Abstract
Endocytic internalization of G protein-coupled receptors (GPCRs) plays a critical role in down-regulation of GPCR signaling. The yeast mating pheromone receptor Ste2p has been used as a model to investigate mechanisms of signal transduction, modification, and endocytic internalization of GPCRs. We previously used a fluorescently labeled mating pheromone derivative to reveal unappreciated molecular and spatiotemporal features of GPCR endocytosis in budding yeast. Here, we identify recruitment of Ste2p to preexisting clathrin-coated pits (CCPs) as a key step regulated by receptor phosphorylation and subsequent ubiquitination upon ligand binding. The yeast casein kinase I homologue Yck2p directly phosphorylates six serine residues located in the C-terminal tail of Ste2p, and mutation of these serine residues to alanine significantly decreased recruitment of Ste2p to CCPs. We also found that the clathrin adaptors Ent1p, Ent2p, and Ede1p work cooperatively to recruit ubiquitinated Ste2p to CCPs. In addition, ubiquitination has a role in ligand-independent constitutive recruitment of Ste2p to CCPs, although this process is much slower than ligand-induced recruitment. These results suggest that ubiquitination of Ste2p is indispensable for recruiting Ste2p to CCPs in both ligand-dependent and ligand-independent endocytosis.
INTRODUCTION
G protein-coupled receptors (GPCRs) are heptahelical membrane proteins that comprise one of the largest families of cell surface signaling receptors in the human genome (Lander et al., 2001; Venter et al., 2001; Takeda et al., 2002; Fredriksson et al., 2003). They are involved in various important physiological processes, including cell growth; morphological changes; blood pressure control; and sensing taste, odor, and light (Fredriksson et al., 2003). In addition, many GPCRs are overexpressed in human cancers and fundamentally involved in tumor progression (Dorsam and Gutkind, 2007). GPCRs are the targets of ∼30% of the drugs currently used for the treatment of a wide range of human diseases (Flower, 1999; Lagerstrom and Schioth, 2008). Thus, elucidating the mechanism of regulation of GPCR signaling is essential for the development of more effective and safer therapeutic agents.
Biochemical desensitization and subsequent endocytic internalization of GPCRs are the predominant mechanisms controlling GPCR signaling. In mammalian cells, most activated GPCRs are rapidly phosphorylated by G protein-coupled receptor kinases (GRKs) and bind β-arrestin, which facilitates receptor uncoupling from G proteins and receptor internalization through clathrin-coated pits (CCPs) (Kohout and Lefkowitz, 2003; Ribas et al., 2007; Marchese et al., 2008). In addition to the involvement of GRKs, recent studies have revealed a role of the casein kinase family in GPCR phosphorylation in both yeast and mammalian cells (Tobin, 2008). Studies on GPCRs (Ste2p and Ste3p) from budding yeast have demonstrated an essential role for the yeast casein kinase I homologues (Yck1p and Yck2p) in the phosphorylation of the cytoplasmic C-terminal regions (Hicke et al., 1998; Feng and Davis, 2000). For example, phosphorylation of the Ste2p receptor seems to trigger mono-ubiquitination of lysine residues located around the phosphorylation sites, and this in turn mediates receptor internalization (Hicke et al., 1998). However, whether these yeast casein kinases directly phosphorylate Ste2p, and why receptor phosphorylation is required for endocytosis, have not been clarified.
There has been lengthy controversy as to whether ligand-activated GPCRs initiate the formation of new CCPs, or whether they are recruited to pre-existing CCPs. Recent evidence obtained in studies of mammalian cells, however, supports the idea that GPCRs are recruited to pre-existing CCPs (Santini et al., 2002; Scott et al., 2002; Ehrlich et al., 2004). In yeast, this issue had not been resolved because of the lack of a suitable fluorescent cargo for analysis of receptor-mediated endocytosis. In a previous study, we synthesized a novel fluorescently labeled mating pheromone derivative (Alexa Fluor-labeled α-factor), which specifically binds to Ste2p GPCR, and is used as an endocytic cargo. Using this fluorescent cargo molecule, we demonstrated that CCPs first assemble at the plasma membrane, and then α-factor-bound Ste2p is recruited to CCPs (Toshima et al., 2006). The questions that arose from this observation are how the GPCR-ligand complexes are recruited to CCPs, and which proteins are involved in the recruitment.
We address these questions here by using fluorescently labeled α-factor to visualize recruitment of GPCRs to CCPs on the plasma membrane. We show that phosphorylation and subsequent ubiquitination of Ste2p facilitates α-factor recruitment to CCPs, and that the mono-ubiquitin binding proteins Ent1/2p and Ede1p mediate this recruitment. In addition, we demonstrate that ubiquitination of Ste2p is also necessary for ligand-independent recruitment of the GPCR to CCPs.
MATERIALS AND METHODS
Yeast Strains, Growth Conditions, and Plasmids
The yeast strains used in this study are listed in Table 1. All strains were grown in standard rich media (YPD) or synthetic media (SD) supplemented with the appropriate amino acids. ste2 mutants were integrated as follows: A 3.9-kb BamHI-EcoRI fragment containing the STE2 gene was cloned into pBluescript II SK (pBS-STE2). ste2Δ300 was generated by digesting the polymerase chain reaction (PCR) product (nt 1150-2014), amplified by primers 5′-TCACAGGCGACAACTTC-3′ and 5′-CGGAATTCCCCGGGAGCAGCCGTGGCCCACATTGA-3′, with AatII and EcoRI, and ligating the fragment into AatII- and EcoRI-digested pBS-STE2 (pBS-ste2Δ300). Plasmids for the point mutants of STE2 were constructed by using a site-directed mutagenesis kit (Stratagene, La Jolla, CA). To integrate each ste2 mutant into the URA3 gene of JTY528, BamHI-EcoRI fragments of each ste2 mutant were cloned into the pRS306 vector, digested with StuI, and transformed into JTY528. Integrated ste2 mutants were selected on SC plates lacking uracil. The Ste2p expression plasmid was constructed by inserting the STE2 open reading frame into BamHI- and EcoRI-digested pRS426 containing the GAL-10 promoter and the ADH1 terminator. Green fluorescent protein (GFP) and monomeric red fluorescent protein (mRFP) tags were integrated at the C terminus of each gene.
Table 1.
Yeast strains
| Strain | Genotype | Source |
|---|---|---|
| JTY0528 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 | This study |
| JTY0569 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 sla2Δ::URA3 SLA1-GFP::HIS3 | This study |
| JTY0718 | Matahis3-Δ200 leu2-3, 112 ura3-52 lys2–801 bar1Δ::LEU2 ede1Δ::URA3 SLA1-GFP::HIS3 | This study |
| JTY0727 | Matahis3 leu2 ura3-52 bar1-1 yck1Δ yck2-2 SLA1-Cherry::HIS3 GFP-YCK2::URA3 | This study |
| JTY0739 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 ste2-6SA::URA3 | This study |
| JTY0743 | Matahis3 leu2 ura3-52 bar1-1 yck1Δ yck2-2 SLA1-GFP::HIS3 YCK2::URA3 | This study |
| JTY0744 | Matahis3 leu2 ura3-52 bar1-1 yck1Δ yck2-2 SLA1-GFP::HIS3 yck2-Cys545,546Ser::URA3 | This study |
| JTY0754 | Matahis3 leu2 ura3-52 bar1-1 yck1Δ yck2-2 SLA1-GFP::HIS3 | This study |
| JTY0760 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 STE2::URA3 | This study |
| JTY0761 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 ste2Δ300::URA3 | This study |
| JTY0762 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 ste2-3SA::URA3 | This study |
| JTY0763 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 ste2-6SD::URA3 | This study |
| JTY0810 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 ste2-7KR-G392A::URA3 | This study |
| JTY0812 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 ste2-7KR::URA3 | This study |
| JTY0825 | Matahis3 leu2 ura3-52 lys2 trp1 bar1-1 ent1Δ::HIS3 ent2Δ::KanMX6 bar1 SLA1-GFP::URA3 [pRS315-ent1ΔUIM] | This study |
| JTY0870 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 ste2-6SD-7KR::URA3 | This study |
| JTY0872 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 Ste2-6SA-UBI4::URA3 | This study |
| JTY1035 | Matahis3 leu2 ura3-52 lys2 trp1 bar1-1 ent1Δ::HIS3 ent2Δ::KanMX6 ede1Δ::KanMX6 SLA1-GFP::URA3 [pRS315-ent1ΔUIM] | This study |
| JTY1036 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 STE2-GFP::URA3 | This study |
| JTY1037 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 ste2-7KR-GFP::URA3 | This study |
| JTY1038 | Matahis3-Δ200 leu2-3, 112 ura3-52 bar1Δ::LEU2 ste2Δ::KanMX6 SLA1-GFP::HIS3 ste2-7KR-G392A-GFP::URA3 | This study |
| JTY1233 | Mata/Matαhis3-Δ200/his3-Δ200 leu2-3, 112/leu2-3, 112 ura3-52/ura3-52 LYS2/ lys2-801 ADE2/ade2-1 SLA1-GFP::HIS3 [pRS426-STE2] | This study |
| JTY1234 | Matahis3 leu2 ura3-52 bar1-1 yck1Δ yck2-2 ste2Δ::KanMX6 SLA1-GFP::HIS3 ste2-6SD::URA3 | This study |
All strains are derived from strain S288C.
Fluorescence Labeling of α-Factor and Endocytosis Assays
Fluorescence labeling of α-factor was performed as described previously (Toshima et al., 2006). Alexa-594 maleimide (Invitrogen, Carlsbad, CA) was coupled to the synthesized α-factor and purified by reverse-phase high-performance liquid chromatography; structure and purity (>95%) were assessed by electrospray ionization-Fourier transform ion cyclotron resonance mass spectrometry (Bruker 9.4T spectrometer; Bruker, Newark, DE). For endocytosis assays, cells were grown to an OD600 of 0.5 in 0.4 ml of YPD. After treatment with a final concentration of 200 μM latrunculin A (LatA) for 30 min, the cells were centrifuged briefly and resuspended in 20 μl of synthetic media (SM) (lacking glucose) with 1% (wt/vol) bovine serum albumin (BSA) and 5 μM Alexa-α-factor in the presence of 200 μM LatA. After incubation on ice for 30 min, cells were washed into ice-cold SM containing 1% BSA. Recruitment of Alexa-α-factor to CCPs was initiated by the addition of SM containing 4% glucose and amino acids and by allowing the sample to warm to room temperature in the continuous presence of 200 μM LatA. Alexa Fluor-594 α-factor (A594-α-factor) imaging was done using a rhodamine/Texas-Red filter, and images were acquired with an IX81 microscope equipped with a 100×/numerical aperture 1.40 (Olympus, Tokyo, Japan) objective and Orca-AG cooled charge-coupled device camera (Hamamatsu, Bridgewater, NJ), using MetaMorph software (Molecular Devices, Sunnyvale, CA).
35S-Labeled α-Factor Internalization Assay
Preparation and internalization of 35S-labeled α-factor was performed as described previously (Toshima et al., 2005). In brief, cells were grown to an OD600 of 0.3 in 50 ml of YPD, centrifuged briefly, and resuspended in 4 ml of YPD containing 1% (wt/vol) BSA; 50 mM KH2PO4, pH 6.0; and 20 μg/ml uracil, adenine, and histidine. After adding 35S-labeled α-factor, cell aliquots were withdrawn at various time points and subjected to a wash in pH 1.0 buffer to remove surface-bound α-factor so internal α-factor could be measured, or in pH 6.0 buffer to determine the total (internal and bound) α-factor. The amount of cell-associated radioactivity after each wash was determined by scintillation counting. Each experiment was performed at least three times.
Protein Expression and Purification
Tobacco etch virus (TEV)-myc-tagged Yck2p was purified as follows: DDY1810 strains expressing TEV-myc-tagged Yck2p were grown to saturation in 20 ml of SD supplemented with all amino acids except uracil. This inoculum was added to 1.5 l of synthetic media containing 2% (wt/vol) raffinose but lacking uracil, and it was grown to OD600 of 1.0. Protein expression was induced upon addition of 30 g of bacto-peptone, 15 g of yeast extract, and a final concentration of 2% (wt/vol) galactose for 8–12 h, as described previously (Rodal et al., 2003). Cells were harvested by centrifugation, washed with 500 ml of water, resuspended at a 1:5 (wt/vol) ratio of yeast to water, and drop frozen in liquid N2. Cells were lysed by homogenization five times in a Waring blender with liquid N2. TEV-myc-tagged Yck2p was purified as described previously (Rodal et al., 2003). Glutathione transferase (GST) fusion proteins were expressed in Escherichia coli and purified on a glutathione-Sepharose (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), as described previously (Toshima et al., 2001).
In Vitro Kinase Assay and Immunoblotting
In vitro kinase assays were performed as described previously (Toshima et al., 2001). Samples were separated on SDS-polyacrylamide gel electrophoresis (PAGE) and analyzed by autoradiography, amido black staining, and immunoblotting with an anti-Myc antibody. Immunoblot analysis was performed as described previously (Toshima et al., 2001). Immunoreactive protein bands were visualized using a SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL).
RESULTS
Ste2p Receptor Is Recruited to Preexisting CCPs in a Time-dependent Manner after Ligand Stimulation
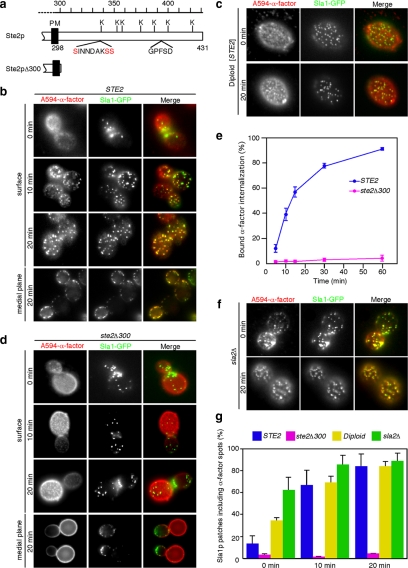
Using A594-α-factor as a marker for ligand-bound Ste2p receptor, we first examined how Ste2p is recruited to CCPs. Treatment of cells with 200 μM LatA, which leads to the complete disassembly of cortical actin, blocks the endocytic pathway at the internalization step. As we showed previously, in LatA-treated cells A594-α-factor accumulates with components of the endocytic machinery in puncta on the plasma membrane (Figure 1b) (Toshima et al., 2006). This accumulation is time-dependent in that A594-α-factor was observed as dispersed, homogeneous faint spots when cells were first labeled (Figure 1b, 0 min), but the fluorescence gradually coalesced into discernible spots after 10 min (Figure 1b, 10 min), and finally concentrated into clear punctate spots after 20 min (Figure 1b, 20 min). Approximately 14% of the Sla1p patches, which correspond to CCPs arrested before the internalization step, colocalized with the A594-α-factor spots at 0 min, but after 20 min ∼84.3% of the patches colocalized with the spots, as visualized in surface and medial focal planes of cells (Figure 1, b and g). This observation clearly indicates that Ste2p is recruited to CCPs upon ligand binding. We next examined whether G protein mediated signaling is responsible for Ste2p recruitment to CCPs. A previous study clearly showed that Ste2p internalization is independent of G protein-mediated signal transduction by using diploid cells expressing Ste2p (Zanolari et al., 1992). Diploid cells lack pheromone receptors and the tripartite G protein and thus do not respond to α-factor, even when Ste2p is expressed artificially (Zanolari et al., 1992). In agreement with these previous observations, Ste2p was efficiently recruited to CCPs in diploid cells expressing Ste2p (Figure 1, c and g), indicating that Ste2p recruitment to CCPs is independent of G protein-mediated signaling.
Figure 1.
The C-terminal cytoplasmic region of Ste2p mediates α-factor recruitment to endocytic sites. (a) Diagrams of the cytoplasmic C termini of wild-type Ste2p and Ste2pΔ300. The potential ubiquitination sites (K), phosphorylation sites (red), and GPFSD sequence [similar to the NPFX(1,2)D targeting signal] are indicated. The residue numbers are indicated on the top. PM, plasma membrane. (b, d, and f) Localization of A594-α-factor and Sla1-GFP in wild-type (b), ste2Δ300 (d), and sla2Δ (f) cells treated with LatA. After incubating cells expressing Sla1-GFP with 200 μM LatA at 25°C for 30 min, they were labeled with A594-α-factor in the presence of LatA. The images were acquired at 0, 10, 20, or 30 min after washing out unbound A594-α-factor and warming the cells to 25°C and incubating them with glucose-containing medium in the continuous presence of 200 μM LatA. (c) Localization of A594-α-factor and Sla1-GFP treated with LatA in diploid cells expressing Ste2p from the Gal promoter. Cells were grown for 4 h in YP medium containing 2% galactose. (e) Internalization of 35S-labeled α-factor was measured. (g) Quantification of colocalization of A594-α-factor and Sla1-GFP in individual cells. Error bars represent the SD from at least three experiments.
The C-terminal cytoplasmic tail of the Ste2p receptor contains several serine and lysine residues that are potential sites for ubiquitination or phosphorylation (Figure 1a) (Hicke and Riezman, 1996, 1998). To identify the region required for Ste2p recruitment to CCPs, we first determined whether A594-α-factor is recruited to CCPs when the C terminus of Ste2p is truncated (Ste2pΔ300) (Figure 1a). In contrast to the wild-type cells, little colocalization of A594-α-factor with Sla1p patches was observed at any time point (Figure 1, d and g). This result indicates that the cytoplasmic region is indispensable for Ste2 recruitment to CCPs. We next assayed 35S-labeled α-factor internalization and confirmed a severe endocytic defect in cells expressing Ste2pΔ300 compared with wild-type cells (Figure 1e). Because it has been reported that several endocytic proteins accumulate at CCPs in sla2Δ cells (Kaksonen et al., 2003), we next examined A594-α-factor localization in sla2Δ cells in the presence of LatA. Interestingly, A594-α-factor showed extensive colocalization with Sla1p patches at 0 min (∼62.5%), and this colocalization increased over time (Figure 1, f and g). Ste2p has been shown previously to be highly phosphorylated and ubiquitinated in sla2Δ cells (Hicke et al., 1998). These results therefore indicate that Ste2p receptors are randomly distributed on the plasma membrane and that subsequent modification of the cytoplasmic tail of Ste2p mediates the recruitment of this receptor and its associated A594-α-factor to the site of endocytosis.
Phosphorylation of the Ste2p Cytoplasmic Tail Is Required for Ligand-Induced Receptor Recruitment to CCPs
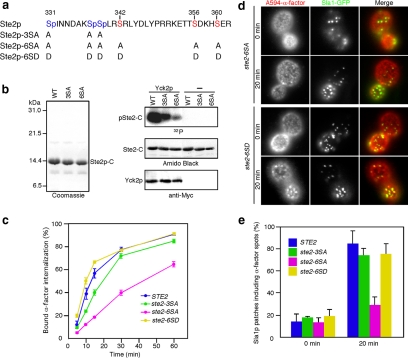
The C-terminal tail region of Ste2p contains several potential phosphorylation sites (Figure 1a), and phosphorylation of these sites facilitates ubiquitination of the neighboring lysines (Hicke and Riezman, 1996; Hicke et al., 1998). Previous studies showed that the three serine residues within the SINNDAKSS internalization signal are important for endocytic internalization of the receptor (Hicke et al., 1998). However, mutation of these serine residues (S331, S338, and S339) to alanine (ste2-3SA) only slightly slowed ligand-stimulated receptor internalization (Figure 2c) (Hicke et al., 1998). However, in Ste2-7KR cells, in which the potential ubiquitination sites are all mutated to arginine, showed a marked reduction in receptor internalization (see Figure 4c) (Howard et al., 2002). These findings indicate that other phosphorylation site(s) might exist in the C-terminal tail region of Ste2p. Yeast casein kinase I homologues (Yck1p and Yck2p) were shown previously to be required for phosphorylation of Ste2p, although direct phosphorylation of Ste2p by these kinases has not been examined. We identified three more serine residues (S342, S356, and S360) in the Ste2p C-terminal region that correspond to the reported consensus sequences (D/E-X-X-S/T and S/T(P)-X-X-S/T) recognized by casein kinase (Figure 2a) (Flotow et al., 1990; Knippschild et al., 2005). To determine whether Yck2p is able to phosphorylate Ste2p, we purified GST-fused C-terminal fragments of Ste2p, Ste2p-3SA, and Ste2p-6SA (Figure 2b, left) and performed in vitro kinase assays on them (Figure 2b, right). The C-terminal fragments of wild-type Ste2p and Ste2p-3SA were efficiently phosphorylated in the presence of Yck2p, with a modest reduction in Ste2p-3SA phosphorylation level compared with wild-type (Figure 2b, right). In contrast, Ste2p-6SA, in which all serine residues that correspond to the Yckp consensus sequence are replaced by alanine (Figure 2a), showed significantly reduced phosphorylation by Yck2p (Figure 2b, right). We then tested whether the decreased level of phosphorylation influences uptake of 35S-labeled α-factor. As shown in Figure 2c, ste2-6SA cells internalized 35S-labeled α-factor more slowly than did wild-type or ste2-3SA cells, reflecting the reduced phosphorylation level of ste2-6SA cells (Figure 2c). This decrease in 35S-labeled α-factor internalization was similar to that observed in ste2-7KR cells, indicating that most of the phosphorylation sites important for internalization are disrupted in the ste2-6SA mutant (Figures 2c and 4c). Interestingly, 35S-labeled α-factor internalization was almost restored in cells expressing Ste2p-6SD, in which phosphorylation sites have been replaced by aspartic acid to mimic the phosphorylation state of native Ste2p (Figure 2, a and c).
Figure 2.
Phosphorylation of Ste2p receptor is important for α-factor recruitment to endocytic sites. (a) Phosphorylation sites in the cytoplasmic tail of Ste2p are shown. Blue indicates known previously phosphorylation sites, and red indicates phosphorylation sites newly found in this study. The position and amino acid substitutions for each mutant are shown below. (b) In vitro phosphorylation of Ste2p C-terminal fragment by purified Yck2p. Left, Coomassie Blue-stained gels of purified C-terminal fragments of wild-type Ste2p, Ste2-3SAp, and Ste2-6SAp. Right, in vitro kinase assay using purified Ste2p fragments and Yck2p. Ste2p fragments were incubated with [γ-32P]ATP and Yck2p at 30°C, separated by SDS-PAGE, and analyzed using autoradiography and amido black staining. Yck2p was analyzed by immunoblotting with an anti-Myc antibody. (c) Internalization of 35S-labeled α-factor was measured in the Ste2 phosphorylation-site mutants. (d) Localization of A594-α-factor and Sla1-GFP in ste2-6SA (top) or ste2-6SD (bottom) cells treated with LatA. Assays were performed as described in text. (e) Quantification of colocalization of A594-α-factor and Sla1-GFP in Ste2p phosphorylation-site mutants. Error bars represent the SD from at least three experiments.
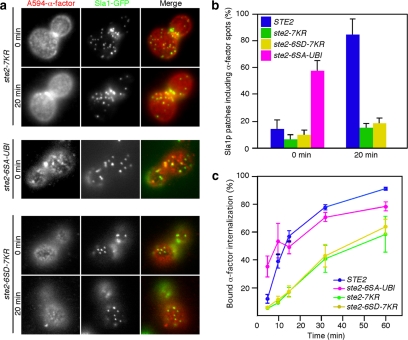
Figure 4.
Ubiquitination is of primary importance for recruitment of α-factor to CCPs. (a) Localization of A594-α-factor and Sla1-GFP in ste2-7KR, ste2-6SA-UBI, or ste2-6SD-7KR cells treated with LatA. The images were acquired at 0 or 20 min after washing out unbound Alexa-α-factor and warming the cells to 25°C. (b) Quantification of colocalization of A594-α-factor and Sla1-GFP in the indicated strains. (c) Internalization of 35S-labeled α-factor was performed on the indicated strains. Error bars represent the SD from at least three experiments.
Next, we examined the contribution of Ste2p phosphorylation to A594-α-factor recruitment to CCPs. Compared with wild-type cells, little colocalization of A594-α-factor spots with Sla1p patches was observed either at 0 min (∼13.5%) or at 20 min (∼28.8%) in the ste2-6SA mutant (Figure 2d, top; and e). Conversely, recruitment of A594-α-factor was restored in cells expressing Ste2p-6SD (Figure 2d, bottom; and e). These results support of the conclusion that phosphorylation of Ste2p receptor is important for recruitment of A594-α-factor to CCPs.
Proper Localization and Activity of Yck2p Are Required for Ligand-induced Receptor Recruitment to CCPs
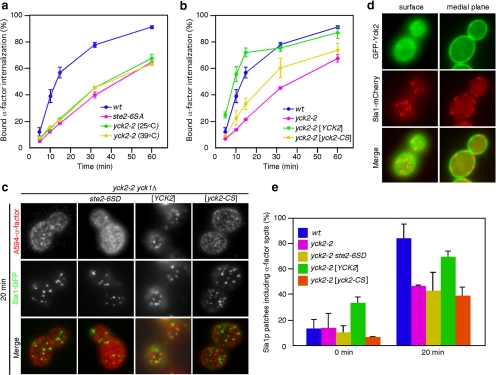
Next, we examined the involvement of Yck2p in A594-α-factor recruitment to CCPs. For this purpose we used yck1Δyck2-2 cells, which lack the YCK1 gene and carry temperature-sensitive alleles of the functionally redundant YCK2 gene (Panek et al., 1997). Although an earlier study reported that yck1Δyck2-2 cells failed to internalize 35S-labeled α-factor at 37°C (Hicke et al., 1998), we observed slow internalization of 35S-labeled α-factor by these cells at both 25 and 39°C, similar to the behavior of ste2-6SA cells (Figure 3a). This observation is plausible given the expected similarity in the phosphorylation state of Ste2p in yck1Δ yck2-2 cells and ste2-6SA cells, because Ste2p-6SA is hardly phosphorylated by Yck2 (Figure 2b). In yck1Δ yck2-2 cells treated with LatA, colocalization of A594-α-factor spots with Sla1p patches was reduced at 20 min (∼47.4%), confirming the requirement for Yck2p (and Yck1p) in Ste2p recruitment to CCPs (Figure 3c, left; and e). We also found that the recruitment of A594-α-factor to CCPs was not restored in yck1Δ yck2-2 cells expressing Ste2p-6SD (∼43.4%) (Figure 3, c and e). This result suggests that additional phosphorylation on Ste2p-6SD may be required for efficient recruitment of Ste2p to CCPs. Yck2p localizes at the plasma membrane through its palmitoylated C-terminal Cys residues (Roth et al., 2002). We found that GFP-Yck2 localized as faint spots on the plasma membrane and that the spots are highly motile (Figure 3d). In addition, GFP-Yck2p did not colocalize with Sla1p patches (Figure 3d), suggesting that phosphorylation of Ste2p by Yck2p occurs before Ste2p recruitment to CCPs. To further clarify the requirement for localization of Yck2p on the plasma membrane for Ste2p recruitment, we examined 35S-labeled α-factor internalization in yck1Δyck2-2 cells expressing yck2-C545,546S (yck2-CS), in which the C-terminal palmitoylated cysteine has been replaced by serine. Unlike yck1Δyck2-2 cells expressing Yck2p, yck-CS cells showed defects in 35S-labeled α-factor internalization (Figure 3b) and A594-α-factor recruitment (Figure 3, c and e). This observation supports the conclusion that proper localization of Yck2p at the plasma membrane is required for efficient phosphorylation and recruitment of ligand-bound Ste2p receptor to CCPs.
Figure 3.
Plasma membrane localization of functional Yck2p is required for the recruitment of α-factor to CCPs. (a) Internalization of 35S-labeled α-factor was measured in wild-type, ste2-6SA, and yck1Δ yck2-2 strains. yck1Δ yck2-2 strains were preincubated for 1 h at 25 or 39°C before the assay. (b) Internalization of 35S-labeled α-factor was measured in wild-type, yck1Δ yck2-2, or yck1Δ yck2-2 strains expressing wild-type Yck2p or Yck2p-C545,546S (Yck2-CS) at 25°C. (c) Localization of A594-α-factor and Sla1-GFP in yck1Δ yck2-2 cells, or yck1Δ yck2-2 cells expressing Ste2p-6SD, Yck2p, or Yck2p-CS, treated with LatA. The images were acquired at 20 min after washing out unbound Alexa-α-factor and warming the cells to 25°C. (d) Localization of GFP-Yck2 and Sla1-mCherry visualized at a surface (left) or medial focal plane (right) of wild-type cells. (e) Quantification of colocalization of A594-α-factor and Sla1-GFP in yck1Δ yck2-2 cells, or yck1Δ yck2-2 cells expressing Ste2p-6SD, Yck2p, or Yck2p-CS. Error bars represent the SD from at least three experiments.
Ubiquitination of Ste2p Receptor Is Critical for Its Ligand-induced Recruitment
Next, we used ste2-7KR cells to investigate the role of ubiquitination in Ste2p recruitment to CCPs. In ste2-7KR cells, colocalization of A594-α-factor spots with Sla1p patches was rarely observed at 20 min (Figure 4a, top). As shown in Figure 4b, whereas ∼84.3% of A594-α-factor spots colocalized with Sla1p patches in wild-type cells at 20 min, a significant reduction of colocalization of A594-α-factor spots with Sla1p patches was observed in ste2-7KR cells (∼15.6%). This result indicates that ubiquitination is indispensable for Ste2p recruitment to CCPs. To test the conclusion that the inability of the ste2-6SA mutant to be ubiquitinated accounts for the defect in accumulation of α-factor-bound receptor, we prepared the ste2-6SA-UBI mutant in which ubiquitin is fused to the C terminus of Ste2p-6SA. Interestingly, marked colocalization of A594-α-factor spots with Sla1p patches was observed even at 0 min (∼57.3%) in ste2-6SA-UBI cells (Figure 4a, middle; and b). These results demonstrate that ubiquitinated receptor can be recruited to CCPs without phosphorylation. Furthermore, the fusion of ubiquitin to Ste2p-6SA in the ste2-6SA-UBI cells also restored the ability of ste2-6SA cells to internalize 35S-labeled α-factor (Figure 4c). To further confirm the contribution of phosphorylation and ubiquitination to A594-α-factor recruitment, we also prepared the ste2-6SD-7KR mutant. As expected, neither accumulation of A594-α-factor on Sla1p patches nor internalization of 35S-labeled α-factor was observed in the ste2-6SD-7KR mutant (Figure 4a, bottom; b and c). These results clearly indicate that ubiquitination, rather than phosphorylation, of Ste2p is critical for recruitment of A594-α-factor to CCPs.
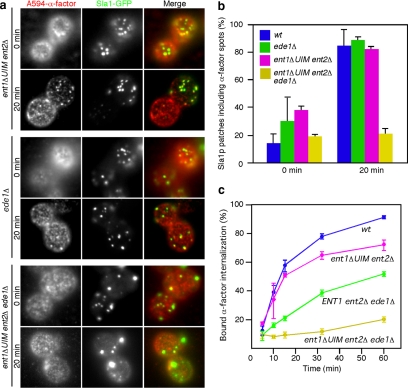
Ent1p UIM and Ede1p Work Cooperatively to Recruit Ubiquitinated Ste2p to CCPs
We next sought to identify protein(s) that mediate the recruitment of ubiquitinated receptor to CCPs. A previous study reported that Ent1p (yeast Epsin) and Ede1p (yeast Eps15) contain ubiquitin-binding domains that directly interact with monoubiquitin and function in receptor internalization (Shih et al., 2002). The ent1ΔUIM ent2Δ ede1Δ strain was shown previously to be unable to internalize α-factor (Shih et al., 2002). We therefore examined the behavior of A594-α-factor in ent1ΔUIM ent2Δ, ede1Δ or ent1ΔUIM ent2Δ ede1Δ strains. LatA treatment of ent1ΔUIM ent2Δ or ede1Δ mutants resulted in clear colocalization of A594-α-factor spots with Sla1p patches at 20 min (∼81.8% and ∼88.2%, respectively) (Figure 5a, top and middle; and b). In contrast, colocalization of A594-α-factor with Sla1p patches was significantly decreased in the ent1ΔUIM ent2Δ ede1Δ strain at 20 min (∼21.1%) (Fig. 5a, bottom; and b). This result indicates that Ent1p, Ent2p, and Ede1p participate in recruitment of Ste2p to CCPs. Consistent with this conclusion, the ent1ΔUIM ent2Δ ede1Δ mutant showed a significant defect in 35S-labeled α-factor internalization, whereas the ent1ΔUIM ent2Δ and ENT1 ent2Δ ede1Δ mutants had only a slight defect (Figure 5c). These results suggest that the Ent1p UIM and Ede1p might work cooperatively to recruit ubiquitinated receptor to CCPs.
Figure 5.
Ent1p and Ede1p recruit Ste2p to endocytic sites. (a) Localization of A594-α-factor and Sla1-GFP in ent1ΔUIM ent2Δ, ede1Δ, or ent1ΔUIM ent2Δ ede1Δ cells treated with LatA. The images were acquired at 0 or 20 min after washing out unbound Alexa-α-factor and warming the cells to 25°C. (b) Quantification of colocalization of A594-α-factor and Sla1-GFP in the indicated strains. (c) Internalization of 35S-labeled α-factor was measured in the indicated strains. Error bars represent the SD from at least three experiments.
Ste2p Ubiquitination Is Required for Ligand-independent Receptor Recruitment to CCPs
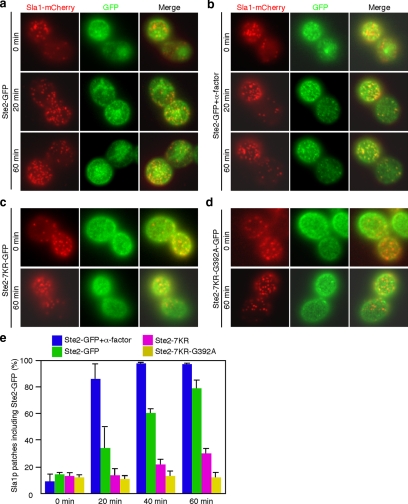
Ste2-GFP has been shown to localize to endocytic compartments in the absence of ligand when expressed in cells at steady state (Chang et al., 2005), suggesting that Ste2p may be constitutively endocytosed and transported to the vacuole in the absence of ligand, albeit at a low rate. We therefore examined how Ste2p is recruited to CCPs in the absence of ligand by imaging the behavior of Ste2-GFP on the plasma membrane. Treatment of cells expressing Ste2-GFP with LatA caused recruitment of Ste2-GFP to Sla1p-mCherry–labeled CCPs (Figure 6, a and e). This recruitment was much slower than ligand-stimulated recruitment (Figures 1b and 6a). Consistent with the results obtained with A594-α-factor, recruitment of Ste2-GFP was markedly facilitated at 20 min in the presence of unlabeled α-factor (Figure 6, b and e). In contrast, and intriguingly, Ste2p-7KR-GFP showed only ∼34.5% colocalization with Sla1p at 60 min (Figure 6, c and e). This result indicates that ligand-independent recruitment of Ste2p also requires ubiquitination of the cytoplasmic region.
Figure 6.
Ubiquitination, but not the GPFSD motif, is necessary for constitutive recruitment of Ste2p to CCPs. (a–d) Comparison of localization of Sla1-mCherry and Ste2-GFP (a and b), Ste2-7KR-GFP (c), or Ste2-7KR-G392A-GFP (d) in cells treated with LatA. The images were acquired at 0, 20, and 60 min after LatA treatment. (e) Quantification of colocalization of Sla1-mCherry and the above-mentioned GFP-fused proteins at the indicated times.
The NPFSD-related motif GPFSD, present in the C-terminal region of Ste2p (Figure 1a), has been identified as another endocytic targeting signal that works separately from the ubiquitin signal (Howard et al., 2002). Therefore, we examined the relevance of the GPFSD sequence to ligand-independent recruitment of Ste2p to CCPs. In ste2-G392A cells, in which the glycine in the GPFSD sequence is mutated to alanine, Ste2p is recruited to CCPs as effectively as in wild-type cells (data not shown). We further created Ste2-7KR-G392A-GFP, in which both the ubiquitination sites and the GPFSD sequence are mutated, and examined its colocalization with Sla1p patches. The colocalization of Ste2-7KR-G392A-GFP with Sla1p patches was slightly reduced compared with that of Ste2-7KR-GFP (Figure 6, d and e). Thus, we concluded that ubiquitination is primarily required for both ligand-dependent and ligand-independent Ste2p recruitment to CCPs.
DISCUSSION
Internalization of many GPCRs in both yeast and mammals is mediated by clathrin-dependent endocytosis (Kaksonen et al., 2006; Toshima et al., 2006; Marchese et al., 2008). Clathrin, adaptor proteins, and many other proteins coordinate assembly and invagination of CCPs, which are pinched off to form CCVs concomitant with a burst of actin polymerization (Merrifield, 2004; Kaksonen et al., 2006). In recent years, the budding yeast Saccharomyces cerevisiae has emerged as an important organism for studies of endocytic mechanisms because of its advantageous properties. First, there seems to be only one pathway for endocytic internalization, which greatly simplifies studies (Kaksonen et al., 2005). Second, yeast genetics has revealed in vivo functions for many endocytic proteins (Engqvist-Goldstein and Drubin, 2003; Kaksonen et al., 2003, 2005; Perrais and Merrifield, 2005). Third, real-time visualization of endocytic events is more straightforward than in more complex cell types. Using these properties of budding yeast, we dissected receptor endocytosis into a recruitment step and an internalization step, and succeeded in visualizing time-dependent constitutive and ligand-induced recruitment of GPCR to CCPs. On the basis of the data presented in this study and in previous studies, we propose the following model for GPCR internalization in budding yeast. First, clathrin and its adaptor proteins, such as Ede1p, appear at the plasma membrane and aid in initiating CCP assembly (Kaksonen et al., 2005; Toshima et al., 2006). Then, upon binding of α-factor, most Ste2p receptors are recruited to CCPs within 10 min in wild-type cells, whereas they are recruited much more slowly without ligand binding. Because α-factor-Ste2p complexes seem to move on the plasma membrane randomly and to become immobilized when they encounter CCPs (Toshima et al., 2006), binding of ligand could facilitate association of the receptor with CCPs by increasing their affinity for each other. A short sequence motif, GFPSD, which binds directly to the Sla1p homology domain 1 of Sla1p (Howard et al., 2002), might also participate in this step, but it seems to have only an auxiliary role. Activated Ste2p is rapidly phosphorylated by Yck1p and Yck2p, and this in turn mediates receptor ubiquitination. Both phosphorylation and ubiquitination are essential for receptor recruitment to CCPs, but ubiquitination is the primary requirement because Ste2-6SA-UBI, in which ubiquitin is fused to the C terminus of Ste2-6SA, an unphosphorylatable form of Ste2p, is efficiently recruited to CCPs. The clathrin adaptors Ent1p and Ent2p (yeast Epsin homologues) and Ede1p (a yeast Eps15 homologue), work cooperatively to bind and immobilize randomly moving Ste2p at CCPs, which finally internalize the receptors into CCVs.
Yeast do not contain homologues of the GRKs that phosphorylate many mammalian GPCRs. Although yeast casein kinases have previously been suggested to phosphorylate Ste2p (Hicke et al., 1998), direct phosphorylation by these kinases had not been demonstrated. A recent study in mammalian cells demonstrated that casein kinases 1 and 2 are able to phosphorylate the M3-muscarinic receptor, indicating a role for the casein kinase family in GPCR phosphorylation (Tobin, 2008). Here, we showed that Ste2p is directly phosphorylated by Yck2p, and we demonstrated a specific role for yeast casein kinases in Ste2p association with CCPs during receptor down-regulation. Interestingly, we found that Yck2p is present as highly motile spots on the plasma membrane, supporting our conclusion that phosphorylation of Ste2p occurs before its recruitment to CCPs. How does Yck2p associate with Ste2p and regulate Ste2p phosphorylation after ligand binding? It is widely believed that agonist binding induces a conformational change in GPCRs, which allows these receptors to associate with G proteins (Tobin, 2008). In the case of β2-adrenoceptor, agonist binding results in a change in transmembrane α-helices III and VI, which causes a reorientation of the third intracellular loop in a manner that allows for G protein coupling (Kobilka, 2002). This agonist-induced conformational change is also thought to unmask sites on the intracellular domains that can be modified by phosphorylation (Pitcher et al., 1998). Binding of α-factor to Ste2p is also reported to cause a conformational change of the third intracellular loop and the C-terminal cytoplasmic domain of the receptor (Bukusoglu and Jenness, 1996), suggesting that these conformational changes might permit Yck2p to associate with and phosphorylate Ste2p.
Although the NPFXD-like motif has been reported to be important for constitutive internalization of Wsc1p (Tan et al., 1996; Howard et al., 2002; Piao et al., 2007), whether this sequence plays a critical role in Ste2p is not clear, because the effect of mutating the sequence (GPFSD) in Ste2p was not apparent unless ubiquitination sites were removed (Tan et al., 1996; Howard et al., 2002; Piao et al., 2007). In this study, we also observed that the Ste2-G392A mutant has no effect on its ligand-independent recruitment to CCPs and that the Ste2-7KR-G392A mutant showed only a slightly enhanced defect. Thus, it seems that the GPFSD sequence is not essential for constitutive recruitment of Ste2p to CCPs. In contrast, ubiquitination seems to play an important role in both constitutive and ligand-induced recruitment of Ste2p. Several previous studies support this idea. First, studies using yeast strains that lack specific ubiquitin-conjugating enzymes or that contain ubiquitination-defective Ste2 mutants or chimeras indicate that monoubiquitination is necessary for constitutive and ligand-induced receptor internalization (Hicke and Riezman, 1996; Terrell et al., 1998). Second, Ste3p, another pheromone receptor in budding yeast, has also been shown to be phosphorylated and ubiquitinated, and both of these modifications are required for constitutive and ligand-induced internalization (Roth and Davis, 1996; Panek et al., 1997). These observations, together with our results, provide evidence for the importance of ubiquitination for both ligand-dependent and ligand-independent recruitment of Ste2p to CCPs. These results also could explain the previous observation that Ste2p is endocytosed 5- to 10-fold more rapidly after binding of α-factor compared with constitutive internalization (Jenness and Spatrick, 1986). A low level of Ste2p ubiquitination mediates slow constitutive receptor recruitment to CCPs. On ligand binding, the recruitment is facilitated by increased levels of receptor ubiquitination. Whether ubiquitination is required only for receptor recruitment to CCPs, or whether it is also required for receptor internalization, is the next important question.
Why receptor phosphorylation induced by the ligand is required for ubiquitination is not yet fully understood in yeast. For many mammalian GPCRs, phosphorylation of the C-terminal region of the GPCR triggers arrestin binding. Arrestin 2 (β-arrestin 1) and arrestin 3 (β-arrestin 2) are proteins that are well established to function in regulation of GPCR desensitization and internalization. These arrestins bind activated and phosphorylated GPCRs, which promotes receptor uncoupling from G proteins and internalization through CCPs (Kohout and Lefkowitz, 2003; Lefkowitz and Shenoy, 2005; Marchese et al., 2008; Tobin, 2008). A conformational change in arrestin induced by binding to GPCRs exposes C-terminal domains that interact with clathrin and/or the β2-adaptin subunit of the clathrin adaptor AP-2 complex, resulting in GPCR internalization (Goodman et al., 1996). Although it was believed previously that yeast do not have arrestin proteins, recent work has identified a family of arrestin-related proteins (ARTs) in budding yeast that target specific plasma membrane proteins for endocytic internalization (Lin et al., 2008). In addition to their similarity to arrestins, these ARTs contain multiple PY motifs that are required for recruitment of Rsp5p, a Nedd4-like ubiquitin ligase, which ubiquitinates many yeast endocytic cargo proteins, including Ste2p (Dunn and Hicke, 2001). Together, phosphorylation of Ste2p might be required for association with these arrestin-related proteins that in turn recruit Rsp5p, thereby ubiquitinating Ste2p. The existence and details of this putative process need to be investigated. F-box proteins, which are components of the SCF (Skp1p-cullin-F-box proteins) complex, are also candidates to regulate phospho-dependent Ste2p ubiquitination. Generally, the targets of F-box proteins are first phosphorylated before being recognized and ubiquitinated by the SCF complex (Skowyra et al., 1997). In budding yeast, ∼20 F-box proteins were identified and 13 of them were found to bind Skp1 (Skowyra et al., 1997). Although no interaction between an F-box protein and Ste2p has been reported, we cannot exclude this possibility because the functions of some of these proteins are not well characterized.
In conclusion, we have demonstrated that the yeast G protein-coupled Ste2p receptor is recruited to preexisting CCPs by its phosphorylation and subsequent ubiquitination. The clathrin adaptors Ent1p, Ent2p, and Ede1p seem to work cooperatively to recruit ubiquitinated Ste2p. Mammalian GPCRs, such as thyrotropin-releasing hormone receptor-1 and β1-adrenergic receptor, have been shown to be recruited to CCPs in a similar manner (Scott et al., 2002; Puthenveedu and von Zastrow, 2006). Furthermore, a recent study using electron microscope analysis showed that small interfering RNA knockdown of Epsin1 inhibited epidermal growth factor-induced recruitment of the epidermal growth factor receptor (EGFR) to CCPs, suggesting a possible role for Epsin in recruitment of the ubiquitinated EGFR into CCPs (Kazazic et al., 2009). Epsin and Eps15 interact with each other and are likely to function cooperatively. Therefore, yeast and mammals seem to share similar mechanisms for GPCR recruitment. Knowledge of this mechanism could also explain more clearly how GPCR internalization is facilitated upon binding of its ligand.
ACKNOWLEDGMENTS
We thank Gregory S. Payne (University of California, Los Angeles) for the ste2 strains, Linda Hicke (Northwestern University) for the ent1 and ede1 strains, Lucy C. Robinson (Louisiana State University) for the yck2 strains and plasmids, and Jeremy Thorner for helpful advice. We also thank the members of the Drubin/Barnes laboratories and Mizuno laboratory for sharing materials and for helpful discussions. This work was supported by the Novartis Foundation (Japan), the Mitsubishi foundation, the Naito Foundation, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research (to J. T.); the Japan Society for the promotion of Science (to J.Y.T.); and National Institutes of Health grant GM-R0150399 (to D.G.D.).
Abbreviations used:
- CCV
clathrin-coated vesicle
- CCP
clathrin-coated pit
- LatA
latrunculin A
- GPCR
G protein-coupled receptor
- GFP
green fluorescent protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-07-0541) on October 14, 2009.
REFERENCES
- Bukusoglu G., Jenness D. D. Agonist-specific conformational changes in the yeast alpha-factor pheromone receptor. Mol. Cell. Biol. 1996;16:4818–4823. doi: 10.1128/mcb.16.9.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F. S., Han G. S., Carman G. M., Blumer K. J. A WASp-binding type II phosphatidylinositol 4-kinase required for actin polymerization-driven endosome motility. J. Cell Biol. 2005;171:133–142. doi: 10.1083/jcb.200501086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsam R. T., Gutkind J. S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- Dunn R., Hicke L. Domains of the Rsp5 ubiquitin-protein ligase required for receptor-mediated and fluid-phase endocytosis. Mol. Biol. Cell. 2001;12:421–435. doi: 10.1091/mbc.12.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M., Boll W., Van Oijen A., Hariharan R., Chandran K., Nibert M. L., Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E., Drubin D. G. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- Feng Y., Davis N. G. Akr1p and the type I casein kinases act prior to the ubiquitination step of yeast endocytosis: Akr1p is required for kinase localization to the plasma membrane. Mol. Cell. Biol. 2000;20:5350–5359. doi: 10.1128/mcb.20.14.5350-5359.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotow H., Graves P. R., Wang A. Q., Fiol C. J., Roeske R. W., Roach P. J. Phosphate groups as substrate determinants for casein kinase I action. J. Biol. Chem. 1990;265:14264–14269. [PubMed] [Google Scholar]
- Flower D. R. Modelling G-protein-coupled receptors for drug design. Biochim. Biophys. Acta. 1999;1422:207–234. doi: 10.1016/s0304-4157(99)00006-4. [DOI] [PubMed] [Google Scholar]
- Fredriksson R., Lagerstrom M. C., Lundin L. G., Schioth H. B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Goodman O. B., Jr, Krupnick J. G., Santini F., Gurevich V. V., Penn R. B., Gagnon A. W., Keen J. H., Benovic J. L. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Hicke L., Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- Hicke L., Zanolari B., Riezman H. Cytoplasmic tail phosphorylation of the alpha-factor receptor is required for its ubiquitination and internalization. J. Cell Biol. 1998;141:349–358. doi: 10.1083/jcb.141.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. P., Hutton J. L., Olson J. M., Payne G. S. Sla1p serves as the targeting signal recognition factor for NPFX(1,2)D-mediated endocytosis. J. Cell Biol. 2002;157:315–326. doi: 10.1083/jcb.200110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness D. D., Spatrick P. Down regulation of the alpha-factor pheromone receptor in S. cerevisiae. Cell. 1986;46:345–353. doi: 10.1016/0092-8674(86)90655-0. [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Sun Y., Drubin D. G. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–487. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–320. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- Kazazic M., Bertelsen V., Pedersen K. W., Vuong T. T., Grandal M. V., Rodland M. S., Traub L. M., Stang E., Madshus I. H. Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic. 2009;10:235–245. doi: 10.1111/j.1600-0854.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- Knippschild U., Gocht A., Wolff S., Huber N., Lohler J., Stoter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K. Agonist-induced conformational changes in the beta2 adrenergic receptor. J. Pept. Res. 2002;60:317–321. doi: 10.1034/j.1399-3011.2002.21062.x. [DOI] [PubMed] [Google Scholar]
- Kohout T. A., Lefkowitz R. J. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol. Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- Lagerstrom M. C., Schioth H. B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- Lander E. S., et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Shenoy S. K. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Lin C. H., MacGurn J. A., Chu T., Stefan C. J., Emr S. D. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135:714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Marchese A., Paing M. M., Temple B. R., Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield C. J. Seeing is believing: imaging actin dynamics at single sites of endocytosis. Trends Cell Biol. 2004;14:352–358. doi: 10.1016/j.tcb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Panek H. R., Stepp J. D., Engle H. M., Marks K. M., Tan P. K., Lemmon S. K., Robinson L. C. Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J. 1997;16:4194–4204. doi: 10.1093/emboj/16.14.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D., Merrifield C. J. Dynamics of endocytic vesicle creation. Dev. Cell. 2005;9:581–592. doi: 10.1016/j.devcel.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Piao H. L., Machado I. M., Payne G. S. NPFXD-mediated endocytosis is required for polarity and function of a yeast cell wall stress sensor. Mol. Biol. Cell. 2007;18:57–65. doi: 10.1091/mbc.E06-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher J. A., Freedman N. J., Lefkowitz R. J. G protein-coupled receptor kinases. Annu. Rev. Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Puthenveedu M. A., von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Ribas C., Penela P., Murga C., Salcedo A., Garcia-Hoz C., Jurado-Pueyo M., Aymerich I., Mayor F., Jr The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim. Biophys. Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Rodal A. A., Manning A. L., Goode B. L., Drubin D. G. Negative regulation of yeast WASp by two SH3 domain-containing proteins. Curr. Biol. 2003;13:1000–1008. doi: 10.1016/s0960-9822(03)00383-x. [DOI] [PubMed] [Google Scholar]
- Roth A. F., Davis N. G. Ubiquitination of the yeast a-factor receptor. J. Cell Biol. 1996;134:661–674. doi: 10.1083/jcb.134.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A. F., Feng Y., Chen L., Davis N. G. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 2002;159:23–28. doi: 10.1083/jcb.200206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini F., Gaidarov I., Keen J. H. G protein-coupled receptor/arrestin3 modulation of the endocytic machinery. J. Cell Biol. 2002;156:665–676. doi: 10.1083/jcb.200110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. G., Benmerah A., Muntaner O., Marullo S. Recruitment of activated G protein-coupled receptors to pre-existing clathrin-coated pits in living cells. J. Biol. Chem. 2002;277:3552–3559. doi: 10.1074/jbc.M106586200. [DOI] [PubMed] [Google Scholar]
- Shih S. C., Katzmann D. J., Schnell J. D., Sutanto M., Emr S. D., Hicke L. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 2002;4:389–393. doi: 10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- Skowyra D., Craig K. L., Tyers M., Elledge S. J., Harper J. W. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- Takeda S., Kadowaki S., Haga T., Takaesu H., Mitaku S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. doi: 10.1016/s0014-5793(02)02775-8. [DOI] [PubMed] [Google Scholar]
- Tan P. K., Howard J. P., Payne G. S. The sequence NPFXD defines a new class of endocytosis signal in Saccharomyces cerevisiae. J. Cell Biol. 1996;135:1789–1800. doi: 10.1083/jcb.135.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell J., Shih S., Dunn R., Hicke L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol. Cell. 1998;1:193–202. doi: 10.1016/s1097-2765(00)80020-9. [DOI] [PubMed] [Google Scholar]
- Tobin A. B. G-protein-coupled receptor phosphorylation: where, when and by whom. Br. J. Pharmacol. 2008;153(suppl 1):S167–S176. doi: 10.1038/sj.bjp.0707662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima J., Toshima J. Y., Amano T., Yang N., Narumiya S., Mizuno K. Cofilin phosphorylation by protein kinase testicular protein kinase 1 and its role in integrin-mediated actin reorganization and focal adhesion formation. Mol. Biol. Cell. 2001;12:1131–1145. doi: 10.1091/mbc.12.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima J., Toshima J. Y., Martin A. C., Drubin D. G. Phosphoregulation of Arp2/3-dependent actin assembly during receptor-mediated endocytosis. Nat. Cell Biol. 2005;7:246–254. doi: 10.1038/ncb1229. [DOI] [PubMed] [Google Scholar]
- Toshima J. Y., Toshima J., Kaksonen M., Martin A. C., King D. S., Drubin D. G. Spatial dynamics of receptor-mediated endocytic trafficking in budding yeast revealed by using fluorescent alpha-factor derivatives. Proc. Natl. Acad. Sci. USA. 2006;103:5793–5798. doi: 10.1073/pnas.0601042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter J. C., et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Zanolari B., Raths S., Singer-Kruger B., Riezman H. Yeast pheromone receptor endocytosis and hyperphosphorylation are independent of G protein-mediated signal transduction. Cell. 1992;71:755–763. doi: 10.1016/0092-8674(92)90552-n. [DOI] [PubMed] [Google Scholar]