Abstract
The venoarteriolar response (VAR) increases vascular resistance upon increases in venous transmural pressure in cutaneous, subcutaneous, and muscle vascular beds. During orthostasis, it has been proposed that up to 45% of the increase in systemic vascular tone is due to VAR-related local mechanism(s). The objective of this project was to test the hypothesis that heat stress attenuates VAR-mediated cutaneous and whole leg vasoconstriction. During normothermic conditions, measurements of cutaneous blood flow (laser-Doppler flowmetry) and femoral artery blood flow (Doppler ultrasound) were obtained from both legs during supine and leg-dependent conditions. These measurements were repeated following a whole body heat stress (increase in internal temperature of 1.4 ± 0.2°C). Before leg dependency, cutaneous (CVC) and femoral vascular conductances (FVC) were significantly elevated in both legs during heat stress relative to normothermia (P < 0.001). During leg dependency the absolute decrease in CVC was attenuated during heat stress (P < 0.01) while the absolute decrease in FVC was unaffected (P = 0.90). When CVC and FVC data were analyzed as a relative change from their respective baseline values, heat stress significantly attenuated the magnitude of vasoconstriction due to leg dependency in the cutaneous and femoral circulations (P < 0.001 for both variables). These data suggest that an attenuated local vasoconstriction, evoked via the venoarteriolar response, may contribute to reduced blood pressure control and thus reduced orthostatic tolerance that occurs in heat-stressed individuals.
Keywords: hyperthermia, orthostatic tolerance, blood pressure
in humans, an elevation in internal temperature imposes a significant stress to the cardiovascular system (26). To optimize heat dissipation, cutaneous blood flow increases to values as high as 7,500 ml/min compared with ∼300 ml/min when individuals are normothermic (25, 26). Maintenance of arterial blood pressure during heat stress conditions, in the face of robust increases in cutaneous vascular conductance (CVC), requires profound increases in resistance of noncutaneous vascular beds (24, 25, 27).
The control of blood pressure becomes compromised when an orthostatic challenge is superimposed with elevated internal temperatures (5, 25, 38, 39). The mechanism(s) resulting in reduced blood pressure control during heat stress is multifaceted, most likely owing to reductions in cerebral blood flow (20, 38), cutaneous vascular sensitivity to sympathetic nerve activity (18, 37), central blood volume (4), and ventricular filling pressures (2, 22, 26, 41).
A primary component of blood pressure regulation during an orthostatic challenge is an appropriate increase in vascular resistance. Vascular resistance can be elevated through a variety of methods, including increased sympathetic efferent outflow, as well as local mechanisms such as the venoarteriolar response (VAR) (12, 14, 15). Vasoconstriction evoked by the VAR occurs when venous transmural pressure is elevated in cutaneous, subcutaneous, and muscle vascular beds, secondary to venous stasis, which occurs during orthostasis (1, 13, 31). As a result of this increase in venous pressure, local vascular resistance is elevated and thus blood flow decreases within that region (15). During orthostasis, it has been suggested that the VAR contributes up to 45% of the increase in systemic vascular tone, while the central reflex response to unloading of the baroreceptors contributes the other 55% (12, 15). An attenuated VAR may contribute, in part, to the decreases in orthostatic tolerance that occur in individuals following head-down-tilt bed rest (40) and patients with postural orthostatic tachycardia syndrome (POTS) (33), while a preserved VAR response in individuals with a spinal cord injury likely contributes to their maintenance of orthostatic tolerance (9, 32, 35).
Two studies have investigated the effects of heating on cutaneous vasoconstrictor responsiveness, both of which reported the heating stimulus attenuated cutaneous vasoconstriction to the VAR. The first investigated the effect of local heating on forearm skin responsiveness to the VAR (7). Aside from this assessment being on forearm skin, which would be less likely to be exposed to pressures that engage the VAR, the effect of local cutaneous heating on the VAR would likely be different relative to cutaneous vasodilation due to indirect whole body heating (i.e., heating the core but not the area where skin blood flow is assessed). The second study investigated the effects of a relatively minor whole body heat stress (i.e., increase in internal temperature of ∼0.5°C) on VAR-mediated vasoconstriction in the lower leg (42). The results of the second study are also clouded by the possibility that vasoconstrictor responses to the mode utilized to engage the VAR (i.e., proximal cuff inflation) were due to a combination of the VAR and reduced perfusion pressure, as recently proposed (23).
An attenuated VAR from cutaneous vascular beds of the lower limbs during an orthostatic stress of profoundly heat-stressed subjects may contribute to attenuated increases in vascular resistance, thereby predisposing subjects to reduced orthostatic tolerance while in this thermal condition. Moreover, the effects of heat stress on the VAR of an entire limb, comprising both skin and muscle vascular beds, are unknown. Since reduced vasoconstrictive responses of the lower extremities could have important implications with respect to orthostatic tolerance, and the femoral artery is a large-conduit vessel serving both muscle and skin of the legs, identification of the effects of heat stress on the VAR of the entire lower limbs could provide further mechanistic insight regarding the etiology of heat stress-induced reductions in orthostatic tolerance. Given this background, the aim of this study was to test the hypothesis that whole body heat stress attenuates the cutaneous and whole leg vasoconstrictor response evoked by the VAR relative to when subjects are normothermic.
METHODS
Sixteen healthy normotensive subjects (10 men and 6 women) participated in this study. Average subject characteristics were: age, 35 ± 10 yr; height, 172 ± 8 cm; and weight, 68 ± 9 kg (mean ± SD). Subjects were not taking medications and were free of any known cardiovascular, metabolic, or neurological diseases. The phase of the menstrual cycle of the female subjects was not taken into account with the collection of these data. Subjects were informed of the purpose and risks of the study before providing their informed written consent. The protocol and consent were approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas. Subjects refrained from alcohol, caffeine, and exercise for 24 h before the study.
Instrumentation and Measurements
Each subject swallowed an ingestible telemetry pill for the measurement of intestinal temperature (HQ, Palmetto, FL). Subjects were fitted with a water-perfused, tube-lined suit (Med-Eng, Ottawa, Canada) that covered the entire body except for the head, face, hands, one leg, and feet. One leg, randomized between subjects, was not covered by the suit, thereby permitting the evaluation of the VAR in a leg that was only subjected to reflex-induced cutaneous vasodilation (i.e., exposed leg) and a leg subjected to both reflex and local heating-induced cutaneous vasodilation (i.e., covered leg). The suit permitted the control of skin and internal temperatures by adjusting the temperature of the water perfusing the suit. Mean skin temperature was measured from the weighted average of six thermocouples attached to the skin under the water-perfused suit (34). An additional thermocouple was attached to the shin of the exposed leg to monitor local skin temperature. Heart rate was continuously obtained from an electrocardiogram (HP Patient Monitor, Agilent, Santa Clara, CA) interfaced with a cardiotachometer (CWE, Ardmore, PA). Intermittent blood pressure measurements were obtained by auscultation of the brachial artery via electrosphygmomanometry (SunTech, Raleigh, NC). Skin blood flow was indexed at the top of the ankle and the top of the shin (approximately midway between the ankle joint and the knee joint) on both the covered and exposed legs (total of 4 sites) via laser-Doppler flowmetry using integrating flow probes (MoorLab Laser-Doppler Perfusion Monitor, Moor Instruments, Wilmington, DE).
Femoral blood flow.
Femoral artery diameter and femoral blood velocity were obtained using commercially available Doppler ultrasound equipment and were used for subsequent calculation of femoral blood flow. Ultrasound imaging of femoral artery diameter was performed from the perpendicular image along the central axis of the scanned area and was accomplished using a linear array transducer at a site ∼2 cm proximal to the femoral artery bifurcation where the best spatial resolution was achieved. The blood velocity profiles were obtained using the same transducer with a sample volume depth of 3.5–4 mm and size adjusted to cover the entire width of the artery. During all blood velocity measurements the probe was appropriately positioned in a fixed manner to maintain an insonation angle of ≤60°. Femoral artery diameter images were stored onto the hard drive of the Doppler ultrasound machine and were later measured, using on-screen calipers. Angle-corrected, time- and space-averaged, and intensity-weighted mean femoral artery blood velocities were calculated using on screen calipers immediately following each data collection period.
Experimental protocol.
All experiments were performed in a temperature-controlled laboratory (∼26°C) in the morning or early afternoon. Following instrumentation, subjects rested on a patient table in the supine position while thermoneutral water (34°C) circulated through the suit. The patient table was adapted with two planks each attached to the table by hinges. With this set-up the upper portion of the subject (from the hips up) rested on the patient table while the lower portion (from the hips down) rested on the planks with each leg on a separate plank. This configuration enabled one leg to be lowered at ∼45° at the hip with the leg remaining on the plank, while the remainder of the body, including the contralateral leg, remained in the horizontal position, thus minimizing/eliminating the effects of unloading baroreceptors. During leg dependency, the dependent ankle was ∼41 cm below the contralateral (i.e., supine) ankle. Data collection during normothermic baseline included 6 min of continuous assessment of heart rate, internal temperature, mean skin temperature, skin blood flows, and skin temperatures. Blood pressure was measured intermittently via auscultation of the brachial artery. Following this baseline period Doppler ultrasound assessment of femoral artery diameter and velocities were performed on one leg while in the horizontal position. Immediately after completion of this measurement, that leg was lowered at the hip, and following a 60-s stabilization period the aforementioned Doppler ultrasound measurements were repeated, along with continuous measures of skin blood flow. The leg was in the dependent position for ∼4 min. On completion of these measurements the lowered leg was returned to horizontal. The aforementioned measures and procedures were then repeated from the opposite leg. Following completion of normothermic data collection, whole body heating began by circulating 49°C water through the suit until internal temperature increased ∼1.3°C above baseline temperature. Once this increase in internal temperature was attained, the temperature of the water circulating the suit was slightly decreased (typically decreased to ∼47.5°C) in an effort to attenuate the rate of rise in internal temperature during data collection. Following a 6-min baseline heat stress data collection period, the aforementioned femoral artery diameter, femoral artery velocity, and skin blood flow data were obtained from both legs separately in the horizontal and dependent positions as outlined above.
Data Analysis
Thermal and hemodynamic data were sampled at 50 Hz via a data-acquisition system (Biopac System, Santa Barbara, CA). Data from the last 60 s of the 6-min baseline period were averaged and compared between thermal conditions. The effect of leg dependency on cutaneous blood flow and conductance was always calculated at 2 min post-leg lowering and was compared relative to the last minute before leg lowering. There were no differences in CVC responses to leg lowering between the distal and the proximal sites of either the covered or exposed legs regardless of the thermal condition. Additionally, the effects of heat stress on the cutaneous vasoconstrictor responsiveness were similar between sites; therefore, cutaneous blood flow and conductance data were analyzed using the average of the ankle and shin sites for each leg. The average of six measurements of femoral artery diameter and an average of 20–30 s of intensity-weighted time-averaged mean femoral blood velocities (Vmean) were used for subsequent blood flow determination. Femoral artery blood flow was calculated as: femoral blood flow (ml/min) = Vmean·π·(femoral artery diameter/2)2·60. Leg vascular conductance was calculated as: femoral blood flow/MAP where MAP is mean arterial pressure. CVC was calculated as: skin blood flux/MAP.
Statistical comparisons between steady state (i.e., last minute of the 6-min baseline data collection period) thermal (i.e., core and skin temperatures) and hemodynamic (i.e., MAP and heart rate) data during normothermic and heat stress conditions were performed using paired t-tests. The effects of core body temperature (normothermia vs. heat stress) and local skin temperature (exposed leg vs. covered leg) on femoral and cutaneous blood flows and vascular conductances before the VAR were evaluated using a two-way ANOVA. Likewise, a two-way ANOVA was used to evaluate the effects of core body temperature and local skin temperature on the magnitude of the reduction of those variables to the VAR (i.e., calculated delta from pre-VAR). For each thermal condition, the effect of the VAR on cutaneous blood flow and vascular conductance of the limb that remained horizontal was analyzed using a paired-samples t-test (i.e., supine vs. lowered of the contralateral limb). All statistical analyses were performed using a commercially available statistical software package (SigmaStat 3.11, Chicago, IL). All values are reported as means ± SD. P values < 0.05 were considered statistically significant.
RESULTS
Before the whole body heat stress, mean skin and internal temperatures were 34.5 ± 0.6°C and 37.0 ± 0.2°C, respectively. Whole body heat stress increased mean skin temperature by 4.3 ± 0.8°C (P < 0.001) and internal temperature by 1.4 ± 0.2°C (P < 0.001). MAP was not altered by heat stress relative to normothermia (P = 0.78), while heart rate was increased (P < 0.001; Table 1).
Table 1.
Baseline thermal and hemodynamic values during normothermia and heat stress
| Normothermia | Heat Stress | P Value | |
|---|---|---|---|
| Mean arterial pressure, mmHg | 80±7 | 80±6 | 0.78 |
| Heart rate, beats/min | 58±8 | 99±16 | <0.001 |
| Internal temperature, °C | 37.0±0.2 | 38.4±0.2 | <0.001 |
| Mean skin temperature, °C | 34.5±0.6 | 38.8±0.6 | <0.001 |
Values are means ± SD.
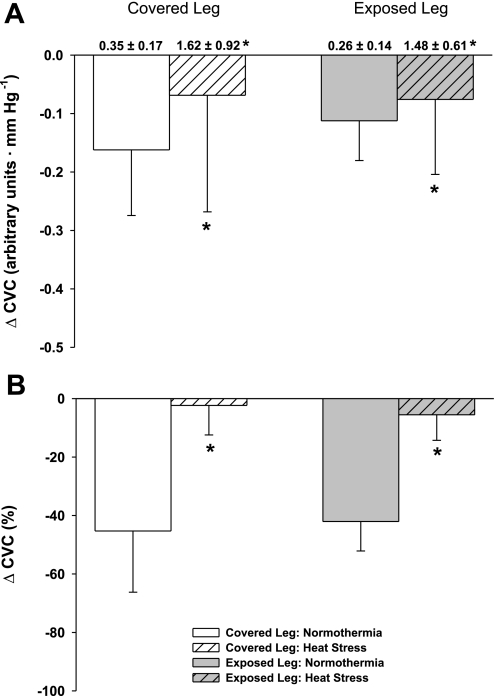
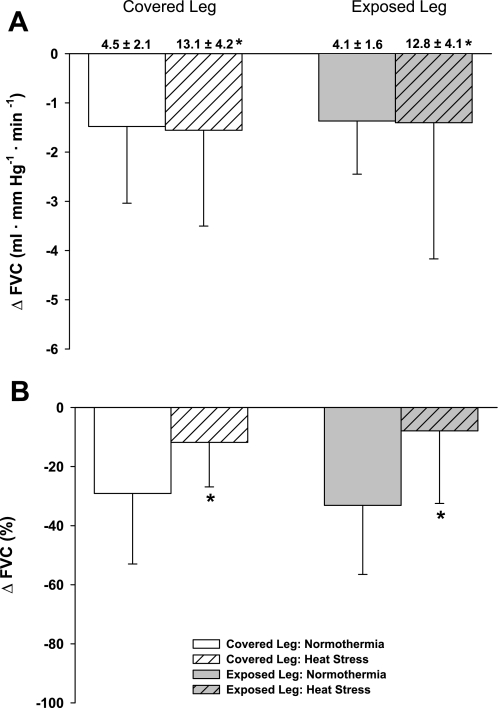
Baseline (i.e., before leg lowering) CVC and femoral vascular conductance (FVC) were significantly increased in both legs during heat stress relative to each respective normothermic condition (P < 0.001). There was no difference in baseline CVC (P = 0.41) or FVC (P = 0.46) between the covered and exposed leg within each thermal condition (Figs. 1A and 2A). While normothermic, leg lowering reduced CVC (∼45%) and FVC (∼35%) relative to the supine condition, indicating successful activation of the VAR. During leg lowering, the absolute decrease in CVC was similar between covered and uncovered legs (Fig. 1A: P = 0.64) but was attenuated during heat stress, relative to normothermia, for both legs (Fig. 1A: P < 0.01). The absolute decrease in FVC was similar between legs (Fig. 2A: P = 0.79) and thermal conditions (Fig. 2A: P = 0.90). When CVC and FVC data were analyzed as a relative change from the respective baseline condition (i.e., before leg lowering), the VAR-mediated vasoconstriction was attenuated during heat stress from both the covered and exposed legs (P < 0.001 for both CVC and FVC), with equal attenuation of these responses between legs (CVC: P = 0.11; FVC: P = 0.53).
Fig. 1.
Cutaneous vascular conductance (CVC) responses to leg dependency. Baseline (i.e., before leg dependency) absolute CVC values for each condition are shown above their respective bars in A. Heat stress significantly increased baseline CVC in both the covered and exposed leg with respect to normothermia, and the magnitude of this increase was similar between legs. Panel A illustrates the absolute decrease in CVC during leg dependency, which was similar between the covered and exposed legs (P = 0.64); however, the response was attenuated in both legs during heat stress (P > 0.01). When the CVC data are represented as a percent change (B), there was a significant attenuation of vasoconstriction in both legs due to leg dependency during heat stress relative to normothermia (P < 0.001); however, the effect of heat stress was not different between the covered and exposed legs (P = 0.99). *Heat stress significantly different relative to the corresponding normothermic condition.
Fig. 2.
Femoral vascular conductance (FVC) response to leg dependency. Baseline (i.e., before leg dependency) absolute FVC values for each condition are shown above their respective bars in A. Heat stress significantly increased baseline FVC in both the covered and exposed leg with respect to normothermia, and the magnitude of this increase was similar between legs. Panel A illustrates the absolute decrease in FVC during leg dependency, which was similar between thermal conditions (P = 0.90) regardless of whether the leg was covered or exposed (P = 0.79). When the FVC data are represented as a percent change (B), there was a significant attenuation of vasoconstriction in both legs due to leg dependency during heat stress relative to normothermia (P > 0.001); however, the effect of heat stress was not different between the covered and exposed legs (P = 0.99). *Heat stress significantly different relative to the corresponding normothermic condition.
The cutaneous and femoral blood flow responses to leg lowering followed similar patterns to that observed with CVC and FVC, i.e., while normothermic, in that leg dependency reduced cutaneous blood flow (∼45%) and femoral blood flow (∼35%) relative to the supine condition, again indicating successful activation of the VAR. The absolute decrease in cutaneous blood flow to leg lowering was similar between covered and uncovered legs (P = 0.60) but was greater during normothermia for both legs (P < 0.01), whereas there was no difference between legs (P = 0.92) or thermal conditions (P = 0.90) in the absolute reduction in femoral blood flow to leg lowering. When analyzed as a relative change, the reduction in cutaneous and femoral blood flows was attenuated during heat stress (P < 0.01 for both variables). There was no effect of thermal condition or leg dependency on femoral artery diameter from either leg, indicating that the observed changes in femoral blood flow were attributed to downstream vascular adaptations. There was no effect of leg lowering on CVC or cutaneous blood flow in the leg that remained horizontal, indicating that the effects of limb dependency were localized to the leg being lowered. Femoral blood flow from the leg that remained horizontal, when the opposing leg was lowered, was not measured.
DISCUSSION
The primary findings of this study are that the decreases in CVC and FVC during the engagement of the VAR via leg dependency are significantly attenuated when subjects are heat stressed to a magnitude that is associated with an increased incidence of orthostatic intolerance (5, 25, 38, 39). The observed CVC responses are generally in agreement with a previous report of an attenuated VAR response during a less severe whole body heat stress (42), which is unlikely to impair orthostatic tolerance to the same extent relative to the degree of heating for the present study. Finally, to our knowledge, the present study is the first to investigate the effects of heat stress on VAR-mediated vasoconstriction of the legs via assessment of flow through the femoral artery. The present findings suggest that an attenuated cutaneous and whole leg VAR could contribute to reduced orthostatic tolerance in heat-stressed individuals.
A reduction in blood pressure control and thus orthostatic tolerance has been extensively demonstrated in individuals with elevated internal temperature (5, 24, 38, 39). During heat stress conditions cardiac output can more than double, approaching values as high as 13 l/min, with 50% or more of that value being directed toward skin (25, 26). In the present study femoral blood flow was used to calculate vascular conductance of the whole leg. While it is understood that a large portion of femoral blood flow during heat stress is directed toward the cutaneous circulation, femoral blood flow represents the total amount of blood perfusing the leg. This point is particularly important given the small area of skin evaluated with the laser-Doppler probes (<0.3 cm2). A reduction in whole leg vasoconstriction during leg dependency associated with standing could have major implications for blood pressure control during orthostasis. The present findings indicate that the VAR associated with leg dependency only reduced FVC by ∼10% when subjects were heat stressed, whereas during normothermia FVC was reduced by ∼35%. An example of the importance of this inadequate vasoconstriction during orthostasis of heat-stressed subjects is as follows. If cardiac output were elevated to 10 l/min during supine conditions in a heat-stressed individual and MAP were 80 mmHg, then systemic vascular conductance would be 125 ml·mmHg−1·min−1, ignoring the effect of heat stress on central venous pressure. On standing, or a similar orthostatic challenge, if cardiac output dropped by 35% (39), and the 55% contribution of increased vascular tone via central reflex mechanisms (12, 15) was fully effective while the VAR only reduced vascular conductance by 10%, then the resulting MAP would be ∼69 mmHg. If this same scenario were to occur during normothermia in an individual with a supine cardiac output of 5 l/min, a MAP of 80 mmHg, and a fully effective VAR, then MAP would be maintained on orthostasis. The above-mentioned scenario is based on several assumptions that are recognized to not be physiologically accurate. For example, the magnitude of decrease in cardiac output that occurs during orthostasis in nonsyncopal normothermic subjects is closer to 25% (10); however, during heat stress the reduction is ∼35% (39). Another potentially inaccurate assumption is that the magnitude of change in the VAR in the femoral artery is reflective of the systemic VAR. Last, it may be that in the aforementioned scenario other redundant blood pressure control mechanisms (i.e., baroreflex-mediated vasoconstriction) would assume a greater contributory role, thereby minimizing reductions in arterial pressure to an attenuated VAR. Despite these potential caveats, the point remains that a significant attenuation of the VAR during heat stress may have a profound impact on blood pressure control and thus may contribute to reduced orthostatic tolerance while individuals are in this thermal condition (5, 24, 38, 39).
During heat stress, due to large increases in CVC and the fraction of cardiac output to the skin, control of the cutaneous circulation becomes increasingly more critical for blood pressure control. Absolute cutaneous blood flow is reduced in the forearm circulation of heat-stressed individuals on exposure to an orthostatic challenge (i.e., lower-body negative pressure). However, the relative change is greatly attenuated during an orthostatic challenge, even at the onset of syncopal symptoms, when the individual is heat stressed (17). While in the cited study (17) the forearm was not exposed to increased distending pressures, and thus was not exposed to the VAR, it is possible that heat stress-induced attenuation of the VAR in the regions exposed to elevated venous pressures (i.e., the legs and lower torso) could contribute to compromised blood pressure control while in this thermal condition. In this regard, and in contrast to the present findings, Yamazaki et al. (42) reported greater reductions in absolute cutaneous blood flow in the calf during incremental increases in thigh cuff pressure following an increase in internal temperature of ∼0.5°C. However, the relative reduction was attenuated while heat stressed during moderate increases in thigh cuff pressure (i.e., 30 and 50 mmHg), whereas the effect of greater levels of thigh cuff pressure was similar between thermal conditions (42). This is in stark contrast to the present protocol where we observed less of a decrease in absolute CVC to the VAR during heat stress compared with during normothermia. Furthermore, the relative decrease in CVC to the VAR, in the present study, was ∼45% during normothermia whereas during heat stress this reduction was ∼5%. It is likely that differences in the magnitude of the imposed heat stress in the study by Yamazaki et al. (42) (0.5°C increase in core temperature) and the present protocol (1.4°C increase in core temperature) contributed to the contrasting findings. Additionally, with the thigh cuff inflation protocol utilized by Yamazaki et al. (42) the reduction in blood flow occurs through both the VAR and through a decrease in perfusion gradient, given the elevation in venous pressure without a corresponding increase in arterial pressure (23), whereas the reductions in flow due to leg dependency occur only through the VAR. Thus the differences in the technique to evoke the VAR between studies may have also contributed to these contrasting observations. Nonetheless, an attenuated VAR in the cutaneous circulation of the lower leg, and perhaps other regions of the body, following heat stress implicates a heat stress-induced attenuation of the VAR as a possible mechanism contributing to reduced blood pressure control.
Davison et al. (7) found that the VAR was attenuated when the skin was locally heated to 42°C. In the present study the cutaneous and whole limb VAR was evaluated from the leg that was and was not exposed to the tube-lined suit. Thus local skin temperature was different between these legs. While we did not independently assess skin temperature from the covered leg, it is assumed that this temperature was relatively close to mean skin temperature (∼38°C). Skin temperature on the exposed leg during the heat stress was 33.1 ± 1.4°C. Despite local temperature being elevated on the covered leg, the ANOVA revealed that the magnitude of the reductions in both CVC and FVC while subjects were heat stressed was not different between the uncovered and covered legs, although this finding was not unexpected given that attenuation of the cutaneous VAR did not occur when the skin was locally heated to 38 °C in the study by Davison et al. (7).
In the present protocol, leg dependency was chosen over upright tilting to engage the VAR given that upright tilting decreases central blood volume, which unloads baroreceptors, resulting in central activation of the sympathetic nervous system, which itself could be a mechanism for reductions in CVC and FVC. Thus upright tilting does not exclusively isolate the VAR. The absence of a reduction in arterial blood pressure or an elevation in heart rate during leg dependency in the present study strongly suggests that this perturbation caused little to no reduction in baroreceptor loading status. That being said, it is possible that VAR-mediated vasoconstriction during leg dependency occurs through, or in conjunction with, local myogenic mechanisms (6, 16). With the present study design it is difficult if not impossible to decipher contributory differences between VAR- and myogenic-mediated vasoconstriction. Another common difficulty encountered in these types of studies involves the comparison of blood flow or conductance responses between conditions where baseline values are dramatically different, as is the case in the present study in which cutaneous and femoral blood flows and conductances were elevated by approximately 3.5- to 4-fold during heat stress. In these scenarios it is common that the absolute reduction is greater in the high-flow/conductance scenario (i.e., heat stress) whereas the relative reduction is significantly attenuated. Interestingly, despite these large elevations in blood flow and vascular conductance, during leg dependency the absolute reduction in cutaneous blood flow was attenuated during heat stress, whereas the absolute reduction in femoral blood flow was similar between thermal conditions. When taken together, attenuated or similar absolute reductions in CVC and FVC between normothermia and heat stress conditions, coupled with significantly attenuated relative reductions in these variables during the engagement of the VAR, strongly suggest that pronounced heat stress attenuates the VAR.
It has been hypothesized that the VAR occurs through activation of stretch receptors in small veins, which results in vasoconstriction of upstream arterioles (12), although the mechanisms through which this occurs remain unknown. The findings that the response persists in sites distal to acute spinal and sympathetic neural blockade (11, 12, 14, 15, 31, 36), in denervated skin flaps (43), in areas distal to the lesion in spinal cord-injured individuals (1, 9, 32, 35), and is abolished following local anesthesia at the site of measurement suggest that the VAR is a locally mediated response (11, 12, 14, 15, 36). Additionally, the VAR is not mediated by α-adrenergic mechanisms, given that in cutaneous tissue this response was preserved at sites receiving α-adrenergic antagonists (3). Thus the VAR is mediated either by nonadrenergic but neurally mediated mechanisms or by local myogenic mechanisms within the vasculature (6, 16). Furthermore, the mechanism(s) resulting in attenuated vasoconstriction to the VAR during heat stress are unknown. Shibasaki et al. (29) proposed that variables associated with cutaneous active vasodilation have the capability to attenuate cutaneous vasoconstrictor responsiveness. Similarly, nitric oxide, which contributes to cutaneous vasodilation during heat stress (19, 21, 28, 30), has been shown to attenuate vasoconstrictor responses in a variety of tissues, including the skin (8). On the basis of those data, it may be that attenuated skin, and thus whole limb, vasoconstriction in response to limb dependency during the heat stress was due to neurotransmitter(s) associated with cutaneous active vasodilation, and/or nitric oxide, inhibiting VAR-mediated vasoconstriction. Alternatively, attenuated VAR responsiveness may simply be due to an effect of cutaneous vasodilation overriding a VAR-mediated cutaneous vasoconstrictor stimulus.
In summary, the vasocontrictive response that occurs during the engagement of the VAR via leg dependency is significantly attenuated when evaluated at the femoral artery as well as the cutaneous vasculature of the lower leg following pronounced whole body heating. Reduced vasoconstrictor responses, and thereby attenuated reductions in vascular conductance to the VAR in heat-stressed subjects, likely contribute to compromised blood pressure control and thus reduced orthostatic tolerance that occurs when individuals are in this thermal state.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL-61388 and HL-84072 and by The Research and Education Institute of Texas Health Resources.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We express appreciation to Jena Porterfield for technical assistance and to the subjects for their willing participation in this project.
REFERENCES
- 1. Andersen EB, Boesen F, Henriksen O, Sonne M. Blood flow in skeletal muscle of tetraplegic man during postural changes. Clin Sci (Colch) 70: 321–325, 1986. [DOI] [PubMed] [Google Scholar]
- 2. Crandall CG, Levine BD, Etzel RA. Effect of increasing central venous pressure during passive heating on skin blood flow. J Appl Physiol 86: 605–610, 1999. [DOI] [PubMed] [Google Scholar]
- 3. Crandall CG, Shibasaki M, Yen TC. Evidence that the human cutaneous venoarteriolar response is not mediated by adrenergic mechanisms. J Physiol 538: 599–605, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crandall CG, Wilson TE, Marving J, Vongelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol 586: 293–301, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci 116: 54–61, 2004. [DOI] [PubMed] [Google Scholar]
- 6. Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. [DOI] [PubMed] [Google Scholar]
- 7. Davison JL, Short DS, Wilson TE. Effect of local heating and vasodilation on the cutaneous venoarteriolar response. Clin Auton Res 14: 385–390, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Durand S, Davis SL, Cui J, Crandall CG. Exogenous nitric oxide inhibits sympathetically mediated vasoconstriction in human skin. J Physiol 562: 629–634, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Groothuis JT, Boot CR, Houtman S, van Langen H, Hopman MT. Leg vascular resistance increases during head-up tilt in paraplegics. Eur J Appl Physiol 94: 408–414, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Harms MP, van Lieshout JJ, Jenstrup M, Pott F, Secher NH. Postural effects on cardiac output and mixed venous oxygen saturation in humans. Exp Physiol 88: 611–616, 2003. [DOI] [PubMed] [Google Scholar]
- 11. Hassan AA, Tooke JE. Mechanism of the postural vasoconstrictor response in the human foot. Clin Sci (Colch) 75: 379–387, 1988. [DOI] [PubMed] [Google Scholar]
- 12. Henriksen O. Local sympathetic reflex mechanism in regulation of blood flow in human subcutaneous adipose tissue. Acta Physiol Scand Suppl 450: 1–48, 1977. [PubMed] [Google Scholar]
- 13. Henriksen O, Nielsen SL, Paaske WP, Sejrsen P. Autoregulation of blood flow in human cutaneous tissue. Acta Physiol Scand 89: 538–543, 1973. [DOI] [PubMed] [Google Scholar]
- 14. Henriksen O, Sejrsen P. Local reflex in microcirculation in human cutaneous tissue. Acta Physiol Scand 98: 227–231, 1976. [DOI] [PubMed] [Google Scholar]
- 15. Henriksen O, Sejrsen P. Local reflex in microcirculation in human skeletal muscle. Acta Physiol Scand 99: 19–26, 1977. [DOI] [PubMed] [Google Scholar]
- 16. Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J Appl Physiol 91: 973–983, 2001. [DOI] [PubMed] [Google Scholar]
- 17. Johnson JM, Niederberger M, Rowell LB, Eisman MM, Brengelmann GL. Competition between cutaneous vasodilator and vasoconstrictor reflexes in man. J Appl Physiol 35: 798–803, 1973. [DOI] [PubMed] [Google Scholar]
- 18. Kellogg DL, Jr, Johnson JM, Kosiba WA. Baroreflex control of the cutaneous active vasodilator system in humans. Circ Res 66: 1420–1426, 1990. [DOI] [PubMed] [Google Scholar]
- 19. Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vasculature effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 20. Low DA, Wingo JE, Keller DM, Davis SL, Zhang R, Crandall CG. Cerebrovascular responsiveness to steady-state changes in end-tidal CO2 during passive heat stress. J Appl Physiol 104: 976–981, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 22. Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters cardiovascular response to direct passive heating. J Appl Physiol 84: 1323–1332, 1998. [DOI] [PubMed] [Google Scholar]
- 23. Okazaki K, Fu Q, Martini ER, Shook R, Conner C, Zhang R, Crandall CG, Levine BD. Vasoconstriction during venous congestion: effects of venoarteriolar response, myogenic reflexes, and hemodynamics of changing perfusion pressure. Am J Physiol Regul Integr Comp Physiol 289: R1354–R1359, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension 15: 505–507, 1990. [DOI] [PubMed] [Google Scholar]
- 25. Rowell LB. Thermal stress. In: Human Circulation Regulation During Physical Stress. New York: Oxford Univ. Press, 1986, p. 174–212 [Google Scholar]
- 26. Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 27: 673–680, 1969. [DOI] [PubMed] [Google Scholar]
- 27. Rowell LB, Detry JR, Profant GR, Wyss C. Splanchnic vasoconstriction in hyperthermic man—role of falling blood pressure. J Appl Physiol 31: 864–869, 1971. [DOI] [PubMed] [Google Scholar]
- 28. Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85: 830–834, 1998. [DOI] [PubMed] [Google Scholar]
- 29. Shibasaki M, Davis SL, Cui J, Low DA, Keller DM, Durand S, Crandall CG. Neurally mediated vasoconstriction is capable of decreasing skin blood flow during orthostasis in the heat-stressed human. J Physiol 575: 953–959, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J Appl Physiol 93: 1947–1951, 2002. [DOI] [PubMed] [Google Scholar]
- 31. Skagen K, Bonde-Petersen F. Regulation of subcutaneous blood flow during head-up tilt (45°) in normals. Acta Physiol Scand 114: 31–35, 1982. [DOI] [PubMed] [Google Scholar]
- 32. Skagen K, Jensen K, Henriksen O, Knudsen L. Sympathetic reflex control of subcutaneous blood flow in tetraplegic man during postural changes. Clin Sci (Colch) 62: 605–609, 1982. [DOI] [PubMed] [Google Scholar]
- 33. Stewart JM, Medow MS, Montgomery LD. Local vascular responses affecting blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 285: H2749–H2756, 2003. [DOI] [PubMed] [Google Scholar]
- 34. Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989. [DOI] [PubMed] [Google Scholar]
- 35. Theisen D, Vanlandewijck Y, Sturbois X, Francaux M. Blood distribution adaptations in paraplegics during posture changes: peripheral and central reflex responses. Eur J Appl Physiol 81: 463–469, 2000. [DOI] [PubMed] [Google Scholar]
- 36. Vissing SF, Secher NH, Victor RG. Mechanisms of cutaneous vasoconstriction during upright posture. Acta Physiol Scand 159: 131–138, 1997. [DOI] [PubMed] [Google Scholar]
- 37. Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci 97: 122–128, 2002. [DOI] [PubMed] [Google Scholar]
- 38. Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol 291: R1443–R1448, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol 93: 85–91, 2002. [DOI] [PubMed] [Google Scholar]
- 40. Wilson TE, Shibasaki M, Cui J, Levine BD, Crandall CG. Effects of 14 days of head-down tilt bed rest on cutaneous vasoconstrictor responses in humans. J Appl Physiol 94: 2113–2118, 2003. [DOI] [PubMed] [Google Scholar]
- 41. Wilson TE, Tollund C, Yoshiga CC, Dawson EA, Nissen P, Secher NH, Crandall CG. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. J Physiol 585: 279–285, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamazaki F, Nakayama Y, Sone R. Whole-body heating decreases skin vascular response to low orthostatic stress in the lower extremities. J Physiol Sci 56: 157–164, 2006. [DOI] [PubMed] [Google Scholar]
- 43. Zoltie N, Young C, Faris I, Tan E. The veno-arteriolar reflex in free skin flaps. Clin Physiol 9: 183–188, 1989. [DOI] [PubMed] [Google Scholar]