Abstract
Prostaglandin I2 (PGI2) has been shown to attenuate vascular constriction, hyperpermeability, inflammation, and acute lung injury. However, molecular mechanisms of PGI2 protective effects on pulmonary endothelial cells (EC) are not well understood. We tested a role of cAMP-activated Epac-Rap1 pathway in the barrier protective effects of PGI2 analog iloprost in the murine model of ventilator-induced lung injury. Mice were treated with iloprost (2 μg/kg) after onset of high tidal volume ventilation (30 ml/kg, 4 h). Bronchoalveolar lavage, histological analysis, and measurements of Evans blue accumulation were performed. In vitro, microvascular EC barrier function was assessed by morphological analysis of agonist-induced gap formation and monitoring of Rho pathway activation and EC permeability. Iloprost reduced bronchoalveolar lavage protein content, neutrophil accumulation, capillary filtration coefficient, and Evans blue albumin extravasation caused by high tidal volume ventilation. Small-interfering RNA-based Rap1 knockdown inhibited protective effects of iloprost. In vitro, iloprost increased barrier properties of lung microvascular endothelium and alleviated thrombin-induced EC barrier disruption. In line with in vivo results, Rap1 depletion attenuated protective effects of iloprost in the thrombin model of EC permeability. These data describe for the first time protective effects for Rap1-dependent signaling against ventilator-induced lung injury and pulmonary endothelial barrier dysfunction.
Keywords: prostacyclin, cytoskeleton, vascular permeability, lung endothelium
although ventilator support is an indispensable treatment for critically ill patients, ventilator-induced lung injury (VILI), with the associated multiorgan dysfunction, may lead to significant morbidity and mortality and thus remains one of the most important problems in the management of patients in the intensive care unit (45, 48).
Prostaglandins (PGs) are products of arachidonic acid metabolic pathway and synthesized by many tissues, including vascular endothelial cells (ECs). PGs have pleiotropic roles in physiological and pathophysiological processes, including inflammation and allergic response (21, 52). Stable PGI2 analog iloprost is currently used for treatment of pulmonary hypertension (41). An increasing body of evidence indicates anti-inflammatory and endothelium dependent anti-edemagenic effects of PGs in several models of acute lung injury (ALI) (20, 25, 30, 47). These PG effects involved cAMP and protein kinase A (PKA) mediated signaling. However, molecular mechanisms of pulmonary endothelial barrier protection by PGs are not fully understood.
PGI2 binds specific G protein-coupled receptor IP, leading to elevation of intracellular cAMP (19). Observed PG-induced EC barrier enhancement and anti-inflammatory effects were explained by cAMP- and PKA-dependent mechanisms, which reduce activation of endothelial myosin light chain (MLC) kinase (MLCK) and Rho GTPase, decrease a pool of phosphorylated MLC, and lead to relaxation of actomyosin complex and strengthening of cell-matrix adhesions (9, 37, 42). However, pharmacological inhibitors of PKA do not completely reverse EC barrier enhancement caused by cAMP elevation, suggesting the existence of PKA-independent pathways (14, 22, 33).
Recent studies described a novel mechanism of small GTPase regulation by cAMP. Increased intracellular cAMP levels directly activate the nucleotide exchange protein Epac (exchange protein directly activated by cAMP) (24), which triggers the activity of small GTPase Rap1 in a cAMP-dependent, PKA-independent manner (17). Rap1 is activated by extracellular signals through several regulatory proteins, and it might function in diverse processes, ranging from modulation of growth and differentiation to cell adhesion and endothelial permeability (18, 27, 35). Our laboratory (14) and others (22, 27, 54) have recently shown that barrier-protective effects of PGE2 and PGI2 on pulmonary EC are mediated by PKA and cAMP-Epac-Rap1 pathways, which converge on Rac activation and lead to enhancement of peripheral actin cytoskeleton and adherens junctions.
Small GTPase Rho and Rac play important roles in the regulation of cytoskeletal remodeling and EC permeability (6, 10, 12, 14, 15). Rac-mediated formation of a peripheral F-actin rim, increased association of focal adhesion proteins, and enhancement of intercellular adherens junctions are associated with EC barrier enhancement (4, 10, 11, 13), whereas Rho and Rho-associated kinase may directly catalyze MLC or act indirectly via inactivation of MLC phosphatase (MYPT-1), and cause actomyosin-driven cell contraction and EC barrier dysfunction (49, 50).
This study investigated the involvement of Rap1 in the endothelial barrier protective mechanisms induced by iloprost in human lung microvascular EC (HLMVEC) and in the murine model of VILI.
MATERIALS AND METHODS
Reagents.
Primary antibodies to Rap1 and VE-cadherin were purchased from BD Transduction Laboratories (San Diego, CA). Phospho-MLC antibody, horseradish peroxidase-linked anti-mouse and anti-rabbit IgG were obtained from Cell Signaling (Beverly, MA). Phospho-MYPT and cortactin antibodies were obtained from Upstate Biotechnology (Lake Placid, NY). Texas Red phalloidin and Alexa Flour 488 conjugated secondary antibodies were purchased from Molecular Probes (Eugene, OR). Unless otherwise specified, biochemical reagents were obtained from Sigma (St. Louis, MO). HLMVEC were obtained from Lonza (Walkersville, MD), cultured according to the manufacturer's protocol, and used at passages 5–9.
Mechanical ventilation protocol.
Adult male C57BL/6J mice, 8–10 wk old, with average weight 20–25 g (Jackson Laboratories, Bar Harbor, ME) were bred at the University of Chicago animal care center. All experimental protocols involving the use of animals were approved by the University of Chicago Institutional Animal Care and Use Committee for the humane treatment of experimental animals. C57BL/6J mice were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and acepromazine (1.5 mg/kg). Tracheotomy was performed, and the trachea was cannulated with a 20-gauge 1-in. catheter (Penn-Century, Philadelphia, PA), which was tied into place to prevent air leak. The animals have been placed on a mechanical ventilator (Harvard Apparatus, Boston, MA) for 4 h with high tidal volume (HTV; 30 ml/kg) ventilation. Mice were randomized to concurrently receive sterile saline solution or iloprost (2 μg/kg iv) at three time points (0, 40, and 80 min) during mechanical ventilation. Control animals were anesthetized and allowed to breathe spontaneously. After the experiment, animals were killed by exsanguination under anesthesia. Bronchoalveolar lavage (BAL) was performed using 1 ml of sterile Hanks' balanced saline buffer. The BAL protein concentration was determined by a modified Lowry colorimetric assay using a Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). BAL cell count was performed with a hemacytometer.
Assessment of pulmonary vascular leakage by Evans blue.
Evans blue dye (EBD; 30 ml/kg) was injected into the external jugular vein 2 h before termination of ventilation to assess vascular leak, as described previously (39). In brief, at the end of the experiment, thoracotomy was performed, and the lungs were perfused free of blood with PBS containing 5 mM EDTA. Both left lung and right lung were excised and imaged by a Kodak digital camera, after imaging lungs were blotted dry, weighed, and homogenized in PBS (1 ml/100 μg tissue). Homogenized tissue was incubated with 2 volume formamide (18 h, 60°C) and centrifuged at 12,000 g for 20 min. Optical density of the supernatant was determined by spectrophotometry at 620 and 740 nm. EBD accumulation (micrograms of EBD per gram lung) in lung homogenates was calculated against a standard curve.
Measurement of capillary filtration coefficient.
Pulmonary capillary filtration coefficient (Kf,c) was measured to determine microvascular permeability to liquid, as previously described (31). In brief, mice were anesthetized, and lungs were removed, ventilated, and perfused ex vivo to obtain stable pulmonary artery pressure (7 cmH2O) and lung wet weight over a minimum of a 90-min period. Kf,c was measured by applying a 10-cmH2O brief step increase in left atrial pressure at 20 min postextraction (during the isogravimetric period). After 5 min, the slope was measured over a 20-min period of elevated pressure.
Histological assessment for lung injury.
At death, the lungs were harvested without lavage collection and fixed in 4% paraformaldehyde. After fixation, the lungs were embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin. Sections were evaluated at ×40 magnification.
In vivo and in vitro depletion of endogenous Rap1.
To deplete endogenous Rap1, predesigned standard purity Stealth small-interfering RNA (siRNA) (Homo sapiens) sets were used, which possess the same sequences with mouse Rap1 corresponding segments (Invitrogen, Carlsbad, CA). In vitro transfection with siRNA was performed as previously described (13). In brief, HLMVEC were grown to 70% confluence; siRap1 (final concentration 50 nM) was mixed gently with Dharma FECT1 transfection reagent (Dharmacon, Lafayette, CO) at room temperature for 20 min and applied to cells, according to the manufacturer's protocol. For in vivo depletion, we used the TransIT-QR (Quick Recovery) Hydrodynamic Delivery Solution (Mirus Bio, Madison, WI), designed specifically for the safe and efficient delivery of nucleic acids into live laboratory mice. The required total injection volume is determined by the following formula: total volume needed per mouse (in ml) = mouse weight (g)/10 + 0.1 ml delivery solution (represents the void volume that remains in the syringe and needle after injection). Thus, for in vivo Rap1 depletion, the same set of siRap1 (100% homology between human and mouse Rap1 sequences) or nonspecific RNA (both 4 mg/kg) was premixed with corresponding volume of TransIT-QR Hydrodynamic Delivery Solution (≈2 ml) at room temperature and used within 30 min of mixing. Mice were anesthetized and injected with the premixed solution into the jugular vein. Seventy-two hours posttransfection, mice were subjected to mechanical ventilation; after ventilation the lungs were flushed free of blood cells with cold PBS buffer and harvested for Western blot verification of Rap1 depletion. Nonspecific, nontargeting siRNA (Dharmacon, Lafayette, CO) was used as a control treatment for both in vitro and in vivo experiments.
Measurement of transendothelial electrical resistance.
The cellular barrier properties were analyzed by measurements of transendothelial electrical resistance across confluent EC monolayer using the electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY), as previously described (4, 8).
Immunofluorescence staining.
ECs plated on glass coverslips were grown to confluence, stimulated with iloprost, or left untreated, and immunofluorescence double staining for F-actin and cortactin was performed, as described elsewhere (3). Likewise, after 48 h of transfection with siRap1, EC were stimulated with thrombin with or without iloprost pretreatment, followed by double immunofluorescence staining for F-actin and VE-cadherin. Images were processed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) software.
Western blot analysis.
Protein extracts from mice lungs or EC were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes, and the membranes were incubated with specific antibodies of interest. Equal protein loading was verified by reprobing membranes with β-actin or β-tubulin antibodies. Immunoreactive proteins were detected with the enhanced chemiluminescent detection system, according to the manufacturer's protocol (Amersham, Little Chalfont, UK).
Statistical analysis.
Results are expressed as means ± SD of three to nine independent experiments. Experimental samples were compared with controls by unpaired Student's t-test. For multiple-group comparisons, a one-way variance analysis (ANOVA) and post hoc multiple-comparisons tests were used. P < 0.05 was considered statistically significant.
RESULTS
Protective effects of iloprost against VILI.
Effects of iloprost on the lung barrier function were studied in murine model of VILI. Experimental animals were exposed to HTV mechanical ventilation (30 ml/kg) and treated with iloprost or sterile saline at three time points (0, 40, and 80 min of HTV). After 4 h of ventilation, BAL fluid was collected, and protein concentration and cell counts were analyzed, as described in materials and methods. The results demonstrate that, compared with control animals, mechanical ventilation at HTV significantly increased BAL cell count and protein concentration (Fig. 1A). These effects were attenuated by injection of iloprost (1.56 ± 0.52 vs. 2.6 ± 0.49 × 105/ml, P < 0.001; 0.21 ± 0.04 vs. 0.32 ± 0.04 mg/ml, P < 0.001, respectively). There was no significant difference in BAL cell count and protein between iloprost-treated animals and controls in the absence of HTV (1.15 ± 0.21 vs. 1.18 ± 0.38 × 105/ml and 0.08 ± 0.05 vs. 0.12 ± 0.06 mg/ml, respectively). Histological analysis of hematoxylin and eosin-stained lung sections revealed parenchymal inflammatory cell infiltration and alveolar hemorrhage caused by HTV, which were significantly attenuated by iloprost treatment (Fig. 1B). There were no significant histological changes in the lungs from iloprost-treated mice, compared with control animals.
Fig. 1.
Effects of iloprost (ILO) on ventilator-induced acute lung injury (VILI). Mice were subjected to high tidal volume (HTV; 30 ml/kg, 4 h). Spontaneously ventilated animals served as controls (Con). ILO (2 μg/kg iv) was given at 0, 40, and 80 min during mechanical ventilation. Sterile saline was given as a control. A: cell count and protein concentration were measured in bronchoalveolar lavage (BAL) samples from Con and experimental animals. Results are representative of 5 independent experiments; 2–4 animals per condition. Values are means ± SD. *P < 0.01. B: lung specimens were obtained from Con, HTV, ILO, and HTV + ILO groups. Lungs were fixed in 4% parafomaldehyde, embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin. Images are representative of 6–9 lung specimens for each condition. Veh, vehicle.
Iloprost improves lung endothelial barrier function in vivo.
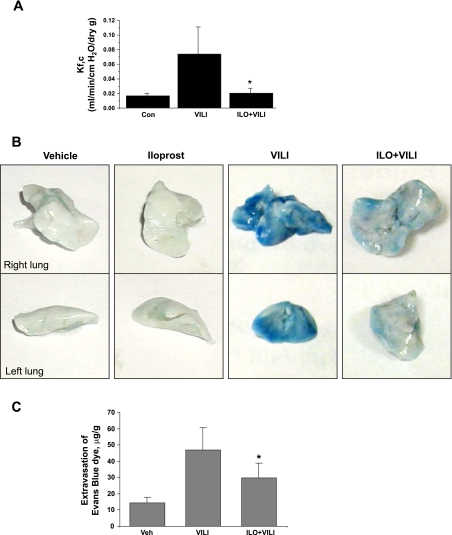
In the following experiments, we characterized effects of iloprost on pulmonary vascular endothelial barrier function. First, microvascular permeability was evaluated by measurements of Kfc. HTV dramatically increased Kfc compared with control animals (0.075 ± 0.035 vs. 0.016 ± 0.0042 ml·min−1·cmH2O−1·dry g−1, P < 0.01) (Fig. 2A). Importantly, iloprost decreased the Kfc value compared with the HTV group (0.022 ± 0.0063 vs. 0.075 ± 0.035 ml·min−1·cmH2O−1·dry g−1, P < 0.02).
Fig. 2.
Effects of ILO on HTV-induced lung microvascular permeability. A: pulmonary capillary filtration coefficient (Kf,c) was measured to determine microvascular permeability, as described in materials and methods. B: Evans blue dye (30 ml/kg) was injected into the external jugular vein 2 h before termination of ventilation to assess vascular leak. Lungs were harvested and imaged against white background. Images are representative of three independent experiments. C: Evans blue extravasation was measured in Con and treated animals by spectrophotometry, as described in materials and methods. Values are means ± SD; n = 4 per condition. *P < 0.05.
Protective effects of iloprost against vascular leak were further assessed by measurement of Evans blue leakage into the lung tissue, as described in materials and methods. HTV caused Evans blue leakage from the vascular space into the lung parenchyma, which was significantly decreased by iloprost treatment (Fig. 2B). Quantitative analysis of Evans blue-labeled albumin extravasation (Fig. 2C) shows that, compared with control animals, Evans blue accumulation in the lungs from HTV-exposed animals was dramatically increased. Iloprost treatment significantly decreased HTV-induced extravasation of EBD in the lung parenchyma. Taken together, our data clearly demonstrate protective effects of iloprost in the mouse model of ALI induced by mechanical ventilation at HTV.
Depletion of Rap1 abolishes the protective role of iloprost.
To investigate whether Rap1 is involved in iloprost-mediated protective effects against HTV-induced lung injury, endogenous Rap1 was depleted using the siRNA approach. Mice were transfected with nonspecific or Rap1-specific siRNA for 72 h, followed by HTV mechanical ventilation, with or without iloprost treatment. After 4 h of ventilation, BAL and lung tissue were collected, as described above. Rap1 protein depletion was confirmed by Western blot analysis of lung tissue (Fig. 3A, inset). Knockdown of Rap1 did not affect elevation of BAL cell count and protein content caused by HTV ventilation (Fig. 3, A and B, left). However, Rap1 knockdown significantly attenuated protective effects of iloprost against HTV-induced increases in BAL cell count (Fig. 3A, right) and protein concentration (Fig. 3B, right), compared with control animals transfected with nonspecific siRNA. Histological analysis of lung sections confirmed that Rap1 knockdown abrogated the ability of iloprost to inhibit HTV-mediated alveolar fluid accumulation and leukocyte infiltration (Fig. 3C).
Fig. 3.
Effects of Rap1 depletion on protective effects by ILO. Mice were tranfected with nonspecific (NS) or Rap1-specific small-interfering RNA (siRap1) for 72 h, followed by HTV (30 ml/kg, 4 h), with or without ILO treatment. Cell count (A) and protein concentration (B) in BAL samples were analyzed. For each bar graph, cell counts or protein content determined in BAL from NS RNA (nsRNA)-treated animals were taken as 100%. Values are means ± SD; n = 4 per condition. *P < 0.05. Inset: Rap1 depletion in lung samples assessed by Western blot. Reprobing of the membrane with actin antibody served as a loading control. C: lung tissues from mice treated with NS RNA or siRap1 were collected and stained with hematoxylin and eosin. Images are representative of 5–7 lung specimens for each condition.
Pulmonary endothelium plays a critical role in control of lung vascular permeability for macromolecules and leukocyte transmigration, and the next studies examined in more detail the role of Rap1 signaling in protective effects of iloprost in pulmonary microvascular EC.
Protective effect of iloprost on endothelial barrier dysfunction in vitro.
Thrombin is an edemagenic agonist released by activated platelets, which triggers Rho- and MLCK-dependent mechanisms of EC permeability (12, 28). On the other hand, Rho pathway is also involved in HTV-induced lung vascular leak and ALI (39). In the following experiments, we utilized the thrombin model of EC permeability to evaluate barrier-protective effects of iloprost on HLMVEC in ALI-related conditions.
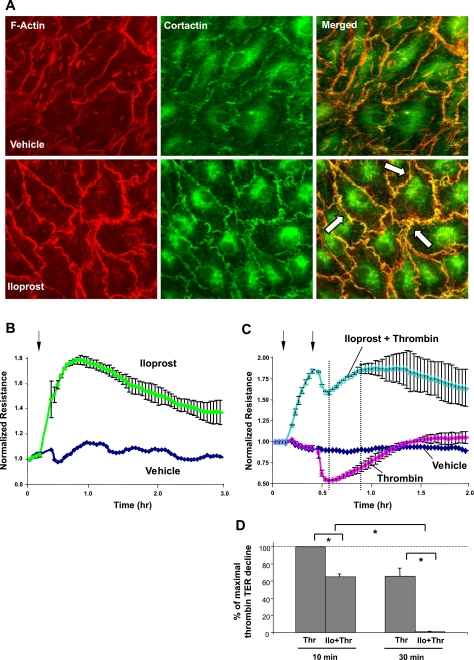
Treatment of HLMVEC with iloprost caused enhancement of cortical actin ring formation and increased peripheral accumulation of actin-binding protein cortactin detected by double immunofluorescence staining with Texas Red phalloidin and cortactin antibody (Fig. 4A). Merged images show areas of increased peripheral actin polymerization outlined by F-actin and cortactin colocalization. In consistence with morphological changes indicating enhancement of EC monolayer integrity, iloprost induced a rapid and sustained increase in transendothelial electrical resistance, reflecting enhanced EC barrier properties (Fig. 4B). Maximal barrier protective response was observed at 15–20 min of iloprost stimulation, slightly declined during the next 2 h, but remained elevated above baseline at 3 h. It is important to note that iloprost pretreatment protected against thrombin-induced EC hyperpermeability (Fig. 4C). These data demonstrate that, in microvascular EC, iloprost exhibits strong barrier-enhancing properties and protects EC monolayer against thrombin-induced barrier failure.
Fig. 4.
Effects of ILO on thrombin (Thr)-induced endothelial cell (EC) barrier dysfunction. A: human lung microvascular EC (HLMVEC) were plated onto glass coverslips and grown to confluence followed by ILO (200 ng/ml, 20 min) treatment. Effects of ILO on EC actin cytoskeleton were monitored by double immunofluorescence staining using cortactin antibody and Texas Red phalloidin. Right column shows merged images of F-actin and cortactin staining (areas of actin and cortactin colocalization appear in yellow and are marked by arrows). Results are representative of 3 independent experiments. B–D: HLMVEC plated onto gold microelectrodes were grown to confluence. B: at the time point indicated by arrow, EC were stimulated with ILO (200 ng/ml) or left untreated, and transendothelial electrical resistance (TER) measurements were performed over the time. C: EC were stimulated with Thr (0.2 U/ml), with or without ILO pretreatment (200 ng/ml, 20 min, indicated by arrows), and TER were monitored over 2 h. D: bar graph depicts statistical analysis of TER measurements performed at the time point marked by the dotted lines in C. TER decline induced by Thr was taken as 100%. Values are means ± SD; n = 5 per condition. *P < 0.001.
Rap1 mediates protective effects of iloprost against thrombin-induced barrier disruption.
To investigate the role of Rap1 in the mediation of iloprost barrier-protective effects in vitro, we performed siRNA-based knockdown of endogenous Rap1. In HLMVEC, nearly complete Rap1 knockdown was observed after 72 h of transfection (data not shown); it significantly decreased basal levels of electrical resistance (741 ± 249 Ω for EC transfected with Rap1-specific siRNA compared with 1,354 ± 94 Ω for nonspecific RNA controls) and even caused cell detachment in select experiments, which is not surprising, given the critical role of Rap1 for the maintenance of cell adhesions and EC monolayer integrity. Therefore, the following experiments were performed after 48 h of siRNA transfection. These conditions provided significant target protein depletion, verified by Western blot analysis (data not shown), but caused only modest reduction in basal resistance (1,036 ± 162 Ω for EC transfected with Rap1-specific siRNA compared with 1,366 ± 133 Ω for nonspecific RNA controls). EC responses to iloprost and thrombin were characterized in Rap1-depleted and control cells transfected with nonspecific RNA. Rap1 depletion abolished EC barrier enhancement by iloprost (Fig. 5A). Similar to nontransfected cells, iloprost stimulation of EC treated with nonspecific RNA not only attenuated thrombin-induced drop in resistance during acute phase, but also accelerated EC monolayer recovery phase after thrombin challenge. Knockdown of Rap1 did not affect acute phase of thrombin-induced permeability, but suppressed the phase of EC barrier restoration. More importantly, Rap1 inhibition markedly decreased the protective effect of iloprost against thrombin-induced permeability during both acute and recovery phases of thrombin stimulation (Fig. 5, B and C).
Fig. 5.
Effect of Rap1 depletion on ILO-mediated protection against Thr-induced EC barrier dysfunction. HLMVEC were transfected with nsRNA or siRap1 for 72 h. A: at the time point indicated by arrow, cells were treated with 200 ng/ml ILO, followed by permeability measurements. B: transfected cells were treated with ILO (200 ng/ml, 20 min) or left untreated before Thr (0.2 U/ml) challenge, and TER was monitored over the time. C: bar graph represents statistical analysis of TER measurements performed at the indicated time points after Thr treatment. TER decline induced by Thr was taken as 100%. Values are means ± SD; n = 5 per condition. *P < 0.05. D: transfected EC were subjected to Thr (0.2 U/ml, 5 min) treatment, with or without ILO (100 ng/ml or 200 ng/ml, 20 min) stimulation. Phosphorylation of myosin light chain (p-MLC) and MLC phosphatase (p-MYPT) was detected by Western blot with specific antibodies. Probing for β-tubulin was used as internal control for equal protein loading. Results are representative of 3–5 independent experiments.
Rho pathway of endothelial permeability involves Rho-kinase-mediated phosphorylation of MYPT, leading to increased phospho-MLC levels, actomyosin contraction, and barrier compromise (12). We examined effects of iloprost and thrombin treatment on phosphorylation profile of MYPT and MLC in control and Rap1-depleted EC using diphospho-MLC (Thr18/ Ser19) and phospho-MYPT (Thr850) antibodies (Fig. 5C). Thrombin induced similar increases in MLC and MYPT phosphorylation in both groups treated with nonspecific or Rap1-specific siRNA. Iloprost inhibited MLC and MYPT phosphorylation in a dose-dependent fashion in control EC transfected with nonspecific RNA. However, iloprost failed to inhibit thrombin-induced phosphorylation of MYPT and MLC in Rap1-depleted cells (Fig. 5C, right).
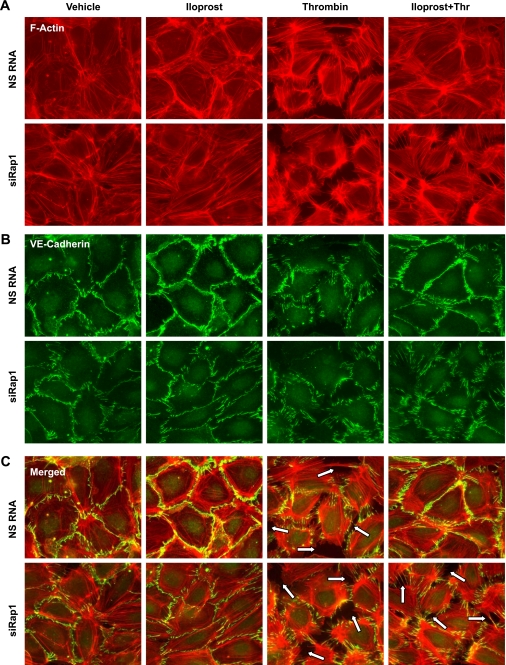
EC barrier dysfunction is accompanied by cytoskeletal remodeling and manifested by formation of actin stress fibers and disruption of intercellular contacts. Analysis of actin rearrangement showed that, in control EC transfected with nonspecific RNA, iloprost induced significant reduction in central stress fibers, promoted accumulation of peripheral F-actin, and increased VE-cadherin immunoreactivity at the areas of intercellular junctions (Fig. 6). Stimulation with thrombin increased accumulation of stress fibers, paracellular gap formation, and disrupted adherens junctions detected by VE-cadherin staining. These cytoskeletal effects were significantly attenuated by iloprost (Fig. 6, right). In contrast to control cells treated with nonspecific RNA, Rap1 depletion suppressed iloprost-induced actin peripheral rim formation and enhancement of adherens junctions. Furthermore, Rap1 knockdown abolished protective effects of iloprost against thrombin-induced barrier disruption. Taken together, these results strongly suggest the critical role of Rap1 in the pulmonary EC cytoskeletal remodeling and barrier enhancement by iloprost.
Fig. 6.
Effect of Rap1 knockdown on ILO-induced cytoskeletal remodeling and adherns junction integrity. HLMVEC were transfected with NS RNA or Rap1-specific siRNA for 72 h. Double immunofluorescence staining was performed using Texas Red phalloidin to detect F-actin (A) and VE-cadherin antibodies to visualize adherens junctions (B). C: merged images depict ILO-induced peripheral accumulation of F-actin in the areas adjacent to adherens junctions in Con cells stimulated with ILO and attenuation of Thr-induced disruption of cell junctions by ILO. This protective effect was abolished in Rap1-depleted EC monolayers. Paracellular gaps and disrupted intercellular contacts are marked by arrows. Results are representative of three independent experiments.
DISCUSSION
Clinical observations and animal studies show beneficiary effects of PGs in pathological settings, such as ischemia-reperfusion, ALI, pulmonary hypertension, and asthma (2, 32, 41). Inhaled iloprost improved gas exchange in the model of ALI induced by repeated lung lavage by decreasing the pulmonary shunt and reduced lung edema formation (25). Iloprost attenuated remote lung injury caused by ischemia-reperfusion-induced damage of hindlimb (34). In a murine model of doxorubicin-induced cardiac injury, iloprost attenuated parameters of injury and dysfunction without compromising doxorubicin chemotherapeutic effect, suggesting potent anti-inflammatory and organ-protective effects of iloprost (38). However, despite the observations suggesting protective effects of iloprost in vivo, signaling mechanisms underlying protective effects of PGs on the pulmonary EC barrier dysfunction associated with ALI are not well understood.
It was suggested that PG exerts its protective effect on physiological processes through cAMP-mediated activation of PKA. In turn, activated PKA inhibits MLCK and suppresses Rho activity by activating Rho-GDP dissociation inhibitor (42) or by direct phosphorylation of RhoA (26, 36). Recent studies have described a novel PKA-independent pathway responsible for cAMP-mediated endothelial barrier enhancement (16, 17, 27). Elevation of intracellular cAMP activates nucleotide exchange factor Epac, which, in turn, activates Rap1 GTPase. Rap1 stimulates Rac-specific nucleotide exchange factors Tiam1 and Vav2, leading to activation of small GTPase Rac (14, 17). Our previous studies show that Rap1 stimulates Rac signaling and enhances endothelial barrier properties in vitro (13, 14). In microvascular endothelium, suppression of Rap1 significantly attenuated restoration of EC monolayer integrity after thrombin challenge (Fig. 5B), possibly via suppression of Rap1-mediated Rac activation during recovery phase. Importantly, Rap1 knockdown abolished enhancement of VE-caherin-containing adherens junctions and suppressed protective effects of iloprost against thrombin-mediated EC barrier disruption (Fig. 6).
This study shows for the first time the Rap1 mechanism of the iloprost-induced lung vascular barrier protection in the animal model of VILI. The data show that iloprost attenuated VILI-induced lung vascular permeability monitored by Evans blue extravasation into the lung parenchyma and Kf,c measurements and markedly decreased leukocyte infiltration. Protective effects of iloprost against vascular hyperpermeability and leukocyte infiltration were attenuated by Rap1 knockdown. Similar to in vivo effects, in the culture of HLMVEC, Rap1 knockdown suppressed iloprost-induced barrier enhancement, blocked inhibitory effect of iloprost on Rho activation by thrombin, and abolished EC protective response against thrombin-induced permeability.
Interestingly, in contrast to in vitro studies, administration of iloprost alone did not change the basal lung permeability. Although the nature of this phenomenon is not completely clear, we speculate that further decrease of basal permeability in healthy lungs may cause a dysfunction of normal fluid/molecule exchange. Therefore, at the organ level, iloprost-mediated permeability decrease may be adjusted by physiological compensatory mechanisms, which maintain parameters of permeability at physiological levels. In contrast, under pathological conditions, for example during VILI, attenuation of hyperpermeability by barrier-protective mediators is an essential step for restoration of lung homeostasis. Under these conditions, iloprost suppresses signaling pathways of barrier dysfunction, leading to permeability decrease and restoration of vascular barrier.
Disregulation of coagulation, thrombin activation, and fibrinolysis are frequent complications of ALI/adult respiratory distress syndrome. The important role of pulmonary coagulation was also recognized in the development of VILI (40, 44). Clinical investigations and experimental models of ALI/adult respiratory distress syndrome, VILI, and pneumonia demonstrated beneficial effects of anticoagulant therapy (40, 43, 44, 51). Our recent study described protective effect of Rho kinase inhibitor in the murine model of VILI (39). These data are highly consistent with previous reports showing that suppression of Rho kinase in pulmonary EC monolayers abrogates disruption of monolayer integrity induced by pathological cyclic stretch and thrombin (5, 7). Thus the thrombin model of Rho activation in pulmonary EC used in this study reproduces, at least in part, signaling events associated with VILI.
This study shows that iloprost-induced Rap1 activation leads to downregulation of Rho signaling. One potential mechanism of Rap1-induced Rho inhibition may be associated with activation of Rac, which may negatively regulate Rho in several ways (46). Another mechanism involves Rap1-Rac-induced association of Rho inhibitor p190RhoGAP with adherens junction protein p120-catenin, leading to local inhibition of Rho at the cell junction areas (53). Whether Rap1 directly downregulates Rho activity remains to be investigated. Interestingly, published data indicate that Rap1 may also be activated by disengagement of VE-cadherin during dissociation of adherens junctions (1). These data suggest a positive feedback recovery mechanism of disrupted EC monolayers by Rap1 activation and subsequent stimulation of Rac pathways of peripheral cytoskeletal remodeling and restoration of adherens junctions. Together with published data, our results suggest a parallel mechanism of iloprost-induced EC barrier protection via Rap1-dependent inhibition of barrier-disruptive Rho signaling and Rap1-mediated strengthening of intercellular contacts and peripheral actin cytoskeleton.
Although a main focus of the present study is the Rap1 signaling pathway, both Epac-Rap1- and PKA-dependent mechanisms coordinate endothelial barrier functions. Previous studies discussed the differences in cAMP concentrations required for Epac and PKA activation as potential switch between Epac and PKA signaling. PKA was considered to be activated by lower cAMP concentration than Epac. For example, it was thought that the inhibition of platelet-derived growth factor-induced ERK activation by forskolin is a PKA-mediated event because it occurs at much lower cAMP concentration than suggested for activation of Rap1 (56). This conclusion was recently challenged by results demonstrating that the binding affinity of cAMP for PKA and Epac is similar (23). Our laboratory's published data show that PGE2 and PGI2 increase EC barrier function via both PKA-dependent and Epac-Rap1-dependent pathways (14). In many processes, Epac and PKA pathways are interconnected. For example, Rap1-induced activation of Rac by Tiam1 may be additionally modulated by PKA-dependent phosphorylation of Tiam1 at PKA consensus site (55). Thus cross talk between Epac/Rap1 and PKA signaling is involved in Rac activation and EC barrier protective response to prostacyclin and iloprost. Interestingly, the protective effects of iloprost on EC barrier were not totally inhibited by Rap1 depletion. This finding can be explained by iloprost-mediated activation of PKA-dependent pathways, such as direct or indirect suppression of Rho GTPase (26, 36, 42) and EC MLCK (29) activities, which result in enhanced endothelial barrier function.
In conclusion, this study provides a new insight into mechanisms of pulmonary endothelial barrier regulation and demonstrates a role of Rap1 pathway in protective effects of iloprost against VILI and EC hyperpermeability. Our data show that, in addition to activation of PKA-dependent mechanisms, iloprost inhibits Rho pathway of vascular barrier dysfunction via Rap1-dependent mechanism. These pathways activated by iloprost lead to attenuation of vascular leak and leukocyte infiltration and may accelerate recovery of pulmonary endothelial barrier in the course of ALI. Thus results support clinical data suggesting beneficial effects of prostacyclin analogs in the treatment of ALI.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL87823, HL76259, and HL58064 to K. G. Birukov; and Grant HL89257 and the American Lung Association Biomedical Research Grant to A. A. Birukova.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. Richard Minshall and Dr. Stephen Vogel (Univ. of Illinois at Chicago) for valuable advice and assistance with measurements of Kf,c. The authors also thank Nurgul Moldobaeva for superb laboratory assistance.
REFERENCES
- 1. Asuri S, Yan J, Paranavitana NC, Quilliam LA. E-cadherin dis-engagement activates the Rap1 GTPase. J Cell Biochem 105: 1027–1037, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baltalarli A, Ozcan V, Bir F, Aybek H, Sacar M, Onem G, Goksin I, Demir S, Teke Z. Ascorbic acid (vitamin C) and iloprost attenuate the lung injury caused by ischemia/reperfusion of the lower extremities of rats. Ann Vasc Surg 20: 49–55, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol 26: 453–464, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res 95: 892–901, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–L797, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AA. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 290: L540–L548, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol 168: 1749–1761, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Birukova AA, Cokic I, Moldobaeva N, Birukov KG. Paxillin is involved in the differential regulation of endothelial barrier by HGF and VEGF. Am J Respir Cell Mol Biol 40: 99–107, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Birukova AA, Liu F, Garcia JG, Verin AD. Protein kinase A attenuates endothelial cell barrier dysfunction induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 287: L86–L93, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Birukova AA, Malyukova I, Mikaelyan A, Fu P, Birukov KG. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. J Cell Physiol 211: 608–617, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin-β-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 293: L199–L211, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67: 64–77, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol 215: 715–724, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Birukova AA, Zagranichnaya T, Alekseeva E, Fu P, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res 313: 2504–2520, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 348: 241–255, 2000. [PMC free article] [PubMed] [Google Scholar]
- 16. Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 4: 733–738, 2003. [DOI] [PubMed] [Google Scholar]
- 18. Bos JL, de Rooij J, Reedquist KA. Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol 2: 369–377, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 41: 661–690, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Brigham KL, Stecenko AA. Gene therapy for acute lung injury. Intensive Care Med 26, Suppl 1: S119–S123, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carey MA, Germolec DR, Langenbach R, Zeldin DC. Cyclooxygenase enzymes in allergic inflammation and asthma. Prostaglandins Leukot Essent Fatty Acids 69: 157–162, 2003. [DOI] [PubMed] [Google Scholar]
- 22. Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105: 1950–1955, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Dao KK, Teigen K, Kopperud R, Hodneland E, Schwede F, Christensen AE, Martinez A, Doskeland SO. Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J Biol Chem 281: 21500–21511, 2006. [DOI] [PubMed] [Google Scholar]
- 24. de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998. [DOI] [PubMed] [Google Scholar]
- 25. Dembinski R, Brackhahn W, Henzler D, Rott A, Bensberg R, Kuhlen R, Rossaint R. Cardiopulmonary effects of iloprost in experimental acute lung injury. Eur Respir J 25: 81–87, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Dong JM, Leung T, Manser E, Lim L. cAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROK alpha. J Biol Chem 273: 22554–22562, 1998. [DOI] [PubMed] [Google Scholar]
- 27. Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25: 136–146, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 163: 510–522, 1995. [DOI] [PubMed] [Google Scholar]
- 29. Garcia JG, Lazar V, Gilbert-McClain LI, Gallagher PJ, Verin AD. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol 16: 489–494, 1997. [DOI] [PubMed] [Google Scholar]
- 30. Howard LS, Morrell NW. New therapeutic agents for pulmonary vascular disease. Paediatr Respir Rev 6: 285–291, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Hu G, Ye RD, Dinauer MC, Malik AB, Minshall RD. Neutrophil caveolin-1 expression contributes to mechanism of lung inflammation and injury. Am J Physiol Lung Cell Mol Physiol 294: L178–L186, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Idzko M, Hammad H, van Nimwegen M, Kool M, Vos N, Hoogsteden HC, Lambrecht BN. Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. J Clin Invest 117: 464–472, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ishizaki T, Chiba H, Kojima T, Fujibe M, Soma T, Miyajima H, Nagasawa K, Wada I, Sawada N. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp Cell Res 290: 275–288, 2003. [DOI] [PubMed] [Google Scholar]
- 34. Koksel O, Ozdulger A, Aytacoglu B, Tamer L, Polat A, Sucu N, Yildirim C, Degirmenci U, Kanik A. The influence of iloprost on acute lung injury induced by hind limb ischemia-reperfusion in rats. Pulm Pharmacol Ther 18: 235–241, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett 579: 4966–4972, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J 15: 510–519, 1996. [PMC free article] [PubMed] [Google Scholar]
- 37. Liu F, Verin AD, Borbiev T, Garcia JG. Role of cAMP-dependent protein kinase A activity in endothelial cell cytoskeleton rearrangement. Am J Physiol Lung Cell Mol Physiol 280: L1309–L1317, 2001. [DOI] [PubMed] [Google Scholar]
- 38. Neilan TG, Jassal DS, Scully MF, Chen G, Deflandre C, McAllister H, Kay E, Austin SC, Halpern EF, Harmey JH, Fitzgerald DJ. Iloprost attenuates doxorubicin-induced cardiac injury in a murine model without compromising tumour suppression. Eur Heart J 27: 1251–1256, 2006. [DOI] [PubMed] [Google Scholar]
- 39. Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, Leitinger N, Garcia JG, Birukov KG. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care 12: R27, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oeckler RA, Hubmayr RD. Ventilator-associated lung injury: a search for better therapeutic targets. Eur Respir J 30: 1216–1226, 2007. [DOI] [PubMed] [Google Scholar]
- 41. Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J, Winkler J, Sitbon O, Popov W, Ghofrani HA, Manes A, Kiely DG, Ewert R, Meyer A, Corris PA, Delcroix M, Gomez-Sanchez M, Siedentop H, Seeger W. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 347: 322–329, 2002. [DOI] [PubMed] [Google Scholar]
- 42. Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 284: L972–L980, 2003. [DOI] [PubMed] [Google Scholar]
- 43. Russell JA. Management of sepsis. N Engl J Med 355: 1699–1713, 2006. [DOI] [PubMed] [Google Scholar]
- 44. Schultz MJ, Haitsma JJ, Zhang H, Slutsky AS. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia–a review. Crit Care Med 34: 871–877, 2006. [PubMed] [Google Scholar]
- 45. Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 157: 1721–1725, 1998. [DOI] [PubMed] [Google Scholar]
- 46. Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev 81: 153–208, 2001. [DOI] [PubMed] [Google Scholar]
- 47. Ueno Y, Koike H, Annoh S, Nishio S. Anti-inflammatory effects of beraprost sodium, a stable analogue of PGI2, and its mechanisms. Prostaglandins 53: 279–289, 1997. [DOI] [PubMed] [Google Scholar]
- 48. Uhlig S. Ventilation-induced lung injury and mechanotransduction: stretching it too far? Am J Physiol Lung Cell Mol Physiol 282: L892–L896, 2002. [DOI] [PubMed] [Google Scholar]
- 49. van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 87: 335–340, 2000. [DOI] [PubMed] [Google Scholar]
- 50. Vouret-Craviari V, Boquet P, Pouyssegur J, Van Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell 9: 2639–2653, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ware LB, Camerer E, Welty-Wolf K, Schultz MJ, Matthay MA. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am J Physiol Lung Cell Mol Physiol 291: L307–L311, 2006. [DOI] [PubMed] [Google Scholar]
- 52. Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J 18: 790–804, 2004. [DOI] [PubMed] [Google Scholar]
- 53. Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-Catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell 127: 1027–1039, 2006. [DOI] [PubMed] [Google Scholar]
- 54. Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem 280: 11675–11682, 2005. [DOI] [PubMed] [Google Scholar]
- 55. Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci 26: 724–732, 2001. [DOI] [PubMed] [Google Scholar]
- 56. Zwartkruis FJ, Wolthuis RM, Nabben NM, Franke B, Bos JL. Extracellular signal-regulated activation of Rap1 fails to interfere in Ras effector signalling. EMBO J 17: 5905–5912, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]