutrophils. In contrast, MMP-2/-9-positive cells were infrequently observed in rac2−/− ALI. These findings suggest that Rac2 has a previously unrecognized role in generating MMP-2 and MMP-9 from alveolar macrophages and neutrophils that may contribute to lung injury. Rac2 contributes to macrophage phagocytosis and superoxide generation (49). In addition, as with rac2−/− ALI, MMP-2- and MMP-9-deficient mice exhibit accumulation of neutrophils in the lung interstitium, but not in BAL samples, in a model of allergic lung inflammation (12, 13). This suggests a defect in transepithelial migration of leukocytes in both rac2−/− mice and MMP-2/-9-deficient mice. Indeed, Rac2-mediated production of MMP-2/-9 may be critical for injury as well as neutrophil transmigration across basement membranes and the epithelial barrier (15).
There are several possible mechanisms by which gene deletion of Rac2 could lead to reduced injury in ALI. The restricted expression of Rac2 to hematopoietic tissues and our finding regarding the reconstitution of injury in WT/Rac2−/− chimeras with ALI suggest that Rac2 mainly functions in macrophages, neutrophils, and potentially other cells derived from bone marrow. Based on our immunohistochemistry for MMP-2/-9 in lung sections (Fig. 4F), we speculate that Rac2 may be a regulatory GTPase for exocytosis of secretory products including MMP-2/-9 from macrophages and neutrophils. Other possible mechanisms for Rac2 in ALI include F-actin-mediated neutrophil chemotaxis and recruitment as well as NADPH oxidase-associated oxidant production from neutrophils. All of these events may collectively serve to diminish recruitment and activation of inflammatory cells to the airways in response to immune complexes.
In neutrophils, the function of Rac2 is related to adhesion, chemotaxis, activation of NADPH oxidase, and primary granule release (2, 9, 10, 41). In the present study, neutrophil numbers were increased in lung interstitium, although they were not elevated in the alveolar spaces in rac2−/− mice with ALI. Since rac2−/− mice have a 2.5- to 3-fold higher level of circulating neutrophils than their WT counterparts (41), reduced neutrophil numbers in BAL samples in rac2−/− mice are not likely related to lower circulation numbers. It is conceivable that altered neutrophil actin remodeling, an expected phenotype of Rac2 deficiency, might cause dysregulated neutrophil retention in pulmonary capillaries (48). There are three possible stages that could be affected in transepithelial migration of neutrophils: adhesion, transmigration, and migration. Rac2−/− neutrophil adhesion has been shown to be reduced to glycosylation-dependent cell adhesion molecule 1 (GlyCAM-1) and P-selectin, and Rac2−/− cells show defective chemotactic responses to formyl-Met-Leu-Phe and IL-8 (41). A limitation of the findings in this study is that we could not determine where the defect in the recruitment cascade occurred in rac2−/− mice with ALI.
Similar levels of proinflammatory cytokines (TNF and IL-17) and chemokines (CCL3, CXCL1, and CXCL2) were found in the BAL fluid of immune complex-treated WT and rac2−/− mice, with the exception of IL-1β. Neutrophil retention in the lung parenchyma of rac2−/− mice was not likely to be caused by deficient IL-1β production, since antibody to IL-1β prevented neutrophil accumulation in the lungs, thereby producing a different phenotype from that of rac2−/− mice with ALI (39).
Rac2 is an essential regulatory protein for NADPH oxidase activation in neutrophils (41). The model of IgG immune complex-mediated ALI used in this study is specifically dependent on production of toxic oxygen metabolites from neutrophils (27). Application of intratracheal catalase to scavenge H2O2 from alveolar spaces prevented alveolar hemorrhage and edema in ALI but did not reduce neutrophil recruitment to interstitial tissues. In contrast, SOD, a highly specific scavenger for superoxide, did not reduce lung injury in this model (27). We could not detect oxygen metabolites in lung tissue from animals with ALI by nitrotyrosine labeling (generated from peroxynitrite reaction with tyrosine residues) or assay for oxidative stress products. Evidence from our study suggests that bone marrow neutrophils from rac2−/− mice were deficient in generating superoxide, in agreement with previous reports (40, 41). Therefore, it is possible that reduced injury in rac2−/− mice with ALI is related to the defect in superoxide release from neutrophils, although we could not detect products of oxidant-related activity in the lungs to confirm this. The inability to detect oxidant-related injury in this mouse model differs from previous studies using a rat model, on which this study was based, by Gao and colleagues (20). These findings suggest that there may be a species difference in the dependency of lung injury on the production of toxic oxygen metabolites, which has not been reported before. Of interest, clinical findings demonstrate that antioxidants fail to reverse or attenuate ALI (17).
We found significantly diminished levels of MPO in BAL from rac2−/− ALI compared with ALI in WT mice. In contrast, secondary granule lactoferrin was elevated in WT and rac2−/− mice with ALI, suggesting that Rac2−/− neutrophils in the parenchyma were releasing secondary granules in response to immune complex stimulation. Although it is possible that MPO and MMP levels were diminished due to fewer neutrophils in the BAL, our finding of high levels of lactoferrin suggest that this mediator diffused from activated tissue neutrophils into alveolar spaces where it was detected in BAL samples. These observations correlate with in vitro findings showing a dependency of primary granule exocytosis on Rac2, whereas secondary granule release of lactoferrin was independent of Rac2 function (2).
Bone marrow chimera data generated from WT and rac2−/− mice with ALI demonstrated that reduced lung injury was associated with bone marrow-derived, Rac2-expressing hematopoietic cells. The number of BAL cells in WT/Rac2−/− chimera, compared with WT/WT ALI, was significantly diminished during lung injury. However, these chimera do not distinguish between the various hematopoietic cell types that express Rac2, including macrophages and neutrophils, which are fully reconstituted by donor cells in bone marrow chimeras by 6–8 wk after irradiation (37). With regard to the apparent increase in BAL MPO in Rac2−/−/Rac2−/− chimeras with ALI, it is important to note that total cells and neutrophils were also slightly elevated in rac2−/− mice with ALI at the same dose of antibody used for the chimera experiments (160 μg).
In chimera experiments using irradiated animals, WT/WT and WT/Rac2−/− animals with ALI showed an increased density of total cells and neutrophils, along with elevated albumin and cytokine levels in BAL, compared with nonirradiated WT and rac2−/− mice with ALI. It is speculated that these increased levels were related to radiation treatment, which systemically enhances proinflammatory cytokine expression.
In summary, Rac2 gene deletion significantly attenuated lung inflammation and injury induced by immune complex deposition. A salient finding in this study is that Rac2 may be important for MMP-2 and MMP-9 generation by alveolar macrophages and neutrophils as well as for neutrophil transmigration into the alveolar spaces during injury. Based on our observations, we have shown that Rac2-mediated injury in this model may not be dependent on the production of toxic oxygen metabolites in the lungs. These findings suggest a novel function for Rac2 in acute inflammatory processes and provide potential targets for therapeutic intervention in ALI and its associated complications.
GRANTS
This study was supported by Canada Foundation for Innovation, the Canadian Institutes of Health Research, SickKids Foundation, Alberta Heritage Foundation for Medical Research, Natural Sciences and Engineering Research Council of Canada, National Institutes of Health Grant HL-027068, and the Canadian Lung Association.
DISCLOSURES
The authors have no conflict of interest to declare for this study.
ACKNOWLEDGMENTS
We thank Robin Stocks and Tom Turner in the Department of Pathology for their assistance with immunohistochemistry and generating images of lung sections.
REFERENCES
- 1.Abdel-Latif D, Steward M, Lacy P. Neutrophil primary granule release and maximal superoxide generation depend on Rac2 in a common signalling pathway. Can J Physiol Pharmacol 83: 69– 75, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P. Rac2 is critical for neutrophil primary granule exocytosis. Blood 104: 832– 839, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Abraham E. Neutrophils and acute lung injury. Crit Care Med 31: S195– S199, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Atabai K, Matthay MA. Acute lung injury and the acute respiratory distress syndrome: definitions and epidemiology. In: Respiratory Management in Critical Care, edited by Griffiths JD, Evans TW. London: BMJ Books, 2004, p. 31– 37 [Google Scholar]
- 5.Baumann U, Kohl J, Tschernig T, Schwerter-Strumpf K, Verbeek JS, Schmidt RE, Gessner JE. A codominant role of Fc gamma RI/III and C5aR in the reverse Arthus reaction. J Immunol 164: 1065– 1070, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bless NM, Huber-Lang M, Guo RF, Warner RL, Schmal H, Czermak BJ, Shanley TP, Crouch LD, Lentsch AB, Sarma V, Mulligan MS, Friedl HP, Ward PA. Role of CC chemokines (macrophage inflammatory protein-1 beta, monocyte chemoattractant protein-1, RANTES) in acute lung injury in rats. J Immunol 164: 2650– 2659, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Bless NM, Smith D, Charlton J, Czermak BJ, Schmal H, Friedl HP, Ward PA. Protective effects of an aptamer inhibitor of neutrophil elastase in lung inflammatory injury. Curr Biol 7: 877– 880, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol 15: 163– 171, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Carstanjen D, Yamauchi A, Koornneef A, Zang H, Filippi MD, Harris C, Towe J, Atkinson S, Zheng Y, Dinauer MC, Williams DA. Rac2 regulates neutrophil chemotaxis, superoxide production, and myeloid colony formation through multiple distinct effector pathways. J Immunol 174: 4613– 4620, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med 189: 179– 185, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corry DB, Kiss A, Song LZ, Song L, Xu J, Lee SH, Werb Z, Kheradmand F. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J 18: 995– 997, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corry DB, Rishi K, Kanellis J, Kiss A, Song Lz LZ, Xu J, Feng L, Werb Z, Kheradmand F. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol 3: 347– 353, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.d'Ortho MP, Clerici C, Yao PM, Delacourt C, Delclaux C, Franco-Montoya ML, Harf A, Lafuma C. Alveolar epithelial cells in vitro produce gelatinases and tissue inhibitor of matrix metalloproteinase-2. Am J Physiol Lung Cell Mol Physiol 273: L663– L675, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Delclaux C, Delacourt C, d'Ortho MP, Boyer V, Lafuma C, Harf A. Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am J Respir Cell Mol Biol 14: 288– 295, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 118: 3301– 3315, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esper AM, Martin GS. Evolution of treatments for patients with acute lung injury. Expert Opin Investig Drugs 14: 633– 645, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Fernandez N, Renedo M, Garcia-Rodriguez C, Sanchez CM. Activation of monocytic cells through Fc gamma receptors induces the expression of macrophage-inflammatory protein (MIP)-1 alpha, MIP-1 beta, and RANTES. J Immunol 169: 3321– 3328, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Filippi MD, Harris CE, Meller J, Gu Y, Zheng Y, Williams DA. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat Immunol 5: 744– 751, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Gao H, Neff T, Ward PA. Regulation of lung inflammation in the model of IgG immune-complex injury. Annu Rev Pathol 1: 215– 242, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Gibbs DF, Shanley TP, Warner RL, Murphy HS, Varani J, Johnson KJ. Role of matrix metalloproteinases in models of macrophage-dependent acute lung injury. Evidence for alveolar macrophage as source of proteinases. Am J Respir Cell Mol Biol 20: 1145– 1154, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 302: 445– 449, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Guo RF, Ward PA. Mediators and regulation of neutrophil accumulation in inflammatory responses in lung: insights from the IgG immune complex model. Free Radic Biol Med 33: 303– 310, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Haataja L, Groffen J, Heisterkamp N. Characterization of RAC3, a novel member of the Rho family. J Biol Chem 272: 20384– 20388, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Hazenbos WL, Heijnen IA, Meyer D, Hofhuis FM, Renardel de Lavalette CR, Schmidt RE, Capel PJ, van de Winkel JG, Gessner JE, van den Berg TK, Verbeek JS. Murine IgG1 complexes trigger immune effector functions predominantly via Fc gamma RIII (CD16). J Immunol 161: 3026– 3032, 1998 [PubMed] [Google Scholar]
- 26.Johnson KJ, Ward PA. Acute immunologic pulmonary alveolitis. J Clin Invest 54: 349– 357, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson KJ, Ward PA. Role of oxygen metabolites in immune complex injury of lung. J Immunol 126: 2365– 2369, 1981 [PubMed] [Google Scholar]
- 28.Johnson KJ, Wilson BS, Till GO, Ward PA. Acute lung injury in rat caused by immunoglobulin A immune complexes. J Clin Invest 74: 358– 369, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity 21: 467– 476, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Lacy P. The role of Rho GTPases and SNAREs in mediator release from granulocytes. Pharmacol Ther 107: 358– 376, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Lacy P, Abdel-Latif D, Steward M, Musat-Marcu S, Man SF, Moqbel R. Divergence of mechanisms regulating respiratory burst in blood and sputum eosinophils and neutrophils from atopic subjects. J Immunol 170: 2670– 2679, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Lacy P, Mahmudi-Azer S, Bablitz B, Hagen SC, Velazquez JR, Man SF, Moqbel R. Rapid mobilization of intracellularly stored RANTES in response to interferon-γ in human eosinophils. Blood 94: 23– 32, 1999 [PubMed] [Google Scholar]
- 33.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111: 635– 646, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. J Clin Invest 116: 1615– 1623, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukacs NW, Glovsky MM, Ward PA. Complement-dependent immune complex-induced bronchial inflammation and hyperreactivity. Am J Physiol Lung Cell Mol Physiol 280: L512– L518, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 167: 1027– 1035, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Matute-Bello G, Lee JS, Frevert CW, Liles WC, Sutlief S, Ballman K, Wong V, Selk A, Martin TR. Optimal timing to repopulation of resident alveolar macrophages with donor cells following total body irradiation and bone marrow transplantation in mice. J Immunol Methods 292: 25– 34, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Mehta S. The effects of nitric oxide in acute lung injury. Vascul Pharmacol 43: 390– 403, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Mulligan MS, Ward PA. Immune complex-induced lung and dermal vascular injury. Differing requirements for tumor necrosis factor-alpha and IL-1. J Immunol 149: 331– 339, 1992 [PubMed] [Google Scholar]
- 40.Price MO, Atkinson SJ, Knaus UG, Dinauer MC. Rac activation induces NADPH oxidase activity in transgenic COSphox cells, and the level of superoxide production is exchange factor-dependent. J Biol Chem 277: 19220– 19228, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity 10: 183– 196, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685– 1693, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Shanley TP, Schmal H, Friedl HP, Jones ML, Ward PA. Role of macrophage inflammatory protein-1 alpha (MIP-1 alpha) in acute lung injury in rats. J Immunol 154: 4793– 4802, 1995 [PubMed] [Google Scholar]
- 44.Shanley TP, Schmal H, Warner RL, Schmid E, Friedl HP, Ward PA. Requirement for C-X-C chemokines (macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant) in IgG immune complex-induced lung injury. J Immunol 158: 3439– 3448, 1997 [PubMed] [Google Scholar]
- 45.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood 105: 2266– 2273, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Sylvestre DL, Ravetch JV. Fc receptors initiate the Arthus reaction: redefining the inflammatory cascade. Science 265: 1095– 1098, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Ward PA, Lentsch AB. Endogenous regulation of the acute inflammatory response. Mol Cell Biochem 234–235: 225– 228, 2002 [PubMed] [Google Scholar]
- 48.Worthen GS, Schwab B, 3rd, Elson EL, Downey GP. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science 245: 183– 186, 1989 [DOI] [PubMed] [Google Scholar]
- 49.Yamauchi A, Kim C, Li S, Marchal CC, Towe J, Atkinson SJ, Dinauer MC. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J Immunol 173: 5971– 5979, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Zeiher BG, Artigas A, Vincent JL, Dmitrienko A, Jackson K, Thompson BT, Bernard G. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med 32: 1695– 1702, 2004 [DOI] [PubMed] [Google Scholar]