Abstract
The clarification of mechanisms that negatively regulate the invasive behavior of human glioma cells is of great importance in order to find new methods of treatment. In this study, we have focused on the negative regulation of lysophosphatidic acid (LPA)-induced migration in glioma cells. Using small interference RNA and dominant-negative gene strategies in addition to pharmacological tools, we found that isoproterenol (ISO) and sphingosine-1-phosphate (S1P) negatively but differently regulate the LPA-induced migration. ISO-induced suppression of the migration of glioma cells occurs via β2-adrenergic receptor/cAMP/Epac/Rap1B/inhibition of Rac, whereas S1P has been shown to suppress the migration of the cells through S1P2 receptor/Rho-mediated down-regulation of Rac1. The expression of tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is required for the inhibitory ISO-induced and Rap1B-mediated actions on the migration, Rac1 activation, and Akt activation in response to LPA. Thus, the PTEN-mediated down-regulation of phosphatidylinositol 3-kinase activity may be involved in the regulation of Rap1B-dependent inhibition of Rac1 activity. These findings suggest that there are at least two distinct inhibitory pathways, which are mediated by the S1P2 receptor and β2-adrenergic receptor, to control the migratory, hence invasive, behavior of glioma cells.
INTRODUCTION
The malignant astrocytomas, the anaplastic astrocytoma and glioblastoma multiforme, are the most common glial tumors, with an annual incidence of three to four per 100,000 of population (DeAngelis, 2001). Because radical surgical treatment of malignant glial tumors is impossible because of the highly expressed migratory and invasive features of glioma cells, further understanding of the complex regulation and signaling molecules involved in glioma invasion is still needed. Lysophosphatidic acid (LPA) has been shown to play a significant role in human tumorigenesis as a factor to increase the motility and invasiveness of different cell types (Imamura et al., 1993; Stam et al., 1998). In glioma cells as well, extracellularly added LPA induced the proliferation and migration of stimulated cells (Malchinkhuu et al., 2005). A major part of extracellular LPA is thought to be produced from lysophosphatidylcholine by autotaxin/lysophospholipase D (Tokumura et al., 2002; Umezu-Goto et al., 2002). In the CNS as well, LPA production seems to occur in a similar way (Sato et al., 2005). Recent evidence suggested that autotaxin is overexpressed in glioblastoma multiforme and contributes to the cell motility of glioblastomas (Kishi et al., 2006). For this reason, revealing new substances or mechanisms that could negatively regulate the LPA-induced invasive behavior is of great importance for devising new and effective treatment modalities to counteract tumor cells.
Our previous studies (Malchinkhuu et al., 2005, 2008) and those from other laboratories (Lepley et al., 2005) suggest that sphingosine 1-phosphate (S1P) and its receptors are candidates for the treatment of glioblastomas. Cyclic AMP–increasing agents have been shown to regulate cell migration (Howe, 2004; Howe et al., 2005) and to exert the inhibitory effect through cAMP-dependent protein kinase A (PKA) in fibroblasts (Kohyama et al., 2001) and through cAMP response element–binding protein in vascular smooth muscle cells (Ono et al., 2004). However, there are increasing numbers of reports showing that cAMP can also activate small GTPase Rap1 through stimulating Epac, an exchange protein directly activated by cAMP (de Rooij et al., 1998; Kawasaki et al., 1998; Enserink et al., 2002). Rap1 activation seems to play a crucial role in the control of cell adhesion and migration through the inside-out activation of integrins (Rangarajan et al., 2003). Thus, cAMP-increasing agents, whether their mechanism is through PKA or Epac/Rap1, may be effective tools to regulate the invasiveness of glioblastomas. In this study, we have aimed to elucidate the effect of cAMP on glioma cell movement. In particular, we have tried to clarify the mechanisms whereby a cAMP-increasing β-adrenergic agonist, isoproterenol (ISO), regulates the LPA-induced migratory response, and compared them to those of S1P, which inhibits the migration through S1P2-mediated Rho-dependent suppression of Rac (Sugimoto et al., 2003; Sanchez et al., 2005; Malchinkhuu et al., 2008). Here, we report that, unlike S1P action, β2-adrenergic receptor–mediated ISO action utilizes small GTPase Rap1B to inhibit glioma cell migration. Furthermore, the activation of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) that leads to the suppression of phosphatidylinositol 3-kinase (PI3K)-dependent pathway may be critical for the ISO-induced inhibitory action.
MATERIALS AND METHODS
Materials
1-Oleoyl-sn-glycero-3-phosphate (lysophosphatidic acid; LPA) and sphingosine 1-phosphate (S1P) were purchased from Cayman Chemical Co. (Ann Arbor, MI); anti-PTEN antibody (2B1) and anti-Rap1GAP antibody (H-93) were from Santa Cruz Biotechnology (Santa Cruz, CA); anti-phospho-Akt antibody (Ser473) and anti-Akt antibody were from Cell Signaling Technology (Beverly, MA); 8-(4-chlorophenylthio)-2′-O-methyladenosine 3′, 5′-cyclic monophosphate (8CPT-2Me-cAMP), isoproterenol, CGP-20712A, ICI 118,551, forskolin, anti-FLAG M2 antibody, and anti-β-actin antibody were from Sigma-Aldrich (St. Louis, MO); EZ-Detect Activation Kit for Rap1, Rac1, and Rho were from Pierce (Rockford, IL); nonsilencing RNAs (si-NS, D-001206-13), and siRNAs specific to Rap1A (M-003623-01), Rap1B (M-010364-02) and PTEN (M-003023-01) were from Dharmacon (Lafayette, CO); RNAiFect reagent was from QIAGEN (Valencia, CA); dipotassium bisperoxo(picolinato)oxovanadate (V) [bpV(pic), a PTEN inhibitor], and fatty acid–free BSA were from Calbiochem-Novabiochem (San Diego, CA); myristoylated PKA inhibitor (myr-PKI) was from BIOMOL Research Laboratories (Plymouth Meeting, PA); and AKAP St-Ht31 inhibitor peptide and St-Ht31P control peptide were from Promega (Madison, WI). The cDNA coding T19N-RhoA was a gift from Prof. Kozo Kaibuchi (Nagoya University, Nagoya, Japan) and G12VRap1 and Rap1GAPII were from Prof. Naoki Mochizuki (National Cardiovascular Center Research Institute, Osaka, Japan). Cilostazol, an antiplatelet agent was provided by Otsuka Pharmaceutical (Tokyo, Japan). The sources of all other reagents were the same as those described previously (Malchinkhuu et al., 2005; Sato et al., 2005).
Cell Culture
GNS-3314 cells and CGNH-89 cells (Ishiuchi et al., 2002), established from human glioblastoma surgical specimens, were grown in MEM containing 10% FBS. Human U87MG cells were grown in DMEM supplemented with 10% FBS. Rat C6 glioma and human 1321N1 astrocytoma cells were grown in DMEM supplemented with 10% FBS (Malchinkhuu et al., 2005). For serum-free studies, the culture medium was changed to MEM or DMEM containing 0.1% BSA, and incubated for 24–48 h.
Construction of Adenoviral Vector and Infection of Recombinant Adenovirus
The cDNAs for human PTEN (C124S), a PTEN mutant that lacks both lipid and protein phosphatase activity (Maehama and Dixon, 1998), were used for recombinant adenovirus construction according to the instruction manual of AdEasy XL Adenoviral Vector System (Stratagene, La Jolla, CA). To construct recombinant adenovirus for human Rap1GAPII, the entire coding region of Rap1GAPII (Fukuhara et al., 2005) was subcloned into pShuttle-CMV according to the instruction manual of AdEasy XL Adenoviral Vector System. The cDNA coding G12VRap1, a constitutively active form of Rap1, containing FLAG in Adeno-X Expression System (Fukuhara et al., 2005) was used for recombinant adenovirus construction. The recombinant adenovirus for T19NRhoA, a dominant-negative form of RhoA, and p115RGS, an RGS domain of p115RhoGEF, were constructed as described previously (Arai et al., 2003). The adenovirus expressing green fluorescent protein (GFP) was used for evaluation of the expression by visualization. For the infection with recombinant adenoviruses, glioma cells were plated on 6- or 10-cm dishes. When the cells had become 90% confluent, the culture medium was changed to DMEM with 5% FBS containing adenovirus at a multiplicity of infection of 100. After incubation for 1 h at 37°C, the infection was terminated by adding an excess amount of a fresh medium containing 0.1% BSA and further cultured for 24–48 h. The cells were used for subsequent application as shown below.
Transfection of siRNA and Plasmid DNA
When the glioblastoma cells reached ∼75–80% confluency, the amount (total 50–100 nM) of siRNAs specific to Rap1A, Rap1B, PTEN, and nonsilencing RNA (si-NS) were transfected using an RNAiFect reagent as described previously (Malchinkhuu et al., 2005). After 48-h transfection, knockdown of targets was assessed by RT-PCR or Western blot, and a cell migration assay was performed 24 h after serum starvation. For the transfection experiments with pEGFP-C1 (Clontech, Palo Alto, CA) containing PTEN, U87MG cells were harvested with trypsin and washed with PBS. The cell suspension (∼5 × 106 cells in 0.1 ml of U87MG Nucleofector Kit T Solution) was mixed with plasmid DNA (2 μg), transfected by Nucleofector (Amaxa, Gaithersburg, MD), and cultured for 24 h on 6-cm dishes in DMEM containing 10% FBS. The overexpression of PTEN was assessed by GFP expression (∼70%), and a cell migration assay was performed 24 h after serum starvation.
Cell Migration Assay
The cell migration was quantified using a blind Boyden chamber apparatus (Neuro Probe, Gaithersburg, MD) as described previously (Kon et al., 1999). Briefly, the lower chambers were filled with the indicated concentrations of test agents and subsequently were covered with 8-μm pore filters coated with type I collagen. The cells were trypsinized and washed once with DMEM containing 0.1% BSA. Resuspended cells, after being incubated for 30 min with or without different inhibitors at 37°C, were loaded into the upper chamber. Migration was allowed to proceed for 4 h at 37°C under a humidified air/CO2 (19:1) atmosphere. Cells that had migrated to the lower surface were counted in four microscopic fields at 400× magnification. Results were expressed as the number of migrated cells per fields.
Quantitative RT-PCR Using Real-Time TaqMan
The isolated total RNA was treated with DNase I to remove genomic DNA contaminating in RNA preparations. The total RNA (5 μg) was subjected to the quantitative RT-PCR using TaqMan technology as described previously (Yamada et al., 2004). The human Rap1A (Hs002324123)-, Rap1B (Hs00763004)-, PTEN (Hs00829813)-, and GAPDH (Hs99999905)-specific probes were obtained from Assay-on-Demand products (Applied Biosystems, Foster City, CA). The thermal cycling conditions were as follows: 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 s at 95°C, and 1 min at 60°C. The expression level of the target mRNA was normalized to the relative ratio of the expression of GAPDH mRNA.
Estimation of Rap1, Rho, and Rac1 Activation by the Pulldown Reaction
The 24-h serum-starved cells were washed once, preincubated for 10 min at 37°C in a HEPES-buffered medium composed of 20 mM HEPES, pH 7.4, 134 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2 mM CaCl2, 2.5 mM NaHCO3, 5 mM glucose, and 0.1% BSA and then incubated for 2.5 min with test agents at 37°C. The incubation was terminated by washing twice with ice-cold PBS and adding 0.8 ml of a lysis buffer according to the instruction manual of EZ-Detect Rap1, Rac1, and Rho Activation Kit (Pierce).
Estimation of Akt Activation
Anti-phosphorylated Akt antibody and anti-Akt antibody were used. The cells were serum-starved for 24 h before incubation with the HEPES-buffered medium containing test substances for the indicated times at 37°C. Reactions were terminated by washing twice with PBS and adding 0.2 ml of a lysis buffer composed of 50 mM HEPES, pH 7.0, 250 mM NaCl, 0.1% Nonidet P-40, 1% phosphatase inhibitor cocktail 2 (Sigma-Aldrich), and 1% proteinase inhibitor cocktail (Sigma-Aldrich). Cytosol fractions were prepared and subjected to the Western blot analysis.
Western Blot Analysis
For analyses of cellular proteins, the 24-h serum-starved cells were washed with PBS and harvested by adding a lysis buffer composed of PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, and 1% proteinase inhibitor cocktail. The recovered lysate was centrifuged at 14,000 × g for 20 min. The supernatant was resolved by 12.5% SDS-PAGE, and the protein bands were detected by alkaline phosphatase method, as described previously (Sato et al., 2005).
Measurement of cAMP Accumulation
The 24-h serum-starved glioma cells were washed once and preincubated for 10 min at 37°C in the HEPES-buffered medium, pH 7.4. The cells were then incubated for 5 min in the presence of 0.5 mM IBMX in a final volume of 0.4 ml. The reaction was terminated by adding 50 μl of 1N HCl. Cyclic AMP in the acid extract was measured by radioimmunoassay as described previously (Kon et al., 1999).
Data Presentation
All experiments were performed in duplicate or triplicate. The results of multiple observations are presented as the mean ± SEM or as representative results from more than three different batches of cells unless otherwise stated. Statistical significance was assessed by the Student's t test; values were considered significant at p <0.05 (*).
RESULTS
ISO Inhibits LPA-induced Glioma Cell Migration and Rac1 Activation in a Manner Independent of Rho Activity, Whereas S1P Does So through a Mechanism Involving Rho
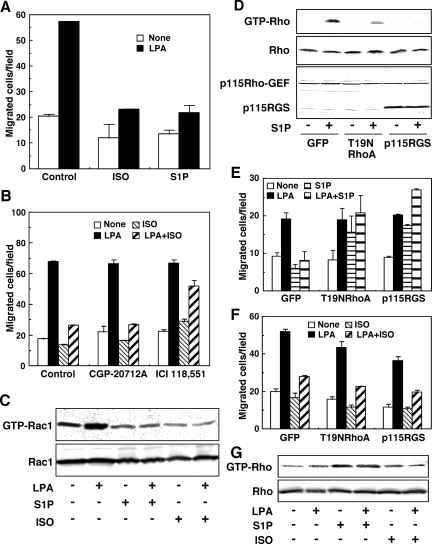
S1P has been shown to inhibit cell migration through S1P2 receptor/Rho signaling pathways in a variety of nontumor (Sugimoto et al., 2003; Sanchez et al., 2005) and tumor cells (Arikawa et al., 2003), including glioblastoma cells (Lepley et al., 2005; Malchinkhuu et al., 2005, 2008). In the present study, we examined the effect of ISO, a β-adrenergic receptor agonist, on glioma cell migration and compared it with that of S1P. In GNS-3314 human glioblastoma cells, ISO attenuated the migration response to LPA to the same level as S1P (Figure 1A), probably through a β2-adrenergic receptor (Figure 1B). Thus, the ISO-induced action was attenuated by ICI 118,551, a β2-adrenergic receptor antagonist, but not by CGP-20712A, a specific antagonist for the β1 receptor (Figure 1B). As we show in Figure 1C, the inhibitory action of ISO on migration was associated with the suppression of LPA-induced Rac1 activation, as was the case for the S1P action. Thus, either ISO or S1P inhibited LPA-induced migration of glioblastoma cells, in association with inhibition of Rac1 activity.
Figure 1.
S1P and ISO inhibited the LPA-induced migratory response by suppressing Rac1 activity in GNS-3314 cells via Rho-dependent and -independent pathways, respectively. (A) The cells were serum-starved and subjected to the migration assay. The migration was allowed to proceed for 4 h at 37°C. The serum-starved cells were stimulated with or without 100 nM LPA in the presence or absence of 1 μM ISO or 100 nM S1P. Results are expressed as the number of migrated cells per field. Data are the mean ± SEM of three determinations of a representative experiment. Other two separate experiments gave similar results. (B) Effect of adrenergic-receptor antagonists on migration. The serum-starved cells were preincubated for 30 min at 37°C with 300 nM CGP-20712A and 300 nM ICI 118,551, selective antagonists for β1- and β2-receptor, respectively. The cells were then allowed to migrate toward a vehicle, 100 nM LPA and/or 1 μM ISO. Results are expressed as described in A. (C) The serum-starved cells were stimulated for 2.5 min at 37°C with or without 100 nM LPA in the presence or absence of 100 nM S1P or 1 μM ISO and then Rac1 activity was estimated by the pulldown reaction. (D–F) Cells were infected with adenovirus containing GFP, T19NRhoA, or p115RGS and cultured in 0.1% BSA/MEM. The cells were then incubated for 2.5 min and 4 h with the indicated test agents, concentrations of which were the same as those in A, to measure Rho activity by the pulldown assay (D) and migration activity (E and F), respectively. The protein bands (bottom panel in D) were also detected with an antibody recognizing both p115Rho-GEF (wild type, 115 kDa) and p115RGS (a RGS domain of p115Rho-GEF, 35 kDa). (G) Cells were incubated for 2.5 min with or without 100 nM LPA in the presence or absence of 100 nM S1P and 1 μM ISO, and then cell lysates were probed for Rho activation. Data are representative results from three separate experiments.
We next examined the role of Rho in the negative regulation of LPA-induced actions. As shown in Figure 1D, a dominant-negative mutant T19NRho (for RhoA) or p115RGS (for p115Rho-GEF) clearly inhibited S1P-induced Rho activation. Consistently with a previous study (Malchinkhuu et al., 2008), the inhibitors of Rho signaling pathways reversed the inhibitory action caused by S1P (Figure 1E). Under the strong inhibition of Rho activation by p115RGS treatment, the inhibitory action of S1P on Rac1 activation in response to LPA was also reversed (Supplemental Figure S1). The significant increase in migration (Figure 1E) and small increase in Rac1 activity (Supplemental Figure S1) by S1P in the absence of LPA under the conditions of knockdown of Rho signaling pathways may be explained by unmasking the S1P1 receptor–mediated stimulatory activity on migration and Rac1 (Malchinkhuu et al., 2008). In contrast, the inhibitory action by ISO was hardly affected by the inhibitors of the Rho signaling pathways (Figure 1F). Indeed, in contrast to S1P, ISO did not appreciably affect the Rho activity (Figure 1G). S1P-related findings from previous (Malchinkhuu et al., 2005, 2008) and current studies suggest that, in human glioblastoma cells, S1P inhibits LPA-induced migration through S1P2 receptor-mediated and Rho-dependent suppression of Rac1 activity. However, the mechanism whereby ISO down-regulates Rac1 seems to be distinct from that of S1P.
Small G-Protein Rap1B, But Not PKA, Is Involved in ISO Inhibitory Action
As shown in Supplemental Figure S2, cAMP-increasing agents, including forskolin, an activator of adenylyl cyclase, and cilostazol, an inhibitor of phosphodiesterase, also inhibited LPA-induced migration, suggesting that cAMP mediates ISO-induced inhibitory action on the migration response to LPA. Indeed, ISO, but neither S1P nor LPA, induced a remarkable cAMP accumulation (Supplemental Figure S3). The cAMP-increasing agent, ISO, potentially utilizes both the PKA pathway and the PKA-independent Epac-Rap1 pathway to inhibit glioma cell migration. To determine the way ISO works in our case, we used specific inhibitors for PKA and found that the inhibitory action of ISO and forskolin was not appreciably affected by PKA-specific inhibitors (Supplemental Figure S2). The concentrations of PKA inhibitors used in our cell system appeared to be effective. As evidenced by the finding that the effect of cilostazol, a phosphodiesterase inhibitor, was reversed in cells pre-treated with these PKA inhibitors (Supplemental Figure S2). Thus, the activation of the PKA pathway potentially inhibits LPA-induced cell migration when cAMP degradation was inhibited by the phosphodiesterase inhibitor, but ISO and forskolin, both of which lead to cAMP accumulation by stimulating adenylyl cyclase, inhibited the migration response to LPA in a manner independent of PKA. Further experiments are necessary to determine whether the PKA-dependent and -independent mechanisms are attributed to different mechanisms of cAMP accumulation.
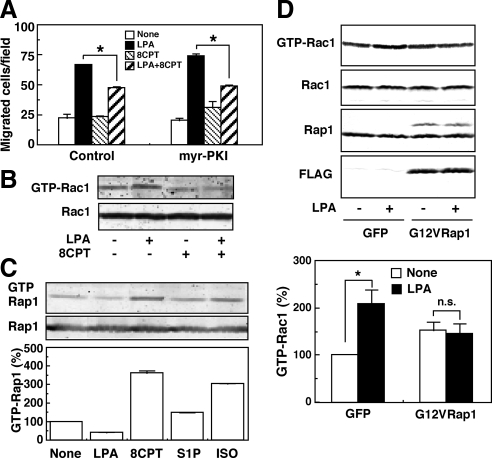
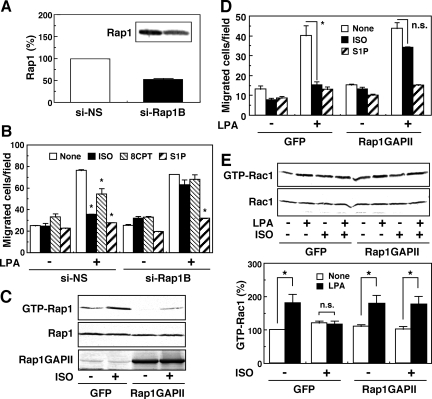
The ISO-induced inhibition of migration may be mediated by the Epac-Rap1 signaling pathway. Thus, an Epac-specific cAMP analogue, 8CPT-2Me-cAMP, was effective in inhibiting the LPA action on migration in a manner insensitive to the PKA inhibitor (Figure 2A). In this experiment, we used 8CPT-2Me-cAMP at 30 μM, which concentration is effective in other cell system with the specificity (Rangarajan et al., 2003). We also used 300 μM of the cAMP derivative and observed essentially the same results (data not shown). To exclude the possible inhibition of PKA, however, we used the cAMP derivative at 30 μM throughout the present study. The suppression of Rac1 activity by 8CPT-2Me-cAMP was also observed (Figure 2B). Moreover, we proved that ISO and 8CPT-2Me-cAMP activated the Rap1 protein (Figure 2C) and that the overexpression of an active-form of Rap1, G12VRap1, resulted in the abolishment of the Rac1 activation in response to LPA (Figure 2D). To further confirm the role of Rap1 in the inhibitory action of ISO, we used siRNA strategies. Transfection of siRNAs specific to Rap1B into GNS-3314 cells resulted in a 50–60% reduction of the expression of the Rap1B protein (Figure 3A). The decrease of Rap1B on the mRNA level was also confirmed (data not shown). Under the knockdown of Rap1B, a significant recovery of the migratory response to LPA, which was suppressed by ISO or 8CPT-2Me-cAMP, was observed without any significant change in S1P-induced inhibition (Figure 3B). In GNS-3314 cells, we could scarcely detect the expression of Rap1A mRNA (data not shown). Moreover, overexpression of Rap1GAPII, which inhibited the ISO-induced activation of Rap1 (Figure 3C), reversed ISO-induced inhibition of the migration response to LPA without any significant effect on the S1P-induced inhibitory action (Figure 3D). The appearance of LPA-induced migration under the ISO treatment by Rap1GAPII was associated with the activation of Rac1 (Figure 3E). Our findings are consistent with previous studies by another research group (Gutmann et al., 1997) showing that increased expression of the Rap1B protein is a common occurrence in human gliomas. Taken together, these results suggest that the PKA-independent Epac/Rap1B pathway may be important for ISO-induced inhibition of glioma cell migration and Rac1 activation.
Figure 2.
Rap1 and cAMP inhibited LPA-induced migration of GNS-3314 cells. (A) A PKA inhibitor myr-PKI failed to attenuate the inhibitory action of 8CPT-2Me-cAMP. The serum-starved cells were pretreated with 10 μM myr-PKI for 30 min and then stimulated with or without 100 nM LPA in the presence or absence of 30 μM 8CPT-2Me-cAMP (8CPT) for the migration assay. The asterisk (*) indicates a significant difference. (B) Rac1 activity assay was performed with or without 100 nM LPA in the presence or absence of 30 μM 8CPT-2Me-cAMP (8CPT). (C) The cells were stimulated with a vehicle, 100 nM LPA, 30 μM 8CPT-2Me-cAMP (8CPT), 100 nM S1P, or 1 μM ISO to measure Rap1 activity. Data are representative results (top panel) and active Rap1 activity (bottom panel) was expressed as percentages of the basal activity (None), which was quantified by densitometry. (D) Cells were infected with adenovirus containing GFP or G12VRap1, cultured in 0.1% BSA/MEM and then stimulated for the estimation of Rac1 activity by the pulldown reaction. The blotting data are representative results (top panels). Rac1 activities were quantified by densitometry and were expressed as percentages of control value (None in GFP; bottom panel). The effect of LPA was significant (*) or not significant (n.s.). The expression levels of wild-type Rap1 and the mutated Rap1 (G12VRap1; as a band with slightly slower migration than wild-type Rap1) were assessed with specific antibodies for Rap1 and/or FLAG.
Figure 3.
Involvement of Rap1B in the inhibitory action of ISO on LPA-induced migration in GNS-3314 cells. (A) After transfection with 50 nM siRNAs for nonsilencing (NS) and Rap1B, cell lysates were subjected to Western blot analysis for detection of Rap1 protein. The results are expressed as a percentage of the basal expression of Rap1 (NS), which was evaluated by densitometry. (B) The siRNA-transfected cells were stimulated with or without 100 nM LPA in the presence or absence of 1 μM ISO, 100 nM S1P, or 30 μM 8CPT-2Me-cAMP (8CPT) for migration assay. Data are the mean ± SEM of four separate experiments performed in duplicates. The asterisk (*) indicates a significant difference from None. (C–E) The cells were infected with adenovirus carrying GFP or Rap1GAPII and cultured in 0.1% BSA/MEM. The cells were then incubated with the indicated agents, concentrations of which are the same as those for B, to measure Rap1 activity by the pulldown assay in C, migration activity in D, and Rac1 activity by the pulldown assay in E. The expression level of Rap1GAPII (lower blot) was assessed with an antibody for Rap1GAP in C. Other experimental conditions are the same as those for Figure 2D. The effect of ISO in D and LPA in E was significant (*) or not significant (n.s.).
Involvement of PTEN in the Inhibitory Actions of ISO on LPA-induced Akt Activation, Rac1 Activation, and the Migration Response
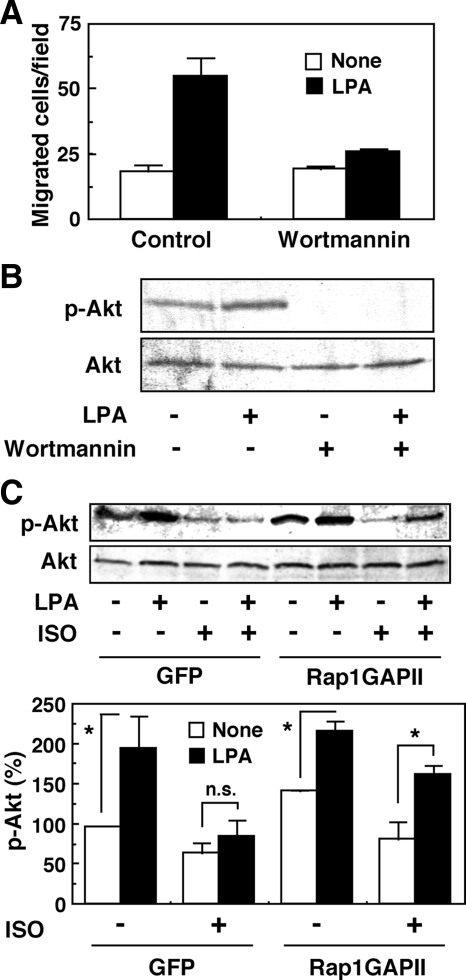
We have previously reported that, in human glioma cells, PI3K transmits the LPA1 receptor–mediated migratory response (Malchinkhuu et al., 2005). We assumed that, in some way, ISO may regulate the PI3K-dependent pathway. If this were the case, we could assess it by examining the phosphorylation of the PI3K downstream target, Akt. Consistently with a previous study (Malchinkhuu et al., 2005), wortmannin, an inhibitor of PI3K, at 100 nM attenuated the migration response to LPA (Figure 4A). The concentration of wortmannin was chosen based on the original article (Okada et al., 1994) and appears to be effective in our system, with specificity as well. Thus, 100 nM of wortmannin almost completely inhibited the LPA-induced Akt activation (Figure 4B) without any appreciable effect on S1P-induced RhoA activation (Supplemental Figure S4A). The inhibitory effect of wortmannin on the migration response to LPA was also observed in other glioma cells: C6 cells (Malchinkhuu et al., 2005), U87MG cells, and 1321N1 astrocytoma cells (Supplemental Figure S4, B and C). Although ISO has been shown to activate Akt in different types of cells (Morisco et al., 2005), in our cell system, we observed that ISO abrogates the LPA-induced Akt phosphorylation and that the ISO-induced inhibitory action was reversed by down-regulation of Rap1 activity with Rap1GAPII overexpression (Figure 4C).
Figure 4.
Attenuation by ISO of LPA-induced Akt phosphorylation in GNS-3314 cells seems to be Rap1-dependent. (A) The serum-starved cells were treated with 100 nM wortmannin for 15 min and applied to migration assay toward a vehicle and 100 nM LPA. (B) The cells were pre-treated with 100 nM wortmannin for 15 min, stimulated with or without 1 μM LPA, and then analyzed by Western blot for detection of phosphorylated Akt. Data are representative results from two separate experiments. (C) Cells were infected with adenovirus carrying GFP or Rap1GAPII, cultured in 0.1% BSA/MEM and then treated with a vehicle, 1 μM LPA, and/or ISO for 2.5 min at 37°C to measure phosphorylation of Akt protein. Data are representative results (top panels). Akt phosphorylation activities were quantified by densitometry and were expressed as percentages of control value (None without ISO in GFP treatment; bottom panel). The effect of LPA was significant (*) or not significant (n.s.).
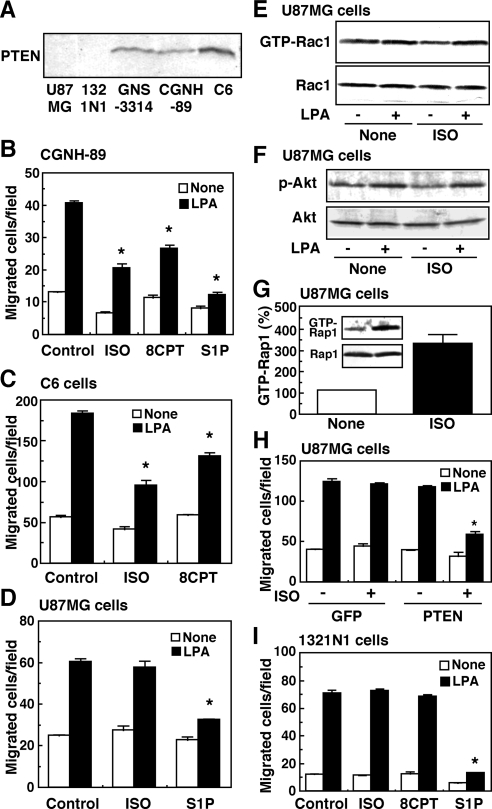
Because PI3K activity is counteracted by a lipid phosphatase, PTEN, inhibition of LPA-induced Akt activation by ISO can be achieved by the activation of PTEN and/or the inhibition of PI3K. It is well known that alteration of the PTEN tumor suppressor gene is associated with glioblastomas (Rasheed et al., 1997). We, therefore, examined ISO action on migration in astrocytoma and glioma cells other than GNS-3314 in relation to PTEN expression. Consistently with a previous study (Li et al., 1997), PTEN was null in U87MG cells (Figure 5A). PTEN was not detected in 1321N1 astrocytoma cells either, although a significant level of PTEN protein was detected in GNS-3314, CGNH-89, and C6 cells. In CGNH-89 and C6 cells expressing the PTEN protein, ISO and 8CPT-2Me-cAMP effectively inhibited LPA-induced migration (Figure 5, B and C), as in GNS-3314 cells. However, ISO or 8CPT-2Me-cAMP, but not S1P, was ineffective in inhibiting LPA-induced migration of PTEN-null U87MG cells and 1321N1 astrocytoma cells (Figure 5, D and I). Consistent with the result of the insignificant effect of ISO on the migration response, Rac1 activation and Akt phosphorylation in response to LPA were not affected by the ISO treatment in U87MG cells (Figure 5, E and F), although Rap1 activation by ISO was detected (Figure 5G), suggesting the presence of ISO-sensitive receptor-mediated Rap1 signaling pathways. Nevertheless, when wild-type PTEN was introduced into U87MG cells, the inhibitory action by ISO on LPA-induced migration could be reconstituted (Figure 5H). These results raised the possibility that PTEN is required for the ISO-induced inhibition of cell migration.
Figure 5.
The ISO- and 8CPT-2Me-cAMP–induced action on cell motility is dependent on PTEN expression in glioma cells. (A) Detection of PTEN by Western blot analysis. Cell extracts were prepared from human U87MG cells, 1321N1 astrocytoma cells, GNS-3314 cells, CGNH-89 cells, and rat C6 glioma cells. (B) CGNH-89 cells were serum-starved and subjected to migration assay. The cells were stimulated with or without 100 nM LPA in the presence or absence of 1 μM ISO, 30 μM 8CPT-2Me-cAMP (8CPT), and 100 nM S1P. (C) C6 cells were serum-starved and stimulated with or without 100 nM LPA in the presence or absence of 1 μM ISO and 30 μM 8CPT-2Me-cAMP (8CPT). (D–G) U87MG cells were serum-starved and stimulated with the indicated agents to measure migration activity (D), Rac1 activity by the pulldown assay (E), Akt phosphorylation activity in (F), and Rap1 activity by the pulldown assay (G). The concentrations of test agents and other experimental conditions were the same as those of B for D, Figure 1C for E, Figure 4C for F, and Figure 2C for G. (H) U87MG cells were transfected with pEGFP-C1 containing PTEN or plasmid alone (GFP) according to Nucleofector Kit T, cultured in 0.1% BSA/DMEM, and then incubated to measure the migratory response to a vehicle and 100 nM LPA in the presence or absence of 1 μM ISO. (I) 1321N1 cells were serum-starved and treated with or without 100 nM LPA in the presence or absence of 1 μM ISO, 30 μM 8CPT-2Me-cAMP (8CPT), and 100 nM S1P. The LPA effect was significantly lower than that in control cells (*) in B–D, H, and I.
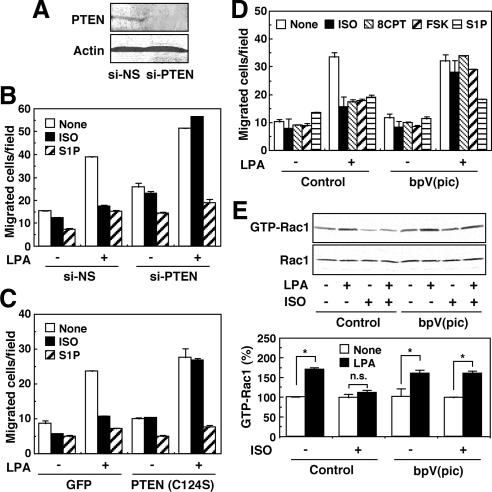
To address this possibility, we used siRNA specific to PTEN in PTEN-expressing GNS-3314 cells. As shown in Figure 6A, siRNA treatment resulted in a remarkable decrease in PTEN expression, which was associated with a reversal of ISO-induced inhibition of the migration response to LPA (Figure 6B). Infection of an adenovirus containing a dominant-negative C124S mutant, which may lead to a complete loss of phosphatase activity (Maehama and Dixon, 1998; Figure 6C), or treatment with a selective PTEN inhibitor, bpV(pic) (Figure 6D; Schmid et al., 2004), was also effective for the suppression of the ISO-induced inhibitory action on migration in GNS-3314 cells. The reversal of the ISO-induced inhibitory migration response to LPA by bpV(pic) was associated with an appearance of Rac1 response to LPA which was inhibited by ISO (Figure 6E). In the cells treated with the PTEN inhibitor forskolin, and 8CPT-2Me-cAMP–induced inhibition of migration response to LPA was also reversed (Figure 6D). As shown in Supplemental Figure S5, the siRNA against PTEN or the PTEN inhibitor also reversed ISO- or 8CPT-2Me-cAMP–induced inhibition of the migration response to LPA in the PTEN-expressing CGNH-89 and C6 cells. However, S1P-induced suppression of migration was not recovered by the treatments (Figure 6 and Supplemental Figure S5B). We finally examined the possibility of whether ISO and other test agents affect the expression of PTEN, because it has been reported that altered expression of PTEN may play a significant role in the progression of glioblastoma multiforme (Sano et al., 1999). As shown in Supplemental Figure S6, however, ISO, S1P, and LPA did not significantly alter the PTEN expression, at least under the experimental conditions used. In any event, the present results strongly suggest that PTEN is critically required for the Rap1B-dependent negative regulation of migration by ISO.
Figure 6.
Involvement of PTEN in ISO action on migration of GNS-3314 cells. (A) After transfection of the cells with 100 nM siRNAs for nonsilencing (NS) and PTEN, cell lysates were prepared and subjected to Western blot analysis for detection of PTEN and β-actin. (B–D) The cells were similarly treated with siRNAs in B, infected with adenovirus containing GFP or C124S mutant of PTEN in C, and treated with or without 300 nM bpV(pic) for 30 min in D. The cells were then incubated with or without 100 nM LPA in the presence or absence of 1 μM ISO, 100 nM S1P, 30 μM 8CPT-2Me-cAMP (8CPT), or 300 nM forskolin (FSK) to measure migration activity. (E) Cells were pretreated with 300 nM bpV(pic) for 30 min and then stimulated with or without 100 nM LPA in the presence or absence of 1 μM ISO. Cell lysates were subjected to the Rac1 activation assay by the pulldown reaction. Data are representative results from three separate experiments (top panels). Rac1 activities were quantified by densitometry and were expressed as percentages of control value (None; bottom panel). The asterisk (*) indicates that LPA effect is statistically significant.
DISCUSSION
In glioma cells, PI3K/Rac1 pathways are important for LPA1 receptor-mediated migration (Malchinkhuu et al., 2005). Our present results demonstrated that S1P and ISO both negatively but differently regulate LPA-induced glioma cell migration. Consistently with previous studies of glioma cells (Lepley et al., 2005; Malchinkhuu et al., 2005, 2008) and other cell types (Arikawa et al., 2003; Sugimoto et al., 2003; Sanchez et al., 2005), the present study suggests that S1P suppresses the migration through S1P2 receptor/Rho-mediated down-regulation of Rac1. In contrast, ISO-induced suppression occurs via β2-adrenergic receptor/cAMP/Epac/Rap1B-dependent down-regulation of Rac1 activation. Thus, we speculate that there are at least two distinct inhibitory pathways, which are mediated by the S1P2 receptor and the β2-adrenergic receptor, to control the migration of glioma cells. Furthermore, on the basis of the findings from the experiments in which PTEN expression was down-regulated genetically or pharmacologically, we suggest that activated PTEN may be crucial for ISO inhibitory action on the PI3K-dependent pathway and, consequently, cell migration. Thus, the inhibitory effect of ISO was coincident with the expression of PTEN protein in glioma cells. To our knowledge, this is the first observation showing cAMP stimulators, such as ISO, to regulate endogenous PTEN activity through the activation of small GTPase Rap1B for the inhibition of Rac1 activity and glioma cell motility.
The Rho-like G-proteins, i.e., Rho and Rac, are known principally for their pivotal role in regulating the actin cytoskeleton in cell migration (Burridge and Wennerberg, 2004). PI3K has been thought to be a key molecule in the activation of cell migration through Rac signaling (Chandrasekar et al., 2003; van Leeuwen et al., 2003). The Rho-like G-proteins seem to play a dichotomous cell type–specific role in the regulation of motility (Malchinkhuu et al., 2005). In some tumor cells, S1P inhibits cell migration, and this inhibitory effect of S1P occurs through the S1P2/Rho-mediated suppression of Rac (Arikawa et al., 2003; Lepley et al., 2005). Consistent with this, the present and previous results showed that S1P inhibited the migration via Rho-dependent suppression of Rac1 activity in human glioma cells, whereas the inhibitory action of ISO on migration was Rho-independent. Thus, these data indicate the differential role of small G-proteins in the negative migratory response to S1P and ISO in glioma cells. Recently, two models have been proposed to account for the ability of Rho effectors in the downstream of Rho signaling in the S1P-induced inhibitory action. The first model suggests that an additional mechanism other than that of Rho-kinase may be required for the down-regulation of Rac activity (Sugimoto et al., 2003). The second shows that the S1P-induced inhibitory action is completely abolished by Y-27632, a specific inhibitor for Rho-kinase, suggesting that the pathway is Rho-kinase-dependent (Sanchez et al., 2005). Further experiments are necessary to clarify which molecular mechanism downstream of Rho is involved in the inhibition of glioma cell migration by S1P.
The activation of Ras-like G-proteins, e.g., Rap1, has been reported to play a crucial role in the control of cell adhesion and migration through the inside-out activation of integrins (Rangarajan et al., 2003). Cyclic AMP–mediated Rap1 stimulation exhibited Akt phosphorylation in thyroid cells (Tsygankova et al., 2001). We observed the opposite suppressive effect on Akt activation by treatment of cells with ISO, which stimulated Rap1 in an Epac-dependent manner (Figures 3 and 4C). These findings were supported by notable studies in B-lymphocytes, in which the activation of Rap GTPases modulated the B-cell antigen receptor-mediated activation of Akt (Christian et al., 2003). A controversy in the Rap1 effect on Akt from the studies could be explained by the difference in the type of Rap1 protein expressed in one or another cell type. We have shown that ISO-induced β2-adrenergic receptor stimulation in glioma cells suppressed the migratory response to LPA through the Epac/Rap1B-dependent pathway (Figures 1B and 3). Our results are consistent with the findings from other groups. The expression of Rap1 or Epac has been shown to result in the suppression of accelerated migration in C3G-deficient fibroblasts (Ohba et al., 2003). Recent findings have also shown that constitutively active RapV12 transfected into epithelial carcinoma cells led to down-regulated Rac1 expression and, hence, abrogated migration (Valles et al., 2004). However, the manner in which Epac/Rap1 participates in the regulation of PI3K/Rac-dependent cytoskeleton rearrangement and cell migration remains unclear. The possibility that Rap1 modulates PI3K itself and thereby inhibits the activity of Rac1-mediated migration may be excluded. Thus, ISO failed to inhibit LPA-induced and PI3K-dependent Akt phosphorylation in PTEN-null U87MG cells (Figure 5F).
Several in vitro studies have shown that PTEN expression level is critical for the migration and invasion of glioma cells. For example, Tamura et al. (1999) and Koul et al. (2001) showed that the introduction of PTEN gene into human PTEN-null glioma U251 and/or U87 cells significantly inhibited in vitro invasion. Clinical data also indicated that patients with glioblastoma multiforme express PTEN at lower levels than those with lower grades of gliomas (Sano et al., 1999). Moreover, Kaplan-Meier survival plots, adjusted for age and tumor grade, showed a significantly better prognosis for patients whose tumors expressed high levels of PTEN (Sano et al., 1999). Thus, these results suggest that PTEN expression might be inversely correlated with the invasiveness of glioma cells. However, the inhibition of migration has been explained in widely different ways. Raftopoulou et al. (2004) have shown that exogenously expressed PTEN can inhibit cell migration through its C2 domain, independently of its lipid phosphatase activity in PTEN-null glioma cells. On the other hand, migration was abrogated by prostaglandin E2-induced PTEN lipid-phosphatase activation in fibroblasts, in which PKA was suggested to mediate the increase in agonist-stimulated PTEN activity (White et al., 2005). Consistent with this report, our results indicate that PTEN is required for the suppression of glioma cell migration. However, PKA signaling is insufficient to account for the ISO action on migration via PTEN. Two lines of evidence support the role of PTEN without involvement of PKA in the inhibition of glioma cell motility by ISO. First, ISO-induced suppression of migration was not affected by the treatment of cells with PKA inhibitors, AKAP St-Ht31 and myr-PKI (Supplemental Figure S2). Rap1B, rather than PKA, seems to be involved in the ISO actions, as described above. Second, the ISO action was reversed by the knockdown of PTEN with siRNA, the C124S mutant of PTEN expression, and the inhibition of PTEN activity with bpV(pic) (Figure 6 and Supplemental Figure S5). Another research group has also shown that PTEN activity was required for S1P2 receptor-dependent inhibition of cell movement in HUVEC cells (Sanchez et al., 2005). However, according to our previous report, S1P was able to attenuate the migration even in cells with null expression of PTEN, such as U87MG (Li et al., 1997) and 1321N1 astrocytoma (Malchinkhuu et al., 2008).
Although our present study strongly suggests the involvement of PTEN in the ISO/cAMP/Rap1B-mediated inhibition of glioma cell migration, the molecular mechanism by which Rap1B activation leads to changes in the PTEN activity remains unknown. In our preliminary study, we measured PTEN phosphorylation at Ser-380/Thr-382/Thr-383 but failed to detect any significant change in the activity by either LPA or ISO (data not shown). Moreover, we have not yet detected any change in the translocation of PTEN by any agonist stimulation (data not shown). However, there are other potential Ser, Thr, and Tyr residues besides Ser-380 that could be phosphorylated and modulate the enzyme activity in PTEN (Raftopoulou et al., 2004; White et al., 2005). The clarification of the mechanism whereby Rap1B regulates PTEN activity is our current subject of investigation.
Our present study raised the possibility that expression level of invasive LPA1 receptor and suppressive S1P2 receptor/β2 receptor and/or their receptor-mediated signaling activities may be critical factors to determine the invasiveness of glioma cells in addition to the expression level of PTEN. As for the expression level of invasive LPA1 receptors, we have previously shown that the LPA1 receptor is highly expressed in glioma cells, whereas its expression is marginal in normal astrocytes (Malchinkhuu et al., 2005). Indeed, the expression of LPA1 receptors in highly invasive glioblastomas (grade IV) has been shown to be much higher than that in grade II astrocytomas (Kishi et al., 2006). As for the suppressive receptor system, the S1P2 receptor is also more highly expressed in glioma cell lines than in normal astrocytes (Van Brocklyn et al., 2003; Malchinkhuu et al., 2005). Thus, the expression level of the S1P2 receptor does not seem to explain the invasiveness of glioma cells. It should be noted, however, the expression profile of the S1P receptors in glioma cells is complex. Thus, the invasive S1P1 receptor-mediated migration response to S1P was unmasked under the conditions where the S1P2 receptor/Rho signaling pathway was inhibited (Figure 1E and Supplemental Figure S1). However, there seems to be no correlation between the expression levels of S1P1 and S1P2 receptors in the individual glioma cells (Van Brocklyn et al., 2003). Moreover, there is no previous information concerning the relation of PTEN loss with the expression profile of S1P receptor subtypes. On the other hand, cAMP accumulating activity linked to β-adrenergic receptors might be involved in the invasiveness of glioma cells. Thus, it has been reported that the levels of cAMP and β-adrenergic receptor–mediated adenylyl cyclase activity are inversely related to the degree of malignancy of gliomas (Furman and Shulman, 1977). In summary, in highly invasive glioma cells, invasive LPA1 receptor expression is up-regulated, and suppressive β-adrenergic receptor/cAMP systems are down-regulated. Thus, the balance of invasive and suppressive signals might also be important to determine the invasiveness of glioma cells, although we have to await the further information on the expression levels of S1P receptor subtypes. Rasheed et al. (1997) have reported that mutations of the PTEN gene are restricted to high-grade adult gliomas, and no mutations were seen in low-grade adult and childhood gliomas. As is evident from previous studies, PTEN activity seems to be regulated to inhibit cell migration through multiple mechanisms; in the present study, we suggest the participation of the novel cAMP-induced Epac/Rap1B/PTEN pathway. Thus, cAMP-induced PTEN stimulation may provide a novel therapeutic means against gliomas without PTEN mutations. In relation to this, several experiments using animal models to inhibit the tumorigenesis of glioblastomas have been tried. For example, Li et al. (2007) showed that injection of cholera toxin, a stimulator of Gs protein, into brain tumors induced their differentiation. A phosphodiesterase inhibitor, rolipram, suppressed glioma cell growth in vitro and, upon oral administration, inhibited intracranial growth in xenograft models of malignant brain tumors (Yang et al., 2007). The present study suggests that cilostazol is another potential drug with an inhibitory activity of phosphodiesterase for glioblastomas as well. Thus, many potential tools are available to increase cAMP in vivo, i.e., agonists for Gs protein–coupled receptors, adenylyl cyclase stimulators, inhibitors of phosphodiesterase, and cAMP derivatives, some of which are now used for the clinical treatment of various disorders. In addition to the modulators of cAMP levels, the control of LPA1 receptor activity may be another way to treat malignant brain tumors. For example, the LPA receptor antagonist, Ki16425 (Ohta et al., 2003; Malchinkhuu et al., 2005), is a potential drug for this purpose. However, the presence of blood brain barriers may be a problem that needs to be overcome for the chemotherapy against brain tumors.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Prof. Kozo (Kaibuchi of Nagoya University) for T19NRhoA, Prof. Naoki Mochizuki of (National Cardiovascular Center Research Institute) for G12VRap1 and Rap1GAPII, and Mr. Yoshihiro Sugiyama (Otsuka Pharmaceutical Co.) for cilostazol. We also thank Chisuko Uchiyama and Mutsumi Takano for their technical assistance. This work was supported by a Grants-in-Aid for scientific research from the Japan Society for the Promotion of Science (K.S., H.T., and F.O.); a grant of the Global COE Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (K.S. and C.M.); and grants from Takeda Science Foundation (K.S. and F.O.). E.M. is a Japan Society for the Promotion of Science Fellow.
Abbreviations used:
- 8CPT-2Me-cAMP
8-(4-chlorophenylthio)-2′-O-methyladenosine 3′, 5′-cyclic monophosphate
- Epac
exchange protein directly activated by cAMP
- G-protein
GTP-binding regulatory protein
- ISO
isoproterenol
- LPA
1-oleoyl-sn-glycero-3-phosphate or lysophosphatidic acid
- PI3K
phosphatidylinositol 3-kinase
- PKA
cAMP-dependent protein kinase
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- siRNA
small interfering RNA
- S1P
sphingosine 1-phosphate.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-08-0692) on October 28, 2009.
REFERENCES
- Arai K., Maruyama Y., Nishida M., Tanabe S., Takagahara S., Kozasa T., Mori Y., Nagao T., Kurose H. Differential requirement of Gα12, Gα13, Gαq, and Gβγ for endothelin-1-induced c-Jun NH2-terminal kinase and extracellular signal-regulated kinase activation. Mol. Pharmacol. 2003;63:478–488. doi: 10.1124/mol.63.3.478. [DOI] [PubMed] [Google Scholar]
- Arikawa K., Takuwa N., Yamaguchi H., Sugimoto N., Kitayama J., Nagawa H., Takehara K., Takuwa Y. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor. Requirement of inhibition of cellular RAC activity. J. Biol. Chem. 2003;278:32841–32851. doi: 10.1074/jbc.M305024200. [DOI] [PubMed] [Google Scholar]
- Burridge K., Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Chandrasekar N., Mohanam S., Lakka S. S., Dinh D. H., Olivero W. C., Gujrati M., Rao J. S. Glial cell-induced endothelial morphogenesis is inhibited by interfering with extracellular signal-regulated kinase signaling. Clin. Cancer Res. 2003;9:2342–2349. [PubMed] [Google Scholar]
- Christian S. L., Lee R. L., McLeod S. J., Burgess A. E., Li A. H., Dang-Lawson M., Lin K. B., Gold M. R. Activation of the Rap GTPases in B lymphocytes modulates B cell antigen receptor-induced activation of Akt but has no effect on MAPK activation. J. Biol. Chem. 2003;278:41756–41767. doi: 10.1074/jbc.M303180200. [DOI] [PubMed] [Google Scholar]
- DeAngelis L. M. Brain tumors. N. Engl. J. Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- de Rooij J., Zwartkruis F. J., Verheijen M. H., Cool R. H., Nijman S. M., Wittinghofer A., Bos J. L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Enserink J. M., Christensen A. E., de Rooij J., van Triest M., Schwede F., Genieser H. G., Doskeland S. O., Blank J. L., Bos J. L. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Sakurai A., Sano H., Yamagishi A., Somekawa S., Takakura N., Saito Y., Kangawa K., Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol. Cell. Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman M. A., Shulman K. Cyclic AMP and adenyl cyclase in brain tumors. J. Neurosurg. 1977;46:477–483. doi: 10.3171/jns.1977.46.4.0477. [DOI] [PubMed] [Google Scholar]
- Gutmann D. H., Saporito-Irwin S., DeClue J. E., Wienecke R., Guha A. Alterations in the rap1 signaling pathway are common in human gliomas. Oncogene. 1997;15:1611–1616. doi: 10.1038/sj.onc.1201314. [DOI] [PubMed] [Google Scholar]
- Howe A. K. Regulation of actin-based cell migration by cAMP/PKA. Biochim. Biophys. Acta. 2004;1692:159–174. doi: 10.1016/j.bbamcr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Howe A. K., Baldor L. C., Hogan B. P. Spatial regulation of the cAMP-dependent protein kinase during chemotactic cell migration. Proc. Natl. Acad. Sci. USA. 2005;102:14320–14325. doi: 10.1073/pnas.0507072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura F., Horai T., Mukai M., Shinkai K., Sawada M., Akedo H. Induction of in vitro tumor cell invasion of cellular monolayers by lysophosphatidic acid or phospholipase D. Biochem. Biophys. Res. Commun. 1993;193:497–503. doi: 10.1006/bbrc.1993.1651. [DOI] [PubMed] [Google Scholar]
- Ishiuchi S., et al. Blockage of Ca2+-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat. Med. 2002;8:971–978. doi: 10.1038/nm746. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Springett G. M., Mochizuki N., Toki S., Nakaya M., Matsuda M., Housman D. E., Graybiel A. M. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Kishi Y., Okudaira S., Tanaka M., Hama K., Shida D., Kitayama J., Yamori T., Aoki J., Fujimaki T., Arai H. Autotaxin is overexpressed in glioblastoma multiforme and contributes to cell motility of glioblastoma by converting lysophosphatidylcholine to lysophosphatidic acid. J. Biol. Chem. 2006;281:17492–17500. doi: 10.1074/jbc.M601803200. [DOI] [PubMed] [Google Scholar]
- Kohyama T., Ertl R. F., Valenti V., Spurzem J., Kawamoto M., Nakamura Y., Veys T., Allegra L., Romberger D., Rennard S. I. Prostaglandin E2 inhibits fibroblast chemotaxis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L1257–L1263. doi: 10.1152/ajplung.2001.281.5.L1257. [DOI] [PubMed] [Google Scholar]
- Kon J., et al. Comparison of intrinsic activities of the putative sphingosine 1-phosphate receptor subtypes to regulate several signaling pathways in their cDNA-transfected Chinese hamster ovary cells. J. Biol. Chem. 1999;274:23940–23947. doi: 10.1074/jbc.274.34.23940. [DOI] [PubMed] [Google Scholar]
- Koul D., et al. Suppression of matrix metalloproteinase-2 gene expression and invasion in human glioma cells by MMAC/PTEN. Oncogene. 2001;20:6669–6678. doi: 10.1038/sj.onc.1204799. [DOI] [PubMed] [Google Scholar]
- Lepley D., Paik J. H., Hla T., Ferrer F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005;65:3788–3795. doi: 10.1158/0008-5472.CAN-04-2311. [DOI] [PubMed] [Google Scholar]
- Li J., et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Li Y., Yin W., Wang X., Zhu W., Huang Y., Yan G. Cholera toxin induces malignant glioma cell differentiation via the PKA/CREB pathway. Proc. Natl. Acad. Sci. USA. 2007;104:13438–13443. doi: 10.1073/pnas.0701990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T., Dixon J. E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Malchinkhuu E., Sato K., Horiuchi Y., Mogi C., Ohwada S., Ishiuchi S., Saito N., Kurose H., Tomura H., Okajima F. Role of p38 mitogen-activated kinase and c-Jun terminal kinase in migration response to lysophosphatidic acid and sphingosine-1-phosphate in glioma cells. Oncogene. 2005;24:6676–6688. doi: 10.1038/sj.onc.1208805. [DOI] [PubMed] [Google Scholar]
- Malchinkhuu E., Sato K., Maehama T., Mogi C., Tomura H., Ishiuchi S., Yoshimoto Y., Kurose H., Okajima F. S1P2 receptors mediate inhibition of glioma cell migration through Rho signaling pathways independent of PTEN. Biochem. Biophys. Res. Commun. 2008;366:963–968. doi: 10.1016/j.bbrc.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Morisco C., Condorelli G., Trimarco V., Bellis A., Marrone C., Sadoshima J., Trimarco B. Akt mediates the cross-talk between beta-adrenergic and insulin receptors in neonatal cardiomyocytes. Circ. Res. 2005;96:180–188. doi: 10.1161/01.RES.0000152968.71868.c3. [DOI] [PubMed] [Google Scholar]
- Ohba Y., Kurokawa K., Matsuda M. Mechanism of the spatio-temporal regulation of Ras and Rap1. EMBO J. 2003;22:859–869. doi: 10.1093/emboj/cdg087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H., et al. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol. Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- Okada T., Sakuma L., Fukui Y., Hazeki O., Ui M. Blockage of chemotactic peptide-induced stimulation of neutrophils by wortmannin as a result of selective inhibition of phosphatidylinositol 3-kinase. J. Biol. Chem. 1994;269:3563–3567. [PubMed] [Google Scholar]
- Ono H., Ichiki T., Fukuyama K., Iino N., Masuda S., Egashira K., Takeshita A. cAMP-response element-binding protein mediates tumor necrosis factor-alpha-induced vascular smooth muscle cell migration. Arterioscler. Thromb. Vasc. Biol. 2004;24:1634–1639. doi: 10.1161/01.ATV.0000138052.86051.0d. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M., Etienne-Manneville S., Self A., Nicholls S., Hall A. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science. 2004;303:1179–1181. doi: 10.1126/science.1092089. [DOI] [PubMed] [Google Scholar]
- Rangarajan S., Enserink J. M., Kuiperij H. B., de Rooij J., Price L. S., Schwede F., Bos J. L. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J. Cell Biol. 2003;160:487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed B. K., Stenzel T. T., McLendon R. E., Parsons R., Friedman A. H., Friedman H. S., Bigner D. D., Bigner S. H. PTEN gene mutations are seen in high-grade but not in low-grade gliomas. Cancer Res. 1997;57:4187–4190. [PubMed] [Google Scholar]
- Sanchez T., Thangada S., Wu M. T., Kontos C. D., Wu D., Wu H., Hla T. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc. Natl. Acad. Sci. USA. 2005;102:4312–4317. doi: 10.1073/pnas.0409784102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T., et al. Differential expression of MMAC/PTEN in glioblastoma multiforme: relationship to localization and prognosis. Cancer Res. 1999;59:1820–1824. [PubMed] [Google Scholar]
- Sato K., et al. Identification of autotaxin as a neurite retraction-inducing factor of PC12 cells in cerebrospinal fluid and its possible sources. J. Neurochem. 2005;92:904–914. doi: 10.1111/j.1471-4159.2004.02933.x. [DOI] [PubMed] [Google Scholar]
- Schmid A. C., Byrne R. D., Vilar R., Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–38. doi: 10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- Stam J. C., Michiels F., van der Kammen R. A., Moolenaar W. H., Collard J. G. Invasion of T-lymphoma cells: cooperation between Rho family GTPases and lysophospholipid receptor signaling. EMBO J. 1998;17:4066–4074. doi: 10.1093/emboj/17.14.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N., Takuwa N., Okamoto H., Sakurada S., Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol. Cell. Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Gu J., Takino T., Yamada K. M. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130Cas. Cancer Res. 1999;59:442–449. [PubMed] [Google Scholar]
- Tokumura A., Majima E., Kariya Y., Tominaga K., Kogure K., Yasuda K., Fukuzawa K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 2002;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- Tsygankova O. M., Saavedra A., Rebhun J. F., Quilliam L. A., Meinkoth J. L. Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol. Cell. Biol. 2001;21:1921–1929. doi: 10.1128/MCB.21.6.1921-1929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu-Goto M., et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles A. M., Beuvin M., Boyer B. Activation of Rac1 by paxillin-Crk-DOCK180 signaling complex is antagonized by Rap1 in migrating NBT-II cells. J. Biol. Chem. 2004;279:44490–44496. doi: 10.1074/jbc.M405144200. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn J. R., Young N., Roof R. Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Lett. 2003;199:53–60. doi: 10.1016/s0304-3835(03)00334-3. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F. N., Olivo C., Grivell S., Giepmans B. N., Collard J. G., Moolenaar W. H. Rac activation by lysophosphatidic acid LPA1 receptors through the guanine nucleotide exchange factor Tiam1. J. Biol. Chem. 2003;278:400–406. doi: 10.1074/jbc.M210151200. [DOI] [PubMed] [Google Scholar]
- White E. S., Atrasz R. G., Dickie E. G., Aronoff D. M., Stambolic V., Mak T. W., Moore B. B., Peters-Golden M. Prostaglandin E2 inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am. J. Respir. Cell. Mol. Biol. 2005;32:135–141. doi: 10.1165/rcmb.2004-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., et al. Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1. J. Biol. Chem. 2004;279:6595–6605. doi: 10.1074/jbc.M308133200. [DOI] [PubMed] [Google Scholar]
- Yang L., Jackson E., Woerner B. M., Perry A., Piwnica-Worms D., Rubin J. B. Blocking CXCR4-mediated cyclic AMP suppression inhibits brain tumor growth in vivo. Cancer Res. 2007;67:651–658. doi: 10.1158/0008-5472.CAN-06-2762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.