Abstract
The role of gastroesophageal reflux and micro-aspiration as a trigger of airways hyperresponsiveness (AHR) in patients with asthma is controversial. The role of acid reflux and aspiration as a direct cause of AHR in normal subjects is also unclear. We speculated that aspiration of a weak acid with a pH (1.8) equivalent to the upper range of typical gastric contents would lead to AHR in naive mice. We further speculated that modest reductions in aspirate acidity to a level expected during gastric acid suppression therapy (pH 4.0) would impede aspiration-induced AHR. BALB/c female mice were briefly anesthetized with isoflurane and allowed to aspirate 75 μl of saline with HCl (pH 1.8, 4.0, or 7.4) or underwent sham aspiration. Mice were re-anesthetized 2 or 24 h later, underwent tracheostomy, and were coupled to a mechanical ventilator. Forced oscillations were used to periodically measure respiratory impedance (Zrs) following aerosol delivery of saline and increasing doses of methacholine to measure for AHR. Values for elastance (H), airways resistance (RN), and tissue damping (G) were derived from Zrs. Aspirate pH of 1.8 led to a significant overall increase in peak RN, G, and H compared with pH 4.0 and 7.4 at 2 and 24 h. Differences between pH 7.4 and 4.0 were not significant. In mice aspirating pH 1.8 compared with controls, airway lavage fluid contained more neutrophils, higher protein, and demonstrated higher permeability. We conclude that acid aspiration triggers an acute AHR, driven principally by breakdown of epithelial barrier integrity within the airways.
Keywords: lung mechanics, respiratory impedance, forced oscillations, gastroesophageal reflux, asthma
gastroesophageal reflux disease and asthma are two common ailments in Western society that contribute to an enormous public health burden (30, 34). Although considered two separate diseases, there is widespread belief that reflux disease may contribute to airways hyperresponsiveness (AHR) in patients with and without asthma (9, 21, 22, 41). For instance, esophageal pH monitoring has documented esophageal acid reflux in 30–80% of asthmatics, many of whom often report no reflux-related symptoms (20, 48, 52). Equally compelling evidence for a causal link between these conditions is the near twofold risk of self-reported asthma and threefold risk of nocturnal asthma symptoms in patients with reflux disease (17). Indeed, this link has been the primary motivation for empiric treatment of patients with cough or difficult-to-control asthma with pharmacological gastric acid suppression, sometimes even in the absence of overt reflux or heartburn symptoms (23, 28).
The confusion that surrounds the link between reflux disease and asthma may be in part due to the manner in which many patients are labeled as having “asthma,” a diagnosis that requires either a demonstrated response to inhaled bronchodilators or an exaggerated decline in airflow following provocation with exercise or inhaled methacholine (10). The latter criterion, termed AHR, is a highly sensitive test for the presence of asthma. However, conditions other than asthma may also cause AHR (10). In particular, we hypothesized reflux and aspiration of gastric acid could damage the airway epithelium in a manner that would impair its ability to serve as a barrier between the airway lumen and the underlying smooth muscle. This would in turn allow provocative agents such as methacholine aerosol freer access to the smooth muscle, resulting in hyperreactivity via the same mechanism we have previously demonstrated to be responsible for the development of AHR following instillation of cationic protein (5, 26). Certainly, epithelial barrier disruption is known to occur following concentrated acid instillation (16), but whether this occurs with a more clinically relevant aspirate pH has not been explored.
Accordingly, in the present study, we tested the hypothesis that aspiration of fluid with a pH at the upper end of normal gastric acidity causes disruption of airway epithelial barrier function, resulting in AHR to methacholine challenge (38, 39). We further determined whether such AHR could be attenuated by raising the pH of the aspirate to that of gastric contents in patients taking gastric acid-suppressing medication (13, 44).
METHODS
Aspiration protocol.
All protocols were approved following review by the Institutional Animal Care and Uses Committee at the University of Vermont, and all animals were habituated and housed in a pathogen-free facility at the University. Eight to ten-week-old female BALB/c mice (JAX Labs, ME) were studied at either an acute (2 h) or a delayed (24 h) interval following aspiration. For the 24-h interval, mice aspirated 75 μl of nonbuffered normal saline combined with HCl to a pH of 1.8, 4.0, or 7.4 (n = 7 per group). Mice received similar exposures for the 2-h interval, but an additional group was assigned to sham aspiration to control for effects of residual airway fluid. Mice were deeply anesthetized in a plexiglass chamber with 5% inhaled isoflurane until respirations slowed to 1 breath/s. They were then hung semi-recumbently by their teeth, and their tongue was retracted with padded forceps to allow for visualization of the vocal cords. The solution (or nothing in the case of sham) was then slowly delivered to the deep posterior oropharynx with a plastic-tipped pipette during deep agonal breathing. The tongue remained retracted to prevent swallowing and to allow for audible passage through the vocal cords until the volume was fully aspirated. Mice recovered from anesthesia within seconds.
Methacholine aerosol challenge.
At either 2 or 24 h following aspiration, mice were anesthetized with intraperitonal (IP) pentobarbital (90 mg/kg), tracheostomized with an 18-gauge metal cannula, and connected to a flexiVent (SCIREQ, Montreal, Canada) computer-controlled small animal ventilator. Mice received a tidal volume (Vt) of 10 ml/kg at 180 breaths/min with 3-cmH2O positive end-expiratory pressure. The mice were paralyzed with IP pancuronium bromide (0.5 mg/kg) and were allowed 5 min for full effect of the drug. Heart rate was monitored by electrocardiogram to ensure proper anesthesia. Lung volume history was normalized by delivering two deep nonsustained lung inflations (DIs) (1.0 ml, constant flow, pressure-limited to 35 cmH2O) over 4 s, allowing for complete passive exhalation between DIs. This was followed by two baseline Zrs measurements during regular sinusoidal ventilation. Aerosol challenge was delivered by channeling inspiratory flow from the ventilator through a Beetle-Neb ultrasonic nebulizer, during which time the piston delivered a Vt of 40 ml/kg at 30 breaths/min. At completion of aerosol delivery, rate, and Vt returned to 180/min and 10 ml/kg, and Zrs was measured every 15 s over 5 min, followed by two DIs to re-open the lung. Following saline aerosol challenge, the protocol was repeated with methacholine (MCh) concentrations of 3.125, 12.5, and 50 mg/ml (Fig. 1).
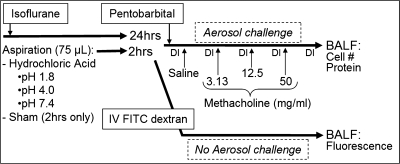
Fig. 1.
Schematic outline of aspiration and methacholine challenge protocol. Hrs, hours; DI, deep inflation; HCl, hydrochloric acid; BAL, bronchoalveolar lavage; IV FITC, intravenous fluorescein isothiocyanate.
Impedance data analysis.
Zrs was determined via Fourier transform from the signals of ventilator piston volume displacement and cylinder pressure, measured during 2-s oscillatory volume perturbations. The perturbations were composed of 13 superimposed sine waves with frequencies ranging between 1 and 20.5 Hz and mutually primed to reduce harmonic distortion (2, 4). Zrs itself was interpreted by being fit to the constant phase model of the viscoelastic lung, from which the parameters RN, G, and H were derived. The parameter RN characterizes airways resistance, whereas G and H characterize the dissipative (tissue damping) and elastic properties of the lung tissues, respectively (19). Successive measurements of Zrs using this 2-s perturbation did not demonstrate any significant effect of start-up transients on the parameter values.
BALF collection and analysis.
Following MCh challenge, the mice were euthanized with pentobarbital, and bronchoalveolar lavage fluid (BALF) was obtained by instilling 1 ml of PBS through the tracheal cannula and suctioning back for a return of ∼0.9 ml. Immediately following collection, the BALF was centrifuged, and the supernatant was stored at −80°C. The cell pellet was resuspended, and the total cell count was determined by an Advia 120 hematology analyzer (Bayer, Tarrytown, NY). Cytospun slides were stained with hematoxylin and eosin for differential count determination. Protein content was calculated using a colorimetric assay (Bio-Rad Laboratories, Hercules, CA), standardized to graded concentrations of bovine serum albumin (BSA). To provide an additional index of epithelial barrier integrity at 2 and 24 h, a separate set of mice not undergoing MCh challenge (n = 6 per group) were analyzed for airway permeability to FITC-dextran following acid aspiration (FD4, Sigma Labs) as previously described (2, 18). Briefly, FITC dextran solution (2.5 mg/ml) was injected (200 μl) at 2 or 24 h following acid aspiration and measured via fluorescence of the BALF 30 min after tail vein delivery.
All graphing and statistical analyses were performed using Origin software (version 7.5, Northampton, MA). ANOVA was used to compare differences in the magnitude of response to MCh, cell counts, and BAL protein values among all groups, followed by post hoc Bonferonni tests for means comparison between groups.
RESULTS
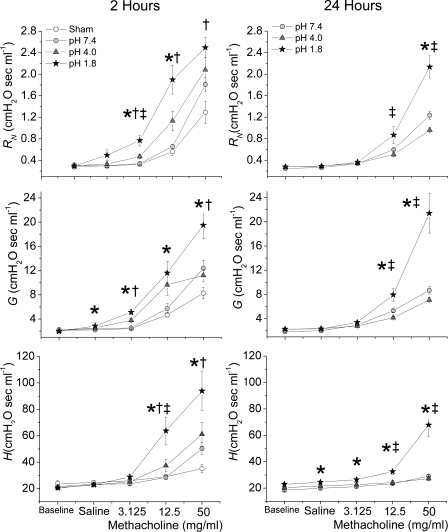
Figure 2 plots the complete time courses of RN, G, and H at the 2-h time point measured in all experimental groups following each challenge. The responses to each challenge varied substantially in terms of magnitude but in most cases exhibited a common temporal pattern beginning with a rapid rise to a sharp peak (with the exception of H at low MCh doses) that rapidly descended to an intermediate plateau. The plateaus were generally quite stable, although most exhibited an increasing trend at the highest dose of MCh. These plateaus of all three parameter measurements remained elevated until airways were reopened with two deep inspirations, after which they returned essentially to baseline. The responses obtained at the 24-h time point were similar in nature to those in Fig. 2 but were reduced in magnitude.
Fig. 2.
Mean values for airways resistance (RN), tissue damping (G), and tissue elastance (H) 2 and 24 h following aspiration, plotted against time at baseline and following normal saline and methacholine (MCh) aerosol challenge. Open circles represent sham (2 h only); shaded circles represent aspirate pH 7.4; shaded triangles represent pH 4.0; and shaded stars represent pH 1.8. Values are means ± SE.
To quantify the various responses in RN, G, and H, we had to extract a single value from the postchallenge time courses shown in Fig. 2. In the case of RN and G, we used the value of the earliest peak as a measure of the degree of smooth muscle contraction, whereas for H we used the early peak value, and if no obvious peak was present, we used the high point within the first six measurements. The results for all study groups at both 2 and 24 h are shown in Fig. 3. There were no significant differences between the baseline values of RN, G, and H, indicating negligible disruption of baseline lung mechanics at any pH. As expected, all parameters increased progressively with each increasing MCh dose. Most importantly, however, in many instances, for a given concentration of MCh, the parameter values increased significantly as the pH of the aspirate decreased. At the 2-h time point, the pH effect on RN was particularly significant at lower MCh concentrations, whereas at the highest dose of MCh the elevation in peak RN in the pH 1.8 group only reached statistical significance compared with that of the sham group (Fig. 3, top left). Thus acid aspiration led to an apparent leftward shift in the dose response curve at 2 h (hypersensitivity), with a lesser effect on the maximal response (hyperreactivity) (15, 49). In contrast, at 24 h, decreasing pH increased maximal RN reactivity with little effect on sensitivity (Fig. 3, top right). Decreasing pH also caused the peaks in RN to be achieved slightly earlier at both 2 h (Fig. 2, top left) and 24 h (Fig. 2, top right). The picture for H was somewhat different with pH having a less robust effect on hypersensitivity at 2 h and a predominant effect on maximal reactivity at 24 h (Fig. 3, bottom). The effect of pH on G (Fig. 3, middle) was intermediate between that on RN and H but again predominantly affected hypersensitivity at 2 h and maximal reactivity at 24 h.
Fig. 3.
Mean peak values for RN, G, and H obtained following normal saline and MCh aerosol challenge. Left and right columns values were obtained at 2 and 24 h following aspiration, respectively. Open circles represent sham (2 h only); shaded circles represent aspirate pH 7.4; shaded triangles represent pH 4.0; shaded stars represent pH 1.8. Values are means ± SE. *Significant difference between pH 1.8 and pH 7.4 (P < 0.05). †Significant difference between pH 1.8 and sham (P < 0.05). ‡Significant difference between pH 1.8 and pH 4.0 (P < 0.05).
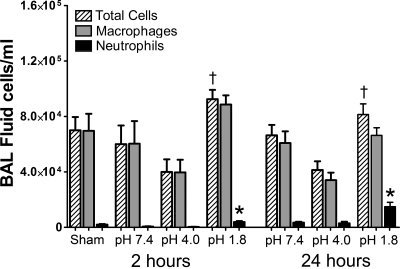
Analysis of the cells in the BALF indicated that acid aspiration caused a mild inflammatory reaction in the lungs of the mice, as shown in Fig. 4. BALF total cell counts were significantly increased in the pH 1.8 group relative to the pH 4.0 group at both the 2- and 24-h time points. Neutrophil counts were significantly greater in the pH 1.8 group compared with both pH 4.0 and pH 7.4 groups, the effect being somewhat more pronounced at 24 h.
Fig. 4.
Means ± SE for total cell counts (hatched bars), total macrophages (shaded bars), and neutrophil counts (filled bars) in BALF (in cells/ml) for all experimental groups. Groups from 2-h time point are plotted on left and from 24-h time point are plotted on right. *Significant difference in counts between pH 1.8 and both pH 4.0 and pH 7.4 (P < 0.05). †Significant difference in counts between pH 1.8 and pH 4.0 (P < 0.05).
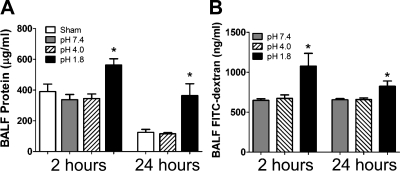
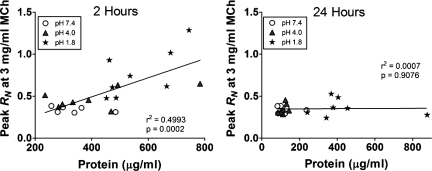
The concentration of protein in BALF, serving as a marker of barrier disruption averaged over the time since aspiration, was significantly increased in the pH 1.8 group compared with the pH 4.0 and pH 7.4 groups, as shown in Fig. 5A. Figure 5B shows that similar differences were observed in BALF concentrations of FITC-dextran, serving as an indicator of epithelial permeability at the time of measurement. Figure 6 shows that BALF protein levels in individual mice correlated significantly with their respective peak values of RN following challenge with 3 mg/ml MCh (sensitivity) at 2 h (r2 = 0.4993, P = 0.0002) but not at 24 h (r2 = 0.0007, P = 0.91).
Fig. 5.
A: means ± SE at 2 and 24 h for total protein (μg/ml) in BALF from sham aspiration mice (open bars, 2 h only) and mice aspirating saline at pH 7.4 (shaded bars), pH 4.0 (hatched bars), and pH 1.8 (filled bars). B: means ± SE at 2 and 24 h for FITC-dextran in BALF (ng/ml) from mice aspirating saline at pH 7.4 (shaded bars), pH 4.0 (hatched bars), and pH 1.8 (filled bars). *Significant difference between pH 1.8 group and pH 4.0, and pH 7.4 (and sham for protein) (P < 0.05).
Fig. 6.
Individual values for BALF protein levels (μg/ml), plotted against peak RN following low-dose MCh aerosol exposure (3 mg/ml) from mice aspirating saline at pH 7.4 (shaded circles), pH 4.0 (shaded triangles), and pH 1.8 (filled stars). Groups from 2-h time point are plotted on left and from 24-h time point are plotted on right. Correlation coefficient (r2) and P values are listed at bottom right of each panel.
DISCUSSION
We have shown that aspiration of fluid with a pH comparable to that of normal gastric contents can elicit both an acute and sustained increase in AHR in otherwise naive mice without significantly altering baseline lung mechanical function. Furthermore, increasing the pH of the aspirate to a level comparable to that of gastric contents in patients on gastric acid-suppressing medication (13, 44) reduces airway responsiveness to a level comparable to that following aspiration of fluid at a normal physiological airway pH of 7.4 (29). This suggests that a critical level of aspirate pH exists, below which significant AHR is induced. Importantly, our results indicate that this critical pH level lies within the range that can be manipulated by current acid-suppressing medications (13, 44).
The patterns of responsiveness we observed at 2 and 24 h after aspiration suggest that the mechanisms involved in acid-induced AHR change with time. At 2 h, there was a leftward shift in the RN dose-response relationship to saline and MCh, with a less significant pH effect at the maximal dose of 50 mg/ml (Fig. 3, top left). Admittedly, this lesser difference between groups at 50 mg/ml could have simply been due to added variation in measurement or having actually missed the true peak by the time of our first sampled values. This leftward shift in the dose response curve at 2 h represents a pattern commonly referred to as “hypersensitivity” or a proclivity to react to doses at which normal subjects will not typically respond (15, 49). This is typically differentiated from “hyperreactivity,” which is defined by a greater (and possibly unlimited) maximal response to escalating doses at which even normal subjects respond (15, 49). Since RN is a measure of airways resistance (19), this suggests that the acute aspiration of acid led to a hypersensitivity of the conducting airways. The mechanism responsible is suggested by our measurements of BALF protein (Fig. 5A) and FITC-dextran (Fig. 5B). Both markers of epithelial permeability were increased significantly at 2 h in the most acidic group compared with the others, suggesting that aspirating pH 1.8 fluid compromised epithelial barrier function. A reduction in the epithelial barrier would be expected to promote a more rapid and complete egress of MCh aerosol to the underlying smooth muscle, leading to a more robust accumulation of MCh at its muscarinic receptor and a more rapid onset to peak response (37). Indeed, we have previously found a similar effect to occur following intratracheal instillation of poly-l-lysine, a cationic protein known to disrupt epithelial barrier integrity (5, 26). The peak and plateau values for the parameters G and H also increased inversely with pH at 2 h, the effect being most pronounced at the highest MCh concentration (Fig. 2, left middle and bottom). The MCh dose-dependent increases in plateau values for G and H are likely due to a progressively increasing degree of peripheral airway closure (40) that persists beyond the immediate effect of MCh on airway narrowing and secretions. This is supported by the drop in these values below the plateau following airway reopening with deep inflation at the end of each measurement period (Fig. 2). Alternatively, the plateau in G could also represent an increase in heterogeneity imposed by patchy peripheral airway narrowing (31), which would also be expected to drop in response to DI.
At 24 h following aspiration, the picture is somewhat different. Here, the MCh responses in RN, G, and H were noticeably reduced compared with 2 h in mice aspirating a pH of 7.4 and 4.0, but less so in mice aspirating pH 1.8 (Fig. 3, right). Furthermore, the peak RN and G responses at 24 h in the pH 1.8 group were manifest primarily as an increased reactivity to high MCh concentrations, with none of the hypersensitivity to saline and lower doses that was observed at 2 h. This suggests that barrier function and airway responsiveness following aspiration of less acidic fluids were in the process of recovering toward normal by 24 h. However, even at the lowest pH of 1.8, markers of epithelial permeability were somewhat reduced at 24 h compared with 2 h (Fig. 5), suggesting that even this degree of barrier disruption was beginning to recover by 24 h. Interestingly, neutrophil counts were increased at 24 h compared with 2 h in the pH 1.8 group (Fig. 4), and this may perhaps account for the persistent response in RN, G, and H to maximal dose MCh observed at 24 h. We have previously shown that allergic inflammation in mice can cause a substantial increase in the MCh responsiveness of H due to peripheral airway closure (40). However, the lack of any commensurate increase in peak impedance response to low and intermediate doses of MCh to match the increase in airspace neutrophils between 2 and 24 h further supports the notion that the AHR at 2 h was not the effect of inflammation but rather due to a disruption in airway barrier integrity.
One question that arises from our observations is why airways responsiveness was elevated above that of controls with the weaker acid of pH 4.0, as occurred at intermediate MCh doses at 2 h (Fig. 3, left). One potential explanation is that there was a small degree of barrier disruption at pH 4.0 that was not detectable by BALF protein or FITC-dextran. However, even the sham group had elevated protein at 2 h compared with 24 h (Fig. 5A), suggesting that the mere airway manipulations involved in delivering an aspirate may have imparted some degree of transient airway damage. Also, in the animals that aspirated fluid, the decrease in AHR between 2 and 24 h in the higher pH groups may have been in part due to a clearance of airway lining fluid over time. Such residual airway fluid in these groups early on would accentuate liquid cross-bridge formation and distal airway closure during the process of acute MCh-induced airways constriction (7, 25). Indeed, an effect of residual airway fluid on AHR at 2 h is implied by the elevation in MCh response in mice aspirating saline (pH 7.4) compared with sham, particularly in the plateaus of impedance parameters following 50 mg/ml MCh (Fig. 2).
The effect of residual airway fluid on AHR, although not entirely accounting for the pH-dependent increase in AHR at 2 h compared with sham (Fig. 3, left), also raises the question of whether other phenomenon such as protein inhibition of surfactant could lead to enhanced surface tension and distal airway closure (46). Although we cannot discount this as a potential contributing factor in the pH 1.8 group (higher protein at 2 and 24 h), the lack of any differences in BALF protein between the sham and pH 4.0 and 7.4 groups (Fig. 5) suggests this to be of negligible consequence for differences in AHR observed between these groups. Furthermore, although we acknowledge that other models of more acidic aspiration injury demonstrate injury to the alveolar-capillary barrier (1, 14), there are a number of reasons why we assert that our findings are more likely to demonstrate a disruption of the proximal airway epithelium. First, whereas BAL fluid is typically bloody in models of ALI involving a disrupted alveolar-capillary barrier, the spun cell pellets from the BALF in this study were free of blood, both grossly and microscopically. In addition, in ALI models that involve direct injury to the alveolar-capillary barrier, the resulting pulmonary edema that ensues will lead to lower baseline aerated lung with rapid accrual of atelectasis following DI, respectively leading to elevated baseline values and progressive rises in G and H over time (1, 3). This was not the case in the present study since G and H remained quite stable after saline challenge once the small peak effect resolved. In fact, the rapid speed with which impedance parameters initially achieved their peak and quickly dropped back down to a lower plateau level suggests a rapid and transient central airways response and would not be expected to recover so quickly or respond so readily to deep inflation if the response were due to a disruption of the alveolar-capillary barrier.
Our data thus support the hypothesis that the primary mechanism responsible for the hypersensitivity to MCh at 2 h following acid aspiration is a disruption of epithelial barrier function. Studies using acid aspiration to induce acute lung injury (ALI) have demonstrated significant disruption of epithelial barrier function manifest as increased permeability (2, 16, 33, 35), but these models have employed considerably stronger preparations of acid than that used in the present study. These previous studies also did not investigate the effects of acid on airway responsiveness. Nevertheless, prior in vitro work with confluent bronchial epithelial layers have demonstrated that weaker preparations of acid are capable of increasing paracellular permeability (51, 53) with a steep dose response to acidity (51). Notably, the ALI that follows aspiration of stronger acid preparations has been shown in some models to be biphasic in nature, with an early direct disruption in permeability and a later phase characterized by recovery in barier function accompanied by an influx of neutrophils (33), which is highly analogous to the results of the present study (Figs. 4 and 5).
Our study has important clinical implications for patients with active reflux disease who present with “asthma-like” symptoms and are subsequently tested for asthma via MCh challenge, but do not have bona fide allergic asthma. If such patients have neither atopy nor allergic airways disease, our results suggest that gastric acid suppression alone may be sufficient to control their AHR and asthma-like symptoms. This may help to resolve what has thus far been a controversial issue. Many experts continue to recommend the use of gastric acid-suppressing medications for the control of cough and difficult-to-control asthma (22, 27), even in the setting of unreported reflux symptoms (21), but this has recently drawn greater controversy. Such practice has been substantiated in some small clinical studies (12, 23, 24, 54) but not in others (50). For example, a large multicenter trial recently demonstrated that the use of acid-suppressing medications does not improve control of asthma in patients with silent (asymptomatic) reflux (42). However, patients with overt reflux symptoms, the same patients likely to be suffering from micro-aspiration, were excluded from this trial. In another recent study of 63 children with nonatopic asthma, esophageal pH monitoring revealed evidence of reflux in 44 of them (42). Among this group, anti-reflux therapy led to an improvement in lung function and a reduction in the number of exacerbations. Our results support these findings and imply that gastric acid suppression may be justified in a certain subset of patients with active GERD and “asthma-like” symptoms.
An important caveat of the inferences drawn from the present study is the somewhat simplified nature of our model. Although pharmacological agents such as histamine-2 receptor antagonists and proton pump inhibitors are quite effective at reducing gastric and esophageal acidity (13, 32, 43), there exist other constituents of gastric juice aside from acid that could be playing a role in reflux-mediated cough and AHR. For example, aspiration of acid can synergistically promote airway injury when accompanied by particulate matter (11, 36). Furthermore, it has been shown that the gastric digestive enzyme trypsin, a powerful serine protease, can directly induce intrapulmonary bronchospasm through its activity at protease-activated receptors (45). However, trypsin may also confer a degree of bronchoprotection (47), possibly by inducing release of prostaglandin E-2 from airway smooth muscle cells (8). We must thus be cautious in making the inferential jump from a mouse model of acid aspiration to conclusions regarding acid suppression therapy in patients with AHR and reflux disease. Nevertheless, by showing how isolated acid aspiration can directly lead to in vivo AHR in a pH-dependent manner, we believe our results challenge what is precisely implied by a positive MCh challenge test and have helped broaden the differential list of potential causes of AHR during MCh challenge in the diagnostic evaluation of “asthma-like” symptoms.
We conclude that the aspiration of a weak acid with a pH comparable to that of normal gastric contents induces an acute central airway hypersensitivity pursuant to a disruption in airway epithelial barrier function. This barrier disruption facilitates the egress of MCh from the airway lumen to the underlying smooth muscle, resulting in an exaggerated and accelerated airways response (6). Furthermore, the persistence of this airway hypersensitivity, over the 24 h following aspiration, is a function of the aspirate pH and is eliminated altogether if pH is raised to that of gastric contents found in patients on acid-suppressing medication. These findings support the notion that AHR discovered in patients with cough or asthma-like symptoms may in some cases be due to reflux disease alone. This lends credibility to current expert guidelines that recommend use of acid-suppressing medications for control of cough and asthma-like symptoms in patients with symptomatic or suspected reflux disease (22, 27).
GRANTS
This study was funded by National Institutes of Health Grants K08 HL-074107, R01 HL75593, and COBRE P20 RR-15557.
REFERENCES
- 1. Allen GB, Cloutier ME, Larrabee YC, Tetenev K, Smiley ST, Bates JH. Neither fibrin nor plasminogen activator inhibitor-1 deficiency protects lung function in a mouse model of acute lung injury. Am J Physiol Lung Cell Mol Physiol 296: L277–L285, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen GB, Leclair T, Cloutier M, Thompson-Figueroa J, Bates JH. The response to recruitment worsens with progression of lung injury and fibrin accumulation in a mouse model of acid aspiration. Am J Physiol Lung Cell Mol Physiol 292: L1580–L1589, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Allen GB, Pavone LA, DiRocco JD, Bates JHT, Nieman GF. Pulmonary impedance and alveolar instability during injurious ventilation in rats. J Appl Physiol 99: 723–730, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Allen GB, Suratt BT, Rinaldi L, Petty JM, Bates JH. Choosing the frequency of deep inflation in mice: balancing recruitment against ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 291: L710–L717, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Bates JH, Cojocaru A, Haverkamp HC, Rinaldi LM, Irvin CG. The synergistic interactions of allergic lung inflammation and intratracheal cationic protein. Am J Respir Crit Care Med 177: 261–268, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bates JH, Wagers SS, Norton RJ, Rinaldi LM, Irvin CG. Exaggerated airway narrowing in mice treated with intratracheal cationic protein. J Appl Physiol 100: 500–506, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Cassidy KJ, Halpern D, Ressler BG, Grotberg JB. Surfactant effects in model airway closure experiments. J Appl Physiol 87: 415–427, 1999. [DOI] [PubMed] [Google Scholar]
- 8. Chambers LS, Black JL, Ge Q, Carlin SM, Au WW, Poniris M, Thompson J, Johnson PR, Burgess JK. PAR-2 activation, PGE2, and COX-2 in human asthmatic and nonasthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 285: L619–L627, 2003. [DOI] [PubMed] [Google Scholar]
- 9. Chernow B, Castell DO. Asthma and gastroesophageal reflux. JAMA 237: 2379, 1977. [DOI] [PubMed] [Google Scholar]
- 10. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 161: 309–329, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Davidson BA, Knight PR, Wang Z, Chess PR, Holm BA, Russo TA, Hutson A, Notter RH. Surfactant alterations in acute inflammatory lung injury from aspiration of acid and gastric particulates. Am J Physiol Lung Cell Mol Physiol 288: L699–L708, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Depla AC, Bartelsman JF, Roos CM, Tytgat GN, Jansen HM. Beneficial effect of omeprazole in a patient with severe bronchial asthma and gastro-oesophageal reflux. Eur Respir J 1: 966–968, 1988. [PubMed] [Google Scholar]
- 13. Fackler WK, Ours TM, Vaezi MF, Richter JE. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology 122: 625–632, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med 165: 242–249, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Fredberg JJ. Bronchospasm and its biophysical basis in airway smooth muscle. Respir Res 5: 2, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldman G, Welbourn R, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Reactive oxygen species and elastase mediate lung permeability after acid aspiration. J Appl Physiol 73: 571–575, 1992. [DOI] [PubMed] [Google Scholar]
- 17. Gunnbjornsdottir MI, Omenaas E, Gislason T, Norrman E, Olin AC, Jogi R, Jensen EJ, Lindberg E, Bjornsson E, Franklin K, Janson C, Gulsvik A, Laerum B, Svanes C, Toren K, Tunsater A, Lillienberg L, Gislason D, Blondal T, Bjornsdottir US, Jorundsdottir KB, Talvik R, Forsberg B, Franklin K, Lundback B, Soderberg M, Ledin MC, Boman G, Norback D, Wieslander G, Spetz-Nystrom U, Cashelunge KS, Ryden E. Obesity and nocturnal gastro-oesophageal reflux are related to onset of asthma and respiratory symptoms. Eur Respir J 24: 116–121, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Han X, Fink MP, Uchiyama T, Yang R, Delude RL. Increased iNOS activity is essential for pulmonary epithelial tight junction dysfunction in endotoxemic mice. Am J Physiol Lung Cell Mol Physiol 286: L259–L267, 2004. [DOI] [PubMed] [Google Scholar]
- 19. Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992. [DOI] [PubMed] [Google Scholar]
- 20. Harding SM, Guzzo MR, Richter JE. 24-h esophageal pH testing in asthmatics: respiratory symptom correlation with esophageal acid events. Chest 115: 654–659, 1999. [DOI] [PubMed] [Google Scholar]
- 21. Harding SM, Guzzo MR, Richter JE. The prevalence of gastroesophageal reflux in asthma patients without reflux symptoms. Am J Respir Crit Care Med 162: 34–39, 2000. [DOI] [PubMed] [Google Scholar]
- 22. Harding SM, Richter JE. The role of gastroesophageal reflux in chronic cough and asthma. Chest 111: 1389–1402, 1997. [DOI] [PubMed] [Google Scholar]
- 23. Harding SM, Richter JE, Guzzo MR, Schan CA, Alexander RW, Bradley LA. Asthma and gastroesophageal reflux: acid suppressive therapy improves asthma outcome. Am J Med 100: 395–405, 1996. [DOI] [PubMed] [Google Scholar]
- 24. Harper PC, Bergner A, Kaye MD. Antireflux treatment for asthma. Improvement in patients with associated gastroesophageal reflux. Arch Intern Med 147: 56–60, 1987. [DOI] [PubMed] [Google Scholar]
- 25. Heil M, Hazel AL, Smith JA. The mechanics of airway closure. Respir Physiol Neurobiol 163: 214–221, 2008. [DOI] [PubMed] [Google Scholar]
- 26. Homma T, Bates JH, Irvin CG. Airway hyperresponsiveness induced by cationic proteins in vivo: site of action. Am J Physiol Lung Cell Mol Physiol 289: L413–L418, 2005. [DOI] [PubMed] [Google Scholar]
- 27. Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, Brown KK, Canning BJ, Chang AB, Dicpinigaitis PV, Eccles R, Glomb WB, Goldstein LB, Graham LM, Hargreave FE, Kvale PA, Lewis SZ, McCool FD, McCrory DC, Prakash UB, Pratter MR, Rosen MJ, Schulman E, Shannon JJ, Smith Hammond C, Tarlo SM. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 129: 1S–23S, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Irwin RS, Curley FJ, French CL. Difficult-to-control asthma. Contributing factors and outcome of a systematic management protocol. Chest 103: 1662–1669, 1993. [DOI] [PubMed] [Google Scholar]
- 29. Jack CI, Calverley PM, Donnelly RJ, Tran J, Russell G, Hind CR, Evans CC. Simultaneous tracheal and oesophageal pH measurements in asthmatic patients with gastro-oesophageal reflux. Thorax 50: 201–204, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Joish VN, Donaldson G, Stockdale W, Oderda GM, Crawley J, Sasane R, Joshua-Gotlib S, Brixner DI. The economic impact of GERD and PUD: examination of direct and indirect costs using a large integrated employer claims database. Curr Med Res Opin 21: 535–544, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Kaczka DW, Ingenito EP, Israel E, Lutchen KR. Airway and lung tissue mechanics in asthma. Effects of albuterol. Am J Respir Crit Care Med 159: 169–178, 1999. [DOI] [PubMed] [Google Scholar]
- 32. Katz PO, Koch FK, Ballard ED, Bagin RG, Gautille TC, Checani GC, Hogan DL, Pratha VS. Comparison of the effects of immediate-release omeprazole oral suspension, delayed-release lansoprazole capsules and delayed-release esomeprazole capsules on nocturnal gastric acidity after bedtime dosing in patients with night-time GERD symptoms. Aliment Pharmacol Ther 25: 197–205, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Kennedy TP, Johnson KJ, Kunkel RG, Ward PA, Knight PR, Finch JS. Acute acid aspiration lung injury in the rat: biphasic pathogenesis. Anesthesia Analgesia 69: 87–92, 1989. [PubMed] [Google Scholar]
- 34. Kinoshita Y, Sato S. Burden and cost of treatment for GERD. J Gastroenterol 40: 1083–1084, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Knight PR, Druskovich G, Tait AR, Johnson KJ. The role of neutrophils, oxidants, and proteases in the pathogenesis of acid pulmonary injury. Anesthesiology 77: 772–778, 1992. [DOI] [PubMed] [Google Scholar]
- 36. Knight PR, Rutter T, Tait AR, Coleman E, Johnson K. Pathogenesis of gastric particulate lung injury: a comparison and interaction with acidic pneumonitis. Anesthesia Analgesia 77: 754–760, 1993. [DOI] [PubMed] [Google Scholar]
- 37. Lauzon AM, Bates JH. Kinetics of respiratory system elastance after airway challenge in dogs. J Appl Physiol 89: 2023–2029, 2000. [DOI] [PubMed] [Google Scholar]
- 38. Leclair TR, Bates JHT, Allen GB. Airways hyperresponsiveness in a mouse model of aspiration injury (Abstract). Am J Respir Crit Care Med 137: A823, 2006. [Google Scholar]
- 39. Leclair TR, Cojocaru A, Bates JHT, Allen GB. Airways hyperresponsiveness in a mouse model of aspiration injury due to bronchial epithelial barrier disruption (Abstract). Am J Respir Crit Care Med 175: A155, 2007. [Google Scholar]
- 40. Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, Bates JH. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med 175: 768–774, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mansfield LE, Stein MR. Gastroesophageal reflux and asthma: a possible reflex mechanism. Ann Allergy 41: 224–226, 1978. [PubMed] [Google Scholar]
- 42. Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, Teague WG, Wise RA. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med 360: 1487–1499, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miehlke S, Madisch A, Kirsch C, Lindner F, Kuhlisch E, Laass M, Knoth H, Morgner A, Labenz J. Intragastric acidity during treatment with esomeprazole 40 mg twice daily or pantoprazole 40 mg twice daily: a randomized, two-way crossover study. Aliment Pharmacol Ther 21: 963–967, 2005. [DOI] [PubMed] [Google Scholar]
- 44. Ours TM, Fackler WK, Richter JE, Vaezi MF. Nocturnal acid breakthrough: clinical significance and correlation with esophageal acid exposure. Am J Gastroenterol 98: 545–550, 2003. [DOI] [PubMed] [Google Scholar]
- 45. Ricciardolo FL, Steinhoff M, Amadesi S, Guerrini R, Tognetto M, Trevisani M, Creminon C, Bertrand C, Bunnett NW, Fabbri LM, Salvadori S, Geppetti P. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am J Respir Crit Care Med 161: 1672–1680, 2000. [DOI] [PubMed] [Google Scholar]
- 46. Seeger W, Grube C, Gunther A, Schmidt R. Surfactant inhibition by plasma proteins: differential sensitivity of various surfactant preparations. Eur Respir J 6: 971–977, 1993. [PubMed] [Google Scholar]
- 47. Sharma A, Goh HL, Asokananthan N, Bakker A, Stewart GA, Mitchell HW. Delayed and persistent suppression of bronchoconstriction by trypsin in the airway lumen. Eur Respir J 27: 20–28, 2006. [DOI] [PubMed] [Google Scholar]
- 48. Sontag SJ, O'Connell S, Khandelwal S, Miller T, Nemchausky B, Schnell TG, Serlovsky R. Most asthmatics have gastroesophageal reflux with or without bronchodilator therapy. Gastroenterology 99: 613–620, 1990. [DOI] [PubMed] [Google Scholar]
- 49. Sterk PJ, Bel EH. Bronchial hyperresponsiveness: the need for a distinction between hypersensitivity and excessive airway narrowing. Eur Respir J 2: 267–274, 1989. [PubMed] [Google Scholar]
- 50. Stordal K, Johannesdottir GB, Bentsen BS, Knudsen PK, Carlsen KC, Closs O, Handeland M, Holm HK, Sandvik L. Acid suppression does not change respiratory symptoms in children with asthma and gastro-oesophageal reflux disease. Arch Dis Child 90: 956–960, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Venglarik CJ, Giron-Calle J, Wigley AF, Malle E, Watanabe N, Forman HJ. Hypochlorous acid alters bronchial epithelial cell membrane properties and prevention by extracellular glutathione. J Appl Physiol 95: 2444–2452, 2003. [DOI] [PubMed] [Google Scholar]
- 52. Vincent D, Cohen-Jonathan AM, Leport J, Merrouche M, Geronimi A, Pradalier A, Soule JC. Gastro-oesophageal reflux prevalence and relationship with bronchial reactivity in asthma. Eur Respir J 10: 2255–2259, 1997. [DOI] [PubMed] [Google Scholar]
- 53. Welsh MJ, Shasby DM, Husted RM. Oxidants increase paracellular permeability in a cultured epithelial cell line. J Clin Invest 76: 1155–1168, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wong CH, Chua CJ, Liam CK, Goh KL. Gastro-oesophageal reflux disease in ‘difficult-to-control’ asthma: prevalence and response to treatment with acid suppressive therapy. Aliment Pharmacol Ther 23: 1321–1327, 2006. [DOI] [PubMed] [Google Scholar]