Abstract
Impaired cerebral autoregulation during marked reductions in arterial blood pressure may contribute to heat stress-induced orthostatic intolerance. This study tested the hypothesis that passive heat stress attenuates dynamic cerebral autoregulation during pronounced swings in arterial blood pressure. Mean arterial blood pressure (MAP) and middle cerebral artery blood velocity were continuously recorded for ∼6 min during normothermia and heat stress (core body temperature = 36.9 ± 0.1°C and 38.0 ± 0.1°C, respectively, P < 0.001) in nine healthy individuals. Swings in MAP were induced by 70-mmHg oscillatory lower body negative pressure (OLBNP) during normothermia and at a sufficient lower body negative pressure to cause similar swings in MAP during heat stress. OLBNP was applied at a very low frequency (∼0.03 Hz, i.e., 15 s on-15 s off) and a low frequency (∼0.1 Hz, i.e., 5 s on-5 s off). For each thermal condition, transfer gain, phase, and coherence function were calculated at both frequencies of OLBNP. During very low-frequency OLBNP, transfer function gain was reduced by heat stress (0.55 ± 0.20 and 0.31 ± 0.07 cm·s−1·mmHg−1 during normothermia and heat stress, respectively, P = 0.02), which is reflective of improved cerebrovascular autoregulation. During low-frequency OLBNP, transfer function gain was similar between thermal conditions (1.19 ± 0.53 and 1.01 ± 0.20 cm·s−1·mmHg−1 during normothermia and heat stress, respectively, P = 0.32). Estimates of phase and coherence were similar between thermal conditions at both frequencies of OLBNP. Contrary to our hypothesis, dynamic cerebral autoregulation during large swings in arterial blood pressure during very low-frequency (i.e., 0.03 Hz) OLBNP is improved during heat stress, but it is unchanged during low-frequency (i.e., 0.1 Hz) OLBNP.
Keywords: brain blood flow, hyperthermia, transfer function, orthostasis
in humans, elevated internal temperature induces a variety of cardiovascular changes aimed toward optimizing temperature regulation while simultaneously maintaining arterial blood pressure, despite pronounced reductions in systemic vascular resistance (34). Ultimately, however, orthostatic tolerance is reduced while individuals are in this thermal condition (2, 8, 18, 20, 21, 39). Adequate cerebral blood flow, and thus oxygenation, is critical in the regulation of brain function, and syncope will occur if cerebral perfusion is not sufficiently maintained (35). During heat stress, cerebral perfusion and cerebral vascular conductance decrease by as much as 30% relative to normothermia (23, 39, 40). This alone reduces the range over which these variables can subsequently decrease before the onset of syncopal symptoms. Furthermore, during an orthostatic challenge, the decrease in cerebral vascular conductance and the accompanying reduction in cerebral perfusion are greater when subjects are heat stressed (39). Clearly, these combined responses likely contribute to reduced orthostatic tolerance in heat-stressed individuals.
Maintenance of cerebral perfusion during large changes in systemic blood pressure is accomplished by a variety of mechanisms, including cerebral autoregulation, which acts to offset changes in perfusion pressure by adjusting the resistance of the cerebral vasculature (17, 33). Impaired cerebral autoregulation has been reported during orthostatic stress in healthy individuals (6, 44), in patients with neurogenic orthostatic hypotension (27), and in patients with postural tachycardia syndrome (28). Doering et al. (13) reported an increase in a cerebral autoregulatory index during a brief hypotensive challenge following a mild heat stress (∼0.4°C increase in internal temperature), whereas recent reports indicate improvements in dynamic cerebral autoregulation following a more severe heat stress (i.e., ∼1.1°C increase in internal temperature) during spontaneous changes in arterial blood pressure or during an acute hypotensive challenge (22). Primary limitations of these studies are the use of a mild heat stress (13), small oscillations in blood pressure (22), and brevity of the hypotensive challenge (13, 22).
Reduced cerebral perfusion is more common, and thus effective cerebral autoregulation becomes more important, during large reductions in blood pressure that occur during a change from the supine to the upright posture or sudden blood loss. However, evaluation of the effects of heat stress on cerebral autoregulation during an orthostatic challenge [e.g., continuous upright tilt or lower body negative pressure (LBNP)] is not practical, because subjects become syncopal rapidly in this thermal state (2, 8, 18, 20, 21, 39), resulting in analysis of data only from individuals who are able to tolerate these gravitational loads for an adequate period to obtain the necessary data. An alternative approach to evaluate the effects of heat stress on cerebral autoregulation during pronounced fluctuations in arterial blood pressure, but without subjects becoming syncopal, may be the application of continuous oscillatory LBNP (OLBNP), as performed by Hamner et al. (16) in normothermic subjects. Within this context, the aim of the present study was to test the hypothesis that dynamic cerebral autoregulation during large fluctuations in arterial blood pressure induced by OLBNP is attenuated during heat stress.
METHODS
Nine healthy normotensive subjects (7 men, 2 women) participated in the study. Average subject characteristics (mean ± SD) were as follows: 37 ± 12 yr, 176 ± 10 cm, and 75 ± 9 kg. Subjects were not taking medications and were free of any known cardiovascular, metabolic, or neurological diseases. The phase of the menstrual cycle was recorded but not controlled. Subjects were informed of the purpose and risks of the study before providing their informed written consent. The consent and protocol were approved by the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas. Subjects abstained from alcohol and caffeine and refrained from exercise for 24 h before the study.
Instrumentation and measurements.
After arrival at the laboratory, each subject swallowed a telemetry pill for the measurement of intestinal temperature (HQ, Palmetto, FL). Mean skin temperature was measured from the weighted average of six thermocouples attached to the skin. Each subject wore a water-perfused tube-lined suit (Med-Eng, Ottawa, ON, Canada) and was placed inside an LBNP chamber, sealed at the iliac crest, while in the supine position. The suit covered the entire body, except for the head, face, hands, one forearm, and feet. The suit permitted the control of skin and internal temperatures by adjustment of the temperature of the water perfusing the suit. Heart rate was continuously obtained from an electrocardiogram (HP Patient Monitor, Agilent, Santa Clara, CA) interfaced with a cardiotachometer (CWE, Ardmore, PA). Continuous beat-by-beat arterial blood pressure was recorded from a finger by the Penaz method (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands). Intermittent blood pressure measurements were obtained by auscultation of the brachial artery via electrosphygmomanometry (SunTech, Raleigh, NC), with mean arterial blood pressure (MAP) being calculated as one-third pulse pressure + diastolic blood pressure. Skin blood flow was indexed from the exposed forearm via laser-Doppler flowmetry using an integrating probe (MoorLab Laser Doppler Perfusion Monitor, Moor Instruments, Wilmington, DE). Cutaneous vascular conductance was calculated from the ratio of laser-Doppler flux to MAP. The velocity of blood from the middle cerebral artery (MCA Vmean) was continuously measured using transcranial Doppler ultrasonography. A 2-MHz Doppler probe (Multi-flow, DWL Elektronische Systeme, Singen, Germany) was adjusted over the temporal window of the right middle cerebral artery until an optimal signal was identified. The probe was then fixed and held in place using headgear. A cerebral vascular resistance index was calculated from the ratio of MAP to MCA Vmean. End-tidal Pco2 (PetCO2) was monitored using a nasal cannula (VitalCap Capnograph Monitor, Oridion, Needham, MA).
Experimental protocol.
After instrumentation, subjects rested in the supine position for ∼30 min while thermoneutral water (34°C) circulated through the suit. After this resting period, baseline measurements of thermal and hemodynamic data, including continuous measurements of arterial blood pressure, MCA Vmean, and PetCO2, were obtained during ∼5 min of spontaneous respiration followed by measurement of arterial blood pressure by arm cuff. Subjects were then exposed to a 6-min challenge of 70-mmHg OLBNP at a very low frequency (∼0.03 Hz, i.e., 15 s on-15 s off) and another 6-min challenge during low-frequency OLBNP (∼0.1 Hz, i.e., 5 s on-5 s off), while the aforementioned thermal and hemodynamic data were obtained. Subjects were then heat stressed by perfusion of 49°C water through the suit until an increase in internal temperature of ∼1.0°C was obtained. Once the desired increase in internal temperature was obtained, the temperature of the water circulating the suit was slightly decreased to attenuate the rate of rise in internal temperature during data collection. The aforementioned data were then obtained during ∼5 min of resting baseline (0 mmHg LBNP), 6 min of very high-frequency OLBNP, and 6 min of low-frequency OLBNP. For the heat stress challenges, the level of OLBNP was selected for each individual so that the magnitude of the reduction in MAP was similar to that during normothermia. For both thermal conditions, the order of the OLBNP frequency was randomized, and there was an ∼5-min break between OLBNP challenges.
Data analysis.
Thermal and hemodynamic data were sampled at 50 Hz via a data-acquisition system (Biopac System, Santa Barbara, CA). Steady-state data from the last 60 s of each 5-min baseline period before OLBNP, as well as 60 s of data after 1 min following the final OLBNP during heat stress, were obtained. The magnitude of the change in MAP due to OLBNP was calculated from the mean difference between the maximum and minimum values obtained during each on-off cycle throughout the 6 min of OLBNP. Additionally, mean PetCO2 during each OLBNP condition was obtained.
Transfer function analysis.
Beat-to-beat MAP and MCA Vmean were obtained by integration of analog signals within each cardiac cycle during the 6-min OLBNP periods and then linear interpolation and resampling of these data at 2 Hz for spectral analysis (44). Resampling and linear interpolation are necessary to convert the unequally spaced beat-to-beat data to a uniformly spaced time series for spectral and transfer function analyses. These data were analyzed via a 256-point fast-Fourier transformation, with 50% overlap between sequential segments for Welch spectral estimation, and smoothed using the Hanning window, as previously described (44). For transfer function estimation, the cross-spectrum between changes in MAP and MCA Vmean was estimated and then divided by the autospectrum of MAP. In contrast to prior experiments in which the frequency range was 0–0.5 Hz (42), in the present protocol the power spectral density of MAP and MCA Vmean and mean values of transfer gain, phase, and coherence functions were evaluated solely at the applied OLBNP frequency. Data were included for analysis only if peak MAP and MCA Vmean power spectrum occurred at the same frequency and coherence between MAP and MCA Vmean was ≥0.5. Coherence of 0.5 was chosen, because this is typically the lower limit for reliable estimates of transfer gain and phase functions (30). Although data from nine subjects were obtained, data from two subjects were not included during very low-frequency OLBNP because of low coherence, and data from one subject were not included during low-frequency OLBNP because of an unexplainable mismatch between the peak frequency of arterial blood pressure and MCA Vmean. The transfer gain and phase reflect the relative amplitude and the time relationship between changes in MAP and MCA Vmean, respectively. Furthermore, the coherence function was calculated to assess the linear relationship between these two variables. A coherence approaching 1 in a specific frequency range suggests a linear relationship between two signals within that frequency range, whereas a coherence approximating 0 may indicate a nonlinear relationship, severe extraneous noise in the signals, or simply no relationship between signals (16).
The effect of heat stress on baseline steady-state (i.e., non-OLBNP) thermal and hemodynamic responses before the onset of the OLBNP challenges, as well as after the final OLBNP challenge, was analyzed via one-way repeated-measures ANOVA followed by a Tukey's post hoc analysis when a main effect was identified. For each OLBNP frequency, the effect of heat stress on the magnitude of the change in MAP, power spectral densities of MAP and MCA Vmean, and transfer gain, phase, and coherence functions was statistically compared with the effect of normothermia using a paired t-test. Statistical comparisons were not made between the OLBNP frequencies, because the swings in MAP (i.e., input signal) were much greater during very low-frequency OLBNP (see results). The α-level for all analyses was set at 0.05. Results are reported as means ± SD. Transfer function analyses were performed with DADiSP software (DSP Development, Cambridge, MA).
RESULTS
Baseline thermal and hemodynamic data.
Before the onset of OLBNP, heat stress caused expected thermal and hemodynamic responses (Table 1). Core and skin temperatures increased by 1.1 ± 0.1 and 3.8 ± 0.7°C, respectively (P > 0.05 for both variables). Heart rate increased by 30 ± 10 beats/min (P > 0.05), and MAP was well maintained (−2 ± 6 mmHg, P > 0.05). Forearm cutaneous vascular conductance increased nearly sevenfold (1.64 ± 0.66 arbitrary units/mmHg, P > 0.05). MCA Vmean (−11 ± 5 cm/s, P > 0.05) and PetCO2 (−4 ± 2 Torr, P = 0.05) decreased, whereas cerebral vascular resistance index increased (0.28 ± 0.21 mmHg·cm·s−1, P > 0.05). After both bouts of OLBNP during heat stress, MAP remained similar (P > 0.05), MCA Vmean remained reduced (P > 0.05), and the other variables remained elevated (P > 0.05) relative to normothermia. Furthermore, values obtained for all variables after heat stress OLBNP were not different from values obtained before heat stress OLBNP (P > 0.05).
Table 1.
Cardiovascular and thermal responses during normothermia and heat stress before and 1 min after final OLBNP challenge
| Heat Stress |
|||
|---|---|---|---|
| Normothermia | Pre OLBNP | Post OLBNP | |
| Core temperature, °C | 36.9±0.1 | 38.0±0.1* | 38.1±0.2* |
| Mean skin temperature, °C | 34.6±0.5 | 38.4±0.5* | 38.1±0.6* |
| Heart rate, beats/min | 66±7 | 96±15* | 96±13* |
| MAP, mmHg | 85±7 | 84±7 | 84±10 |
| Forearm CVC, AU/mmHg | 0.27±0.15 | 1.9±0.74* | 2.1±0.80* |
| MCA Vmean, cm/s | 62±12 | 51±10* | 49±12* |
| End-tidal Pco2, Torr | 39±2 | 35±3* | 34±3* |
| CVRi, mmHg·cm·s−1 | 1.4±0.2 | 1.7±0.4* | 1.8±0.4* |
Values are means ± SD. OLBNP, oscillatory lower body negative pressure; CVC, cutaneous vascular conductance; AU, arbitrary units; MCA Vmean, middle cerebral artery mean blood velocity; CVRi, cerebral vascular resistance index.
P < 0.05 vs. normothermia.
Throughout OLBNP, PetCO2 remained reduced, relative to normothermia, during 0.03-Hz (38 ± 2 and 34 ± 2 Torr during normothermia and heat stress, respectively, P > 0.001) and 0.1-Hz (38 ± 1 and 34 ± 2 Torr during normothermia and heat stress, respectively, P > 0.001) OLBNP.
Very low-frequency (0.03 Hz) OLBNP.
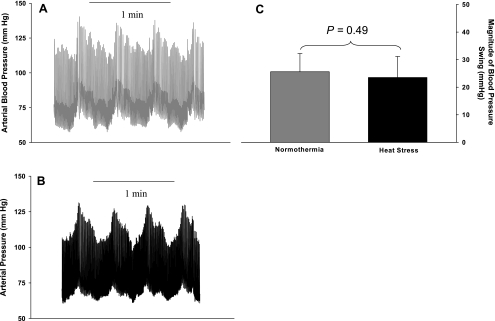
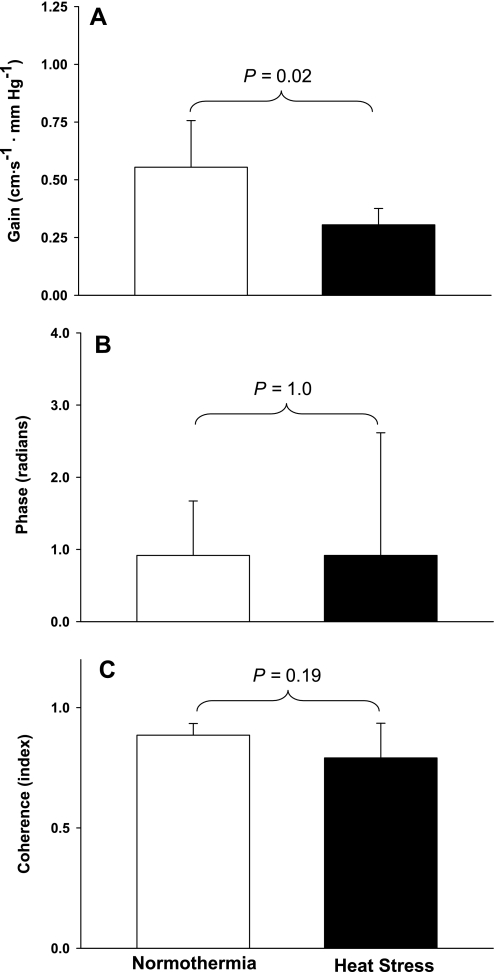
Representative arterial blood pressure traces during 2 min of OLBNP for a subject in both thermal conditions are shown in Fig. 1, A and B. OLBNP caused pronounced swings in MAP that were similar in magnitude between thermal conditions (Fig. 1C; 26 ± 7 and 24 ± 8 mmHg during normothermia and heat stress, respectively, P = 0.49) and power spectral densities (3,408 ± 2,527 and 3,168 ± 2,570 mmHg2/Hz during normothermia and heat stress, respectively, P = 0.82). There was no statistical difference in the power spectral density of MCA Vmean between thermal conditions; however, there was a tendency for smaller fluctuations in MCA Vmean during heat stress than during normothermia [402 ± 458 vs. 1,113 ± 918 (cm·s−1)2·Hz−1, P = 0.13]. The estimates of transfer gain, phase, and coherence functions during 0.03-Hz OLBNP are displayed in Fig. 2. Transfer gain was reduced by heat stress (Fig. 2A; 0.55 ± 0.20 and 0.31 ± 0.07 cm·s−1·mmHg−1 during normothermia and heat stress, respectively, P = 0.02), whereas phase (Fig. 2B; 0.92 ± 0.76 and 0.92 ± 1.7 rad during normothermia and heat stress, respectively, P = 1.0) and coherence (Fig. 2C; 0.89 ± 0.05 and 0.79 ± 0.14 during normothermia and heat stress, respectively, P = 0.19) were similar between thermal conditions.
Fig. 1.
Arterial blood pressure during very low-frequency (0.03 Hz) oscillatory lower body negative pressure (OLBNP). A and B: representative blood pressure traces during very low-frequency OLBNP for a subject during normothermia and heat stress, respectively. C: group average change in mean arterial blood pressure during 6 min of OLBNP was similar between thermal conditions.
Fig. 2.
Very low-frequency OLBNP transfer function data. A–C: group-averaged estimates of transfer gain, phase, and coherence, respectively, between changes in mean arterial blood pressure and middle cerebral artery (MCA) mean blood velocity (MCA Vmean) during very low-frequency OLBNP. Although estimates of phase and coherence were similar between thermal conditions, there was a significant reduction of transfer gain during heat stress, indicating that heat stress improves cerebral autoregulation in this frequency range.
Low-frequency (0.1 Hz) OLBNP.
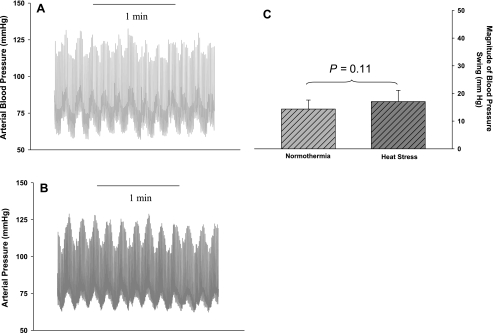
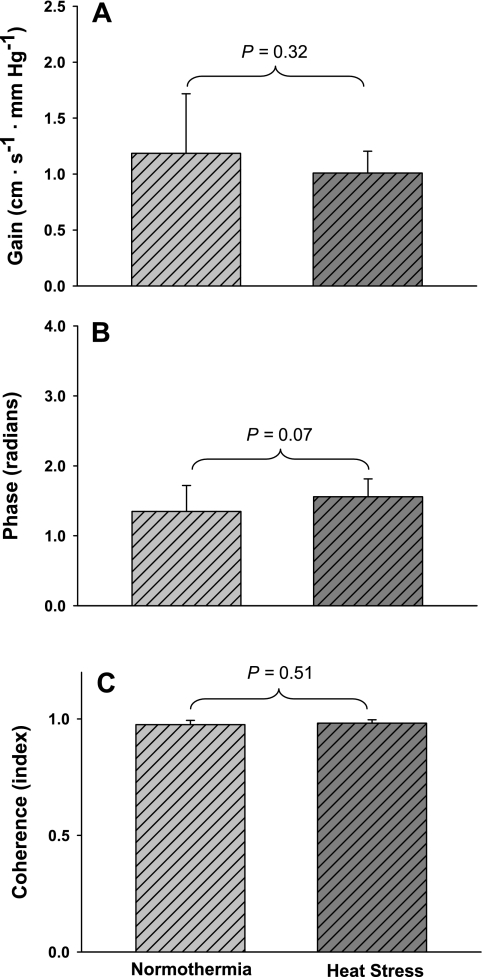
Representative blood pressure traces during 2 min of OLBNP for a subject during both thermal conditions are illustrated in Fig. 3, A and B. As described above for very low-frequency OLBNP, low-frequency OLBNP produced large changes in MAP that were similar in magnitude between thermal conditions, as indicated by similar fluctuations in MAP (Fig. 3C; 14 ± 3 and 17 ± 4 mmHg during normothermia and heat stress, respectively, P = 0.11) and power spectral densities (1,303 ± 567 and 1,709 ± 936 mmHg2/Hz during normothermia and heat stress, respectively, P = 0.30). Also, the power spectral density of MCA Vmean was similar between thermal conditions [1,839 ± 1,240 and 1,867 ± 1,207 (cm·s−1)2·Hz−1 during normothermia and heat stress, respectively, P = 0.96]. There was no effect of heat stress on transfer gain (Fig. 4A; 1.19 ± 0.53 and 1.01 ± 0.20 cm·s−1·mmHg−1 during normothermia and heat stress, respectively, P = 0.32), phase (Fig. 4B; 1.35 ± 0.37 and 1.56 ± 0.23 rad during normothermia and heat stress, respectively, P = 0.07), or coherence (Fig. 4C; 0.98 ± 0.02 and 0.98 ± 0.01 during normothermia and heat stress, respectively, P = 0.51) during low-frequency OLBNP.
Fig. 3.
Arterial blood pressure during low-frequency (0.1 Hz) OLBNP. A and B: representative blood pressure traces during low-frequency OLBNP for a subject during normothermia and heat stress, respectively. C: group average change in mean arterial blood pressure during 6 min of OLBNP was similar between thermal conditions.
Fig. 4.
Low-frequency OLBNP transfer function data. A–C: group averaged estimates of transfer gain, phase, and coherence, respectively, between changes in mean arterial blood pressure and MCA Vmean during low-frequency OLBNP. Estimates of transfer gain, phase, and coherence were similar between thermal conditions, indicating that heat stress has no effect on cerebral autoregulation in this frequency range.
DISCUSSION
The aim of this study was to test the hypothesis that dynamic cerebral autoregulation during pronounced reductions in blood pressure, which is reflective of orthostasis, is reduced during heat stress. This was accomplished by assessment of dynamic cerebral autoregulation via transfer function analyses during oscillatory changes in arterial blood pressure induced by two frequencies of OLBNP. The primary findings are that heat stress reduced transfer function gain when the swings in blood pressure occurred at a very low frequency (0.03 Hz), whereas this gain was unaffected when the swings occurred at a low frequency (0.1 Hz). Contrary to the proposed hypothesis, these data indicate that dynamic cerebral autoregulation during large swings in MAP at a very low frequency is improved with whole body heating, whereas autoregulation is similar between thermal conditions when swings in MAP occur at a low frequency.
Orthostatic tolerance is reduced in heat-stressed individuals, (2, 8, 18, 20, 21, 39), and an impairment in cerebral autoregulation would contribute to this reduced tolerance (39). Contrary to that hypothesis, dynamic cerebral autoregulation is either unaffected or improved by heat stress, depending on the index evaluated, during relatively small spontaneous oscillations in arterial blood pressure (22). Although Low et al. (22) did not report the magnitude of swing in MAP, our laboratory routinely has observed ∼5-mmHg swings in MAP within the assessed frequency range of the present study during spontaneous breathing in the supine position under both thermal conditions (unpublished observations). Because reduced cerebral perfusion is more common, and thus cerebral autoregulation becomes more important, during larger reductions in blood pressure, a criticism of the study of Low et al. was the relatively small blood pressure variability and, thus, subsequent challenge to cerebral autoregulatory mechanisms. To address that limitation, OLBNP was utilized in the present study, which has the benefit of forcing large blood pressure oscillations (e.g., 14–26 mmHg) focused within a specific frequency (16, 43) while simultaneously producing high estimates of coherence between arterial blood pressure (i.e., input signal) and MCA Vmean (i.e., output signal; Figs. 2C and 4C). Importantly, the magnitude of the swings in MAP for a given OLBNP was matched between thermal conditions (Figs. 1C and 3C).
Heat stress-induced reductions in transfer gain during very low-frequency OLBNP indicate that dynamic cerebral autoregulation is improved at this low frequency. The mechanism(s) leading to this response is unknown. In normothermic individuals, various indexes of cerebral autoregulation are enhanced by hypocapnia (1, 11, 14, 31). The applied heat stress caused reductions in PetCO2, perhaps contributing to elevated cerebral autoregulation, although it is unclear why similar reductions in PetCO2 during low-frequency OLBNP did not similarly improve cerebral autoregulation. Autonomic nervous system activity has also been suggested to contribute to cerebral autoregulation. Ogoh et al. (29) reported that α1-adrenoreceptor blockade with prazosin decreased the rate of regulation of cerebral vascular conductance, which is an index of cerebral autoregulation, and resulted in greater decreases in MCA Vmean during an acute hypotensive challenge induced by bilateral cuff deflation. Morita et al. (24) reported that cerebral blood flow decreased with stepwise reductions in MAP following sympathetic denervation in a rat model. Zhang et al. (43) reported an increase in transfer function gain and diminished phase in humans after ganglionic blockade with trimethaphan, suggestive of reduced cerebral autoregulation upon removal of the systemic inhibition of the autonomic nervous system. Together, these results suggest that cerebral autoregulation may be impaired after removal of sympathetic tone. In contrast, heat stress results in pronounced increases in muscle and skin sympathetic nerve activities (3, 7–9, 19, 26) and may increase sympathetic activity to the cerebral circulation (39). Therefore, it is plausible to speculate that an increase in cerebral sympathetic activity due to whole body heat stress may contribute to the improvements in cerebral autoregulation during very low-frequency OLBNP. However, if elevated cerebral sympathetic activity is the mechanism by which heat stress improved cerebral autoregulation, it is unclear why this did not occur during low-frequency OLBNP.
It is interesting to note that the lack of change in dynamic cerebral autoregulation during low-frequency OLBNP occurred despite similar elevations in internal temperature, reductions in PetCO2, and, presumably, sympathetic activation relative to very low-frequency OLBNP. Cerebral autoregulation displays properties of a high-pass filter (12, 16, 25, 32, 42). This refers to the frequency-dependent nature of cerebral autoregulation, which effectively buffers the effects of oscillations in MAP in the very low-frequency ranges on cerebral perfusion, whereas oscillations in blood pressure in higher-frequency ranges pass through, thereby resulting in large oscillations in cerebral perfusion (42). This observation suggests that cerebral autoregulation is absent or weak in the higher-frequency ranges. An improved cerebral autoregulation in heat-stressed subjects only in the very low-frequency range, coupled with an absence of a change in autoregulation in the higher-frequency range, is consistent with this high-pass filter characteristic of cerebral autoregulation. Put another way, given that the cerebral circulation cannot buffer, or is less effective in buffering, oscillations in arterial blood pressure within the higher-frequency ranges, it is not surprising that heat stress did not alter transfer function gain at the higher of the two imposed OLBNP frequencies.
Alternatively, cerebral autoregulation still may be effective at 0.1 Hz, as indicated by a positive phase during normothermia and heat stress (Fig. 4). If this is the case, the absence of a change in transfer function gain and phase at 0.1 Hz by heat stress may reflect a potential interaction of changes in baroreflex function and cerebral autoregulation. It is possible that a stronger baroreflex modulation of vascular tone than that at 0.03-Hz OLBNP may attenuate heat stress-induced changes in cerebral autoregulation at 0.1 Hz (38). However, in the present study, there was no effect of heat stress (main effect of thermal condition: P > 0.05), nor was there an interaction between OLBNP and thermal condition (P > 0.05), in baroreflex control of heart rate, as identified by transfer function gain between changes in arterial blood pressure and R-R interval (data not shown). Therefore, the discrepancy between OLBNP frequencies and the effects of heat stress on cerebral autoregulation is unlikely to be related to a difference in baroreflex modulation of vascular tone during 0.1-Hz OLBNP.
A lack of change in dynamic cerebral autoregulation at ∼0.1-Hz oscillations in MAP during heat stress is in contrast to the observations of Low et al. (22), who reported that phase was increased (suggestive of improved cerebral autoregulation) during heat stress in the frequency range of 0.07–0.2 Hz. Apart from the possible effects of different study designs (i.e., OLBNP relative to spontaneous respiration), increases in the magnitude of swings in arterial blood pressure and coherence during OLBNP may contribute to this discrepancy (43, 44).
Finally, improvements in dynamic cerebral autoregulation during heat stress, as identified by a reduced transfer function gain at the very low frequency (Fig. 2A), may be mediated by hypocapnia and/or enhanced sympathetic activity (or perhaps other mechanisms), whereas these mechanisms may have less effect or are counterbalanced by other vascular regulatory mechanisms at high frequencies (42). Alternatively, heat stress-induced improvements in cerebral autoregulation also could be related to the greater magnitude of fluctuation in MAP between the imposed OLBNP frequencies. The magnitude of fluctuations in MAP was ∼40% greater during very low-frequency OLBNP (24 ± 8 mmHg) than during low-frequency OLBNP (17 ± 4 mmHg). However, counter to that speculation, our laboratory also reported heat stress-induced improvements in cerebral autoregulation (i.e., reduced transfer function gain) within the very low-frequency range (i.e., <0.07 Hz), despite relatively small spontaneous oscillations in MAP (22). Thus improved cerebral autoregulation during heat stress described by transfer function gain appears to be independent of the magnitude of blood pressure oscillations.
Regardless of the frequency of OLBNP, counter to the proposed hypothesis, the present data clearly demonstrate that dynamic cerebral autoregulation is not attenuated in the heat-stressed human. Why then is orthostatic tolerance greatly reduced in the heat-stressed human? A likely possibility is that other mechanisms are responsible and that improved dynamic cerebral autoregulation associated with heat stress is a protective mechanism serving to minimize reductions in cerebral perfusion associated with large reductions in arterial blood pressure. Without improvements in dynamic cerebral autoregulation, it may be that reductions in orthostatic tolerance would be even more pronounced in heat-stressed humans.
Methodological considerations.
Indexes of dynamic cerebral autoregulation are typically assessed across a wide range of frequencies [very low frequency (≤0.07 Hz), low frequency (0.07–0.20 Hz), and high frequency (≥0.2 Hz) (42)], which allows for consideration of natural variability of physiological responses within these ranges, likely to be mediated by different regulatory mechanisms. The present protocol “manipulated the system” using OLBNP (16, 43), which augmented blood pressure oscillations specifically at the imposed frequencies (Figs. 1C and 3C) and generated high coherence between changes in blood pressure and MCA Vmean (Figs. 2C and 4C). For this reason, the data were analyzed only at 0.03 and 0.01 Hz, rather than averaged across relatively larger frequency ranges. Consistent with our expectations, data from both frequencies of OLBNP generated a high estimate of coherence between MAP and MCA Vmean (Figs. 2C and 4C), which indicates high statistical reliability for the transfer gain and phase estimates (16). This is in contrast to a low estimate of coherence often observed in the very low-frequency range for spontaneous oscillations in arterial blood pressure, which makes it difficult to evaluate the dynamic pressure-flow relationship of the cerebral circulation (15, 16, 42).
MCA Vmean was used as an index of cerebral perfusion. Although it is recognized that MCA Vmean is not the same as cerebral blood flow, a linear relationship exists between flow and MCA Vmean if the diameter of the insonated vessel does not change. Importantly, the diameter of large cerebral vessels, such as the middle cerebral artery, does not change during a variety of perturbations, including LBNP and hyper/hypocapnia (36, 37). Thus MCA Vmean provides a reliable and quantifiable index of changes in blood flow through cerebral vessels that has been validated against other techniques (4, 10).
We analyzed absolute transfer function gain (cm·s−1·mmHg−1), rather than normalized transfer function gain (%MCA Vmean/mmHg), which is often analyzed (5, 14). The reason for the use of absolute gain in the present study is that changes in cerebral blood velocity were compared between thermal conditions from the same subject, with position and angle of the Doppler probe carefully locked under all experimental conditions, which is in contrast to other studies where cerebral blood velocity was compared between groups of subjects, thereby requiring normalization. Given that the Doppler probe was not removed from the subjects during the heat stress, coupled with the expectation that the diameter of the insonated vessel does not change due to heat stress, changes in MCA Vmean in response to changes in arterial blood pressure (i.e., the absolute transfer function gain) more accurately reflect the physiological responses to the heat stress than do normalized changes in MCA Vmean.
It has been proposed that increases in steady-state cerebral vascular resistance, or decreases in vascular compliance, are capable of reducing transfer function gain independent of dynamic cerebral autoregulation (41). Cerebral vascular resistance was elevated ∼20% during heat stress (Table 1). However, transfer function gain was decreased during very low-frequency, but not low-frequency, OLBNP. These data suggest that reductions in transfer function gain during very low-frequency OLBNP reflect improved dynamic cerebral autoregulation and are unlikely to be due solely to changes in cerebral vascular resistance.
Conclusion.
The present data indicate that dynamic cerebral autoregulation is improved during large swings in MAP at a very low frequency (0.03 Hz) by heat stress; however, autoregulation is similar between thermal conditions when these swings occur at a low frequency (0.1 Hz). Taken together, it is unlikely that altered dynamic cerebral autoregulation by whole body heat stress contributes to reduced orthostatic tolerance. On the contrary, the present data suggest that, at the lower frequencies, heat stress-induced improvements in dynamic cerebral autoregulation preserve cerebral perfusion by buffering the effects of reduced arterial blood pressure on cerebral perfusion.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL-61388 and HL-84072 and by the Research and Education Institute of Texas Health Resources.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Jena Porterfield for technical assistance and the subjects for their willing participation in this project.
REFERENCES
- 1. Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke 20: 45–52, 1989. [DOI] [PubMed] [Google Scholar]
- 2. Allan JR, Crossley RJ. Effect of controlled elevation of body temperature on human tolerance to +Gz acceleration. J Appl Physiol 33: 418–420, 1972. [DOI] [PubMed] [Google Scholar]
- 3. Bini G, Hagbarth KE, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol 306: 537–552, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke 17: 913–915, 1986. [DOI] [PubMed] [Google Scholar]
- 5. Blaber AP, Bondar RL, Stein F, Dunphy PT, Moradshahi P, Kassam MS, Freeman R. Transfer function analysis of cerebral autoregulation dynamics in autonomic failure patients. Stroke 28: 1686–1692, 1997. [DOI] [PubMed] [Google Scholar]
- 6. Bondar RL, Kassam MS, Stein F, Dunphy PT, Fortney S, Riedesel ML. Simultaneous cerebrovascular and cardiovascular responses during presyncope. Stroke 26: 1794–1800, 1995. [DOI] [PubMed] [Google Scholar]
- 7. Cui J, Wilson TE, Crandall CG. Baroreflex modulation of sympathetic nerve activity to muscle in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol 282: R252–R258, 2002. [DOI] [PubMed] [Google Scholar]
- 8. Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci 116: 54–61, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Cui J, Zhang R, Wilson TE, Crandall CG. Spectral analysis of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 286: H1101–H1106, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Dahl A, Lindegaard KF, Russell D, Nyberg-Hansen R, Rootwelt K, Sorteberg W, Nornes H. A comparison of transcranial Doppler and cerebral blood flow studies to assess cerebral vasoreactivity. Stroke 23: 15–19, 1992. [DOI] [PubMed] [Google Scholar]
- 11. Diehl RR, Linden D, Lucke D, Berlit P. Phase relationship between cerebral blood flow velocity and blood pressure. A clinical test of autoregulation. Stroke 26: 1801–1804, 1995. [DOI] [PubMed] [Google Scholar]
- 12. Diehl RR, Linden D, Lucke D, Berlit P. Spontaneous blood pressure oscillations and cerebral autoregulation. Clin Auton Res 8: 7–12, 1998. [DOI] [PubMed] [Google Scholar]
- 13. Doering TJ, Aaslid R, Steuernagel B, Brix J, Niederstadt C, Breull A, Schneider B, Fischer GC. Cerebral autoregulation during whole-body hypothermia and hyperthermia stimulus. Am J Phys Med Rehabil 78: 33–38, 1999. [DOI] [PubMed] [Google Scholar]
- 14. Edwards MR, Shoemaker JK, Hughson RL. Dynamic modulation of cerebrovascular resistance as an index of autoregulation under tilt and controlled PetCO2. Am J Physiol Regul Integr Comp Physiol 283: R653–R662, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Giller CA, Mueller M. Linearity and non-linearity in cerebral hemodynamics. Med Eng Phys 25: 633–646, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Hamner JW, Cohen MA, Mukai S, Lipsitz LA, Taylor JA. Spectral indices of human cerebral blood flow control: responses to augmented blood pressure oscillations. J Physiol 559: 965–973, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heistad D, Kontos H. Cerebral circulation. In: Handbook of Physiology. The Cardiovascular System. Bethesda, MD: Am. Physiol. Soc., 1983, sect. 2, p. 137–182 [Google Scholar]
- 18. Johnson JM, Niederberger M, Rowell LB, Eisman MM, Brengelmann GL. Competition between cutaneous vasodilator and vasoconstrictor reflexes in man. J Appl Physiol 35: 798–803, 1973. [DOI] [PubMed] [Google Scholar]
- 19. Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445–451, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keller DM, Low DA, Wingo JE, Brothers RM, Hastings J, Davis SL, Crandall CG. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. J Physiol 587: 1131–1139, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol 25: 268–276, 1968. [DOI] [PubMed] [Google Scholar]
- 22. Low DA, Wingo JE, Keller DM, Davis SL, Cui J, Zhang R, Crandall CG. Dynamic cerebral autoregulation during passive heat stress in humans. Am J Physiol Regul Integr Comp Physiol 296: R1598–R1605, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Low DA, Wingo JE, Keller DM, Davis SL, Zhang R, Crandall CG. Cerebrovascular responsiveness to steady-state changes in end-tidal CO2 during passive heat stress. J Appl Physiol 104: 976–981, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morita Y, Hardebo JE, Bouskela E. Influence of cerebrovascular sympathetic, parasympathetic, and sensory nerves on autoregulation and spontaneous vasomotion. Acta Physiol Scand 154: 121–130, 1995. [DOI] [PubMed] [Google Scholar]
- 25. Newell DW, Grady MS, Sirotta P, Winn HR. Evaluation of brain death using transcranial Doppler. Neurosurgery 24: 509–513, 1989. [DOI] [PubMed] [Google Scholar]
- 26. Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman ASM, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst 63: 61–67, 1997. [DOI] [PubMed] [Google Scholar]
- 27. Novak V, Novak P, Spies JM, Low PA. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke 29: 104–111, 1998. [DOI] [PubMed] [Google Scholar]
- 28. Ocon AJ, Medow MS, Taneja I, Clark D, Stewart JM. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 297: H664–H673, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogoh S, Brothers RM, Eubank WL, Raven PB. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke 39: 1979–1987, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Panerai RB. Cerebral autoregulation: from models to clinical applications. Cardiovasc Eng 8: 42–59, 2008. [DOI] [PubMed] [Google Scholar]
- 31. Panerai RB, Deverson ST, Mahony P, Hayes P, Evans DH. Effects of CO2 on dynamic cerebral autoregulation measurement. Physiol Meas 20: 265–275, 1999. [DOI] [PubMed] [Google Scholar]
- 32. Panerai RB, Rennie JM, Kelsall AW, Evans DH. Frequency-domain analysis of cerebral autoregulation from spontaneous fluctuations in arterial blood pressure. Med Biol Eng Comput 36: 315–322, 1998. [DOI] [PubMed] [Google Scholar]
- 33. Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2: 161–192, 1990. [PubMed] [Google Scholar]
- 34. Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension 15: 505–507, 1990. [DOI] [PubMed] [Google Scholar]
- 35. Rowell LB. Orthostatic intolerance. In: Human Cardiovascular Control. New York: Oxford University Press, 1993, p. 118–161 [Google Scholar]
- 36. Schreiber SJ, Gottschalk S, Weih M, Villringer A, Valdueza JM. Assessment of blood flow velocity and diameter of the middle cerebral artery during the acetazolamide provocation test by use of transcranial Doppler sonography and MR imaging. AJNR Am J Neuroradiol 21: 1207–1211, 2000. [PMC free article] [PubMed] [Google Scholar]
- 37. Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000. [DOI] [PubMed] [Google Scholar]
- 38. Stauss HM, Rarick KR, Deklotz RJ, Sheriff DD. Frequency response characteristics of whole body autoregulation of blood flow in rats. Am J Physiol Heart Circ Physiol 296: H1607–H1616, 2009. [DOI] [PubMed] [Google Scholar]
- 39. Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol 291: R1443–R1448, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol 93: 85–91, 2002. [DOI] [PubMed] [Google Scholar]
- 41. Zhang R, Behbehani K, Levine BD. Dynamic pressure-flow relationship of the cerebral circulation during acute increase in arterial pressure. J Physiol 587: 2567–2577, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 274: H233–H241, 1998. [DOI] [PubMed] [Google Scholar]
- 43. Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 106: 1814–1820, 2002. [DOI] [PubMed] [Google Scholar]
- 44. Zhang R, Zuckerman JH, Levine BD. Deterioration of cerebral autoregulation during orthostatic stress: insights from the frequency domain. J Appl Physiol 85: 1113–1122, 1998. [DOI] [PubMed] [Google Scholar]