Abstract
The c-Myc promoter binding protein 1 (MBP-1) is a transcriptional suppressor of c-myc expression and involved in control of tumorigenesis. Gastric cancer is one of the most frequent neoplasms and lethal malignancies worldwide. So far, the regulatory mechanism of its aggressiveness has not been clearly characterized. Here we studied roles of MBP-1 in gastric cancer progression. We found that cell proliferation was inhibited by MBP-1 overexpression in human stomach adenocarcinoma SC-M1 cells. Colony formation, migration, and invasion abilities of SC-M1 cells were suppressed by MBP-1 overexpression but promoted by MBP-1 knockdown. Furthermore, the xenografted tumor growth of SC-M1 cells was suppressed by MBP-1 overexpression. Metastasis in lungs of mice was inhibited by MBP-1 after tail vein injection with SC-M1 cells. MBP-1 also suppressed epithelial-mesenchymal transition in SC-M1 cells. Additionally, MBP-1 bound on cyclooxygenase 2 (COX-2) promoter and downregulated COX-2 expression. The MBP-1-suppressed tumor progression in SC-M1 cells were through inhibition of COX-2 expression. MBP-1 also exerted a suppressive effect on tumor progression of other gastric cancer cells such as AGS and NUGC-3 cells. Taken together, these results suggest that MBP-1–suppressed COX-2 expression plays an important role in the inhibition of growth and progression of gastric cancer.
INTRODUCTION
Gastric cancer is one of the most frequent neoplasms and leading causes of cancer-related mortality worldwide (Terry et al., 2002; Executive Yuan, 2006). At present, curative surgery of its primary tumor and control of lymph node metastasis are still the mainstay of treatment for gastric cancer without distant metastasis (Wu et al., 2006). However, gastric cancer with distant metastasis remains incurable now. More than 95% of malignancies of the stomach are adenocarcinomas (Smith et al., 2006). The risk factors of human gastric cancer include diet, Helicobacter pylori infection, and accumulation of specific genetic alterations (Gonzalez et al., 2002; Ushijima and Sasako, 2004; Zheng et al., 2004). To date, the regulatory mechanism of aggressiveness in gastric cancer has not yet been clearly characterized. Therefore, it is essential to gain further insights into the physiology of gastric cancer and its accumulated genetic alterations.
The inducible cyclooxygenase, COX-2, catalyzes the rate-limiting step in conversion of arachidonate into prostaglandin E2 (PGE2). It was shown that COX-2 expression is upregulated in gastric cancer (Ristimäki et al., 1997; Uefuji et al., 1998; Yamamoto et al., 1999; Lim et al., 2000). COX-2 expression is also correlated with depth of invasion, lymphatic vessel invasion, lymph node metastasis, and poor prognosis of human gastric carcinoma (Murata et al., 1999; Ohno et al., 2001; Shi et al., 2003; Chen et al., 2006). Epithelial-mesenchymal transition (EMT) plays a key role in development and tumorigenesis (for a review, see Thiery and Sleeman, 2006). In gastric cancer cells with fibroblastoid morphological changes, EMT signaling was suggested to promote motility and invasiveness through decreasing cell–cell adhesion (Katoh, 2005). Recently, COX-2 expression was found to enhance EMT stimulated by TGF-β through a PGE2-dependent manner in breast cancer (Neil et al., 2008). In this scenario, the induction of COX-2 expression in gastric cancer could further induce EMT to promote metastasis.
The c-Myc promoter binding protein 1 (MBP-1), a negative regulator of c-myc expression, is ubiquitously expressed in normal human tissues (Ray et al., 1994). Although MBP-1 does not contain a known DNA-binding domain, it and TATA-binding protein simultaneously bind in the minor groove of the major c-Myc promoter, the P2 promoter (Chaudhary and Miller, 1995). The 37-kDa MBP-1 is produced by alternative translation initiation from α-enolase gene but without enzyme activity of enolase (Feo et al., 2000; Subramanian and Miller, 2000). So far, several MBP-1–associating proteins were identified, including histone deacetylase HDAC1 (Ghosh et al., 1999), MIP2A/sedlin (Ghosh et al., 2001), MEK5α (Ghosh et al., 2005a), NS1-BP (Perconti et al., 2007), and Notch1 receptor intracellular domain (Hsu et al., 2008). The downstream target genes of MBP-1 remain unclear exclusive of c-myc. It was reported that MBP-1 could regulate target genes at least through p53–p21 pathway (Ghosh et al., 2008).
Mounting evidence indicates that both MBP-1 and α-enolase are involved in tumorigenesis of breast carcinoma (Ray et al., 1995), nonsmall cell lung cancer (Chang et al., 2003; Ghosh et al., 2006b), hepatitis C virus–related hepatocellular carcinoma (Takashima et al., 2005), prostate tumor (Ghosh et al., 2005a; Ghosh et al., 2005b; Ghosh et al., 2006a), and neuroblastoma (Ejeskar et al., 2005). It was also suggested that MBP-1 expression reduces the invasive ability of breast cancer (Ray et al., 1995), and α-enolase may participate in control of EMT (Demir et al., 2005) and metastasis (Chang et al., 2003). Therefore, we sought to evaluate whether MBP-1 exhibits potential avenues for the development of novel therapeutic strategies against gastric cancer. We also further investigated underlying mechanisms of the MBP-1–modulated effect on tumor progression of gastric cancer in the present study.
MATERIALS AND METHODS
Plasmids and Plasmid Construction
Both pcDNA-HA-MBP-1 and pcDNA-HA-α-enolase expression constructs contain cDNAs of MBP-1 and α-enolase with N-terminal HA tags (Hsu et al., 2008). The fusion protein plasmids pcDNA-HA-MBP-1 (1-178), pcDNA-HA-MBP-1 (190-338), and pcDNA-HA-MBP-1 (232-338) direct the expression of HA-fusion proteins with amino acid residues 1-178, 190-338, and 232-338 of MBP-1, respectively. Construct pVP16-MBP-1 contains cDNA encoding MBP-1 with VP16 transactivation domain at N terminus. The pcDNA-COX-2 contains cDNA of human COX-2 (Su et al., 2004). The siRNA vectors were constructed in pLKO.1 siRNA vector to knockdown the endogenous MBP-1 and α-enolase (#22 and #24) (Hsu et al., 2008). The pLKO.1-shLuc siRNA vector against luciferase was a control for knockdown validation in this study. Reporter plasmid pCOX-2-Luc (−1334/−1) contains human COX-2 promoter in front of the luciferase gene in pGL3-basic vector (a kind gift from Dr. L.-F. Shyur, Academia Sinica, Taipei, Taiwan).
Cell Culture and Transfection
Human embryonic kidney (HEK) 293T cells, stomach carcinoma SC-M1, AGS, AZ521, NUGC-3, and KATO III cells, or erythroleukemia K562 cells were cultured in DMEM or RPMI 1640 medium with 10% FBS. For the establishment of stable SC-M1 cells expressing HA-MBP-1 fusion protein (SC-M1/HA-MBP-1 #2, #3, #5, and #6 cells), SC-M1 cells (2 × 106) were seeded and then transfected with lineralized pcDNA-HA-MBP-1 expression plasmid as described previously (Yeh et al., 2003). The stable clones derived from single cells were screened for the constitutive expression of HA-MBP-1 fusion protein by Western blot analysis using both anti-HA (Santa Cruz Biotechnology, Santa Cruz, CA) and anti–MBP-1 antibodies. The linearized pcDNA3-HA plasmid was also electroporated into SC-M1 cells to establish the stable SC-M1/pcDNA3 cells for the control.
For evaluation of colony formation, migration, and invasion abilities, cells were transiently transfected by electroporation and then seeded after transfection for two days. For transient transfection of luciferase reporter assay, K562 cells (5 × 105) were seeded onto 6-well plates and transfected using SuperFect transfection reagent, (Qiagen, Valencia, CA) for two days. Luciferase activities were measured using the Dual-Luciferase reporter assay system (Promega, Madison, WI) and Renilla luciferase activity was used to normalize transfection efficiency (Wang et al., 2009).
Fifty μM NS-398 (Sigma-Aldrich, St. Louis, MO) in DMSO and 2 μg/ml PGE2 (Sigma-Aldrich) in ethanol or an equal volume of vehicles were used in the present study.
Generation of Anti–MBP-1 Antibody
Aligning amino acid sequences of MBP-1 (accession No. M55914) and α-enolase (accession No. AK222517), the N-terminal amino acid sequence of MBP-1 is different from that of α-enolase. To generate antibodies against MBP-1 but not α-enolase, the synthetic peptide GCPLPSAKLVPLRRG (amino acid residues 16-30 of MBP-1) was processed to immunize rabbits by Genemed Synthesis.
Western Blot Analysis
Whole-cell lysates were prepared and analyzed by SDS-PAGE as previously described (Yeh et al., 2003). Then Western blot analysis was performed with anti–MBP-1, anti–α-enolase, anti-plakoglobin (Santa Cruz Biotechnology), anti–E-cadherin (Cell Signaling Technology, Beverly, MA), anti–N-cadherin (BD Biosciences, Franklin Lakes, NJ), anti-vimentin (Sigma-Aldrich), anti–c-Myc, anti–COX-1 (Santa Cruz), anti–COX-2 (Cayman Chemical, Ann Arbor, MI), and anti-GAPDH antibodies (Biogenesis, Poole, United Kingdom).
Colony-Forming Assay
As Hsu et al. described previously (Hsu et al., 2008), 4000 parental cells (SC-M1, AGS, AZ521, NUGC-3, and KATO III cells) and 8000 SC-M1/pcDNA3 or SC-M1/HA-MBP-1 cells were used for assay of anchorage-independent growth in soft agar. Then cells were incubated at 37°C for 14 d and 200 μl of medium was added every 3 d to prevent desiccation. These cells were stained and colonies were counted from 10 random fields under microscope.
Xenografted Tumorigenicity Assay
All animal experiments were carried out with the approval of ethical committee. Five-week-old BALB/c nu/nu mice were purchased from National Science Council Animal Center (Taipei, Taiwan) and allowed free access to food and water. Nude mice were inoculated with 6 × 106 viable SC-M1/HA-MBP-1 or SC-M1/pcDNA3 control cells in a total volume of 0.1 ml of PBS by subcutaneous injection into both hind limbs. The length and width of tumors were measured with calipers every 3 d to estimate tumor volume as previously described (Liao et al., 2007).
Migration and Invasion Assays
Migration and invasion abilities of cells (1 × 104 for migration assay, 5 × 104 for invasion assay) were analyzed in 24-well plates by Millicell tissue culture plate well inserts (Millipore, Bedord, MA) for 12 h and BD BioCoat Matrigel Invasion Chambers (Becton Dickson, Mountain View, CA) for 20 h, respectively. Then cells on the upper surface of membrane were removed with a cotton swab after incubation. After fixation with methanol, cells in the lower surface of membrane were stained with 0.005% crystal violet in PBS for 1 h and the number of migrated or invaded cells was counted from 10 random fields under microscope.
In Vivo Tail Vein Metastasis Assay
Female nonobese diabetic severe-combined immunodeficiency (NOD-SCID) mice (National Taiwan University, Taipei, Taiwan) aged 6 wk were inoculated with 1 × 106 viable SC-M1/HA-MBP-1 or SC-M1/pcDNA3 cells in a total volume of 0.1 ml of PBS by tail vein injection. The mice were killed 9 weeks later, and the metastatic nodules in lungs of mice were counted by gross and microscopic examination.
Immunofluorescence Staining
Immunofluorescence staining was performed as described previously (Hsu et al., 2008). After fixation, cells grown on coverslips were incubated with primary rabbit anti–E-cadherin antibody or mouse anti-vimentin antibody and subsequently with secondary Alexa Fluor 568-conjugated donkey anti-rabbit IgG or Alexa Fluor 488-conjugated donkey anti-mouse IgG (Molecular Probes, Eugene, OR). For nuclear staining, cells were further incubated with 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma-Aldrich). After staining, cells were mounted with anti-bleaching reagent (DAKO) and their localizations of E-cadherin and vimentin were examined by immunofluorescence microscope.
Real-Time PCR Analysis
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA), and cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (New England BioLabs, Beverly, MA) with an oligo (dT)18 primer (Wang et al., 2009). The 305-base pairs COX-2 cDNA was amplified with primers 5′-TTCAAATGAGATTGTGGGAAAAT-3′ and 5′-AGATCATCTCTGCCTGAGTATCTT-3′. The 478-base pairs c-Myc cDNA was amplified with primers 5′-TACCCTCTCAACGACAGCAG-3′ and 5′-TCTTGACATTCTCCTCGGTG-3′. The 86-base pairs cyclin D1 cDNA was amplified with primers 5′-CCGTCCATGCGGAAGATC-3′ and 5′-ATGGCCAGCGGGAAGAC-3′. The 176-base pairs GAPDH cDNA was amplified with primers 5′-AAATCCCATCACCATCTTCC-3′ and 5′-TCACACCCATGACGAACA-3′. Quantitative real-time PCR was performed using a LightCycler system with LightCycler FastStart DNA MasterPLUS SYBR Green I (Roche, Indianapolis, IN) (Wang et al., 2009). All data are shown as mean values and standard deviations from at least 3 independent experiments.
Chromatin Immunoprecipitation (ChIP) Assay
As described before (Hsu et al., 2008), nuclear extracts of SC-M1 cells were prepared for ChIP assay using protein A Sepharose-bound antibodies of anti–MBP-1, anti–α-enolase, and anti-IgG antibodies. By PCR amplification, the 125-base pairs DNA fragment of COX-2 promoter was amplified with primers 5′-TAAGGGGAGAGGAGGGAAAAAT-3′ and 5′-ACAATTGGTCGCTAACCGAG-3′. The 231-base pairs DNA fragment of COX-1 promoter was amplified with primers 5′-GTGAGTTTCTCATCTAGG-3′ and 5′-CGTCTGAACCACATACC-3′. The 210-base pairs DNA fragment of c-Myc promoter was amplified with primers 5′-GAGGAGCAGCAGAGAAAGG-3′ and 5′-TCCCCCACGCCCTCTGC-3′. Furthermore, percentages of immunoprecipitated promoter fragments were quantified by real-time PCR using SYBR green as described elsewhere (Hsu et al., 2008) and normalized to total input DNA.
Statistical Analysis
Statistical calculations were performed using Student t test for simple comparison of two values. The difference was considered to be statistically significant when the P value was <0.05. Survival rate of mice inoculated with MBP-1–expressing SC-M1/HA-MBP-1 cells or their control cells by tail vein injection was analyzed using the Kaplan–Meier method.
RESULTS
Tumor Growth of SC-M1 Cells Is Suppressed by MBP-1
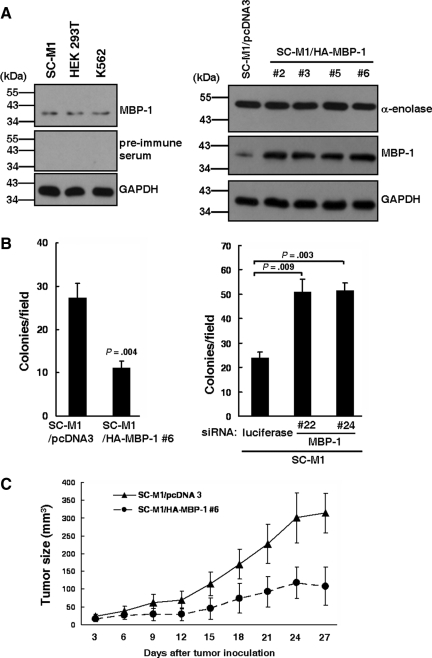
Owing to similarity of amino acid sequence at the C-terminal region of MBP-1 to α-enolase sequence (Feo et al., 2000), both MBP-1 and α-enolase can be detected by polyclonal anti–α-enolase antibody simultaneously (Ito et al., 2007; Hsu et al., 2008). To investigate role of endogenous MBP-1 in tumorigenesis of gastric cancer, antibodies against MBP-1 but not α-enolase were generated from rabbits immunized with the synthetic peptide of MBP-1. The generated antibody could detect MBP-1 but not α-enolase in SC-M1, HEK 293T, and K562 cells by Western blot analysis (Figure 1A, left).
Figure 1.
Tumor growth of SC-M1 cells is suppressed by MBP-1. (A) Whole-cell extracts of SC-M1, HEK 293T, and K562 cells were prepared for Western blot analysis using rabbit anti–MBP-1 antibody or its pre-immune serum and anti-GAPDH antibody (left). Note the abundant α-enolase (48-kDa) was not recognized by anti–MBP-1 antibody. Whole-cell extracts of SC-M1/HA-MBP-1 cells and control cells were also used to examine expressions of α-enolase, MBP-1, and GAPDH by Western blot analysis (right). (B) SC-M1/HA-MBP-1 #6 cells and control cells were seeded for colony-forming assay (left). SC-M1 cells were transfected with siRNA vectors against MBP-1 (#22 and #24) or luciferase. The transfected SC-M1 cells were seeded for colony-forming assay (right). Means of three independent experiments performed in triplicate are shown. (C) Viable SC-M1/HA-MBP-1 #6 cells and control cells were subcutaneously inoculated into nude mice (n = 7 per group) for measurement of tumor sizes at the time indicated. Data are representative of 3 experiments with similar results.
Because more than 95% of malignancies of the stomach are adenocarcinomas (Smith et al., 2006), the MBP-1–expressing gastric cancer cells were established in human stomach adenocarcinoma SC-M1 cells. Using Western blot analysis with anti–MBP-1 antibody, we found that MBP-1 was overexpressed in SC-M1/HA-MBP-1 #2, #3, #5, and #6 cells (Figure 1A, right). To clarify whether MBP-1 regulates growth of SC-M1 cells, trypan blue exclusion method was performed. All cumulative numbers of SC-M1/HA-MBP-1 cells (#2, #3, #5, and #6) were lower than control cells (Supplemental Figure S1A). Additionally, MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide) assay results showed that the viability of SC-M1 cells was decreased by exogenous MBP-1 (Supplemental Figure S1B).
To investigate the role of MBP-1 in tumorigenesis of SC-M1 cells, colony-forming assay was performed. The colony-forming ability of SC-M1 cells was suppressed by MBP-1 overexpression (Figure 1B, left). To check whether endogenous MBP-1 is involved in control of anchorage-independent growth of SC-M1 cells, colony-forming ability was also determined after MBP-1 knockdown. Expressions of endogenous MBP-1 in SC-M1 cells were knocked down after transfection with siRNA vectors (#22 and #24) against MBP-1 or α-enolase (Supplemental Figure S2). Colony-forming ability of SC-M1 cells was promoted by MBP-1 knockdown (Figure 1B, right).
We further assessed effect of MBP-1 on tumor growth in a xenografted tumor model in which nude mice were subcutaneously implanted with MBP-1–expressing gastric cancer cells. After inoculation, the xenografted tumor sizes of MBP-1–expressing SC-M1/HA-MBP-1 #6 cells were smaller than those of control cells (Figure 1C).
Tumor Progression of SC-M1 Cells Is Suppressed by MBP-1
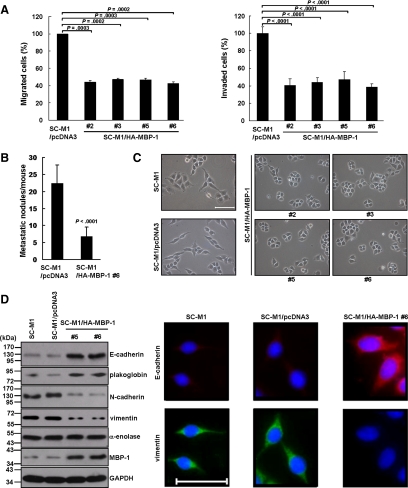
To explore whether MBP-1 participates in metastasis of gastric cancer, migration and invasion abilities were evaluated. Migration and invasion abilities of SC-M1 cells were suppressed by MBP-1 overexpression (Figure 2A).
Figure 2.
Tumor progression ability of SC-M1 cells are suppressed by MBP-1. (A) SC-M1/HA-MBP-1 cells and control cells were seeded to evaluate migration (left) and invasion (right) abilities. Means of three independent experiments performed in triplicate are shown. (B) The viable SC-M1/HA-MBP-1 #6 cells and control cells were inoculated into NOD-SCID mice (n = 9 per group) by tail vein injection for measurement of metastatic nodules in lungs. Data are representative of 3 experiments with similar results. (C) SC-M1 cells, SC-M1/pcDNA3 control cells, and SC-M1/HA-MBP-1 cells were seeded onto 6-well plates for 48 h for morphological examination. Bar, 100 μm. (D) Whole-cell extracts were prepared for Western blot analysis using anti–E-cadherin, anti-plakoglobin, anti–N-cadherin, anti-vimentin, anti–α-enolase, anti–MBP-1, and anti-GAPDH antibodies (left). Cells were seeded on coverslips for immunofluorescence staining with primary anti–E-cadherin or anti-vimentin antibodies (right). Bar, 40 μm.
Effect of MBP-1 on metastatic colonization was also determined by intravenous injection of MBP-1–expressing SC-M1/HA-MBP-1 #6 cells into lateral tail vein of NOD-SCID mice. Nine weeks later, mice injected with control cells had numerous large metastases in lung, whereas those injected with SC-M1/HA-MBP-1 #6 cells had fewer and smaller lung metastatic nodules (Figure 2B and Supplemental Figure S3A). Moreover, survival rate of the mice injected with SC-M1/HA-MBP-1 #6 cells was increased compared with those injected with control cells (Supplemental Figure S3B). Therefore, MBP-1 overexpression attenuated ability of SC-M1 cells to form metastatic nodules in lungs.
To evaluate whether MBP-1 affects angiogenesis in metastatic tumors of lungs after injection with MBP-1–expressing SC-M1/HA-MBP-1 #6 cells or their control cells, the mRNA expressions of VEGF-A and PDGF-B were detected by real-time PCR. The results showed that expressions of VEGF-A and PDGF-B mRNAs were suppressed by MBP-1 in the metastatic nodules (Supplemental Figure S3C). Thus, MBP-1 could inhibit angiogenesis in metastatic nodules of SC-M1 cells.
MBP-1 Overexpression Suppresses EMT in SC-M1 Cells
Next, we sought to examine whether MBP-1 inhibits metastasis of gastric cancer through suppression of EMT. Both parental SC-M1 and SC-M1/pcDNA3 control cells dispersedly grew and had a little spindle- and fibroblast-like morphology, whereas MBP-1–expressing SC-M1/HA-MBP-1 cells (#2, #3, #5, and #6) grew as clusters of cells (Figure 2C). Thus, MBP-1 overexpression induced morphological change in SC-M1 cells from the more extended and elongated shape to tightly packed colonies.
Furthermore, epithelial markers including E-cadherin and plakoglobin were upregulated, whereas mesenchymal markers such as N-cadherin and vimentin were downregulated by MBP-1 overexpression (Figure 2D, left). Consistently, level of E-cadherin was enhanced along with the decreased vimentin expression in MBP-1–expressing SC-M1/HA-MBP-1 #6 cells using immunofluorescence staining (Figure 2D, right).
MBP-1 Binds on Human COX-2 Promoter and Downregulates COX-2 Expression
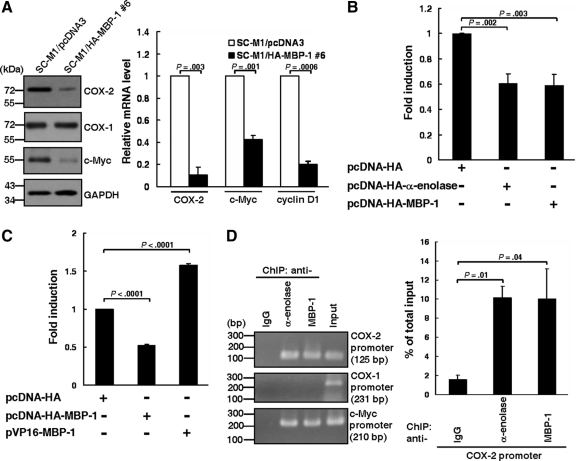
To address whether MBP-1 modulates tumorigenesis of SC-M1 cells through COX-2, Western blot analysis was performed. As shown in Figure 3A (left), MBP-1 overexpression inhibited expressions of COX-2 and c-Myc, a downstream target gene of MBP-1, but not COX-1.
Figure 3.
MBP-1 binds on human COX-2 promoter and downregulates COX-2 expression. (A) Whole-cell extracts of SC-M1/HA-MBP-1 #6 cells and control cells were prepared for Western blot analysis using anti–COX-2, anti–COX-1, anti–c-Myc, and anti-GAPDH antibodies (left). The transcript levels of COX-2, c-Myc, and cyclin D1 in SC-M1/HA-MBP-1 #6 cells or control cells were measured by quantitative real-time PCR (right). (B) Reporter plasmid pCOX-2-Luc (−1334/−1) containing full-length COX-2 promoter was cotransfected with MBP-1–expressing construct (pcDNA-HA-MBP-1), α-enolase–expressing construct (pcDNA-HA-α-enolase), or control vector (pcDNA-HA) into K562 cells for reporter gene assay. (C) Reporter plasmid pCOX-2-Luc (−1334/−1) was cotransfected with MBP-1–expressing construct, VP16-MBP-1 fusion protein–expressing construct (pVP16-MBP-1), or control vector into K562 cells for reporter gene assay. (D) SC-M1 cells were harvested for ChIP assay using anti-IgG, anti–α-enolase, and anti–MBP-1 antibodies. The immunoprecipitated DNA was used to amplify PCR products of COX-2, COX-1, and c-Myc promoters (left). Percentages of immunoprecipitated DNAs of COX-2 promoter were also quantified by real-time PCR and normalized to total input DNA (right). Means of three independent experiments performed in triplicate are shown.
In addition to c-Myc, cyclin D1 expression is also suppressed by MBP-1 (Ghosh et al., 2005a). Therefore, effects of MBP-1 on mRNA expressions of COX-2, c-Myc, and cyclin D1 were also evaluated in SC-M1/HA-MBP-1 #6 cells by real-time PCR. Results showed that MBP-1 overexpression diminished mRNA expressions of COX-2, c-Myc, and cyclin D1 (Figure 3A, right). The mRNA expression of COX-2 was also lowered in the xenografted tumors in nude mice injected with MBP-1-expressing SC-M1/HA-MBP-1 #6 cells (Supplemental Figure S4).
Then reporter gene assay was performed to check whether MBP-1 can block COX-2 expression through inhibiting COX-2 promoter activity. Reporter plasmid containing human COX-2 promoter (pCOX-2-Luc [−1334/−1]) was cotransfected with expression constructs of α-enolase or MBP-1. Data showed that COX-2 promoter activity was attenuated after transfection with α-enolase or MBP-1 expression constructs (Figure 3B).
Alternatively, the fusion protein of VP16 transactivation domain and MBP-1 (VP16-MBP-1) was expressed to assess whether COX-2 promoter activity is induced after cotransfection with reporter plasmid pCOX-2-Luc (-1334/-1) and expression construct pVP16-MBP-1 (Figure 3C). Results showed that COX-2 promoter activity was enhanced by VP16-MBP-1 fusion protein.
We hypothesized that MBP-1 might bind to COX-2 promoter of chromosomal DNA to modulate promoter activity in the context of living cells. To clarify this possibility, we examined the DNA-binding ability of MBP-1 on COX-2 promoter by ChIP assay using anti-IgG, anti–α-enolase, and anti–MBP-1 antibodies in SC-M1 cells. The immunoprecipitated DNA was used to amplify PCR products of COX-2, COX-1, and c-Myc promoters. The amplified PCR products of COX-2 and c-Myc promoters were present in the samples immunoprecipitated with anti–α-enolase and anti–MBP-1 antibodies, but not with anti-IgG antibody (Figure 3D). No amplified PCR products of COX-1 promoter were found in those immunoprecipitated with anti–α-enolase, anti–MBP-1, and anti-IgG antibodies. These results suggest that MBP-1 binds to COX-2 and c-Myc promoters in SC-M1 cells.
MBP-1 Suppresses Tumor Progression of SC-M1 Cells Through COX-2
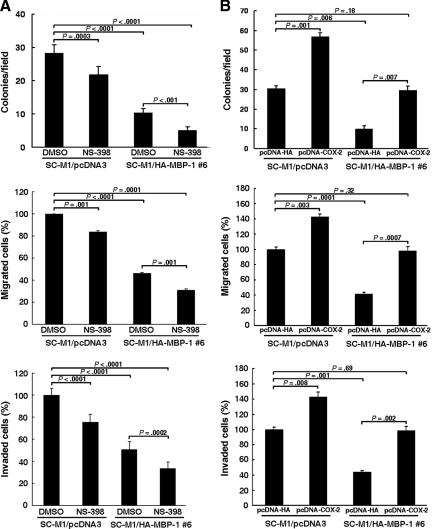
To delineate whether the MBP-1–suppressed tumor progression in gastric cancer is through decreasing COX-2 expression, MBP-1–expressing SC-M1/HA-MBP-1 cells were treated with 50 μM NS-398 to block COX-2 activity. Colony formation, migration, and invasion abilities of SC-M1/pcDNA3 control cells were attenuated after NS-398 treatment (Figure 4A). The MBP-1–suppressed abilities of colony formation, migration, and invasion were further inhibited by NS-398 in SC-M1/HA-MBP-1 #6 cells.
Figure 4.
MBP-1 suppresses tumor progression of SC-M1 cells through COX-2. (A) SC-M1/HA-MBP-1 #6 cells were treated with 50 μM NS-398 (A) or transfected with COX-2-expressing construct pcDNA-COX-2 (B) for colony-forming (upper), migration (middle), and invasion (lower) assays. Means of three independent experiments performed in triplicate are shown.
Additionally, we evaluated whether the MBP-1–suppressed tumor progression in SC-M1 cells is reversed by PGE2, a major product of COX-2. Results showed that ability of tumor progression was elevated in control cells after PGE2 treatment (Supplemental Figure S5). The MBP-1–suppressed tumor progression was reversed by PGE2 in SC-M1/HA-MBP-1 #6 cells.
We also checked whether the MBP-1–suppressed tumor progression in SC-M1 cells is affected by exogenous COX-2. Results showed that ability of tumor progression was elevated in control cells after transfection with COX-2–expressing construct (Figure 4B). The MBP-1–suppressed tumor progression was restored by transfection with COX-2–expressing construct in SC-M1/HA-MBP-1 #6 cells.
Furthermore, the enhancement of tumor progression in SC-M1 cells by MBP-1 knockdown was evaluated after treatment with COX-1 or COX-2 inhibitors. The colony formation, migration, and invasion abilities of SC-M1 cells transfected with siRNA vector (#22) to knock down endogenous MBP-1 were examined after treatment with valeryl salicylate or NS-398. The elevation of tumor progression by MBP-1 knockdown in SC-M1 cells was inhibited by NS-398 but not valeryl salicylate (Supplemental Figure S6A).
The effect of COX-1 and COX-2 knockdowns on the enhanced tumor progression by MBP-1 knockdown in SC-M1 cells was also assessed. Colony formation, migration, and invasion abilities of SC-M1 cells were analyzed after cotransfection with siRNA vectors against MBP-1 (#22) and COX-1 or COX-2 (#1 and #5). The enhanced tumor progression by MBP-1 knockdown in SC-M1 cells was abrogated by knockdown of COX-2 but not COX-1 (Supplemental Figure S6B). Collectively, these data suggest that COX-2 is essential for the suppression of tumor progression by MBP-1 in SC-M1 cells.
COX-2 Restores the MBP-1–Suppressed EMT in SC-M1 Cells
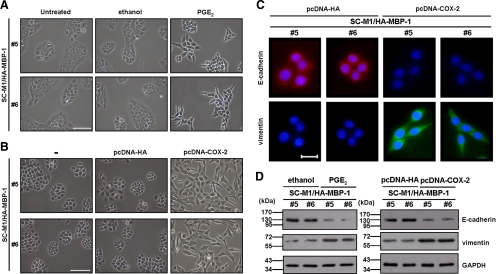
As demonstrated in Figure 2D, MBP-1 overexpression suppressed EMT in SC-M1 cells. To further check whether MBP-1 suppresses EMT through decreasing COX-2 expression, MBP-1–expressing SC-M1/HA-MBP-1 cells were treated with PGE2 or transfected with COX-2–expressing construct. The morphology of MBP-1–expressing SC-M1/HA-MBP-1 cells (#5 and #6) was restored from tightly packed colonies to more extended and elongated shape after 48 h of PGE2 treatment (Figure 5A) or COX-2–expressing construct transfection (Figure 5B). By immunofluorescence staining, E-cadherin expression was suppressed and vimentin expression was enhanced in MBP-1–expressing SC-M1/HA-MBP-1 cells (#5 and #6) after transfection with COX-2–expressing construct for 48 h (Figure 5C). Consistently, the same results were obtained by Western blot analysis after PGE2 treatment or COX-2–expressing construct transfection (Figure 5D).
Figure 5.
COX-2 restores the MBP-1–suppressed EMT in SC-M1 cells. (A and B) Morphology of SC-M1/HA-MBP-1 cells (#5 and #6) treated with 2 μg/ml PGE2 (A) or transfected with COX-2–expressing construct (B) for 48 h were examined. −, cells without transfection. Bar, 100 μm. (C) After transfection with COX-2–expressing construct or control vector, SC-M1/HA-MBP-1 cells (#5 and #6) were seeded for immunofluorescence staining to detect E-cadherin and vimentin. Bar, 20 μm. (D) SC-M1/HA–MBP-1 cells (#5 and #6) were treated with 2 μg/ml PGE2 (left) or transfected with COX-2–expressing construct (right) for 48 h for Western blot analysis.
MBP-1 Is Also Involved in Tumor Progression of AGS and NUGC-3 Gastric Cancer Cells
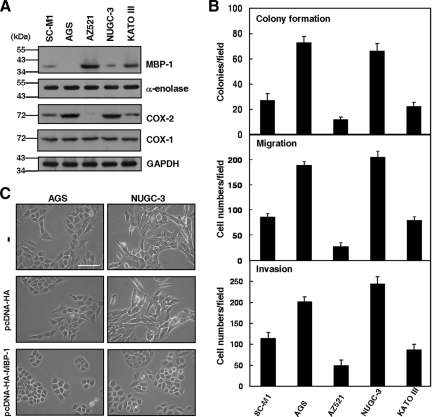
In addition to SC-M1 cells, we also checked whether MBP-1 participates in tumor progression of the other gastric cancer cells. The endogenous MBP-1 could be detected in SC-M1, AZ521, NUGC-3, and KATO III gastric cancer cells but not AGS cells by Western blot analysis (Figure 6A). Level of MBP-1 was inversely proportional to expression of COX-2 but not COX-1 in these cells. Furthermore, colony formation, migration, and invasion abilities of these cells were associated with COX-2 expression but inversely correlated with MBP-1 expression (Figure 6B).
Figure 6.
MBP-1 is also involved in tumor progression of AGS and NUGC-3 gastric cancer cells. (A) Whole-cell extracts of SC-M1, AGS, AZ521, NUGC-3, and KATO III cells were prepared for Western blot analysis using anti–MBP-1, anti–α-enolase, anti–COX-2, anti–COX-1, and anti-GAPDH antibodies. (B) SC-M1, AGS, AZ521, NUGC-3, and KATO III cells were seeded for colony-forming (upper), migration (middle), and invasion (lower) assays. (C) Morphology of AGS and NUGC-3 cells transfected with MBP-1–expressing construct or control vector for 48 h were examined. −, cells without transfection. Bar, 100 μm.
As shown in Figure 6A, both AGS and NUGC-3 cells that negatively or weakly expressed MBP-1 were used to evaluate the effect of MBP-1 on tumor progression. Results showed that colony formation, migration, and invasion abilities of these cells were suppressed after transfection with MBP-1–expressing construct (Supplemental Figure S7). Moreover, morphological changes in AGS and NUGC-3 cells from more extended and elongated shape to tightly packed colonies were also observed after transfection with MBP-1–expressing construct (Figure 6C).
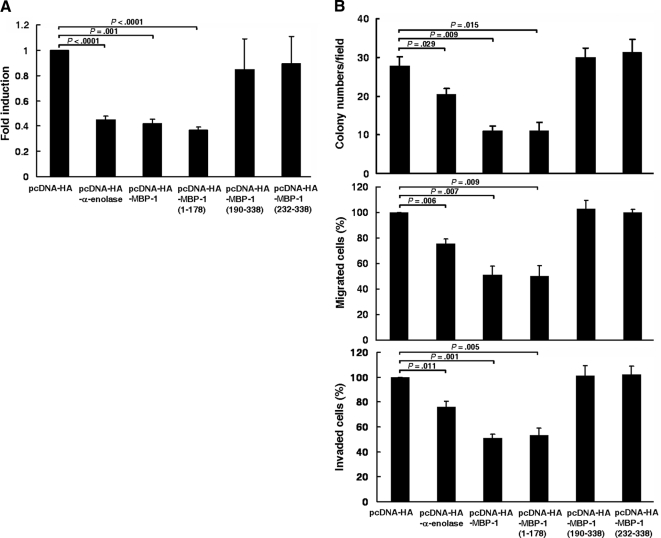
The Region of Amino Acid Residues 1-178 in MBP-1 Is Sufficient to Suppress COX-2 Promoter Activity and Gastric Cancer Progression
To dissect the region(s) essential for inhibition of COX-2 promoter activity and gastric cancer progression, expression plasmids harboring various lengths (amino acid residues 1-178, 190-338, and 232-338) of MBP-1 were transfected into K562 cells for reporter gene assay or SC-M1 cells for colony-forming, migration, and invasion assays. The results showed that COX-2 promoter activity was significantly suppressed after transfection with expression construct of truncated MBP-1 containing amino acid residues 1-178, but not 190-338 or 232-338 (Figure 7A). Additionally, the region of amino acid residues 1-178 in MBP-1 also inhibited gastric cancer progression including colony formation, migration, and invasion abilities (Figure 7B). Therefore, the region of N-terminal amino acid residues 1-178 in MBP-1 is essential for exhibiting the suppressive effects on COX-2 promoter activity and gastric cancer progression.
Figure 7.
The region of amino acid residues 1-178 in MBP-1 is sufficient to suppress COX-2 promoter activity and gastric cancer progression. (A) Reporter plasmid pCOX-2-Luc (−1334/−1) containing full-length COX-2 promoter was cotransfected with MBP-1–expressing construct (pcDNA-HA-MBP-1), α-enolase–expressing construct (pcDNA-HA-α-enolase), expression constructs of various lengths of MBP-1 (pcDNA-HA-MBP-1 [1-178], pcDNA-HA-MBP-1 [190-338], and pcDNA-HA-MBP-1 [232-338]), or control vector (pcDNA-HA) into K562 cells for reporter gene assay. (B) SC-M1 cells were transfected with MBP-1–expressing construct (pcDNA-HA-MBP-1), α-enolase-expressing construct (pcDNA-HA-α-enolase), expression constructs of various lengths of MBP-1 (pcDNA-HA-MBP-1 [1-178], pcDNA-HA-MBP-1 [190-338], and pcDNA-HA-MBP-1 [232-338]), or control vector (pcDNA-HA) for colony-forming (upper), migration (middle), and invasion (lower) assays. Means of three independent experiments performed in triplicate are shown.
DISCUSSION
The regulatory mechanisms of aggressiveness in gastric cancer still remain obscure. To unravel the molecular basis for gastric cancer progression, the role of MBP-1 in tumor growth and metastasis was investigated herein. We show that MBP-1 exerts a tumor-suppressive effect on gastric cancer progression by decreasing COX-2 expression. To our knowledge, this is the first report regarding the role of MBP-1 in modulation of tumorigenesis in gastric cancer through COX-2 expression.
In gastric cancer, COX-2 expression is correlated with tumor progression (Murata et al., 1999; Ohno et al., 2001; Shi et al., 2003). We found that MBP-1 may regulate tumor progression in gastric cancer through the MBP-1–COX-2 signaling axis. In addition to gastric cancer, MBP-1 was shown to participate in tumorigenesis of several tumors (Ray et al., 1995; Chang et al., 2003; Ejeskar et al., 2005; Ghosh et al., 2005a; Ghosh et al., 2005b; Takashima et al., 2005; Ghosh et al., 2006a; Ghosh et al., 2006b). Interestingly, COX-2 overexpression is observed in a variety of malignancies (Sano et al., 1995; Ristimäki et al., 1997; Hida et al., 1998; Zimmermann et al., 1999). Therefore, MBP-1 may also be involved in tumorigenesis of various tumor types through COX-2.
Besides c-Myc P2 promoter (Chaudhary and Miller, 1995), MBP-1 could bind on COX-2 promoter (Figure 3D). However, MBP-1 does not contain the DNA-binding domain. How did it bind on COX-2 promoter to suppress COX-2 promoter activity? Possibly, MBP-1 may bind on COX-2 promoter to modulate COX-2 expression directly. To delineate this possibility, it is indispensable to further map the critical regions of COX-2 promoter regulated by MBP-1. Alternatively, MBP-1 could indirectly bind on COX-2 promoter through directly interacting with transcription factors to regulate COX-2 expression. Therefore, it is important to identify candidates of MBP-1–associated cellular factors.
Interestingly, MBP-1 overexpression exerts suppressive effect on cell growth of prostate cancer (Ghosh et al., 2005a; Ghosh et al., 2005b), whereas MBP-1 knockdown inhibits prostate cancer growth and enlarges cell size (Ghosh et al., 2006a). The endogenous and overexpressed MBP-1s may exhibit opposite biological functions in control of prostate cancer growth (Ghosh et al., 2006a). At present, there are several cellular factors playing the multifaceted roles. For example, YY1 may function as a transcritptional repressor, a transcription activator, or a transcriptional initiator. Depending on tumor types, Notch receptor also may act as an oncogene or tumor suppressor to promote or suppress tumorigenesis (Roy et al., 2007). The mutations of von Hippel–Lindau tumor suppressor gene enhance susceptibility to development of highly vascularized tumors (Mack et al., 2005). However, loss of von Hippel–Lindau tumor suppressor gene was demonstrated to arrest cell growth.
MBP-1 and α-enolase are involved in tumorigenesis (Ray et al., 1995; Chang et al., 2003; Ejeskar et al., 2005; Ghosh et al., 2005a; Ghosh et al., 2005b; Takashima et al., 2005; Ghosh et al., 2006a; Ghosh et al., 2006b) and metastasis (Ray et al., 1995; Chang et al., 2003; Demir et al., 2005). It was proven that MBP-1 has the potential of a candidate for gene therapy against tumor growth (Chang et al., 2003; Ghosh et al., 2005b; Ghosh et al., 2006b). Although the susceptibility of substantial gastrointestinal toxicity and increase of cardiovascular risk, COX-2 inhibitors were shown to have a promising role in prevention and treatment of gastric cancer (Jiang and Wong, 2003; Fujimura et al., 2006; Lee and Moss, 2006). Based on the findings described in Figure 4A, the MBP-1–suppressed tumor progression of SC-M1 cells was further inhibited after treatment with NS-398. Therefore, gene therapy targeting MBP-1 in combination with chemotherapeutic targeting COX-2 could offer a new therapeutic strategy for treatment with gastric cancer in the future.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. L.-F. Shyur for the kind gift of pCOX-2-Luc (−1334/−1) reporter plasmids. RNAi reagents were obtained from the National RNAi Core Facility. This work was supported by grants from National Science Council (NSC 96-3112-B-010-019, NSC 97-3112-B-010-011, and NSC 98-3112-B-010-004) and a grant from Ministry of Education, Aim for the Top University Plan.
Abbreviations used:
- MBP-1
c-Myc promoter binding protein 1
- COX-2
cyclooxygenase 2
- EMT
epithelial-mesenchymal transition
- PGE2
prostaglandin E2
- HEK
human embryonic kidney
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide
- DAPI
4′, 6-diamidino-2-phenylindole dihydrochloride
- ChIP
chromatin immunoprecipitation
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-05-0386) on October 21, 2009.
REFERENCES
- Chang Y. S., Wu W., Walsh G., Hong W. K., Mao L. Enolase-α is frequently down-regulated in non-small cell lung cancer and predicts aggressive biological behavior. Clin. Cancer Res. 2003;9:3641–3644. [PubMed] [Google Scholar]
- Chaudhary D., Miller D. M. The c-myc promoter binding protein (MBP-1) and TBP bind simultaneously in the minor groove of the c-myc P2 promoter. Biochemistry. 1995;34:3438–3445. doi: 10.1021/bi00010a036. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Wu C. W., Kao H. L., Chang H. M., Li A.F.Y., Liu T. Y., Chi C. W. Effects of COX-2 inhibitor on growth of human gastric cancer cells and its relation to hepatocyte growth factor. Cancer Lett. 2006;239:263–270. doi: 10.1016/j.canlet.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Demir A. Y., Groothuis P. G., Dunselman G.A.J., Schurgers L., Evers J.L.H., de Goeij A.F.P.M. Molecular characterization of soluble factors from human menstrual effluent that induce epithelial to mesenchymal transitions in mesothelial cells. Cell Tissue Res. 2005;322:299–311. doi: 10.1007/s00441-005-0002-6. [DOI] [PubMed] [Google Scholar]
- Ejeskar K., Krona C., Caren H., Zaibak F., Li L., Martinsson T., Ioannou P. Introduction of in vitro transcribed ENO1 mRNA into neuroblastoma cells induces cell death. BMC Cancer. 2005;5:161. doi: 10.1186/1471-2407-5-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Executive Yuan. Taiwan: Department of Health, Executive Yuan; 2006. Taiwan area: death rate of ten leading sites of malignant neoplasms; pp. 160–173. [Google Scholar]
- Feo S., Arcuri D., Piddini E., Passantino R., Giallongo A. ENO1 gene product binds to the c-myc promoter and acts as a transcriptional repressor: relationship with Myc promoter-binding protein 1 (MBP-1) FEBS Lett. 2000;473:47–52. doi: 10.1016/s0014-5793(00)01494-0. [DOI] [PubMed] [Google Scholar]
- Fujimura T., Ohta T., Oyama K., Miyashita T., Miwa K. Role of cyclooxygenase-2 in the carcinogenesis of gastrointestinal tract cancers: a review and report of personal experience. World J. Gastroenterol. 2006;12:1336–1345. doi: 10.3748/wjg.v12.i9.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K., Kanda T., Steele R., Ray R. B. Knockdown of MBP-1 in human foreskin fibroblasts induces p53–p21 dependent senescence. PLoS ONE. 2008;3:e3384. doi: 10.1371/journal.pone.0003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K., Majumder M., Steele R., White R. A., Ray R. B. A novel 16-kilodalton cellular protein physically interacts with and antagonizes the functional activity of c-myc promoter-binding protein 1. Mol. Cell. Biol. 2001;21:655–662. doi: 10.1128/MCB.21.2.655-662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K., Steele R., Ray R. B. MBP-1 physically associates with histone deacetylase for transcriptional repression. Biochem. Biophys. Res. Commun. 1999;260:405–409. doi: 10.1006/bbrc.1999.0921. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K., Steele R., Ray R. B. c-myc promoter-binding protein 1 (MBP-1) regulates prostate cancer cell growth by inhibiting MAPK pathway. J. Biol. Chem. 2005a;280:14325–14330. doi: 10.1074/jbc.M413313200. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K., Steele R., Ray R. B. Carboxyl-terminal repressor domain of MBP-1 is sufficient for regression of prostate tumor growth in nude mice. Cancer Res. 2005b;65:718–721. [PubMed] [Google Scholar]
- Ghosh A. K., Steele R., Ray R. B. Knockdown of MBP-1 in human prostate cancer cells delays cell cycle progression. J. Biol. Chem. 2006a;281:23652–23657. doi: 10.1074/jbc.M602930200. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K., Steele R., Ryerse J., Ray R. B. Tumor-suppressive effects of MBP-1 in non-small cell lung cancer cells. Cancer Res. 2006b;66:11907–11912. doi: 10.1158/0008-5472.CAN-06-2754. [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Sala N., Capella G. Genetic susceptibility and gastric cancer risk. Int. J. Cancer. 2002;100:249–260. doi: 10.1002/ijc.10466. [DOI] [PubMed] [Google Scholar]
- Hida T., Yatabe Y., Achiwa H., Muramatsu H., Kozaki K., Nakamura S., Ogawa M., Mitsudomi T., Sugiura T., Takahashi T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- Hsu K. W., Hsieh R. H., Lee Y.H.W., Chao C. H., Wu K. J., Tseng M. J., Yeh T. S. The activated Notch1 receptor cooperates with α-enolase and MBP-1 in modulating c-myc activity. Mol. Cell. Biol. 2008;28:4829–4842. doi: 10.1128/MCB.00175-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Honma T., Ishida K., Wada N., Sasaoka S., Hosoda M., Nohno T. Differential expression of the human α-enolase gene in oral epithelium and squamous cell carcinoma. Cancer Sci. 2007;98:499–505. doi: 10.1111/j.1349-7006.2007.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wong B. Cyclooxygenase-2 inhibition and gastric cancer. Curr. Pharm. Des. 2003;9:2281–2288. doi: 10.2174/1381612033453983. [DOI] [PubMed] [Google Scholar]
- Katoh M. Epithelial-mesenchymal transition in gastric cancer (Review) Int. J. Oncol. 2005;27:1677–1683. [PubMed] [Google Scholar]
- Lee D., Moss S. COX-2 inhibition and the prevention of gastric cancer. Digestion. 2006;74:184–186. doi: 10.1159/000100502. [DOI] [PubMed] [Google Scholar]
- Liao W. R., Hsieh R. H., Hsu K. W., Wu M. Z., Tseng M. J., Mai R. T., Lee Y.H.W., Yeh T. S. The CBF1-independent Notch1 signal pathway activates human c-myc expression partially via transcription factor YY1. Carcinogenesis. 2007;28:1867–1876. doi: 10.1093/carcin/bgm092. [DOI] [PubMed] [Google Scholar]
- Lim H. Y., Joo H. J., Choi J. H., Yi J. W., Yang M. S., Cho D. Y., Kim H. S., Nam D. K., Lee K. B., Kim H. C. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin. Cancer Res. 2000;6:519–525. [PubMed] [Google Scholar]
- Mack F. A., Patel J. H., Biju M. P., Haase V. H., Simon M. C. Decreased growth of Vhl−/− fibrosarcomas is associated with elevated levels of cyclin kinase inhibitors p21 and p27. Mol. Cell. Biol. 2005;25:4565–4578. doi: 10.1128/MCB.25.11.4565-4578.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H., Kawano S., Tsuji S., Tsuji M., Sawaoka H., Kimura Y., Shiozaki H., Hori M. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am. J. Gastroenterol. 1999;94:451–455. doi: 10.1111/j.1572-0241.1999.876_e.x. [DOI] [PubMed] [Google Scholar]
- Neil J. R., Johnson K. M., Nemenoff R. A., Schiemann W. P. COX-2 inactivates Smad signaling and enhances EMT stimulated by TGF-β through a PGE2-dependent mechanisms. Carcinogenesis. 2008;29:2227–2235. doi: 10.1093/carcin/bgn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno R., Yoshinaga K., Fujita T., Hasegawa K., Iseki H., Tsunozaki H., Ichikawa W., Nihei Z., Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876–1881. [PubMed] [Google Scholar]
- Perconti G., Ferro A., Amato F., Rubino P., Randazzo D., Wolff T., Feo S., Giallongo A. The Kelch protein NS1-BP interacts with alpha-enolase/MBP-1 and is involved in c-Myc gene transcriptional control. Biochim. Biophys. Acta. 2007;1773:1774–1785. doi: 10.1016/j.bbamcr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Ray R. B., Sheikh M. S., Fontana J. F., Miller D. M. Human breast carcinoma cells show correlation in expression of c-myc oncogene and the c-myc binding protein (MBP-1) Int. J. Oncol. 1994;5:1433–1436. doi: 10.3892/ijo.5.6.1433. [DOI] [PubMed] [Google Scholar]
- Ray R. B., Steele R., Seftor E., Hendrix M. Human breast carcinoma cells transfected with the gene encoding a c-myc promoter-binding protein (MBP-1) inhibits tumors in nude mice. Cancer Res. 1995;55:3747–3751. [PubMed] [Google Scholar]
- Ristimäki A., Honkanen N., Jänkälä H., Sipponen P., Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276–1280. [PubMed] [Google Scholar]
- Roy M., Pear W. S., Aster J. C. The multifaceted role of Notch in cancer. Curr. Opin. Genet. Dev. 2007;17:52–59. doi: 10.1016/j.gde.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Sano H., Kawahito Y., Wilder R. L., Hashiramoto A., Mukai S., Asai K., Kimura S., Kato H., Kondo M., Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- Shi H., Xu J., Hu N., Xie H. Prognostic significance of expression of cyclooxygenase-2 and vascular endothelial growth factor in human gastric carcinoma. World J. Gastroenterol. 2003;9:1421–1426. doi: 10.3748/wjg.v9.i7.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Hold G., Tahara E., El-Omar E. Cellular and molecular aspects of gastric cancer. World J. Gastroenterol. 2006;12:2979–2990. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. L., Shih J. Y., Yen M. L., Jeng Y. M., Chang C. C., Hsieh C. Y., Wei L. H., Yang P. C., Kuo M. L. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res. 2004;64:554–564. doi: 10.1158/0008-5472.can-03-1301. [DOI] [PubMed] [Google Scholar]
- Subramanian A., Miller D. M. Structural analysis of alpha-enolase: mapping the functional domains involved in down-regulation of the c-myc protooncogene. J. Biol. Chem. 2000;275:5958–5965. doi: 10.1074/jbc.275.8.5958. [DOI] [PubMed] [Google Scholar]
- Takashima M., Kuramitsu Y., Yokoyama Y., Iizuka N., Fujimoto M., Nishisaka T., Okita K., Oka M., Nakamura K. Overexpression of alpha enolase in hepatitis C virus-related hepatocellular carcinoma: association with tumor progression as determined by proteomic analysis. Proteomics. 2005;5:1686–1692. doi: 10.1002/pmic.200401022. [DOI] [PubMed] [Google Scholar]
- Terry M., Gaudet M., Gammon M. The epidemiology of gastric cancer. Semin. Radiat. Oncol. 2002;12:111–127. doi: 10.1053/srao.30814. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Sleeman J. P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Uefuji K., Ichikura T., Mochizuki H., Shinomiya N. Expression of cyclooxygenase-2 protein in gastric adenocarcinoma. J. Surgical Oncol. 1998;69:168–172. doi: 10.1002/(sici)1096-9098(199811)69:3<168::aid-jso9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Ushijima T., Sasako M. Focus on gastric cancer. Cancer Cell. 2004;5:121–125. doi: 10.1016/s1535-6108(04)00033-9. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Ku H. H., Liang Y. C., Chen Y. C., Hwu Y. M., Yeh T. S. The autonomous Notch signal pathway is activated by baicalin and baicalein but is suppressed by niclosamide in K562 cells. J. Cell. Biochem. 2009;106:682–692. doi: 10.1002/jcb.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. W., Hsiung C. A., Lo S. S., Hsieh M. C., Chen J. H., Li A. F., Lui W. Y., Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309–315. doi: 10.1016/S1470-2045(06)70623-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Itoh F., Fukushima H., Hinoda Y., Imai K. Overexpression of cyclooxygenase-2 protein is less frequent in gastric cancers with microsatellite instability. Int. J. Cancer. 1999;84:400–403. doi: 10.1002/(sici)1097-0215(19990820)84:4<400::aid-ijc12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Yeh T. S., Lin Y. M., Hsieh R. H., Tseng M. J. Association of transcription factor YY1 with the high molecular weight Notch complex suppresses the transactivation activity of Notch. J. Biol. Chem. 2003;278:41963–41969. doi: 10.1074/jbc.M304353200. [DOI] [PubMed] [Google Scholar]
- Zheng L., Wang L., Ajani J., Xie K. Molecular basis of gastric cancer development and progression. Gastric Cancer. 2004;7:61–77. doi: 10.1007/s10120-004-0277-4. [DOI] [PubMed] [Google Scholar]
- Zimmermann K. C., Sarbia M., Weber A. A., Borchard F., Gabbert H. E., Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.