Abstract
Two prevailing models have emerged to explain the mechanism of contractile-ring assembly during cytokinesis in the fission yeast Schizosaccharomyces pombe: the spot/leading cable model and the search, capture, pull, and release (SCPR) model. We tested some of the basic assumptions of the two models. Monte Carlo simulations of the SCPR model require that the formin Cdc12p is present in >30 nodes from which actin filaments are nucleated and captured by myosin-II in neighboring nodes. The force produced by myosin motors pulls the nodes together to form a compact contractile ring. Live microscopy of cells expressing Cdc12p fluorescent fusion proteins shows for the first time that Cdc12p localizes to a broad band of 30–50 dynamic nodes, where actin filaments are nucleated in random directions. The proposed progenitor spot, essential for the spot/leading cable model, usually disappears without nucleating actin filaments. α-Actinin ain1 deletion cells form a normal contractile ring through nodes in the absence of the spot. Myosin motor activity is required to condense the nodes into a contractile ring, based on slower or absent node condensation in myo2-E1 and UCS rng3-65 mutants. Taken together, these data provide strong support for the SCPR model of contractile-ring formation in cytokinesis.
INTRODUCTION
Cytokinesis partitions cellular constituents into two daughter cells. When coordinated with the generation of cellular asymmetry, cytokinesis can produce diverse cell types in multicellular organisms. Thus, cytokinesis plays a crucial role in both cell proliferation and cell differentiation. Despite being studied since the 19th century, cytokinesis remains one of the least understood steps of the cell-division cycle. Powerful forward and reverse genetics in yeast have identified and characterized the majority of genes involved in cytokinesis (Balasubramanian et al., 2004; Wolfe and Gould, 2005). Affinity purification (Miller et al., 1989), isolation of cytokinetic structures (Skop et al., 2004), and recent genome-wide RNAi screens have nearly completed the list of genes contributing to cytokinesis in Caenorhabditis elegans (Fraser et al., 2000; Gönczy et al., 2000; Sönnichsen et al., 2005), Drosophila (Echard et al., 2004), and cultured human cells (Kittler et al., 2007). A majority of these genes are conserved among eukaryotes including humans (Balasubramanian et al., 2004; Glotzer, 2005; Barr and Gruneberg, 2007). The current challenge is to determine how these gene products work together and to elucidate the underlying molecular mechanisms of cytokinesis.
The fission yeast Schizosaccharomyces pombe has emerged as an important model system for investigating molecular mechanisms of cytokinesis (Balasubramanian et al., 2004; Wolfe and Gould, 2005; Pollard, 2008). Similar to animal cells, the contractile ring composed of actin, myosin-II, formin, and other associated proteins is essential for cytokinesis in S. pombe. The anillin-like protein Mid1p/Dmf1p is crucial for the positioning and proper assembly of the contractile ring (Sohrmann et al., 1996; Bähler et al., 1998a; Paoletti and Chang, 2000; Motegi et al., 2004; Wu et al., 2003, 2006). Mid1p specifies the cleavage site by a balance of positive and negative signaling cues. The nucleus is positioned medially by dynamic microtubules (Tran et al., 2001), thus determining the initial location of Mid1p once it exits from the nucleus (Paoletti and Chang, 2000; Daga and Chang, 2005). Mid1p then diffuses into the cytoplasm and binds as nodes to the plasma membrane and the kinase Cdr2p (Celton-Morizur et al., 2004; Almonacid et al., 2009), whereas the DYRK-family kinase Pom1p and an unknown inhibitor prevent the binding of Mid1p to the cell poles (Celton-Morizur et al., 2006; Padte et al., 2006; Huang et al., 2007). Thus, Mid1p is concentrated as a broad band of nodes at the equator of the cell.
Several studies agree that Mid1p initiates contractile-ring assembly by recruiting myosin-II to a broad band of ∼65 membrane-bound nodes (Motegi et al., 2000, 2004; Wu et al., 2003, 2006; Vavylonis et al., 2008). However, the distribution of the formin Cdc12p and actin filaments is controversial, primarily because Cdc12p is one of the least abundant cytokinesis proteins (Wu and Pollard, 2005). Cdc12p, a nucleator of unbranched actin filaments (Kovar et al., 2003), is essential for the assembly of the contractile ring (Chang et al., 1997) and is thought to be recruited to the division site by the F-BAR/pombe Cdc15 homology (PCH) protein Cdc15p (Carnahan and Gould, 2003). Cdc12p has been observed arriving at the division site in one or several spots that spread and integrate into the contractile ring (Chang et al., 1997; Chang, 1999; Yonetani et al., 2008). However, Cdc12p has also been found in a broad band of nodes in synchronized cdc25-22 cells (Wu et al., 2006; Huang et al., 2008) and occasionally clustered in a few nodes in wild-type cells before ring formation (Wu et al., 2006).
Currently, two main models have been proposed to explain contractile-ring assembly in wild-type fission yeast cells (reviewed in Mishra and Oliferenko, 2008 and Roberts-Galbraith and Gould, 2008). One is the spot/leading cable model. According to this and similar models, the Cdc12p spot moves to the division site marked by Mid1p and is proposed to nucleate linear actin filaments from a single site to form an aster and one (or two) leading cable(s). The leading cable(s) and other actin filaments grow from the aster and curve around the circumference of the cell to form a contractile ring (Chang et al., 1997; Chang, 1999; Arai and Mabuchi, 2002; Carnahan and Gould, 2003; Motegi et al., 2004; Kamasaki et al., 2007). An alternative model uses a search, capture, pull, and release (SCPR) mechanism to assemble the contractile ring from a broad band of nodes. In contrast to the spot model, the SCPR model assumes that Cdc12p concentrates in >50% of Mid1p-myosin-II nodes and nucleates short actin filaments all over the medial cortex in random directions. Next, transient connections are established between actin filaments and myosin-II, which condense the nodes into a contractile ring by myosin-II motor activity (Bähler et al., 1998a; Wu et al., 2006; Vavylonis et al., 2008). The two models disagree on whether actin filaments for contractile-ring formation initiate from a single Cdc12p spot or from multiple Cdc12p nodes around the cell's equator. In addition, the Myo2p motor activity is required for the condensation of nodes in the SCPR model, whereas in the spot model Myo2p is only needed at later stages to reorient actin filaments and constrict the ring.
In this study, we found that the formin Cdc12p localizes to a broad band of dynamic nodes from which actin filaments are nucleated in random directions. Our data also suggest that Myo2p motor activity is required to condense the nodes into a contractile ring. These results provide strong support for the basic assumptions of the SCPR model of contractile-ring assembly.
MATERIALS AND METHODS
Strains and Genetic, Molecular, and Cell Biology Methods
Table 1 lists the S. pombe strains used in this study. PCR-based gene targeting was performed as described (Bähler et al., 1998b). All tagged genes are under the control of endogenous promoters and integrated at their native chromosomal loci except 3nmt1-GFP-atb2, integrated at the lys1 locus, 41nmt1-GFP-CHD (calponin homology domain) and lifeact (see below), integrated at the leu1 locus. We tested the functionalities of new strains by examining growth and cellular morphology at different temperatures and by crossing tagged strains with mutant strains known to have synthetic interactions with mutations in the tagged gene. The strains cdc12-tdTomato and cdc12-mCherry were crossed with rng2-D5 (Eng et al., 1998), and the double mutants resemble rng2-D5 from 25 to 36°C. Similarly, ain1-3GFP and ain1-3YFP were crossed with fim1Δ (Wu et al., 2001), and cdc12-3GFP and cdc12-3YFP were crossed with ain1Δ (Wu et al., 2001); all tagged strains were found to be functional. Some strains were previously reported to be functional: mYFP-cdc15, GFP-myo2, and sad1-CFP (Wu et al., 2003) and rlc1-tdTomato and rlc1-mCherry (Vavylonis et al., 2008). Conversely, ain1-tdTomato fim1Δ was sicker than fim1Δ, indicating the fusion protein was not fully functional.
Table 1.
S. pombe strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| JW81 | h−ade6-210 ura4-D18 leu1-32 | Wu et al. (2003) |
| JW1054 | h−kans Pcdc15-mYFP-cdc15 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1109 | h+kanMX6-Pmyo2-mEGFP-myo2 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1114 | h+cdc12-3YFP-kanMX6 sad1-CFP-kanMX6 ade6 leu1-32 ura4-D18 | This study |
| JW1136 | kanMX6-Pmyo2-mEGFP-myo2 ain1-Δ1::kanMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1149-1 | h−ain1-3GFP-kanMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1189 | h+mid1-ΔF::ura4+cdc12-3YFP-kanMX6 sad1-CFP-kanMX6 ade6 leu1-32 ura4-D18 | This study |
| JW1340 | h−rlc1-mCherry-natMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1349-2 | h+41nmt1-GFP-CHD (rng2)-leu1+rlc1-tdTomato-natMX6 ade6-M210 leu1-32 ura4-D18 | Vavylonis et al. (2008) |
| JW1351-2 | cdc25-22 41nmt1-GFP-CHD (rng2)-leu1+rlc1-tdTomato-natMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1404-1 | h+cdc12-3YFP-kanMX6 ade6-M216 leu1-32 ura4-D18 | This study |
| JW1405-2 | h+cdc12-3GFP-kanMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1411-1 | h+cdc12-3GFP-kanMX6 rlc1-tdTomato-natMX6 ade6 leu1-32 ura4-D18 | This study |
| JW1413 | cdc12-3YFP-kanMX6 rlc1-mCherry-natMX6 ade6 leu1-32 ura4-D18 | This study |
| JW1423-1 | h−myo2-E1 cdc12-3YFP-kanMX6 ade6 leu1-32 ura4-D18 | This study |
| JW1432 | cdc15-mCherry-natMX6 cdc12-3YFP-kanMX6 ade6 leu1-32 ura4-D18 | This study |
| JW1439-1 | h+ain1-3GFP-kanMX6 rlc1-tdTomato-natMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1443 | ain1-3YFP-kanMX6 sad1-CFP-kanMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1445-1 | h−cdc12-tdTomato-kanMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1449 | ain1-tdTomato-kanMX6 41nmt1-GFP-CHD(rng2)-leu1+ade6-M210 leu1-32 ura4-D18 | This study |
| JW1450 | mid1-ΔF::ura4+ain1-3GFP-kanMX6 ade6 leu1-32 ura4-D18 | This study |
| JW1452-1 | h+cdc12-tdTomato-kanMX6 41nmt1-GFP-CHD(rng2)-leu1+ade6 leu1-32 ura4-D18 | This study |
| JW1456 | h−ain1-Δ1::kanMX6 cdc12-3YFP-kanMX6 ade6 ura4-D18 | This study |
| JW1458 | ain1-3GFP-kanMX6 cdc12-tdTomato-kanMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1464 | h+/h−cdc12-3YFP-kanMX6/cdc12-3YFP-kanMX6 ade6-M216/ade6-M210 his3-D1/his3+leu1-32/leu1-32 ura4-D18/ura4-D18 | This study |
| JW1465 | cdc25-22 cdc12-tdTomato-kanMX6 41nmt1-GFP-CHD(rng2)-leu1+ade6 leu1-32 ura4-D18 | This study |
| JW1488 | for3Δ::kanMX6 cdc25-22 cdc12-tdTomato-kanMX6 41nmt1-GFP-CHD(rng2)-leu1+ade6 leu1-32 ura4-D18 | This study |
| JW1516 | cdc12-tdTomato-kanMX6 kanMX6-Pcdc15-GFP-cdc15 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1561-4 | h−kanMX6-41nmt1-mEGFP-lifeact ade6-M210 leu1-32 ura4-D18 | This study |
| JW1595 | mid1-Δ2::ura4+for3Δ::kanMX6 cdc25-22 cdc12-tdTomato-kanMX6 41nmt1-GFP-CHD (rng2)-leu1+ade6 leu1-32 ura4-D18 | This study |
| JW1627 | kans Pcdc15-GFP-cdc15 cdc12-tdTomato-kanMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1666 | h−kanMX6–3nmt1-mEGFP-lifeact rlc1-tdTomato-natMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1701-1 | h+myo2-E1 41nmt1-GFP-CHD(rng2)-leu1+rlc1-tdTomato-natMX6 ade6 leu1-32 ura4-D18 | This study |
| JW1713 | lys1+::3nmt1-GFP-atb2 cdc12-tdTomato-kanMX6 kanMX6-Pcdc15-GFP-cdc15 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1716 | lys1+::3nmt1-GFP-atb2 ain1-tdTomato-kanMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| JW1720 | rng3-65 kanMX6–3nmt1-mEGFP-lifeact rlc1-tdTomato-natMX6 ade6 leu1-32 | This study |
| JW1722-1 | ain1-Δ1::kanMX6 cdc12-3GFP-kanMX6 ade6 leu1-32 ura4-D18 | This study |
| JW1893 | kanMX6-41nmt1-mEGFP-lifeact cdc12-tdTomato-kanMX6 ade6-M210 leu1-32 ura4-D18 | This study |
| KV346 | h−cdc12-3YFP-kanMX6 ade6-M216 leu1-32 ura4-D18 his3-D1 | Wu and Pollard (2005);Wu et al. (2006) |
The 41nmt1-mEGFP-lifeact and 3nmt1-mEGFP-lifeact strains were constructed using standard PCR gene targeting techniques. Briefly, mEGFP-lifeact was amplified using plasmid pFA6a-kanMX6-Purg1-mEGFP (JQW183-4) as a template and a reverse primer containing the sequence of lifeact (only 51 nucleotides; Riedl et al., 2008). The PCR product was then cut using restriction sites that were incorporated into the primers and inserted into a pFA6a vector containing a kanMX6 marker upstream of the 41nmt1 promoter or the 3nmt1 promoter. The primers used were as follows: forward: 5′-TAACCTGATTAATTAACAGTAAAGGAGAAGAACTTTTCAC-3′ and reverse: 5′-TACATGACGGCGCGCCCTATTCTTCCTTTGAGATGCTTTCGAATTTCTTGATCAAATCTGCGACACCCATAGAACCTTTGTATAGTTCATCCATGCC-3′, where restriction sites are underlined, the sequence of lifeact is bold, and a two-amino acid linker between mEGFP and lifeact sequences is italicized. The 3′ sequences of the reverse primer are complementary to mEGFP. The final plasmids were named JQW204 (pFA6a-kanMX6-P3nmt1-mEGFP-lifeact) and JQW207 (pFA6a-kanMX6-P41nmt1-mEGFP-lifeact). The mEGFP-lifeact constructs were integrated into the S. pombe genome at the leu1 locus downstream of the endogenous leu1-32 allele just after the stop codon. The lifeact labels all actin structures: actin patches, interphase cables, and contractile rings. Strains were tested for lack of cytokinesis defects in crosses to mutants and by growth on different media at different temperatures. Double mutant strains of the 41nmt1-mEGFP-lifeact and ain1Δ or clp1Δ looked similar to the parent deletion strains under inducing conditions. The 41nmt1-mEGFP-lifeact was imaged after inducing expression in EMM5S medium lacking thiamine for 24 h, and 3nmt1-mEGFP-lifeact was grown in repressing YE5S medium.
Cells were restreaked from −80°C stocks, grown 2–3 d on plates, and then inoculated into 5–15 ml YE5S liquid cultures as described (Wu et al., 2006). Cultures were kept in exponential phase for 36–48 h before microscopy. Only mid1Δ strains were grown for 24 h on plates and 24 h in exponential phase liquid cultures, because they tend to pick up suppressors if grown longer. Growth curves were generated by measuring OD595 at 3-h intervals. Strains with 41nmt1-GFP-CHD and 41nmt1-mEGFP-lifeact were grown in YE5S medium for at least 24 h and then induced in EMM5S medium for 12–48 h before microscopy. Strains with 3nmt1-GFP-atb2 and 3nmt1-mEGFP-lifeact were grown in repressing YE5S medium. Temperature-sensitive cdc25-22 cells were arrested at 35.5°C for 4 h and then released to 23°C before imaging. Latrunculin A (Lat-A) treatments were performed at a concentration of 100 μM, added to the synchronized cells before releasing cells to 23°C as described (Wu et al., 2006). Blebbistatin (B-0560, Sigma-Aldrich, St. Louis, MO) treatment at a final concentration of 1 mM from a 100 mM stock in DMSO was performed similar to Lat-A treatment. Briefly the cells were treated with blebbistatin or equal volume of DMSO at 36°C for 30 min before releasing to 25°C, and imaging began immediately after release. To prevent inactivation of the drug, cells were kept in the dark, DIC images were taken with low light, and fluorescent imaging was only done at the 568-nm channel. Cells were prepared for imaging on bare slides without centrifugation by taking samples from the culture every 10 min.
Microscopy and Data Analysis
Cells for microscopy were collected from liquid cultures, centrifuged at 5000 rpm, and then washed into EMM5S for imaging. Live cell microscopy was performed using a thin layer of EMM5S liquid medium with 20% gelatin (G-2500, Sigma-Aldrich) and 0.1 mM n-propyl-gallate, sealed under a coverslip with Valap, and observed at 23–25°C as described (Wu et al., 2006). We used a 100×/1.4 NA objective lens (Nikon, Melville, NY) on a spinning disk confocal microscope (UltraView ERS, Perkin Elmer Life and Analytical Sciences, Waltham, MA) with a 440-nm solid state laser or 488-, 514-, and 568-nm argon ion lasers and a cooled charge-coupled device camera (ORCA-AG, Hamamatsu, Bridgewater, NJ). Microscopy of temperature-sensitive mutants at 36°C was performed using an Objective Heater Controller (Bioptechs, Butler, PA). Cells were collected as above and placed on EMM5S +2% agar pads and sealed under a coverslip with Valap. All slides and cultures were kept at 36°C during preparation of slides.
The cdc12-3YFP strain was also imaged under modified microscopy conditions to confirm that the Cdc12p speckles and nodes were not imaging artifacts. Cells were either centrifuged at 5000 rpm for 30 s or allowed to precipitate and then imaged in YE5S on a bare slide or on YE5S +20% gelatin pad without washing into EMM5S or using n-propyl-gallate in the medium.
Maximum intensity projections of color images were created using UltraView ERS software or by merging grayscale images in ImageJ (http://rsb.info.nih.gov/ij/). All other image analyses were performed using ImageJ. Images in figures are maximum intensity projections of Z-sections spaced at 0.1–0.4 μm unless otherwise noted. Some images were corrected for photobleaching during image acquisition as described (Vavylonis et al., 2008). Cdc12p speckles are less obvious in some figures because of different microscopy settings such as lower laser power or shorter exposure time. Cellular fluorescence intensity was measured using a sum intensity projection in ImageJ as described (Wu and Pollard, 2005). Wild-type autofluorescence and background intensity from surrounding intracellular regions were subtracted from intensity of the whole cell or individual structures. Two tagged proteins were considered in the same nodes if their centers were within one radius of the nodes. For the blebbistatin experiment, cells were counted with nodes if nodes were still separated greater than about one-third of the full width of the broad band along the long axis of cells. Boxed regions in Figure 2C were scaled to 500% in ImageJ using interpolation. Videos were also scaled to 200% (Videos 1–5, 8, and 9) or 400% (Videos 6 and 7) original size with interpolation.
Figure 2.
Cdc12p nodes appear similar to known nodes and are dynamically associated with Rlc1p nodes. (A) Cdc12p-3YFP (KV346) and mYFP-Cdc15p (JW1054) localize to a broad band of nodes (arrowheads) at the equator of cells. Left, wild-type cells (JW81) with low autofluorescence when excited at 514 nm. Note Cdc15p also localizes to patches concentrated at the cell tips. (B) Time-lapse series of lateral condensation of Cdc12p and Cdc15p nodes into contractile rings (see Videos 3 and 4). Time zero is an arbitrary time with nodes present in both cells. (C) Cdc12p colocalizes with Rlc1p in nodes. Cells expressing Cdc12p-3YFP (middle, JW1404), Rlc1p-mCherry (bottom, JW1340), or both (top, JW1413) were imaged using exactly the same settings. The boxed regions in the double-tagged strain are enlarged, and nodes are circled to show the colocalization of nodes. Cdc12p-3YFP was imaged with 488-nm excitation and an emission filter for YFP, resulting in less obvious speckles. (D) Time course of condensation of Rlc1p and Cdc12p nodes (strain JW1411) during contractile-ring assembly. (E and F) Dynamics of Cdc12p nodes and ring revealed by FRAP of Cdc12p-3YFP (JW1404). (E) Montages are from time-lapse microscopy during FRAP (see Materials and Methods). Cells were bleached within the white box at time zero. (i) Middle section of Cdc12p-3YFP ring. (ii) The slice with nodes chosen for FRAP (see Video 5). (iii) Maximum intensity projection (left) of the cell in ii and the chosen slice for bleaching (right). (F) Recovery of the Cdc12p ring (n = 14 cells, red) and nodes (n = 11 cells, gray). Relative intensity is normalized to the average prebleach intensity, and the immobile fraction for both is ∼30%. Bars, 5 μm, except in C enlarged regions, bar, 1 μm.
Actin filaments and bundles that appeared to associate with the nodes at the early stage of condensation were measured using the schematic in Figure 3D. Each Z-section spaced at 0.1–0.4 μm was examined at both 488- and 568-nm channels to find filaments with one end colocalized with a node. The main source of our data are at the top or bottom slices of the stacks because these slices are parallel to the plasma membrane resulting in better visualization of the filaments and nodes in the same plane. Radial projections in Figure 3B were created using a plugin for ImageJ provided by Dimitrios Vavylonis (Lehigh University). The projection shows the outer layer of the cell just inside the unwrapped plasma membrane, and the equator of the cell is horizontal.
Figure 3.
Cdc12p nodes nucleate and elongate actin filaments in random directions during contractile-ring formation. Cdc12p (red) and actin (green) in merged images. (A–E) Cdc12p-tdTomato GFP-CHD (strain JW1452). (A) A time-lapse series of a meshwork of actin filaments condensing into a ring. Only the GFP signal is shown. The meshwork forms at 1–2 min relative to time zero (white arrow), whereas structures similar to the actin aster and leading cable are seen at 5–6 min (red arrowhead). (B) Radial projections (see Materials and Methods) of Cdc12p nodes and GFP-CHD marked actin filaments of Z-sections spaced at 0.2 μm showing random actin-filament orientation (see Video 6). The long axis of the cell is vertical, but only the medial region is shown as indicated in the schematic to the left (red box). Right, enlarged region (white box) with arrows pointing to two nodes connected by a single actin filament. (C) Montage showing elongation of an actin filament/bundle (green) from a Cdc12p node (red, arrowhead) at high temporal resolution (see Video 7). The node signal from the first frame was smudged (averaging the intensity values over a box of 3 × 3 pixels in the x and y directions) and repeated in all frames for better visualization. (D and E) Histograms of orientations and lengths of actin filaments, n = 317 filaments from 14 cells. (D) Filaments from the top (dark) and bottom half (light) of the cells exhibit a random filament angle distribution. (F) Synchronized cdc25-22 cells (JW1465) after releasing to 25°C at the same time that Lat-A was washed out. These cells have nodes and actin filaments at the division site. (G) Histogram of orientations of >500 filaments binned every 10° including all data from asynchronous and synchronized cultures including data from (D). (H) Time course of new actin filaments growing from Cdc12p nodes after Lat-A was washed out in six for3Δ cells with the long axis of the cell positioned vertically (JW1488). The region of the cells that is shown corresponds to the schematic in B, but these are much longer cells after synchronization. Times given are from Lat-A washout. Top indicates maximum intensity projections of slices 3–9 of 26 sections spaced at 0.2 μm (72 min) or slices 1–5 of 11 sections spaced at 0.5 μm (87, 90 min). Bottom, maximum intensity projections of slices 15–21 (72 min) or slices 6–10 (87, 90 min). (I) Kymograph constructed along the length of an actin filament in JW1488 cells from H, showing elongation of an actin filament (green) from a Cdc12p node (red, arrowhead). The node signal from the first frame was smudged and repeated in all frames for better visualization. Bars, 5 μm; except in B enlargement and C, bars, 1 μm.
Fluorescence recovery after photobleaching (FRAP) was performed using the photokinesis unit on the UltraView ERS confocal system. The data were gathered by taking stacks to determine the optimum slice for bleaching, collecting 4–10 prebleach images, and then acquiring postbleach images appropriate to the structure being bleached as described (McNally, 2008). Ain1p or Cdc12p spot images were taken with 30-s delay, Cdc12p node images with 4-s delay, and rings with 2-s delay. A region of interest (ROI) was selected at each site that was bleached >50% of the original signal. Intensity values (after subtracting background) at each ROI were normalized against the average prebleach value, which is set to 100%. Intensity values of postbleaching were averaged from three consecutive images for each ROI to reduce noise (Vavylonis et al., 2008). The average intensity value at each time point across all ROIs was plotted with the bleaching moment defined as time zero. Best-fit curves were obtained from the average of all ROIs and from individual ROIs separately to obtain SDs. The curve equation is y = m1 − m2 * exp(−m3 * x), where m3 is the off rate. The off rate was used to calculate the τ1/2 of the recovery. The additional criteria for choosing ROIs depended on the structure being analyzed. For Cdc12p nodes, the region bleached had to contain several nodes by the time the recovery reached a plateau. Criteria used to select ROIs for other structures were as described (McNally, 2008).
RESULTS
The Formin Cdc12p Concentrates in Speckles during Interphase and a Broad Band of Nodes at the Onset of Mitosis
To assess the SCPR and spot/leading cable models of contractile-ring assembly in S. pombe, we started with establishing the localization of the formin Cdc12p throughout the cell cycle. One fundamental assumption of the SCPR model is that Cdc12p is present in >50% of the ∼65 nodes in wild-type cells (Vavylonis et al., 2008). This has not been established because of the difficulty in detecting Cdc12p, one of the least abundant cytokinesis proteins (Wu and Pollard, 2005; Wu et al., 2006). We observed cells expressing functional Cdc12p-3YFP, Cdc12p-tdTomato, and Cdc12p-3GFP under the endogenous promoter in place of the native protein using a spinning disk confocal system (see Materials and Methods).
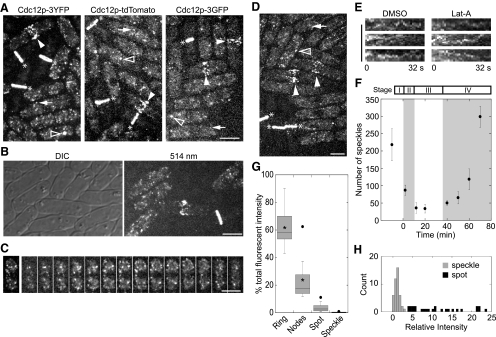
We found that Cdc12p concentrated in distinct cellular locations/structures with different intensities including speckles, spots, nodes, and contractile rings in all three strains although 3YFP provided the best contrast, because of much less autofluorescence when cells were excited at the 514-nm channel (Figure 1, A and B). Speckles, which are obviously brighter than background autofluorescence (Figure 1B), spread throughout the cytoplasm during interphase (Figure 1, A–D, arrow). Present in some cells was one structure much brighter than the speckles (Figure 1A, outlined arrowhead), which we referred to as a spot because it resembled the Cdc12p spot (Chang et al., 1997; Chang, 1999). Nodes appeared near the plasma membrane around the cell equator 1.0 ± 2.5 min (mean ± SD) after spindle pole bodies (SPB) separation (n = 6 cells; strain JW1114). The fluorescence intensity of individual nodes was higher than speckles but lower than most spots. After the nodes condensed together into the contractile ring the total fluorescence intensity was even higher (Figure 1, A and G). Cdc12p-3YFP was also observed in nodes and speckles under modified microscopy conditions (see Materials and Methods) and in diploid cells expressing two copies of cdc12-3YFP (Figure 1D), indicating that these localizations/structures are not artifacts of sample preparation or imaging conditions.
Figure 1.
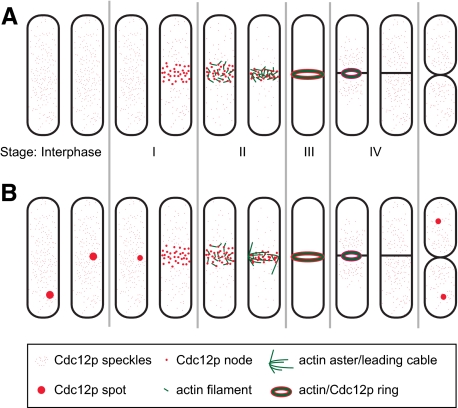
The formin Cdc12p concentrates in hundreds of speckles during interphase and as a broad band of nodes at the onset of mitosis. (A) Localization of Cdc12p-3YFP (left, strain KV346), Cdc12p-tdTomato (middle, JW1445), and Cdc12p-3GFP (right, JW1405) in speckles (arrows), spots (outlined arrowheads), nodes (filled arrowheads), and contractile rings (asterisks) in asynchronous cells. (B, C, and E) cdc12-3YFP (strain KV346). (B) Intensity of speckles and nodes is well above that of background autofluorescence at 514 nm. Wild-type (JW81) and cdc12-3YFP cells were grown in liquid cultures and then mixed just before imaging. DIC is shown to locate wild-type cells that are not visible in the fluorescent image. (C) Cdc12p-3YFP speckles in maximum intensity projection (left panel) or 14 consecutive Z-sections (right panels). (D) Localization of Cdc12p-3YFP to similar structures as in (A) in diploid cells expressing two copies of cdc12-3YFP (JW1464). (E) Actin-independent movement of Cdc12p speckles. Kymographs constructed with a 2-μm slit near the speckle in Lat-A or DMSO control (see Video 1). The x-axis spans 32 s. (F) Number of speckles in cells at each cell cycle stage from asynchronous cell populations (mean ± SD). The onset of mitosis is defined as time zero and the stages of cytokinesis (I–IV) are as described (Wu et al., 2003; Wu and Pollard, 2005). Data were timed and binned based on the morphology of nodes, rings, nuclei, and septa. Data points from left to right are cells at/with: interphase (n = 25 cells); nodes (n = 5); ring with one nucleus (n = 2); full-size ring with two nuclei (n = 5); contracting ring with a diameter of 2.2–3.0 μm (n = 7); ring 1.5–2.2 μm (n = 6); ring < 1.5 μm (n = 4); and complete septum but before cell separation (n = 4). (G) Box plot showing fraction of total cellular fluorescence intensity in each Cdc12p structure. Asterisk indicates the mean for rings and nodes. Left to right, n = 24, 14, and 25 cells, and 20 brightest speckles from two cells. (H) Histogram showing relative intensities of each speckle (gray) and spot (black) with the average speckle intensity set to 1. Bars, 5 μm.
The Cdc12p structures had distinct quantities. In interphase cells, Cdc12p was present in 218 ± 46 speckles, and the number of speckles increased with the cell size. The speckles moved within a localized area of the cytoplasm at 0.9 ± 0.5 μm/s (n = 30 speckles; Video 1) in an actin-independent manner, based on treatment with Lat-A, an inhibitor of actin polymerization (Figure 1E). Despite a constant global concentration of Cdc12p throughout the cell cycle (Chang et al., 1997; Wu and Pollard, 2005), the number of speckles declined dramatically during node and ring formation, held steady during ring maturation, and increased during ring constriction and disassembly (Figure 1F). Thus, it appears that Cdc12p molecules in the speckles are recruited to the nodes and contractile ring and released as the ring disassembles. One minute after SPB separation, a broad band of 43 ± 6 Cdc12p nodes appeared around the equator of the cell (n = 10 cells; Figure 1A, filled arrowhead; Video 2).
The intensity of Cdc12p structures varied. Each speckle contained 0.4 ± 0.2% of the total cellular Cdc12p intensity and the Cdc12p spot contained 3.6 ± 2.7% of total cellular intensity (Figure 1G). Thus Cdc12p was mainly in the speckles or diffused in the cytoplasm as monomers during interphase so that the spot was not the main reservoir of Cdc12p. The distribution of speckle intensities was approximately Gaussian, but the intensity of the spots was outside the Gaussian distribution (Figure 1H), being on average 10 times that of speckles. The broad band of nodes contained 24 ± 14% of the total cellular intensity (Figure 1G). At a later stage of mitosis, a compact Cdc12p-3YFP ring formed (Figure 1A; asterisk), which contained 62 ± 14% of the total cellular intensity (Figure 1G).
Cdc12p Colocalizes in Nodes with Myosin-II and Condenses into the Contractile Ring
Once we found that Cdc12p localized to a broad band of nodes, we investigated whether these nodes are precursors of the contractile ring. Cdc12p nodes resembled nodes of the F-BAR/PCH protein Cdc15p (Figure 2A) that arrives at the division site shortly before Cdc12p (Wu et al., 2003) and is thought to recruit Cdc12p (Carnahan and Gould, 2003). Both Cdc12p and Cdc15p nodes condensed laterally into a contractile ring at early mitosis judged by the presence of a single dark nucleus region (Figure 2B; Videos 3 and 4).
It is established that the anillin Mid1p, myosin-II Myo2p including its light chains Cdc4p and Rlc1p, and IQGAP Rng2p are all components of the same nodes (Motegi et al., 2000, 2004; Paoletti and Chang, 2000; Wu et al., 2003, 2006). However, whether Cdc12p is a component of the anillin-myosin-II nodes remains controversial because of its low copy number (Wu et al., 2006; Huang et al., 2008; Yonetani et al., 2008). We used cells expressing fluorescent Cdc12p and myosin regulatory light chain Rlc1p to clarify the localization (Figure 2, C and D). Rlc1p appears at the division site ∼10 min earlier than Cdc12p (Wu et al., 2003). Consistent with the timing, 60% of cells with Rlc1p-mCherry nodes did not contain Cdc12p-3YFP in the nodes (n = 113 cells). Once Cdc12p was recruited to the division site at 1 min after SPB separation, we found that 70 ± 5% of Cdc12p nodes colocalized with Rlc1p nodes, whereas 50 ± 3% of Rlc1p nodes contained Cdc12p (Figure 2C; n = 6 cells). The percentage of colocalization could be an underestimation because of the weak Cdc12p signal. Imaging of strains expressing each tagged protein indicated that the colocalization was not due to bleed-through of signals (Figure 2C). Consistent with the assumption of the SCPR model (Vavylonis et al., 2008), these data indicate that Cdc12p is present in at least half of the myosin-II nodes before condensation into a ring.
Experiments using Cdc15p-mCherry and Cdc12p-3YFP (strain JW1432) suggest that colocalization of Cdc12p with other node proteins increases during node condensation, though fewer distinct nodes can be counted at later time points (n = 13 cells). We also found that Cdc12p-3GFP and Rlc1p-tdTomato colocalized in nodes and condensed simultaneously during ring assembly although the autofluorescence is high at the GFP channel (Figure 2D).
The partial colocalization of Cdc12p with Rlc1p nodes suggests that Cdc12p might be dynamically associated with nodes. To test this hypothesis, we performed FRAP on Cdc12p-3YFP nodes (Figure 2, E and F; Video 5). It is challenging to investigate the dynamics of Cdc12p nodes because they begin to condense soon after formation. The nodes were bleached near the beginning of node condensation (see Materials and Methods). As a control we bleached the Cdc12p fluorescence in fully formed contractile rings and found a recovery τ1/2 = 41.6 ± 13.8 s, similar to previous studies (Clifford et al., 2008; Yonetani et al., 2008). The dynamics of Cdc12p nodes were similar to that of the ring, with a recovery τ1/2 = 30.3 ± 10.3 s (p = 0.03). This rapid turnover of Cdc12p may be partially responsible for the dynamic interactions between actin filaments and nodes (Vavylonis et al., 2008).
Actin Filaments Are Nucleated by Cdc12p Nodes and Elongate in Random Directions during Contractile-Ring Assembly
To investigate the location of actin nucleation and actin filament orientation during contractile-ring assembly, we simultaneously visualized actin filaments labeled with green fluorescent protein-calponin homology domain (GFP-CHD; Wachtler et al., 2003; Karagiannis et al., 2005; Martin and Chang, 2006) and Cdc12p-tdTomato using time lapse microscopy (Figure 3). We found a meshwork of actin filaments (Vavylonis et al., 2008) at the division site in all cells (n = 25) during ring formation. Similar observations were made with another marker of actin filaments termed mEGFP-lifeact (Riedl et al., 2008; Supplemental Figure S1). In ∼30% of the cells, actin filament structures similar to the aster and/or leading cable (Arai and Mabuchi, 2002) were observed 3–6 min after the appearance of the meshwork of actin filaments (Figure 3A). In only 1 of 25 cells we found that the Cdc12p spot was at the center of the aster during ring formation. These results suggested that the actin aster/leading cable-like structures may not be nucleated by the spot, but instead represent an intermediate stage during actin filament condensation into a contractile ring in some cells.
Next we investigated whether Cdc12p-tdTomato nodes associate with actin filaments/bundles (Figure 3, B and C). We analyzed filaments/bundles that stayed in the same focal plane as the nodes (see Materials and Methods). This method is valid because most filaments are parallel to the plasma membrane. Actin filaments/bundles in the meshwork appeared to elongate from Cdc12p nodes in random directions in asynchronous cells (Figure 3, B–D; Videos 6 and 7). No directional bias was evident in the top half versus the bottom half of the cells or in individual cells. Cdc12p nodes appeared to associate with 25 ± 5 filaments in each cell (n = 11 cells), and ∼50% of Cdc12p nodes were associated with actin filaments at any given time. The filaments were 0.9 ± 0.3 μm in length (Figure 3E). In certain instances, filaments appeared to connect two nodes (Figure 3B, right, white arrows). The logical assumption is that these nodes are nucleating the actin filaments because Cdc12p is known to have this activity in vitro (Kovar et al., 2003; Neidt et al., 2008) and because Cdc12p arrival at the division site precedes the appearance of an actin meshwork (Wu et al., 2006; Vavylonis et al., 2008).
Experiments using the temperature-sensitive cdc25-22 mutant to synchronize cells in G2/M phase confirmed that actin filaments/bundles elongated from a broad band of Cdc12p nodes in random directions (Figure 3, F–I). The filaments and nodes were easier to analyze in synchronized cells released to 25°C, because they were brighter and more numerous (Figure 3F). We performed these experiments in cells with or without For3p, the formin that nucleates interphase actin cables. We found that actin filaments elongated in random directions from Cdc12p nodes in synchronized cells similar to the asynchronous culture. Figure 3G combines data from asynchronous (Figure 3D) and synchronized cells and shows a broad distribution of filament orientation during ring formation, instead of perpendicular to the long axis of the cell as proposed by the spot/leading cable model. In for3Δ cells, all actin filaments at the division site were associated with Cdc12p nodes, in contrast to for3+ cells in which 11.5% of filaments were not associated with nodes (n = 358 filaments).
It is possible that Cdc12p nodes merely associate with actin filaments rather than nucleating them. We tried to distinguish these possibilities by examining regrowth of actin filaments in for3Δ cells, in which Cdc12p is the only formin participating in contractile-ring assembly. After washing out the Lat-A, we observed newly nucleated actin filaments growing from Cdc12p nodes in random directions (Figure 3, H and I). With the elongation of actin filaments, Cdc12p colocalized with filamentous actin structures (Figure 3H, middle and bottom cells). By 90 min after washing out Lat-A, actin networks were nearly condensed into a ring (Figure 3H, bottom right cell).
Thus, these data indicated that most actin filaments are nucleated by Cdc12p in the nodes in random directions. The data support the SCPR model but are contrary to the expectation of the spot/leading cable model.
Spot Characteristics Are Inconsistent with the Assumption That the Spot Is the Origin of the Contractile Ring
The spot/leading cable model proposes that the Cdc12p spot initiates contractile-ring formation (see Introduction). To test this assumption, we further examined the relationship between the spot and the assembly of the contractile ring. It was sometimes difficult to distinguish the elusive spot from speckles and nodes in cells expressing fluorescent Cdc12p (Figure 1A). To overcome this difficulty, we looked for other proteins that colocalized with Cdc12p in spots to use as a marker. Rlc1p spots are induced when cells are grown at 36°C (Wong et al., 2002) but do not colocalize with Cdc12p spots (Figure 4A). Next we tested and found that α-actinin Ain1p (Wu et al., 2001), a putative actin cross-linking protein, colocalized with Cdc12p in spots (Figure 4A) but not in speckles or nodes.
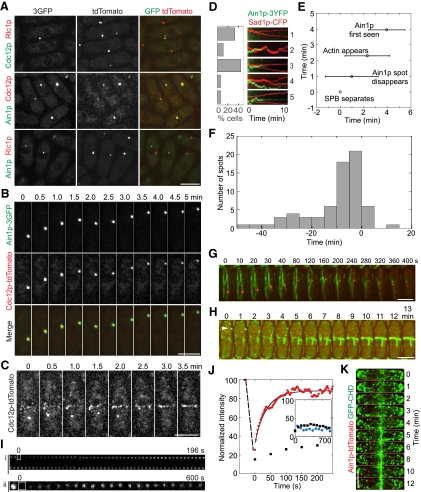
Figure 4.
The stable Cdc12p spot colocalizes with the α-actinin Ain1p spot and usually disappears before contractile-ring formation. (A) Localization of Cdc12p, Ain1p, and Rlc1p to spots. Cells expressing both 3GFP (green) and tdTomato-tagged proteins (red) were imaged. Strains JW1411, JW1458, and JW1439. (B and C) Strain JW1458. (B) The Ain1p spot (green) and Cdc12p spot (red) colocalize and move synchronously in an interphase cell. (C) A time-lapse series of a Cdc12p spot incorporated into the contractile ring after node condensation. Ain1p colocalizes with Cdc12p at the spot but is not shown for clarity. (D) Kymographs (right) and histogram (left) showing the relationship between the Ain1p-3YFP spot (green) and SPB separation (red), n = 18 cells. Kymographs were constructed with a 4-μm slit parallel to the long axis of the cell (JW1443) at the medial region. Images were collected every 20–30 s for 10 min. Representative cells are divided into five categories as described in Results. Graph shows the percentage of cells in each category. (E) Time line (mean ± SD) showing SPB separates (defined as time zero), Ain1p spot disappears, actin accumulates at the division site (Vavylonis et al., 2008), and Ain1p begins to accumulate at the contractile ring; n = 9 cells. (F) Histogram showing the timing that the spot arrived at the division site with spindle appearance defined as time 0 (strain JW1713 GFP-cdc15 cdc12-tdTomato 3nmt1-GFP-atb2). Example of an early arriving spot is shown in G and late-arriving spots are shown in H and K. (G) The Ain1p-tdTomato spot (red) moves to the division site on a MT track (green) well before spindle appearance (at 400 s). GFP-Atb2p as a marker for MTs under the 3nmt1 promoter in repressing condition with thiamine (JW1716). (H) Cdc12p-tdTomato and two times overexpressed GFP-Cdc15p (JW1713) colocalize in a spot (yellow, arrowhead) that moves to the division site on the last interphase MT (green). (I and J) Dynamics of Ain1p spots and ring revealed by FRAP of strain ain1-3GFP (JW1149). Montages are from time-lapse microscopy during FRAP (see Materials and Methods). Cells were bleached at time zero. (I) Middle section of Ain1p-3GFP ring (i; see Video 8) and Ain1p-3GFP spot (ii). (J) Recovery of the Ain1p-3GFP ring (n = 9 cells, red) and lack of recovery of the Ain1p-3GFP spot (n = 10, black and inset). Inset, blue points represent lack of recovery of Cdc12p-tdTomato spots bleached in strain JW1516, similar to Ain1p-3GFP spots (black). Relative intensity is normalized to the average prebleach intensity. (K) An Ain1p spot moves to the division site after a meshwork of actin filaments appeared there (JW1449). Bars, 5 μm except in (I, ii), bar, 1 μm.
In order to characterize the spot, we first validated Ain1p as a marker for the Cdc12p spot. The Ain1p spot contained 13.8 ± 2.7% of the total Ain1p molecules (n = 10 cells), whereas the Cdc12p spot contained only 3.6 ± 2.7% of the total Cdc12p molecules (Figure 1G) in interphase cells. In addition, Ain1p is six times more abundant than Cdc12p (Wu and Pollard, 2005) and does not localize to any other cellular structures during interphase. Thus, Ain1p spots are much brighter and easier to track than Cdc12p spots. We found that 70% of interphase cells lacked both the Ain1p-3GFP and the Cdc12p-tdTomato spots in a double-tagged strain (n = 1717 interphase cells). In cells with spots, 93% of spots contained both Ain1p-3GFP and Cdc12p-tdTomato (Figure 4A; n = 509 spots), whereas 2% cells contained only Cdc12p spots and 5% only Ain1p spots. As a confirmation, we determined that Ain1p and Rlc1p never colocalized in spots (Figure 4A). The spot contained at least three proteins: Cdc15p, Cdc12p, and Ain1p. In ain1Δ cells, Cdc12p-3YFP or Cdc12p-3GFP spots essentially disappeared (see below, Figure 5A). Because 95% of Ain1p spots contained Cdc12p and Cdc12p spot formation and/or maintenance was dependent on Ain1p, Ain1p is a reliable marker for tracking spots during contractile-ring assembly.
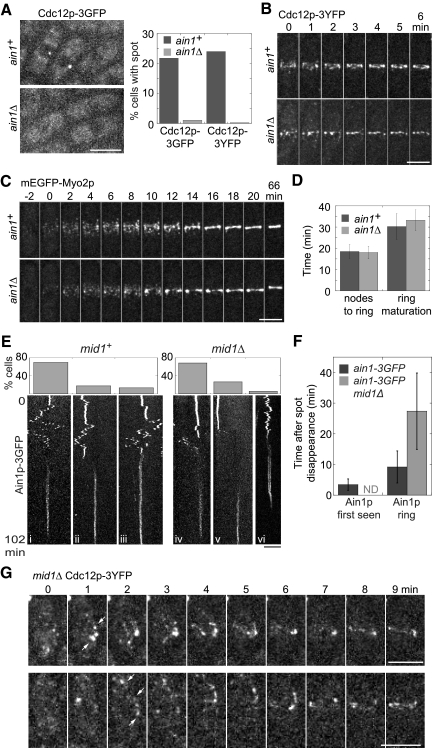
Figure 5.
The contractile ring assembles from nodes in the absence of the spot (A–D) and the spot is dispensable for the node-independent ring assembly pathway (E–G). (A) Localization of Cdc12p to the spot depends on Ain1p. Left, micrographs of maximum intensity projections of 23 sections spaced at 0.2 μm. Right, percentage of ain1+ (strains JW1404 and JW1405) and ain1Δ (JW1456 and JW1722) interphase cells with Cdc12p spots (n > 250 cells each). (B) A time series of ring assembly from Cdc12p-3YFP nodes in ain1+ and ain1Δ cells. Maximum intensity projections of six slices spaced at 0.8 μm resulting in fewer detectable nodes and less homogeneous ring appearance. (C) Time series showing ring assembly from nodes in the absence of a spot (strains JW1109 and JW1136). Maximum intensity projections of nine sections spaced at 0.6 μm. Node appearance is set as time zero. (D) The timing of node condensation into a ring and ring maturation was similar in ain1+ and ain1Δ cells expressing mEGFP-Myo2p (JW1109 and JW1136). The same cells were analyzed at both stages: ain1+, n = 15; ain1Δ, n = 25. (E) Graphs (top) and kymographs (bottom) of Ain1p-3GFP showing the relationship between spot movement (top portion of the kymographs) and contractile-ring formation (bottom portion of the kymographs) in mid1+ and mid1Δ cells. Kymographs were constructed using a slit parallel to the long axis of maximum intensity projections of the cells. Z-sections were collected at 0.8-μm spacing every 30 s for 102 min (see Materials and Methods). The cells from mid1+ (n = 23, JW1149) and mid1Δ (n = 18, JW1450) were grouped into three categories (graphs show the percentage of cells in each): (i and iv) The Ain1p spot moved to the division site and disappeared before ring formation; (ii and v) The Ain1p spot disappeared distant to the division site before ring formation (see Video 9); (iii and vi) The spot was integrated into the contractile ring. (F) Temporal relationship (mean ± SD) between Ain1p-3GFP spot disappearance, the first sign of Ain1p-3GFP accumulation in the contractile ring and the appearance of a sharp Ain1p ring in mid1+ (n = 20) and mid1Δ cells (n = 17). ND, not determined. (G) The Cdc12p filament and ring do not originate from a spot in most mid1Δ cells. Strain JW1189 (mid1Δ cdc12-3YFP) was observed for 30 min. Cdc12p-3YFP appears at several locations near the division site (white arrows) in two representative cells at time 1 min. The signals at 0 min are cytoplasmic speckles. Bars, 5 μm.
The life cycle of the spot corresponds to the S. pombe cell cycle. We found that Ain1p and Cdc12p spots appeared around cell separation in 30% cells, consistent with the timing of an earlier report regarding Cdc12p (Chang, 1999). Cdc12p and Ain1p spots moved together during interphase (Figure 4B) and ring formation (Figure 4C). Seventy-three percent of cells forming contractile rings lacked an obvious spot in either the Cdc12p or Ain1p channels in movies made over 5–10 min (n = 11). The spot with both Ain1p and Cdc12p was recruited to the division site in three of the cells: in one cell, the spot arrived during node formation; the spot in the other two cells arrived at the division site after the broad band of nodes had condensed into a ring (Figure 4C). This suggests that the spot may not be the origin of the actin ring, but delivers its contents to the division site to be used in the ring.
The spot disappears at varied times. The timing of cytokinesis has been mapped out using the SPBs as an internal clock (Wu et al., 2003), so we examined the timing of Ain1p spot disappearance relative to the cytokinesis clock. In 89% of cells, the Ain1p spot did not exist or disappeared before Ain1p ring formation (Figure 4D, Categories 1–3). Of those cells, 38% had no Ain1p spot up to 9.5 min before SPB separation (Figure 4D, Category 1), in 12% the spot disappeared 2.3 ± 0.5 min before SPB separation (Figure 4D, Category 2), and in 50% the spot disappeared 1.9 ± 1.5 min after SPB separation but before ring formation (Figure 4D, Category 3). In 11% of cells the spot persisted for >5 min after SPB separation and in one of them it integrated into the ring (Figure 4D, Category 5), while the other cell did not form a ring during image acquisition (Figure 4D, Category 4). On average the spot disappeared 1.0 ± 2.2 min after SPB separation, which is before a meshwork of actin filaments and Ain1p appear at the division site at 2.3 min (Vavylonis et al., 2008) and 4.0 ± 1.5 min after SPB separation, respectively (Figure 4E). We did not observe that the spot spread into a contractile ring. Although the actin aster frequently located near SPBs in fixed cells (Arai and Mabuchi, 2002), we did not find an obvious correlation between the positions of the spot and the SPBs and between the spot and the contractile-ring assembly. The observation is further supported by using Cdc15p as a marker for the spot. Cdc15p localizes to nodes, the contractile ring, and partially to actin patches (Carnahan and Gould, 2003; Wu et al., 2006). Mild overexpression of Cdc15p resulted in brighter Cdc12p spots (Carnahan and Gould, 2003). Using a strain expressing Cdc12p-tdTomato and two times the native level GFP-Cdc15p (Wu and Pollard, 2005), we analyzed the timing of spot disappearance. We found that in 88% of cells forming rings, the spot disappeared during node formation or condensation (n = 17 cells; Supplemental Figure S2A). However, the spot in cells expressing the native level of GFP-Cdc15p disappeared earlier. The Cdc12p and Cdc15p spot disappeared ∼6 min before Cdc15p appeared in nodes (n = 37 cells; Supplemental Figure S2B). Only 32% of spots in these cells persisted to node formation, and none persisted to ring formation. This is consistent with our Ain1p data.
The spot may travel to the division site through its association with a cytoskeletal track. Although the localization of Ain1p at the contractile ring depends on actin filaments (Wu et al., 2001), the Ain1p spot was not dependent on actin filaments for its maintenance revealed by Lat-A treatment, similar to the Cdc12p spot (Chang, 2000). However, most Ain1p spots stopped moving without actin filaments. This suggests that actin filaments were the main track of spot movement. We observed cells expressing both Ain1p-tdTomato spots and GFP-CHD–labeled actin filaments and noticed a strong bias for spots to move away from the medial region toward cell tips on actin filaments during interphase. Two-thirds of spots moved toward cell tips, whereas the rest moved toward the medial region or in both directions on the same filament (n = 69 spots). Of spots not associated with actin filaments, <10% moved toward the cell tips. Of the remaining 90%, about half moved toward the medial region from the cell tips and the other half moved in both directions successively (n = 71 spots). Because Cdc12p spots cannot move without actin or microtubule (MT) tracks (Chang, 2000), we assumed that the movements independent of actin filaments were on MTs. In cells expressing GFP-Atb2p (the α-tubulin subunit of MTs) as well as the spot markers, spots mostly moved toward the cell middle on MTs and arrived at the division site well before spindle formation (Figure 4F). Just before contractile-ring assembly, 64% of spots traveled to the division site on a MT track (n = 42 cells), 10.7 ± 11.9 min before the formation of the mitotic spindle (Figure 4, F–H). The large variance reflects the fact that some spots arrived on MTs and stayed at the division site as long as 50 min before spindle formation. Only 7% of spots appeared to arrive at the division site on the last interphase MT initiated from the nuclear region before mitosis (Figure 4H), consistent with our previous observation that the location of the spot did not correlate with SPBs.
The dynamics of the spot differ from those of the contractile ring. The contractile ring is a very dynamic structure (Pelham and Chang, 2002; Murthy and Wadsworth, 2005). We used FRAP to provide insights into the protein dynamics of the putative precursor spot. The Ain1p ring was a dynamic structure, with recovery half time τ1/2 = 16.1 ± 6.6 s (Figure 4, I and J; Video 8), similar to other ring proteins (Clifford et al., 2008; Yonetani et al., 2008). The intensity of the Ain1p ring increases during ring maturation (Wu and Pollard, 2005), so the calculated half time is likely an underestimate of the actual recovery half time. In contrast to the ring, the Ain1p and Cdc12p spot was a relatively static structure, with a maximum of 30% recovery in more than 10 min (Figure 4J, inset, black squares for Ain1p and blue circles for Cdc12p). We cannot exclude the possibility that the dynamics of the spot may change just before ring assembly, but these data suggest that the spot is dynamically different from the contractile ring.
The spot is not the site of actin nucleation. We confirmed that the spot did not associate with actin filaments in an actin aster or leading cable during contractile-ring assembly by investigating the distribution of actin filaments relative to the spot using Ain1p as a marker. Ain1p spots were undetectable, dissolved, or not associated with actin filaments in 64% of cells just before contractile-ring assembly (n = 18). In ∼20% of cells with a structure similar to an aster or leading cable, the Ain1p spot persisted and moved to the preformed actin meshwork during filament condensation (Figure 4K). Thus, it seems that the spot does not nucleate an aster or a leading cable.
In summary, spots moved to the division site at various times mainly on MTs, dissolved before ring assembly, turned over protein components slower than nodes and rings, and rarely originated actin asters. Thus, the characteristics of Ain1p, Cdc12p, and Cdc15p spots do not support the hypothesis that the spot nucleates actin filaments and then spreads directly into a contractile ring through a spot/leading cable model.
In Cells Lacking Cdc12p Spots, the Contractile Ring Assembles via Nodes
A possible role of the spot in contractile-ring formation was further investigated in mutant cells lacking the spot. In ain1Δ cells, <2% cells contained Cdc12p-3YFP or Cdc12p-3GFP spots (Figure 5A). Cdc12p contractile rings still formed from nodes in ain1Δ cells (Figure 5B). We confirmed the results using mEGFP-Myo2p in ain1+ and ain1Δ cells. The time between the appearance of Myo2p nodes and formation of a compact contractile ring was unaffected by the lack of a spot (Figure 5, C and D). Ring maturation, from the appearance of a compact ring to the beginning of ring constriction, took slightly longer in ain1Δ cells, but the difference was not significant (p < 0.1). Thus, it seems the spot is dispensable for the assembly of the contractile ring under normal growth conditions.
Node-independent Ring Assembly Might Not Depend on the Cdc12p/Ain1p Spot
Mitotic node formation is dependent on the anillin Mid1p (Wu et al., 2003, 2006; Motegi et al., 2004). It was suggested that the spot might play a more important role in ring assembly in mid1Δ cells lacking nodes (reviewed in Mishra and Oliferenko, 2008). To test this hypothesis, we investigated the movement of Ain1p spots during contractile-ring formation in mid1+ and mid1Δ cells (Figure 5, E and F). Among the mid1+ cells with an Ain1p spot that formed a ring during 102-min movies, in 87% of the cells the spot disassembled and disappeared, usually near the division site, 3.5 ± 1.9 min before the accumulation of Ain1p around the circumference of the cell equator and 9.2 ± 5.2 min before the appearance of a well-formed Ain1p ring (Figure 5, E, i–iii, and F). Because the Cdc12p spot depends on Ain1p for formation and/or maintenance (Figure 5A), Cdc12p spots likely disassemble with Ain1p. Of the mid1Δ cells that formed rings during 102-min movies, in 94% of the cells the spot disappeared 27.4 ± 12.4 min before a well-formed but misplaced ring appeared (Figure 5E, iv–vi, and F).
Cdc12p behavior in mid1Δ cells is consistent with experiments showing that the spot marked by Ain1p-3GFP does not nucleate rings (Figure 5G). Cdc12p filaments appeared to elongate from a starting spot structure in only 23% mid1Δ cells, whereas in 77% of cells Cdc12p was recruited at multiple places at once near the plasma membrane (Figure 5G, white arrows; n = 39 cells forming filaments and/or rings during 15–60 min movies). It is still unknown how some mid1Δ cells manage to form the contractile ring.
Taken together, the spot was recruited to the division site and dispersed well before ring assembly in a majority of cells, thus failing to support the hypothesis that the spot spreads directly into a contractile ring. Therefore, it appears that spots are not crucial for the assembly of the contractile ring, even without Mid1p and nodes.
Myosin Motor Activity Is Required for Condensation of Nodes into a Contractile Ring
The myosin-II motor activity is not essential for the spot/leading cable model (reviewed in Mishra and Oliferenko, 2008 and Roberts-Galbraith and Gould, 2008). However, one key assumption of the SCPR model is that the myosin-II motor drives the condensation of nodes into a ring (Vavylonis et al., 2008). Biochemistry experiments show that UCS protein Rng3p activates the motor activity of Myo2p (Lord and Pollard, 2004; Lord et al., 2008). The myosin motor activity is very weak or abolished when the temperature-sensitive mutants myo2-E1 or rng3-65 are grown at restrictive temperature. Thus, we tested roles of myosin motor activity in node condensation and ring formation using mutants myo2-E1 and rng3-65 (Balasubramanian et al., 1998).
We found that cells with nodes were more abundant in myo2-E1 mutants even at 25°C (permissive temperature) compared with myo2+ cells (Figure 6, A and B). Only 1% of asynchronous control cells contained Cdc12p-3YFP nodes, because Cdc12p nodes begin to condense soon after their appearance (Video 2). However, 5% of myo2-E1 cells contained Cdc12p nodes, suggesting that myo2-E1 cells spent five times longer in node formation and/or condensation stages than myo2+ cells, although the growth rates of these strains were similar at 25°C (p = 0.5; Figure 6C).
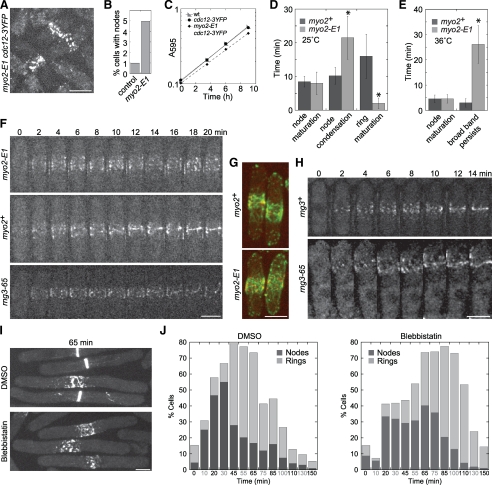
Figure 6.
The myosin-II motor activity is essential for condensation of the broad band of nodes into a contractile ring. (A and B) Localization of Cdc12p-3YFP to the nodes in control myo2+ and myo2-E1 mutant at the permissive temperature of 25°C. Cells (n > 1000 cells each strain) before or at any stage of node condensation were counted as long as nodes could be distinguished. (C) Growth rate of myo2-E1 and myo2+ strains at 25°C. The Y-axis is a logarithmic scale. (A–C) Strains JW81, JW1404, and JW1423 were used. (D–I) The myosin-II motor activity is required for condensation of nodes into a contractile ring. (D and E) Cells expressing both Rlc1p-tdTomato and GFP-CHD in myo2+ (JW1349) and myo2-E1 (JW1701) were imaged at 25 or 36°C. Cytokinesis events were defined as the following: Node maturation, the time between the appearance of Rlc1p nodes and that of actin filaments in the broad band; Node condensation, the time from actin appearance to formation of a compact ring with no lagging nodes; Ring maturation, the time from a compact ring to the beginning of ring constriction; Broad band persists, the time that the broad band of nodes remains uncondensed after the appearance of actin filaments among nodes until the end of 40-min movies. Number of cells analyzed (left to right): (D) 11, 28, 10, 26, 10, and 45; (E) 9, 12, 8, and 22. *p < 0.005. (F) Representative cells from the data in E as well as rng3-65 (JW1720) imaged at 36°C. Only the Rlc1p-tdTomato signal is shown for clarity. The signal is weaker at 36°C than at 25°C, so nodes are somewhat harder to visualize. (G) GFP-CHD–labeled actin filaments associate with Rlc1p-labeled nodes. Maximum intensity projection (seven sections spaced at 0.8 μm) of cells expressing both Rlc1p-tdTomato (red) and GFP-CHD (green) in myo2+ (top, JW1349) or myo2-E1 mutant (bottom, JW1701). (H) The contractile ring assembles from nodes after the recovery of Myo2p motor activity. Cells of rng3+ (JW1666) or rng3-65 (JW1720) were shifted to 36°C for 4 h and then back to 24°C for 4 h before imaging at 24°C. (I and J) cdc25-22 rlc1-tdTomato cells (JW1351) were synchronized at 36°C for 4 h and then treated with 1 mM blebbistatin or an equal volume of DMSO at 36°C for 30 min before releasing to 25°C for imaging at time zero. (I) Representative DMSO and blebbistatin-treated cells at 65 min after release. Maximum intensity projection of eight slices spaced 0.6 μm apart. (J) Quantification of cells in each culture that have Rlc1p nodes (dark) and rings (light) at each time point (n > 45 cells for each column). Bars, 5 μm.
To investigate the roles of the myosin motor activity in node formation and node condensation into a contractile ring, we observed cells expressing both Rlc1p-tdTomato and GFP-CHD in myo2-E1 and myo2+ strains. In both strains at 25°C, Rlc1p nodes persisted for ∼10 min (node formation or node maturation) before actin filaments appeared among the nodes and node condensation began (Figure 6D). However, we found that node condensation in myo2-E1 took twice as long as in myo2+ cells. Interestingly ring constriction began almost immediately after all lagging nodes joined to the ring in myo2-E1. Thus, the total time for ring assembly was very similar (Figure 6D), which may explain why the doubling times were almost identical (Figure 6C). At the restrictive temperature of 36°C, nodes in myo2-E1 cells were able to condense within the first 30 min of the temperature shift. After 1 h at 36°C, the myo2-E1 cells were capable of forming new Rlc1p nodes, but were unable to condense the nodes into a contractile ring, whereas nodes in myo2+ cells began to condense within 5 min after appearance (Figure 6, E and F). One explanation for failure of node condensation is that actin filaments are unable to associate with nodes. However, we found that GFP-CHD labeled actin filaments still associated with nodes in the broad band but were unable to form an actin ring (Figure 6G). These data suggest that the Myo2p motor activity is important for node condensation and contractile-ring formation.
To confirm the importance of myosin motor activity during node condensation into the contractile ring, we utilized rng3-65 cells that also express Rlc1p-tdTomato. After growing asynchronous cultures at 36°C for 90 min, 6.5% cells contained Rlc1p-tdTomato nodes (n = 214 cells), compared with 3.3% of rng3+ cells under the same conditions (n = 180). Similar to myo2-E1, we found that Rlc1p nodes failed to condense into a complete ring (Figure 6F) and a broad band of Rlc1p short filamentous structures was formed instead, indicating that the Myo2p motor activity is required for nodes to condense. In addition, cells incubated for 4 h at 36°C and shifted back to 24°C partially recovered the ability to condense the nodes (Figure 6H). Although cells exhibited evidence of cytokinesis failure from the previous cell cycle and accumulated four nuclei, we observed an imperfect contractile ring assembled from a broad band of nodes after cells recovered for 4 h at 24°C (Figure 6H), suggesting that the recovery of Rng3p restores Myo2p motor activity and can lead to condensation of nodes. Consistent with another study (Wong et al., 2002), the Rlc1p spot was not seen in rng3-65 cells grown at 36°C and not recovered after shifting cells down to the permissive temperature.
Additional evidence for a role of myosin motor activity during node condensation was obtained using blebbistatin to inhibit the motor activity of myosin-II (Straight et al., 2003; Riveline and Nurse, 2009). Although our results suggested that the inhibitor is not very effective in fission yeast since rings still form eventually, it did cause a delay in node condensation into a contractile ring in synchronized cells (Figure 6, I and J). At 65 min after releasing cdc25-22 cells to the permissive temperature, <20% cells treated with DMSO still had uncondensed nodes, whereas the blebbistatin-treated culture still had 40% cells with uncondensed nodes. Taken together, these data suggested that myosin-II motor activity is required for the condensation of the nodes and actin filaments into a contractile ring.
DISCUSSION
An actomyosin contractile ring assembles and constricts in coordination with mitosis to properly segregate genetic materials during cytokinesis. The molecular mechanism of contractile-ring assembly remains poorly understood and controversial. Two major models describe how the contractile ring assembles in wild-type fission yeast cells: the spot/leading cable model and the SCPR model (reviewed in Mishra and Oliferenko, 2008 and Roberts-Galbraith and Gould, 2008). In this study, we test the two models for Mid1p-dependent contractile-ring formation by investigating roles of formin Cdc12p and myosin-II motor activity in contractile-ring assembly.
Contribution of the Formin Spot and Nodes to Contractile-Ring Formation
The spot/leading cable model and the SCPR model differ on how the essential actin nucleator formin Cdc12p is recruited and distributed at the division site and how actin filaments are oriented in the contractile ring.
The spot/leading cable model began with the observation that a Cdc12p spot travels to the division site and then a strand of Cdc12p emanates from the spot to encircle the cell equator to form the ring when Cdc12p is overexpressed (Chang et al., 1997). It was suggested but never observed that the Cdc12p spot may reside at the origin of the actin aster seen before ring formation in fixed cells and that the Cdc12p strand may coincide with the leading actin cable (Arai and Mabuchi, 2002). Orientations of actin filaments in the contractile ring were determined by analyzing electron micrographs of myosin S1-decorated actin filaments in fixed dividing cells (Kamasaki et al., 2007). Two fixed cells in early anaphase were reported to have two semicircles of actin filaments in opposite orientations perpendicular to the long axis of the cell, suggesting a single origin of nucleation by the Cdc12p spot.
In this study, we were able to visualize Cdc12p expressed under its native promoter in speckles, spots, nodes, and contractile rings. Cdc12p has not been observed in speckles or the broad band of nodes in previous studies. We speculate the following possible reasons: 1) Cdc12p-3YFP might be excited with a 488-nm laser in previous reports. The high autofluorescence of S. pombe at 488-nm channel masked the weak signals at nodes and speckles. The speckles remain elusive in the Cdc12p-3GFP strain because of autofluorescence and GFP is less bright than YFP (Figure 1); 2) Our use of a more sensitive spinning disk confocal system might also account for the detection of Cdc12p in nodes and speckles when the same strain was used (Wu et al., 2006). The number of speckles in the cell during interphase is approximately half the number of Cdc12p molecules in the cell as determined by Wu and Pollard (2005). The intensity at speckles is on average half the intensity at the node, which contains about two Cdc12p dimers (Wu and Pollard, 2005). Thus, one Cdc12p speckle might contain only one formin dimer (six YFP molecules).
We found that the spot disassembles/disappears before actin filament accumulation at the division site and ring formation in most cells (Figures 4 and 5), rather than being at the center of actin nucleation as proposed by the spot/leading cable model. Even when the spot persists in some cells, it moves to the preformed actin meshwork nucleated by formin nodes during filament condensation (Figures 3 and 4). We found that the spot is recruited to the division site at various times and places, mainly by traveling on MTs, suggesting its contribution to the contractile ring might be similar to that of the speckles. An ain1Δ mutant, rarely forming the Cdc12p spot, assembles contractile rings normally from nodes (Figure 5), further suggesting that the spot is not essential for contractile-ring assembly, as reported earlier (Yonetani et al., 2008). The discrepancy between previous reports that Cdc12p spots spread into rings, and our data showing that spots disappear before ring assembly can be explained by the localization of Cdc12p to the nodes. Because Cdc12p nodes are visible for no more than 2 min before condensation starts, it may appear that the dissolving of the spot directly correlates with ring formation when nodes are not detectable.
However, it is still possible that the spot may play some roles during contractile-ring assembly. One hypothesis is that the spot dissolves at the division site to produce a higher local concentration of Cdc12p available to nodes. Although the timing of ring assembly and morphology of the ring appear normal in cells without the spot, the spot in some cells does travel to the division site and likely contributes to ring assembly. The second hypothesis is that the spot may be involved in the ring assembly pathway orchestrated by the septation initiation network (SIN) or in mid1Δ cells (reviewed in Mishra and Oliferenko, 2008 and Roberts-Galbraith and Gould, 2008). Activation of the SIN pathway in interphase or late anaphase cells leads to the formation of contractile rings through a filament intermediate, and this is likely the mechanism of ring formation in mid1Δ (Hachet and Simanis, 2008; Huang et al., 2008). The role of the spot in this pathway of ring formation appears to be minimal (Figure 5). In mid1Δ cells, the spot disassembles well before ring formation (Figure 5, E and F). Thus, the spot does not appear to be essential in ring formation in the absence of Mid1p and nodes. Further studies are needed to determine how rings form in these mutants. The third hypothesis is that the spot might become more important for ring formation under nutrition depletion or other stress conditions, which needs to be investigated in the future.
The SCPR model suggests that the contractile ring assembles from a broad band of precursor nodes that contain at least seven cytokinesis proteins (Wu et al., 2006; Vavylonis et al., 2008). However, in the spot/leading cable model, Cdc12p and Cdc15p do not have to colocalize with other node proteins (Chang et al., 1997; Chang, 1999; Arai and Mabuchi, 2002; Carnahan and Gould, 2003; Motegi et al., 2004; Kamasaki et al., 2007). The Cdc12p spot contains Cdc15p (Carnahan and Gould, 2003) and Ain1p (this study), but not myosin-II. In contrast, 70% of Cdc12p nodes contain Rlc1p (Figure 2), which colocalizes with Myo2p, Cdc4p, anillin Mid1p, IQGAP Rng2p, and F-BAR/PCH protein Cdc15p (Wu et al., 2006). The dynamic nature (Figure 2) and sequential assembly of nodes may explain why the node proteins are not colocalized 100% of the time.
The detection of seven critical cytokinesis proteins colocalizing in the nodes in wild-type cells provides strong support for the SCPR model. Cdc12p dynamically localizes to at least half the cytokinetic nodes (Figure 2) by recruitment of molecules from cytoplasmic speckles and possibly the spot (Figures 1 and 4). The possible roles of Cdc12p nodes in contractile-ring assembly include 1) Nucleate actin filaments in random directions necessary to condense nodes via the SCPR mechanism (Figure 3); 2) Anchor actin filaments to the plasma membrane; and 3) Contribute Cdc12p molecules to the contractile ring (Figures 1 and 2). Unlike the contractile ring in wild-type cells, the rings formed in mid1Δ cells or induced by SIN pathway are misplaced and constrict more slowly (Wu et al., 2003; Hachet and Simanis, 2008; Huang et al., 2008). Thus, nodes might be important for conveying the positional cue from Mid1p for timely and efficient assembly of the contractile ring at the right place.
Currently we cannot rule out the possibility that another model may arise that fits all the available data because not all assumptions of SCPR have been tested, but we can say that the Cdc12p spot does not nucleate all the actin filaments in the contractile ring under normal conditions. In the future, the physical interactions among the seven node proteins need to be mapped out to reveal the stepwise and hierarchical assembly of nodes and the contractile ring.
Interactions between a Meshwork of Actin Filaments and Precursor Nodes May Result in Actin Structures Similar to the Aster or Leading Cable
Consistent with the key assumption of the SCPR model, Cdc12p concentrates in nodes from which short actin filaments are nucleated and grow in random directions (Figure 3). We observed that the meshwork of actin filaments and nodes can sometimes condense into structures that appear similar to the aster or leading cable in live cells. These results lead us to the speculation that the aster and leading cable observed by Arai and Mabuchi (2002) in fixed cells may represent an intermediate stage during the condensation of nodes and actin filaments. Appearance of a meshwork of low fluorescence intensity actin filaments and the initial condensation of nodes and the filaments into thicker bundles occur at the same time, 2 min after Cdc12p appears in the nodes (Vavylonis et al., 2008). Because of this narrow window of time, the early actin filaments' nucleation by Cdc12p may have been missed in the studies of fixed cells (Arai and Mabuchi, 2002). Another possibility suggested by the authors is that the deconvolution may cause weaker signal to be lost. Thus, instead of the leading cable extending around the cell equator from the spot, it appears that the aster and leading cable-like structures are intermediates during the formation of the ring, which continues to brighten as it matures. The aster/leading cable-like structure sometimes observed may be a site where condensation of nodes and actin filaments is faster. However, the difference in the orientations of actin filaments during ring formation between the fixed early anaphase cells in Kamasaki et al. (2007), and live imaging of early mitotic cells in this study remains to be solved in the future.
Novel models have been proposed to unify the spot/leading cable model and the SCPR model (Hachet and Simanis, 2008; reviewed in Roberts-Galbraith and Gould, 2008). It was suggested that the Mid1p-myosin-II nodes converge not by coalescence of a meshwork of actin filaments and nodes but by nodes attaching to the filaments nucleated by a few actin asters. Our results lead us to a different integration of the two models for contractile-ring formation in wild-type cells. Figure 7 summarizes our data demonstrating that formin nodes are the main sites of actin nucleation during ring assembly by an SCPR mechanism. The nodes are formed by recruiting formin molecules from speckles and the diffuse cytoplasmic pool and sometimes the formin spot. During the condensation of the meshwork of nodes and actin filaments, formation of the aster and leading cable-like structure as intermediate stages could occur stochastically.
Figure 7.
A summary of our data regarding Mid1p-dependent contractile-ring assembly in fission yeast. (A and B) Contractile rings are assembled from Cdc12p nodes and actin filaments growing in random directions from the nodes through the myosin-II motor activity. Cdc12p molecules in speckles and/or diffused in cytoplasm are gradually recruited into the division machinery as the nodes mature and condense. When the ring constricts, Cdc12p molecules are released back to the cytoplasm as the numbers of speckles increase. The stages of cytokinesis are the same as in Figure 1F. (A) The majority of cells contain neither spots nor aster/leading cable-like structures. (B) Spots in some cells travel to the division site and usually dissolve before node maturation and ring assembly. As nodes condense into a contractile ring, structures like the aster and leading cable are occasionally formed from short actin filaments but not from the spot. Around the time of cell separation, new spots may form.
Myosin Motor Activity Is Required for Mid1p-dependent Contractile-Ring Assembly
The spot/leading cable model and the SCPR model also differ in the roles of myosin-II motor activity during contractile-ring assembly. The myosin-II motor activity is not required for ring formation through the spot/leading cable (reviewed in Mishra and Oliferenko, 2008 and Roberts-Galbraith and Gould, 2008). However, myosin-II motors are necessary for the condensation of nodes into a ring via the SCPR model (Pollard, 2008; Vavylonis et al., 2008).
The involvement of Myo2p motor activity in condensing the nodes into a contractile ring is controversial because the activator of Myo2p motor activity, Rng3p, has been detected at the division site only after the ring has formed (Wong et al., 2002; Lord and Pollard, 2004). It is difficult to detect Rng3p-3GFP or Rng3p-3YFP in the nodes because of their high concentration in the cytoplasm, low concentration at the division site, and substoichiometric interaction with myosin-II (Wu and Pollard, 2005). Nevertheless, the following evidence suggests that the myosin-II motor activity is required for the formation of the contractile ring: 1) In cells with myo2-E1, a mutation that results in very weak or abolished motilities (Lord and Pollard, 2004), node condensation slows or fails although actin filaments are still associated with nodes (Figure 6); 2) A Myo2p mutant with mutation in the ATP-binding site is defective in the assembly of the contractile ring (Naqvi et al., 1999); 3) Blebbistatin treatment causes a delay in node condensation into a contractile ring in synchronized cells (Figure 6); 4) Nodes cannot condense into a contractile ring in rng3-65 mutant at the restrictive temperature (Figure 6), suggesting Rng3p function is required for activating myosin motor activity during node condensation; and 5) Rng3p is detected in the nodes in myo2-E1 mutant background (Lord et al., 2008). This suggests that either Rng3p is present in the nodes but undetectable in wild-type cells or it may activate the myosin motor in the cytoplasm before Myo2p is recruited to the nodes. These two possibilities are difficult to distinguish with available resources, but should be addressed in the future. Consistent with our data, Sladewski et al. (2009) recently reported that a nonphosphorylatable Rlc1p mutant reduces the average rate of Myo2p motility in vitro and results in ring assembly taking 50% longer in vivo.
Contractile-Ring Assembly during Cytokinesis in Animal Cells
The contractile ring is essential for cytokinesis in higher eukaryotes. Recruitment of myosin and actin filaments to the contractile ring has been described in two hypotheses, cortical flow and de novo synthesis (Zhou and Wang, 2008). Myosin filaments are assembled de novo at the cleavage furrow, but actin filaments are both assembled de novo and recruited via cortical flow (Noguchi and Mabuchi, 2001; Zhou and Wang, 2008). In S. pombe, it appears that de novo assembly of myosin and actin filaments is dominant, because cells lacking the formin For3p form a normal contractile ring.
Formins are essential for cytokinesis in animal cells. In C. elegans embryos, node-like myosin-II foci are pulled together in a formin CYK-1–dependent manner (Werner et al., 2007). The Drosophila formin diaphanous is necessary for maintenance of myosin-II in a broad band at the cell equator during cytokinesis (Dean et al., 2005). In murine cells, mDia2 is responsible for de novo actin filament polymerization at the cleavage furrow in addition to recruiting preexisting actin cables via cortical flow (Watanabe et al., 2008).
The accumulation of myosin and anillin in node-like structures in animal cells has been reported in several studies (Maupin et al., 1994; Noguchi and Mabuchi, 2001; Straight et al., 2003; Zhou and Wang, 2008). Although many similarities exist, it is premature to suggest that the SCPR model can explain contractile-ring assembly in animal cells. More studies are needed to investigate the roles of each component and to establish how they work together during cytokinesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tiffany Talabere, Peng-cheng Wu, and Keerthana Bolisetty for technical assistance; David Clever, Stephen Osmani, Thomas Pollard, and Dimitrios Vavylonis for critical reading of the manuscript; Mohan Balasubramanian (Temasek Life Sciences Laboratory), Fred Chang (Columbia University), Da-Qiao Ding and Yasushi Hiraoka (Kansai Advanced Research Center), and David Kovar (University of Chicago) for fission yeast strains; and Roger Tsien (University of California, San Diego) and Ken Sawin (University of Edinburgh) for mCherry and tdTomato plasmids. This work was partially supported by the American Cancer Society, Ohio Pilot Research Grant; American Heart Association, Great Rivers Affiliate; Ohio Cancer Research Associates; Basil O'Connor Starter Scholar Research Award from the March of Dimes Foundation; and National Institute of Health (NIH) Grant R01GM086546.
Abbreviations used:
- CHD
calponin homology domain
- fps
frames per second
- FRAP
fluorescence recovery after photobleaching
- Lat-A
latrunculin A
- MT
microtubule
- PCH
pombe Cdc15 homology
- ROI
region of interest
- SCPR
search, capture, pull, and release
- SPB
spindle pole body.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-05-0428) on October 28, 2009.
REFERENCES
- Almonacid M., Moseley J. B., Janvore J., Mayeux A., Fraisier V., Nurse P., Paoletti A. Spatial control of cytokinesis by Cdr2 kinase and mid1/anillin nuclear export. Curr. Biol. 2009;19:961–966. doi: 10.1016/j.cub.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Arai R., Mabuchi I. F-actin ring formation and the role of F-actin cables in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 2002;115:887–898. doi: 10.1242/jcs.115.5.887. [DOI] [PubMed] [Google Scholar]
- Bähler J., Steever A. B., Wheatley S., Wang Y.-l., Pringle J. R., Gould K. L., McCollum D. Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J. Cell Biol. 1998a;143:1603–1616. doi: 10.1083/jcb.143.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J., Wu J.-Q., Longtine M. S., Shah N. G., McKenzie A., III, Steever A. B., Wach A., Philippsen P., Pringle J. R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998b;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., Bi E., Glotzer M. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr. Biol. 2004;14:R806–R818. doi: 10.1016/j.cub.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., McCollum D., Chang L., Wong K. C., Naqvi N. I., He X., Sazer S., Gould K. L. Isolation and characterization of new fission yeast cytokinesis mutants. Genetics. 1998;149:1265–1275. doi: 10.1093/genetics/149.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr F. A., Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Carnahan R. H., Gould K. L. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J. Cell Biol. 2003;162:851–862. doi: 10.1083/jcb.200305012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celton-Morizur S., Bordes N., Fraisier V., Tran P. T., Paoletti A. C-terminal anchoring of mid1p to membranes stabilizes cytokinetic ring position in early mitosis in fission yeast. Mol. Cell. Biol. 2004;24:10621–10635. doi: 10.1128/MCB.24.24.10621-10635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celton-Morizur S., Racine V., Sibarita J.-B., Paoletti A. Pom1 kinase links division plane position to cell polarity by regulating Mid1p cortical distribution. J. Cell Sci. 2006;119:4710–4718. doi: 10.1242/jcs.03261. [DOI] [PubMed] [Google Scholar]
- Chang F. Movement of a cytokinesis factor cdc12p to the site of cell division. Curr. Biol. 1999;9:849–852. doi: 10.1016/s0960-9822(99)80372-8. [DOI] [PubMed] [Google Scholar]
- Chang F. Microtubule and actin-dependent movement of the formin cdc12p in fission yeast. Microsc. Res. Tech. 2000;49:161–167. doi: 10.1002/(SICI)1097-0029(20000415)49:2<161::AID-JEMT8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Chang F., Drubin D., Nurse P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J. Cell Biol. 1997;137:169–182. doi: 10.1083/jcb.137.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford D. M., Wolfe B. A., Roberts-Galbraith R. H., McDonald W. H., Yates J. R., III, Gould K. L. The Clp1/Cdc14 phosphatase contributes to the robustness of cytokinesis by association with anillin-related Mid1. J. Cell Biol. 2008;181:79–88. doi: 10.1083/jcb.200709060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daga R. R., Chang F. Dynamic positioning of the fission yeast cell division plane. Proc. Natl. Acad. Sci. USA. 2005;102:8228–8232. doi: 10.1073/pnas.0409021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean S. O., Rogers S. L., Stuurman N., Vale R. D., Spudich J. A. Distinct pathways control recruitment and maintenance of myosin II at the cleavage furrow during cytokinesis. Proc. Natl. Acad. Sci. USA. 2005;102:13473–13478. doi: 10.1073/pnas.0506810102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echard A., Hickson G. R., Foley E., O'Farrell P. H. Terminal cytokinesis events uncovered after an RNAi screen. Curr. Biol. 2004;14:1685–1693. doi: 10.1016/j.cub.2004.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng K., Naqvi N. I., Wong K. C., Balasubramanian M. K. Rng2p, a protein required for cytokinesis in fission yeast, is a component of the actomyosin ring and the spindle pole body. Curr. Biol. 1998;8:611–621. doi: 10.1016/s0960-9822(98)70248-9. [DOI] [PubMed] [Google Scholar]
- Fraser A. G., Kamath R. S., Zipperlen P., Martinez-Campos M., Sohrmann M., Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Glotzer M., et al. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- Gönczy P. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature. 2000;408:331–336. doi: 10.1038/35042526. [DOI] [PubMed] [Google Scholar]
- Hachet O., Simanis V. Mid1p/anillin and the septation initiation network orchestrate contractile ring assembly for cytokinesis. Genes Dev. 2008;22:3217–3226. doi: 10.1101/gad.1697208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Chew T. G., Ge W., Balasubramanian M. K. Polarity determinants Tea1p, Tea4p, and Pom1p inhibit division-septum assembly at cell ends in fission yeast. Dev. Cell. 2007;12:987–996. doi: 10.1016/j.devcel.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Huang Y., Yan H., Balasubramanian M. K. Assembly of normal actomyosin rings in the absence of Mid1p and cortical nodes in fission yeast. J. Cell Biol. 2008;183:979–988. doi: 10.1083/jcb.200806151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamasaki T., Osumi M., Mabuchi I. Three-dimensional arrangement of F-actin in the contractile ring of fission yeast. J. Cell Biol. 2007;178:765–771. doi: 10.1083/jcb.200612018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis J., Bimbó A., Rajagopalan R., Liu J., Balasubramanian M. K. The nuclear kinase Lsk1p positively regulates the septation initiation network and promotes the successful completion of cytokinesis in response to perturbation of the actomyosin ring in Schizosaccharomyces pombe. Mol. Biol. Cell. 2005;16:358–371. doi: 10.1091/mbc.E04-06-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler R., et al. Genome-scale RNAi profiling of cell division in human tissue culture cells. Nat. Cell Biol. 2007;9:1401–1412. doi: 10.1038/ncb1659. [DOI] [PubMed] [Google Scholar]
- Kovar D. R., Kuhn J. R., Tichy A. L., Pollard T. D. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 2003;161:875–887. doi: 10.1083/jcb.200211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord M., Pollard T. D. UCS protein Rng3p activates actin filament gliding by fission yeast myosin-II. J. Cell Biol. 2004;167:315–325. doi: 10.1083/jcb.200404045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord M., Sladewski T. E., Pollard T. D. Yeast UCS proteins promote actomyosin interactions and limit myosin turnover in cells. Proc. Natl. Acad. Sci. USA. 2008;105:8014–8019. doi: 10.1073/pnas.0802874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. G., Chang F. Dynamics of the formin for3p in actin cable assembly. Curr. Biol. 2006;16:1161–1170. doi: 10.1016/j.cub.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Maupin P., Phillips C. L., Adelstein R. S., Pollard T. D. Differential localization of myosin-II isozymes in human cultured cells and blood cells. J. Cell Sci. 1994;107:3077–3090. doi: 10.1242/jcs.107.11.3077. [DOI] [PubMed] [Google Scholar]
- McNally J. G. Quantitative FRAP in analysis of molecular binding dynamics in vivo. In: Sullivan K. F., editor. Methods in Cell Biology. Vol. 85. Burlington, MA: Academic Press; 2008. pp. 329–351. Fluorescent Proteins. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Field C. M., Alberts B. M. Actin-binding proteins from Drosophila embryos: a complex network of interacting proteins detected by F-actin affinity chromatography. J. Cell Biol. 1989;109:2963–2975. doi: 10.1083/jcb.109.6.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M., Oliferenko S. Cytokinesis: catch and drag. Curr. Biol. 2008;18:R247–R250. doi: 10.1016/j.cub.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Motegi F., Nakano K., Mabuchi I. Molecular mechanism of myosin-II assembly at the division site in Schizosaccharomyces pombe. J. Cell Sci. 2000;113:1813–1825. doi: 10.1242/jcs.113.10.1813. [DOI] [PubMed] [Google Scholar]
- Motegi F., Mishra M., Balasubramanian M. K., Mabuchi I. Myosin-II reorganization during mitosis is controlled temporally by its dephosphorylation and spatially by Mid1 in fission yeast. J. Cell Biol. 2004;165:685–695. doi: 10.1083/jcb.200402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy K., Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr. Biol. 2005;15:724–731. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- Naqvi N. I., Eng K., Gould K. L., Balasubramanian M. K. Evidence for F-actin-dependent and -independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast. EMBO J. 1999;18:854–862. doi: 10.1093/emboj/18.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidt E. M., Skau C. T., Kovar D. R. The cytokinesis formins from the nematode worm and fission yeast differentially mediate actin filament assembly. J. Biol. Chem. 2008;283:23872–23883. doi: 10.1074/jbc.M803734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Mabuchi I. Reorganization of actin cytoskeleton at the growing end of the cleavage furrow of Xenopus egg during cytokinesis. J. Cell Sci. 2001;114:401–412. doi: 10.1242/jcs.114.2.401. [DOI] [PubMed] [Google Scholar]
- Padte N. N., Martin S. G., Howard M., Chang F. The cell-end factor pom1p inhibits mid1p in specification of the cell division plane in fission yeast. Curr. Biol. 2006;16:2480–2487. doi: 10.1016/j.cub.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Paoletti A., Chang F. Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol. Biol. Cell. 2000;11:2757–2773. doi: 10.1091/mbc.11.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham R. J., Jr, Chang F. Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature. 2002;419:82–86. doi: 10.1038/nature00999. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Progress towards understanding the mechanism of cytokinesis in fission yeast. Biochem. Soc. Trans. 2008;36:425–430. doi: 10.1042/BST0360425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J., et al. Lifeact: a versatile marker to visualize F-actin. Nat. Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveline D., Nurse P. ‘Injecting’ yeast. Nat. Methods. 2009;6:513–514. doi: 10.1038/nmeth.1335. [DOI] [PubMed] [Google Scholar]
- Roberts-Galbraith R. H., Gould K. L. Stepping into the ring: the SIN takes on contractile ring assembly. Genes Dev. 2008;22:3082–3088. doi: 10.1101/gad.1748908. [DOI] [PubMed] [Google Scholar]
- Skop A. R., Liu H., Yates J., III, Meyer B. J., Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladewski T. E., Previs M. J., Lord M. Regulation of fission yeast myosin-II function and contractile ring dynamics by regulatory light-chain and heavy-chain phosphorylation. Mol. Biol. Cell. 2009;20:3941–3952. doi: 10.1091/mbc.E09-04-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrmann M., Fankhauser C., Brodbeck C., Simanis V. The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes Dev. 1996;10:2707–2719. doi: 10.1101/gad.10.21.2707. [DOI] [PubMed] [Google Scholar]
- Sönnichsen B., et al. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434:462–469. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- Straight A. F., Cheung A., Limouze J., Chen I., Westwood N. J., Sellers J. R., Mitchison T. J. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Tran P. T., Doye V., Inoué S., Chang F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J. Cell Biol. 2001;153:397–411. doi: 10.1083/jcb.153.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavylonis D., Wu J.-Q., Hao S., O'Shaughnessy B., Pollard T. D. Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science. 2008;319:97–100. doi: 10.1126/science.1151086. [DOI] [PubMed] [Google Scholar]
- Wachtler V., Rajagopalan S., Balasubramanian M. K. Sterol-rich plasma membrane domains in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 2003;116:867–874. doi: 10.1242/jcs.00299. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Ando Y., Yasuda S., Hosoya H., Watanabe N., Ishizaki T., Narumiya S. mDia2 induces the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol. Biol. Cell. 2008;19:2328–2338. doi: 10.1091/mbc.E07-10-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M., Munro E., Glotzer M. Astral signals spatially bias cortical myosin recruitment to break symmetry and promote cytokinesis. Curr. Biol. 2007;17:1286–1297. doi: 10.1016/j.cub.2007.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe B. A., Gould K. L. Split decisions: coordinating cytokinesis in yeast. Trends Cell Biol. 2005;15:10–18. doi: 10.1016/j.tcb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Wong K.C.Y., D'Souza V. M., Naqvi N. I., Motegi F., Mabuchi I., Balasubramanian M. K. Importance of a myosin II-containing progenitor for actomyosin ring assembly in fission yeast. Curr. Biol. 2002;12:724–729. doi: 10.1016/s0960-9822(02)00790-x. [DOI] [PubMed] [Google Scholar]
- Wu J.-Q., Bähler J., Pringle J. R. Roles of a fimbrin and an alpha-actinin-like protein in fission yeast cell polarization and cytokinesis. Mol. Biol. Cell. 2001;12:1061–1077. doi: 10.1091/mbc.12.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.-Q., Kuhn J. R., Kovar D. R., Pollard T. D. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell. 2003;5:723–734. doi: 10.1016/s1534-5807(03)00324-1. [DOI] [PubMed] [Google Scholar]
- Wu J.-Q., Pollard T. D. Counting cytokinesis proteins globally and locally in fission yeast. Science. 2005;310:310–314. doi: 10.1126/science.1113230. [DOI] [PubMed] [Google Scholar]
- Wu J.-Q., Sirotkin V., Kovar D. R., Lord M., Beltzner C. C., Kuhn J. R., Pollard T. D. Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J. Cell Biol. 2006;174:391–402. doi: 10.1083/jcb.200602032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonetani A., Lustig R. J., Moseley J. B., Takeda T., Goode B. L., Chang F. Regulation and targeting of the fission yeast formin cdc12p in cytokinesis. Mol. Biol. Cell. 2008;19:2208–2219. doi: 10.1091/mbc.E07-07-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Wang Y.-L. Distinct pathways for the early recruitment of myosin II and actin to the cytokinetic furrow. Mol. Biol. Cell. 2008;19:318–326. doi: 10.1091/mbc.E07-08-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.