ABSTRACT
QUESTION One of my pregnant patients came for a routine prenatal visit at 20 weeks’ gestation. Near the end of the consultation, she asked me about “slapped cheek” disease and pregnancy, as her son had been diagnosed with fifth disease the previous week. What is the current guideline for pregnant women exposed to parvovirus B19?
ANSWER The rate of vertical transmission during maternal parvovirus B19 infection is estimated at 33%, with fetal complications occurring in 3% of infected women. Fetal complications comprising hemolysis, anemia, and nonimmune hydrops fetalis and fetal loss are more frequent when maternal infection occurs before 20 weeks of gestation. The first step in the management of this patient would be to obtain immunoglobulin (Ig) M and IgG titres against parvovirus to evaluate if the patient has had previous immunity against the disease. If results are negative for IgG but positive for IgM (ie, primary infection), this patient would need close obstetrical monitoring for the following weeks, including serial ultrasounds to rule out fetal anemia and hydrops fetalis.
RÉSUMÉ
QUESTION Une de mes patientes enceintes est venue me voir pour une visite prénatale ordinaire à 20 semaines de gestation. Vers la fin de la consultation, elle m’a posé des questions sur la cinquième maladie et la grossesse, parce que son fils avait reçu un diagnostic de mégalérythème épidémique la semaine précédente. Quelles sont les lignes directrices actuelles à suivre pour les femmes enceintes exposées au parvovirus B19?
RÉPONSE O n estime à 33 % le taux de transmission verticale durant une infection maternelle au parvovirus B19, et chez les femmes infectées, à 3 % le taux de complications fœtales. Les complications fœtales, notamment l’hémolyse, l’anémie, l’anasarque fœtoplacentaire non immunologique et la perte du fœtus, sont plus fréquentes quand l’infection maternelle se produit avant 20 semaines de gestation. La première étape dans la prise en charge de cette patiente est d’obtenir ses titres d’immunoglobuline (Ig) M et d’IgG contre le parvovirus pour évaluer si la patiente a déjà été immunisée antérieurement contre la maladie. Si les résultats sont négatifs pour l’IgG mais positifs pour l’IgM (c.-à-d. une infection primaire), il faudra exercer une surveillance obstétricale étroite chez cette patiente dans les semaines qui suivent, y compris des échographies en séries, pour écarter la possibilité d’anémie fœtale ou d’anasarque fœtoplacentaire.
The human parvovirus B19 is the most common viral agent associated with rashes in school-aged children.1 Transmission can occur via the respiratory route, hand-to-mouth contact, blood products, and vertically from the mother to the fetus. The virus replicates in rapidly proliferating cells, such as erythroblast precursors. In healthy hosts the virus can cause a range of clinical manifestations, including erythema infectiosum (ie, fifth disease), transient aplastic crisis, chronic red cell aplasia, myocarditis, arthropathy, and nonimmune hydrops fetalis.2
Typically, erythema infectiosum presents with mild and nonspecific symptoms. Approximately 1 week after initial infection a mild illness develops, characterized by headaches, mild fever, malaise, joint pain, and myalgia.3 After 2 to 5 days, a red pruritic rash emerges on the cheeks, causing the typical “slapped-cheek” appearance (Figure 1).4 The rash spares the areas around the nose, mouth, and eyes. It usually disappears within 2 to 4 days and is followed by a second rash on the trunk and extremities.5 The rash consists of pink maculae, which usually undergo a central fading.2 In 20% to 30% of adults, the infection is asymptomatic. At present, there is no vaccine available for this virus.3
Figure 1.
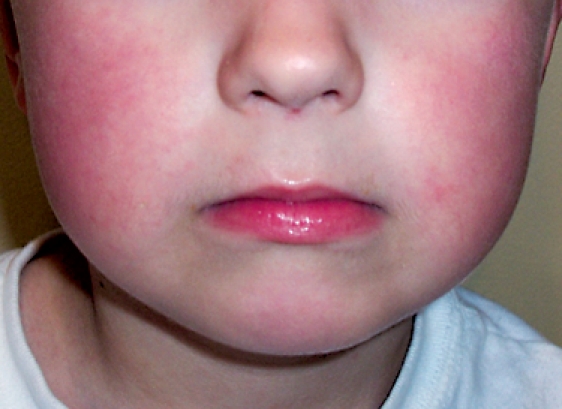
Red, pruritic rash on the cheeks, creating the typical “slapped cheek” appearance of fifth disease
Sixty-five percent of pregnant women in North America have evidence of past infection with parvovirus B19, while 35% to 45% do not possess protective immunoglobulin (Ig) G antibodies. The incidence of acute parvovirus B19 infection in pregnancy is approximately 1% to 2% in endemic periods, but can exceed 10% in epidemics.6
Parvovirus B19 infection during pregnancy is mostly asymptomatic, but in approximately 3% of infected women it might cause a range of complications, including abortion, severe fetal anemia, nonimmune hydrops fetalis, and even fetal demise.3,7 Several factors have been associated with an increased risk of acute parvovirus B19 infection in pregnant women: Women who have only 1 child have a 3-fold greater risk of infection compared with nulliparous women; the risk increases to 7.5-fold in women with 3 or more children. Working in nursery schools or after-school clubs or day care centres also appears to increase the risk.6 Serious medical conditions and stressful jobs have also been identified as risk factors.8 There is approximately a 50% risk of infection from close, frequent interaction with an infected child (eg, in the home).
Parvovirus infection is transmitted across the placenta to the fetus in approximately 30% of pregnant women who contract the infection, with a mean interval of 6 to 7 weeks between maternal exposure and fetal infection. For women who contract parvovirus in the first trimester, the rate of fetal loss can be as high as 10%. The highest risk is between 9 and 16 weeks of gestation. The risk is reduced in the second trimester, and fetal complications are rare during the last 2 months of pregnancy. When hydrops develops, it most frequently occurs 2 to 4 weeks after maternal infection.3,9 Most of the time clinical signs of hydrops are evident in the second trimester (mean gestational age of 22 to 23 weeks).
The severe fetal anemia leads to high-output congestive heart failure and might result in nonimmune hydrops fetalis. In addition, several cases of fetal central nervous system abnormalities, encephalopathy, and neonatal encephalitis or meningitis have been reported.10 The fetus is especially vulnerable to severe anemia and hydrops fetalis at 8 to 20 weeks’ gestational age owing to the shorter erythrocyte half-life at this stage of development compared with later bone marrow and splenic hematopoietic stages.11 Other possible causes include fetal viral myocarditis, leading to cardiac failure, and hepatitis, with impaired hepatic function leading to fetal hypoalbuminemia.3,12
Diagnosis
The characteristic facial rash of erythema infectiosum—also referred to as “slapped cheek” syndrome—and other symptoms of viral disease are suggestive, but not diagnostic, of parvovirus B19 infection. Conclusive diagnosis of parvovirus B19 infection relies mostly on indirect (serologic) and direct (DNA detection) tests.5
Diagnosis of acute parvovirus B19 infection can be made most reliably by the finding of virus-specific IgM antibodies in immunocompetent persons or by the isolation of parvovirus DNA. In the context of a negative IgG test result for the virus, a positive IgM test result indicates recent infection and levels are usually elevated 21 to 24 days after the initial exposure to the virus, or 3 to 4 days after the onset of clinical illness. Elevated IgM levels generally persist for 2 to 3 months, although in some patients they can persist for more than 6 months. Immunoglobulin G levels rise 24 to 28 days after exposure or after 7 days of clinical infection and remain elevated, indicating lifelong immunity. The finding of both IgM and IgG antibodies indicates an infection that began as recently as 7 days to as long ago as 6 months previously.2,13,14 Maternal IgM and viral load values can be used as virological predictors of fetal infection severity.15
Management in pregnancy
There is no specific antiviral drug against parvovirus B19 infection. Nonsteroidal anti-inflammatory drugs and acetaminophen can be used for muscle and joint pain. In pregnant patients, serologic testing should be done to establish immune status. If there is evidence of a past infection (ie, IgG positive, IgM negative) no further tests will be necessary, as the patient is considered immune. In the case of a pregnant woman whose test results are negative for both IgM and IgG, repeated determination of IgM within 2 to 3 weeks after exposure is recommended to exclude seroconversion.3,16 Weekly ultrasound examinations, for up to 12 weeks after maternal exposure, should be performed in all patients with suspected or confirmed infection to evaluate for signs of fetal anemia or hydrops fetalis (ascites, pericardial effusion). Beyond 12 weeks after the potential infection, the risk virtually disappears. Doppler ultrasound measurements showing elevated peak systolic velocity values in the fetal middle cerebral artery accurately predict fetal anemia. Fetal blood sampling is warranted in the present of hydrops to assess the degree of fetal anemia. Fetal management is dependent on gestational age, but intrauterine transfusion via cordocentesis for hydrops might improve fetal outcome. The risk of fetal death appears to be higher in fetuses managed expectantly than in those managed with intrauterine transfusion.3,13 In one study approximately one-third of the cases of parvovirus-induced nonimmune hydrops resolved spontaneously, whereas 83.5% of transfused hydropic fetuses survived.17 The average time for hydrops resolution was 4 weeks.10
Conclusion
Maternal exposure to acute parvovirus B19 infection has the potential risk of adverse fetal outcome. The risk is increased if maternal infection occurs during the first 2 trimesters, but still exists during the third trimester. Despite placental transfer of parvovirus reaching 30% to 50%, most neonates are born normal. Hence, invasive prenatal diagnosis should be considered only if there are clinical signs of fetal anemia or hydrops fetalis. Then, depending on gestational age, induction of labour versus fetal blood sampling and intrauterine transfusion should be considered by a high-risk perinatal team.18
MOTHERISK
Motherisk questions are prepared by the Motherisk Team at the Hospital for Sick Children in Toronto, Ont. Drs Staroselsky, Klieger-Grossmann, and Garcia-Bournissen are members and Dr Koren is Director of the Motherisk Program. Dr Koren is supported by the Research Leadership for Better Pharmacotherapy during Pregnancy and Lactation. He holds the Ivey Chair in Molecular Toxicology in the Department of Medicine at the University of Western Ontario in London.
Do you have questions about the effects of drugs, chemicals, radiation, or infections in women who are pregnant or breastfeeding? We invite you to submit them to the Motherisk Program by fax at 416 813–7562; they will be addressed in future Motherisk Updates.
Published Motherisk Updates are available on the Canadian Family Physician website (www.cfp.ca) and also on the Motherisk website (www.motherisk.org).
Footnotes
Competing interests
None declared
References
- 1.From the Centers for Disease Control and Prevention. Rashes among schoolchildren—14 states, October 4, 2001-February 27, 2002. JAMA. 2002;287(11):1389–91. [PubMed] [Google Scholar]
- 2.Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15(3):485–505. doi: 10.1128/CMR.15.3.485-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ergaz Z, Ornoy A. Parvovirus B19 in pregnancy. Reprod Toxicol. 2006;21(4):421–35. doi: 10.1016/j.reprotox.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Servey JT, Reamy BV, Hodge J. Clinical presentations of parvovirus B19 infection. Am Fam Physician. 2007;75(3):373–6. [PubMed] [Google Scholar]
- 5.Young NS, Brown KE. Parvovirus B19. N Engl J Med. 2004;350(6):586–97. doi: 10.1056/NEJMra030840. [DOI] [PubMed] [Google Scholar]
- 6.Valeur-Jensen AK, Pedersen CB, Westergaard T, Jensen IP, Lebech M, Andersen PK, et al. Risk factors for parvovirus B19 infection in pregnancy. JAMA. 1999;281(12):1099–105. doi: 10.1001/jama.281.12.1099. [DOI] [PubMed] [Google Scholar]
- 7.Koch WC, Harger JH, Barnstein B, Adler SP. Serologic and virologic evidence for frequent intrauterine transmission of human parvovirus B19 with a primary maternal infection during pregnancy. Pediatr Infect Dis J. 1998;17(6):489–94. doi: 10.1097/00006454-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Jensen IP, Thorsen P, Jeune B, Moller BR, Vestergaard BF. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG. 2000;107(5):637–43. doi: 10.1111/j.1471-0528.2000.tb13306.x. [DOI] [PubMed] [Google Scholar]
- 9.Goff M. Parvovirus B19 in pregnancy. J Midwifery Womens Health. 2005;50(6):536–8. doi: 10.1016/j.jmwh.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 10.De Jong EP, de Haan TR, Kroes AC, Beersma MF, Oepkes D, Walther FJ. Parvovirus B19 infection in pregnancy. J Clin Virol . 2006;36(1):1–7. doi: 10.1016/j.jcv.2006.01.004. Epub 2006 Feb 20. Erratum in: J Clin Virol 2007 Feb;38(2):188. [DOI] [PubMed] [Google Scholar]
- 11.Chisaka H, Morita E, Yaegashi N, Sugamura K. Parvovirus B19 and the pathogenesis of anaemia. Rev Med Virol. 2003;13(6):347–59. doi: 10.1002/rmv.395. [DOI] [PubMed] [Google Scholar]
- 12.Garcia AG, Pegado CS, Cubel Rde C, Fonseca ME, Sloboda I, Nascimento JP. Fetoplacentary pathology in human parvovirus B19 infection. Rev Inst Med Trop Sao Paulo. 1998;40(3):145–50. doi: 10.1590/s0036-46651998000300003. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Raff TC, Muallem NS, Neubert AG. Hydrops fetalis secondary to parvovirus B19 infections. J Am Board Fam Pract. 2003;16(1):63–8. doi: 10.3122/jabfm.16.1.63. [DOI] [PubMed] [Google Scholar]
- 14.De Haan TR, Beersma MF, Claas EC, Oepkes D, Kroes AC, Walther FJ. Parvovirus B19 infection in pregnancy studied by maternal viral load and immune responses. Fetal Diagn Ther . 2007;22(1):55–62. doi: 10.1159/000095845. Epub 2006 Sep 22. [DOI] [PubMed] [Google Scholar]
- 15.De Haan TR, Beersma MF, Oepkes D, de Jong EP, Kroes AC, Walther FJ. Parvovirus B19 infection in pregnancy: maternal and fetal viral load measurements related to clinical parameters. Prenat Diagn. 2007;27(1):46–50. doi: 10.1002/pd.1619. [DOI] [PubMed] [Google Scholar]
- 16.Crane J. Parvovirus B19 infection in pregnancy. J Obstet Gynaecol Can. 2002;24(9):727–43. [PubMed] [Google Scholar]
- 17.Rodis JF, Borgida AF, Wilson M, Egan JF, Leo MV, Odibo AO, et al. Management of parvovirus infection in pregnancy and outcomes of hydrops: a survey of members of the Society of Perinatal Obstetricians. Am J Obstet Gynecol. 1998;179(4):985–8. doi: 10.1016/s0002-9378(98)70203-0. [DOI] [PubMed] [Google Scholar]
- 18.Crane J Society of Obstetricians and Gynaecologists of Canada. Parvovirus B19 infection in pregnancy. J Obstet Gynaecol Can. 2002;24(9):727–43. [PubMed] [Google Scholar]


