Abstract
We previously demonstrated that skeletal muscle blood flow is restored in the exercising forearm during experimental hypoperfusion via local dilator and/or myogenic mechanisms. This study examined the role of nitric oxide (NO) in the restoration of blood flow to the active muscles during hypoperfusion. Eleven healthy subjects (10 men/1 woman; 25 ± 1 yr of age) performed rhythmic forearm exercise (10% and 20% of maximum) while hypoperfusion was evoked by balloon inflation in the brachial artery above the elbow. Each trial included baseline, exercise, exercise with inflation, and exercise after deflation (3 min each). Forearm blood flow (FBF; ultrasound) and local (brachial artery catheter pressure, BAP) and systemic arterial pressure [mean arterial pressure (MAP); Finometer] were measured. The exercise bouts were repeated during NG-monomethyl-l-arginine (l-NMMA) infusion (NO synthase inhibition). Forearm vascular conductance (FVC; ml·min−1·100 mmHg−1) was calculated from BF (ml/min) and BAP (mmHg). FBF and FVC fell acutely with balloon inflation during all trials (P < 0.01). Recovery of FBF and FVC [(inflation − nadir)/(steady-state exercise − nadir)] with l-NMMA administration was reduced during 20% exercise (FBF = 77 ± 7% vs. 88 ± 8%; FVC = 71 ± 8% vs. 90 ± 9%; P < 0.01) but not 10% exercise (FBF = 83 ± 4% vs. 81 ± 5%, P = 0.37; FVC = 75 ± 10% vs. 76 ± 7%; P = 0.44) compared with the respective control trial. The time to steady-state vasodilator response was substantially longer during the l-NMMA trials (10% = 74 ± 4 s vs. 61 ± 6 s; 20% = 53 ± 4 s vs. 41 ± 4 s; P < 0.05). Thus the magnitude and timing of the NO contribution to compensatory dilation during forearm exercise with hypoperfusion was dependent on exercise intensity. These observations suggest that NO is released by contracting muscles or that a portion of the dilation caused by ischemic metabolites is NO dependent.
Keywords: exercise hyperemia, nitric oxide, vasodilation, hypoperfusion
during dynamic exercise increased metabolic demand is matched closely by increases in skeletal muscle blood flow and oxygen delivery. During conditions of insufficient oxygen delivery to the active muscle (e.g., hypoperfusion or ischemia), metabolites can accumulate that either stimulate blood pressure-raising sensory nerves within the muscle or evoke vasodilation (1, 7, 17, 18, 40, 48, 49). Either of these mechanisms might act to restore blood flow to the contracting muscle and relieve the flow/metabolism mismatch.
We previously demonstrated that skeletal muscle blood flow is restored in the exercising forearm following experimental hypoperfusion caused by inflation of a balloon in the brachial artery in the absence of a pressor response (4). This suggests forearm blood flow (FBF) is restored during exercise with hypoperfusion via local dilator mechanisms and/or a myogenic response. Although nitric oxide (NO) synthase (NOS) inhibition has a modest effect on skeletal muscle blood flow during exercise in humans (15, 36, 39), the involvement of NO may be enhanced under conditions of impaired oxygen delivery (2, 10, 50). Along these lines, a number of the substances implicated in the dilator responses to ischemia or hypoxia including adenosine and ATP act in part through NO (27, 29, 50). Therefore, we tested the hypothesis that NO contributes to the compensatory vasodilation observed during acute hypoperfusion in exercising human skeletal muscle.
METHODS
Subjects.
Eleven young healthy subjects (10 men; 1 woman) volunteered to participate in the study. Subjects gave written informed consent, were nonobese and nonsmokers, and were not taking any medications. Studies were performed after an overnight fast and after the subjects refrained from exercise and caffeine for at least 24 h. The female subject was studied during the early follicular phase of the menstrual cycle. All study protocols were approved by Institutional Review Board and in accordance with the Declaration of Helsinki.
Heart rate and systemic blood pressure.
Heart rate (HR) was measured by three-lead electrocardiography (ECG). Systemic blood pressure was assessed (beat to beat) with a finger plethysmograph (Finometer) on the nonexercising hand and verified with an automated cuff on the same arm. The systemic pressure was used as an index of pressure proximal (upstream) from the balloon. Cardiac output (CO) was estimated using the Modelflow technique, which has been validated against other techniques and used in exercise studies (31, 51).
Arterial catheterization and balloon placement.
Brachial catheter placement and balloon insertion have been described in detail previously (4). Briefly, a 20-gauge, 5-cm catheter was placed in the brachial artery in the experimental arm under aseptic conditions after local anesthesia (2% lidocaine). A guide wire was then placed in the artery which was then cannulated with a 4-Fr introducer (Cook, Bloomington, IN) that permitted insertion of a 2-Fr Fogarty balloon catheter into the brachial artery. A port and stopcock system allowed the measurement of arterial pressure, administration of study drugs, and drawing of arterial blood samples. The system was continuously flushed (3 ml/h) with heparinized saline. The configuration of the balloon upstream from the lumen of the introducer allowed measurement of the arterial pressure distal to the balloon that was perfusing the contracting forearm muscles.
FBF.
Brachial artery mean blood velocity (MBV) and brachial artery diameter were determined with a 12-MHz linear-array Doppler probe (model M12L, Vivid 7, General Electric, Milwaukee, WI). Brachial artery blood velocity was measured throughout each condition with a probe insonation angle previously calibrated to 60°. Brachial artery and balloon diameter measurements were obtained at end diastole and between contractions during steady-state conditions. Diameter measurement typically results in the loss of the pulse wave signal for 15–20 s. Velocity and diameter measurements were made 2–3 cm proximal to the balloon. FBF was calculated as the product of MBV (cm/s) and brachial artery cross-sectional area (cm2) and multiplied by 60 to present as milliliters per minute.
Forearm exercise.
Rhythmic forearm exercise was performed with a handgrip device by the nondominant arm at 10% and 20% of each subject's maximal voluntary contraction (MVC, mean 46 ± 2 kg, range 35–61 kg). The weight was lifted 4–5 cm over a pulley at a duty cycle of 1-s contraction and 2-s relaxation (20 contractions per minute) using a metronome to ensure correct timing. The average weight used for forearm exercise was 4.6 ± 0.2 and 9.3 ± 0.4 kg for 10 and 20% MVC, respectively.
Brachial artery balloon inflation.
To reduce FBF the brachial artery was partially occluded via inflation of the Fogarty balloon catheter with saline using a calibrated microsyringe for tight control of balloon volume. Balloon inflations were targeted to reduce blood velocity by 40–50%.
Catecholamine analysis.
Arterial plasma catecholamine (epinephrine and norepinephrine) levels were determined by HPLC with electrochemical detection.
Pharmacological infusions.
l-NMMA (NOS inhibitor) was infused at a loading dose of 5 mg/min for 5 min and then at a maintenance dose of 1 mg/min for the remainder of the study. To test the efficacy of the NOS inhibition with l-NMMA, acetylcholine (ACh; a nonspecific muscarinic agonist) was infused intra-arterially at 2.0, 4.0, and 8.0 μg·dl forearm volume−1·min−1 for 2 min each before and after l-NMMA administration. Intra-arterial infusion of sodium nitroprusside (NTP) was used to determine whether l-NMMA administration has any nonspecific effects on forearm vasodilation. NTP was infused at 0.5, 1.0, and 2.0 μg·dl forearm volume−1·min−1 for 2 min each before and after l-NMMA administration (11).
Experimental protocol.
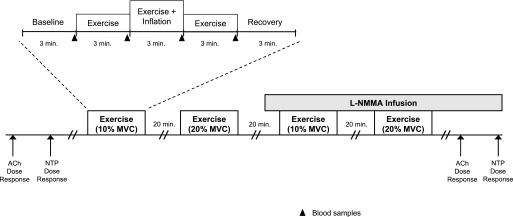
A schematic of the general experimental design is illustrated in Fig. 1. Each subject completed four exercise trials (2 each at 10% and 20% MVC). Each exercise trial consisted of 3 min of rest, exercise, exercise with balloon inflation, exercise following balloon deflation, and recovery (15 min total; 9 min of total exercise). The l-NMMA trials were always completed last due to the drug's long half-life. Each trial was separated by 20 min of rest to allow FBF to return to baseline. Arterial blood samples were obtained for plasma catecholamine determination during the last 30 s of each phase of the exercise trials.
Fig. 1.
Schematic diagram of experimental protocol. Subjects completed 4 exercise trials. Each exercise trial consisted of baseline, exercise (control), exercise during inflation, exercise following deflation, and recovery measurements (3 min each). Exercise trials were performed during control (no drug) and NG-monomethyl-l-arginine (l-NMMA) infusions. Each trial was separated by at least 20 min of rest to allow forearm blood flow (FBF) to return to baseline values. Acetylcholine (ACh) and sodium nitroprusside (NTP) dose-response infusions were performed at the start and end of the study. MVC, maximal voluntary contraction.
Data analysis and statistics.
Data were collected at 200 Hz, stored on a computer, and analyzed offline with signal processing software (WinDaq, DATAQ Instruments, Akron, OH). Local mean arterial pressure (BAP) was determined from the brachial artery pressure waveform measured distal to the balloon, systemic MAP (e.g., pressure proximal to the balloon) was derived from the Finometer pressure waveform, and HR was determined from the electrocardiogram. FBF, BAP, MAP, CO, and HR were determined by averaging values during the last 30–45 s of rest, exercise, exercise with inflation, exercise following deflation, and recovery. In addition, all values were analyzed and averaged during the first 10 s of target balloon inflation (nadir) and the first 10 s immediately following balloon deflation. Forearm vascular conductance (FVC) was calculated as (FBF/BAP) × 100 and expressed as milliliters per minute per 100 mmHg.
All values are expressed as means ± SE. Within a given protocol, the FBF, FVC, BAP, systemic MAP, HR, CO, and plasma catecholamines during rest, exercise, the nadir after balloon inflation, exercise at the end of the balloon inflation, exercise following deflation, and recovery were analyzed by repeated-measures ANOVA. When significance was detected, Tukey's post hoc test was used to identify individual differences and adjust P values to account for multiple comparisons to preserve an overall type I error rate of 0.05.
Percent recovery in FBF and FVC was calculated as (steady-state inflation plus exercise value − nadir)/[steady-state exercise (i.e., control) value − nadir]. To investigate the role of NO on percent recovery of blood flow and conductance, paired t-tests were performed between drug conditions (with and without l-NMMA) at each exercise intensity. To further explore the contribution of local vasodilatation to any restoration of flow, we analyzed balloon resistance and forearm vascular resistance and considered them individually and in series (4, 30). Using systemic arterial pressure (SAP; Finometer), brachial artery pressure distal to the balloon (BAP; catheter) and brachial artery blood flow, we calculated the resistance of the balloon [(SAP − BAP)/flow] and vascular resistance (BAP/flow). The total resistance was calculated as the sum of these two resistors. Changes in vascular and balloon resistance were analyzed from the onset of balloon inflation (nadir) until the end of the inflation period and expressed as a percent change. Paired t-tests were used to compare the percent change in resistance between drug conditions for each exercise intensity. Statistical significance was set a priori at P < 0.05.
RESULTS
All eleven subjects (10 men, 1 woman) completed the study protocol. The subjects were 25 ± 1 yr of age, 181 ± 2 cm in height, and weighed 79 ± 2 kg (body mass index:: 24 ± 1 kg/m2).
Forearm blood flow and vasodilatation during exercise with balloon inflation.
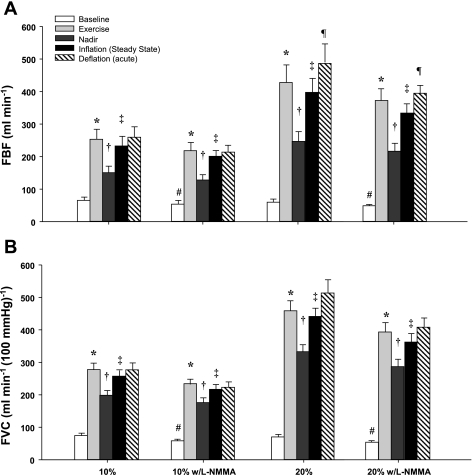
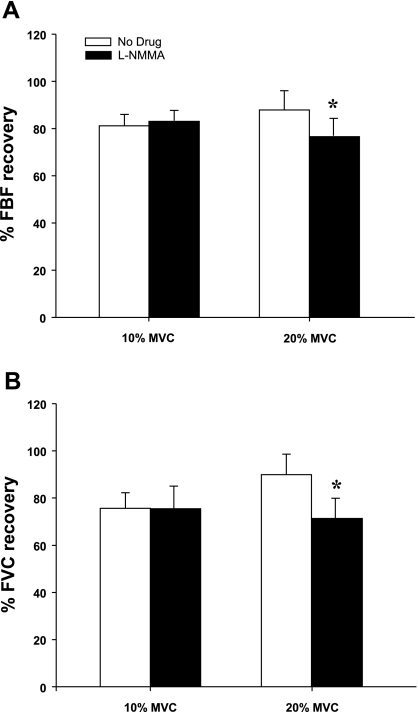
Figure 2 is a fully analyzed record of the FBF and BAP responses to exercise with balloon-induced hypoperfusion. Group mean data for FBF and FVC responses are presented in Fig. 3, A and B. As expected, exercise increased FBF and FVC in all four exercise trials (P < 0.001). Balloon inflation (nadir) during the exercise trials with no drug acutely reduced FBF by 41% and 42% and FVC by 28% and 28% at 10% and 20% MVC, respectively (P < 0.001). FBF and FVC at the end of inflation were partially restored to exercise (control) levels at 10% and 20% MVC, which were substantially higher than their respective nadir values (P < 0.001). The percent recovery of FBF and FVC during the exercise trials are presented in Fig. 4, A and B.
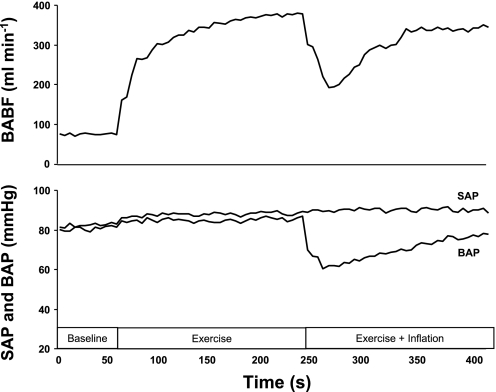
Fig. 2.
Typical blood flow and brachial (BAP) and systemic arterial pressure (SAP) response to hypoperfusion during forearm exercise. A sample tracing of FBF [i.e., brachial artery blood flow (BABF)] (top) and BAP and SAP (bottom) in 1 subject during dynamic forearm exercise (20% MVC).
Fig. 3.
Effect of balloon-induced hypoperfusion on FBF (A) and forearm vascular conductance (FVC; B) during exercise. Balloon inflation resulted in an acute reduction in FBF and FVC (nadir), which were partially restored in all 4 exercise trials. *P < 0.001 vs. baseline; †P < 0.001 vs. exercise; ‡P < 0.001 vs. nadir; ¶P < 0.05 vs. steady-state inflation; #P < 0.05 vs. baseline control (no drug) trial.
Fig. 4.
Percent recovery in FBF (A) and FVC (B) during balloon inflation at 10% and 20% exercise. At 10% forearm exercise, nitric oxide synthase inhibition (l-NMMA) did not reduce the percent recovery of FBF and FVC compared with the respective percent recovery during the control (no drug) trial. At 20% forearm exercise, nitric oxide synthase inhibition reduced the percent recovery of FBF and FVC compared with the control (no drug) trial. *P < 0.01 vs. control (no drug).
Impact of NOS inhibition on vascular response.
l-NMMA administration decreased baseline (resting) blood flow below values observed during control (no drug) trials (P < 0.05). Additionally, l-NMMA administration attenuated the blood flow response to exercise (change from baseline) by 12.3% and 11.3% at 10% and 20% MVC, respectively (P < 0.05). Balloon inflation (nadir) during the exercise trials with NOS inhibition acutely reduced FBF by 42% and 42% and FVC by 25% and 27% at 10% and 20% MVC, respectively (P < 0.001). Similar to the control trials the FBF and FVC at the end of inflation were partially restored to exercise (control) levels at 10% and 20% MVC, which were substantially higher than their respective nadir values (P < 0.001). The percent recovery of FBF and FVC during the 10% trial with l-NMMA did not differ from the respective percent recovery during the control (no drug) trial. However, the percent recovery of FBF and FVC during the 20% trial following l-NMMA was substantially lower than the percent recovery values observed during the control (no drug) trial (Fig. 4, A and B).
Vascular resistance during balloon inflation (from nadir to end of inflation) decreased in the 10% MVC trial with no drug (0.53 ± 0.05 vs. 0.40 ± 0.04 mmHg·ml·min−1; P < 0.01) and with NOS inhibition (0.61 ± 0.06 vs. 0.47 ± 0.03 mmHg·ml·min−1; P < 0.01). The percent reduction in vascular resistance was not different between NOS inhibition and no drug at 10% MVC (−23 ± 3% vs. −24 ± 3%; P = 0.62). Vascular resistance decreased during the 20% MVC trial with no drug (0.33 ± 0.04 vs. 0.22 ± 0.02 mmHg·ml·min−1; P < 0.01) and with NOS inhibition (0.36 ± 0.06 vs. 0.28 ± 0.03 mmHg·ml·min−1; P < 0.05). Consequently, the percent reduction in vascular resistance was less with NOS inhibition (−21 ± 5% vs. −29 ± 4%; P < 0.01). Balloon resistance decreased (from nadir to end of inflation) in the 10% MVC trial with no drug (0.09 ± 0.02 vs. 0.04 ± 0.01 mmHg·ml·min−1; P < 0.01) and with NOS inhibition (0.10 ± 0.01 vs. 0.04 ± 0.01 mmHg ml min-1; P < 0.01). However, the absolute (0.05 ± 0.01 vs. 0.06 ± 0.01; P = 0.31) and relative (55 ± 8 vs. 57 ± 8%; P = 0.54) change in balloon resistance was not different between drug conditions for the 10% exercise trials. Balloon resistance decreased during the 20% MVC trial with no drug (0.7 ± 0.01 vs. 0.04 ± 0.01 mmHg·ml·min−1; P < 0.01) and with NOS inhibition (0.08 ± 0.01 vs. 0.04 ± 0.01 mmHg·ml·min−1; P < 0.01). The absolute (0.04 ± 0.01 vs. 0.04 ± 0.01; P = 0.53) and relative (52 ± 8 vs. 56 ± 6%; P = 0.51) change in balloon resistance was not different between drug conditions for the 20% exercise trials.
Rapid deflation of the balloon during exercise resulted in an acute elevation (reactive hyperemia) in FBF compared with steady-state inflation values at 20% MVC (P < 0.05) but not at 10% MVC (P = 0.48). The magnitude of reactive hyperemia following balloon deflation was not different during NOS inhibition at 10% MVC (P = 0.08) or 20% MVC (P = 0.14).
The vasodilator response (change in FVC from baseline) to ACh infusion was significantly lower at all three doses (2.0, 4.0, and 8.0 μg·dl forearm volume−1·min−1) in the presence of l-NMMA (109 ± 24, 130 ± 25, and 160 ± 35 ml·min−1·100 mmHg−1, respectively) compared with no drug (210 ± 37, 223 ± 49, and 283 ± 66 ml·min−1·100 mmHg−1, respectively; P < 0.05), thus confirming effective NOS inhibition. The vasodilator response to NTP was not different at any dose (0.5, 1.0, and 2.0 μg·dl forearm volume−1·min−1) following l-NMMA administration (142 ± 29, 196 ± 42, and 302 ± 69 ml·min−1·100 mmHg−1, respectively) compared with no drug (149 ± 31, 192 ± 38, and 286 ± 52 ml·min−1·100 mmHg−1, respectively; P = 0.32–0.42).
Timing of compensatory vasodilatation.
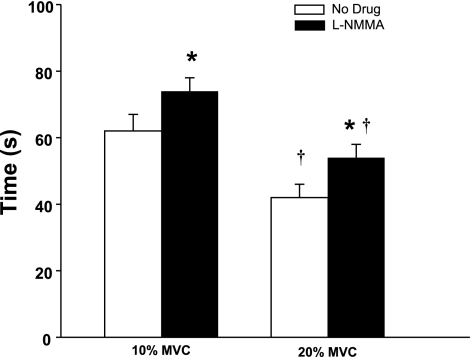
The time required to reach a steady-state FBF and FVC during balloon inflation was longer during the 10% compared with 20% MVC, independent of drug condition. Additionally, NOS inhibition increased the time to reach steady-state FBF and FVC during balloon inflation at 10% and 20% MVC (Fig. 5).
Fig. 5.
Timing of flow restoration. The time to reach steady-state blood flow during balloon inflation under nitric oxide synthase inhibition is delayed at 10% and 20% MVC. Independent of drug condition, the time to steady-state compensation was faster at higher exercise intensities (20% MVC). *P < 0.05 vs. control (no drug); †P < 0.01 vs. 10% MVC.
Hemodynamic changes.
Systemic hemodynamic responses during exercise are presented in Table 1. Exercise resulted in an increase in MAP in all trials, except the 10% with l-NMMA trial (P < 0.05). MAP remained elevated above baseline values throughout each trial (P < 0.05). Estimated CO and HR did not change with exercise (control) in any of the four trials. MAP, HR, and CO did not change with balloon inflation compared with exercise (control) values. Systemic arterial catecholamine levels did not change throughout any of the trials (data not shown).
Table 1.
Systemic hemodynamic responses
| Baseline | Exercise (Control) | Inflation (Nadir) | Inflation (Steady State) | Deflation (Acute) | Deflation (Steady State) | |
|---|---|---|---|---|---|---|
| 10% MVC (control) | ||||||
| Mean arterial pressure, mmHg | 89±3 | 96±3* | 95±3* | 97±4* | 96±4* | 98±4* |
| Brachial artery pressure, mmHg | 89±2 | 91±2 | 75±4*‡ | 88±2† | 93±2† | 93±3† |
| Heart rate, beats/min | 64±1 | 64±1 | 64±1 | 64±1 | 64±1 | 64±1 |
| Cardiac output, l/min | 4.9±0.3 | 5.0±2 | 5.1±0.2 | 5.1±0.3 | 5.1±0.3 | 5.1±0.4 |
| 20% MVC (control) | ||||||
| Mean arterial pressure, mmHg | 91±2 | 99±2* | 100±3* | 103±3* | 103±3* | 102±3* |
| Brachial artery pressure, mmHg | 89±2 | 93±3* | 76±3*‡ | 87±3†‡ | 94±3† | 95±3† |
| Heart rate, beats/min | 63±1 | 64±1 | 64±1 | 65±1 | 65±1 | 65±1 |
| Cardiac output, l/min | 4.6±0.3 | 4.9±3 | 5.0±0.3* | 5.1±0.3* | 5.2±0.3* | 5.1±0.3* |
| 10% MVC (l-NMMA) | ||||||
| Mean arterial pressure, mmHg | 91±3 | 95±2 | 94±2 | 96±3* | 97±3* | 96±3* |
| Brachial artery pressure, mmHg | 93±2 | 93±2 | 75±3*‡ | 90±2† | 95±2† | 95±3† |
| Heart rate, beats/min | 63±1 | 63±1 | 63±1 | 64±1* | 64±1 | 64±1 |
| Cardiac output, l/min | 4.8±0.2 | 4.9±0.3 | 4.9±0.3 | 4.8±0.3 | 4.8±0.3 | 5.0±0.4 |
| 20% MVC (l-NMMA) | ||||||
| Mean arterial pressure, mmHg | 95±2 | 101±3* | 102±4* | 104±3* | 104±4* | 105±4* |
| Brachial artery pressure, mmHg | 92±3 | 95±3 | 77±4*‡ | 90±3†‡ | 96±3† | 98±3† |
| Heart rate, beats/min | 63±1 | 64±1 | 64±1 | 64±1 | 64±1 | 64±2 |
| Cardiac output, l/min | 4.6±0.3 | 5.0±0.4 | 5.0±0.4* | 5.1±0.3* | 5.1±0.4* | 5.2±0.3* |
Values are means ± SE; n = 11. l-NMMA, NG-monomethyl-l-arginine; MVC, maximal voluntary contraction.
P < 0.05 vs. baseline;
P < 0.05 vs. nadir;
P < 0.05 vs. exercise (control).
DISCUSSION
The novel findings from our study are 1) NO contributes to the compensatory vasodilation in the contracting human forearm subjected to acute hypoperfusion during higher intensity exercise, 2) NOS inhibition slows the compensatory vasodilator response to hypoperfusion in the contracting human forearm, and 3) similar to our previous findings (4) the compensatory vasodilation and partial flow restoration occurs without a marked pressor response.
In most human studies NOS inhibition has a modest effect on skeletal muscle blood flow during dynamic exercise (15, 23, 35, 39). In the present study the blood flow response to exercise was 12.3% and 11.3% lower following l-NMMA administration at 10 and 20% MVC, respectively. In theory, the stimuli for NO production during exercise could come from numerous sources, including shear stress or metabolites stimulating the vascular endothelium, vasodilator nerves, contracting skeletal muscle, and/or red blood cells. To our knowledge this is the first study to examine the role of NO in the regulation of skeletal muscle blood flow during exercise with hypoperfusion in humans or animal models. Impaired oxygen delivery to respiring tissues has been proposed to result in an increased NO release from the endothelium and/or erythrocytes (34, 42, 45). In this context, NOS inhibition reduces the skeletal muscle vasodilator response to systemic hypoxia in humans (2, 50). It is currently unclear whether the mechanisms that promote compensatory vasodilation in skeletal muscle during hypoperfusion are the same or different from those that cause vasodilation and increase blood flow when oxygen delivery is reduced via hypoxia in humans.
In the coronary circulation of dogs inhibition of NO synthesis had no effect on coronary flow at normal pressures, but when coronary artery pressure was reduced below the autoregulatory range, coronary flow rates were lower following NOS inhibition than during control conditions (43). Additionally, and of particular interest to the findings of the present study, Duncker and Bache (10) assessed the contribution of NO to the vasodilation of coronary resistance arteries during exercise in the presence of a flow-limiting coronary stenosis. Inhibition of NO production during partial inflation of a hydraulic occluder in dogs performing moderate treadmill exercise resulted in a decreased mean myocardial blood flow in the region perfused by the stenotic artery, but not in the normally perfused control region. Taken together, these results support a role for NO in the compensatory vasodilator responses to exercise with hypoperfusion.
Blunting of flow restoration following l-NMMA.
The present study demonstrates that NO contributes modestly to the compensatory dilator response to hypoperfusion in contracting skeletal muscle at higher exercise intensities. This finding is evidenced by the reduced percent restoration of blood flow (Fig. 4) and attenuated drop in vascular resistance during balloon inflation following l-NMMA administration.
However, there was still a substantial recovery in FBF following NOS inhibition (77%), thus suggesting that there is likely an additional vasodilator mechanism involved. In this context, adenosine released from the active muscles is thought to be a primary compensatory vasodilator signal during periods of reduced oxygen availability (16, 19–21). Indeed, Koch et al. (20) demonstrated that antagonism of adenosine receptors via aminophylline blunted the recovery of hindlimb blood flow in dogs during high-intensity exercise in response to partial vascular occlusion. Additionally, there is evidence that adenosine production is positively correlated with the degree of hypoperfusion in the coronary circulation of dogs (8), and adenosine receptor blockade decreases mean myocardial blood flow in the region perfused by a stenotic artery in exercising dogs (22). Although adenosine may be a major compensatory vasodilator signal, recent evidence in humans, consistent with our observations, suggests that adenosine contributes to the regulation of skeletal muscle blood flow by stimulating NO and prostaglandin mediated vasodilation (29).
Available evidence suggests that prostaglandins normally contribute to the regulation of skeletal muscle blood flow during exercise but are not obligatory (3, 28, 39). Whether the role of prostaglandins becomes enhanced during exercise under conditions of altered flow and/or impaired oxygen delivery is unknown. Under conditions of chronic ischemia vasodilator prostaglandins appear to contribute to the regulation of coronary vascular tone in patients with coronary artery disease (9). However, vasodilator prostaglandins do not appear to contribute to the dilation observed in resistance vessels distal to an experimentally induced flow-limiting coronary artery stenosis in pigs (38).
ATP released from oxygen-sensitive erythrocytes or endothelial cells may have also contributed to some of the local vasodilator response during periods of hypoperfusion. By binding to the purinergic P2y receptors located on the vascular endothelial cells, ATP can induce the release of NO, prostaglandins, and/or endothelium-derived hyperpolarization factors (12, 52). In vitro studies suggest that ATP-induced vasodilation is mediated through an NO pathway (6, 24). A similar interaction between ATP and NO has been reported to exist in the human leg (27). In contrast, ATP-induced vasodilation is unaltered following NOS inhibition in the human forearm (37, 41, 47), thus suggesting that the actions of ATP are not mediated through a NO pathway. These discrepant findings make it difficult to speculate about whether the vasodilatory actions of ATP are conducted through an NO-mediated mechanism in a way that might explain the effects of NOS inhibition on the compensatory dilator response we observed. On a more general note, the absence of P2y antagonists approved for human use limits our ability to determine the role of ATP in the regulation of skeletal muscle blood flow.
Endothelin-mediated vasoconstriction has been shown to be enhanced at lower perfusion pressures in coronary arteries of dogs (5). Furthermore, endothelin-induced vasoconstriction has been shown to increase with NOS inhibition in swine (26). Taken together, these findings in the coronary circulation of dogs suggest that the lower percent recovery of flow during the l-NMMA trails may have been a result of increased endothelin-mediated vasoconstrictor tone. However, it is also possible that the withdrawal of an endothelin-mediated vasoconstriction might actually explain some of the vasodilation and flow restoration. In this context, a substantial metabolic attenuation or “lysing” of endothelin-mediated vasoconstriction during dynamic exercise occurs in the coronary circulation of dogs (46) and swine (25) and the leg of humans (53).
The incomplete blockade of the compensatory vasodilator response to acute hypoperfusion may also be explained by myogenic mechanisms. Evidence from animal muscle preparations indicates that in response to acute reductions in intravascular pressure, vessels dilate, and flow is restored via an enhanced myogenic response (14, 33). Unfortunately, our present model does not allow us to separate and quantify the relative contributions of vasodilator metabolites vs. the myogenic mechanism. Finally, NOS inhibition had no effect on the reactive hyperemia response following balloon deflation. This is consistent with responses seen in the resting forearm where NOS inhibition has minimal impact on the peak blood flow response during reactive hyperemia (13).
Role of NO at the onset of the compensatory response.
Unlike our previous study (4), there was a difference in the timing of compensation between exercise intensities in the present study (Fig. 5). Compensation occurred at a rate that was ∼20 s faster during heavier exercise (20% MVC) compared with lighter exercise (10% MVC). This faster compensation is not surprising since an error signal for flow restoration would likely be enhanced at higher metabolic rates (44). Of particular interest to the present study, NOS inhibition delayed the compensatory vasodilation and flow restoration at both exercise intensities (Fig. 5). Interestingly, these observations are consistent with findings in isolated coronary arteries of guinea pigs in which NOS inhibition limited the amount of vasodilation in the early phase (within 1 min) of acute exposure to hypoxia (32).
Experimental considerations.
There are four potential limitations to our study that should be mentioned. First, we were concerned that the attenuated flow restoration and smaller reduction in vascular resistance during the 20% exercise trial following NOS inhibition might have been due to less pronounced changes in balloon resistance. Although the calculated balloon resistance decreased in all four trials, NOS inhibition did not alter the absolute or relative change in balloon resistance. The decrease in balloon resistance during inflation was not surprising to us. Similar changes were observed in our previous study (4), despite no changes in the diameter of the balloon or the brachial artery around the balloon. Therefore, changes in balloon resistance and the corresponding increase in distal pressure are likely explained by dilation in the collateral vessels around the elbow. Interestingly, decreases in occluder resistance have been shown to occur in dogs during exercise using the Seattle model (30, 54). Therefore changes in balloon resistance are not responsible for the differences in flow recovery and reductions in vascular resistance during NOS inhibition. Second, the dilation of the collateral vessels around the elbow and subsequent partial restoration of distal pressure during balloon inflation might have contributed to the restoration of FVC via flow-mediated mechanisms. In this context, the blood flow responses observed in our model would reflect a vasodilator response of collateral vessels and not truly an autoregulatory response of the microcirculation in the presence of a significant reduction in perfusion pressure. However, several subjects demonstrated a substantial recovery in flow despite little to no change in balloon resistance and minimal increases in distal pressure, thus suggesting that flow restoration occurs in the absence of collaterals.
Third, the experimental model used in the present study does not allow us to differentiate whether the flow restoration was due to metabolic or myogenic mechanisms. It is likely that the regulation of flow in response to changes in arterial perfusion pressure during exercise is shared between vasodilator metabolites, produced during periods of decreased oxygen supply to the muscle (balloon inflation), and myogenic mechanisms responding to changes in perfusion pressure. However, the faster flow compensation observed at higher-exercise intensity supports the notion that that at least a portion of the response is mediated by a metabolic mechanism and that this increases with exercise intensity. Fourth, administration of l-NMMA altered baseline flows and slightly decreased the absolute flow response to exercise (11–12%). It could be argued that these small changes in flows before balloon inflation may explain the attenuated flow recovery following NOS inhibition. However, the use of percent recovery {(steady-state inflation plus exercise value − nadir)/[steady-state exercise (i.e., control) value − nadir]} in the comparison between drug trials clearly accounts for the differences in flow before balloon inflation.
Perspectives and conclusion.
We believe our findings provide important information about the role of NO in the regulation of skeletal muscle blood flow during conditions of reduced oxygen delivery.
Findings from our previous work (4) and the present study demonstrate that there is only partial compensation of flow (<100% recovery) in response to acute hypoperfusion in young healthy subjects. A further attenuation of the already existing “less than perfect” compensatory flow response following NOS inhibition raises concerns that the ability to restore flow might be impaired to a greater extent in conditions with endothelial dysfunction and/or reduced NO bioavailability (i.e., aging, hypertension, vascular disease). In this context, patients with impaired endothelium-dependent dilation are likely to be more susceptible to hypoperfusion and/or ischemia during exercise, especially in vascular regions distal to a stenosis.
Our findings also demonstrate that the contribution of NO to the overall magnitude of compensatory dilation observed during forearm exercise with hypoperfusion is dependent on exercise intensity. Last, the modest attenuation of flow restoration by NOS inhibition suggests that additional vasodilator signals or myogenic mechanisms are likely involved in this response. In future studies it will be important to identify these other potential signals and examine how they interact with one another to regulate flow to the underperfused exercising muscles.
GRANTS
This study was supported by National Institutes of Health Research Grants HL-46493 (M. J. Joyner), AR-55819 (D. P. Casey), and CTSA RR-024150. The Caywood Professorship via the Mayo Foundation also supported this research.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Branton Walker, Shelly Roberts, Jean Knutson, Christopher Johnson, Pam Engrav, and Karen Krucker for technical assistance. We also thank the volunteers for their time.
REFERENCES
- 1. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blitzer ML, Lee SD, Creager MA. Endothelium-derived nitric oxide mediates hypoxic vasodilation of resistance vessels in humans. Am J Physiol Heart Circ Physiol 271: H1182–H1185, 1996. [DOI] [PubMed] [Google Scholar]
- 3. Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol 543: 691–698, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casey DP, Joyner MJ. Skeletal muscle blood flow responses to hypoperfusion at rest and during rhythmic exercise in humans. J Appl Physiol 107: 429–437, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clozel JP, Sprecher U. Influence of low perfusion pressure on effect of endothelin on coronary vascular bed. Am J Physiol Heart Circ Physiol 260: H893–H901, 1991. [DOI] [PubMed] [Google Scholar]
- 6. Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res 56: 43–53, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Daley JC, 3rd, Khan MH, Hogeman CS, Sinoway LI. Autonomic and vascular responses to reduced limb perfusion. J Appl Physiol 95: 1493–1498, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Deussen A, Borst M, Kroll K, Schrader J. Formation of S-adenosylhomocysteine in the heart. II. A sensitive index for regional myocardial underperfusion. Circ Res 63: 250–261, 1988. [DOI] [PubMed] [Google Scholar]
- 9. Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation 100: 1951–1957, 1999. [DOI] [PubMed] [Google Scholar]
- 10. Duncker DJ, Bache RJ. Inhibition of nitric oxide production aggravates myocardial hypoperfusion during exercise in the presence of a coronary artery stenosis. Circ Res 74: 629–640, 1994. [DOI] [PubMed] [Google Scholar]
- 11. Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JL, Fealey RD, Dietz NM, Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol 92: 2019–2025, 2002. [DOI] [PubMed] [Google Scholar]
- 12. Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995. [DOI] [PubMed] [Google Scholar]
- 13. Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol 81: 1807–1814, 1996. [DOI] [PubMed] [Google Scholar]
- 14. Faber JE, Meininger GA. Selective interaction of α-adrenoceptors with myogenic regulation of microvascular smooth muscle. Am J Physiol Heart Circ Physiol 259: H1126–H1133, 1990. [DOI] [PubMed] [Google Scholar]
- 15. Frandsenn U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with NG-nitro-l-arginine methyl ester in humans. J Physiol 531: 257–264, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation 98: 6–8, 1998. [DOI] [PubMed] [Google Scholar]
- 17. Joyner MJ. Muscle chemoreflexes and exercise in humans. Clin Auton Res 2: 201–208, 1992. [DOI] [PubMed] [Google Scholar]
- 18. Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002. [DOI] [PubMed] [Google Scholar]
- 19. Klabunde RE. Conditions for dipyridamole potentiation of skeletal muscle active hyperemia. Am J Physiol Heart Circ Physiol 250: H62–H67, 1986. [DOI] [PubMed] [Google Scholar]
- 20. Koch LG, Strick DM, Britton SL, Metting PJ. Reflex versus autoregulatory control of hindlimb blood flow during treadmill exercise in dogs. Am J Physiol Heart Circ Physiol 260: H436–H444, 1991. [DOI] [PubMed] [Google Scholar]
- 21. Laughlin MH, Klabunde RE, Delp MD, Armstrong RB. Effects of dipyridamole on muscle blood flow in exercising miniature swine. Am J Physiol Heart Circ Physiol 257: H1507–H1515, 1989. [DOI] [PubMed] [Google Scholar]
- 22. Laxson DD, Homans DC, Bache RJ. Inhibition of adenosine-mediated coronary vasodilation exacerbates myocardial ischemia during exercise. Am J Physiol Heart Circ Physiol 265: H1471–H1477, 1993. [DOI] [PubMed] [Google Scholar]
- 23. Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Bimodal distribution of vasodilator responsiveness to adenosine due to difference in nitric oxide contribution: implications for exercise hyperemia. J Appl Physiol 101: 492–499, 2006. [DOI] [PubMed] [Google Scholar]
- 24. McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol Heart Circ Physiol 272: H1886–H1891, 1997. [DOI] [PubMed] [Google Scholar]
- 25. Merkus D, Houweling B, Mirza A, Boomsma F, van den Meiracker AH, Duncker DJ. Contribution of endothelin and its receptors to the regulation of vascular tone during exercise is different in the systemic, coronary and pulmonary circulation. Cardiovasc Res 59: 745–754, 2003. [DOI] [PubMed] [Google Scholar]
- 26. Merkus D, Sorop O, Houweling B, Boomsma F, van den Meiracker AH, Duncker DJ. NO and prostanoids blunt endothelin-mediated coronary vasoconstrictor influence in exercising swine. Am J Physiol Heart Circ Physiol 291: H2075–H2081, 2006. [DOI] [PubMed] [Google Scholar]
- 27. Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol 296: R1140–R1148, 2009. [DOI] [PubMed] [Google Scholar]
- 28. Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53: 993–999, 2009. [DOI] [PubMed] [Google Scholar]
- 30. O'Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995. [DOI] [PubMed] [Google Scholar]
- 31. Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park KH, Rubin LE, Gross SS, Levi R. Nitric oxide is a mediator of hypoxic coronary vasodilatation. Relation to adenosine and cyclooxygenase-derived metabolites. Circ Res 71: 992–1001, 1992. [DOI] [PubMed] [Google Scholar]
- 33. Ping P, Johnson PC. Role of myogenic response in enhancing autoregulation of flow during sympathetic nerve stimulation. Am J Physiol Heart Circ Physiol 263: H1177–H1184, 1992. [DOI] [PubMed] [Google Scholar]
- 34. Pohl U, Busse R. Hypoxia stimulates release of endothelium-derived relaxant factor. Am J Physiol Heart Circ Physiol 256: H1595–H1600, 1989. [DOI] [PubMed] [Google Scholar]
- 35. Radegran G. Limb and skeletal muscle blood flow measurements at rest and during exercise in human subjects. Proc Nutr Soc 58: 887–898, 1999. [DOI] [PubMed] [Google Scholar]
- 36. Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1951–H1960, 1999. [DOI] [PubMed] [Google Scholar]
- 37. Rongen GA, Smits P, Thien T. Characterization of ATP-induced vasodilation in the human forearm vascular bed. Circulation 90: 1891–1898, 1994. [DOI] [PubMed] [Google Scholar]
- 38. Ruocco NA, Most AS, Sasken H, Steiner M, Gewirtz H. Role of endogenous prostacyclin in myocardial blood flow regulation distal to a severe coronary stenosis. Cardiovasc Res 22: 511–519, 1988. [DOI] [PubMed] [Google Scholar]
- 39. Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shepherd JT. Circulation to skeletal muscle. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. Bethesda, MD: Am. Physiol. Soc, 1983, sect. 2, vol. III, pt. 1, chapt. 11, p. 319–370 [Google Scholar]
- 41. Shiramoto M, Imaizumi T, Hirooka Y, Endo T, Namba T, Oyama J, Hironaga K, Takeshita A. Role of nitric oxide towards vasodilator effects of substance P and ATP in human forearm vessels. Clin Sci (Lond) 92: 123–131, 1997. [DOI] [PubMed] [Google Scholar]
- 42. Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 67: 99–145, 2005. [DOI] [PubMed] [Google Scholar]
- 43. Smith TP, Jr, Canty JM., Jr Modulation of coronary autoregulatory responses by nitric oxide Evidence for flow-dependent resistance adjustments in conscious dogs. Circ Res 73: 232–240, 1993. [DOI] [PubMed] [Google Scholar]
- 44. Stainsby WN. Autoregulation of blood flow in skeletal muscle during increased metabolic activity. Am J Physiol 202: 273–276, 1962. [DOI] [PubMed] [Google Scholar]
- 45. Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276: 2034–2037, 1997. [DOI] [PubMed] [Google Scholar]
- 46. Takamura M, Parent R, Cernacek P, Lavallee M. Influence of dual ETA/ETB-receptor blockade on coronary responses to treadmill exercise in dogs. J Appl Physiol 89: 2041–2048, 2000. [DOI] [PubMed] [Google Scholar]
- 47. van Ginneken EE, Meijer P, Verkaik N, Smits P, Rongen GA. ATP-induced vasodilation in human skeletal muscle. Br J Pharmacol 141: 842–850, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Victor RG, Seals DR. Reflex stimulation of sympathetic outflow during rhythmic exercise in humans. Am J Physiol Heart Circ Physiol 257: H2017–H2024, 1989. [DOI] [PubMed] [Google Scholar]
- 49. Wallin BG, Victor RG, Mark AL. Sympathetic outflow to resting muscles during static handgrip and postcontraction muscle ischemia. Am J Physiol Heart Circ Physiol 256: H105–H110, 1989. [DOI] [PubMed] [Google Scholar]
- 50. Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol 537: 613–621, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993. [DOI] [PubMed] [Google Scholar]
- 52. Wihlborg AK, Malmsjo M, Eyjolfsson A, Gustafsson R, Jacobson K, Erlinge D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br J Pharmacol 138: 1451–1458, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wray DW, Nishiyama SK, Donato AJ, Sander M, Wagner PD, Richardson RS. Endothelin-1-mediated vasoconstriction at rest and during dynamic exercise in healthy humans. Am J Physiol Heart Circ Physiol 293: H2550–H2556, 2007. [DOI] [PubMed] [Google Scholar]
- 54. Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983. [DOI] [PubMed] [Google Scholar]