Abstract
Despite years of antidepressant drug development and patient and provider education, suboptimal medication dosing and duration of exposure resulting in incomplete remission of symptoms remains the norm in the treatment of depression. Additionally, since no one treatment is effective for all patients, optimal implementation focusing on the measurement of symptoms, side effects, and function is essential to determine effective sequential treatment approaches. There is a need for a paradigm shift in how clinical decision making is incorporated into clinical practice and for a move away from the trial-and-error approach that currently determines the “next best” treatment. This paper describes how our experience with the Texas Medication Algorithm Project (TMAP) and the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial has confirmed the need for easy-to-use clinical support systems to ensure fidelity to guidelines. To further enhance guideline fidelity, we have developed an electronic decision support system that provides critical feedback and guidance at the point of patient care. We believe that a measurement-based care (MBC) approach is essential to any decision support system, allowing physicians to individualize and adapt decisions about patient care based on symptom progress, tolerability of medication, and dose optimization. We also believe that successful integration of sequential algorithms with MBC into real-world clinics will facilitate change that will endure and improve patient outcomes. Although we use major depression to illustrate our approach, the issues addressed are applicable to other chronic psychiatric conditions including comorbid depression and substance use disorder as well as other medical illnesses.
Keywords: Measurement-Based Care, Decision Support Systems, Adaptive Treatment Strategies, Depression
1. Introduction
Depression is a common and often chronic and recurrent disease affecting a vast number of people worldwide (Kessler et al., 2003; Murray and Lopez 1996). Currently, depression ranks fourth for disability-adjusted life-years worldwide (World Health Organization 2001). Substance use disorder (SUD) and major depressive disorder (MDD) commonly co-occur (Kelly et al., 2003). Both disorders pose a particularly complex treatment challenge when they coexist. Just as chronically medically ill patients vary over time in their need for different treatments, so do those with chronic psychiatric disorders. In this context, awareness of strategies to improve fidelity to evidence-based treatment guidelines for depression and SUD should be particularly useful to clinicians working with patients with co-occurring MDD and SUD. For the purpose of the following discussion, we will focus on the treatment of depression and provide examples of potential uses in the treatment of comorbid SUD. The goal of the following paper is to introduce the reader to the concept of measurement-based care as an adaptive treatment strategy, as well as to provide the rationale for using this approach in conjunction with a computerized, evidence-based, medication algorithm when tailoring treatment for individuals with MDD.
Despite the high prevalence of MDD and the availability of new and effective treatments over the last 20 years, recent evidence in practice settings continues to demonstrate high rates of inadequate antidepressant medication treatment in terms of dose and duration, inappropriate and frequent changes in treatment, as well as low adherence and high drop out rates, all contributing to low rates of remission for depression (Kessler et al., 2003). For example, in routine practice patients often do not receive a large enough dose of medication during a treatment trial, suggesting that lack of remission (i.e., the absence of symptoms) may be a result of an inadequate dose rather than an ineffective treatment. Similarly, there is also evidence that many patients do not receive therapeutic doses of medication for sufficient duration. Furthermore, inconsistency of treatment from physician to physician is common, suggesting a practice bias rather than a tailored, individualized treatment approach. The number of patients achieving symptom remission to initial antidepressant treatment is no more than 35% among all patients treated, with the remaining requiring at least two or more steps in pharmacotherapy (Rush et al., 2006c; Trivedi et al., 2004b; Trivedi et al., 2006c; Trivedi et al., 2006a). Considering the progress made in psychiatric care and treatment, this outcome is unsatisfactory and increases the risk of developing treatment resistant depression (TRD).
In clinical practice, when a patient does not achieve remission following an initial adequate antidepressant trial, several treatment choices are currently deemed possible. One, switch to an antidepressant within the same class; two, switch to an antidepressant from another class; or three, augment the current antidepressant with another medication. Given that significant variation in treatment choices and actual outcomes continues, sequenced algorithms and measurement-based treatment may be the most justifiable, cost-effective method to provide a structured synthesis of relevant data and a detailed set of strategies and tactics to achieve full remission.
To date, as opposed to efficacy trials, effectiveness trials conducted in “real world” clinical practice settings reveal even lower remission rates (15% to 35%) in primary and specialty care even with the addition of depression care specialists to pharmacotherapy (Katon et al., 2004; Trivedi et al., 2004b; Trivedi et al., 2006c; Unützer et al., 2002). Previous attempts to implement guidelines for MDD using a paper-and-pencil format in clinical settings (Trivedi et al., 2004b; Trivedi et al., 2006c; Trivedi et al., 2007) have revealed problems with fidelity in terms of dosing, adequate duration of treatment, and visit frequency. One possible solution to this problem is to couple guidelines with a measurement-based, computerized approach (Hunt et al., 1998; Trivedi et al., 2000; Trivedi et al., 2001; Trivedi et al., 2002; Trivedi et al., 2004a). There is already evidence of improved physician fidelity to guidelines using this approach, suggesting that providing immediate decision support at the point of care via a comprehensive electronic health record could improve physician adherence and clinical outcomes (Trivedi et al., 2000; Trivedi et al., 2002; Trivedi et al., 2004a).
2. Background
2.1. Major Depressive Disorder
Major depressive disorder (MDD) is a serious, debilitating illness that affects persons of all ages, races, and socioeconomic backgrounds. MDD occurs in up to one in eight individuals during their lifetime, making it one of the most prevalent of all medical illnesses. The point prevalence rates for MDD are approximately 2.3%-3.2% in men and 4.5%-9.3% in women, with a lifetime risk for developing an episode of 7-12% for men and 20-25% for women (Diagnostic and Statistical Manual-Fourth Edition Text Revision (DSM-IV TR;(American Psychiatric Association 2000).
2.2. Substance Use Disorder and Comorbid Depression
As mentioned in our introduction, SUD and MDD commonly co-occur (Kelly et al., 2003) and when occurring simultaneously are particularly difficult to treat. Data suggest that as many as 40-50% of those presenting to primary care with depression may have co-occurring SUD (Mallin et al., 2002). Furthermore, it has also been theorized that relapsing substance abuse could be a consequence of MDD (Hauenstein 2003).
In the past there may have been reluctance to use antidepressants to treat MDD in patients with SUD (Pettinati 2004); however, there is now evidence that patients who have been dually diagnosed have less success in treatment programs targeting their substance use. Similarly, dual diagnosis research has also shown that these individuals use more psychiatric and medical care resources than persons with depression alone (Mark 2003), with evidence that individuals with co-occurring depression and alcoholism, for example, had 55% more hospital days and 13% more visits than those with depression alone (Fortney et al., 1999). Research also shows that a relapsing course is of particular concern in both the treatment of depression and substance abuse (Fortney et al., 1999; Kelly et al., 2003), with evidence suggesting that depressed people are more likely to relapse due to substance abuse (Greenfield et al., 1998).
2.3 Residual Symptoms and Functional Impairment in Major Depression
The goal of depression treatment is now full remission of symptoms (i.e., absence of symptoms), rather than response (Bakish 2001; Rush et al., 2006b). Traditional definitions of outcomes for depression only focus on symptomatic improvement or response, rather than full remission of symptoms and fail to emphasize the substantial impact of depression on social function (Rush et al., 2006b; Trivedi et al., 2006d; Wisniewski et al., 2006). There is considerable evidence that even with treatment, residual symptoms often persist, leading to psychosocial dysfunction (Bakish 2001; Judd et al., 1998; Trivedi et al., 2006d), and the longer a patient remains symptomatic, the lower the chances of a complete recovery (Keller et al., 1992). The co-occurrence of MDD and substance abuse or other comorbidities intensifies the degree of medical and psychosocial impairment, resulting in significant suffering and degradation in global function.
2.4. Current Pharmacological Treatment of Depression
As mentioned in our introduction, disparity between “best practice” and actual practice in the treatment of depression is found abundantly in the literature (Katon et al., 1995), with studies revealing that too often patients do not get the correct medications, the correct dose, or treatment for an adequate duration to yield maximal clinical benefit (Ford 2000; Katon et al., 1995; Lin et al., 1995; Simon et al., 1993). As a result, the desired goal of full remission of depressive symptoms and return to premorbid level of functioning is not commonly achieved. Given this background, medication guidelines with appropriate cues about critical times for reevaluation and medication changes should improve both the quality of care and patient outcomes.
2.5. Guidelines for the Treatment of Depression
Evidence-based clinical practice guidelines are the foundation for developing more specific, clinician-friendly, recommended treatment sequences — algorithms (Depression Guideline Panel 1993a; Depression Guideline Panel 1993b; Schulberg et al., 1998). Treatment algorithms recommend specific treatment sequences and provide tactical recommendations (at what dose, over what period of time should particular strategies be used), while employing a format that results in consistency in treatment across physicians, leading to better treatments at lower costs. Although one algorithm will not suffice for all patients, it should produce a reduction of symptoms in most. Medication algorithms should lead to a more rapid and/or more thorough (complete) response as well as bringing appropriate uniformity to effective treatments at predictable costs, thereby improving the quality of care and potentially the overall cost of care.
2.6. Barriers to Dissemination and Implementation of Guidelines
While efforts to promote current advances in pharmacology have resulted in a surge of consensus guidelines, finding an effective dissemination method continues to be the greatest challenge (Davis et al., 1995; Gorton et al., 1995; Trivedi et al., 2000; Trivedi et al., 2002; Trivedi et al., 2004a). Despite wide promotion, clinical practice guidelines have had limited effect in changing physician behavior (Hayward 1997; Lomas et al., 1989). Simply publishing treatment guidelines or providing them through continuing medical education programs have not been shown to improve and update physicians' practice patterns (Deyo and Carter 1992; Evans et al., 1986; Gemson et al., 1995).
Physician adherence is critical not only in translating recommendations into improved care and achieving fidelity to treatment algorithms, but also in adapting treatments to the needs of individual patients. It has been argued that guideline development and implementation should be theory-driven and evidence-based (Bauer 2002; Smith 2000), and that changing health care provider behavior is the necessary step to ensure improved outcomes (Rubenstein et al., 2000).
In an effort to evaluate guideline adherence, Chen and Rosenheck (Chen and Rosenheck 2001) examined the translation of Agency for Health Care Policy and Research (AHCPR) guidelines for MDD using computerized patient database-derived measures to assess guideline performance and patterns of care for depression in a mental health clinic setting. Conformance was measured for three guideline recommendations. Of patients on a single antidepressant, 87% received dosages within the recommended range. Sixty-nine percent received the recommended number of follow-up visits. Specific condition-related treatment interventions were identified in 32% of patients with comorbid alcoholism. Even though the data relating to medication dosing appear to suggest reasonable adherence, these data should be interpreted with care, due to limitations noted by the authors including incomplete administrative data and use of a patient population who had recently been discharged from hospital (Chen and Rosenheck 2001).
Research demonstrates improved outcomes when guidelines are better adhered to during the research project, with benefits dissipating after the study is over (Katon et al., 1995; Lin et al., 1997). For example, using a collaborative care program, Katon and colleagues achieved AHCPR treatment guidelines for depression by improving the therapeutic practices of primary care physicians, resulting in better adherence to antidepressant therapy and enhanced clinical outcomes (Katon et al., 1995). Unfortunately, however, there was no enduring effect observed in patterns of treating depression after the discontinuation of the multifaceted program (Lin et al., 1997). Specifically, there was no change in adequacy of dosage and duration of pharmacotherapy at the end of the 2-year period.
Barriers to guideline adherence have been identified, including lack of awareness, agreement, self-efficacy, outcome expectancy, and the inertia of previous practice (Cabana and Clark 2003), as well as lack of time, insufficient staff/support, and patient-related factors. To be successful, algorithms must be easy to use and to understand, as well as time efficient. Although physicians acknowledge making suboptimal decisions prior to the algorithm implementation, they report adhering to it to be too tedious (Margolis et al., 1992).
3. Measurement-Based Treatment of Depression
Even in guideline-driven practice, clinical treatment of depression is often associated with wide variations among practitioners. Clinicians often change from one antidepressant to another too quickly or, conversely, conduct an unnecessarily prolonged treatment trial with an obviously unsuccessful medication or psychotherapy (Rush et al., 2003a; Trivedi et al., 2006c).
Practitioners also differ in how they assess the outcomes of treatment (symptoms, function, side-effect frequency and burden), with global judgments often used instead of specific symptom assessments, even though the former are less accurate (Biggs et al., 2000). These differences lead to wide variability in treatment implementation and likely also result in wide variations in outcomes in typical practice.
Specific recommendations to guide treatment decisions have been shown to be most effective when they are tailored to individual patients and provided while the clinical decision is being made (Trivedi et al., 2000; Trivedi et al., 2002; Trivedi et al., 2004a). Treatment trials for the management of major chronic medical illnesses, such as diabetes mellitus, utilize laboratory as well as symptom and function measures in research settings that are readily usable in clinical practice. To our knowledge, however, no system to provide specific feedback or prompts related to symptoms, side effects, and recommended tactics (i.e., when and by how much to change the dose) during treatment has been successfully used in a large clinical trial for patients with psychiatric disorders.
We believe that a measurement-based care approach is an essential component to any adaptive decision support system, allowing the physician to individualize decisions about care for the patient based on their progress and their ability to tolerate the medication (Trivedi et al., 2006c). The algorithms developed by our group allow for sequential adaptive treatment approaches including switching or augmenting antidepressant treatment in the case of patients who do not fully remit following an adequate trial (at an adequate dose) of an antidepressant. To date, our group has used paper-and-pencil-based clinical decision support systems in two hybrid efficacy-effectiveness algorithm studies (Rush et al., 2004; Trivedi et al., 2004b; Trivedi et al., 2006c). Both the Texas Medication Algorithm Project (TMAP) and Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trials occurred in real-world clinical settings and emphasized the importance of a measurement-based treatment approach (Trivedi et al., 2006c) – wherein the physician assessed depression symptom severity, adherence to treatment, and side effects at each visit and used this information when following the medication treatment protocol. Despite the obvious advantages of this approach, results using the paper-and-pencil format have shown only modest improvement in depression outcomes.
3.1. The Texas Medication Algorithm Project (TMAP)
TMAP evaluated the clinical and economic impact of medication treatment algorithms for MDD compared with treatment as usual (Crismon et al., 1999). Recommendations were based on published evidence and when necessary, by clinical judgment. The algorithm treatment steps guided physicians in determining medication treatment plans and were designed with the objectives of long-term safety, tolerability, and full symptom remission.
In total, 547 patients with MDD were enrolled, of which 181 were treated using the MDD algorithm (ALGO), and 366 followed in treatment as usual. Using the clinician-rated Inventory of Depressive Symptomatology (IDS-C30) (Rush et al., 1996; Rush et al., 2000; Trivedi et al., 2004c) as the primary outcome measure and the self-rated version (IDS-SR30) as the secondary outcome, results showed that treatment with the MDD algorithm was superior to treatment as usual based on both clinician and self-reported symptoms (P= 0.002) (Trivedi et al., 2004b). Similarly, functional improvement (as measured by the Medical Outcomes Study 12-item Short-Form Health Survey - SF-12) (Ware et al., 1996) was also superior with ALGO treatment compared to treatment as usual (P< .046). ALGO patients with MDD also had lower side effects as compared to treatment as usual. Despite the benefits associated with algorithm care, inspection of TMAP data suggests only moderate adherence to the algorithms, with problems identified in terms of staging, dosing, and adherence to visit frequency. While response and remission rates increased from 3-12 months, the one-year last observation carried forward (LOCF) response (26.3%) and remission (11.0%) rates for the ALGO group were not impressive (sustained response = 14.4%; sustained remission = 5.1%) (Rush et al., 2004). The outcomes at 12 months using LOCF were also poor for those treated with treatment as usual (response 19.4%; remission 7.8%; and sustained response and remission 9.1% and 3.6%, respectively). Overall, although the results of the study do suggest favorable outcomes, problems relating to guideline adherence were identified that emphasize the need for better and perhaps electronic approaches to ensure higher levels of physician adherence to algorithms.
3.2. Sequenced Treatment Alternatives to Relieve Depression (STAR*D)
Specific recommendations for dosing tactics were provided in the form of a treatment manual. Unlike standard double-blind efficacy trials, the STAR*D treatment protocol was designed for adaptation to specific clinical situations in order to define optimal care for individual participants at specified times. A Clinical Research Coordinator monitored and prompted the treatment decisions with post visit monitoring to ensure that true treatment resistance was present before proceeding to the next treatment step.
The STAR*D monitoring scheme provided feedback in a combination of Web reports and paper-pencil decision support to the clinical team to ensure that the pharmacotherapies were delivered at optimal doses and for adequate durations, as gauged by the symptomatic status and side-effect burden at clinic visits. Clinically useful tools were developed that promoted the use of maximally effective, well-tolerated medication dosages for a sufficient time period to ensure that the recognition of treatment failure was neither premature nor inappropriately delayed. These easy-to-use tools and methods were designed to provide feedback to, and corrective action by, physicians, and are easily transferable to routine practice.
STAR*D enrolled a total of 4041 patients. Those patients with chronic recurrent MDD (80% of the sample) had the most disabling form of nonpsychotic MDD, with higher rates of prior suicide attempts, early onset MDD, and more comorbid general medical and psychiatric disorders. STAR*D provided prospective, randomized, controlled evidence to compare different treatments following inadequate benefit from initial (Level 1) treatment with citalopram (Trivedi et al., 2006c). The second step (Level 2) compared several switch or augment antidepressant treatments. Remission rates for Level 1 were 28% (17-item Hamilton Rating Scale for Depression - HRSD17) (Hamilton 1960; Hamilton 1967) and 33% (16-item self-report Quick Inventory for Depressive Symptomatology - QIDS-SR16) (Rush et al., 2003b; Rush et al., 2006a; Trivedi et al., 2004c). The response rate was 47% (QIDS-SR16) (Trivedi et al., 2006c). Primary and psychiatric care settings did not differ in either remission or response rates. A substantial portion of participants who did achieve either response or remission at study exit did so at or after 8 weeks of treatment.
Table 1 provides an example of an operationalized treatment algorithm similar to those developed for the STAR*D study. To ensure that each medication was optimally used in terms of dose and duration, the STAR*D treatment manual identified critical decision points (weeks 4, 6, 9, and 12) for each medication stage. At each critical decision point, changes in either the treatment strategy or tactic were recommended based on the current dose and duration of the particular medication together with the degree of symptom change and side-effect burden. The clinician, using the decision rules provided, used this data (obtained as part of measurement-based care) to drive their clinical treatment at that point in time, thereby tailoring treatment to the individual patient. Critical decision points provide a means of implementing adaptive treatment strategies in real-world practice. In the case of STAR*D, the 16-item QIDS-C (Rush et al., 2003b; Rush et al., 2006a; Trivedi et al., 2004c) was used to measure depression symptom severity. For example, if at week 8 the patient continued to show little or no improvement (i.e., QIDS ≥ 9), the decision rules suggested that the patient be switched to a new treatment (i.e., changes stage). In terms of side effects, STAR*D developed the self-report Frequency, Intensity, and Burden of Side Effects Rating scale (FIBSER) (Wisniewski et al., 2006) to assess for the burden associated with any side effects present.
Table 1. Measurement-Based Care and Tactics for Acute Phase Treatment of Major Depressive Disorder.
| Critical Decision Point (CDP) | Clinical Status | Plan | |
|---|---|---|---|
| Week 0 (CDP #1) | HAM-D17 ≥ 14 | Symptomatic |
|
| Week 4 (CDP #2) | QIDS-C16 ≤5 | Remission |
|
| QIDS-C16 = 6-8 | Partial Response |
|
|
| SEs intolerable |
|
||
| QIDS-C16 ≥9 | Nonresponse |
|
|
| SEs intolerable |
|
||
| Week 6 (CDP #3) | QIDS-C16 ≤5 | Remission |
|
| QIDS-C16 = 6-8 | Partial Response |
|
|
| SEs intolerable |
|
||
| QIDS-C16 ≥9 | Nonresponse |
|
|
| SEs intolerable |
|
||
| Week 9 CDP #4) | QIDS-C16 ≤5 | Remission |
|
| QIDS-C16 = 6-8 | Partial Response |
|
|
| QIDS-C16 ≥9 | Nonresponse or SEs intolerable |
|
|
| Week 12 (CDP #5) | QIDS-C16 ≤5 | Remission |
|
| QIDS-C16 = 6-8 | Partial Response |
|
|
| QIDS-C16 ≥9 | Nonresponse or SEs intolerable |
|
|
If after 12 weeks the patient has not remitted, but the clinician feels that 2 more weeks of treatment would be beneficial, treatment may be extended.
QIDS-C16, 16-item Quick Inventory of Depressive Symptomatology – Clinician-rated; SEs, side effects; HAM-D17, 17-item Hamilton Depression Rating Scale
4. Conclusions drawn from paper-and-pencil clinical support
Even though both TMAP and the STAR*D trials showed improvement in outcomes, overall results achieved were still less than optimal with evidence of problems with guideline adherence in terms of dosing, stage changes, and visit frequency (Trivedi et al., 2004b). The fact that guidelines were in a paper-and-pencil format may have further hindered implementation. In addition, the presence of an on-site clinical research coordinator to encourage physicians to follow guidelines was an added expense.
4.1. Adherence to Treatment Guidelines
TMAP did not directly measure adherence at the point of care. Instead, clinical data were reviewed for guideline adherence for each patient after the visit in order to assess the degree of algorithm concordant care. As the TMAP methodology included assessment of symptoms and side effects at each physician visit, these data could be used to assess physician behaviors at each medication visit. Our group is currently working on a manuscript in which we will report on findings following a retrospective review of adherence from the TMAP data. This retrospective review identified specific measures of algorithm adherence and explored how the degree of deviation from the algorithm recommendations may have impacted clinical outcomes in this population. Preliminary evidence showed that only 18.8% of patients had algorithm-concordant care based on three specific guideline rules throughout their treatment. Poorer outcomes were most clearly associated with a failure to change medication after an extended (12-24 weeks) treatment trial that had not produced a meaningful benefit, as well as with a failure to increase the medication dose in a timely manner within the first 12 weeks in spite of a lack of significant side effects.
STAR*D was developed after TMAP and used a Web-based treatment monitoring system to provide feedback retrospectively to clinical research coordinators regarding adherence to the treatment recommendations for each patient after the visit had taken place (Trivedi et al., 2006c). Preliminary data evaluating adherence based on the first treatment step with citalopram show that over 85% of treatment encounters had appropriate fidelity to treatment recommendations (Trivedi et al., 2006b). In addition, most deviations from treatment recommendations occurred late in treatment and were often justifiable. Unlike TMAP, STAR*D used an innovative systematic prompt and feedback system to monitor adequacy of treatment, thereby further facilitating effective guideline-driven care.
Our experience with the issue of adherence to paper-and-pencil algorithms in both TMAP and STAR*D, as well as data supporting the importance of providing clinician feedback at the time of the patient visit, further illustrates the potential benefits of a state-of-the-art computerized decision support system developed by our group for sequenced treatment approaches. Unlike a paper-and-pencil format, such a computerized system would have the advantage of being able to provide evidence-based recommendations regarding medication dosing at the point of care, based on the clinician's assessment of symptoms and side effects at that clinic visit. Additionally, the system prompts the clinician to schedule the next appointment based on the recommended visit frequency, thereby potentially directly improving adherence to treatment guidelines. Such a system would also have the advantage of not requiring the presence and added expense of an on-site coordinator.
5. A Computerized Decision Support for Depression
Recommendations have already been made for accelerating the development and adoption of a computerized decision support system (CDSS) for evidence-based medicine (Sim et al., 2001). By using novel health information technology, researchers can bring guideline information to the point where decisions are being made (Deering 2002; Tierney 2001).
Some attempts, in various medical fields, have been made to implement algorithms via computer systems. Implementation, acceptance, and adherence are generally more successful in settings where computerized systems, such as computerized medical record systems or computerized physician orders, are already in place. One review of computer-based CDSSs concluded that these systems are increasing rapidly, and their quality is improving (Hunt et al., 1998). The authors go on to say that these algorithms “can enhance clinical performance for drug dosing, preventive care and other aspects of medical care.” A more recent systematic review examined controlled trials assessing the effects of computerized clinical decision support systems (CDSSs) (Garg et al., 2005). Of the 100 trials using a CDSS, many improved clinician performance, but effects on patient outcomes were inconsistent and understudied. The authors did find further evidence, however, that studies in which users were automatically prompted reported better performance than those in which users had to actively initiate the system.
Further validation for employing a computerized, clinical decision making system can be found by considering the conclusions drawn in a study by Tierney and colleagues (Tierney 2001). They noted that guidelines regarding patient care were more likely to be followed by the physician when they were presented while the patient was at the clinic, rather than afterwards. Physicians were required to make a response to follow or not follow each computer suggestion, a process which not only assures that the physician actually considers information/recommendation presented, but also that their clinical judgment remains primary in determining patient care. This study models the dual task theory, which stresses the importance of timing in relaying critical information to physicians (Buff et al., 1986). The Tierney study is an exception. Most current strategies for shaping physician behavior fall short of their desired outcome. Our computer platform lends itself to provide instant consultation during the care of patients with MDD.
5.1 Barriers to Implementation of a Computerized Decision Support System
Despite the advantages, significant barriers continue in terms of implementing computerized guidelines. Key issues identified to date include the relevance and accuracy of the messages, as well as flexibility (Rousseau et al., 2003). In a recent review of 70 trials using clinical decision support systems, 75% of interventions succeeded when decision support was provided to clinicians automatically, whereas none succeeded when clinicians were required to seek out the advice of the decision support system (Kawamoto et al., 2005), suggesting effective clinical decision support systems must provide decision support automatically as part of the clinician's workflow, deliver decision support at the time and location of the decision making, provide actionable recommendations, and use a computer to generate the decision support.
It is clear that the central issue is how to incorporate efficacious treatments in real-world clinical settings, such that they will be utilized and remain a part of the system of care. Physician adherence is critical in translating recommendations into improved care. As seen in Table 2, other barriers to automated feedback systems have already been identified – including physician resistance. Other examples of barriers seen in practice to date with automated feedback systems include varying degrees of computer literacy, lack of technical support, and patient-related factors. These barriers emphasize that to be successful a computerized decision support system must be easy to use and feasible for use in real-world practice settings. In the next section, we discuss how a computerized decision support system developed by our group potentially addresses these concerns.
Table 2. Barriers to Automated Feedback Systems in Practice.
|
5.2 Description of the CDSS-D
Based on the problems discussed, we have developed a computerized clinical support system for depression (CDSS-D) for use by physicians to enhance their ability to provide the best evidence-based treatment for depression (Trivedi et al., 2000; Trivedi et al., 2002; Trivedi et al., 2004a). Given the wide variation in computer literacy and lack of technical support, we have designed the CDSS-D so that it is user-friendly, with only five main screens to be navigated during a normal follow-up clinical visit. This technology incorporates the most current information about the treatment of depression, as well as providing an easy-to-use interface allowing physicians to use this decision support tool within the context of a routine clinical visit. CDSSs, as opposed to a paper-and-pencil format, have the advantage of allowing the practicing physician to have timely and efficient access to information at the time of treatment decisions, as well as the added benefit of integrating new findings into the decision support system immediately.
In addition, our CDSS-D provides all the prompts necessary to implement the algorithm without the need of additional personnel. In contrast to a paper-and-pencil format, the computerized algorithm should facilitate the process of following the suggested dosing schedules and tactical recommendations by displaying the recommended dosage and treatment options at that point in time according to the decision rules. Additionally, all patient information, medication information, medication dosages, next appointments, and progress notes are accessible with a click of the computer mouse and recorded electronically, thereby reducing time-consuming paperwork for both physicians and nonphysician staff. The program also provides a recommended time frame for the patient to return based on algorithm stage. By suggesting the next appointment automatically via computer, the CDSS-D should assure that the appropriate time between visits is achieved. The most significant advantage is that feedback from the CDSS-D is ongoing and available during visits rather than before or after the visits.
In terms of feasibility in real-world clinical settings, we are currently comparing the effectiveness and feasibility of a computerized depression algorithm compared to the paper-and-pencil format and usual care in an ongoing, large, multisite NIMH-funded study (5R01MH064062-2 (Computerized Decision Support System for Depression) in public mental health tertiary care settings (Trivedi et al., 2007). This study will examine the effect of the CDSS-D on physician adherence to the algorithm, as well as on patient outcomes compared to the paper-and-pencil format and usual care.
Figure 1 shows the treatment evaluation screen seen by the clinician when using the CDSS-D. As the name suggests, this is the stage at which the clinician addresses the issues of the patient's level of depressive symptoms, adherence to medication, and side-effect burden at that point in time. The data entered into the computer are used to drive the clinical decision, thereby facilitating measurement-based care. This screen also provides information regarding the treatment to date – with both a graphical presentation of progress and a copy of the last visit note available. As can be seen, the CDSS-D has been designed to be user-friendly and easy to navigate.
Figure 1.
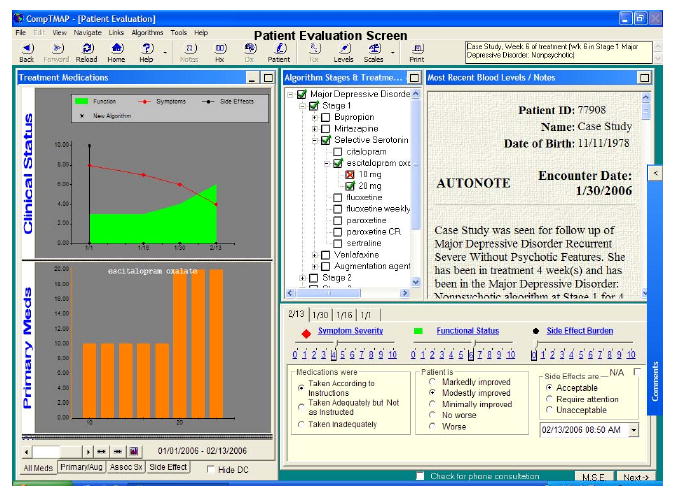
A Sample Computerized Decision Support System for Depression Enabling Physician Decision Making Based on the Symptom Severity and Tolerability of Medication
In much the same way as STAR*D implementation was described in section 3.2, an operationalized treatment algorithm based on TMAP is used by the CDSS-D program. To ensure that medication was optimally used in terms of dose and duration, the program uses preidentified critical decision points (weeks 4, 6, 8, 10, and 12) for each medication stage. As with the STAR*D algorithm, at each critical decision point changes in either the treatment strategy or tactic are recommended based on the current dose and duration of the particular medication together with the degree of symptom change and side-effect burden. The clinician, using the decision rules provided, uses this data (obtained as part of measurement-based care) to drive clinical treatment at that point in time, thereby tailoring treatment to the individual patient. For example if after 6 weeks, a patient has shown minimal or no response to a subtherapeutic dose of sertraline (e.g. 50mg QD) but is tolerating the medication, then the decision support function would recommend that the dose of the antidepressant be increased. As stated later, this strategy has implications not only for other psychiatric disorders, but also for general medical disorders. Once a treatment guideline is established it can be programmed into the computer in a similar fashion as used in the CDSS-D.
6. Conclusion
We believe that by incorporating measurement-based algorithm care in a comprehensive electronic medical record program, we may more successfully integrate algorithm-driven care into busy, real-world clinics and thus facilitate change that will endure and ultimately improve patient outcomes. This approach signals a paradigm shift toward the use of measurement-based clinical decisions, both at the point of care and following each visit, to deliver optimal pharmacotherapy for depression. This concept is not only novel for clinical research but is also likely to improve the clinical care of patients with chronic and/or recurrent major psychiatric disorders.
Although we use major depression to illustrate our approach, the issues addressed are applicable to depression co-occurring with SUD, as well as to other chronic psychiatric and medical illnesses. As mentioned earlier, SUD and MDD commonly co-occur (Kelly et al., 2003) and when occurring simultaneously are particularly difficult to treat. Furthermore, despite the large number of individuals with substance abuse and depression, until now many researchers have focused on depression alone, often specifically excluding individuals with substance abuse from clinical studies. However, there is evidence that “self-medication” with legal and illegal drugs is very common among depressed patients (Helzer and Pryzbeck 1988; Khantzian 1985), and recovery rates from substance abuse are lower when patients have co-occurring depressive disorders (Ouimette et al., 1999).
We believe that the model discussed above has the potential to be adapted for the treatment of co-occurring MDD and SUD in routine clinical care. Just as the CDSS-D uses an evidence-based medication algorithm for the treatment of MDD alone, so also could an algorithm for co-occurring MDD and SUD (adapted using evidence-based research in this specific patient population) be incorporated into a decision support system similar to that described above. A full discussion regarding pharmacotherapy for depression and comorbid substance abuse is beyond the scope of this article; however, there is evidence that antidepressant use in a population of patients with comorbid SUD is helpful. For example, in one placebo-controlled trial, Cornelius et al. found at one-year follow-up that alcohol-dependent patients treated with fluoxetine had significantly better depression ratings and significantly fewer days intoxicated than those who originally received placebo (Cornelius et al., 2000). In another study in a similar population, Pettinati et al.(Pettinati et al., 2001) did not find an effect of sertraline (vs. placebo) on depressive symptoms, but did report higher rates of abstinence and longer time to relapse. In contrast, Roy found that sertraline improved depression ratings, but did not report on substance use outcomes (Roy 1998).
Any decision support system developed for this patient population would need to simultaneously address the treatment for the substance abuse as well as MDD, with warnings and recommendations built in regarding pharmacotherapy. As with the CDSS-D, this program could be designed to facilitate clinician adherence to treatment guidelines using an MBC approach, while providing evidence-based recommendations in terms of dosing, visit frequency, and sequential treatments should they be required.
Finally, one limitation of previous studies looking at antidepressant treatment in patients with substance abuse is that they were of short duration and only examined the potential benefits of a single antidepressant. However, as with patients with any chronic illness, patients with chronic or recurrent depression, with or without SUD, may vary over time in terms of their need for different treatments. In terms of sequential treatment approaches, there are no randomized studies suggesting which treatment choice is best, and further studies are clearly needed to evaluate the comparative efficacy and tolerability of different approaches. Adaptive strategies to date rely primarily on consensus-based, clinical decision making rather than on innovative study designs that address the identification of the best sequence for individual or groups of patients. Traditional approaches have considered each step in the sequence as a new trial, but we know that each treatment step builds on the previous treatment and that resistance to one step increases the chances of resistance to subsequent steps. In addition, despite patient and provider education, suboptimal medication dosing and duration of exposure remain the norm. These difficulties herald the need for a paradigm shift in how clinical decision making is incorporated into clinical practice and research study designs.
Acknowledgments
This project has been funded with Federal funds from the National Institute of Mental Health, National Institutes of Health, under 5R01MH064062-2 (Computerized Decision Support System for Depression - IMPACTS) and NIMH 5R01MH067692-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Press; Washington DC: 2000. Text Revision. [Google Scholar]
- Bakish D. New standard of depression treatment: remission and full recovery. J Clin Psychiatry. 2001;62 26:5–9. [PubMed] [Google Scholar]
- Bauer MS. A review of quantitative studies of adherence to mental health clinical practice guidelines. Harv Rev Psychiatry. 2002;10:138–153. doi: 10.1080/10673220216217. [DOI] [PubMed] [Google Scholar]
- Biggs MM, Shores-Wilson K, Rush AJ, Carmody TJ, Trivedi MH, Crismon ML, Toprac MG, Mason M. A comparison of alternative assessments of depressive symptom severity: a pilot study. Psychiatry Res. 2000;96:269–279. doi: 10.1016/s0165-1781(00)00235-3. [DOI] [PubMed] [Google Scholar]
- Buff KR, Kaufman L, Thomas JP. Handbook of Perception and Human Performance Vol 11 Cognitive Processes and Performanc. John Wiley; New York, NY: 1986. [Google Scholar]
- Cabana MD, Clark N. Challenges in evaluating methods to improve physician practice. J Pediatr. 2003;143:413–414. doi: 10.1067/S0022-3476(03)00438-4. [DOI] [PubMed] [Google Scholar]
- Chen RS, Rosenheck R. Using a computerized patient database to evaluate guideline adherence and measure patterns of care for major depression. J Behav Health Serv Res. 2001;28:466–474. doi: 10.1007/BF02287776. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Haskett RF, Daley DC, Cornelius MD, Thase ME, Perel JM. Fluoxetine versus placebo in depressed alcoholics: a 1-year follow-up study. Addict Behav. 2000;25:307–310. doi: 10.1016/s0306-4603(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Crismon ML, Trivedi M, Pigott TA, Rush AJ, Hirschfeld RM, Kahn DA, DeBattista C, Nelson JC, Nierenberg AA, Sackeim HA, Thase ME. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder. J Clin Psychiatry. 1999;60:142–156. [PubMed] [Google Scholar]
- Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA. 1995;274:700–705. doi: 10.1001/jama.274.9.700. [DOI] [PubMed] [Google Scholar]
- Deering MJ. Developing the health information infrastructure in the United States. Stud Health Technol Inform. 2002;80:121–128. [PubMed] [Google Scholar]
- Depression Guideline Panel. Clinical Practice Guideline, Number 5: Depression in Primary Care, Volume 1: Detection and Diagnosis. U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; Rockville, MD: 1993a. [Google Scholar]
- Depression Guideline Panel. Clinical Practice Guideline, Number 5: Depression in Primary Care: Volume 2 Treatment of Major Depression. U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; Rockville, MD: 1993b. [Google Scholar]
- Deyo RA, Carter WB. Strategies for improving and expanding the application of health status measures in clinical settings. A researcher-developer viewpoint. Med Care. 1992;30:MS176–MS186. doi: 10.1097/00005650-199205001-00015. [DOI] [PubMed] [Google Scholar]
- Evans CE, Haynes RB, Birkett NJ, Gilbert JR, Taylor DW, Sackett DL, Johnston ME, Hewson SA. Does a mailed continuing education program improve physician performance? Results of a randomized trial in antihypertensive care. JAMA. 1986;255:501–504. [PubMed] [Google Scholar]
- Ford DE. Managing patients with depression: is primary care up to the challenge? J Gen Intern Med. 2000;15:344–345. doi: 10.1046/j.1525-1497.2000.03011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortney JC, Booth BM, Curran GM. Do patients with alcohol dependence use more services? A comparative analysis with other chronic disorders. Alcohol Clin Exp Res. 1999;23:127–133. [PubMed] [Google Scholar]
- Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, Sam J, Haynes RB. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293:1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- Gemson DH, Ashford AR, Dickey LL, Raymore SH, Roberts JW, Ehrlich MH, Foster BG, Ganz ML, Moon-Howard J, Field LS. Putting prevention into practice. Impact of a multifaceted physician education program on preventive services in the inner city. Arch Intern Med. 1995;155:2210–2216. doi: 10.1001/archinte.155.20.2210. [DOI] [PubMed] [Google Scholar]
- Gorton TA, Cranford CO, Golden WE, Walls RC, Pawelak JE. Primary care physicians' response to dissemination of practice guidelines. Arch Fam Med. 1995;4:135–142. doi: 10.1001/archfami.4.2.135. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Weiss RD, Muenz LR, Vagge LM, Kelly JF, Bello LR, Michael J. The effect of depression on return to drinking: a prospective study. Arch Gen Psychiatry. 1998;55:259–265. doi: 10.1001/archpsyc.55.3.259. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hauenstein EJ. Depression in adolescence. J Obstet Gynecol Neonatal Nurs. 2003;32:239–248. doi: 10.1177/0884217503252133. [DOI] [PubMed] [Google Scholar]
- Hayward RS. Clinical practice guidelines on trial. Can Med Assoc J. 1997;156:1725–1727. [PMC free article] [PubMed] [Google Scholar]
- Helzer JE, Pryzbeck TR. The co-occurrence of alcoholism with other psychiatric disorders in the general population and its impact on treatment. J Stud Alcohol. 1988;49:219–224. doi: 10.15288/jsa.1988.49.219. [DOI] [PubMed] [Google Scholar]
- Hunt DL, Haynes RB, Hanna SE, Smith K. Effects of computer-based clinical decision support systems on physician performance and patient outcomes: a systematic review. JAMA. 1998;280:1339–1346. doi: 10.1001/jama.280.15.1339. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55:694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- Katon W, Von Korff M, Lin E, Walker E, Simon GE, Bush T, Robinson P, Russo J. Collaborative management to achieve treatment guidelines. Impact on depression in primary care. JAMA. 1995;273:1026–1031. [PubMed] [Google Scholar]
- Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, Ciechanowski P, Walker E, Bush T. The pathways study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61:1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330:765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RM, Shea T. Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry. 1992;49:809–816. doi: 10.1001/archpsyc.1992.01820100053010. [DOI] [PubMed] [Google Scholar]
- Kelly JF, McKellar JD, Moos R. Major depression in patients with substance use disorders: relationship to 12-Step self-help involvement and substance use outcomes. Addiction. 2003;98:499–508. doi: 10.1046/j.1360-0443.2003.t01-1-00294.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Lin EH, Katon WJ, Simon GE, Von KM, Bush TM, Rutter CM, Saunders KW, Walker EA. Achieving guidelines for the treatment of depression in primary care: is physician education enough? Med Care. 1997;35:831–842. doi: 10.1097/00005650-199708000-00008. [DOI] [PubMed] [Google Scholar]
- Lin EHB, Von Korff M, Katon W, Bush T, Simon GE, Walker E, Robinson P. The role of the primary care physician in patients' adherence to antidepressant therapy. Med Care. 1995;33:67–74. doi: 10.1097/00005650-199501000-00006. [DOI] [PubMed] [Google Scholar]
- Lomas J, Anderson GM, Domnick-Pierre K, Vayda E, Enkin MW, Hannah WJ. Do practice guidelines guide practice? The effect of a consensus statement on the practice of physicians. N Engl J Med. 1989;321:1306–1311. doi: 10.1056/NEJM198911093211906. [DOI] [PubMed] [Google Scholar]
- Mallin R, Slott K, Tumblin M, Hunter M. Detection of substance use disorders in patients presenting with depression. Subst Abus. 2002;23:115–120. doi: 10.1080/08897070209511481. [DOI] [PubMed] [Google Scholar]
- Margolis CZ, Warshawsky SS, Goldman L, Dagan O, Wirtschafter D, Pliskin JS. Computerized algorithms and pediatricians' management of common problems in a community clinic. Acad Med. 1992;67:282–284. doi: 10.1097/00001888-199204000-00021. [DOI] [PubMed] [Google Scholar]
- Mark TL. The costs of treating persons with depression and alcoholism compared with depression alone. Psychiatr Serv. 2003;54:1095–1097. doi: 10.1176/appi.ps.54.8.1095. [DOI] [PubMed] [Google Scholar]
- Murray C, Lopez A. Global Health Statistics: A Compendium of Incidence, Prevalence and Mortality Estimates for over 2000 Conditions. Harvard School of Public Health; Cambridge: 1996. [Google Scholar]
- Ouimette PC, Gima K, Moos RH, Finney JW. A comparative evaluation of substance abuse treatment IV. The effect of comorbid psychiatric diagnoses on amount of treatment, continuing care, and 1-year outcomes. Alcohol Clin Exp Res. 1999;23:552–557. [PubMed] [Google Scholar]
- Pettinati HM. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56:785–792. doi: 10.1016/j.biopsych.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Luck G, Kranzler HR, Rukstalis MR, Cnaan A. Double-blind clinical trial of sertraline treatment for alcohol dependence. J Clin Psychopharmacol. 2001;21:143–153. doi: 10.1097/00004714-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Rousseau N, McColl E, Newton J, Grimshaw J, Eccles M. Practice based, longitudinal, qualitative interview study of computerised evidence based guidelines in primary care. BMJ. 2003;326:314. doi: 10.1136/bmj.326.7384.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. Placebo-controlled study of sertraline in depressed recently abstinent alcoholics. Biol Psychiatry. 1998;44:633–637. doi: 10.1016/s0006-3223(97)00509-x. [DOI] [PubMed] [Google Scholar]
- Rubenstein LV, Mittman BS, Yano EM, Mulrow CD. From understanding health care provider behavior to improving health care: the QUERI framework for quality improvement. Quality Enhancement Research Initiative. Med Care. 2000;38:I129–I141. [PubMed] [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, Shores-Wilson K, Biggs MM, Woo A, Nierenberg AA, Fava M. Biol Psychiatry. Vol. 59. 2006a. An evaluation of the Quick Inventory of Depressive Symptomatology and the Hamilton Rating Scale for Depression: a Sequenced Treatment Alternatives to Relieve Depression trial report; pp. 493–501. Epub 2005 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Carmody TJ, Reimitz PE. The Inventory of Depressive Symptomatology (IDS): clinician (IDS-C) and self-report (IDS-SR) ratings of depressive symptoms. Int J Methods Psychiatr Res. 2000;9:45–59. [Google Scholar]
- Rush AJ, Crismon ML, Kashner TM, Toprac MG, Carmody TJ, Trivedi MH, Suppes T, Miller AL, Biggs MM, Shores-Wilson K, Witte BP, Shon SP, Rago WV, Altshuler KZ. Texas Medication Algorithm Project, phase 3 (TMAP-3): rationale and study design. J Clin Psychiatry. 2003a;64:357–369. doi: 10.4088/jcp.v64n0402. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, Regier DA, Rosenbaum JF, Ray O, Schatzberg AF. Report by the ACNP Task Force on Response and Remission in Major Depressive Disorder. Neuropsychopharmacology. 2006b;31:1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi M, Carmody TJ, Biggs MM, Shores-Wilson K, Ibrahim H, Crismon ML. One-year clinical outcomes of depressed public sector outpatients: a benchmark for subsequent studies. Biol Psychiatry. 2004;56:46–53. doi: 10.1016/j.biopsych.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003b;54:573–583. 585. doi: 10.1016/s0006-3223(02)01866-8. Erratum. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, Ritz L, Biggs MM, Warden D, Luther JF, Shores-Wilson K, Niederehe G, Fava M. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006c;354:1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- Schulberg HC, Katon W, Simon GE, Rush AJ. Treating major depression in primary care practice: an update of the Agency for Health Care Policy and Research Practice Guidelines. Arch Gen Psychiatry. 1998;55:1121–1127. doi: 10.1001/archpsyc.55.12.1121. [DOI] [PubMed] [Google Scholar]
- Sim I, Gorman P, Greenes RA, Haynes RB, Kaplan B, Lehmann H, Tang PC. Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc. 2001;8:527–534. doi: 10.1136/jamia.2001.0080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, VonKorff M, Wagner EH, Barlow W. Patterns of antidepressant use in community practice. Gen Hosp Psychiatry. 1993;15:399–408. doi: 10.1016/0163-8343(93)90009-d. [DOI] [PubMed] [Google Scholar]
- Smith WR. Evidence for the effectiveness of techniques to change physician behavior. Chest. 2000;118:8S–17S. doi: 10.1378/chest.118.2_suppl.8s. [DOI] [PubMed] [Google Scholar]
- Tierney WM. Improving clinical decisions and outcomes with information: a review. Int J Med Inform. 2001;62:1–9. doi: 10.1016/s1386-5056(01)00127-7. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Claassen CA, Grannemann BD, Kashner TM, Carmody TJ, Daly E, Kern JK. Assessing Physicians' Use of Treatment Algorithms: Project IMPACTS Study Design and Rationale. Contemp Clin Trials. 2007;28:192–212. doi: 10.1016/j.cct.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006a;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Kern JK, Baker SM, Altshuler KZ. Computerizing medication algorithms and decision support systems for major psychiatric disorders. J Psychiatr Pract. 2000;6:237–246. doi: 10.1097/00131746-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Kern JK, Grannemann BD, Altshuler KZ, Sunderajan P. A computerized clinical decision support system as a means of implementing depression guidelines. Psychiatr Serv. 2004a;55:879–885. doi: 10.1176/appi.ps.55.8.879. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Kern JK, Marcee A, Grannemann B, Kleiber B, Bettinger T, Altshuler KZ, McClelland A. Development and implementation of computerized clinical guidelines: barriers and solutions. Methods Inf Med. 2002;41:435–442. [PubMed] [Google Scholar]
- Trivedi MH, Kern JK, Voegtle TM, Baker SM, Altshuler KZ. Computerized medication algorithms in behavioral health care. In: Dewan NA, et al., editors. Behavioral Health Care Informatics. Springer-Verlag New York Incin cooperation with Springer-Verlag GmbH & Co.; KG Berlin Heidelberg: 2001. [Google Scholar]
- Trivedi MH, Rush AJ, Crismon ML, Kashner TM, Toprac MG, Carmody TJ, Key T, Biggs MM, Shores-Wilson K, Witte B, Suppes T, Miller AL, Altshuler KZ, Shon SP. Clinical results for patients with major depressive disorder in the Texas Medication Algorithm Project. Arch Gen Psychiatry. 2004b;61:669–680. doi: 10.1001/archpsyc.61.7.669. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Gaynes BN, Stewart JW, Wisniewski SR, Warden D, Ritz L, Luther J, Stegman D, DeVeaugh-Geiss J, Howland RH. Maximizing the adequacy of medication treatment in controlled trials and clinical practice: STAR*D measurement-based care. Neuropsychopharmacology. 2006b doi: 10.1038/sj.npp.1301390. submitted. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004c;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006c;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Warden D, McKinney W, Downing M, Berman SR, Farabaugh A, Luther JF, Nierenberg AA, Callan JA, Sackeim HA. Factors associated with health-related quality of life among outpatients with major depressive disorder: a STAR*D report. J Clin Psychiatry. 2006d;67:185–195. doi: 10.4088/jcp.v67n0203. [DOI] [PubMed] [Google Scholar]
- Unützer J, Katon W, Callahan CM, Williams JW, Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. A 12-item Short-Form Health Survey. Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA. Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract. 2006;12:71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World Health Report 2001. Mental Health: New Understanding, New Hope. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]


