Abstract
All forms of chronic pulmonary hypertension (PH) are characterized by structural remodeling of the pulmonary artery (PA) media, a process previously attributed solely to changes in the phenotype of resident smooth muscle cells (SMC). However, recent experimental evidence in both systemic and pulmonary circulations suggests that other cell types, including circulating and local progenitors, contribute significantly to this process. The goal of this study was to determine if hypoxia-induced remodeling of distal PA (dPA) media involves the emergence of cells with phenotypic and functional characteristics distinct from those of resident dPA SMC and fibroblasts. In vivo, in contrast to the phenotypically uniform SMC composition of dPA media in control calves, the remodeled dPA media of neonatal calves with severe hypoxia-induced PH comprised cells exhibiting a distinct phenotype, including the expression of hematopoetic (CD45), leukocytic/monocytic (CD11b, CD14), progenitor (cKit), and motility-associated (S100A4) cell markers. Consistent with these in vivo observations, primary cell cultures isolated from dPA media of hypertensive calves yielded not only differentiated SMC, but also smaller, morphologically rhomboidal (thus termed here “R”) cells that transiently expressed CD11b, constitutively expressed the mesenchymal cell marker type I procollagen, expressed high mRNA levels of progenitor cell markers cKit, CD34, CD73, as well as for inflammatory mediators, IL-6 and MCP-1, and, with time in culture, gained expression of a myofibroblast marker, α-SM-actin. R cells exhibited highly augmented proliferative, migratory, invasive, and potent promitogenic capabilities, which were due, at least in part, to the production of PDGFs, SDF-1/CXCL12, and S100A4. These data suggest that the cellular mechanisms of dPA remodeling include the emergence of cells with phenotypic and functional characteristics markedly distinct from those of resident dPA cells.
Keywords: pulmonary hypertension, vascular remodeling, progenitor cells, inflammation, S100A4
chronic pulmonary hypertension (PH) is characterized by profound remodeling of the pulmonary artery (PA) wall (29, 64, 73). The remodeling is complex, and all three vascular layers (intima, media, and adventitia) can be involved, albeit in different ways and degrees depending on the location of the vessel studied, the injurious stimulus involved, and the age of the patient. In neonatal forms of PH, remodeling of the PA tunica media is prominent and was traditionally believed to be the result of dedifferentiation and subsequent hyperplasia of arterial smooth muscle cells (SMC) (31, 66). However, the additional possibility that cells other than resident differentiated SMC contribute to medial remodeling has been raised in a variety of experimental vascular injury settings. These possibilities include, but are not limited to: 1) recruitment and differentiation of circulating progenitor cells (6, 9, 17, 22, 24, 41, 52, 57, 61, 63, 70, 75, 79), 2) activation and differentiation of resident vascular progenitor cells (13, 26, 28, 32, 47, 81), 3) activation and migration of adventitial fibroblasts to the media and even intima (56, 60, 62, 80), and 4) transdifferentiation of endothelial-like cells into mesenchymal cells (1, 20). At present, it remains unclear if any of these cellular mechanisms contribute to PA media remodeling in PH, but a number of studies have recently emerged in both humans and animal models documenting the presence of cells expressing hematopoietic, progenitor-like, and motility-associated antigens in the intima and media of remodeled pulmonary vessels (21–23, 38, 42, 74).
The goal of the present study was to, first, evaluate in vivo, in the setting of neonatal hypoxia-induced PH, whether remodeling of the distal PA (dPA) media is associated with emergence of cells exhibiting phenotypic characteristics distinct from those of resident dPA cells. If so, our goal was to compare, in vitro, their functional capabilities to those of resident dPA wall cells. As the animal model of neonatal hypoxic PH, we used neonatal calves, which develop severe PH and marked PA medial thickening in response to chronic hypoxia (17, 64, 65). We focused our analysis on distal elastic PAs of 600–1,500 μm in diameter, since we have previously shown that the tunica media of these PAs is normally composed of a uniform population of differentiated SMCs (67), and, therefore, any changes in the cellular composition of the vessel wall (such as emergence of cells phenotypically distinct from resident PA cells) would be noticeable. We performed immunostaining of dPA tissues from control and hypertensive calves to detect the appearance of hematopoetic, leukocytic, and progenitor-like cells. Ex vivo, cell cultures of dPA media from the same animals were isolated, and subsequent morphological, immunohistochemical, RT-PCR, protein, and functional (proliferative, promitogenic, and migratory) assessments were performed.
METHODS
Animal model.
The neonatal calf model of severe hypoxic pulmonary hypertension has been described previously (1, 15, 20) and includes the development of PA pressure equal to, or exceeding, systemic pressure as well as remarkable PA remodeling with medial and adventitial thickening being prominent along with periadventitial inflammation, resembling that of human neonatal PH (22, 29, 31, 73). One-day-old male Holstein calves were purchased from Laluna Dairy Farm (Fort Collins, CO). The experimental group (n = 7) was exposed to hypobaric hypoxia (PB = 445 mmHg) for 2 wk, whereas age-matched controls (n = 6) were kept at ambient altitude (PB = 640 mmHg) (64). Standard veterinary care was used following institutional guidelines, and the procedure was Institutional Animal Care and Use Committee approved (Dept. of Physiology, School of Veterinary Medicine, Colorado State Univ., Fort Collins, CO). Animals were euthanized by overdose of pentobarbital sodium (160 mg/kg body wt).
Immunofluorescent analysis.
Immunofluorescent staining was performed as previously described (18). Antibodies (Abs) against the following antigens were used: CD45, CD11b, CD14 (15 μg/ml; VMRD, Pullman, WA), CD68 (clone EBM11) and cKit (rabbit polyclonal Abs) (both at 1:100, from Dako, Carpinteria, CA), S100A4 (rabbit polyclonal Abs, Ab-8, 1:100; Thermo Fisher Scientific, Fremont, CA), α-SM-actin (clone 1A4, Sigma-Aldrich, St. Louis, MO), SM-myosin heavy chains (rabbit polyclonal Abs, a generous gift from Dr. R. Adelstein, National Institutes of Health), procollagen type I (clone Sp1.D8, Developmental Studies Hybridoma Bank, Iowa City, IA), heat-shock protein 47 (Hsp47; M16.10A1, Calbiochem, San Diego, CA), BrdU (clone BU-33, 1:200, Sigma Aldrich). For enhanced immunostaining of α-SM-actin, biotin-streptavidin system was used (Invitrogen, Carlsbad, CA). Immunolabeled sections were mounted in VectaShield with DAPI (Vector Labs) and examined under a Ziess fluorescent microscope with an AxioVision digital imaging system.
Isolation of cells.
Isolation of cells was performed from dPAs of an external diameter of 670 μm-1,340 μm (means 994 ± 25 μm) as previously described (65) from both control normotensive and chronically hypoxic hypertensive calves using explant techniques (65). Briefly, the bronchus entering the lung lobe was cut open and followed (under the dissecting microscope) through several branching points all the way to the tip of the lung. Next, the bronchus was removed and the underlying PA was exposed. The smaller PA branches were followed until the desired size (∼1 mm in diameter) was identified. These dPAs were dissected out and placed into PBS for further size evaluation and processing. The external diameter was measured under the microscope, and the selected dPAs were pinned to the bottom of a polymer-coated Petri dish for further cleaning of tunica media. First, all the remaining pieces of lung parenchyma were removed. Next the adventitia was thoroughly removed so that the artery appeared “smooth” on the outside. These dPAs were cut into pieces of ∼1/16 in length, and each piece was placed on the bottom of a well into a 24-well cell culture plate for the explant technique method of cell isolation (15). Briefly, after arterial pieces appeared adhered to plastic, growth medium was gently added, and explants were left undisturbed in the incubator (5% CO2) for a period of 5–10 days (cells from the dPAs of hypertensive calves were found to migrate out from the tissue pieces faster than those from control animals). When a substantial number of cells had migrated out, the tissue piece was removed, and cells were allowed to expand until ready for passaging. Cells were expanded in complete DMEM (Sigma-Aldrich) with 10% calf serum (CS; HyClone Laboratories, Logan, UT). Colonies of cells morphologically distinct from dPA-SMC were selectively isolated using Teflon “cloning” rings (15). All experimental assays were performed on cells at passages 4–6.
Cell size analysis.
Cell size analysis was performed by forward scatter expressed as channel number (33). Cell cultures were analyzed in a log phase of growth (10% CS) and under growth arrest (0.1% CS, 72 h). Cells were diluted in Isoton reagent (Beckman-Coulter), and relative size distribution was measured on a Coulter EPICS Flow Cytometer (Hialeah, FL).
Cell proliferation analyses.
Cell proliferation analyses were performed either in the format of a growth curve (16) or as assessment of BrdU incorporation. Generation of a growth curve was performed as previously described (16). Briefly, cells were plated onto 24-multiwell plates at initial density of 20 × 103 cells per well in DMEM supplemented with 10% CS. For the growth curve under serum-supplemented conditions, cells were maintained in 10% CS throughout the time course. For the growth curves under serum-free conditions, cells, plated in 10% CS-DMEM on day 0, were rinsed in PBS, and medium was replaced with DMEM supplemented with 0.2% plasma-derived serum (PDS; Cocalico Biologicals, Reamstown, PA), which is depleted of growth factors present in serum via charcoal stripping. During the 2-wk period, with the intervals of 2–4 days, cells in four wells were tripsinized and counted in a standard Spotlite hemacytometer (Baxter). Data were expressed as cell number × 103 per well. All experiments were repeated with cells derived from five to seven calves in both control and hypoxic groups.
ELISA assay of conditioned medium samples.
To collect conditioned medium, cells were plated in 100-mm Petri dishes in serum-containing DMEM and grown until confluent. Next, cells were rinsed with PBS, and growth medium was replaced with serum-deprived (0.2% PDS) DMEM. In 72 h, conditioned medium was collected, aliquoted, and snap-frozen for ELISA analysis.
Immunoreactive PDGF-BB was quantified using a commercially available ELISA kit (900-K04 PeproTech) according to the manufacturer's instructions, using Nunc Maxisorp plates. Capture antibody was bound overnight at 1 μg/ml, washed 4× in PBS/0.05% Tween 20, and blocked with 1% BSA/PBS for 1 h. Samples and standards were applied in triplicate for 2 h. Detection antibody (0.25 μg/ml) was incubated for 2 h. Avidin-HRP conjugate (1:2,000) was applied for 30 min and washed, and ABTS substrate was applied until color developed. Plates were immediately read at 405 nm in an ELISA plate reader. Wavelength correction was set at 650 nm.
Inhibition assays.
Inhibition assays were performed to test a potential role of PDGF, SDF-1, and S100A4 in autocrine growth of d“R” cells and in paracrine effect of dR cell-conditioned medium (R-CM). For testing the potential role of these mediators on autocrine growth of dR cells, the latter were plated at 10 × 103 cells/well onto a 24-well plate in complete 10% CS-DMEM. The next day, cells were washed with PBS, and serum-deprived (0.2% PDS) DMEM was added for 10 days with or without antibodies. On days 4 and 8, one-half of culture medium was replaced, and antibodies were re-added. The following antibodies were used in these experiments: goat anti-human PDGF-BB/AB neutralizing IgG, goat anti-human SDF-1/CXCL12 neutralizing IgG (both from R&D Systems, Minneapolis, MN), rabbit anti-S100A4 IgG (Thermo Fisher Scientific). Concentrations of antibodies/reagents are specified in the figure legends. For assaying paracrine effects of R-CM, target cells (d“S”-SMC and/or dPA adventitial fibroblasts) were plated at 20 × 103 cells per well onto 24-well culture plates in complete 10% CS-DMEM. The next day, cells were washed with PBS and growth arrested in serum-deprived (0.2% PDS) DMEM for 72 h. Next, R-CM was added with or without “inhibitory” agents or antibodies to be tested. BrdU was added 24 h before termination of the experiment. Cell numbers were acquired at time points specified in the figure legends.
Coculture experiments.
Coculture experiments were performed using a “source/target” design (16) to determine if a certain cell population secreted either growth-promoting or growth-inhibitory factors. Briefly, target cells (in which the effect of the conditioned medium from the source cells was tested) were plated inside a 15-mm plastic ring, which was greased to the center of a 35-mm Petri dish. The source cells (whose conditioned medium is to be tested for production of paracrine factors) were plated outside the plastic ring (with target cells inside the ring). When cell cultures displayed sufficient confluency, culture medium for both cell types was replaced with 0.1% CS-DMEM for 72 h. All the medium of the target cells was then withdrawn, and the ring separating the two cell types was removed, allowing the medium, conditioned by the source cells for 72 h, to spread over the target cells. After 16 h, BrdU was added to culture medium, and, after additional 24 h, cells were fixed, immunostained for BrdU, and counterstained with hematoxylin, and BrdU nuclear incorporation was quantified by a manual count and normalized per total number of cell nuclei.
DNA synthesis analysis.
DNA synthesis analysis was determined by measuring [3H]thymidine incorporation in growth-arrested cells isolated from control or hypertensive calves and exposed in vitro to either normoxic (21% O2) or hypoxic (3% O2) conditions as previously described (15). Briefly, cells were plated at 20 × 103 cells/well onto 24-multiwell plates in 10% CS-DMEM and rinsed with PBS within 24 h, and growth medium was replaced with serum-deprived (0.1% CS) DMEM for 72 h. Cells were then placed in air-tight sealed humidified modular incubator gas chambers (Billups-Rothenberg, Del Mar, CA), which were purged for 20 min with the defined gas mixture of either 21% O2 or 3% O2 and with 5% CO2 balanced with N2 (AirGas, Denver, CO) and placed in the 37°C incubator for 48 h. pH of the culture medium was monitored by the color of phenol red. Next, chambers were opened, [3H]thymidine (0.5 μCi/ml) was added to wells, and chambers were sealed and gassed again. After an additional 24 h of incubation, measurement of [3H]thymidine incorporation was performed and expressed as dpm per cell.
Cell migration.
Cell migration was assessed using a “scrape” assay in complete DMEM supplemented with 5 μM hydroxyurea to inhibit cell proliferation. Briefly, in subconfluent cell cultures, growth medium was replaced with 0.2% PDS-DMEM for 72 h, and hydroxyurea (5 μM, Sigma) was added for 2 h to inhibit cell replication, after which a 2-mm scrape was performed (that time point was considered 0 h). The borders of the wound (scrape) were labeled with a permanent marker (on the bottom of the Petri dish), and the number of cells migrating past the drawn line (into the “scrape area”) was counted at 24 and 48 h. Cell migration analysis was also performed in the presence of human recombinant S100A4 (2.5 μg/ml) and soluble RAGE (5 μg/ml) as well as with antibodies against S100A4 (1.25 μg/ml) and against RAGE (6 μg/ml). All these reagents were purchased from Abcam (Cambridge, MA).
Quantitative real-time PCR.
Total cellular RNA from each sample was extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany). Complementary DNA was synthesized from 1 μg of total cellular RNA using iScript cDNA Synthesis Kit (BioRad, Hercules, CA). Quantitative real-time RT-PCR was performed in triplicate with the iCycler My iQ with iQ SYBR Green Supermix (BioRad). Primers were designed using Primer 3 Software (53).
Genes were normalized to the housekeeping gene hypoxanthine-xanthine phosphoribosyl transferase (HPRT). The sequences for primers were as follows: PDGF-B (fwd 5′-AGTGACCACTCCATCCGTTC-3′, rev 5′-TCAGCCCCGTCTTCATCTAC-3′), PDGF-A (fwd 5′-CCAACCAGATGTGAGGTGAA-3′, rev 5′-CTGGCAGTAAGCACCGTACA-3′), SDF-1/CXCL12 (fwd 5′-CCTTGCCGATTCTTTGAGAG-3′, rev 5′-CTGAAGGGAGCAGTTTGGAG-3′), CXCR4 (fwd 5′-TCAAGGAGGTGGATGAGAGG-3′, rev 5′-AGGATGACGATACCAGGCAG), S100A4 (fwd 5′-CAAGGAGGGTGACAAGTTC-3′, rev 5′-TCGTTGTCCTTGTTGCAGTC-3′), RAGE (fwd 5′-TGATGGCAAAGGAGTGTCAG-3′, rev 5′-CAGGGGTGAAGCTACAGGAG), cKIT (fwd 5′-TCCTGATTGACCTTCCCT TG-3′, rev 5′-TGTCAAATCCTTGGGGAGAG-3′), CD34 (fwd 5′-AACCCATCTCCGTTATTCCC-3′, rev 5′-TCGGGGAACTTAGAGAGCAA-3′), CD73 (fwd 5′-GTGTCGTGTGCCCAGTTATG-3′, rev 5′-AATCCGTCTCCACCACTGAC-3′), IL-6 (fwd 5′-GTGAAAGCAGCAAGGAGACA-3′, rev 5′-ATCCGTCCTTTTCCTCCATT-3′), MCP-1 (fwd 5′-CGCCTGCTGCTATACATTCA-3′, rev 5′-ACACTTGCTGCTGGTGACTC-3′), Flt-1 (fwd 5′-CTCCCGAGTCCA TCTTTGAC-3′, rev 5′-GGGACCCACCTAAGGAGAAG-3′), Tie-2 (fwd 5′-CCTGTACCTGGCCATTGAGT-3′, rev 5′-CGTCTCTAGCACTCGGCTCT-3′), PECAM-1 (fwd 5′-GCAGGGTGTTCAAGAGAAGC-3′, rev 5′-TAATCACCTCGGACCTGGAG-3′), VE-cadherin (fwd 5′-GTACTCCCCAAATGCTGGAA-3′, rev 5′-CTTTGAGTTGGACCCG TGAT-3′). Results are presented either as relative expression to HPRT using delta CT method or as fold increase relative to S cells using ΔΔCT method (59).
SDS-PAGE and Western blotting.
SDS-PAGE and Western blotting were performed as previously described (18) using S100A4 Abs (Ab-8, 1:500, Thermo Fisher Scientific) or MMP-2 Abs (42-5D11, 1:400, Calbiochem).
Cell invasion.
Cell invasion capacity was tested in a Matrigel (BD Pharmingen) assay as per the manufacturer's instructions with or without the general MMP inhibitor GM6001 (20 μM).
Gelatin zymography.
Gelatin zymography was performed as described elsewhere (35). Briefly, cultured cells were lysed in cold lysis buffer (50 mM Tris, 250 mM NaCl, 0.5% Triton X-100, 0.1% SDS). Lysates were concentrated depending on the protein amounts (determined by Bradford and BCA assay) using Centricon-10 centrifugal filter devices. Samples were mixed with 2× sample buffer (63 mM Tris·HCl, 10% glycerol, 2% SDS, 0.0025% bromphenol blue, pH 6.8), and protein (10.5 μg/lane) was separated by electrophoresis under nonreducing conditions, using precast gelatin-embedded 10% polyacrylamide gels (Novex) in zymogram running buffer (25 mM Tris base, 192 mM glycine, 0.1% SDS, pH 8.3). Gels were renatured at room temperature for 30 min with gentle agitation in zymogram-renaturing buffer [25% (vol/vol) Triton X-100] followed by 30-min incubation in zymogram-developing buffer [50 mM Tris base, 40 mM HCl, 20 mM NaCl, 5 mM CaCl2, 0.02% (wt/vol) Brig 35-Novex]. Gels were then incubated overnight at 37°C in fresh developing buffer. To confirm the presence of MMPs, duplicate gels were incubated in developing buffer containing 20 mM EDTA to chelate the zinc ions and inactivate the MMPs. Gels were rinsed, stained with Simply Blue SafeStain, and destained until clear bands, indicative of gelatinase activity, were evident. Precision Plus Dual Color Protein Standards (BioRad Laboratories) were used on every gel.
Data analysis.
Data are presented as means ± SE. Student's t-test and one-way ANOVA were used for statistical analysis; significance was accepted at P < 0.05.
RESULTS
In vivo, phenotypically distinct cells emerge within the remodeled dPA media.
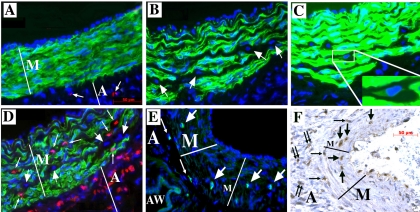
Two-week exposure of neonatal calves to hypobaric hypoxia consistently resulted in marked PA media thickening (Fig. 1 and Refs. 64 and 65). As previously reported (67), in control normoxic calves, the media of dPAs appears composed of a phenotypically uniform population of differentiated SMC, expressing α-SM-actin, SM-myosin, calponin, and meta-vinculin (α-SM-actin is shown in Fig. 1A). In contrast, the dPA media of chronically hypoxic hypertensive calves consistently contained a number of cells that either did not express any SM-related markers (Fig. 1B, staining for α-SM-actin is shown) or expressed very low levels of α-SM-actin only, i.e., exhibited a “myofibroblast” phenotype (Fig. 1C). Interestingly, cells were observed within the dPA media of hypertensive, but not control, animals, which expressed hematopoietic (CD45) and leukocytic/monocytic (CD11b, CD14) markers, as well as a progenitor-like cell marker (cKit) and a marker associated with enhanced cell motility (S100A4) (Fig. 1, D–F, and Supplemental Fig. 1. Supplemental data for this article is available online at the AJP-Lung web site.). Notably, CD45+, CD11b+, CD14+ and/or S100A4+ cells were observed throughout the dPA media (Fig. 1, D and F), whereas cKit+ cells were localized primarily to the outer media and medial-adventitial border (Fig. 1E), and practically all coexpressed CD45 (not shown). No coexpression of CD45, CD11b, CD14 and/or cKit with α-SM-actin by cells within the media was identified.
Fig. 1.
Chronic hypoxia induces the appearance of cells, phenotypically distinct from SMCs, within the media of distal pulmonary arteries (dPAs). A: in control normoxic calves, a phenotypically uniform SMC population (all cells express α-SM-actin; green) comprises the tunica media (M) of dPAs. Only in the adventitia (A) are there a few cells that express CD45 (red, arrows). B–F: in chronically hypoxic calves, the dPA media is markedly thickened and is composed of a phenotypically heterogeneous cell population, where some cells do not express α-SM-actin (arrows in B), some express low levels of α-SM-actin (as visualized in C, inset) through the “enhanced” immunostaining method (see methods), and some cells express CD45 (as shown in D, small arrows pointing to cells stained in red; large arrows point to cells that do not express either α-SM-actin or CD45). There are a few cells that express cKit (E, green) either within the outer media (large arrows) or at the medial-adventitial border (small arrows). Cell nuclei are labeled with DAPI (blue). AW, airway. Several cells within the dPA media (M) express S100A4 [F, large arrows pointing to brown cells; small single arrows point to S100A4-positive cells at the medial-adventitial border; small double-arrows point to S100A4-positive cells within the adventitia (A)]. Control arteries lacking cKit+ and/or S100A4+ cells within the media are shown in Supplemental Fig. 1, available online at the AJP-Lung web site.
Phenotypically distinct cells emerge in cultures from dPA media of hypertensive calves.
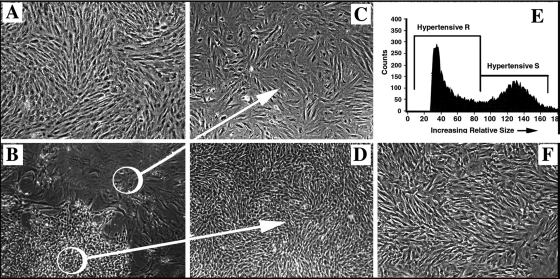
Primary cell cultures isolated from the dPA media of control calves were consistently composed of a morphologically and phenotypically uniform population of large spindle-shaped SMC that grew in a “hill-and-valley” pattern (Fig. 2A) and expressed a number of SM-related markers, α-SM-actin, calponin, SM-myosin, meta-vinculin, findings consistent with those previously described by us (67). In contrast, cell cultures isolated from the remodeled dPA media of hypoxic hypertensive calves consistently yielded two morphologically distinct cell populations (Fig. 2B). One of these populations (Fig. 2C) represented SMC (as defined by α-SM-actin and SM-myosin expression, Fig. 3, B and C), which were phenotypically similar to SMC obtained from control calves, yet significantly hypertrophied, as analyzed by forward scatter (Supplemental Fig. 2). Another population (Fig. 2D) was composed of very small cells (as analyzed by forward scatter, Fig. 2E) of a rhomboidal morphological appearance that were growing without cell-cell contact inhibition. They always demonstrated a delayed appearance in primary culture, but subsequently expanded rapidly and formed dense cell colonies. For reference purposes, we termed the larger spindle-shaped SMC as dS-SMC (“d” for distal PA), and the small rhomboidal cells as dR cells. Importantly, adventitial fibroblast isolated from the same vessel were morphologically (Fig. 2F) and functionally (see below) different from the dR cells.
Fig. 2.
In primary culture, dPA media of chronically hypoxic calves yields phenotypically distinct cell populations. A: primary cultures isolated from the dPA media of control calves consistently yield a morphologically uniform population of cells. B: in contrast, primary cultures obtained from the dPA media of chronically hypoxic calves consistently yield 2 morphologically distinct cell types. C and D: the 2 morphologically distinct cell types [termed dS-SMC (C) and dR cells (D)] observed in primary cultures of chronically hypoxic calves (B) can be selectively isolated as uniform populations (see methods). When expanded in subculture, both cell populations maintain their morphological differences (passage 4 is shown) as well as the differences in cell size (E; passage 5 is shown) as demonstrated by FACS-based analysis performed by forward scatter (dR cells are significantly smaller in size than dS-SMC). F: fibroblasts, obtained from the same dPA used for isolation of medial cells, appear morphologically different from dR cells (shown in D).
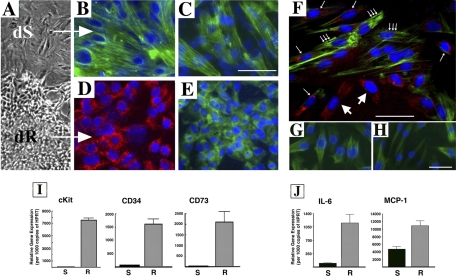
Fig. 3.
Cultured dR cells express an immunophenotype distinct from that of dS-SMC and mRNA for progenitor-associated markers and inflammatory mediators. A: morphological differences between dS-SMC and dR cells are apparent in primary culture of dPA media. B and C: dS-SMC express SM-related markers, α-SM-actin (B) and SM-myosin heavy chains (C), not only in primary culture but also at subsequent passages in subculture (passage 5 is shown). D and E: dR cell colonies express CD11b in early primary culture (D) and consistently (at all passages in culture) express type I procollagen-associated enzyme Hsp47 (E). F: in early subculture (passage 1), CD11b expression (large arrows, red staining) is gradually lost, whereas expression of α-SM-actin is gradually acquired (small single arrows point at cells that coexpress both antigens, whereas small triple arrows point at cells that express only α-SM-actin). G and H: clonally derived dR cells express myofibroblast-related markers α-SM-actin (G) and calponin (H). I: dR cells express high levels of mRNA for progenitor cell-associated antigens cKit, CD34, and CD73. Fold-change dR vs. dS: cKit 68.03 ± 13.79; CD34 20.59 ± 2.35; CD73 42.02 ± 13.28. J: dR cells express higher levels of mRNA for inflammatory mediators IL-6 and MCP-1 compared with dS-SMC. Fold-change dR vs. dS: IL-6 11.54 ± 2.29-fold and MCP-1 2.29 ± 0.14-fold, respectively.
In primary culture, some dR cell colonies transiently expressed CD11b (Fig. 3D) and consistently expressed mesenchymal cell markers, type I procollagen, and the type I collagen-associated enzyme heat shock protein 47 (Hsp47) (Fig. 3E, Hsp47 is shown), but initially did not express α-SM-actin. With time in early subculture (passage 1), cells gradually lost expression of CD11b and gained immunoreactivity for α-SM-actin (Fig. 3F). The acquisition of α-SM-actin reactivity was also confirmed by analysis of clonally derived R cells (all obtained from single-cell clones) that gained expression of α-SM-actin and calponin (Fig. 3, G and H). Importantly, no phenotypic switch of dPA SMC into R cells was observed under any conditions/stimuli tested, including hypoxia, PDGF, M-CSF, EBM-2 medium, MSC medium, coculture with endothelial cells (EC), etc. (not shown).
To further characterize dR cell phenotype, we compared these cells to EC isolated from main pulmonary artery (MPA-EC) and from dPA (1,000 μm in diameter, dPA-EC) of control calves. dR cells expressed markedly lower mRNA levels of endothelial markers, Tie2, PECAM-1/CD31, and VE-cadherin than MPA-EC and dPA-EC. The exception was observed for Flt-1 mRNA, which was expressed by dR cells and adventitial fibroblasts (Fibs) at levels comparable to those of dPA-EC, yet lower than those of MPA-EC (Supplemental Fig. 3).
dR cells express mRNA for progenitor cell markers and for inflammatory mediators.
Notably, dR cells expressed high mRNA levels for antigens commonly expressed by progenitor-like cells cKit, CD34, and CD73 (68.03 ± 13.79-fold, 20.59 ± 2.35-fold, 42.02 ± 13.28-fold, respectively, compared with dS-SMC) (Fig. 3I). Furthermore, dR cells exhibited higher than dS-SMC mRNA expression levels for inflammatory mediators IL-6 and MCP-1 (11.54 ± 2.29-fold and 2.29 ± 0.14-fold, respectively, Fig. 3J).
dR cells exhibit augmented proliferative capabilities.
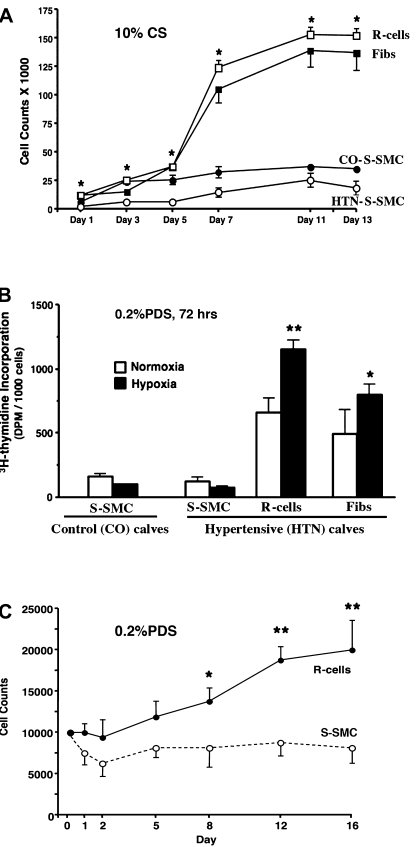
dR cell and dS-SMC populations exhibited markedly different growth capabilities under both serum-stimulated (10% calf serum) (Fig. 4A) and serum-deprived (Fig. 4, B and C) conditions. dR cells proliferated far more rapidly and plateaued at higher cell densities upon serum stimulation (Fig. 4A), as well as exhibited augmented DNA synthesis under serum-deprived (0.2% PDS) conditions (Fig. 4B). Interestingly, hypoxia (3% O2) further augmented DNA synthesis in dR cells (Fig. 4B), which was far more significant than the response of adventitial fibroblasts to hypoxia. Notably, DNA synthesis of dS-SMC was not increased and was rather attenuated in response to hypoxia. Furthermore, dR cells exhibited serum-independent autocrine growth (Fig. 4C), thus suggesting secretion of promitogenic factors that contributed to this growth pattern.
Fig. 4.
Compared with dS-SMC, dR cells exhibit augmented proliferative capabilities under both serum-stimulated and serum-deprived conditions. A: growth curves under serum-stimulated (10% CS) conditions demonstrate that dR cells proliferate far more rapidly than dS-SMC [isolated from either control (CO) or hypoxic hypertensive (HTN) calves] and plateau at higher cell densities. B: under serum-deprived conditions (0.2% PDS, 72 h), dR cells exhibit augmented DNA synthesis compared with dS-SMC (open bars, **P < 0.01). Twenty-four hour exposure to hypoxia (3% O2, closed bars) further augments [3H]thymidine incorporation in dR cells and makes it even higher than that of adventitial fibroblasts (*P < 0.05). This is a representative graph from 6 independent experiments (in which cells from 6 different control and 6 hypoxic calves were used). Samples for each data point were run in triplicate. C: under serum-deprived conditions (0.2% PDS), dR cells exhibit autocrine, serum-independent growth, whereas dS-SMC do not proliferate (*P < 0.05, **P < 0.01).
Autocrine growth of dR cells is due, in part, to production of PDGFs and SDF-1.
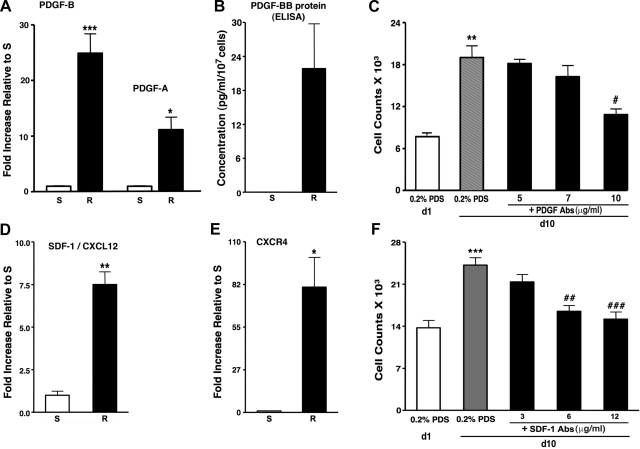
To test the hypothesis that dR cells secreted promitogenic factors, we first performed RT-PCR analysis of “candidate” mitogens, PDGFs and SDF-1/CXCL12. dR cells exhibited high mRNA expression levels of PDGF-B and -A compared with dS-SMC (PDGF-B 26.4 ± 1.6-fold; PDGF-A 13.2 ± 1.3-fold, respectively) (Fig. 5A). ELISA assay of serum-deprived medium conditioned by dR cells (R-CM) for 72 h demonstrated PDGF-BB protein at concentrations of 21.84 ± 7.92 pg/ml/107 cells (Fig. 5B). Furthermore, dR cells expressed higher mRNA levels of SDF-1/CXCL12 and its receptor CXCR4 compared with dS-SMC (SDF-1/CXCL12: 10.0 ± 1.2-fold; CXCR4: 80.84 ± 19.19-fold, respectively) (Fig. 5, D and E).
Fig. 5.
Autocrine growth of dR cells is due, in part, to PDGF-BB and SDF-1/CXCL12. A: real-time PCR analysis demonstrates that dR cells express higher levels of mRNA for PDGF-B and PDGF-A than dS-SMC (26.4 ± 1.6-fold and 13.2 ± 1.3-fold, respectively). Specific messages were normalized to HPRT. Data are presented as fold-change of relative gene expression in dR cells compared with that of dS-SMC. ***P < 0.0003, *P < 0.05. B: dR cells secrete PDGF-BB at concentrations of 21.84 ± 7.92 pg/ml/107 cells, as detected by ELISA in serum-free medium conditioned by cells for 72 h (samples from 5 dR cell populations were analyzed). S-SMC do not make PDGF-BB at detectable levels by ELISA (samples of conditioned medium from 3 dS-SMC populations were analyzed). C: autocrine, serum-independent growth of dR cells (gray bar) is partially attenuated by neutralizing PDGF-BB/AB antibodies (added at 5, 7, 10 ng/ml). Cell counts were performed on day 1 (d1) and day 10 (d10). **P < 0.05 compared with d1 cell counts; #P < 0.05 compared with d10 untreated (0.2% PDS) cell counts. D and E: real-time PCR analysis demonstrates that dR cells express higher levels of mRNA for SDF-1/CXCL12 (D) and its receptor CXCR4 (E) (10.0 ± 1.2-fold and 80.84 ± 19.19-fold, respectively) compared with dS-SMC. Specific messages were normalized to HPRT. Data are presented as fold-change of relative gene expression in dR cells compared with that of dS-SMC. **P < 0.005, *P < 0.05. F: autocrine, serum-independent growth of dR cells (gray bar) is attenuated by neutralizing SDF-1/CXCL12 antibodies (added at 3, 6, 12 ng/ml) in a dose-dependent manner. ***P < 0.0005 compared with 0.2% PDS; ##P < 0.005 and ###P < 0.001 compared with the effect of R-CM without SDF-1 antibodies.
We then tested if autocrine, serum-independent growth of dR cells was due, at least in part, to production of PDGFs and SDF-1. Neutralizing antibodies against PDGF-BB, -AB and/or against SDF-1/CXCL12 inhibited autocrine growth of dR cells in a dose-dependent manner (Fig. 5, C and F, respectively).
dR cells exhibit augmented migratory capabilities due to S100A4/RAGE axis.
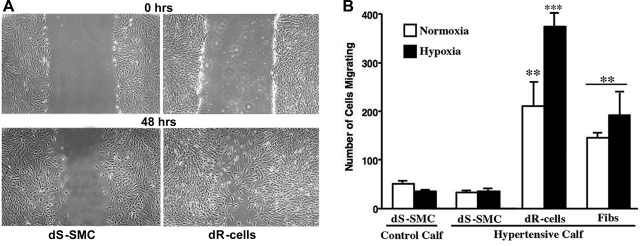
Findings on high proliferative potential of dR cells led us to test the migratory capabilities of these cells. “Wound/scrape” assay (Fig. 6A), using hydroxyurea (5 μM) to exclude contribution of cell proliferation demonstrated that, under normoxic (21% O2) conditions, dR cells exhibited sixfold higher migratory capabilities than the dS-SMC and migrated into the scrape even faster than the adventitial fibroblasts (Fig. 6B). Hypoxia (3% O2) further augmented the migratory potential of dR cells (up to 9-fold compared with dS-SMC) (Fig. 6B) but not that of dS-SMC.
Fig. 6.
dR cells exhibit augmented migratory capabilities compared with dS-SMC. A: migratory capabilities of dR cells and dS-SMC were compared using the “scrape” assay (see methods and Supplemental material) under both normoxic (21% O2) and hypoxic (3% O2, shown) conditions. Hydroxyurea (5 μM) was added to inhibit (and thus exclude contribution of) cell replication. B: statistical analysis demonstrates that 48 h after initiation of the scrape, the number of dR cells that migrated into the scrape area under normoxic conditions was 6-fold higher than that of the dS-SMC. Hypoxia (3% O2) further augmented the migratory capabilities of dR-cells (up to 9-fold compared with dS-SMC). Shown is a representative graph from 4 independent experiments (in which cells from 3 different control and 4 hypoxic calves were used). Samples for each data point were run in triplicate. **P < 0.01; ***P < 0.01 compared with dS-SMC.
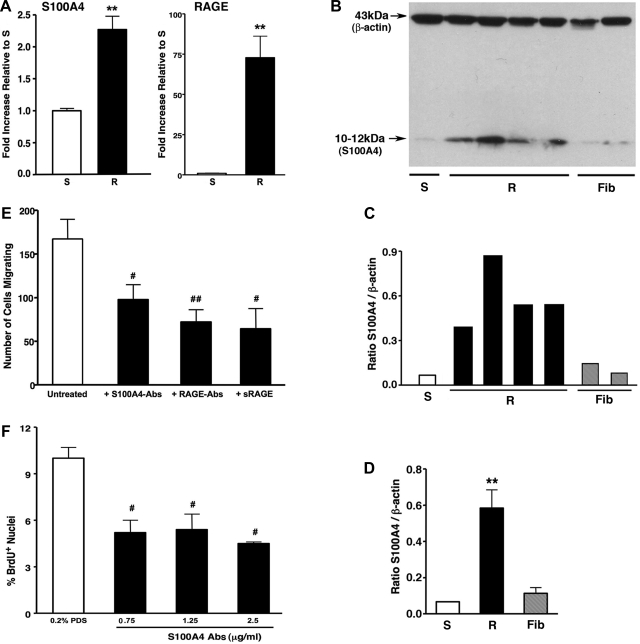
Since S100A4 has been shown to be associated with enhanced cell motility (5, 71), we analyzed mRNA expression of S100A4 and of its receptor, RAGE (receptor for advanced glycation end products), and found that dR cells expressed higher than dS-SMC mRNA levels of S100A4 (2.27 ± 0.21-fold) and of RAGE (72.88 ± 13.36-fold, respectively) (Fig. 7A). At the protein level, S100A4 was expressed by dR cells at markedly higher levels than by dS-SMC (8.98 ± 2.12-fold) or even dPA adventitial fibroblasts (5.29 ± 1.25-fold) (Fig. 7, B–D).
Fig. 7.
Augmented migration of dR cells is due, in part, to the S100A4/RAGE axis. A: real-time PCR analysis demonstrates that dR cells express higher levels of mRNA for S100A4 and its receptor RAGE compared with those of dS-SMC (2.27 ± 0.21-fold and 72.88 ± 13.36-fold, respectively). Specific messages were normalized to HPRT. Data are presented as fold-change of relative gene expression in dR cells compared with that of dS-SMC. **P < 0.005. B–D: Western blot analysis (B), using the S100A4-specific antibodies (see methods) and cell extracts, and its quantification (C and D, average data for R cells and Fibs), demonstrates that dR cells express more S100A4 protein than dS-SMC (8.98 ± 2.12-fold) or even dPA fibroblasts (Fib) (5.29 ± 1.25-fold). **P < 0.05. E: augmented promigratory capabilities of dR cells were attenuated by antibodies against S100A4 (S100A4-Abs, 1.25 μg/ml) against RAGE (RAGE-Abs, 6.25 μg/ml) and by soluble RAGE (sRAGE, 5 μg/ml). #P < 0.05, ##P < 0.01. F: elevated DNA synthesis (identified by BrdU nuclei incorporation) of dR cells is inhibited by S100A4 antibodies at as low as 0.75 μg/ml. #P < 0.01.
To test the potential role of S100A4 and RAGE in conferring high migratory capabilities to dR cells, we utilized several inhibitory strategies and found that antibodies against S100A4 and/or against RAGE, as well as soluble RAGE, all markedly attenuated high migratory capabilities of dR cells (Fig. 7E). Interestingly, autocrine, serum-independent growth of dR cells was partially inhibited by S100A4 Abs (Fig. 7F), thus suggesting a potential role of S100A4 not only in cell motility but in cell replication as well.
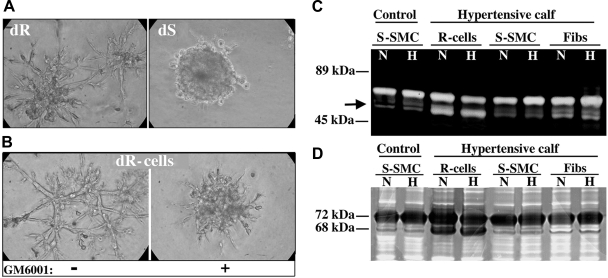
We also assessed the invasive capabilities of dR cells using a Matrigel assay. dR cells demonstrated a robust outgrowth and sprouting in Matrigel, whereas dS-SMC remained as compact cell clusters, as has been reported for SMC (Fig. 8A). Since the ability of cells to form sprouts in Matrigel has been attributed, in part, to MMP production (72), we tested the effect of the general MMP inhibitor GM6001 (20 μM) and observed a substantial inhibition of dR cell outgrowth in Matrigel (Fig. 8B), thus suggesting a role for MMPs in this process. Assessment of MMP activity in gelatin zymography assay showed that dR cells produced a pattern of gelatinolytic activity markedly distinct from that of dS-SMC and adventitial fibroblasts (Fig. 8C). Western blot analysis demonstrated that the observed enhanced gelatinolytic activity in dR cell extracts was specific for the active (68-kDa isoform) MMP-2 (Fig. 8D).
Fig. 8.
dR cells exhibit augmented invasive capabilities and produce active MMP-2. A: dR cells demonstrate a vigorous outgrowth/sprouting in Matrigel (72-h time point is shown), whereas dS-SMC remain as compact cell clusters. B: addition of the general MMP inhibitor GM6001 (20 μM) significantly inhibited dR cell outgrowth in Matrigel. C: gelatin zymography shows that, under both normoxic (N) and hypoxic (H) conditions, dR cells produce a pattern of gelatinolytic activity that is apparently distinct (note the bottom band, marked by an arrow) from that of dS-SMC and even that of adventitial fibroblasts. D: Western blot analysis, using the MMP-2-specific antibodies, demonstrates the 68-kDa bands in the dR cell protein extracts that correlate with the active (68-kDa) form of MMP-2 (72-kDa bands correlate with the latent MMP-2).
dR cells produce potent paracrine promitogenic activity, which is due, in part, to production of PDGFs, SDF-1, and S100A4.
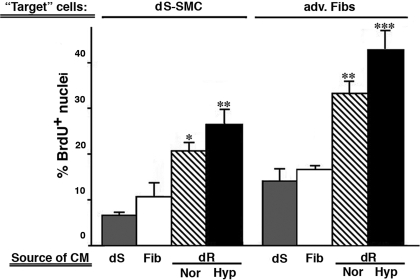
To test if dR cells produced factors that could exert paracrine mitogenic effects on dPA wall cells, we performed coculture experiments (see methods) using dR cells as the source of conditioned medium and as target cells, dPA SMC and adventitial fibroblasts. As shown in Fig. 9, serum-deprived (0.2% PDS) medium conditioned by dR cells for 72 h markedly augmented BrdU incorporation in target dS-SMCs (3-fold) and target adventitial fibroblasts (2-fold). Importantly, serum-deprived medium conditioned by SMC and fibroblasts had no to minimal effect, respectively, on the target cells. Of interest, when dR cells were exposed to hypoxia (3% O2, 24 h) the promitogenic effect of their conditioned medium was further augmented compared with normoxic conditions (Fig. 9).
Fig. 9.
dR cells produce potent paracrine promitogenic activity. The potential of different PA wall cells (dS-SMC, dR cells, or fibroblasts) to secrete promitogenic factors was tested by assessing nuclear BrdU incorporation in the coculture experiment using the “source” cells [those from which conditioned medium (CM) was tested] and the “target” cells (those exposed to CM from the source cells and in which BrdU incorporation was evaluated; see methods). After 24 h, serum-free medium conditioned by the dR cells (hatched bars) exhibited the highest promitogenic effect on dS-SMC and adventitial fibroblasts (adv. Fibs) compared with CM from other cell types tested (dS, gray bars; Fib, open bars). Hypoxia (3% O2, Hyp, closed bars) further augmented this effect [P < 0.05 compared with normoxia (Nor)]. ***P < 0.001, **P < 0.01, *P < 0.05 compared with the effect of Fib-CM or dS-CM.
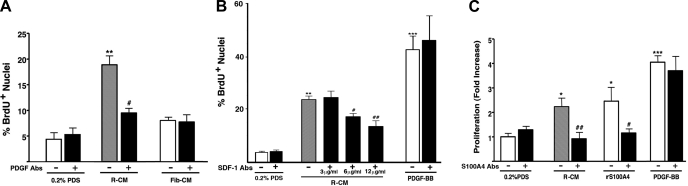
We then tested if potent paracrine promitogenic activity of dR cells was due to production of PDGFs, SDF-1/CXCL12, and S100A4. Neutralizing antibodies against PDGF-BB/-AB, against SDF-1/CXCL12, and against S100A4 all inhibited paracrine promitogenic effect of dR cell-conditioned medium on dS-SMC (Fig. 10, A–C, respectively) and adventitial fibroblasts (not shown). Fibroblast-conditioned medium did not significantly stimulate BrdU incorporation in S-SMC (Fig. 10A), and, importantly, PDGF-BB (10 ng/ml)-stimulated BrdU incorporation in dS-SMC was not affected by either SDF-1 or S100A4 neutralizing Abs.
Fig. 10.
Potent paracrine promitogenic effects of dR-conditioned medium are due, in part, to PDGFs, SDF-1, and S100A4. A: paracrine promitogenic effect of dR cell-conditioned medium (R-CM) on target dS-SMC (gray bar) is partially attenuated by neutralizing PDGF-BB/AB antibodies (10 ng/ml). Serum-free medium conditioned by control adventitial fibroblasts (Fib-CM) had minimal promitogenic effect on S-SMC. The data are presented as the ratio of BrdU-positive cell nuclei vs. total nuclei number. **P < 0.001 compared with 0.2% PDS; #P < 0.05 compared with the effect of R-CM without PDGF antibodies. B: paracrine promitogenic effect of R-CM on target dS-SMC (gray bar) is partially attenuated by neutralizing SDF-1/CXCL12 antibodies (added at 3, 6, 12 ng/ml) in a dose-dependent manner. **P < 0.001 compared with 0.2% PDS; #P < 0.05 and ##P < 0.005 compared with the effect of R-CM without SDF-1 antibodies. PDGF-BB (10 ng/ml)-stimulated BrdU incorporation in target dS-SMC is not affected by SDF-1 neutralizing antibodies (12 ng/ml). ***P < 0.0001 compared with 0.2% PDS. C: R-CM-induced proliferation of target S-SMC (gray bar) is inhibited by incubation with S100A4-antibodies (1.25 μg/ml). Specificity of the effect was tested by adding human recombinant S100A4 (rS100A4, 2.5 μg/ml), which also exhibited promitogenic effect on S-SMC, and this effect was inhibited by S100A4 antibodies. PDGF-BB (10 ng/ml)-stimulated proliferation of target dS-SMC was not affected by S100A4 antibodies. Cell counts were done on day 5. The data are expressed as fold-increase in cell numbers compared with untreated (0.2% PDS) cells. ***P < 0.0001 compared with 0.2% PDS; *P < 0.05 compared with 0.2% PDS; ##P < 0.005 compared with R-CM-treated cells; #P < 0.05 compared with rS100A4-treated cells. Gray and open bars, untreated cells; closed bars, antibody-treated cells. For all experiments, cells growing in 4 wells were analyzed for each data point.
DISCUSSION
Based on emerging experimental data demonstrating that, in addition to phenotypic modulation of resident SMC, other cellular mechanisms contribute to remodeling of vascular tunica media in response to injury, we sought to determine, using both in vivo and ex vivo approaches, if the cellular composition of the remodeled dPA media in calves with severe hypoxia-induced PH has been altered. In vivo, we observed that dPA media of chronically hypoxic hypertensive calves contained, in addition to differentiated SMC, phenotypically distinct cells that expressed hematopoietic (CD45), leukocytic/monocytic (CD11b, CD14), progenitor (cKit), and motility-associated (S100A4) cell markers, which were not observed within the dPA media of control animals. Ex vivo experiments with cell cultures obtained from dPA media of hypertensive calves showed the emergence of cells (termed dR), which exhibited phenotypic and functional characteristics distinct from those of resident SMC, including augmented growth, migratory and invasive potentials, production of potent promitogenic activity, and expression of mRNA for inflammatory mediators and for progenitor-like cells. No phenotypic switch of dPA SMC into R cells was observed under any conditions/stimuli tested. Thus the data of the present study demonstrate a significant alteration in the cellular composition of the remodeled dPA media and suggest that the newly emerging cells could exhibit profound modulatory effects on the vascular microenvironment.
Our in vivo data, showing the emergence of cells expressing antigens not usually observed in differentiated SMC (cKit, CD45, CD11b, CD14), are consistent with observations in human and animal models of PH. In human PH patients, the presence of cells, expressing hematopoietic and inflammatory markers, has been documented both within and around the pulmonary vessel wall (22, 23, 42, 74). In PAs of infants and children with PH, expression of S100A4 (also known as mts1), a calcium-binding protein associated with invasion, metastasis of cancer cells, and with augmented cell motility (5, 45, 54, 71), has been documented, and the numbers of S100A4-expressing cells markedly increased with the severity of pulmonary vascular lesions (21, 22). Recent studies in adults with idiopathic pulmonary arterial hypertension and familial pulmonary arterial hypertension have reported the presence of cells expressing hematopoietic and progenitor cell markers within the vessel wall, and cells exhibiting mesenchymal progenitor characteristics have been reported in remodeled PAs of patients with COPD (2, 38, 39, 48, 49, 75). We have previously reported a marked increase in accumulation of CD45+, CD11b+, CD68+, and cKit+ cells in the adventitia of chronically hypoxic hypertensive calves (9, 17). Others have also found increased numbers of cKit+ cells at the medial-adventitial border of chronically hypoxic mice, and demonstrated that, in vitro, these cells were capable of differentiating into α-SM-actin-expressing cells (58, 79). Bone marrow-derived cells have also been shown to accumulate in the PAs of chronically hypoxic chimeric mice and to differentiate into α-SM-actin-expressing cells (24, 79). Thus our findings regarding the presence of cells expressing hematopoietic/leukocytic (CD45/CD11b), progenitor-like (cKit), and motility-associated (S100A4) markers in the dPA media of hypertensive calves support and extend the idea that multiple cell types and/or cellular mechanisms may contribute to PA medial remodeling in the setting of hypoxia-induced PH.
Our ex vivo findings demonstrated consistently the outgrowth of at least two cell populations (dS-SMC and dR cells) exhibiting distinct and stable phenotypic differences in primary cultures obtained from dPA of hypertensive, but not control, calves. dR cells exhibited augmented growth capabilities under both serum-stimulated and serum-deprived conditions, as well as augmented migratory and invasive potentials. Autocrine (serum-independent) growth of dR cells was due, at least in part, to secretion of PDGFs, SDF-1/CXCL12, and S100A4. Vascular cells with autocrine growth capabilities, obtained from injured adult systemic vessels, have been previously reported, and several factors, including those described here, have been suggested as conferring autocrine growth capabilities to those cells (34, 43, 44, 76, 77). Our study also indicates that the augmented migratory capabilities of dR cells were mediated, at least in part, through the S100A4/RAGE pathway (11, 14, 25). These findings are compatible with a recent report in systemic circulation where diseased porcine and human coronary arteries yielded in culture, besides traditional differentiated SMC, smaller morphologically rhomboid cells that exhibited augmented growth and migratory capabilities, partially due to high expression levels of S100A4 (5). Other studies have shown that S100A4 expression may confer invasive properties to the cell by inducing MMP expression (19, 54, 55, 69), which is consistent with our data demonstrating that dR cells express S100A4 and produce active MMP-2, and that both play a role in augmented growth and/or invasive capabilities of dR cells. Factors participating in regulation of S100A4/RAGE axis in mesenchymal cells have not been delineated, but serotonin (5-HT), frequently implicated in PH remodeling, has been shown to upregulate S100A4 mRNA/protein expression and to stimulate release of S100A4 (36). Hypoxia has also been shown to upregulate RAGE and S100A4 (8, 27, 37, 40). It is possible that other factors implicated in pulmonary hypertension could also operate through S100/RAGE signaling axis.
The present study also demonstrates that dR cells produce potent paracrine promitogenic activity for resident PA wall cells (medial SMC and adventitial fibroblasts), which is due, at least in part, to secretion of PDGFs, SDF-1/CXCL12, and S100A4. Moreover, dR cells have high mRNA expression levels of inflammatory cytokines, IL-6 and MCP-1. Release of these growth factors and inflammatory mediators would have major effects on the pulmonary vascular microenvironment, and the data thus suggest that dR cells can contribute to pulmonary vascular remodeling by modulating the proliferative and inflammatory status of resident PA wall cells. Recent reports have shown that mesenchymal precursor cells, recruited to the site of tissue injury, exert major effects on tissue remodeling through release of paracrine factors, thus altering the local microenvironment (6, 12, 30, 44, 50). For instance, circulating fibrocytes, being critical in the wound healing process, have been reported to secrete both growth-promoting and proangiogenic factors (4, 51). PDGFs are well known to be secreted by monocytes and progenitor cells and to stimulate proliferation of SMC and fibroblasts (3, 46). SDF-1/CXCL12 is secreted by several cell types in response to hypoxia and has been reported to stimulate SMC proliferation (7, 45). Consistent with this observation, we found that hypoxia further induces the potency of promitogenic activity of dR cells. Of relevance to the potential importance of SDF-1 production by the dR cells is the report by Young et al. (79), which demonstrated that inhibition of SDF-1/CXCR4 axis attenuated neonatal hypoxia-induced PH in mice.
The emergence of dR cells, markedly distinct from the resident PA wall cells, raises questions regarding the origin/source of these cells as well as other phenotypically distinct cells that accumulate in the remodeled blood vessels in both pulmonary and systemic circulations. Besides the traditional hypothesis of phenotypic modulation of vascular medial SMCs, possibilities that have been recognized include fibroblasts, local tissue progenitor cells, circulating (hematopoietic or non-hematopoietic) mesenchymal progenitors (including fibrocytes), or even transdifferentiation of endothelial cells (1, 13, 28, 32, 41, 47, 56, 57, 60, 62, 63, 80). Our in vivo data demonstrate that some of the newly emerging cells within the dPA media of hypertensive calves express the hematopoietic/leukocytic markers CD45, CD11b, and CD14, which could potentially be ascribed to the recruitment of circulating hematopoietic cells as described by others (57, 58, 61, 70). We have recently documented the robust recruitment of circulating fibrocytes to the remodeled pulmonary perivascular adventitia of chronically hypoxic calves and rats (17). Circulating fibrocytes are monocytic cells that are recruited to the site of tissue injury where they acquire a mesenchymal (collagen-producing) and even a myofibroblast (α-SM-actin+) phenotype (4, 51, 75). Although we were unable to identify CD45+/collagen+ or CD11b+/collagen+ fibrocyte-like cells in the dPA media in vivo, dR cell colonies in primary culture transiently expressed CD11b, and consistently expressed type I procollagen and mRNA for CXCR4 (receptor for SDF-1/CXCL12), an expression pattern consistent with a fibrocyte phenotype. As suggested by others, this may be due to rapid differentiation (loss of hematopoietic/leukocytic antigens) of fibrocytes into mesenchymal cells under specific pathological environments (68). We also documented the presence of cKit+ (CD45+) cells within the media (notably, closer to the medial-adventitial border). Recent reports in chimeric mice suggest that the appearance of cKit+ cells in the PAs of chronically hypoxic animals is likely due to recruitment of bone marrow-derived cells, which are capable of differentiating into mesenchymal α-SM-actin+ cells (24, 58, 79). In fact, cKit function may be necessary for myogenic differentiation of bone marrow-derived cells (78). Yet another possibility, raised by the findings of Zengin et al. (81) and Passman et al. (47), suggests that there is a subset of resident vascular progenitor cells that reside at the medial-adventitial border (similar to the pattern of cKit+ cells reported here) and have the potential of differentiating into mesenchymal (endothelial-like or SMC-like) cells. Ex vivo data of the present study demonstrate that dR cells exhibited high mRNA expression levels of progenitor-associated cell markers cKit, CD34, and CD73 and had the potential of differentiating into α-SM-actin+, calponin+ spindle-shaped mesenchymal cells. In addition, the possibility that cKit may be expressed under certain conditions by activated fibroblasts, which can migrate into the media and even intima in response to injury, needs to be considered (10). Future studies are necessary to determine the origin(s) of the cells described here within.
In conclusion, in vivo and in vitro experiments of the present study demonstrate that chronic hypoxia induces the emergence of cells within the remodeled dPA media, that are phenotypically and functionally distinct from medial SMC, exhibit augmented proliferative (including autocrine), migratory and invasive capabilities, and secreted potent paracrine promitogenic factors for resident PA wall cells. These data suggest that accumulation of dR cells could contribute to the remodeling process not only directly through differentiation into mesenchymal/myofibroblast-like cells, but also indirectly through secretion of promitogenic and inflammatory mediators, which can modulate the pulmonary vessel wall microenvironment and the proliferative status of resident PA wall cells.
GRANTS
This work was supported by National Institutes of Health Grants HL-084923-1 (SCCOR) and PPG-HL-014985-35 and American Heart Association Grant 0550056Z.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We are thankful to Drs. Russ Anthony and Jan Rhodes for assistance in the neonatal bovine model, and to Marcia McGowan, Stephen Hofmeister, and Dr. Suzette Riddle for help in preparing the manuscript. We acknowledge the Developmental Studies Hybridoma Bank for providing antibodies against procollagen (clone SP1.D8) generated by Dr. Heinz Furthmayr and developed under the auspices of the National Institute of Child Health and Human Development (Univ. of Iowa, Dept. of Biological Sciences, Iowa City, IA).
REFERENCES
- 1.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 293: L1– L8, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C, Comhair SA, Xu W, Licina L, Huang L, Anand-Apte B, Yoder MC, Tuder RM, Erzurum SC. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol 172: 615– 627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball SG, Shuttleworth CA, Kielty CM. Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors. J Cell Mol Med 11: 1012– 1030, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 87: 858– 870, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Brisset AC, Hao H, Camenzind E, Bacchetta M, Geinoz A, Sanchez JC, Chaponnier C, Gabbiani G, Bochaton-Piallat ML. Intimal smooth muscle cells of porcine and human coronary artery express S100A4, a marker of the rhomboid phenotype in vitro. Circ Res 100: 1055– 1062, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci USA 100: 4754– 4759, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10: 858– 864, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Chang JS, Wendt T, Qu W, Kong L, Zou YS, Schmidt AM, Yan SF. Oxygen deprivation triggers upregulation of early growth response-1 by the receptor for advanced glycation end products. Circ Res 102: 905– 913, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Davie NJ, Crossno JT, Jr, Frid MG, Hofmeister SE, Reeves JT, Hyde DM, Carpenter TC, Brunetti JA, McNiece IK, Stenmark KR. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol 286: L668– L678, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Dolgachev VA, Ullenbruch MR, Lukacs NW, Phan SH. Role of stem cell factor and bone marrow-derived fibroblasts in airway remodeling. Am J Pathol 174: 390– 400, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donato R. RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Curr Mol Med 7: 711– 724, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Eddahibi S, Guignabert C, Barlier-Mur AM, Dewachter L, Fadel E, Dartevelle P, Humbert M, Simonneau G, Hanoun N, Saurini F, Hamon M, Adnot S. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle hyperplasia. Circulation 113: 1857– 1864, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Ergun S, Tilki D, Hohn HP, Gehling U, Kilic N. Potential implications of vascular wall resident endothelial progenitor cells. Thromb Haemost 98: 930– 939, 2007 [PubMed] [Google Scholar]
- 14.Farmer DG, Kennedy S. RAGE, vascular tone and vascular disease. Pharmacol Ther 124: 185– 194, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Frid MG, Aldashev AA, Dempsey EC, Stenmark KR. Smooth muscle cells isolated from discrete compartments of the mature vascular media exhibit unique phenotypes and distinct growth capabilities. Circ Res 81: 940– 952, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Frid MG, Aldashev AA, Nemenoff RA, Higashito R, Westcott JY, Stenmark KR. Subendothelial cells from normal bovine arteries exhibit autonomous growth and constitutively activated intracellular signaling. Arterioscler Thromb Vasc Biol 19: 2884– 2893, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659– 669, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frid MG, Moiseeva EP, Stenmark KR. Multiple phenotypically distinct smooth muscle cell populations exist in the adult and developing bovine pulmonary arterial media in vivo. Circ Res 75: 669– 681, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Gao XN, Tang SQ, Zhang XF. S100A4 antisense oligodeoxynucleotide suppresses invasive potential of neuroblastoma cells. J Ped Surg 40: 648– 652, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming growth factor beta-induced endothelial-to-mesenchymal transition: a switch to cardiac fibrosis? Trends Cardiovasc Med 18: 293– 298, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Greenway S, van Suylen RJ, Du Marchie Sarvaas G, Kwan E, Ambartsumian N, Lukanidin E, Rabinovitch M. S100A4/Mts1 produces murine pulmonary artery changes resembling plexogenic arteriopathy and is increased in human plexogenic arteriopathy. Am J Pathol 164: 253– 262, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall SM, Brogan P, Haworth SG, Klein N. Contribution of inflammation to the pathology of idiopathic pulmonary arterial hypertension in children. Thorax 64: 778– 783, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 54: S10– S19, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Hayashida K, Fujita J, Miyake Y, Kawada H, Ando K, Ogawa S, Fukuda K. Bone marrow-derived cells contribute to pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension. Chest 127: 1793– 1798, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Heizmann CW, Ackermann GE, Galichet A. Pathologies involving the S100 proteins and RAGE. Subcell Biochem 45: 93– 138, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 170: 1807– 1816, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiwatashi K, Ueno S, Kubo F, Sakoda M, Tateno T, Hayashi T, Abeyama K, Natsugoe S. Relevance of apoptosis and tolerance to hypoxic stress in cells transfected with receptor for advanced glycation end products (RAGE). Anticancer Res 29: 1287– 1294, 2009 [PubMed] [Google Scholar]
- 28.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest 113: 1258– 1265, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: 13S– 24S, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther 8: 569– 581, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Jeffery TK, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis 45: 173– 202, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Jones R, Jacobson M, Steudel W. α-Smooth-muscle actin and microvascular precursor smooth-muscle cells in pulmonary hypertension. Am J Respir Cell Mol Biol 20: 582– 594, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Jovin TM, Morris SJ, Striker G, Schultens HA, Digweed M, Arndt-Jovin DJ. Automatic sizing and separation of particles by ratios of light scattering intensities. J Histochem Cytochem 24: 269– 283, 1976 [DOI] [PubMed] [Google Scholar]
- 34.Kaplan-Albuquerque N, Bogaert YE, Van Putten V, Weiser-Evans MC, Nemenoff RA. Patterns of gene expression differentially regulated by platelet-derived growth factor and hypertrophic stimuli in vascular smooth muscle cells: markers for phenotypic modulation and response to injury. J Biol Chem 280: 19966– 19976, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Lantz MS, Ciborowski P. Zymographic techniques for detection and characterization of microbial proteases. Methods Enzymol 235: 563– 594, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Lawrie A, Spiekerkoetter E, Martinez EC, Ambartsumian N, Sheward WJ, MacLean MR, Harmar AJ, Schmidt AM, Lukanidin E, Rabinovitch M. Interdependent serotonin transporter and receptor pathways regulate S100A4/Mts1, a gene associated with pulmonary vascular disease. Circ Res 97: 227– 235, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Liao SH, Zhao XY, Han YH, Zhang J, Wang LS, Xia L, Zhao KW, Zheng Y, Guo M, Chen GQ. Proteomics-based identification of two novel direct targets of hypoxia-inducible factor-1 and their potential roles in migration/invasion of cancer cells. Proteomics 9: 3901– 3912, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Majka SM, Skokan M, Wheeler L, Harral J, Gladson S, Burnham E, Loyd JE, Stenmark KR, Varella-Garcia M, West J. Evidence for cell fusion is absent in vascular lesions associated with pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 295: L1028– L1039, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L548– L554, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Merklinger SL, Wagner RA, Spiekerkoetter E, Hinek A, Knutsen RH, Kabir MG, Desai K, Hacker S, Wang L, Cann GM, Ambartsumian NS, Lukanidin E, Bernstein D, Husain M, Mecham RP, Starcher B, Yanagisawa H, Rabinovitch M. Increased fibulin-5 and elastin in S100A4/Mts1 mice with pulmonary hypertension. Circ Res 97: 596– 604, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Metharom P, Caplice NM. Vascular disease: a new progenitor biology. Curr Vasc Pharmacol 5: 61– 68, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20– S31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mourani PM, Garl PJ, Wenzlau JM, Carpenter TC, Stenmark KR, Weiser-Evans MC. Unique, highly proliferative growth phenotype expressed by embryonic and neointimal smooth muscle cells is driven by constitutive Akt, mTOR, and p70S6K signaling and is actively repressed by PTEN. Circulation 109: 1299– 1306, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Nemenoff RA, Simpson PA, Furgeson SB, Kaplan-Albuquerque N, Crossno J, Garl PJ, Cooper J, Weiser-Evans MC. Targeted deletion of PTEN in smooth muscle cells results in vascular remodeling and recruitment of progenitor cells through induction of stromal cell-derived factor-1alpha. Circ Res 102: 1036– 1045, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Oslejskova L, Grigorian M, Gay S, Neidhart M, Senolt L. The metastasis associated protein S100A4: a potential novel link to inflammation and consequent aggressive behaviour of rheumatoid arthritis synovial fibroblasts. Ann Rheumatic Dis 67: 1499– 1504, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Ostman A. PDGF receptors-mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine Growth Factor Rev 15: 275– 286, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA 105: 9349– 9354, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peinado VI, Pizarro S, Barbera JA. Pulmonary vascular involvement in COPD. Chest 134: 808– 814, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Peinado VI, Ramirez J, Roca J, Rodriguez-Roisin R, Barbera JA. Identification of vascular progenitor cells in pulmonary arteries of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 34: 257– 263, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin Pharmacol Ther 82: 241– 243, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep 8: 145– 150, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Ross JJ, Hong Z, Willenbring B, Zeng L, Isenberg B, Lee EH, Reyes M, Keirstead SA, Weir EK, Tranquillo RT, Verfaillie CM. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J Clin Invest 116: 3139– 3149, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365– 386, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, Bin Hafeez B, Bhat KM, Sarfaraz S, Reagan-Shaw S, Spiegelman VS, Setaluri V, Mukhtar H. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc Natl Acad Sci USA 103: 14825– 14830, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samolov B, Steen B, Seregard S, van der Ploeg I, Montan P, Kvanta A. Delayed inflammation-associated corneal neovascularization in MMP-2-deficient mice. Exp Eye Res 80: 159– 166, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res 89: 1111– 1121, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med 8: 403– 409, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Satoh K, Fukumoto Y, Nakano M, Sugimura K, Nawata J, Demachi J, Karibe A, Kagaya Y, Ishii N, Sugamura K, Shimokawa H. Statin ameliorates hypoxia-induced pulmonary hypertension associated with down-regulated stromal cell-derived factor-1. Cardiovas Res 81: 226– 234, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols 3: 1101– 1108, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Shi Y, O'Brien JE, Jr, Ala-Kokko L, Chung W, Mannion JD, Zalewski A. Origin of extracellular matrix synthesis during coronary repair. Circulation 95: 997– 1006, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell RN. Host bone-marrow cells are a source of donor intimal smooth-muscle-like cells in murine aortic transplant arteriopathy. Nat Med 7: 738– 741, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Sobin SS, Tremer HM, Hardy JD, Chiodi HP. Changes in arteriole in acute and chronic hypoxic pulmonary hypertension and recovery in rat. J Appl Physiol 55: 1445– 1455, 1983 [DOI] [PubMed] [Google Scholar]
- 63.Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, Ylostalo J, Prockop DJ. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J 22: 1226– 1236, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675– 691, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Stenmark KR, Fasules J, Hyde DM, Voelkel NF, Henson J, Tucker A, Wilson H, Reeves JT. Severe pulmonary hypertension and arterial adventitial changes in newborn calves at 4,300 m. J Appl Physiol 62: 821– 830, 1987 [DOI] [PubMed] [Google Scholar]
- 66.Stenmark KR, Orton EC, Reeves JT, Voelkel NF, Crouch EC, Parks WC, Mecham RP. Vascular remodeling in neonatal pulmonary hypertension. Role of the smooth muscle cell. Chest 93: 127S– 133S, 1988 [PubMed] [Google Scholar]
- 67.Stiebellehner L, Frid MG, Reeves JT, Low RB, Gnanasekharan M, Stenmark KR. Bovine distal pulmonary arterial media is composed of a uniform population of well-differentiated smooth muscle cells with low proliferative capabilities. Am J Physiol Lung Cell Mol Physiol 285: L819– L828, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J Leukocyte Biol . In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takenaga K, Kozlova EN. Role of intracellular S100A4 for migration of rat astrocytes. Glia 53: 313– 321, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res 93: 783– 790, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Tarabykina S, Griffiths TR, Tulchinsky E, Mellon JK, Bronstein IB, Kriajevska M. Metastasis-associated protein S100A4: spotlight on its role in cell migration. Curr Cancer Drug Targets 7: 217– 228, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Tolboom TC, Huizinga TW. In vitro matrigel fibroblast invasion assay. Methods Mol Med 135: 413– 421, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 28: 23– 42, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tuder RM, Yun JH, Bhunia A, Fijalkowska I. Hypoxia and chronic lung disease. J Mol Med 85: 1317– 1324, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Varcoe RL, Mikhail M, Guiffre AK, Pennings G, Vicaretti M, Hawthorne WJ, Fletcher JP, Medbury HJ. The role of the fibrocyte in intimal hyperplasia. J Thromb Haemost 4: 1125– 1133, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Weiser-Evans MC, Quinn BE, Burkard MR, Stenmark KR. Transient reexpression of an embryonic autonomous growth phenotype by adult carotid artery smooth muscle cells after vascular injury. J Cell Physiol 182: 12– 23, 2000 [DOI] [PubMed] [Google Scholar]
- 77.Weiser-Evans MC, Schwartz PE, Grieshaber NA, Quinn BE, Grieshaber SS, Belknap JK, Mourani PM, Majack RA, Stenmark KR. Novel embryonic genes are preferentially expressed by autonomously replicating rat embryonic and neointimal smooth muscle cells. Circ Res 87: 608– 615, 2000 [DOI] [PubMed] [Google Scholar]
- 78.Xaymardan M, Cimini M, Fazel S, Weisel RD, Lu WY, Martin U, Harvey RP, Li RK. c-Kit function is necessary for in vitro myogenic differentiation of bone marrow hematopoietic cells. Stem Cells 27: 1911– 1920, 2009 [DOI] [PubMed] [Google Scholar]
- 79.Young KC, Torres E, Hatzistergos KE, Hehre D, Suguihara C, Hare JM. Inhibition of the SDF-1/CXCR4 axis attenuates neonatal hypoxia-induced pulmonary hypertension. Circ Res 104: 1293– 1301, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zalewski A, Shi Y. Vascular myofibroblasts. Lessons from coronary repair and remodeling. Arterioscler Thromb Vasc Biol 17: 417– 422, 1997 [DOI] [PubMed] [Google Scholar]
- 81.Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 133: 1543– 1551, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.