Abstract
Singlet oxygen is one of several reactive oxygen species that can destroy biomolecules, microorganisms and other cells. Traditionally, the response to singlet oxygen has been termed photo-oxidative stress, as light-dependent processes in photosynthetic cells are major biological sources of singlet oxygen. Recent work identifying a core set of singlet oxygen stress response genes across various bacterial species highlights the importance of this response for survival by both photosynthetic and non-photosynthetic cells. Here, we review how bacterial cells mount a transcriptional response to photo-oxidative stress in the context of what is known about bacterial stress responses to other reactive oxygen species.
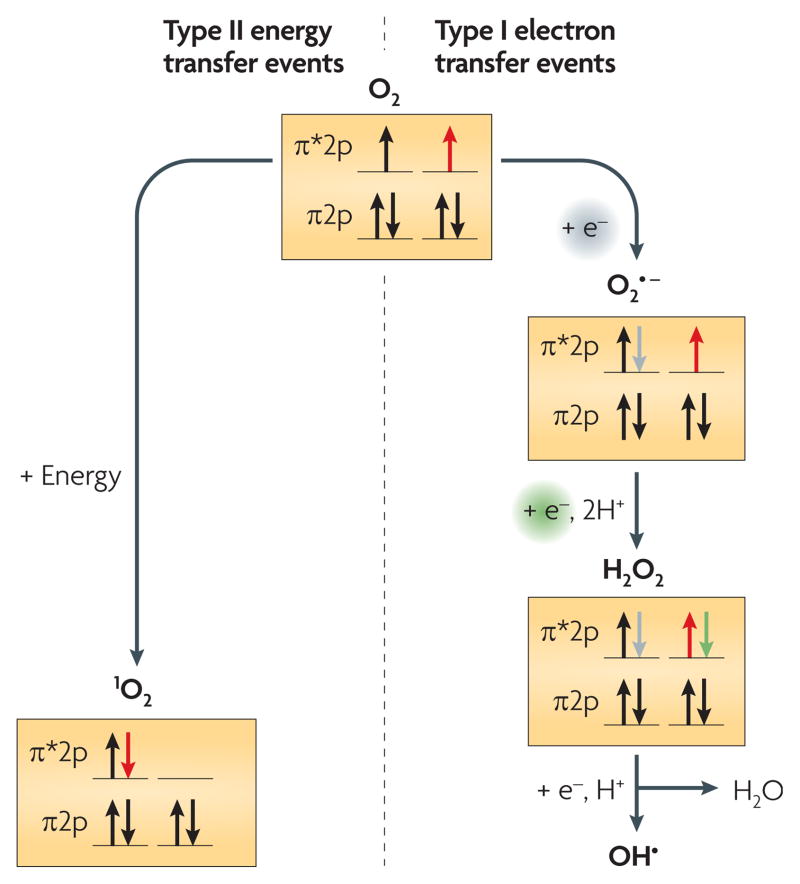
Many key biological processes are dependent on molecular oxygen (O2). A consequence of the use of O2 in bioenergetic or other metabolic pathways is the formation of reactive oxygen species (ROS). There are two classes of ROS, created through either electron transfer (type I) or energy transfer (type II) reactions1,2 (FIG. 1). Electron transfer to O2 can produce superoxide, hydrogen peroxide and hydroxyl radicals, all of which are toxic to cells. Study of the cellular responses to type I ROS has provided considerable information on the mechanisms that cells use to survive in their presence3. In the second type of reaction, energy transfer to O2 results in the formation of singlet oxygen (1O2). In contrast to the responses to the first class of ROS, the cellular responses to 1O2 have only recently begun to be analysed. Here, we review recent investigations that have led to the identification of genes and proteins that are involved in 1O2-dependent stress responses.
Figure 1. Types of reactive oxygen species.
Electron or energy transfer events generate the two main types of reactive oxygen species. The schematic shows the changes in occupancy of the outer p orbitals of molecular oxygen (O2) during the formation of these reactive oxygen species1,2.
H2O2, hydrogen peroxide;1O2, singlet oxygen; O2•, superoxide; OH•, hydroxyl radical.
Photosynthesis and evolution of oxygen
When photosynthetic organisms acquired the ability to produce O2, they substantially altered the Earth’s atmosphere and the forms of life that could be sustained4,5. In particular, the accumulation of atmospheric O2 allowed the evolution of bioenergetic pathways, such as aerobic respiration, that couple the reduction of O2 to the formation of a proton gradient4,6. The advent of aerobic respiration was followed by the evolution of complex organisms, including animals and plants.
ROS
Despite the energetic gains from the presence of atmospheric O2, serious consequences also arose owing to the adventitious formation of ROS in respiratory and other reactions1,3. O2, or dioxygen, is a stable diradical containing two unpaired, spin-aligned electrons in its outer p molecular orbitals1,2 (FIG. 1). This spin-aligned configuration constrains the reactivity of O2 with most non-radical molecules. However, these unpaired, spin-aligned electrons make O2 receptive to accepting electrons1,2. A one-electron transfer reduction of O2 produces a superoxide radical anion (O2•−), commonly referred to as superoxide. An electron transfer reaction to superoxide produces a peroxide anion (O22−), which exists as hydrogen peroxide (H2O2) in biological systems. In turn, H2O2 reacts with ferrous iron (Fe2+) in the Fenton reaction to produce a hydroxyl radical (OH•).
The transfer of excitation energy to ground state (or triplet) O2 can cause a rearrangement of the outermost electrons, producing one of two forms of 1O2. The biologically relevant state of 1O2, designated 1Δg, results when the spin-aligned, unpaired electrons of O2 pair with each other, thereby producing an outer p orbital structure in which one orbital has paired electrons and the other orbital is empty1,2,7 (FIG. 1). This electron rearrangement removes the spin restriction, converting O2 to 1O2, and makes 1O2 the most reactive of oxygen species.
In vitro studies have shown that ROS cause damage to various biomolecules. Primarily, O2− and H2O2 damage proteins through oxidation1,2, whereas OH• damages DNA1,2, resulting in various lesions that are potentially mutagenic and may even be lethal1. Cells can be killed by 1O2, which has been shown to react with numerous cellular components in vitro, including membranes, proteins and DNA1,7–9. Each of these ROS (FIG. 1) can damage cells, but there is some disagreement as to which chemical reactions occur, and at what rates individual reactions occur, in vivo1,10.
Sources of 1O2
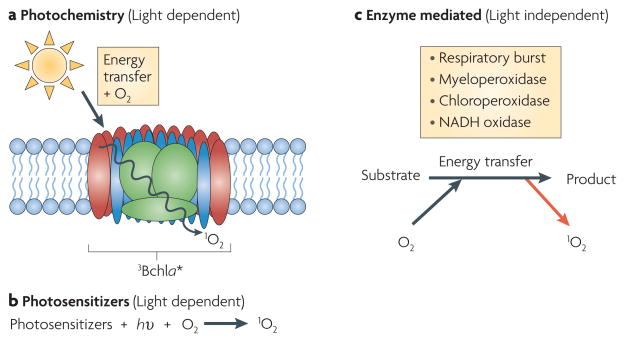
1O2 can be formed during solar energy capture, when photosynthetic pigments (in particular, chlorophyll) are raised to a higher energy level and reach a triplet excited state owing to the absorption of light energy11,12 (FIG. 1). The pigments can then transfer energy to O2 to produce 1O2 (REFS 11,12). Because of this well-known mechanism of forming 1O2, the terms ‘photo-oxidative stress’ and ‘singlet oxygen stress’ are often used interchangeably. Other biological sources of 1O2 production include energy transfer from excited photosensitizers, from the activity of several peroxidases, from the macrophage respiratory burst and from other enzymatic reactions7,13 (FIG. 2).
Figure 2. Sources of singlet oxygen.
a,b. Major light-dependent sources of singlet oxygen (1O2) include energy transfer to molecular oxygen (O2) from excited triplet state pigments such as bacteriochlorophyll a (3Bchla★) in photosynthetic membranes (part a) and energy transfer from excited natural or exogenous photosensitizers, such as tetrapyrroles and methylene blue or rose bengal, respectively (part b). c. Light-independent pathways that generate 1O2 in biological systems include enzymes (such as myeloperoxidase, chloroperoxidase and NADH oxidase) that generate this type of reactive oxygen species as a consequence of catalysis7,13. hυ, light energy.
Oxidative stress responses
The ROS molecules H2O2 and O2•− can be produced in aerobically grown bacteria by the auto-oxidation of respiratory chain components, by various endogenous activities or by exogenous agents1–3. Because ROS can cause damage to DNA, proteins and membranes, mounting a rapid response is crucial for survival1.
Bacterial adaptive or stress responses to H2O2 and O2•− were identified over 20 years ago following the observation that low doses of either H2O2 or O2•− (generated by the biological activation of paraquat) could protect Escherichia coli and Salmonella enterica subsp. enterica serovar Typhimurium from subsequent exposure to higher doses that would otherwise have been lethal14–16. Using two-dimensional gel electrophoresis, it was shown that O2•− and H2O2 induced the synthesis of approximately 30–40 proteins when compared with non-challenged cells15,17–20.
Further studies identified the transcription factors involved (OxyR for H2O2 and SoxRS for O2•−), their target genes and how their activity is regulated3. OxyR activity is increased by H2O2. The OxyR regulon includes genes that are involved in oxidant elimination (katG, ahpC and ahpF), in maintenance of the balance between thiol groups and disulphide bonds (gorA, grxA and trxC) and in limiting Fe2+ availability (dps and fur) to minimize the occurrence of the Fenton reaction3. Once SoxR is activated by O2•−, it directly stimulates transcription of soxS. SoxS acts in a second cascade of the transcriptional response to O2•− to directly activate gene transcription. The SoxRS regulon includes genes that are involved in O2− elimination (manganese superoxide dismutase (sodA)), in DNA repair (endonuclease IV (nfo)) and in increasing cellular pools of reduced pyridine nucleotides for glutathione-dependent repair reactions (glucose-6-phosphate dehydrogenase (zwf)). Additional SoxRS regulon members include fur, superoxide-resistant isozymes of fumarase (fumC) and aconitase (acnA), which allow continued Krebs cycle function, and several flavodoxin genes (fpr, fldA and fldB), the products of which are presumably important for reducing Fe–S clusters3. Both the OxyR and SoxRS regulons also protect cells from other noxious chemicals, including reactive nitrogen species and organic solvents3.
Photo-oxidative stress in bacteria
Most studies on the microbial or cellular response to ROS have centred on protection from O2•− and H2O2. However, in the past decade our understanding of the response to 1O2 (or to photo-oxidative stress) has greatly improved.
Evidence for photo-oxidative stress in bacteria
Photo-oxidative stress and its consequences have been observed in numerous biological systems. The presence of photo-oxidative stress in bacteria was reported over 50 years ago, when the analysis of wild-type and carotenoid-deficient strains of the purple photosynthetic bacterium Rhodobacter sphaeroides showed that the presence of carotenoids protected the cells (BOX 1) from so-called photodynamic damage that was caused by the introduction of air to photosynthetically growing cultures21. Decades later, the identity of the toxic byproduct of photodynamic damage was discovered when triplet excited bacteriochlorophyll a(3Bchla★) was identified as the light-excited sensitizer that reacts with O2 to produce 1O2 (REFS 11,12,22). Carotenoids, along with Bchla, are components of the photosynthetic apparatus, both in photosynthetic reaction centres (the bacterial ancestors of photosystems in O2-evolving phototrophs) and light-harvesting complexes12. Carotenoids have been shown to directly quench 1O2, but their primary protective effect in vivo is likely to be the rapid quenching of 3Bchla★ to reduce 1O2 formation12,22.
Box 1. Rhodobacter sphaeroides as a model system.
Rhodobacter sphaeroides is a Gram-negative, purple non-sulphur bacterium that has a long history of serving as a model system for studies of photosynthesis and other metabolic processes63,64. This alphaproteo-bacterium is normally found in either freshwater or marine environments. R. sphaeroides is a facultative bacterium with the ability to grow using respiratory and photosynthetic lifestyles. This diversity of lifestyles makes R. sphaeroides the model system of choice in many studies, particularly regarding the process and control of photosynthesis. Results from bioenergetic, biochemical, genomic and other studies have provided unparalleled insight into mechanistic, structural and chemical details of the photosynthetic apparatus of R. sphaeroides11,12,21. Furthermore, the ease with which substantial amounts of singlet oxygen (1O2) are formed during light energy capture by the photosynthetic apparatus, the availability of systems to monitor 1O2 effects in vivo and the ability to use genomic approaches make this bacterium the best system for defining the response to this reactive oxygen species.
Detection of 1O2
Several methods have been used to detect 1O2. In R. sphaeroides cultures, the change in Dane-Py fluorescence23 was used to measure the amounts of 1O2 in vivo24. As expected, 1O2 was detected in both wild-type and carotenoid-deficient cells that had been exposed to high light levels, but more 1O2 was detected in the carotenoid-deficient strain24. Both electron paramagnetic resonance spin trapping with 2,2,6,6-tetramethylpiperidine25 and direct near-infrared (NIR) emission at 1,270 nm26,27 have been used to detect 1O2 in R. sphaeroides reaction centres that were isolated from carotenoid-deficient strains. By contrast, 1O2 was not detectable in wild-type reaction centres. Recently, a sensitive NIR emission method was used to provide the first in vitro detection of 1O2 that was generated in wild-type R. sphaeroides reaction centres28. Given the high reactivity of 1O2 and the sensitivity of the assays, it is possible that each of these methods underestimates the actual amount of ROS generated in vivo or in vitro, as each method only reports on the amount of ROS that interact with the probe. Conversely, when using exogenous photosensitizers or other chemical agents as sources of 1O2 with whole cells, the effective concentration or primary site of action (for example, whether it is extracellular or in a subcellular compartment) of ROS is often unknown. Therefore, it is frequently difficult to determine the cellular concentration of 1O2.
Responding to photo-oxidative stress
The ease with which 1O2 is generated through photochemistry has made R. sphaeroides the bacterial model system to study the response to this ROS (BOX 1). To begin to investigate the genes that might be regulated by 1O2, real-time PCR was used to examine the transcriptional activity of genes that were reasoned to have possible roles in the response24. Two candidate genes in this study were activated under photo-oxidative conditions (light plus methylene blue): RSP_2389, which encodes a putative glutathione peroxidase, and RSP_0799, which encodes a putative Zn-dependent hydrolase of the glyoxalase II family24,29. Oxidative damage to proteins can result in the production of protein peroxides, and glutathione peroxidase activity has been shown to detoxify protein peroxides in vitro through degradation30. Glyoxalase enzymes can cleave thioesters that are accumulated during oxidative stress; therefore, RSP_0799 may have a general role in removing several classes of glutathione adducts that are caused by 1O2 stress29. Activation of a glutathione peroxidase-like gene is consistent with studies in the unicellular alga Chlamydomonas reinhardtii, in which transcription of a glutathione peroxidase homologue (GPX5; also known as GPXH) is increased following 1O2 stress31 (see below). Interestingly, expression of the R. sphaeroides carotenoid biosynthesis genes crtA and crtI did not increase under photo-oxidative stress conditions24.
Given the importance of carotenoids during photo-oxidative stress in R. sphaeroides21,25–27, when it was found that inactivation of an early enzyme in carotenoid biosynthesis, phytoene synthase (CrtB), slightly increased the activity of the R. sphaeroides alternative σ-factor, σE (also known as RpoE) (BOX 2), researchers proposed a connection between 1O2 stress and this transcriptional regulator32. Using the promoter from rpoE (which is dependent on its own gene product, σE) fused to the β-galactosidase gene (lacZ), increased rates of β-galactosidase activity were measured after exposing anaerobic, photosynthetic cells to O2 and light — conditions known to cause the production of 1O2. A sustained increase in β-galactosidase activity from this reporter gene was observed only when both O2 and light (at wavelengths known to excite pigments of the photosynthetic apparatus) were introduced to the pho-tosynthetic cultures, consistent with the hypothesis that 1O2 is an inducer of σE activity32. To provide independent support for this hypothesis, cultures grown aerobically (which therefore lack photosynthetic pigments) were exposed to light and methylene blue to induce 1O2 generation. This confirmed that 1O2 causes a marked increase in σE activity, as a high rate of β-galactosidase activity from the rpoE–lacZ reporter was only observed in the presence of oxygen, light and methylene blue. Control experiments in which one of the components needed to generate 1O2 was omitted resulted in no significant increase in σE activity, further indicating that this ROS was responsible for the increase in σE-dependent transcription32. In addition, activation of this σE-dependent response did not occur when cells were exposed to other ROS32, suggesting that 1O2 is a specific activator of this transcriptional pathway. This σE-dependent transcriptional response was crucial to survival in the presence of 1O2, as generation of 1O2 rapidly killed σE-deficient cells that were grown under conditions that restrict carotenogenesis32. Therefore, in R. sphaeroides loss of the quenching ability of carotenoids combined with the lack of the σE-dependent transcriptional pathway creates a synthetic lethal pair in the response to 1O2.
Box 2. σ-factors.
Bacterial transcription occurs through the function of RNA polymerase. Core RNA polymerase is a multiprotein complex composed of a β-, a β′- and two α-subunits. Promoter specificity is achieved when core RNA polymerase associates with one of many σ-subunits to form a holoenzyme65. The primary σ-factor is a member of the so-called σ70 superfamily and is referred to as the housekeeping σ-factor66. Alternative σ-factors provide a mechanism by which bacteria can control gene expression in response to certain environmental or developmental cues, including stress conditions. One class of σ-factor is the group IV σ-factors50,51. Rhodobacter sphaeroides σE (also known as RpoE) is a group IV σ-factor and, like many group IV σ-factors, it is typically maintained in an inactive state by forming a complex with its cognate anti-σ factor, ChrR41–44. σE–ChrR forms a heterodimeric complex from which σE is released on reception of the singlet oxygen signal44. Once released, σE positively autoregulates its own expression (by activating transcription of the rpoEchrR operon) and also activates transcription of additional target genes41–44,47.
Other bacteria have been reported to mount a transcriptional response to 1O2. Carotenogenesis is increased during photo-oxidative stress in Myxococcus xanthus33–35. In this non-photosynthetic bacterium, the tetrapyrrole protoporphyrin IX acts as an endogenous photosensitizer as it accumulates to high levels during stationary phase33. It has been shown that the activity of M. xanthus CarQ, an alternative σ-factor (BOX 2), is increased by photo-oxidative stress. In turn, CarQ increases the expression of the carQRS operon to initiate a gene expression cascade that promotes carotenogenesis and potentially other currently undiscovered functions that protect against 1O2 (REFS 34,35). Photo-oxidative stress conditions in M. xanthus carotenoid-deficient mutants result in cell lysis33, presumably owing to 1O2 toxicity. In the eukaryotic microorganism Phaffia rhodozyma, 1O2 also controls carotenoid biosynthesis. Generation of 1O2 in P. rhodozyma (using exogenous photosensitizers) caused a modest but sustained increase in carotenoid content when compared with that of non-challenged cultures36. Although the mechanism that is used to increase carotenoid content in P. rhodozyma has not been described, a possible explanation is that 1O2 stress activates the expression of carotenoid genes.
In E. coli, forming 1O2 through thermodissociation of exogenous disodium 3,3′-(1,4-naphthylidene) diproprionate (NDPO2) endoperoxide activated expression of the SoxRS regulon in a soxR-dependent manner37. The effect of exogenous NDPO2 was not altered by antioxidants, such as mannitol, glutathione, catalase or superoxide dismutase (SOD), suggesting that the response might be specific to 1O2. In another study, overexpression of E. coli OxyR decreased the bactericidal effect of 1O2 (which was generated by methylene blue plus light), diminished protein oxidation as measured by carbonyl content, and increased catalase and SOD-specific activities38. Consistent with this observation, an oxyR deletion mutant is hypersensitive to 1O2 and exhibits increased protein damage in the presence of this ROS38, suggesting that OxyR protects cells from photo-oxidative damage by increasing the expression of antioxidant enzymes.
In Agrobacterium tumefaciens, three iron-dependent SODs are reported to protect against 1O2 that is generated by the photosensitizer rose bengal39,40. Each of these SODs is reported to have a different expression pattern and cellular localization39, but studies with strains containing single or multiple mutations indicate that SodBI has the largest protective role40.
In considering some of the above work, the chemical differences between 1O2 and O2•− or H2O2 (FIG. 1) make it difficult to explain how SOD activity or members of the OxyR or SoxRS regulons protect cells against photo-oxidative stress. For example, it is unclear whether there is specificity in the response to each type of ROS, whether there is a cross-protective ability of proteins in each stress response or whether cellular damage caused by 1O2 can increase the production of other ROS.
1O2 stress response regulons
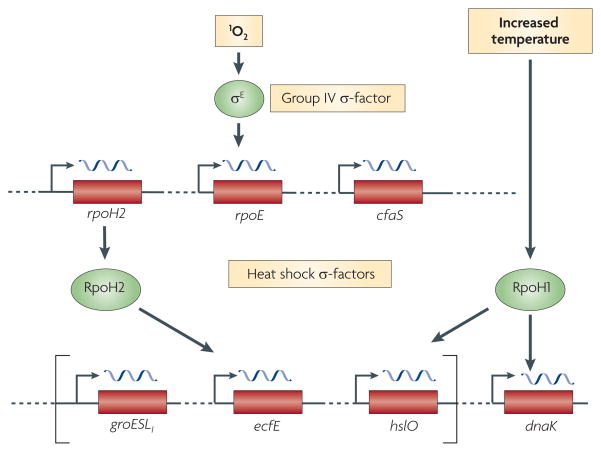
The finding that σE is a required part of the transcriptional response to 1O2 in R. sphaeroides led to the investigation of target genes that are directly and indirectly activated by this ROS32. Wild-type cells have low σE activity owing to the sequestering function of ChrR, the anti-σ factor of σE (REFS 41–44) (BOX 2). On the basis of this, expression profiles for wild-type and ΔChrR cells (which have constitutively increased σE activity) were compared to identify genes that are involved in this response32. As expected, rpoE showed increased expression in ΔChrR cells. In addition, ~180 genes, corresponding to ~60 operons, had a three-fold or more increase in expression in ΔChrR cells. When upstream promoter DNA from nearly half of these operons was tested, only four additional σE-dependent operons were identified (RSP_1091–1087, RSP_2143–2144, RSP_0601 and RSP_1409; TABLE 1)32, suggesting that the presence of 1O2 had many indirect effects on gene expression. One of the direct targets of σE, RSP_0601, encodes an alternative σ-factor of the heat shock family (RpoH2)45, showing that 1O2 activates a transcriptional cascade32 (FIG. 3). In an independent microarray study, these genes were also found to have increased expression in wild-type R. sphaeroides that was grown under semi-aerobic conditions in the presence of light46. On the basis of these observations, the challenge became to develop methods that could distinguish 1O2-induced genes that were directly σE-dependent from those that were part of the response but dependent on RpoH2 or other unknown cellular adaptations to this ROS. The practical nature of this challenge is illustrated by the fact that genes (namely RSP_2389 and RSP_0799) that were previously identified as being 1O2 responsive24,29 are not direct σE targets32,47 (see below).
Table 1.
Members of the Rhodobacter sphaeroides σE–ChrR regulon*
| locus designation | gene name | Annotation‡ |
|---|---|---|
| RSP_1092§ | rpoE | RNA polymerase σ70 factor|| |
| RSP_1093§ | chrR | Anti-σ factor (ChrR)|| |
| RSP_2144§ | cfaS | Cyclopropane fatty acyl-phospholipid synthase (CfaS) |
| RSP_2143§ | phrA | DNA photolyase; cryptochrome 1 apoprotein (blue-light photoreceptor) |
| RSP_1091§ | Unknown | Putative cyclopropane or cyclopropene fatty acid synthesis protein; flavin amine oxidase |
| RSP_1090§ | Unknown | Putative cyclopropane or cyclopropene fatty acid synthesis protein |
| RSP_1089§ | Unknown | Sugar or cation symporter; from the GPH family |
| RSP_1088§ | Unknown | Hypothetical protein |
| RSP_1087§ | Unknown | Short-chain dehydrogenase or reductase family member |
| RSP_0601 | rpoH2 | RNA polymerase σ-factor|| |
| RSP_1409 | Unknown | β-Ig-H3 or fasciclin |
| RSP_1852 | Unknown | Hypothetical protein |
| RSP_0296 | cycA | Cytochrome c2|| |
| RSP_3336 | Unknown | ABC spermidine or putrescine transporter; inner-membrane subunit |
| RSP_6222 | Unknown | Hypothetical protein |
Product name from the IMG website: http://img.jgi.doe.gov/cgi-bin/pub/main.cgi.
Gene known or predicted to be encoded in a polycistronic operon.
Gene product for which a biochemical function has been shown in vitro. ABC, ATP-binding cassette; Ig, immunoglobulin.
Figure 3. Singlet oxygen activates a gene expression cascade in bacteria.
The cascade of transcriptional circuits that is activated by singlet oxygen (1O2) in bacteria is shown. In Rhodobacter sphaeroides, the formation of 1O2 increases the activity of the group IV alternative σ-factor, σE (BOX 2). Genes that are directly transcribed by σE encode proteins that are predicted to protect cells from 1O2 and repair damage caused by this reactive oxygen species (see text), as well as RpoH2, one of two homologues of proteins from the heat shock (σ32) family of alternative σ-factors45,47. Although RpoH2 and RpoH1 can transcribe promoters upstream of common genes (shown in square brackets), there are other genes containing promoters that are recognized by only one of these two related σ-factors45. cfaS, cyclopropane fatty acyl-phospholipid synthase.
Direct σE target genes
To distinguish between direct and indirect σE targets, publicly available R. sphaeroides gene expression microarray data sets were analysed using hierarchical clustering to identify patterns of co-regulated genes. Of the genes that showed differential expression between wild-type and ΔChrR cells, one cluster contained the previously identified members of this regulon (including the rpoE operon) and RSP_1852 (REFS 32,46,47). As expected, when the upstream DNA sequences for each operon were aligned, a sequence motif with features that are typical of bacterial σ-factor binding sites was discovered. When this motif was converted into a position-specific weighted matrix (PSWM) and used to query a putative promoter library, 15 potential σE promoters were identified (TABLE 1) when at least 75% correspondence to the PSWM was required47. In vivo and in vitro testing of the new σE candidates indicated that RSP_1852, RSP_3336 and RSP_6222 are direct σE targets47. Weak activities of the RSP_3336 and RSP_6222 promoters may explain why they were not identified as candidates in the clustering of gene expression data47.
To test whether this approach was successful in finding most or all of the σE target genes, chromatin immuno-precipitation microarray (ChIP-chip) assays were carried out to monitor the occupancy of σE or the β′ subunit of RNA polymerase (as a reporter of active transcription) across the R. sphaeroides genome47. As expected, the known strong σE-dependent promoters showed enrichment for σE and β′ in ΔChrR cells47. Although a small number of additional potential σE binding sites were found in this analysis, none of these was located in upstream promoter DNA, suggesting that all of the major direct σE targets (15 genes in 9 potential operons; TABLE 1) have been discovered47.
Indirect targets of σE
Interestingly, none of the other R. sphaeroides genes found to be differentially expressed when comparing wild-type and ΔChrR cells contained promoters matching the σE motif47. As RpoH2 is essential for viability during the 1O2 response48, some of these differentially expressed genes are likely to be targets of this alternative σ-factor. Results that are consistent with this hypothesis were reported recently for some of these genes48. In addition, the same high-throughput approaches have identified dozens of RpoH2 target genes in R. sphaeroides, some of which have been confirmed as direct targets by in vitro transcription assays (T.J.D., Y.S. Dufour and H.A. Green, unpublished observations). Some of these genes contain promoters that are only recognized by RpoH2 whereas others contain promoters that are also recognized either by RpoH1 (FIG. 3), a second heat shock σ-factor in R. sphaeroides49, or by RNA polymerase containing the housekeeping σ-factor (T.J.D., Y.S. Dufour and H.A. Green, unpublished observations). RpoH1 and RpoH2 have previously been shown to transcribe promoters upstream of the same genes, but there are also gene promoters that are specific to one or the other of these heat shock family σ-factors45 (FIG. 3).
σE–ChrR orthologues
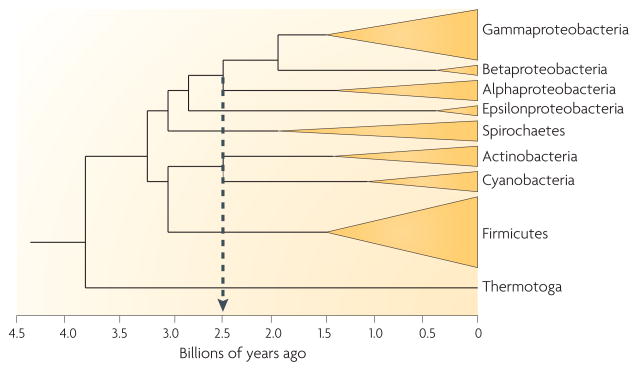
Linking the knowledge discussed above about the R. sphaeroides 1O2 stress response to the large microbial genome database provides an opportunity to test the evolutionary conservation of this system among bacteria. In R. sphaeroides, rpoE is co-transcribed with chrR41 (BOX 2). Seventy-three bacteria containing a group IV σ-factor gene50,51 (such as rpoE) adjacent to a chrR homologue were analysed, most of which were alphaproteobacteria and gammaproteobacteria, with only one betaproteobacterium and one deltaproteobacterium being found to contain σE and ChrR homologues47. A phylogenetic analysis of the σE and ChrR proteins across bacterial divisions also suggested that σE–ChrR evolved before the divergence of the alphaproteobacteria and gammaproteobacteria47. Although Cyanobacteria, which are predicted to be close relatives of the original oxygenic phototrophs4,5, contain multiple group II alternative σ-factors51, isolates with sequenced genomes do not contain group IV σ-factors51 or homologues of σE and ChrR47. This has been taken as evidence that the σE-dependent bacterial response to 1O2 did not precede the accumulation of atmospheric O2 (FIG. 4) from the water-splitting activity of photosystem II from ancestors of modern-day cyanobacteria52. Therefore, the natural history of σE and ChrR suggests that they, and also this photo-oxidative stress response (see below), originated later than the Great Oxidation Event that gave rise to molecular oxygen52.
Figure 4. Natural history of the σ-E-dependent bacterial response to singlet oxygen.
The phylogenetic tree traces the approximate time of branching among selected important microbial groups. Most organisms that are known or predicted to contain the σE-dependent transcriptional response to singlet oxygen are members of the alphaproteobacteria or gammaproteobacteria. The dotted grey line indicates the time when the water-splitting activity of photosystem II by ancestors of modern-day cyanobacteria led to the accumulation of atmospheric O2. Image modified from REF. 52.
It should be noted that the genome sequence data set used for this analysis could be biased towards alphaproteobacteria and gammaproteobacteria, as most of the bacterial genome sequences that are currently available are from these groups. However, the failure to identify σE and ChrR homologues in the large number of enteric bacteria that were included in this analysis is evidence that this system is not present in all members of the gammaproteobacteria47. Until genome sequences from a more representative set of other bacterial divisions become available, the full extent of the conservation of the σE–ChrR pair or the transcriptional response to 1O2 in the bacterial kingdom will not be known.
The core σE–ChrR regulon
To explore the extent and content of the photo-oxidative stress networks across bacterial species, the same 73 genomes were analysed for genes that are orthologous to those in the R. sphaeroides σE–ChrR regulon and for genes with promoters that contain the σE motif. When a phylogenetically determined PSWM was used to score the promoter regions of annotated genes in the various genomes to identify possible σE homologue binding sites, many of the known R. sphaeroides σE–ChrR regulon members were identified in members of the alphaproteobacteria and gammaproteobacteria. The most conserved σE targets, which constitute a so-called core σE–ChrR regulon across species (TABLE 1), include the rpoE–chrR operon (loci RSP_1092–RSP_1093 in R. sphaeroides), phrA (RSP_2143 in R. sphaeroides), cyclopropane fatty acyl-phospholipid synthase (cfaS; RSP_2144 in R. sphaeroides) and RSP_1091–RSP_1087 (REF. 47). Therefore, it is not unreasonable to propose that these photosynthetic and non-photosynthetic bacteria encounter 1O2 in their environment47. Alternatively, some bacterial species might use the σE–ChrR system to respond to other stresses. Recent studies of the σE–ChrR system in Caulobacter crescentus have shown a rapid response to 1O2 and organic hydroperoxides and a slower response to both ultraviolet A and cadmium exposure53, suggesting that there are either additional signals in some bacteria or methods by which one stress can activate other systems.
The predicted core σE–ChrR regulon includes the positive master regulator (rpoE) and its inhibitor (chrR) and encodes proteins that are proposed to be involved in the cellular response to 1O2 (REF. 47). In the presence of 1O2, the integrity of the membrane fatty acid bilayer may be maintained by cfaS, which encodes a putative cyclopropane fatty acid synthase. Using its substrate, S-adenosylmethionine, as a methyl donor, CfaS creates a methylene bridge across unsaturated fatty acid double bonds54. In the case of 1O2 assault, this modification would make the membrane less accessible to chemical modification, thereby minimizing susceptibility to any further damage that could result in increased membrane permeability in the presence of 1O2. RSP_1091 and RSP_1090 have unknown functions but do have limited amino acid similarity to cyclopropane fatty acid synthetases. Repair of light-induced damage to DNA may be accomplished by PhrA, a predicted DNA photolyase55 that could repair pyrimidine dimers. Additional important functions are likely to be carried out by proteins that are encoded by the core σE–ChrR regulon, as the functions of some of these genes are unknown, and there are few clues regarding their putative roles47.
The extended σE–ChrR regulon
A group of genes that are not as prevalent among the 73 bacterial species that were analysed seem to constitute an extended σE–ChrR regulon. As with the core regulon, the extended regulon contains several genes of unknown function47. There are also gene sets that are found mostly in specific groups or genera of bacteria, suggesting that there are functions in this response that may be associated with the environment in which these organisms are found47.
The so-called extended σE–ChrR regulon often contains RSP_0601 (REF. 47), which encodes RpoH245. RpoH2 is found in only a few alphaproteobacteria and in none of the gammaproteobacteria for which genome sequences are currently available47. Most alphaproteobacteria that have RpoH2 homologues also have σE binding motifs in the RpoH2 promoter region47. This suggests that the σE–RpoH2 cascade is a conserved transcriptional network in this group of alphaproteobacteria for the response to 1O2. One of the genes that is directly transcribed by RpoH2 is RSP_2389, which encodes a putative glutathione peroxidase (T.J.D., Y.S. Dufour and H.A. Green, unpublished observations), a class of enzymes that is known to be a key part of the defence against ROS in animals and plants56,57.
Interestingly, RSP_2389 homologues in several gammaproteobacteria that lack RpoH2 but contain σE–ChrR homologues have a conserved σE motif in their promoter regions47. This suggests that RSP_2389 is directly activated by σE in some organisms that lack the second, RpoH2-dependent part of the photo-oxidative stress cascade. These two transcriptional network schemes for the activation of bacterial RSP_2389 homologues, together with studies examining the role of the homologous GPX5 in algae31 (see below), indicate that glutathione peroxidase is a part of the photo-oxidative stress response that is conserved across kingdoms.
1O2 and oxygenic phototrophs
Photo-oxidative stress responses have also been studied in eukaryotic phototrophs. Unlike purple photosynthetic bacteria (which are anaerobic phototrophs that do not form O2 during photochemistry), eukaryotic systems are continually generating 1O2, as photosystem II activity both generates triplet state chlorophyll pigments and releases O2 (REFS 5,58).
The first demonstration of gene induction by 1O2 in a photosynthetic organism was that of GPX5 in C. reinhardtii31. This induction was rapid, robust and showed specificity for 1O2, with O2•− and H2O2 having only small effects on GPX5 expression31,59,60. Further studies provided evidence that C. reinhardtii can acclimatize to 1O2 stress with the help of GPX5 and glutathione S-transferase61, highlighting the importance of these detoxifying functions in the 1O2 response. In recent C. reinhardtii studies, 1O2 was detected in the cytoplasm62, providing evidence for either a signalling pathway between the thylakoid membrane (which is the source of this ROS) and the nucleus or a second pathway that generates 1O2 outside the chloroplast.
Conclusions and future directions
Determining how cells sense and respond to 1O2 stress is necessary for a complete understanding of cellular survival to this toxic ROS. The recent use of traditional, high-throughput and genomic approaches has greatly advanced our understanding of photo-oxidative stress responses in both photosynthetic and non-photosynthetic bacteria. The prevalence of the σE–ChrR proteins that control this response and of the conserved core members of the σE regulon across diverse bacterial species suggest that 1O2, generated by either light-dependent or light-independent processes, is encountered more widely in nature than has previously been appreciated. In the future, it will be interesting to identify the sources of 1O2, particularly in the many non-photosynthetic bacterial species that are predicted to contain the regulators and target genes of a response that was initially identified in the photosynthetic bacterium R. sphaeroides. Much work remains to be carried out to determine the mechanistic details of how cells sense, respond to and protect themselves from 1O2, both in bacteria and eukaryotes. Currently, it is also unclear whether bacteria and eukaryotes use similar gene products or processes to combat 1O2 stress. Some of the genes that are induced by 1O2 stress have proposed protective or repair functions, providing some insight into the nature of this response. However, many genes encode proteins of unknown function, which is currently preventing us from comprehending the full extent of the cellular response to 1O2.
Acknowledgments
Work on the singlet oxygen stress response has been supported by the Department of Energy (DE-FG02-05ER15653) and, more recently, by the National Institutes of General Medical Sciences (GM075273). The authors thank Y.S. Dufour and H.A. Green for allowing us to discuss their unpublished results. We also thank Y. Dufour for advice on the production of Figure 4. Furthermore, we recognize past and current members of the Donohue laboratory for their contribution to the observations summarized in this work.
- Diradical
A molecular species with two electrons occupying two degenerate molecular orbitals, typified by high reactivity and a short lifespan
- Fenton reaction, The reaction in which free ferrous iron (Fe2+) transfers an electron to hydrogen peroxide (H2O2) to produce a hydroxyl radical (OH•)
H2O2 + Fe2+ → OH− + FeO2+ + H+ → Fe3+ + OH− + OH•
- Triplet excited state
The higher energy state of a molecule that is characterized by an electron paramagnetic resonance spectrum with three peaks
- Photosensitizer
A chemical compound that readily undergoes photoexcitation and then transfers its energy to other molecules, resulting in a reaction mixture that is sensitive to light
- Respiratory burst
The rapid release of reactive oxygen species from neutrophils and macrophages of the immune system
- Paraquat
A redox cycling agent that can be oxidized by dioxygen, resulting in the production of superoxide; also called methyl viologen
- Isozymes
Any of the electrophoretically distinct forms of an enzyme, which represent different polymeric states but have the same function
- Carotenoids
A class of pigments that are widely distributed in nature
- pigments can be yellow
orange, red or purple
- Photosynthetic reaction centre
A site where molecular excitations originating from light are transformed into a series of electron transfer reactions. It is composed of a multiprotein complex containing pigmented cofactors (such as chlorophyll)
- Phototroph
An organism that uses light as a source of metabolic energy
- Dane-Py
(3-(N-diethylaminoethyl)-N-dansyl-aminomethyl-2,5-dihydro- 2,2,5,5-tetramethyl-1-H-pyrrole.) A fluorescent molecular trap that is specific for singlet oxygen
- excitation occurs at 337 nm
with emission at 545 nm
- Electron paramagnetic resonance spin trapping
Experiments in which a particular electron spin state of a molecule is isolated to measure its spectrum
- σ-factor
A specificity subunit of prokaryotic RNA polymerase that directs the RNA polymerase holoenzyme to the promoter DNA of specific genes. The primary σ-factor in cells is responsible for the recognition of so-called housekeeping genes, and alternative σ-factors are devoted to specialized functions
- Methylene blue
A photosensitizer that is used to generate singlet oxygen when it is exposed to both oxygen and light
- Rose Bengal
A widely used stain that also functions as a photosensitizer, specifically to generate singlet oxygen from ground state triplet oxygen
- Position-specific weighted matrix
A statistical representation of patterns in biological sequences, composed of a matrix of score values that gives a weighted match to any given substring of fixed length
- Photolyase
An enzyme that repairs DNA that has been damaged by ultraviolet light exposure by breaking pyrimidine dimers
- Thylakoid
An internal membrane system occupying the main body of a plastid; particularly well-developed in chloroplasts.
References
- 1.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Storz G, Zheng M. In: Bacterial Stress Responses. Storz G, Hengge-Aronis R, editors. ASM; Washington: 2000. pp. 47–59. Provides a comprehensive summary of bacterial responses to ROS created by type I reactions, with particular emphasis on the role of the oxyR and soxRS regulons. [Google Scholar]
- 4.Blankenship RE, Hartman H. The origin and evolution of oxygenic photosynthesis. Trends Biochem Sci. 1998;23:94–97. doi: 10.1016/s0968-0004(98)01186-4. [DOI] [PubMed] [Google Scholar]
- 5.Raymond J, Zhaxybayeva O, Gogarten JP, Blankenship RE. Evolution of photosynthetic prokaryotes: a maximum-likelihood mapping approach. Phil Trans R Soc Lond B Biol Sci. 2003;358:223–230. doi: 10.1098/rstb.2002.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gennis RB, Stewart V. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt FC, editor. ASM; Washington: 1999. pp. 217–261. [Google Scholar]
- 7.Briviba K, Klotz L-O, Sies H. Toxic and signaling effects of photochemically or chemically generated singlet oxygen in biological systems. Biol Chem. 1997;378:1259–1265. Summarizes how 1O2 can be formed both in vivo and in vitro, the types of damage that it can inflict on molecules and cells and the types of cellular signalling events that it promotes. [PubMed] [Google Scholar]
- 8.Sies H, Menck CFM. Singlet oxygen induced DNA damage. Mutat Res. 1992;275:367–375. doi: 10.1016/0921-8734(92)90039-r. [DOI] [PubMed] [Google Scholar]
- 9.Epe B, Pflaum M, Boiteux S. DNA damage induced by photosensitizers in cellular and cell-free systems. Mutat Res. 1993;299:135–145. doi: 10.1016/0165-1218(93)90091-q. [DOI] [PubMed] [Google Scholar]
- 10.Davies MJ. The oxidative environment and protein damage. Biochim Biophys Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Borland CF, McGarvey DJ, Truscott TG, Cogdell RJ, Land EJ. Photophysical studies of bacteriochlorophyll a and bacteriopheophytin a – singlet oxygen generation. J Photochem Photobiol B. 1987;1:93–101. [Google Scholar]
- 12.Cogdell RJ, et al. How carotenoids protect bacterial photosynthesis. Phil Trans R Soc Lond B. 2000;355:1345–1349. doi: 10.1098/rstb.2000.0696. Discusses structural studies of bacterial photosynthetic pigments in reaction centres and light-harvesting complexes combined with kinetic studies showing the decay of 3Bchla*and the concomitant formation of triplet state carotenoids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryter SW, Tyrrell RM. Singlet oxygen (1O2): a possible effector of eukaryotic gene expression. Free Radic Biol Med. 1998;24:1520–1534. doi: 10.1016/s0891-5849(97)00461-9. [DOI] [PubMed] [Google Scholar]
- 14.Demple B, Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983;304:466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- 15.Christman MF, Morgan RW, Jacobson FS, Ames BN. Positive control of a regulon for defences against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 16.Farr SB, Natvig DO, Kogoma T. Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli. J Bacteriol. 1985;164:1309–1316. doi: 10.1128/jb.164.3.1309-1316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan RW, Christman MF, Jacobson FS, Storz G, Ames BN. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci USA. 1986;83:8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanBogelen RA, Kelley PM, Neidhardt FC. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987;169:26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg JT, Demple B. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J Bacteriol. 1989;171:3933–3939. doi: 10.1128/jb.171.7.3933-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walkup LKB, Kogoma T. Escherichia coli proteins inducible by oxidative stress mediated by the superoxide radical. J Bacteriol. 1987;171:1476–1484. doi: 10.1128/jb.171.3.1476-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiths M, Sistrom WR, Cohen-Bazire G, Stanier RY. Function of carotenoids in photosynthesis. Nature. 1955;176:1211–1214. doi: 10.1038/1761211a0. This groundbreaking study revealed that carotenoids protect cells from photodynamic destruction. [DOI] [PubMed] [Google Scholar]
- 22.Cogdell RJ, Frank HA. How carotenoids function in photosynthetic bacteria. Biochim Biophys Acta. 1987;895:63–79. doi: 10.1016/s0304-4173(87)80008-3. [DOI] [PubMed] [Google Scholar]
- 23.Kalai T, Hideg E, Vass I, Hideg K. Double (fluorescent and spin) sensors for detection of reactive oxygen species in the thylakoid membrane. Free Radic Biol Med. 1998;24:649–652. doi: 10.1016/s0891-5849(97)00339-0. [DOI] [PubMed] [Google Scholar]
- 24.Glaeser J, Klug G. Photo-oxidative stress in Rhodobacter sphaeroides: protective role of carotenoids and expression of selected genes. Microbiology. 2005;151:1927–1938. doi: 10.1099/mic.0.27789-0. [DOI] [PubMed] [Google Scholar]
- 25.Tandori J, Hideg E, Nagy L, Maroti P, Vass I. Photoinhibition of carotenoidless reaction centers from Rhodobacter sphaeroides by visible light. Effect on protein structure and electron transport. Photosyn Res. 2001;70:175–184. doi: 10.1023/A:1017907404325. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, et al. Magnetic field effect on singlet oxygen production in a biochemical system. Chem Commun (Camb) 2005:174–176. doi: 10.1039/b413489c. [DOI] [PubMed] [Google Scholar]
- 27.Arellano JB, et al. Formation and geminate quenching of singlet oxygen in purple bacterial reaction center. J Photochem Photobiol B. 2007;87:105–112. doi: 10.1016/j.jphotobiol.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Uchoa AF, Knox PP, Turchielle R, Seifullina NK, Baptista MS. Singlet oxygen generation in the reaction centers of Rhodobacter sphaeroides. Eur Biophys J. 2008;37:843–850. doi: 10.1007/s00249-008-0287-y. [DOI] [PubMed] [Google Scholar]
- 29.Glaeser J, Zobawa M, Lottspeich F, Klug G. Protein synthesis patterns reveal a complex regulatory response to singlet oxygen in Rhodobacter. J Proteome Res. 2007;6:2460–2471. doi: 10.1021/pr060624p. [DOI] [PubMed] [Google Scholar]
- 30.Morgan PE, Dean RT, Davies MJ. Protective mechanisms against peptide and protein peroxides generated by singlet oxygen. Free Radic Biol Med. 2004;36:484–496. doi: 10.1016/j.freeradbiomed.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Leisinger U, et al. The glutathione peroxidase homologous gene from Chlamydomonas reinhartdtii is transcriptionally up-regulated by singlet oxygen. Plant Mol Biol. 2001;46:395–408. doi: 10.1023/a:1010601424452. [DOI] [PubMed] [Google Scholar]
- 32.Anthony JR, Warczak KL, Donohue TJ. A transcriptional response to singlet oxygen, a toxic byproduct of photosynthesis. Proc Natl Acad Sci USA. 2005;102:6502–6507. doi: 10.1073/pnas.0502225102. Identified a transcriptional response to 1O2 in R. sphaeroides, in which σE is a key regulator. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burchard RP, Dworkin M. Light-induced lysis and carotenogenesis in Myxococcus xanthus. J Bacteriol. 1966;91:535–545. doi: 10.1128/jb.91.2.535-545.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorham HC, McGowan SJ, Robson PRH, Hodgson DA. Light-induced carotenogenesis in Myxococcus xanthus: light-dependent membrane sequestration of ECF sigma factor CarQ by anti-sigma factor CarR. Mol Microbiol. 1996;19:171–186. doi: 10.1046/j.1365-2958.1996.360888.x. [DOI] [PubMed] [Google Scholar]
- 35.Browning DF, Whitworth DE, Hodgson DA. Light-induced carotenogenesis in Myxococcus xanthus: functional characterization of the ECF sigma factors CarQ and antisigma factor CarR. Mol Microbiol. 2003;48:237–251. doi: 10.1046/j.1365-2958.2003.03431.x. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder WA, Johnson EA. Singlet oxygen and peroxyl radicals regulate carotenoid biosynthesis in Phaffia rhodozyma. J Biol Chem. 1995;270:18374–18379. doi: 10.1074/jbc.270.31.18374. [DOI] [PubMed] [Google Scholar]
- 37.Agnez-Lima LF, Di Mascio P, Demple B, Menck CFM. Singlet molecular oxygen triggers the soxRS regulon of Escherichia coli. Biol Chem. 2001;382:1071–1075. doi: 10.1515/BC.2001.134. [DOI] [PubMed] [Google Scholar]
- 38.Kim SY, Kim EJ, Park JW. Control of singlet oxygen-induced oxidative damage in Escherichia coli. J Biochem Mol Biol. 2002;35:353–357. doi: 10.5483/bmbrep.2002.35.4.353. [DOI] [PubMed] [Google Scholar]
- 39.Saenkham P, Eiamphungporn W, Farrand SK, Vattanaviboon P, Mongkolsuk S. Multiple superoxide dismutases in Agrobacterium tumefaciens: functional analysis, gene regulation, and influence on tumorigenesis. J Bacteriol. 2007;189:8807–8817. doi: 10.1128/JB.00960-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saenkham P, Utamapongchai S, Vattanaviboon P, Mongkolsuk S. Agrobacterium tumefaciens iron superoxide dismutases have protective roles against singlet oxygen toxicity generated from illuminated Rose Bengal. FEMS Microbiol Lett. 2008;289:97–103. doi: 10.1111/j.1574-6968.2008.01382.x. [DOI] [PubMed] [Google Scholar]
- 41.Newman JD, Falkowski MJ, Schilke BA, Anthony LC, Donohue TJ. The Rhodobacter sphaeroides ECF sigma factor, σE, and the target promoters cycA P3 and rpoE P1. J Mol Biol. 1999;294:307–320. doi: 10.1006/jmbi.1999.3263. [DOI] [PubMed] [Google Scholar]
- 42.Newman JD, Anthony JR, Donohue TJ. The importance of zinc-binding to the function of Rhodobacter sphaeroides ChrR as an anti-sigma factor. J Mol Biol. 2001;313:485–499. doi: 10.1006/jmbi.2001.5069. [DOI] [PubMed] [Google Scholar]
- 43.Anthony JR, Newman JD, Donohue TJ. Interactions between the Rhodobacter sphaeroides ECF sigma factor, σE, and its anti-sigma factor, ChrR. J Mol Biol. 2004;341:345–360. doi: 10.1016/j.jmb.2004.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell EA, et al. A conserved structural module regulates transcriptional responses to diverse stress signals in bacteria. Mol Cell. 2007;27:793–805. doi: 10.1016/j.molcel.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green HA, Donohue TJ. Activity of Rhodobacter sphaeroides RpoHII, a second member of the heat shock sigma factor family. J Bacteriol. 2006;188:5712–5721. doi: 10.1128/JB.00405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braatsch S, Moskvin OV, Klug G, Gomelsky M. Responses of the Rhodobacter sphaeroides transcriptome to blue light under semiaerobic conditions. J Bacteriol. 2004;186:7726–7735. doi: 10.1128/JB.186.22.7726-7735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dufour YS, Landick R, Donohue TJ. Organization and evolution of the biological response to singlet oxygen stress. J Mol Biol. 2008;383:713–730. doi: 10.1016/j.jmb.2008.08.017. This article described a new level of understanding of the organization and evolution of 1O2 stress responses in bacteria, provided by high-throughput experimental studies combined with computational tools. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nuss AM, Glaeser J, Klug G. RpoHII activates oxidative-stress defence systems and is controlled by RpoE in the singlet oxygen-dependent response in Rhodobacter sphaeroides. J Bacteriol. 2009;191:220–230. doi: 10.1128/JB.00925-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karls RK, Brooks J, Rossmeissl P, Luedke J, Donohue TJ. Metabolic roles of a Rhodobacter sphaeroides member of the σ32 family. J Bacteriol. 1998;180:10–19. doi: 10.1128/jb.180.1.10-19.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lonetto MA, Brown KL, Rudd KE, Buttner MJ. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial σ factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gruber TM, Gross CA. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol. 2003;57:441–466. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- 52.Battistuzzi FU, Feijao A, Hedges SB. A genomic timescale of prokaryote evolution: insights into the origin of methanogenesis, phototrophy, and the colonization of land. BMC Evol Biol. 2004;4:44–57. doi: 10.1186/1471-2148-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lourenco RF, Gomes SL. The transcriptional response to cadmium, organic hydroperoxide, singlet oxygen and UV-A mediated by the σE-ChrR system in Caulobacter crescentus. Mol Microbiol. 2009;72:1159–1170. doi: 10.1111/j.1365-2958.2009.06714.x. [DOI] [PubMed] [Google Scholar]
- 54.Cronan JE., Jr Phospholipid modifications in bacteria. Curr Opin Microbiol. 2002;5:202–205. doi: 10.1016/s1369-5274(02)00297-7. [DOI] [PubMed] [Google Scholar]
- 55.Hendrischk AK, Braatsch S, Glaeser J, Klug G. The phrA gene of Rhodobacter sphaeroides encodes a photolyase and is regulated by singlet oxygen and peroxide in a σE-dependent manner. Microbiology. 2007;153:1842–1851. doi: 10.1099/mic.0.2006/004390-0. [DOI] [PubMed] [Google Scholar]
- 56.Ursini F, et al. Diversity of glutathione peroxidases. Meth Enzymol. 1995;252:38–53. doi: 10.1016/0076-6879(95)52007-4. [DOI] [PubMed] [Google Scholar]
- 57.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Krieger-Liszkay A. Singlet oxygen production in photosynthesis. J Exp Bot. 2004;56:337–346. doi: 10.1093/jxb/erh237. [DOI] [PubMed] [Google Scholar]
- 59.Fischer BB, Krieger-Liszkay A, Eggen RIL. Oxidative stress induced by the photosensitizers neutral red (type I) or rose Bengal (type II) in the light causes different molecular responses in Chlamydomonas reinhardtii. Plant Sci. 2005;168:747–759. [Google Scholar]
- 60.Fischer BB, Eggen RIL, Trebst A, Krieger-Liszkay A. The glutathione peroxidase homologous gene Gpxh in Chlamydomonas reinhardtii is upregulated by singlet oxygen in photosystem II. Planta. 2006;223:583–590. doi: 10.1007/s00425-005-0108-9. [DOI] [PubMed] [Google Scholar]
- 61.Ledford HK, Chin BL, Niyogi KK. Acclimation to singlet oxygen stress in Chlamydomonas reinhardtii. Eukaryot Cell. 2007;6:919–930. doi: 10.1128/EC.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fischer BB, et al. Role of singlet oxygen in chloroplast to nucleus retrograde signaling in Chlamydomonas reinhardtii. FEBS Lett. 2007;581:5555–5560. doi: 10.1016/j.febslet.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 63.Zeilstra-Ryalls JH, Kaplan S. Oxygen intervention in the regulation of gene expression: the photosynthetic bacterial paradigm. Cell Mol Life Sci. 2004;61:417–436. doi: 10.1007/s00018-003-3242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplan S, Eraso J, Roh JH. Interacting regulatory networks in the facultative photosynthetic bacterium, Rhodobacter sphaeroides 2.4.1. Biochem Soc Trans. 2005;33:51–55. doi: 10.1042/BST0330051. [DOI] [PubMed] [Google Scholar]
- 65.Burgess RR, Travers AA, Dunn JJ, Bautz EKF. Factor stimulating transcription by RNA polymerase. Nature. 1969;221:43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- 66.Lonetto M, Gribskov M, Gross CA. The σ70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]