Abstract
Bone morphogenetic proteins (BMPs) have been implicated in the generation and postnatal differentiation of cerebellar granule cells (CGCs). Here, we examined the eventual role of BMPs on the survival of these neurons. Lack of depolarization causes CGC death by apoptosis in vivo, a phenomenon that is mimicked in vitro by deprivation of high potassium in cultured CGCs. We have found that BMP-6, but not BMP-7, is able to block low potassium–mediated apoptosis in CGCs. The neuroprotective effect of BMP-6 is not accompanied by an increase of Smad translocation to the nucleus, suggesting that the canonical pathway is not involved. By contrast, activation of the MEK/ERK/CREB pathway by BMP-6 is necessary for its neuroprotective effect, which involves inhibition of caspase activity and an increase in Bcl-2 protein levels. Other pathways involved in the regulation of CGC survival, such as the c-Jun terminal kinase and the phosphatidylinositol 3-kinase (PI3K)-Akt/PKB, were not affected by BMP-6. Moreover, failure of BMP-7 to activate the MEK/ERK/CREB pathway could explain its inability to protect CGCs from low potassium–mediated apoptosis. Thus, this study demonstrates that BMP-6 acting through the noncanonical MEK/ERK/CREB pathway plays a crucial role on CGC survival.
INTRODUCTION
Cerebellar granule cells (CGCs) are generated in the external granule layer and migrate to the internal granule layer (Ryder and Cepko, 1994). During their postnatal migration, CGCs require excitatory inputs for proper differentiation and development. Otherwise, CGCs die by apoptosis (Burgoyne and Cambray-Deakin, 1988; Wood et al., 1993). This situation can be mimicked in vitro in primary cultures of CGCs. These neurons undergo spontaneous apoptosis when they grow in the presence of low potassium concentration (5 mM KCl [K5]). By contrast, if they grow in the presence of high potassium concentrations (25 mM KCl [K25]) or N-methyl-d-aspartate (NMDA), they develop and survive (Gallo et al., 1987; Xifro et al., 2005).
BMPs have been described to have an important role during differentiation of CGCs. For instance, BMP-2 and -4 are able to prevent Shh-induced proliferation, thereby allowing granule neuron differentiation (Rios et al., 2004). Expression of granule cell markers such as math1 or Zic has been reported to be controlled by BMPs (Aruga et al., 1994; Ben Arie et al., 1997). Accordingly, Alder et al. (1999) have demonstrated that exposure of neural cells to BMPs induces CGC phenotype, whereas CGC differentiation is greatly impaired in BMP receptors conditional knockout mice (Qin et al., 2006). Moreover, Smad 1 and BMP-4 expression and protein levels peak during CGC differentiation and migration toward the internal granule cell layer (IGL; Angley et al., 2003). Besides its role in CGC differentiation, several reports have suggested that BMPs have an antiapoptotic effect in many cell types (Izumi et al., 2001; Wang et al., 2001; Harvey et al., 2004), which opens the possibility that they could be also involved on regulation of CGC survival (Yabe et al., 2002). Moreover, the eventual antiapoptotic effects of BMPs in the developing cerebellum and the mechanisms involved remain largely unknown.
BMPs signals via two types of receptors (type I, BMPRI; type II, BMPRII) that are expressed as homomeric as well as heteromeric complexes (ten Dijke et al., 1994; Massague, 1998). When BMPRs are activated by BMPs, intracellular signaling is mainly triggered by phosphorylation of the receptor-regulated Smad (R-Smad; Smad 1, 5, or 8) and their subsequent binding to Smad 4. The heterotrimer R/R-Smad/Smad 4 then translocates to the nucleus where it activates the expression of specific genes (Miyazono, 1999). Some reports have suggested that BMPs can also activate other intracellular pathways to exert their cellular functions. For instance, BMP-2 induce the differentiation of osteoblasts by activating the PI3K/Akt pathway (Ghosh-Choudhury et al., 2002). BMP-7 activates the extracellular signal–regulated kinase kinase/extracellular signal–regulated kinases (MEK/ERK) pathway and protein kinase C (PKC) in cortical neurons in vitro (Cox et al., 2004). On the other hand, controversial studies exists about the activation of stress-activated kinases (SAPK) by BMPs in different cell types (Izumi et al., 2001; Hallahan et al., 2003; Lemonnier et al., 2004).
In this study, we show that BMP-6 protects against K5-induced CGC apoptosis through inhibition of caspase-3 activation and elevation of B-cell leukemia/lymphoma-2 (Bcl-2) protein levels, which suggests a novel role for BMPs in developing cerebellum. In addition, we provide strong evidence that this effect is independent of the traditional BMPs signaling pathway associated to Smad activation. Our results show for the first time that BMP-6–mediated antiapoptotic effect is mediated through activation of the MEK/ERK/cAMP-response element-binding protein (CREB) pathway.
MATERIALS AND METHODS
Cell Culture
Granule cell cultures were prepared from dissociated cerebella of 8-d-old Wistar rats as previously described (Balazs et al., 1988). Cells were plated (3 × 103 cells/mm2) in DMEM with GlutaMAX (Invitrogen, Carlsbad, CA) and 25 mM KCl (K25) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 25,000 U penicillin, and 25 mg streptomycin (PAN Biotech, Aidenbach, Germany). Cytosine-β-d-arabinofuranoside, 10 μM, was added to the cultures 24 h after plating to prevent proliferation of nonneuronal cells. Neurons were plated onto poly-l-lysine–coated 24-well plates for measurement of cell viability and immunocytochemistry, on 60-mm culture dishes for RNA extraction and Western blotting, on six-well plates for measurement of caspases-3 activity, and on 100-mm plates for subcellular fractionation. At 6 d in vitro (DIV) neurons were deprived from serum and/or potassium (K5 or K25) and cultured in the absence or presence of BMP-6 (100 ng/ml) or BMP-7 (30 ng/ml; Sigma-Aldrich, St. Louis, MO). In some experiments, cells were preincubated with the MEK inhibitors PD98059 or U0126 (Calbiochem, La Jolla, CA) before replacing the medium. The experimental procedures were performed in accordance with guidelines of the Comissió d'Ètica en l'Experimentació Animal i Humana of the Universitat Autònoma de Barcelona.
Fluorescent Analysis of Apoptotic Nuclei
Apoptosis was assessed by nuclear DNA staining with Hoechst 33258. Twenty-four hours after treatment, cells were washed twice in Tris-buffered saline (TBS) fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 45 min at 4°C and stained with 1 μg/ml Hoechst 33258 (Molecular Probes, Eugene, OR) for 5 min. Nuclear DNA staining was observed with an inverted microscope (Eclipse TE-2000-E; Nikon, Melville, NY). At least 1000 cells were individually examined for each experimental condition. Condensed and/or fragmented nuclei were considered as apoptotic nuclei. Data are given as mean ± SEM of values obtained in three or four independent experiments performed in duplicate. Results are expressed as the percentage of apoptotic nuclei versus total nuclei.
Caspase Activity
Cells were incubated for 10 h in K5 in the presence or absence of BMP-6 (100 ng/ml), BMP-7 (30 ng/ml), staurosporine (1 μM), or K25. Cells were collected and pelleted by centrifugation at 1000 rpm for 3 min at 4°C. The supernatant was discarded, and cells were washed with PBS at 4°C and centrifuged at 12,300 rpm at 4°C for 3 min. Caspase activity was determined following the protocol of ENZCHEK Caspase-3 Assay Kit (Molecular Probes). Fluorescence intensity was measured with a microplate reader (Synergy HT; BioTek Instruments, Burlington, VT). Data are mean ± SEM of the values obtained in four independent experiments performed in duplicate.
Immunocytochemistry
Neurons were incubated in K5 or K25 in the absence or presence of BMP-6 (100 ng/ml) or BMP-7 (30 ng/ml) for 1 h (for Smad localization) or 10 h (active caspase 3 fragment). Then, CGC cultures were washed with PBS and fixed with 4% paraformaldehyde (wt/vol in PBS) at 4°C for 45 min. Afterward, they were washed twice with PBS, blocked with 5% fetal calf serum and 3% bovine serum albumin (BSA) in TBS (blocking buffer), and then incubated overnight at 4°C with antibodies against the active form of caspase-3 (Cell Signaling, Beverly, MA) or Smad 1, Smad 4 (Upstate Biotechnology, Lake Placid, NY), or Smad 5 (Santa Cruz Biotechnology, Santa Cruz, CA; 1:100 dilution in 5% BSA in TBS with 0.1% Tween 20 [TBS/T]). Cultures were then thoroughly washed with TBS/T and incubated with a fluorescein isothiocyanate–conjugated secondary antibody (1:500 dilution) in 5% BSA-TBS/T at room temperature for 1 h. After several washes, fluorescence was visualized under an inverted microscope for cleaved caspases-3 staining or confocal microscope (TCS 4D; Leica, Deerfield, IL) to determine Smad subcellular localization. Three independent experiments were performed in duplicate.
Subcellular Fractionation
CGCs were exposed to a low potassium medium (K5) and with or without BMP-6 (100 ng/ml), BMP-7 (30 ng/ml), or K25 for 1 h. Neurons were washed once with PBS at 4°C, resuspended in PBS, and centrifuged at 1000 rpm at 4°C for 5 min. Pelleted cells were resuspended in buffer containing 0.01 M HEPES, pH 7.9, 0.01 M MgCl2, 0.01 M KCl, 0.5 mM DTT, 0.1% NP-40, 1 mM PMSF, 10 μg/ml aprotinin, 20 μg/ml leupeptin, and 1 mM activated orthovanadate and incubated on ice for 5 min. Afterward, cells were centrifuged at 4000 rpm at 4°C for 30 s. The resulting supernatant was the cytosolic fraction. Pelleted nuclei were incubated at 300 rpm at 4°C for 30 min with buffer containing high salt (0.01 M HEPES, pH 7.9, 25% glycerol, 0.42 M NaCl, 0.2 mM EDTA, 0.5 mM DTT, 1 mM PMSF, 10 μg/ml aprotinin, 20 μg/ml leupeptin, and 1 mM activated orthovanadate) to isolate nuclear proteins. Then, samples were centrifuged at 12,300 rpm at 4°C for 15 min. The resulting supernatant was considered as the nuclear fraction. Proteins were stored at −80°C. Both subcellular fractions were subjected to Western blotting analysis.
Immunoblotting
CGC cultures were washed with PBS, and total protein was extracted by incubating neurons in lysis buffer containing 20 mM Tris, pH 7.5, 1% Nonidet P-40, 150 mM NaCl, 5 mM EDTA, 1 mM PMSF, 10 μg/ml aprotinin, 20 μg/ml leupeptin, and 1 mM activated orthovanadate. Cell lysates were centrifuged at 12,000 rpm for 10 min at 4°C. Proteins (25 μg for total lysates and 5 μg for subcellular fractions) were resolved on 10% SDS-PAGE gels and transferred to nitrocellulose membranes (Amersham Biosciences, Uppsala Sweden). Membranes were washed with TBS/T and incubated in blocking buffer for 1 h (5% of nonfat dry milk in TBS/T) to block nonspecific binding. Blots were washed and incubated at 4°C overnight with primary antibodies against BMPRIa, BMPRIb, Smad 5, c-jun N-terminal kinase (JNK), and histone H1 from Santa Cruz Biotechnology; BMPRII, tubulin, panAkt, Bcl-2, Ras, and Rap-1 from BD Biosciences (San Diego, CA; Smad 1 and 4 from Upstate Biotechnology; GAPDH from Ambion (Austin, TX); phospho Akt (Ser473), phospho-p44/42 MAP kinase and p44/42 MAPK, and phospho-CREB (Ser133) and CREB from Cell Signaling; active JNK from Promega (Madison, WI) and actin from Sigma Aldrich, diluted (1:1000 for all, except Smad 1 and Bcl-2 1:500, active JNK 1:2500, and GAPDH and actin 1:40,000) in blocking buffer. Blots were then washed and incubated at room temperature for 1 h with horseradish peroxidase–conjugated secondary antibodies (BD Biosciences) diluted 1:5000 in blocking buffer. Immunoreactive bands were visualized using the ECL Western blotting detection reagent (Amersham Biosciences) and quantified by a computer-assisted densitometer. Tubulin and actin were used as loading control, and GAPDH and histone H1 were used to assess the purity of cytosolic and nuclear fractions, respectively.
Reverse Transcription–Polymerase Chain Reaction
Total RNA was extracted from CGCs cultured in K25 every 2 DIV with Trizol (Invitrogen) accordingly to manufacturer's instructions. Total RNA, 1 μg, was converted to first-strand cDNA using the SuperScript II Reverse Transcriptase (Invitrogen) following the manufacturer's instructions. The resulting cDNA was subjected to PCR analysis. PCR cycling parameters were as follows: 94°C for 2 min for one cycle, followed by 94°C for 30 s, 60°C for 30 s, and 72°C for 60 s for 40 cycles; 72°C for 5 min with BMPRIa, BMPRIb, BMPRII, and 18s primers and 94°C for 2 min for one cycle, followed by 94°C for 30 s, 60°C for 30 s, and 72°C for 45 s for 35 cycles and 72°C for 5 min with Smad 1, 4, and 5 primers. The PCR products were stained with SYVR Safe (Invitrogen). A sample without RNA was used as negative control, and neonatal rat brain RNA was used as a positive control (results not shown). The sequences of specific primers are as follows: BMPRIa: (sense) 5′ CAG CTA CGC AGG ACA ATA GA 3′, (antisense) 5′ AGC TGA GTC CAG GAA CCA GT; BMPRIb: (sense) 5′ AAG TGT TCT TCA CCA CGG AG 3′, (antisense) 5′ AGG CCG TAA CTT CTT CAT GC 3′; BMPRII: (sense) 5′ GCT TCG CAG AAT CAA GAA CG 3′, (antisense) 5′ GTG GAC TGA GTG GTG TTG TG 3′. 18s: (sense) 5′ TCA AGA ACG AAA GTC GGA GG 3′, (antisense) 5′ GGA CAT CTA AGG GCA TCA CA 3′; Smad 1: (sense) 5′ GAA CTA GAC CAG CCG CTA TG 3′, (antisense) 5′ GTG GTG GTA GTT GCA GTT CC 3′; Smad 4: (sense) 5′ GTT CAG GTA GGA GAG ACC TT 3′, (antisense) 5′ TAA AGG CTG TGG GTC CGC AT 3′; and Smad 5: (sense) 5′ GCC AAG CAA GTG TGT CAC TA 3′, (antisense) 5′ TAG GCA ACA GGC TGA ACA TC.
Ras and Rap1 Activity Assay
Ras and Rap1 activation was determined by measuring p21ras.GTP and Rap1.GTP after a pulldown assay. p21ras-GTP and Rap1-GTP were fished with c-raf-RBD-glutathione S-transferase (GST) and Ral-GDS-GST, respectively (expression plasmids were kindly provided by Dr. Néstor Gómez, Universitat Autònoma de Barcelona, and Dr. Michel Fournier, Université d'Orléans) conjugated to glutathione-Sepharose beads (Amersham Pharmacia Biotech). Cell lysate, 400 μg, was incubated with 30 μl of 50% slurry suspension in binding buffer (20 mM Tris, pH 7.5, 1% NP-40, 200 mM NaCl, 2.5 mM MgCl2, 10 mM NaF, 10 μg/ml aprotinin, 20 μg/ml leupeptin, and 1 mM Na3VO4) at 4°C for 90 min. After incubation, the beads were washed, resuspended in sample buffer, and heated at 95°C for 5 min to release the coupled proteins. Samples were subjected to 12% SDS-PAGE electrophoresis, transferred to nitrocellulose membranes, and detected by immunoblotting using monoclonal anti-Ras (BD Biosciences; 1:1000) and monoclonal anti-Rap1 (BD Biosciences; 1:1000) antibodies.
Electrophoretic Mobility Shift Assay
Cells were processed as described for subcellular fractionation. CREB response element (CRE-like) consensus sequence was labeled with γ-32P using T4 polynucleotide kinase by following Promega′s protocol. Labeled oligonucleotides were purified with Sephadex G-25 column (Sigma-Aldrich). Nuclear extracts, 10 μg, were used for DNA protein-binding assay. Unspecific binding was blocked by using 1 μg of poly(dI·dc) (poly(deoxyinosinic-deoxycytidylic acid; Sigma-Aldrich). The binding medium contained 50 mM HEPES, pH 7.5, 5% glycerol, and 1 mM EDTA. In each reaction 20,000 cpm of radiolabeled probe was included. Samples were incubated for 20 min at room temperature. Bound and unbound probes were resolved on 6% polyacrylamide gels with a running buffer containing 45 mM Tris-borate, pH 8.3, and 1 mM EDTA. Radioactive signals were developed on Hyperfilm ECL films (Amersham Biosciences) after 1–2 d of exposure at 80°C.
Lentiviral Vectors
Constructs for RNA interference experiments were cloned into the pSUPER. retro.puro plasmid (OligoEngine, Seattle, WA). Specific oligonucleotides of the Bcl-2 sequence and the scrambled sequence as a control are as follows: shRNA Bcl-2: (forward) gatccccGAATCAAGTGTTCGTCATAttcaagagaTATGACGAACACTTGATTCttttt, (reverse) agctaaaaaGAATCAAGTGTTCGTCATAtctcttgaaTATGACGAACACTTGATTCggg; and shRNA scramble, which does not recognize any rat coding sequence: (forward) gatccccAGAACACGACGGAACAAGAttcaagagaTCTTGTTCCGTCGTGTTCTttttt, (reverse) agctaaaaaAGAACACGACGGAACAAGAtctcttgaaTCTTGTTCCGTCGTGTTCTggg. Oligonucleotides were obtained from Invitrogen and were cloned between BglII/HindIII sites of pSUPER.retro.puro plasmid. Lentiviral constructs were achieved by digesting EcoRI-ClaI sites from pSUPER-sh to replace H1 promoter with H1-short hairpin RNA (shRNA) cassette in pLVTHM.
Lentiviruses were propagated using methods described previously (Naldini et al., 1996; Zufferey et al., 1998). Briefly, human embryonic kidney 293T (HEK293T) cells were seeded at a density of 2.5 × 106 cells in 100-mm dishes. The next day, cells were transfected with 20 μg pLVTHM derived constructs, 15 μg pSPAX2, and 8 μg pM2G. The transfection was routinely performed by the calcium phosphate transfection method (Cullen, 1987). Cells were allowed to produce lentiviruses for 48 h. After 48 h, the medium was centrifuged at 1200 × g for 5 min, and the supernatant was concentrated at 141,000 × g for 120 min and then resuspended in 50 μl PBS containing 1% BSA. Lentiviruses were stored at −80°C. Biological titers of the viral preparations expressed as the number of transducing units per milliliter (TU/ml) were determined by transducing HEK293T cells in limiting dilutions. After 72 h of infection, the percentage of green fluorescent protein (GFP)-positive cells was determined by using a cytometer, and viruses at 1 × 106 TU/ml were used in the experiments.
Viral Infection
Herpes virus simplex (HSV) with green fluorescent protein (HSV-GFP) or mCREB (a dominant-negative mutant of CREB that lacks Ser 133; tagged with GFP [HSV-MCREBGFP]) were provided by Dr. Nestler (Mount Sinai School of Medicine, New York, NY) (Han et al., 2006). Cells were infected at 4 DIV with HSV-MCREB or HSV-GFP (control), and at 6 DIV cells were deprived from serum and/or potassium (K5 or K25) and treated with or without BMP-6 (100 ng/ml). Nuclei condensation and chromatin fragmentation was monitored at 24 h in neurons expressing GFP.
Lentiviral infection was performed at the day of plating. Cells remained in contact with the lentivirus for 5 h, and then the medium was replaced with DMEM with GlutaMAX (Invitrogen) containing K25 supplemented with 10% heat-inactivated FBS, 25,000 U penicillin, and 25 mg streptomycin. At 6 DIV treatments were performed as described above, and activation of caspase-3 and chromatin condensation was assessed at 10 and 24 h, respectively.
Statistical Analysis
Statistical significance was determined by one-way ANOVA followed by Tukey multiple comparison test. p < 0.05 was considered statistically significant.
RESULTS
BMP-6 Protects against K5-induced Apoptosis in Mature CGCs
Previous studies have described that BMPs are able to promote cell survival in the CNS (Iantosca et al., 1999; Izumi et al., 2001; Wang et al., 2001; Gratacos et al., 2002; Yabe et al., 2002; Chang et al., 2003). Before studying the eventual effect of BMP-6 on survival of cultured CGCs under proapoptotic conditions, we analyzed the expression of BMP receptors and Smad proteins at different days in vitro (DIV). Protein and mRNA for BMPR types Ia, Ib, and II were detected at all DIV in cultured CGCs (Supplemental Figure S1, A and B). When we examined the expression levels of R-Smads (Smad 1 and 5) and Smad 4, we found expression of those Smads at all DIV (Supplemental Figure S1A). We did not find any significant differences in protein levels of those smads when monitored by Western blot (Supplemental Figure S1B). These data suggest that cultured CGCs should be able to form active heterotetrameric receptor complexes capable of mediating BMPs signaling.
To address the eventual effect of BMPs on CGC survival in proapoptotic conditions (K5-containing medium), neurons were grown in K25, and medium was switched to low potassium conditions (K5) in the presence or absence of BMPs at 6 DIV. When cultures were treated with BMP-6, the number of condensed or fragmented nuclei was reduced by 45% (Figure 1). By contrast, no reduction of condensed nuclei was observed in cultures treated with BMP-7 or -2 (Figure 1 and data not shown).
Figure 1.
BMP-6 protects against low potassium–induced apoptosis in CGCs. Cerebellar granule cells cultures (6 DIV) were placed in serum-free medium containing 25 mM (K25) or 5 mM (K5) KCl and when indicated, BMP-6 (100 ng/ml) or BMP-7 (30 ng/ml). Chromatin condensation was assayed 24 h later by staining with Hoechst 33258. (A) Condensed or fragmented nuclei were counted and represented as percentage versus total nuclei. Results are the mean ± SEM from three independent experiments performed in triplicate. **p < 0.01 and *p < 0.05 versus K5. (B) Fluorescence photomicrographs of representative fields from 7 DIV cultures treated as indicated. Yellow arrows show condensed nuclei.
Because caspase-3 is involved in K5-mediated CGC apoptotic death (Armstrong et al., 1997; Moran et al., 1999; Xifro et al., 2006), we wondered whether the prosurvival effect of BMP-6 was due to inhibition of caspase-3 activity. To this aim, we determined the presence of active caspase-3 and the DEVDase activity in our cultures. As shown in Figure 2, the number of immunoreactive cells for active caspase-3 as well as DEVDase activity was increased in CGC cultures when medium was switched from K25 to K5. Treatment with BMP-6 significantly reduced both the number of immunoreactive cells for active caspase-3 (Figure 2, A and B) and caspase activity in the cultures (Figure 2C). By contrast, and in agreement with the absence of effects on chromatin condensation, the addition of BMP-7 neither reduced the number of immunoreactive cells for active caspase-3 (Figure 2, A and B) nor the K5-mediated caspase activation (Figure 2C).
Figure 2.
BMP-6 inhibits K5-induced caspase-3 activation. Cerebellar granule neurons (6 DIV) were placed in serum-free medium containing 25 mM (K25) or 5 mM (K5) KCl and when indicated, with BMP-6 (100 ng/ml) or BMP-7 (30 ng/ml). Activation of caspase-3 was assessed 10 h later by immunocytochemistry against its active fragment (A and B) and by an activity assay (C). Both approaches were performed in three independent experiments by triplicate. **p < 0.01 and *p < 0.05 versus K5.
Inhibition of Caspase Activity by BMPs Is Not Mediated by Nuclear Translocation of Smads
The effects of BMPs are thought to be canonically mediated by nuclear translocation of the complex Smad 4/Smad 1 or 5, which can act as coactivators or corepressors of gene transcription (Miyazono et al., 2005). Because we detected the presence of Smad 1, 4, and 5 proteins in our culture system (Supplemental Figure S1), we then tested whether smad translocation could be observed in mature CGCs after BMPs treatment. For this purpose, we analyzed by confocal microscopy the localization of Smad 1, 4, and 5 in CGCs 1 h after switching the medium to K5 containing vehicle or BMPs. We found the presence of Smad 1, 4, and 5 in the cytoplasm as well as in the nucleus of CGCs in all conditions tested (Figure 3A and Supplemental Figure S2, A and B). These results, which were confirmed by subcellular fractionation and Western blot analysis, revealed no significant differences on nuclear phospho-Smad 1 levels in the presence or absence of BMPs. Similar results were obtained for Smad 4 (Supplemental Figure S2C). Altogether, these data indicate that the antiapoptotic effect of BMP-6 is not due to an increase in the nuclear translocation of Smad proteins, suggesting the involvement of a noncanonical pathway.
Figure 3.
BMP associated Smads are localized in the cytoplasm as well as in the nucleus of cultured CGCs. Cerebellar granule cells cultures (6 DIV) were placed in serum-free medium containing 25 mM (K25) or 5 mM (K5) KCl, and BMP-6 (100 ng/ml) or BMP-7 (30 ng/ml). (A) After 1 h, cells were subjected to immunocytochemistry for Smad 1. Representative microphotographs of confocal microscope images are shown for each treatment. Hoechst staining was used to visualize cell nuclei (merge). Yellow and red arrows, cytoplasmic and nuclear localization, respectively. (B) Representative Western blots of phosphorylated and total Smad 1 in cytosolic (C) and nuclear (N) fractions. Histone H1 and GAPDH were used to monitor the purity of nuclear and cytosolic fractions, respectively. (C) Ratio between phosphorylated and total Smad 1 in the nuclear fraction. Results are the mean ± SEM of three independent experiments. No significant differences were observed.
BMP-6 Induces MEK/ERK Pathway Activation
Because PI3K/Akt has been described to be responsible for the inhibition of K5-mediated apoptosis of CGCs observed with K25, IGF-1, or NMDA (D'Mello et al., 1993; Subramaniam et al., 2003; Xifro et al., 2005), we decided to explore the role of this prosurvival pathway on the antiapoptotic effect of BMP-6 in CGCs. We examined the time course of Akt phosphorylation in CGCs when the medium was switched to K5 in the presence of vehicle or BMP-6. A reduction on Akt phosphorylation was observed in K5-cultured cells, which was not reverted by BMP-6 treatment (Figure 4A). These results suggest that the PI3K/Akt pathway is not involved in the BMP-6 neuroprotective effect in CGCs.
Figure 4.
BMP-6 treatment induces ERK phosphorylation. Cerebellar granule neurons (6 DIV) were switched to a serum free medium with low potassium (K5) and/or BMP-6 (100 ng/ml). Cell lysates were obtained at the indicated times after treatment and subjected to Western blot analysis with (A) phospho-Akt and Akt, (B) phospho-JNK and JNK, and (C) phospho-ERK and ERK antibodies. Results are the mean ± SEM of four independent experiments. Significant increase in ERK phosphorylation induced by BMP-6 treatment was observed at short times (5′ and 15′) and at 8 h. **p < 0. 01; +p < 0.05; ##p < 0.01 versus K5 at 5 and 15 min and 8 h, respectively.
Besides the activation of survival promoting signal pathways, blockade of K5-mediated apoptosis of CGCs have been also associated to inhibition of the JNKs. For instance, pharmacological or synaptic activity-dependent inhibition of JNKs activity is able to reduce K5-mediated apoptosis (Watson et al., 1998; Harris et al., 2002; Xifro et al., 2006). However, inhibition of JNKs activity does not seem to be involved in BMP-6 antiapoptotic effect. Western blot analysis of phospho-JNK showed that treatment with BMP-6 was not able to reduce K5-mediated phosphorylation of JNK (Figure 4B).
The regulation of the MEK/ERK pathway has a more controversial role on the survival of CGCs. Although the MEK/ERK pathway has been described to mediate survival in CGCs, it is still unclear whether K5-induced apoptosis of CGCs is associated to an increase or decrease of MEK/ERK activation (Lafon-Cazal et al., 2002; Subramaniam et al., 2005; Zhu et al., 2005). The activity of the MEK/ERK pathway can be estimated by determining the phosphorylation of ERK. CGCs showed a significant decrease in ERK phosphorylation the first 2 h after switching the medium to K5. BMP-6 was able to increase the phosphorylation of ERK beyond the levels observed in K25 cells. Inhibition of MEK with PD98059 returned pERK to K5 levels (Supplemental Figure S4). That increase was significantly different to the levels of phosphorylated ERK observed in K5 (Figure 4C). By contrast, the activation of MEK/ERK in K5-treated cells was not affected by BMP-7 (Supplemental Figure S3A).
MEK/ERK Pathway Mediates BMP-6 Neuroprotection in CGCs
Because BMP-6 activates the MEK/ERK pathway in CGCs, we next examined whether MEK/ERK activation was involved in its antiapoptotic effect. For this purpose we used a specific MEK inhibitor, PD98059. As shown in Figure 5, preincubation of CGCs with PD98059 for 1 h abolished the antiapoptotic effect of BMP-6, measured as number of cells presenting condensed or fragmented chromatin (Figure 5, A and B). Moreover, the MEK inhibitor was also able to block the BMP-6–mediated reduction of cells immunoreactive for active caspase-3 (Figure 5, C and D) and caspase activity (Figure 5E). Similar results were obtained with another MEK inhibitor U0126 (Supplemental Figure S5). All these data support the notion that MEK/ERK activity is essential for the antiapoptotic effect of BMP-6 in cultured CGCs. Activation of monomeric G-proteins, such as Rap-1 or Ras, have been described to participate in the regulation of the MEK/ERK pathway in response to survival stimuli in CGCs (Obara et al., 2007). Pulldown assay followed by Western blot analysis revealed activation of Rap1 in BMP-6–treated CGC cell lysates (Figure 5F). By contrast, activation of Rap-1 was neither observed in BMP-7 nor in K5 cultured CGCs (Supplemental Figure S3E). Furthermore, we found that Ras was similarly activated by both BMP-6 and -7 but not in K5 conditions (Supplemental Figure S3D).
Figure 5.
ERK pathway activation has a key role in BMP-6 neuroprotection. At 6 DIV, CGC cultures were placed in a serum-free medium and treated with high (K25) or low (K5) potassium with or without BMP-6. The MEK inhibitor PD98059 (50 μM) was added 1 h before the treatment. (A and B) Chromatin condensation was assayed 24 h later by staining with Hoechst 33258. Condensed nuclei were counted and represented as percentage versus total nuclei. Results are the mean ± SEM from three independent experiments performed in triplicate. (C and D) The presence of the active form of caspase-3 was monitored 10 h after treatment by immunocytochemistry (see Materials and Methods). Quantification of positive immunostained cells was performed by triplicate in four experiments. Results are the mean ± SEM. (E) Caspase activity was determined by a fluorometric method after 10 h (see Materials and Methods). Results are the mean ± SEM from three independent experiments performed in duplicate. (F) Cell lysates of indicated treatments were subjected to a pulldown assay with GST-Ral-GDS. Rap1 was detected by Western blotting. Two additional assays gave similar results. **p < 0.01 and *p < 0.05 versus K5; ××p < 0.01 and ×p < 0.05 versus BMP-6 + PD.
cAMP Response Element-Binding Protein Acts Downstream of the MEK/ERK Pathway in BMP-6–treated CGCs
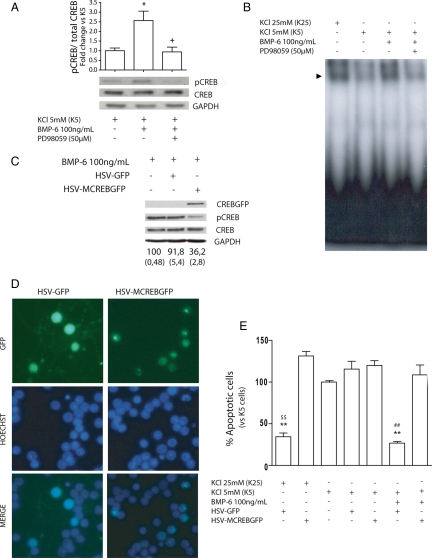
CREB has a key role on the survival of CGCs (Bonni et al., 1999; Monti et al., 2002; Yabe et al., 2002; Zhong et al., 2004; Jia et al., 2007). It is well known that CREB is phosphorylated and activated by the MEK/ERK pathway. In this context, we next addressed the question whether CREB activation was necessary for the survival effect of BMP-6 in CGCs. Therefore, we analyzed CREB phosphorylation in K5 cultures by Western blot in the presence or absence of BMP-6 and/or the MEK inhibitor PD98059. As shown in Supplemental Figure S3B, the level of CREB phosphorylation at serine 133 was significantly decreased after KCl deprivation compared with K25 conditions. Addition of BMP-6 to deprived cultures was able to reverse the K5-mediated decrease on CREB phosphorylation, maintaining the level of phospho-CREB similar to that of K25-treated CGCs (Figure 6A). The effect of BMP-6 was dependent on MEK/ERK activation because the addition of PD 9059 reduced phospho-CREB levels to K5 levels. By contrast, BMP-7 did not affect CREB phosphorylation (Supplemental Figure S3B). These results suggest that MEK/ERK pathway mediates CREB activation in BMP-6–treated cells. Accordingly, when we analyzed the CREB DNA binding activity by electrophoretic mobility shift assay (EMSA) assay with a probe containing a CRE-like consensus sequence, we observed that BMP-6, but not BMP-7, rescued the CREB-binding activity that was abolished in K5 conditions and that inhibition of MEK was able to revert the BMP-6 effect (Figure 6B and Supplemental Figure S3C).
Figure 6.
CREB mediates BMP-6 neuroprotective effect. Mature CGC cultures (6 DIV) were placed in a serum-free medium and treated with high or low potassium (K25 or K5) with or without BMP-6 and the MEK inhibitor, PD98059 (50 μM). (A) Phospho-CREB and total CREB levels were assayed by Western blot at 5 min. Results are the mean ± SEM of four independent experiments. (B) CGCs were treated for 1 h as indicated, and nuclear extracts were prepared for EMSA mobility assay with a CRE probe. A representative assay is shown. Two additional assays gave similar results. (C–E) Cells were infected at 4 DIV with HSV-GFP or HSV-MCREBGFP and treated as described at 6 DIV. A representative Western blot showing the decrease in CREB activation in HSV-MCREBGFP infected CGC cultures is shown in C. Data represent the mean and SEM (between brackets) from four independent Western blots. (D) Representative microphotographs of non-treated and infected cells. Condensed or fragmented nuclei of GFP-expressing cells were determined with Hoechst 33258 24 h after treatment. Data represent the percentage of condensed or fragmented nuclei versus total nuclei in GFP-expressing cells (E). Results are the mean ± SEM from three to five independent experiments performed in duplicate. **p < 0.01 and *p < 0.05 versus K5; +p < 0.05 versus K5 + BMP-6; ##p < 0.01 versus K5 + BMP-6 + HSV-MCREBGFP; $$ K25 + HSV-MCREBGFP.
These experiments suggested that MEK/ERK-mediated activation of CREB could be involved in the neuroprotective effect of BMP-6 in CGCs. Therefore, we asked whether expression of a mutated inactive form of CREB could block the neuroprotective effect of BMP-6. We used an HSV-GFP or HSV-MCREBGFP to infect CGCs (Carlezon et al., 2005). Subcellular localization of HSV-GFP and HSV-MCREBGFP was clearly different. Although GFP alone seemed to be expressed in all subcellular compartments, the MCREBGFP fusion protein seemed to have a more nuclear localization (Figure 6D), suggesting that it was exerting its function as a nuclear CREB inhibitor. To evaluate the role of CREB in the BMP-6–mediated neuroprotection, we infected the cells with the virus at 4 DIV and performed the potassium deprivation at 6 DIV. The number of apoptotic nuclei in cells expressing GFP was determined 24 h later. We found that inhibition of CREB blocked K25- and BMP-6–mediated neuroprotection (Figure 6, D and E), supporting the notion that CREB is involved in the prosurvival effect of BMP-6 in CGCs.
BMP-6 Activation of CREB Mediates Caspase-3 Inhibition by Increasing Bcl-2 Protein Levels
To further investigate the role of CREB activation on BMP-6–mediated neuroprotective effect, we determined the presence of active caspase-3 by Western blot in cells infected with HSV-GFP or HSV-MCREBGFP. As shown in Figure 7A, HSV-MCREBGFP was able to block the effect of BMP-6 on cleaved caspase-3. By contrast, the presence of HSV-GFP did not affect the levels of cleaved caspase-3 observed in BMP-6–treated cells.
Figure 7.
Bcl-2 acts downstream of the MEK/ERK/CREB pathway in the BMP-6 neuroprotective effect. Cerebellar granule neurons were infected at 4 DIV with HSV-GFP or HSV-MCREBGFP or plated in the presence of lentivirus of Bcl-2 shRNA or scramble shRNA as described in Materials and Methods. At 6 DIV cells were switched to K5 and treated with BMP-6 or/and PD98059 as indicated. Cleaved caspase-3 was assessed by Western blot (A and E) or immunocytochemistry (D) 10 h after the treatment. (B and C) Bcl-2 levels were assessed by Western blot 6 h after the treatment. Condensed or fragmented nuclei of treated cells were determined (F) with Hoechst 33258 24 h after treatment. Data represent the percentage of condensed or fragmented nuclei versus total nuclei. Data in K5 were considered as 100%. Actin was used as loading control in Western blot determinations. Results are the mean ± SEM from three to four independent experiments, and representative blots are shown in A, B, C, and E. ***p < 0.001 and **p < 0.01 versus K5; ++p < 0.01 and +p < 0.05 versus K5 + BMP-6; $$p < 0.01 versus K5 + BMP-6 HSV-GFP. ##p < 0.01 vs. K5 + BMP-6 + shRNA scramble; &&p < 0.01 versus K5 + shRNA Bcl-2; n.s., not significant.
The Bcl-2 (Xiang et al., 2006) is a CREB-regulated gene that has been reported to inhibit caspase activation and apoptosis. Western blot analysis indicated that BMP-6 significantly increased Bcl-2 protein levels compared with K5 conditions (Figure 7B). When the MEK/ERK pathway was inhibited with PD98059, a clear reduction in BMP-6–mediated increase in Bcl-2 was observed. A similar inhibition was observed in K5-cultured CGCs treated with BMP-6 after HSV-MCREBGFP infection. To further explore the importance of Bcl-2 in BMP-6–mediated neuroprotection, we used lentiviruses to block the endogenous synthesis of Bcl-2. Although viral-mediated neurotoxicity was kept to minimum levels, an increase in caspase-3 cleavage and apoptosis was observed in K5 and K25 cultures (Figure 7, D and F). We found higher levels of the caspase-3 active form in the presence of Bcl-2 shRNA than in scramble shRNA in BMP-6–treated cells (Figure 7, C–E). In conclusion, silencing Bcl-2 expression reverses the BMP-6–mediated neuroprotective effect in CGCs (Figure 7F).
DISCUSSION
BMPs have previously been proposed to induce the generation of cerebellar granule cells progenitors (Alder et al., 1999) and to regulate their postnatal differentiation during their migration toward the internal granular layer (Qin et al., 2006). Although CGCs differentiate, they require excitatory inputs from the mossy fibers and trophic support from extracellular factors to survive (Gallo et al., 1987; Burgoyne and Cambray-Deakin, 1988; Wood et al., 1993; D'Mello et al., 1997). At present, it is largely unknown the effect of BMPs on CGC survival. Because cultured CGCs provide an in vitro system to explore the molecular factors involved on CGC survival during their postnatal migration from the external to the internal cellular layer in the cerebellum, we took advantage of them to test the effect of BMPs on CGC survival and to explore the molecular mechanisms involved.
Biological actions of BMPs are triggered by binding to homomeric as well as heteromeric receptor complexes containing BMPRI and/or BMPRII and signaling through Smad proteins (Miyazono et al., 2005). Although several reports have shown a role of BMPs on cell fate and development in the cerebellum, few data exist about the expression of both BMPRs and Smad proteins in the postnatal rodent. Angley et al. (2003) showed the presence of BMP-4 and Smad 1 in the postnatal cerebellum (Angley et al., 2003), whereas no reports have addressed the study of BMPR and other Smads. In our CGC cultures we detected the expression of BMPRI, BMPRII, Smad 1, 4, and 5, suggesting that CGC cultures could be sensitive to BMPs.
CGCs in culture need to be depolarized or cultured in the presence of trophic factors to survive. Thus, CGCs die by apoptosis when cultured in a low-potassium (K5; nondepolarizing) medium. When potassium deprivation apoptosis was triggered in mature cultures (6 or 7 DIV), the addition of BMP-6, but not BMP-2 or -7, was able to block the effect of K5 on condensed chromatin nuclei and activation of caspase-3. Importantly, these data show that members from the same BMPs subfamily (BMP-6 and -7) could have different roles on the SNC. By contrast, Yabe et al. (2002) reported that both BMP-6 and -7 were able to promote the survival of CGCs. The fact that they used immature CGCs could explain the discrepancy with our results. In agreement with different effects of BMP-6 and -7 on CGCs, Yabe and coworkers found that BMP-6, but not BMP-7, promoted neurite outgrowth.
Our results may also explain the increase in CGC apoptosis in Bmpr1a and Bmpr1b double knockout mutants (Qin et al., 2006). The neurotrophic effect of BMP-6 on CGCs supports previous reports showing that BMP-6 is a neurotrophic factor for mesencephalic-, septal cholinergic-, and calbindin-positive striatal neurons (Jordan et al., 1997; Nonner et al., 2001; Gratacos et al., 2002). Also, BMP-6 has been reported to reduce ischemia-induced brain damage in rats (Wang et al., 2001). These data indicate that besides their effects on proliferation and differentiation of CGCs (Alder et al., 1999; Angley et al., 2003; Qin et al., 2006), BMPs have also a protective role against apoptosis mediated by synaptic activity deprivation. Thus, the presence of BMPs in the postnatal cerebellum could facilitate the development and maturation of CGCs by acting through different mechanisms.
It is well established that canonical BMPs signaling is mediated by BMP receptors activation followed by nuclear translocation of Smad protein complexes (formed by R-Smads and CoSmad), which in turn modulate gene expression. Unexpectedly, when we analyzed the subcellular distribution of Smads proteins in CGC cultures we observed the presence of both R-Smads and CoSmads in the cytosol and nucleus in all conditions, but their localization was unchanged when cells were treated with BMPs. We could not detect changes on R-Smads and CoSmads protein levels in the nuclear fraction after the addition of BMP-6 or -7. These results contrast with a previous study showing that BMP-4 is able to promote Smad 1 nuclear translocation in CGC cultures (Angley et al., 2003). Thus, the key question was to know the mechanism involved on the neuroprotective effect mediated by BMP-6 in CGCs.
A great insight in the molecular mechanism that control CGC survival has aroused during the last decade from studies using cultured CGCs. Nowadays, it is well accepted that the PI3K-Akt/PKB (Datta et al., 1997; D'Mello et al., 1997), MEK/ERK (Borodinsky et al., 2002; Lafon-Cazal et al., 2002; Xifro et al., 2005) and the JNK pathways are directly involved (Watson et al., 1998; Coffey et al., 2002; Xifro et al., 2006). Our results indicate that neither PI3K nor JNK pathways are involved in neuroprotective effect of BMP-6 because no changes in the activation of both pathways were observed in CGCs in K5 conditions in the presence or absence of BMP-6. Several studies suggest that an early increase in ERK activation is involved in the neuroprotection provided by extracellular factors to CGCs cultured in K5 (Bonni et al., 1999). On the other hand, some reports also suggest that delayed activation of ERK is associated to CGC death in K5 (Subramaniam et al., 2003). Thus, neuroprotection in K5 conditions would probably need an early positive and a delayed negative regulation of the ERK pathway. This has been already described for IGF-1–mediated neuroprotection (Subramaniam et al., 2005). Accordingly, we have observed that addition of BMP-6 induced an early activation of MEK/ERK determined by ERK phosphorylation. Although it remains to be studied whether BMP-6 treatment would block the delayed K5-mediated increase in ERK phosphorylation, the fact that pharmacological inhibition of MEK was able to block the BMP-6–dependent decrease in apoptotic cell number and caspase activity, clearly indicates that BMP-6 protects CGCs from potassium- and serum deprivation–induced apoptosis by a MEK/ERK signaling-dependent mechanism.
However, the link between BMP-6 and MEK/ERK activation remains unclear. Several reports have suggested that activation of the monomeric G-proteins Ras and Rap-1 are involved in MEK/ERK pathway activation (Gao et al., 2006; Je et al., 2006; Marampon et al., 2008). Recently, it has been described that several prosurvival factors activate MEK/ERK in a Ras- and/or Rap-1–dependent manner in cultured CGCs (Obara et al., 2007). A pioneer study (Yue et al., 1999) suggested that BMPs activation of MEK/ERK in epithelial cells was mediated by Ras. In this context we have observed that both BMP-6 and -7 are able to active Ras. By contrast, BMP-6, but not BMP-7, is able to activate Rap-1. The differential activation of Rap-1 by BMP-6 and -7 could explain why BMP-6, but not BMP-7, is able to promote the activation of the MEK/ERK pathway. Our data support a previous report suggesting a selective activation of MEK/ERK by Rap-1 in PC12 cells (York et al., 1998).
One of the main targets of the MEK/ERK pathway is CREB. CREB activation has been implicated in synaptic plasticity, learning, and memory and cell survival (Lonze and Ginty, 2002; Carlezon et al., 2005). Moreover, CREB activation has been reported to be a necessary step in CGC survival and differentiation during the postnatal development (Monti et al., 2002; Zhong et al., 2004). In this context, we have observed that BMP-6, but not BMP-7, activates CREB via the MEK/ERK pathway and this activation is required for the decrease in caspase-3 activity. Several mechanisms could be involved in caspase-3 inhibition. Bcl-2 is an antiapoptotic protein positively regulated by CREB at the transcriptional level (Xiang et al., 2006). It has been also reported that Bcl-2 has a key role preventing cell death in cultured CGCs (Tanabe et al., 1997). Accordingly, we describe that inhibition of caspase-3 activation by BMP-6 is mediated by MEK/ERK/CREB-dependent increase of Bcl-2 levels.
In summary, our results demonstrate that BMP-6 protects CGCs from apoptosis induced by potassium deprivation of excitatory stimuli by a noncanonical pathway involving MEK/ERK/CREB signaling. Activation of this pathway leads to an increase in the antiapoptotic protein Bcl-2 and inhibition of caspase-3 activation (Figure 8, A and B). Our data suggest that BMPs, apart from the previously reported actions on CGC progenitor cells generation and differentiation, has a third biological role as promoters of CGC survival. Moreover, the failure of another BMP subfamily member as BMP-7 to promote survival of CGCs, indicates the functional diversity and complexity of closed-related BMPs in the CNS.
Figure 8.
BMPs role during postnatal cerebellum development. BMPs and their receptors are expressed in embryonic and postnatal cerebellum and have different roles in the generation, migration, and differentiation of CGCs. (A) BMP-6 and -7 are necessary for the generation of CGC precursors at embryonic days 14 and 15 in the proliferation zone of the rhombic limb (Alder et al., 1999; Qin et al., 2006). In the external granule cell layer (EGL), BMP-2 allows CGC precursors to enter their differentiation program by antagonizing sonic hedgehog-mediated signaling (Gao et al., 2006). CGCs complete their differentiation during the migration from the EGL toward the internal granule cell layer (IGL). It has been reported that BMP-4, which is expressed in the EGL and in migrating CGCs, promotes the differentiation of CGCs in culture. Lack of afferent stimulation during CGC migration toward the IGL causes apoptotic death. Our results show that BMP-6 promotes CGC survival in culture, suggesting that it could be an important factor regulating CGC survival during their migration toward IGL. (B) Schematic representation of the mechanisms involved in BMP-6–mediated neuroprotection of K5-mediated CGC apoptosis. BMP-6 activates the MEK/ERK/CREB/Bcl-2 pathway leading to inhibition of caspase activation induced by K5 resulting in protection (reduced) of K5-mediated apoptosis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from Ministerio de Educación y Ciencia (SAF2005-05106), Ministerio de Ciencia e Innovación (SAF2008-01904 and RENEVAS), and CIBERNED (CB06/05/0042) to J.R.A. B.B. was a recipient of a predoctoral fellowship from the Universitat Autònoma de Barcelona, and N.B. was recipient of a predoctoral fellowship from the Gobierno Vasco. R.F, was a recipient of a predoctoral fellowship from the Generalitat de Catalunya.
Abbreviations used:
- CGC
cerebellar granule cell
- DIV
days in vitro
- K5
5 mM KCl
- K25
25 mM KCl.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-05-0424) on October 21, 2009.
REFERENCES
- Alder J., Lee K. J., Jessell T. M., Hatten M. E. Generation of cerebellar granule neurons in vivo by transplantation of BMP-treated neural progenitor cells. Nat. Neurosci. 1999;2:535–540. doi: 10.1038/9189. [DOI] [PubMed] [Google Scholar]
- Angley C., Kumar M., Dinsio K. J., Hall A. K., Siegel R. E. Signaling by bone morphogenetic proteins and Smad1 modulates the postnatal differentiation of cerebellar cells. J. Neurosci. 2003;23:260–268. doi: 10.1523/JNEUROSCI.23-01-00260.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. C., Aja T. J., Hoang K. D., Gaur S., Bai X., Alnemri E. S., Litwack G., Karanewsky D. S., Fritz L. C., Tomaselli K. J. Activation of the CED3/ICE-related protease CPP32 in cerebellar granule neurons undergoing apoptosis but not necrosis. J. Neurosci. 1997;17:553–562. doi: 10.1523/JNEUROSCI.17-02-00553.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruga J., Yokota N., Hashimoto M., Furuichi T., Fukuda M., Mikoshiba K. A novel zinc finger protein, zic, is involved in neurogenesis, especially in the cell lineage of cerebellar granule cells. J. Neurochem. 1994;63:1880–1890. doi: 10.1046/j.1471-4159.1994.63051880.x. [DOI] [PubMed] [Google Scholar]
- Balazs R., Gallo V., Kingsbury A. Effect of depolarization on the maturation of cerebellar granule cells in culture. Brain Res. 1988;468:269–276. doi: 10.1016/0165-3806(88)90139-3. [DOI] [PubMed] [Google Scholar]
- Ben Arie N., Bellen H. J., Armstrong D. L., McCall A. E., Gordadze P. R., Guo Q., Matzuk M. M., Zoghbi H. Y. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- Bonni A., Brunet A., West A. E., Datta S. R., Takasu M. A., Greenberg M. E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Borodinsky L. N., Coso O. A., Fiszman M. L. Contribution of Ca2+ calmodulin-dependent protein kinase II and mitogen-activated protein kinase kinase to neural activity-induced neurite outgrowth and survival of cerebellar granule cells. J. Neurochem. 2002;80:1062–1070. doi: 10.1046/j.1471-4159.2002.00795.x. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cambray-Deakin M. A. The cellular neurobiology of neuronal development: the cerebellar granule cell. Brain Res. 1988;472:77–101. doi: 10.1016/0165-0173(88)90006-9. [DOI] [PubMed] [Google Scholar]
- Carlezon W. A., Jr, Duman R. S., Nestler E. J. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chang C. F., Lin S. Z., Chiang Y. H., Morales M., Chou J., Lein P., Chen H. L., Hoffer B. J., Wang Y. Intravenous administration of bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke. 2003;34:558–564. doi: 10.1161/01.str.0000051507.64423.00. [DOI] [PubMed] [Google Scholar]
- Coffey E. T., Smiciene G., Hongisto V., Cao J., Brecht S., Herdegen T., Courtney M. J. c-Jun N-terminal protein kinase (JNK) 2/3 is specifically activated by stress, mediating c-Jun activation, in the presence of constitutive JNK1 activity in cerebellar neurons. J. Neurosci. 2002;22:4335–4345. doi: 10.1523/JNEUROSCI.22-11-04335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S., Harvey B. K., Sanchez J. F., Wang J. Y., Wang Y. Mediation of BMP7 neuroprotection by MAPK and PKC in rat primary cortical cultures. Brain Res. 2004;1010:55–61. doi: 10.1016/j.brainres.2004.02.068. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- D'Mello S. R., Borodezt K., Soltoff S. P. Insulin-like growth factor and potassium depolarization maintain neuronal survival by distinct pathways: possible involvement of PI 3-kinase in IGF-1 signaling. J. Neurosci. 1997;17:1548–1560. doi: 10.1523/JNEUROSCI.17-05-01548.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello S. R., Galli C., Ciotti T., Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc. Natl. Acad. Sci. USA. 1993;90:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M. E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Gallo V., Kingsbury A., Balázs R., Jorgensen O. S. The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. J. Neurosci. 1987;7:2203–2213. doi: 10.1523/JNEUROSCI.07-07-02203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Feng Y., Bowers R., Becker-Hapak M., Gardner J., Council L., Linette G., Zhao H., Cornelius L. A. Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: two processes important to melanoma tumorigenesis and metastasis. Cancer Res. 2006;66:7880–7888. doi: 10.1158/0008-5472.CAN-06-0254. [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury N., Abboud S. L., Nishimura R., Celeste A., Mahimainathan L., Choudhury G. G. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J. Biol. Chem. 2002;277:33361–33368. doi: 10.1074/jbc.M205053200. [DOI] [PubMed] [Google Scholar]
- Gratacos E., Gavalda N., Alberch J. Bone morphogenetic protein-6 is a neurotrophic factor for calbindin-positive striatal neurons. J. Neurosci. Res. 2002;70:638–644. doi: 10.1002/jnr.10438. [DOI] [PubMed] [Google Scholar]
- Hallahan A. R., Pritchard J. I., Chandraratna R. A., Ellenbogen R. G., Geyer J. R., Overland R. P., Strand A. D., Tapscott S. J., Olson J. M. BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat. Med. 2003;9:1033–1038. doi: 10.1038/nm904. [DOI] [PubMed] [Google Scholar]
- Han M. H., Bolanos C. A., Green T. A., Olson V. G., Neve R. L., Liu R. J., Aghajanian G. K., Nestler E. J. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J. Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C., Maroney A. C., Johnson E. M., Jr Identification of JNK-dependent and -independent components of cerebellar granule neuron apoptosis. J. Neurochem. 2002;83:992–1001. doi: 10.1046/j.1471-4159.2002.01219.x. [DOI] [PubMed] [Google Scholar]
- Harvey B. K., Mark A., Chou J., Chen G. J., Hoffer B. J., Wang Y. Neurotrophic effects of bone morphogenetic protein-7 in a rat model of Parkinson's disease. Brain Res. 2004;1022:88–95. doi: 10.1016/j.brainres.2004.06.072. [DOI] [PubMed] [Google Scholar]
- Iantosca M. R., McPherson C. E., Ho S. Y., Maxwell G. D. Bone morphogenetic proteins-2 and -4 attenuate apoptosis in a cerebellar primitive neuroectodermal tumor cell line. J. Neurosci. Res. 1999;56:248–258. doi: 10.1002/(SICI)1097-4547(19990501)56:3<248::AID-JNR4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Izumi M., et al. Bone morphogenetic protein-2 inhibits serum deprivation-induced apoptosis of neonatal cardiac myocytes through activation of the Smad1 pathway. J. Biol. Chem. 2001;276:31133–31141. doi: 10.1074/jbc.M101463200. [DOI] [PubMed] [Google Scholar]
- Je H. S., Yang F., Zhou J., Lu B. Neurotrophin 3 induces structural and functional modification of synapses through distinct molecular mechanisms. J. Cell Biol. 2006;175:1029–1042. doi: 10.1083/jcb.200603061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Zhou J., Tai Y., Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat. Neurosci. 2007;10:559–567. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- Jordan J., Bottner M., Schluesener H. J., Unsicker K., Krieglstein K. Bone morphogenetic proteins: neurotrophic roles for midbrain dopaminergic neurons and implications of astroglial cells. Eur. J. Neurosci. 1997;9:1699–1710. doi: 10.1111/j.1460-9568.1997.tb01527.x. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M., Perez V., Bockaert J., Marin P. Akt mediates the anti-apoptotic effect of NMDA but not that induced by potassium depolarization in cultured cerebellar granule cells. Eur. J. Neurosci. 2002;16:575–583. doi: 10.1046/j.1460-9568.2002.02124.x. [DOI] [PubMed] [Google Scholar]
- Lemonnier J., Ghayor C., Guicheux J., Caverzasio J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J. Biol. Chem. 2004;279:259–264. doi: 10.1074/jbc.M308665200. [DOI] [PubMed] [Google Scholar]
- Lonze B. E., Ginty D. D. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Marampon F., et al. Nerve growth factor regulation of cyclin D1 in PC12 cells through a p21RAS extracellular signal-related kinase pathway requires cooperative interactions between Sp1 and nuclear factor-kB. Mol. Biol. Cell. 2008;19:2566–2578. doi: 10.1091/mbc.E06-12-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Miyazono K. Signal transduction by bone morphogenetic protein receptors: functional roles of Smad proteins. Bone. 1999;25:91–93. doi: 10.1016/s8756-3282(99)00113-1. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Maeda S., Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Monti B., Marri L., Contestabile A. NMDA receptor-dependent CREB activation in survival of cerebellar granule cells during in vivo and in vitro development. Eur. J. Neurosci. 2002;16:1490–1498. doi: 10.1046/j.1460-9568.2002.02232.x. [DOI] [PubMed] [Google Scholar]
- Moran J., Itoh T., Reddy U. R., Chen M., Alnemri E. S., Pleasure D. Caspase-3 expression by cerebellar granule neurons is regulated by calcium and cyclic AMP. J. Neurochem. 1999;73:568–577. doi: 10.1046/j.1471-4159.1999.0730568.x. [DOI] [PubMed] [Google Scholar]
- Naldini L., Blomer U., Gage F. H., Trono D., Verma I. M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner D., Barrett E. F., Kaplan P., Barrett J. N. Bone morphogenetic proteins (BMP6 and BMP7) enhance the protective effect of neurotrophins on cultured septal cholinergic neurons during hypoglycemia. J. Neurochem. 2001;77:691–699. doi: 10.1046/j.1471-4159.2001.00273.x. [DOI] [PubMed] [Google Scholar]
- Obara Y., Horgan A. M., Stork P. J. The requirement of Ras and Rap1 for the activation of ERKs by cAMP, PACAP, and KCl in cerebellar granule cells. J. Neurochem. 2007;101:470–482. doi: 10.1111/j.1471-4159.2006.04390.x. [DOI] [PubMed] [Google Scholar]
- Qin L., Wine-Lee L., Ahn K. J., Crenshaw E. B., III. Genetic analyses demonstrate that bone morphogenetic protein signaling is required for embryonic cerebellar development. J. Neurosci. 2006;26:1896–1905. doi: 10.1523/JNEUROSCI.3202-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios I., Varez-Rodriguez R., Marti E., Pons S. Bmp2 antagonizes sonic hedgehog-mediated proliferation of cerebellar granule neurones through Smad5 signalling. Development. 2004;131:3159–3168. doi: 10.1242/dev.01188. [DOI] [PubMed] [Google Scholar]
- Ryder E. F., Cepko C. L. Migration patterns of clonally related granule cells and their progenitors in the developing chick cerebellum. Neuron. 1994;12:1011–1028. doi: 10.1016/0896-6273(94)90310-7. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Shahani N., Strelau J., Laliberte C., Brandt R., Kaplan D., Unsicker K. Insulin-like growth factor 1 inhibits extracellular signal-regulated kinase to promote neuronal survival via the phosphatidylinositol 3-kinase/protein kinase A/c-Raf pathway. J. Neurosci. 2005;25:2838–2852. doi: 10.1523/JNEUROSCI.5060-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S., Strelau J., Unsicker K. Growth differentiation factor-15 prevents low potassium-induced cell death of cerebellar granule neurons by differential regulation of Akt and ERK pathways. J. Biol. Chem. 2003;278:8904–8912. doi: 10.1074/jbc.M210037200. [DOI] [PubMed] [Google Scholar]
- Tanabe H., Eguchi Y., Kamada S., Martinou J. C., Tsujimoto Y. Susceptibility of cerebellar granule neurons derived from Bcl-2-deficient and transgenic mice to cell death. Eur. J. Neurosci. 1997;9:848–856. doi: 10.1111/j.1460-9568.1997.tb01434.x. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Yamashita H., Ichijo H., Franzen P., Laiho M., Miyazono K., Heldin C. H. Characterization of type I receptors for transforming growth factor-beta and activin. Science. 1994;264:101–104. doi: 10.1126/science.8140412. [DOI] [PubMed] [Google Scholar]
- Wang Y., et al. Bone morphogenetic protein-6 reduces ischemia-induced brain damage in rats. Stroke. 2001;32:2170–2178. doi: 10.1161/hs0901.095650. [DOI] [PubMed] [Google Scholar]
- Watson A., Eilers A., Lallemand D., Kyriakis J., Rubin L. L., Ham J. Phosphorylation of c-Jun is necessary for apoptosis induced by survival signal withdrawal in cerebellar granule neurons. J. Neurosci. 1998;18:751–762. doi: 10.1523/JNEUROSCI.18-02-00751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. A., Dipasquale B., Youle R. J. In situ labeling of granule cells for apoptosis-associated DNA fragmentation reveals different mechanisms of cell loss in developing cerebellum. Neuron. 1993;11:621–632. doi: 10.1016/0896-6273(93)90074-2. [DOI] [PubMed] [Google Scholar]
- Xiang H., Wang J., Boxer L. M. Role of the cyclic AMP response element in the bcl-2 promoter in the regulation of endogenous Bcl-2 expression and apoptosis in murine B cells. Mol. Cell Biol. 2006;26:8599–8606. doi: 10.1128/MCB.01062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xifro X., Falluel-Morel A., Minano A., Aubert N., Fado R., Malagelada C., Vaudry D., Vaudry H., Gonzalez B., Rodriguez-Alvarez J. N-Methyl-D-aspartate blocks activation of JNK and mitochondrial apoptotic pathway induced by potassium deprivation in cerebellar granule cells. J. Biol. Chem. 2006;281:6801–6812. doi: 10.1074/jbc.M504571200. [DOI] [PubMed] [Google Scholar]
- Xifro X., Malagelada C., Minano A., Rodriguez-Alvarez J. Brief exposure to NMDA produces long-term protection of cerebellar granule cells from apoptosis. Eur. J. Neurosci. 2005;21:827–840. doi: 10.1111/j.1460-9568.2005.03935.x. [DOI] [PubMed] [Google Scholar]
- Yabe T., Samuels I., Schwartz J. P. Bone morphogenetic proteins BMP-6 and BMP-7 have differential effects on survival and neurite outgrowth of cerebellar granule cell neurons. J. Neurosci. Res. 2002;68:161–168. doi: 10.1002/jnr.10210. [DOI] [PubMed] [Google Scholar]
- York R. D., Yao H., Dillon T., Ellig C. L., Eckert S. P., McCleskey E. W., Stork P.J.S. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- Yue J., Frey R. S., Mulder K. M. Cross-talk between the Smad1 and Ras/MEK signaling pathways for TGFbeta. Oncogene. 1999;18:2033–2037. doi: 10.1038/sj.onc.1202521. [DOI] [PubMed] [Google Scholar]
- Zhong J., Deng J., Huang S., Yang X., Lee W. H. High K+ and IGF-1 protect cerebellar granule neurons via distinct signaling pathways. J. Neurosci. Res. 2004;75:794–806. doi: 10.1002/jnr.20024. [DOI] [PubMed] [Google Scholar]
- Zhu D., Wu X., Strauss K. I., Lipsky R. H., Qureshi Z., Terhakopian A., Novelli A., Banaudha K., Marini A. M. N-methyl-D-aspartate and TrkB receptors protect neurons against glutamate excitotoxicity through an extracellular signal-regulated kinase pathway. J. Neurosci. Res. 2005;80:104–113. doi: 10.1002/jnr.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R., Dull T., Mandel R. J., Bukovsky A., Quiroz D., Naldini L., Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.