Abstract
Enzymes of the membrane-bound O-acyltransferase (MBOAT) family add fatty acyl chains to a diverse range of protein and lipid substrates. A chromosomal translocation disrupting human MBOAT1 results in a novel syndrome characterized by male sterility and brachydactyly. We have found that the Drosophila homologues of MBOAT1, Oysgedart (Oys), Nessy (Nes), and Farjavit (Frj), are lysophospholipid acyltransferases. When expressed in yeast, these MBOATs esterify specific lysophospholipids preferentially with unsaturated fatty acids. Generating null mutations for each gene allowed us to identify redundant functions for Oys and Nes in two distinct aspects of Drosophila germ cell development. Embryos lacking both oys and nes show defects in the ability of germ cells to migrate into the mesoderm, a process guided by lipid signals. In addition, oys nes double mutant adult males are sterile due to specific defects in spermatid individualization. oys nes mutant testes, as well as single, double, and triple mutant whole adult animals, show an increase in the saturated fatty acid content of several phospholipid species. Our findings suggest that lysophospholipid acyltransferase activity is essential for germline development and could provide a mechanistic explanation for the etiology of the human MBOAT1 mutation.
INTRODUCTION
The membrane-bound O-acyl transferases (MBOATs) comprise a family of enzymes found in organisms ranging from bacteria to humans (Hofmann, 2000; Shindou and Shimizu, 2008). They catalyze the covalent addition of fatty acid chains to diverse substrates that include sterols, neutral lipids, phospholipids, and proteins, and they are important for such cellular processes as membrane synthesis and remodeling, lipid storage, and signaling (Yang et al., 1996; Cases et al., 1998; Bosson et al., 2006; Miura and Treisman, 2006; Gutierrez et al., 2008; Yang et al., 2008). The defining characteristics of MBOATs are multiple membrane-spanning domains and a conserved histidine residue in the active site (Hofmann, 2000); apart from these features, members of this protein family share only limited homology. Seven predicted MBOATs are encoded by the Drosophila genome (see Figure 1A). Of these, Porcupine (Por) and Rasp have been shown to acylate signaling proteins; Por acylates Wnt family members, whereas Rasp acts on the Hedgehog (Hh) and Epidermal growth factor (EGF) families (Hofmann, 2000; Amanai and Jiang, 2001; Chamoun et al., 2001; Lee and Treisman, 2001; Micchelli et al., 2002; Zhai et al., 2004; Miura et al., 2006; Takada et al., 2006). Midway (Mdy) is a diacylglycerol acyltransferase required for oogenesis, and CG8112 is most similar to acyl-CoA cholesterol acyltransferases (ACATs) from other organisms (Buszczak et al., 2002).
Figure 1.
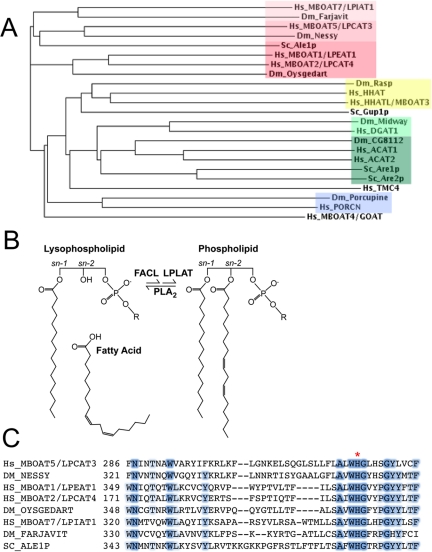
(A) Phylogram of the MBOAT enzymes from S. cerevisiae (Sc), Drosophila melanogaster (Dm), and Homo sapiens (Hs) made using the ClustalW algorithm. Farjavit is the Drosophila homologue of human MBOAT7, a lyso-PI acyltransferase (LPIAT). Nessy is the Drosophila homologue of human MBOAT5, also known as lyso-PC acyltransferase 3 (LPCAT3), and Oysgedart is the Drosophila homologue of human MBOATs 1 and 2. Other MBOAT family members include the Hedgehog acyltransferases (Rasp, HHAT, and HHATL), the Wnt acyltransferases (Porcupine and PORCN), the Ghrelin acyltransferase (GOAT/MBOAT4), the GPI remodeling acyltransferase Gup1p, the diacylglycerol acyltransferases (DGAT1 and Midway), and the cholesterol acyltransferases (ACATs 1 and 2, Are1p and Are2p, and Drosophila CG8112). Transmembrane containing protein 4 (TMC4) appears to be an “orphan” human MBOAT. Color blocks indicate substrate preferences as follows: red, lysophospholipid; yellow, Hedgehog; light green, diacylglycerol; dark green, cholesterol; blue, Wnt. Sequences were collected from NCBI Homologene (http://www.ncbi.nlm.nih.gov/sites/entrez/query.fcgi?db=homologene). (B) Schematic of phospholipid (PL) remodeling via the Lands pathway. Remodeling is initiated by a PLA2, which releases a free fatty acid (FFA) and a lysophospholipid. Acyl-CoA is generated from FFA via a CoA-ligase (FACL), and used by a lysophospholipid acyltransferase (LPLAT) to regenerate the PL. R is the chemical group specific for each phospholipid class: hydroxyl (PA), choline (PC), ethanolamine (PE), glycerol (PG), serine (PS), or inositol (PI). (C) Partial alignment of MBOAT family LPLATs from S. cerevisiae, D. melanogaster, and H. sapiens showing the conserved active site histidine (red asterisk).
The three other Drosophila MBOAT enzymes, encoded by the genes nessy (nes), CG18445, and CG9526, are homologous to the S. cerevisiae broad-specificity lysophospholipid acyltransferase (LPLAT) Ale1p (Benghezal et al., 2007; Chen et al., 2007; Jain et al., 2007; Riekhof et al., 2007a,b; Tamaki et al., 2007). LPLATs are important for remodeling of membrane phospholipids, which involves phospholipase A2 (PLA2)-mediated deacylation at the sn-2 position and subsequent reacylation by LPLATs in a process known as the Lands Cycle (Lands, 1960; Yamashita et al., 1997; see Figure 1B). Acyl-chain remodeling regulates the molecular species distribution of phospholipids and is important for maintenance of membrane fluidity and curvature (van Meer et al., 2008). Because reacylation by LPLATs utilizes lysophospholipids and acyl-CoA as substrates, the Lands Cycle can also regulate the amount of lysophospholipid and free fatty acid available to act as signaling molecules or their precursors (Balsinde et al., 1999; Zarini et al., 2006; Gijón et al., 2008). Lipid signals and second messengers play crucial roles in cell activation events, immune responses, and neuronal function, and they are misregulated in a wide range of human pathologies, including metabolic syndrome, inflammatory diseases, and cancer (Wymann and Schneiter, 2008). However, few studies have addressed the importance of the Lands Cycle for the development of multicellular organisms.
We have named the Drosophila genes CG18445 and CG9526 oysgedart (oys) and farjavit (frj) respectively, two Yiddish words meaning “skinny,” for their role in phospholipid synthesis. Here we use both biochemical and genetic methods to examine the substrate specificities of Oys, Nes, and Frj and their functions in vivo. Our findings implicate phospholipid remodeling in germline development and may provide an explanation for the male sterility associated with a chromosomal translocation disrupting human MBOAT1 (Dauwerse et al., 2007).
MATERIALS AND METHODS
Yeast Genetics and Lysophospholipid Acyltransferase Assays
Full-length cDNAs for nes (RE03440), oys (RE60277), and frj (LD17340) were obtained from the Drosophila Genomics Resource Center (Bloomington, IN). Open reading frames were amplified from these plasmids with the following primer pairs: Oys-Fw, ATGCTAGAACCGCCGAAATT; Oys-Rv, CTTTGCATGACCGTTGCTAA; Nes-Fw, ATGGCGGAATTCGAGGA; Nes-Rv, CTCAGACTTCTTATCTTCTGGTTTCTT; Frj-Fw, ATGAGCATCGACGACGTCAT; and Frj-Rv, CTGCGCCTTCTCCTTCTCTA. Amplified products were cloned as C-terminal V5-His6–tagged fusions in the pYES-2.1-TOPO vector (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions, and diagnostic restriction digestions and DNA sequencing were used to confirm correct orientation and sequence fidelity. Expression of the proteins in the Saccharomyces cerevisiae ale1Δ mutant, microsome preparations, and Western blotting to confirm expression of the proteins were carried out as previously described (Gijón et al., 2008).
Fly Stocks
Stocks used were P{EPgy2}nes[EY22898] (gift of H. Bellen, Baylor College of Medicine, Houston, TX), tubulin::Wun2 (Renault et al., 2004), elav-GAL4, UAS-hmgcr (Van Doren et al., 1998; gifts of R. Lehmann, New York University School of Medicine, New York, NY), P{SUPor-P}KG00817 (oys), P{EPgy2}EY06644 (frj), jar322, Df(3R) crb87-5, Df(2R)X3, ptc-GAL4, c564-GAL4, tub-GAL4, w1118, and Δ2-3, CyO; TM3/T(2;3)apXa (Bloomington Drosophila Stock Center). Mutations in nes, oys, and frj were made by selecting for loss of the w+ eye color marker in the progeny of flies carrying both the respective P element and the Δ2–3 transposase. Approximately 100 independent w− isolates were analyzed for each gene. Putative deletion alleles were analyzed in pools by PCR for loss of DNA in the region of interest, and PCR products were sequenced to determine the extent of the deletion. Precise excisions were also identified by sequencing and used as controls for the phenotypes described.
UAS-Oys-GFP and UAS-Nes-GFP were cloned by PCR amplification from full-length cDNAs and ligation into the Gateway system (Invitrogen), using the entry vector pENTR/D-TOPO (Invitrogen) and the destination vector pTWG (Drosophila Genomics Resource Center), which includes UAS regulatory elements and encodes a C-terminal green fluorescent protein (GFP) tag. Transgenic flies were created by standard methods. The constructs were expressed in S2R+ cultured cells using actin-GAL4 as described in Miura et al. (2006).
Dual-Choice MBOAT Assays and Lipid-Molecular Species Determinations
Enzyme assays and mass-spectrometric analysis of the resulting products to determine the lysophospholipid and fatty acyl-CoA substrate specificities were carried out as previously described for the human MBOAT enzymes (Gijón et al., 2008) using 1–5 μg of yeast microsomal protein containing recombinant Drosophila MBOAT protein. The phospholipid molecular species profiles of mutant flies or testes and the corresponding wild-type controls were determined by liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS) using multiple reaction monitoring. Three frozen flies of each genotype were vortexed with 5-mm glass beads in 0.6 ml of ethanol in a sealed tube to pulverize the tissue, followed by incubation in a boiling water bath for 30 min. After addition of 25 ng of each of the internal standards 17:0/20:4-phosphatidic acid (PA), 17:0/20:4-phosphatidylcholine (PC), 17:0/20:4-phosphatidylethanolamine (PE), 17:0/20:4-phosphatidylglycerol (PG), 17:0/20:4-phosphatidylinositol (PI), and 17:0/20:4-phosphatidylserine (PS), lipids were extracted from this preparation by addition of 4 ml methanol, 4 ml chloroform, and 3.6 ml 0.2 M KCl, followed by vigorous vortexing and centrifugation to effect phase separation. The upper phase was removed and the lower phase was washed twice with 7 ml of theoretical upper phase (methanol/0.2 M KCl/chloroform, 50/45/7, by volume). For testis analysis, 40 adult fly testes were homogenized in 1 ml methanol by microtip sonication for 1 min. Twenty-five nanograms of each of the internal standards [d31]16:0/18:1-PA, [d31]16:0/18:1-PC, [d31]16:0/18:1-PE, [d31]16:0/18:1-PG, [d31]16:0/18:1-PI, and [d31]16:0/18:1-PS (all a generous gift from Avanti Polar Lipids, Alabaster, AL) were added, and extraction proceeded as described above. The washed organic phase was dried under a stream of N2 and dissolved in 500 μl of 25% HPLC solvent A (hexanes/isopropanol 30:40, vol/vol) and 75% solvent B (1 mM ammonium acetate in hexanes/isopropanol/water 30:40:7, vol/vol/vol). A 20-μl aliquot was injected into the LC/MS/MS system. Normal phase chromatography was performed on a silica column (Ascentis, 150 × 2.1 mm, 5 μm, Supelco, Bellefonte, PA) at a flow rate of 200 μl/min. Solvent B was maintained at 25% for 5 min and was increased gradually to 60% in 10 min and then to 95% in 5 min and was held for 20 min before reequilibration for 15 min.
Identification of phospholipids was carried out using an API 3000 triple quadrupole mass spectrometer (Sciex, Thornhill, ON, Canada) in the negative ion mode with multiple reaction monitoring (MRM) of the mass/charge ratio (m/z) transitions shown in Supplemental Table S1, corresponding to combinations of the most common acyl chains in six major phospholipid classes. The amounts of the different phospholipid molecular species were measured by calculating the ratio of the integrated area of the corresponding intensity peaks of the analytes to the integrated areas of the peaks corresponding to the internal standards for the same phospholipid classes. Area integration and calculations were performed using MultiQuant software from Applied Biosystems/MDS Analytical Technologies (Sunnyvale, CA). Analyses were carried out in triplicate for each genotype, and the values reported are means ± SEM.
Neutral Lipid Analysis by TLC
Lipid extraction of whole adult flies was carried out as described above, and TLC was used to assess major changes in neutral lipid abundance. Lipids equivalent to five flies were spotted on silica-60 TLC plates (Electron Microscopy Sciences, Hatfield, PA) and developed in the solvent system hexane/diethylether/acetic acid (60:40:1, vol/vol/vol). Plates were then dried and exposed to I2 vapor to visualize lipids. Standards used for identification were oleic acid, monooleoyl-glycerol, dioleoyl-glycerol, trioleoyl-glycerol, cholesteryl-oleate, and cholesterol.
RNA In Situ Hybridization
Digoxigenin-labeled RNA probes were generated by transcription from either cDNA templates (oys, frj, wunen2) or templates produced by PCR from genomic DNA (nes). In situ hybridization was performed as described (Maurel-Zaffran and Treisman, 2000). Sense probes gave no specific signal when used in parallel.
Immunofluorescence and Immunohistochemistry
Adult testes were dissected in phosphate-buffered saline (PBS) and fixed in 5% formaldehyde for 20 min at room temperature. Imaginal discs and salivary glands were dissected in PBS and fixed in 4% formaldehyde for 30 min at 4°C. Tissues were washed in PBX (PBS + 0.1% Triton X-100) for 15 min and stained with rhodamine-phalloidin (1:200, Sigma-Aldrich, St. Louis, MO) and DAPI (1:4000, Roche, Indianapolis, IN) for 20 min at room temperature. Alternatively, tissues were blocked for 1 h at room temperature in PBS + 1% Triton X-100 supplemented with 5% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) and primary antibody incubations were performed overnight at 4°C. Tissues were subsequently washed in PBX, incubated with Alexa dye–conjugated secondary antibodies (1:1000, Invitrogen) for 2 h at room temperature, washed, counterstained with DAPI and/or rhodamine-phalloidin, and mounted in Aquapolymount (Polysciences, Warrington, PA) or Fluoromount G (Southern Biotechnology, Birmingham, AL). Antibodies used were mouse anti-Hts (1:20, 1B1, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), mouse anti-Myosin VI (1:20, gift of K. Miller, Washington University, St. Louis, MO), rabbit anti-GFP (1:1000, Invitrogen), rabbit anti-active Caspase-3 (1:500, BD Biosciences, San Diego, CA), guinea pig anti-Hsc3 (1:50, gift of H. Ryoo, New York University School of Medicine, New York, NY), and mouse anti-Golgi (1:200, Calbiochem, La Jolla, CA).
Fixed and devitellinized embryos were rehydrated stepwise into PBX, blocked in 5% normal donkey serum in PBS + 1% Triton X-100 and incubated overnight at 4°C in rabbit anti-Vasa primary antibody (1:5000, gift of R. Lehmann). Embryos were washed in PBX, incubated with biotin-coupled anti-rabbit IgG secondary antibody (1:500, Jackson ImmunoResearch) for 2 h at room temperature, washed, and developed using Vectastain ABC Kit (Vector Laboratories, Burlingame, CA). Alternatively, embryos were incubated with HRP-coupled anti-rabbit IgG secondary antibody (1:200, Jackson ImmunoResearch) and developed with 0.5 mg/ml diaminobenzidine. Embryos were dehydrated stepwise into ethanol and mounted in methyl salicylate:Canada Balsam (1:2 vol/vol).
Mitotracker staining of larval fat bodies was performed according to (Frei et al., 2005) with minor modifications. Larvae were fixed in 4% formaldehyde in PBS for 90 min, washed in 1% Tween-20 in PBS for 30 min, and stained with 300 nM Mitotracker Green FM (Invitrogen) in 1% Tween-20 in PBS for 1 h at 37°C.
Electron Microscopy
Testes were dissected from 1- to 2-d-old adult males, fixed in 1.5% glutaraldehyde in PBS, and processed for transmission electron microscopy (EM) according to Noguchi et al. (2006).
Climbing Assay
Climbing assays were adapted from Xu et al. (2006) as follows. Groups of 10–20 flies were tapped to the bottom of a food vial and were given 20 s to climb into a new vial placed on top of the old one (6 cm). This was repeated four more times. Each fly in the group was given one point for every vial that it climbed out of. The total number of points for the group was divided by the number of flies in the group. Six groups (total n = 90 flies) were assayed for each genotype, and the results of all six groups were averaged to yield the climbing index for each genotype.
Rapid Cold Hardening
Flies were subjected to −5°C cold shock for 2 h in Eppendorf tubes using a standard heat block (Fisher Scientific, Pittsburgh, PA) in a −20°C freezer. Prior cold acclimation at ∼5°C was performed in food vials in a water bath placed in a 4°C room.
RESULTS
Nes, Oys, and Frj Encode Lysophospholipid Acyltransferases
In addition to the previously described MBOAT enzymes encoded by por (Zhai et al., 2004), rasp (Amanai and Jiang, 2001; Chamoun et al., 2001; Lee and Treisman, 2001; Micchelli et al., 2002; Miura et al., 2006), and mdy and CG8112 (Buszczak et al., 2002), the Drosophila genome encodes three uncharacterized MBOAT family members. These three MBOATs are homologues of the yeast LPLAT, Ale1p (Riekhof et al., 2007b). Nes is most similar to human MBOAT5, Oys is most similar to human MBOAT1 and MBOAT2, and Frj is most similar to human MBOAT7 (Figure 1, A and C). New techniques for the expression and assay of LPLAT enzymes based on LC/MS/MS were recently developed for characterization of the human MBOAT family (Gijón et al., 2008), and we applied these same techniques to elucidate the functions of the Drosophila enzymes.
We found that Oys, Nes, and Frj could all acylate lysophospholipids when expressed in acyltransferase-deficient ale1 mutant yeast (Riekhof et al., 2007a; Riekhof et al., 2007b). Oys acted as a broad-specificity LPLAT similarly to yeast Ale1p; it was capable of acylating lysophosphatidylcholine (lyso-PC), lysophosphatidylethanolamine (lyso-PE), lysophosphatidylserine (lyso-PS), and lysophosphatidylglycerol (lyso-PG; Figure 2). Nes exhibited the highest activity toward lyso-PC, and Frj preferentially acylated lyso-PI (Figure 2). Oys and Nes preferred unsaturated acyl-CoAs of at least 16 carbons, whereas Frj was highly specific for arachidonoyl-CoA (20:4; Figure 2). These results are consistent with recent reports characterizing the Caenorhabditis elegans and mammalian homologues of the three enzymes (Benghezal et al., 2007; Chen et al., 2007; Jain et al., 2007; Riekhof et al., 2007a,b; Tamaki et al., 2007; Gijón et al., 2008; Hishikawa et al., 2008; Lee et al., 2008; Matsuda et al., 2008; Zhao et al., 2008). Like their mammalian homologues (Hishikawa et al., 2008), GFP-tagged Oys and Nes constructs localized primarily to the ER in a variety of cell types (Supplemental Figure S1, A–I).
Figure 2.
Dual-choice MBOAT assay. The fly MBOAT proteins Oys, Nes, and Frj were expressed in the acyltransferase-deficient ale1Δ yeast strain. Isolated microsomes containing the fly proteins were incubated with a mixture of eight acyl-CoA species and six lysophospholipids, and the products formed by each enzyme were separated and quantified by LC/MS/MS. Scales are adjusted to highlight the substrate preferences of each enzyme. Results given are the mean ± SEM of three experiments. Acyl-CoAs are abbreviated as x:y, where x is the number of carbon atoms in the chain and y is the number of double bonds.
Generation of Oys, Nes, and Frj Mutants
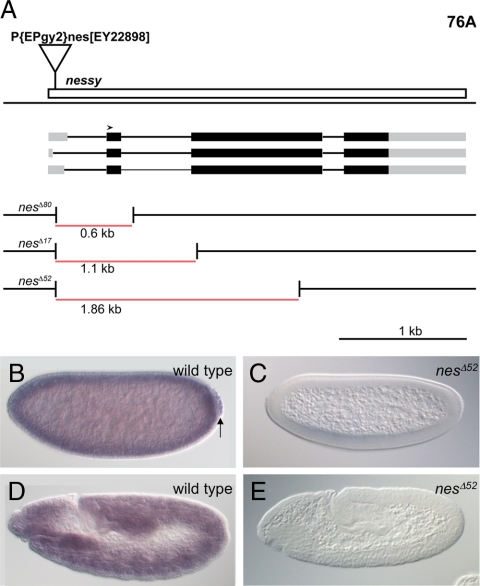
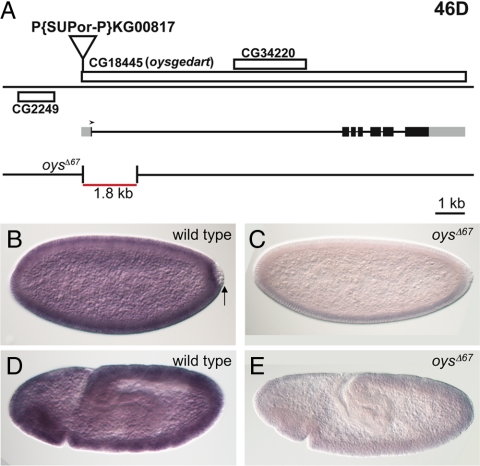
We observed that transcripts of oys, nes, and frj were maternally deposited in the embryo (Figures 3B and 4B, Supplemental Figure S2B) and showed widespread zygotic expression (Figures 3D and 4D, Supplemental Figure S2D). Late embryonic expression was restricted to subdomains of the visceral mesoderm (data not shown), as previously described for nes (Maurel-Zaffran et al., 1999). To examine the roles these LPLATs play in vivo, we created null mutations in all three genes by imprecise excision of upstream P elements (Figures 3A and 4A, Supplemental Figure S2A). We identified three deletions extending into the coding region of nes; the largest, nesΔ52, is a 1.9-kb deletion that removes most of the open reading frame (Figure 3A). nes mRNA was not detectable by in situ hybridization in maternal/zygotic nesΔ52 mutant embryos (Figure 3, C and E). For oys, we isolated a single 1.8-kb deletion, oysΔ67, that removes the first exon including the start codon but does not disrupt the predicted gene CG34220 nested in the first intron (Figure 4A). Embryos maternally and zygotically mutant for oysΔ67 expressed no detectable oys mRNA (Figure 4, C and E). Finally, three deletions extending into the frj coding region were obtained (Supplemental Figure S2A). The absence of mRNA from frjΔ11 (a 1.1-kb deletion) and frjΔ30 (a 1.4-kb deletion) maternal/zygotic mutant embryos was confirmed by in situ hybridization (Supplemental Figure S2, C and E, and data not shown).
Figure 3.
Deletion alleles of nes abolish nes expression. (A) Three independent alleles of nes were identified after imprecise excision of the P{EPgy2}nes[EY22898] element. The three predicted transcripts of nes differ only in the first exon, which constitutes the 5′UTR. Untranslated regions are shown in gray, the open reading frame in black, introns as thinner lines, and the deletions in red. All three deletions extend beyond the second exon, which includes the start codon (arrowhead). (B–E) show in situ hybridization with a nes-specific probe. Wild-type blastoderm embryos show strong maternal nes expression (B); nes RNA is incorporated into the germ cells (arrow). Later in embryogenesis, zygotic nes expression is detected ubiquitously (D). This expression is abolished in embryos produced by nesΔ52 mutant parents, stained in parallel (C and E).
Figure 4.
A deletion of oys abolishes oys expression. (A) A single allele of oys was identified after imprecise excision of the P{SUPor-P}KG00817 element. The oysΔ67 deletion (red) extends past the oys start codon (arrowhead), but does not disrupt the predicted transcription unit CG34220 nested in the first intron of oys. (B–E) In wild-type blastoderm embryos, an oys-specific probe detects strong maternal expression (B); oys RNA is not detectable in the germ cells (arrow). Later in embryogenesis, zygotic oys expression is detected ubiquitously (D). This expression is abolished in embryos produced by oysΔ67 parents (C and E).
Effects of Oys, Nes, and Frj Mutations on Fertility and Lipid Composition
Zygotic loss of oys, nes, or frj did not affect viability or fertility; nesΔ52, oysΔ67, and frjΔ30 flies survived to adulthood with no apparent morphological defects. Although the mammalian Nes homologue has been implicated in Golgi integrity and vesicle trafficking (Hodges et al., 2005), we did not detect any alteration of Golgi morphology in nes mutant flies (Supplemental Figure S1, J and K). To uncover possible functional redundancy between the three enzymes in vivo, we generated all the double and triple mutant combinations of oys, nes, and frj. Although all the mutant combinations allowed adult survival, oysΔ67; nesΔ52 (oys nes) males, as well as triple mutant males, were sterile. The fertility of oys nes mutant females was also reduced; 70% of embryos resulting from a cross to heterozygous males failed to hatch (n = 100), and 24% displayed extensive degeneration of cell membranes (n = 325) (Figure 5, A and B). Removal of zygotic oys and nes further reduced adult viability to 10% of the number of flies carrying wild-type paternal copies of both genes (n = 530). Flies homozygous for precise excisions of both the oys and nes P elements showed normal fertility and viability, indicating that the defects are specifically caused by loss of both oys and nes.
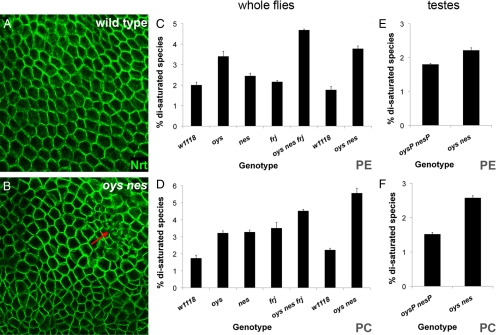
Figure 5.
MBOAT mutants display altered phospholipid composition and membrane defects. (A and B) Cell membranes visualized by staining with an antibody to the transmembrane protein Neurotactin (Nrt, green) show abnormalities in cell size and shape in oys nes mutant embryos (B) compared with wild type (A), as well as degeneration of cell membranes (red arrow). (C and D) Removal of MBOAT proteins increases the amounts of saturated PE and PC species in whole flies. LC/MS/MS was used to quantify the relative amounts of the major molecular species of phospholipids from control (w1118), oys, nes, and frj single mutant, oys nes frj triple mutant, and oys nes double mutant adult flies as described in the text. The relative proportion of molecular species containing two saturated fatty acids is given for PE (C) and PC (D). The data represent the mean ± SEM of three individual lipid extractions, each prepared from three whole flies. (E and F) LC/MS/MS was used to quantify molecular species of phospholipids from testes dissected from control males homozygous for precise excisions of both the oys and nes P elements (oysP nesP) and from oys nes double mutant males. The relative proportion of molecular species containing two saturated fatty acids is given for PE (E) and PC (F). The data represent the mean ± SEM of three individual runs.
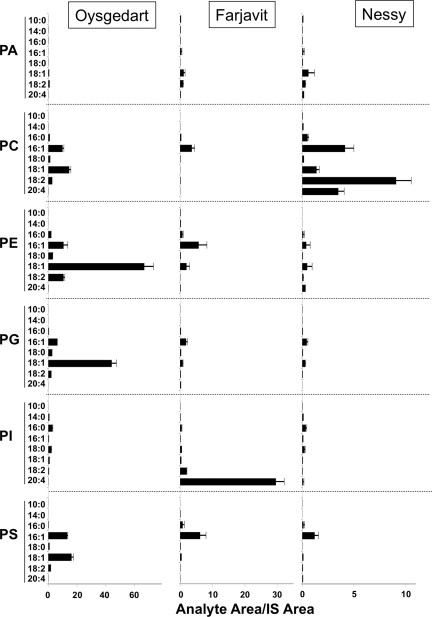
Using LC/MS/MS, we analyzed the lipid composition of adult flies homozygous for each of the three single mutations, as well as oys nes double mutants and oys nes frj triple mutants. Specific phospholipid molecular species were detected by multiple-reaction monitoring using the mass/charge ratio (m/z) transitions included in Supplemental Table S1. We were able to reproducibly measure the relative amounts of 39 different molecular species for each major phospholipid class (PE, PC, PS, PI, PG, and PA) plus odd-chain or deuterated internal standards for each of the classes.
At the level of whole adult animals, the phospholipid molecular species profiles were similar among wild-type flies, each of the single mutants, and the double and triple mutants (Supplemental Table S2 and Supplemental Figure S3). The total amounts of triglycerides, free sterols, and steryl esters measured by TLC also did not vary among the different genotypes (Supplemental Figure S4 and data not shown). Of the more abundant molecular species, only a few differed from wild-type levels by more than 50% (Supplemental Table S2), but there were subtle changes in the molecular species distribution of some phospholipid classes. Notably, an increase in the proportion of molecules bearing two saturated acyl chains was observed for PE in oys, oys nes, and triple mutants (Figure 5C), consistent with the in vitro preference of the Oys enzyme for 18:1-CoA and lyso-PE as substrates. Likewise, each of the single mutants, the double mutant, and the triple mutant showed an increase in the proportion of saturated molecular species of PC (Figure 5D). This change was also consistent with the preference of Nes for unsaturated acyl-CoAs and lyso-PC, and the moderate activity of Oys and Frj toward lyso-PC (Figure 2).
Oys and Nes Are Required for Spermatid Individualization
We examined oys nes mutant testes in order to determine the basis for the male sterility observed in these double mutants. Starting from the apical end of the testis, germline stem cells undergo mitotic and meiotic divisions to generate differentiating cysts each containing 64 spermatids. As they move basally, the spermatids elongate and extend axonemes back toward the apical end (Fuller, 1993; Figure 6A). oys nes mutant testes appeared relatively normal by light and phase-contrast microscopy and contained elongated spermatids (Supplemental Figure S5B). Staining of the nuclei and spectrosomes confirmed that the early stages of spermatogenesis proceeded normally in oys nes mutants (Supplemental Figure S5D and data not shown). After completion of meiosis, the mitochondria of each spermatid fuse to form a Nebenkern, a structure that gives rise to the specialized mitochondrial derivatives that power the motility of the sperm flagellum (Fuller, 1993). In mutants defective in cytokinesis, all the mitochondria that remain together within the spermatids that have failed to divide fuse to form a single large Nebenkern flanked by multiple nuclei (Xu et al., 2002; Farkas et al., 2003). oys nes onion-stage spermatids contained individual Nebenkerne of normal size, indicating normal cytokinesis (Supplemental Figure S5F). Additionally, visualization of the adducin-rich elongation complexes at the tips of the microtubule-based axonemes (Ghosh-Roy et al., 2004) showed no defects in axoneme elongation in mutant testes (Supplemental Figure S5D). EM of preindividualized spermatids from oys nes mutants revealed normal morphology of the major and minor mitochondrial derivatives (Supplemental Figure S6, D and E). Consistent with normal mitochondrial development in other tissues, locomotor activity, a measure of muscle mitochondrial function (Xu et al., 2006), was not altered in oys nes mutant adult flies (Supplemental Figure S6A), and Mitotracker immunofluorescence appeared normal in oys nes mutant larval fat body (Supplemental Figure S6, B and C). Mitochondrial abnormalities are thus unlikely to explain the spermatogenesis defect. Because no mature motile sperm were found in mutant seminal vesicles (data not shown), Oys and Nes must act in the final stages of spermatid differentiation and maturation.
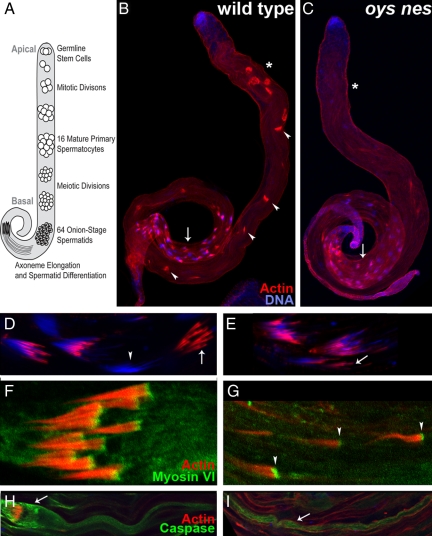
Figure 6.
Spermatid individualization is compromised in the oys nes mutant. (A) Schematic of spermatogenesis. The germline stem cells reside at the apical tip of the testis and progress basally as they divide and undergo meiosis. Once the spermatids have reached the basal region of the testis, differentiation begins and the axonemes extend back toward the apical tip. (B–E) In wild-type testes (B), individualization complexes (ICs, phalloidin, red) form around the nuclei (DAPI, blue) in the basal region (arrow). They progress back (arrowheads) toward the apical tip, individualizing the spermatids as they advance. Once they reach the apical region (asterisk), they are collected as waste bags. In oys nes mutant testes (C), ICs are formed normally around the nuclei (arrow) but fail to advance and are not detected in the apical region (asterisk). Higher magnification views of ICs in the basal region are shown in D and E. In wild type (D), the actin cones remain assembled in the IC (arrow) as they progress away from the nuclei (arrowhead), whereas in the mutant (E), the cones dissociate from each other (arrow) as they progress away from the nuclei. (F and G) Myosin VI (green) localizes to the front of the actin cones in wild type (F) and in the oys nes mutant (G, arrowheads), despite the fact that the mutant actin cones are no longer assembled into ICs. (H and I) Active caspases can be detected in wild-type (H) and oys nes mutant cysts (I, arrow). In wild-type, high levels of active caspase are present in the cystic bulge (arrow) around the IC, whereas no cystic bulges are seen in the mutant.
After elongation of the axonemes is complete, the individual spermatids become separated and most of their cytoplasm is removed. This individualization process depends on an actin-rich individualization complex (IC) that assembles around the spermatid nuclei and traverses the length of the cyst toward the apical end (Tokuyasu et al., 1972; Fabrizio et al., 1998). In wild-type testes, initializing ICs visualized by staining with rhodamine-coupled phalloidin were visible around the spermatid nuclei in the basal testis (Figure 6B, arrow), progressing ICs appeared at different positions along the length of the testis (Figure 6B, arrowheads, mean = 9.95 progressing ICs per testis, n = 20 testes), and completed ICs collected at the end of the cysts in the apical region of the testis (Figure 6B, asterisk). In oys nes double mutants, ICs were visible in the basal region of the testis, in association with the cyst nuclei (Figure 6C, arrow). However, very few progressing ICs were detectable (Figure 6C, mean = 0.58 progressing ICs per testis, n = 19 testes), and no ICs collected in the apical testis (Figure 6C, asterisk). Thus, the individualization process is defective in the absence of oys and nes.
Each IC is composed of 64 actin cones that are thought to push the cytoplasm ahead of them as they traverse the cyst, stripping the spermatids of most of their cellular mass (Tokuyasu et al., 1972; Noguchi and Miller, 2003; Noguchi et al., 2006). In the oys nes double mutant, actin cones assembled normally around the spermatid nuclei (Figure 6, D and E). All 64 wild-type actin cones remained associated in the IC as they moved away from the nuclei (Figure 6, D and F), but oys nes mutant actin cones dissociated from each other as they progressed, disassembling the IC (Figure 6, E and G). Intact ICs were almost never found apical to the nuclei, and individual stray actin cones were frequently observed (Figure 6G). A similar disassembly of the ICs as they progress occurs in myosin VI (jar) mutants (Noguchi et al., 2006). Myosin VI, an actin-binding protein, localizes to the front of the actin cones and is thought to stabilize actin filaments within the cones (Rogat and Miller, 2002; Noguchi et al., 2006; Figure 6F). We found that Myosin VI localization was not disrupted in initializing ICs in the basal region of oys nes mutant testes (not shown). Furthermore, even progressed mutant actin cones that had completely dissociated from ICs displayed normal Myosin VI localization (Figure 6G). Together with the normal morphology of the actin cones, as revealed by phalloidin staining, this suggests that the oys nes phenotype does not arise from defects in cone structure.
As individualization proceeds, the cytoplasm that is extruded from the cyst forms a cystic bulge around the IC that is eventually discarded in a waste bag at the apical end of the cyst (Fuller, 1993). The cystic bulge and the more apical region of the cyst contain activated caspases, which perform a nonapoptotic function in late-stage spermatid differentiation (Arama et al., 2003; Figure 6H). Although oys nes mutant cysts did not form cystic bulges, they contained activated caspases in the apical region (Figure 6I), consistent with other observations showing that individualization is not essential for continued differentiation (Arama et al., 2003). Oys and Nes are thus redundantly required for progression of the ICs during individualization, but not for mitosis, meiosis, cytokinesis, mitochondrial specialization, axoneme elongation, IC formation, or terminal differentiation. The phenotype of the triple oys nes frj mutant was identical to the oys nes double mutant (Supplemental Figure S7). Together with the normal fertility of single frj mutants and oys frj and nes frj double mutants, this indicates that Frj does not play a significant role in spermatogenesis.
We analyzed the testes of oys nes mutant adult males and of control precise excision males to determine their phospholipid molecular species profiles. As shown in Supplemental Table S2, the phospholipid profiles of testes were quite similar to those observed in whole flies, as were the changes in phospholipid species distribution in the mutant testes. Among the more abundant species, only 16:0/18:3PA and 16:0/18:1PS were clearly diminished in the testes of oys nes adult flies, and the relative levels of 18:1/18:2PG were increased. Again, we saw subtle but reproducible increases in the percentage of PC and PE species bearing two saturated acyl chains in the absence of Oys and Nes (Figure 5, E and F), indicating that these proteins are required for normal phospholipid composition in the testis as well as in somatic tissues.
Oys and Nes Act Together to Promote Migration of the Embryonic Germ Cells
The primordial germ cells are formed at the posterior of the embryo during the syncytial blastoderm stage. They are carried inside the embryo by the movements of gastrulation, and they subsequently migrate through the posterior midgut epithelium into the mesoderm and associate with the somatic gonadal precursors (SGPs). A number of genes required for distinct steps in this migration process encode proteins involved in lipid metabolism (Kunwar et al., 2006), prompting us to examine germ cell migration in our MBOAT mutants.
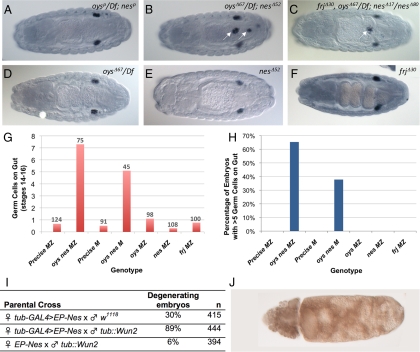
Those embryos laid by oys nes mutant mothers that showed normal membrane structure and embryonic patterning nevertheless frequently contained a significant number of germ cells that failed to reach the gonads (Figure 7, B, G, and H). These germ cells appeared to migrate through the midgut epithelium normally (Supplemental Figure S8), but they remained associated with the basal surface of the gut rather than attaching to the SGPs or scattering throughout the embryo (Figure 7B, Supplemental Figure S8F). This phenotype was not observed in either of the single mutants (Figure 7, D, E, G, and H) or in a strain containing precise excisions of both the oys and nes P elements (Figure 7, A, G, and H), indicating that it can be attributed to the loss of oys and nes rather than to other factors in the genetic background. Both the number of mis-migrating germ cells and the proportion of embryos displaying the defect were increased when the wild-type paternal contributions of oys and nes were removed, suggesting that zygotic transcription can provide partial Oys and Nes function at this stage (Figure 7, G and H). Because embryos from triple mutant mothers did not exhibit a stronger phenotype than those from double mutant mothers (Figure 7C and data not shown), and frj single mutants showed normal germ cell migration (Figure 7, F–H), Frj does not appear to be required for this process.
Figure 7.
oys nes mutants show abnormal germ cell migration. (A–F) By stage 14 of embryogenesis, the germ cells (stained with anti-Vasa in purple) associate with the somatic gonadal precursors and form two bilateral clusters. Embryos from mothers carrying precise excision alleles of oys and nes show no defect in this process (A), in contrast to embryos from oys nes double mutant mothers (B, arrows), or oys nes frj triple mutant mothers (C, arrow), in which some germ cells remain associated with the gut. Mothers singly mutant for oys (D) or nes (E) produce embryos with normal germ cell migration. Embryos from frj mutant mothers are also normal (F). (G and H) Quantification of the germ cell migration phenotype reveals that embryos lacking maternal oys and nes (M) have an average of five germ cells associated with the gut by stage 14, and removing paternally contributed oys and nes from half the embryos (MZ) increases this number to 7 (G). The number of embryos counted is given above each column. Thirty-eight percent of embryos lacking maternal oys and nes (M) have more than five germ cells associated with the gut by stage 14, and the penetrance is increased to 65% when one copy of paternally contributed oys and nes is removed from half the embryos (MZ; H). Control embryos laid by mothers carrying precise excisions of both genes (Precise M, Precise MZ) and single oys, nes or frj mutants show no significant germ cell migration defects (G and H). Embryos showing membrane degeneration were not included in the quantitative analysis. (I and J) nes interacts genetically with wun2. Maternal overexpression of Nes causes 30% of embryos to degenerate, whereas co-overexpression of Nes and Wun2 causes both quantitative and qualitative enhancement of the phenotype. Zygotic overexpression of Wun2 alone causes only 6% of embryos to degenerate (I). An example of a degenerating embryo overexpressing both Nes and Wun2 is shown in J.
Migration of germ cells away from the gut and into the mesoderm is driven by repulsion from regions where the lipid phosphate phosphohydrolases Wunen (Wun) and Wunen2 (Wun2) are expressed at high levels (Zhang et al., 1997; Starz-Gaiano et al., 2001; Sano et al., 2005). In addition to this repulsion, germ cells are attracted to regions with high levels of HMG-CoA reductase (HMGCR) expression (Van Doren et al., 1998). The migration failure in oys nes mutants suggests that germ cells are not repelled normally by Wunens in the absence of Oys and Nes activity. Additionally, the persistent gut association of the germ cells is reminiscent of mutants in the hmgcr pathway (Santos and Lehmann, 2004). To determine whether LPLATs might be involved in one of these pathways, we looked for genetic interactions of oys and nes with wunens or hmgcr. Overexpression of HMGCR in the embryonic CNS induces ectopic migration of the germ cells (Van Doren et al., 1998). This effect was not significantly modified by either the absence of maternal oys and nes or the zygotic overexpression of Nes (Supplemental Figure S8I). In contrast, nes showed genetic interactions with wun2. Maternal overexpression of Nes throughout the embryo induced degeneration similar to that seen in the absence of oys and nes. This effect was greatly exacerbated by the zygotic overexpression of Wun2 (Figure 7, I and J). Overexpression of Wun2 in a wild-type background did not induce degeneration at a significant penetrance. Oys and Nes may thus participate in the same process as Wun and Wun2.
DISCUSSION
Here we present the first comprehensive analysis of MBOAT family LPLATs in an animal model. Using heterologous expression in MBOAT LPLAT-deficient yeast, we have shown that Oys is a broad-specificity LPLAT, Nes preferentially acylates lyso-PC, and Frj preferentially acylates lyso-PI. All three enzymes show a strong preference for unsaturated fatty acyl-CoAs in the yeast system. We have generated Drosophila mutants lacking each MBOAT LPLAT and have performed detailed phospholipid molecular species analyses of our mutants using mass spectrometry. We detected small but significant increases in the percentage of di-saturated phospholipid species in all the mutants, suggesting that LPLATs function to incorporate unsaturated fatty acids into phospholipids in vivo. Our phenotypic analysis has shown that Oys and Nes are redundantly required for two specific aspects of germ cell development: germ cell migration in the embryo and spermatid individualization in the adult. Together, the genetic and biochemical data implicate phospholipids or lysophospholipids in germ cell development, but further studies will be required to identify the specific lipid effectors.
MBOAT LPLATs Are Not Essential for All Phospholipid Remodeling
The Lands Cycle involves the CoA-dependent exchange of acyl chains between different phospholipids. It is thought to play a critical role in the incorporation of polyunsaturated fatty acids into the sn-2 position of membrane phospholipids, which influences the curvature and fluidity of cell membranes (van Meer et al., 2008). The cell membrane defects we observed in embryos lacking both oys and nes provide evidence that these LPLATs are involved in the remodeling of embryonic membranes. However, the adult survival of some double and triple mutant animals, and the relatively small reductions in specific unsaturated phospholipid molecular species in adult mutant flies, suggest that other pathways for introducing unsaturation into membrane phospholipids can compensate for the absence of MBOAT family LPLATs (Yamashita et al., 1997; Shindou and Shimizu, 2008). Similarly, neither ale1 mutations in yeast (Riekhof et al., 2007b) nor cytosolic PLA2 deletions in mice are lethal, although both interfere with the Lands Cycle (Bonventre et al., 1997; Uozumi et al., 1997). Consistent with the existence of multiple parallel pathways for the maintenance of phospholipid compositional homeostasis, we have found that the absence of all three Drosophila LPLATs does not affect rapid cold hardening, a process in which cold temperatures above 0°C induce an increase in membrane desaturation that leads to increased survival on subsequent exposure to subzero temperatures (Watson and Morris, 1987; Czajka and Lee, 1990; Overgaard et al., 2005; Supplemental Figure S9).
Like its orthologues in other species, Frj acts as a lyso-PI acyltransferase when expressed in yeast (Gijón et al., 2008; Lee et al., 2008). However, mass spectrometry did not reveal major changes in PI species in frj mutants. The primary role of the C. elegans Frj ortholog, MBOA-7, appears to be in the incorporation of polyunsaturated fatty acids (PUFAs) from the diet into the PI pool (Lee et al., 2008). Because flies have very little PUFA in their diet, it is not surprising that the relative amounts of the PI molecular species are unchanged. We detected no morphological defects in frj mutants, and we have also found no change in electroretinograms recorded from frj mutants (S. Berger, personal communication), or in their circadian activity patterns (J. Blau, personal communication).
Oys and Nes Contribute to Germ Cell Migration during Embryogenesis
During embryogenesis, the germ cells undergo a series of tightly regulated migration steps to reach their final destination in the mesodermally derived somatic gonad. Several proteins involved in lipid metabolism are required for normal migration, including the Wunen lipid phosphate phosphatases and components of the isoprenoid synthesis pathway (Kunwar et al., 2006). The LPLATs Oys and Nes are likewise required for germ cells to migrate into the mesoderm, and either enzyme is sufficient for this process. Both maternal and zygotic transcripts of both genes appear to contribute to producing the necessary level of LPLAT activity. Because only nes, but not oys, transcripts are detectable in the germ cells (Figures 3B and 4B, arrows), it is likely that Oys and Nes function in the soma to promote germ cell migration, although we cannot rule out the possibility that one or both are required in the germline as well.
Gut specification appears normal in oys nes mutant embryos as judged by morphology and wunen2 expression (Supplemental Figure S8, G and H, and data not shown), and specification of the somatic gonadal precursors is also unlikely to be affected, because some germ cells reach the gonads and condensation occurs normally. Our results thus suggest that Oys and Nes contribute to a signaling pathway involved directly in guidance of the migrating germ cells. The high levels of oys and nes expression in the embryonic mesoderm are consistent with this hypothesis. The in vitro preference of Nes for lyso-PC and the change in PC saturation in oys nes double mutants together suggest that a choline phospholipid such as lyso-PC or PC could be involved in germ cell guidance. Lyso-PC has been shown to act as a guidance cue for mammalian monocytes (Lauber et al., 2003). nes interacts genetically with wunen2, suggesting that Oys and Nes might alter the levels of a Wunen substrate or product. Because LPPs such as Wunen dephosphorylate lyso-PA and PA, but not lyso-PC or PC (Waggoner et al., 1999; Renault et al., 2004), an additional enzymatic step would be required for the two pathways to interact biochemically.
Spermatid Individualization Requires Oys and Nes
Our analysis of oys nes double mutant testes shows that they exhibit a specific defect in spermatid individualization, with mutant ICs dissociating as they progress away from the nuclei. The phenotype is stronger than that of null myosin VI mutants (Morrison and Miller, 2008) and is also distinct in that oys nes mutant actin cones appear normal, as assessed by phalloidin and Myosin VI staining (Noguchi et al., 2006). Several models could explain the function of Oys and Nes in spermatid individualization. It is possible that Oys and Nes are required for the formation of a specific membrane phospholipid necessary for tethering the actin cones to the plasma membrane (Fabrizio et al., 1998; Rogat and Miller, 2002), analogous to the role of the phosphoinositide PI(4,5)P2 in docking the basal body to the nuclear membrane during spermatid elongation (Wei et al., 2008). Alternatively, redistribution of acyl chains catalyzed by Oys and Nes could be required for membrane remodeling around the spermatids as they individualize. A third possibility is that Oys and Nes could regulate the concentration of lipid signals such as lysophospholipids or free fatty acids that might regulate individualization. Caspase activation in apoptotic cells has been shown to regulate the levels of lyso-PC and arachidonic acid (Lauber et al., 2003) and might do so during spermatogenesis as well. Other classes of lipid signals have also been implicated in regulating spermatid individualization (Jung et al., 2007). Finally, Oys and Nes might act nonenzymatically to promote individualization. Although our data do not definitively rule out this possibility, the changes in phospholipid composition we see in mutant testes are consistent with an enzymatic role for these enzymes in spermatogenesis. Successful completion of mammalian spermatogenesis depends on the precise lipid composition of sperm membranes (MacDonald et al., 1984; Hall et al., 1991; Osuga et al., 2000) and an inherited mutation in human MBOAT1, an Oys homologue, also disrupts spermatogenesis (Dauwerse et al., 2007). The role of LPLATs in germline development may thus be conserved throughout metazoa.
Supplementary Material
ACKNOWLEDGMENTS
We thank Hugo Bellen, Ruth Lehmann, Kathryn Miller, Hyung Don Ryoo, the Bloomington Drosophila Stock Center, the Drosophila Genomics Resource Center and the Developmental Studies Hybridoma Bank for fly stocks and reagents. We are grateful to Justin Blau and Shlomo Berger for analysis of circadian rhythm and electroretinogram recordings of frj mutant flies; to Feng-Xia Liang and Eric Roth for EM; to Vitor Barbosa, Kerstin Hofmeyer, Sara Ricardo, Thomas Hurd, and Prashanth Rangan for technical advice; and to Ruth Lehmann and Andrew Renault for advice on the analysis of germ cell migration. The manuscript was improved by the critical comments of Inés Carrera, Kerstin Hofmeyer, Kevin Legent, Jean-Yves Roignant, Prashanth Rangan, and Ruth Lehmann. This work was supported by the National Institutes of Health Grants HD058217 to J.E.T., GM079811 to J.S., GM32453 and GM081461 to D.R.V., GM076798 to W.R.R., and HL025785 to R.C.M. and by the March of Dimes Birth Defects Foundation Grant 1-FY08-509 to J.E.T. W.R.R. is a recipient of the American Cancer Society Great-West Division Post-Doctoral Fellowship Award (PF-06-288-01-CSM).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-05-0382) on October 28, 2009.
REFERENCES
- Amanai K., Jiang J. Distinct roles of Central missing and Dispatched in sending the Hedgehog signal. Development. 2001;128:5119–5127. doi: 10.1242/dev.128.24.5119. [DOI] [PubMed] [Google Scholar]
- Arama E., Agapite J., Steller H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev Cell. 2003;4:687–697. doi: 10.1016/s1534-5807(03)00120-5. [DOI] [PubMed] [Google Scholar]
- Balsinde J., Balboa M. A., Insel P. A., Dennis E. A. Regulation and inhibition of phospholipase A2. Annu. Rev. Pharmacol. Toxicol. 1999;39:175–189. doi: 10.1146/annurev.pharmtox.39.1.175. [DOI] [PubMed] [Google Scholar]
- Benghezal M., Roubaty C., Veepuri V., Knudsen J., Conzelmann A. SLC1 and SLC4 encode partially redundant acyl-coenzyme A 1-acylglycerol-3-phosphate O-acyltransferases of budding yeast. J. Biol. Chem. 2007;282:30845–30855. doi: 10.1074/jbc.M702719200. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Huang Z., Taheri M. R., O'Leary E., Li E., Moskowitz M. A., Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- Bosson R., Jaquenoud M., Conzelmann A. GUP1 of Saccharomyces cerevisiae encodes an O-acyltransferase involved in remodeling of the GPI anchor. Mol. Biol. Cell. 2006;17:2636–2645. doi: 10.1091/mbc.E06-02-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M., Lu X., Segraves W. A., Chang T. Y., Cooley L. Mutations in the midway gene disrupt a Drosophila acyl coenzyme A: diacylglycerol acyltransferase. Genetics. 2002;160:1511–1518. doi: 10.1093/genetics/160.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases S., et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA. 1998;95:13018–13023. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoun Z., Mann R. K., Nellen D., von Kessler D. P., Bellotto M., Beachy P. A., Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- Chen Q., Kazachkov M., Zheng Z., Zou J. The yeast acylglycerol acyltransferase LCA1 is a key component of Lands cycle for phosphatidylcholine turnover. FEBS Lett. 2007;581:5511–5516. doi: 10.1016/j.febslet.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Czajka M. C., Lee R. E., Jr A rapid cold-hardening response protecting against cold shock injury in Drosophila melanogaster. J. Exp. Biol. 1990;148:245–254. doi: 10.1242/jeb.148.1.245. [DOI] [PubMed] [Google Scholar]
- Dauwerse J. G., de Vries B. B., Wouters C. H., Bakker E., Rappold G., Mortier G. R., Breuning M. H., Peters D. J. A t(4;6)(q12;p23) translocation disrupts a membrane-associated O-acetyl transferase gene (MBOAT1) in a patient with a novel brachydactyly-syndactyly syndrome. Eur. J. Hum. Genet. 2007;15:743–751. doi: 10.1038/sj.ejhg.5201833. [DOI] [PubMed] [Google Scholar]
- Fabrizio J. J., Hime G., Lemmon S. K., Bazinet C. Genetic dissection of sperm individualization in Drosophila melanogaster. Development. 1998;125:1833–1843. doi: 10.1242/dev.125.10.1833. [DOI] [PubMed] [Google Scholar]
- Farkas R. M., Giansanti M. G., Gatti M., Fuller M. T. The Drosophila Cog5 homologue is required for cytokinesis, cell elongation, and assembly of specialized Golgi architecture during spermatogenesis. Mol. Biol. Cell. 2003;14:190–200. doi: 10.1091/mbc.E02-06-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei C., Galloni M., Hafen E., Edgar B. A. The Drosophila mitochondrial ribosomal protein mRpL12 is required for Cyclin D/Cdk4-driven growth. EMBO J. 2005;24:623–634. doi: 10.1038/sj.emboj.7600523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M. T. Spermatogenesis. In: Martinez-Arias M.B.a.A., editor. The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 71–148. [Google Scholar]
- Ghosh-Roy A., Kulkarni M., Kumar V., Shirolikar S., Ray K. Cytoplasmic dynein-dynactin complex is required for spermatid growth but not axoneme assembly in Drosophila. Mol. Biol. Cell. 2004;15:2470–2483. doi: 10.1091/mbc.E03-11-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijón M. A., Riekhof W. R., Zarini S., Murphy R. C., Voelker D. R. Lysophospholipid acyltransferases and arachidonate recycling in human neutrophils. J. Biol. Chem. 2008;283:30235–30245. doi: 10.1074/jbc.M806194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J. A., Solenberg P. J., Perkins D. R., Willency J. A., Knierman M. D., Jin Z., Witcher D. R., Luo S., Onyia J. E., Hale J. E. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. USA. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. C., Hadley J., Doman T. Correlation between changes in rat sperm membrane lipids, protein, and the membrane physical state during epididymal maturation. J. Androl. 1991;12:76–87. [PubMed] [Google Scholar]
- Hishikawa D., Shindou H., Kobayashi S., Nakanishi H., Taguchi R., Shimizu T. Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity. Proc. Natl. Acad. Sci. USA. 2008;105:2830–2835. doi: 10.1073/pnas.0712245105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges E., Redelius J. S., Wu W., Hoog C. Accelerated discovery of novel protein function in cultured human cells. Mol. Cell. Proteomics. 2005;4:1319–1327. doi: 10.1074/mcp.M500117-MCP200. [DOI] [PubMed] [Google Scholar]
- Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem. Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- Jain S., Stanford N., Bhagwat N., Seiler B., Costanzo M., Boone C., Oelkers P. Identification of a novel lysophospholipid acyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 2007;282:30562–30569. doi: 10.1074/jbc.M706326200. [DOI] [PubMed] [Google Scholar]
- Jung A., Hollmann M., Schafer M. A. The fatty acid elongase NOA is necessary for viability and has a somatic role in Drosophila sperm development. J. Cell Sci. 2007;120:2924–2934. doi: 10.1242/jcs.006551. [DOI] [PubMed] [Google Scholar]
- Kunwar P. S., Siekhaus D. E., Lehmann R. In vivo migration: a germ cell perspective. Annu. Rev. Cell. Dev. Biol. 2006;22:237–265. doi: 10.1146/annurev.cellbio.22.010305.103337. [DOI] [PubMed] [Google Scholar]
- Lands W. E. Metabolism of glycerolipids. 2. The enzymatic acylation of lysolecithin. J. Biol. Chem. 1960;235:2233–2237. [PubMed] [Google Scholar]
- Lauber K., et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Inoue T., Imae R., Kono N., Shirae S., Matsuda S., Gengyo-Ando K., Mitani S., Arai H. Caenorhabditis elegans mboa-7, a member of the MBOAT family, is required for selective incorporation of polyunsaturated fatty acids into phosphatidylinositol. Mol. Biol. Cell. 2008;19:1174–1184. doi: 10.1091/mbc.E07-09-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Treisman J. E. Sightless has homology to transmembrane acyltransferases and is required to generate active Hedgehog protein. Curr. Biol. 2001;11:1147–1152. doi: 10.1016/s0960-9822(01)00323-2. [DOI] [PubMed] [Google Scholar]
- MacDonald M. L., Rogers Q. R., Morris J. G., Cupps P. T. Effects of linoleate and arachidonate deficiencies on reproduction and spermatogenesis in the cat. J. Nutr. 1984;114:719–726. doi: 10.1093/jn/114.4.719. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Inoue T., Lee H. C., Kono N., Tanaka F., Gengyo-Ando K., Mitani S., Arai H. Member of the membrane-bound O-acyltransferase (MBOAT) family encodes a lysophospholipid acyltransferase with broad substrate specificity. Genes Cells. 2008;13:879–888. doi: 10.1111/j.1365-2443.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- Maurel-Zaffran C., Chauvet S., Jullien N., Miassod R., Pradel J., Aragnol D. nessy, an evolutionary conserved gene controlled by Hox proteins during Drosophila embryogenesis. Mech. Dev. 1999;86:159–163. doi: 10.1016/s0925-4773(99)00105-7. [DOI] [PubMed] [Google Scholar]
- Maurel-Zaffran C., Treisman J. E. pannier acts upstream of wingless to direct dorsal eye disc development in Drosophila. Development. 2000;127:1007–1016. doi: 10.1242/dev.127.5.1007. [DOI] [PubMed] [Google Scholar]
- Micchelli C. A., The I., Selva E., Mogila V., Perrimon N. Rasp, a putative transmembrane acyltransferase, is required for Hedgehog signaling. Development. 2002;129:843–851. doi: 10.1242/dev.129.4.843. [DOI] [PubMed] [Google Scholar]
- Miura G. I., Buglino J., Alvarado D., Lemmon M. A., Resh M. D., Treisman J. E. Palmitoylation of the EGFR ligand Spitz by Rasp increases Spitz activity by restricting its diffusion. Dev. Cell. 2006;10:167–176. doi: 10.1016/j.devcel.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Miura G. I., Treisman J. E. Lipid modification of secreted signaling proteins. Cell Cycle. 2006;5:1184–1188. doi: 10.4161/cc.5.11.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. K., Miller K. G. Genetic characterization of the Drosophila jaguar322 mutant reveals that complete myosin VI loss of function is not lethal. Genetics. 2008;179:711–716. doi: 10.1534/genetics.107.085969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Lenartowska M., Miller K. G. Myosin VI stabilizes an actin network during Drosophila spermatid individualization. Mol. Biol. Cell. 2006;17:2559–2571. doi: 10.1091/mbc.E06-01-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Miller K. G. A role for actin dynamics in individualization during spermatogenesis in Drosophila melanogaster. Development. 2003;130:1805–1816. doi: 10.1242/dev.00406. [DOI] [PubMed] [Google Scholar]
- Osuga J., et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc. Natl. Acad. Sci. USA. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard J., Sorensen J. G., Petersen S. O., Loeschcke V., Holmstrup M. Changes in membrane lipid composition following rapid cold hardening in Drosophila melanogaster. J. Insect Physiol. 2005;51:1173–1182. doi: 10.1016/j.jinsphys.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Renault A. D., Sigal Y. J., Morris A. J., Lehmann R. Soma-germ line competition for lipid phosphate uptake regulates germ cell migration and survival. Science. 2004;305:1963–1966. doi: 10.1126/science.1102421. [DOI] [PubMed] [Google Scholar]
- Riekhof W. R., Wu J., Gijón M. A., Zarini S., Murphy R. C., Voelker D. R. Lysophosphatidylcholine metabolism in Saccharomyces cerevisiae: the role of P-type ATPases in transport and a broad specificity acyltransferase in acylation. J. Biol. Chem. 2007a;282:36853–36861. doi: 10.1074/jbc.M706718200. [DOI] [PubMed] [Google Scholar]
- Riekhof W. R., Wu J., Jones J. L., Voelker D. R. Identification and characterization of the major lysophosphatidylethanolamine acyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 2007b;282:28344–28352. doi: 10.1074/jbc.M705256200. [DOI] [PubMed] [Google Scholar]
- Rogat A. D., Miller K. G. A role for myosin VI in actin dynamics at sites of membrane remodeling during Drosophila spermatogenesis. J. Cell Sci. 2002;115:4855–4865. doi: 10.1242/jcs.00149. [DOI] [PubMed] [Google Scholar]
- Sano H., Renault A. D., Lehmann R. Control of lateral migration and germ cell elimination by the Drosophila melanogaster lipid phosphate phosphatases Wunen and Wunen 2. J. Cell Biol. 2005;171:675–683. doi: 10.1083/jcb.200506038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A. C., Lehmann R. Isoprenoids control germ cell migration downstream of HMGCoA reductase. Dev. Cell. 2004;6:283–293. doi: 10.1016/s1534-5807(04)00023-1. [DOI] [PubMed] [Google Scholar]
- Shindou H., Shimizu T. Acyl-CoA: lysophospholipid acyltransferases. J. Biol. Chem. 2008;284:1–5. doi: 10.1074/jbc.R800046200. [DOI] [PubMed] [Google Scholar]
- Starz-Gaiano M., Cho N. K., Forbes A., Lehmann R. Spatially restricted activity of a Drosophila lipid phosphatase guides migrating germ cells. Development. 2001;128:983–991. doi: 10.1242/dev.128.6.983. [DOI] [PubMed] [Google Scholar]
- Takada R., Satomi Y., Kurata T., Ueno N., Norioka S., Kondoh H., Takao T., Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Tamaki H., Shimada A., Ito Y., Ohya M., Takase J., Miyashita M., Miyagawa H., Nozaki H., Nakayama R., Kumagai H. LPT1 encodes a membrane-bound O-acyltransferase involved in the acylation of lysophospholipids in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2007;282:34288–34298. doi: 10.1074/jbc.M704509200. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T., Peacock W. J., Hardy R. W. Dynamics of spermiogenesis in Drosophila melanogaster. I. Individualization process. Z. Zellforsch. Mikrosk. Anat. 1972;124:479–506. doi: 10.1007/BF00335253. [DOI] [PubMed] [Google Scholar]
- Uozumi N., et al. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- Van Doren M., Broihier H. T., Moore L. A., Lehmann R. HMG-CoA reductase guides migrating primordial germ cells. Nature. 1998;396:466–469. doi: 10.1038/24871. [DOI] [PubMed] [Google Scholar]
- van Meer G., Voelker D. R., Feigenson G. W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner D. W., Xu J., Singh I., Jasinska R., Zhang Q. X., Brindley D. N. Structural organization of mammalian lipid phosphate phosphatases: implications for signal transduction. Biochim. Biophys. Acta. 1999;1439:299–316. doi: 10.1016/s1388-1981(99)00102-x. [DOI] [PubMed] [Google Scholar]
- Watson P. F., Morris G. J. Cold shock injury in animal cells. Symp. Soc. Exp. Biol. 1987;41:311–340. [PubMed] [Google Scholar]
- Wei H. C., Rollins J., Fabian L., Hayes M., Polevoy G., Bazinet C., Brill J. A. Depletion of plasma membrane PtdIns(4,5)P2 reveals essential roles for phosphoinositides in flagellar biogenesis. J. Cell Sci. 2008;121:1076–1084. doi: 10.1242/jcs.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymann M. P., Schneiter R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- Xu H., Brill J. A., Hsien J., McBride R., Boulianne G. L., Trimble W. S. Syntaxin 5 is required for cytokinesis and spermatid differentiation in Drosophila. Dev. Biol. 2002;251:294–306. doi: 10.1006/dbio.2002.0830. [DOI] [PubMed] [Google Scholar]
- Xu Y., Condell M., Plesken H., Edelman-Novemsky I., Ma J., Ren M., Schlame M. A Drosophila model of Barth syndrome. Proc. Natl. Acad. Sci. USA. 2006;103:11584–11588. doi: 10.1073/pnas.0603242103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A., Sugiura T., Waku K. Acyltransferases and transacylases involved in fatty acid remodeling of phospholipids and metabolism of bioactive lipids in mammalian cells. J. Biochem. 1997;122:1–16. doi: 10.1093/oxfordjournals.jbchem.a021715. [DOI] [PubMed] [Google Scholar]
- Yang H., Bard M., Bruner D. A., Gleeson A., Deckelbaum R. J., Aljinovic G., Pohl T. M., Rothstein R., Sturley S. L. Sterol esterification in yeast: a two-gene process. Science. 1996;272:1353–1356. doi: 10.1126/science.272.5266.1353. [DOI] [PubMed] [Google Scholar]
- Yang J., Brown M. S., Liang G., Grishin N. V., Goldstein J. L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Zarini S., Gijon M. A., Folco G., Murphy R. C. Effect of arachidonic acid reacylation on leukotriene biosynthesis in human neutrophils stimulated with granulocyte-macrophage colony-stimulating factor and formyl-methionyl-leucyl-phenylalanine. J. Biol. Chem. 2006;281:10134–10142. doi: 10.1074/jbc.M510783200. [DOI] [PubMed] [Google Scholar]
- Zhai L., Chaturvedi D., Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J. Biol. Chem. 2004;279:33220–33227. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- Zhang N., Zhang J., Cheng Y., Howard K. Identification and genetic analysis of wunen, a gene guiding Drosophila melanogaster germ cell migration. Genetics. 1996;143:1231–1241. doi: 10.1093/genetics/143.3.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Zhang J., Purcell K. J., Cheng Y., Howard K. The Drosophila protein Wunen repels migrating germ cells. Nature. 1997;385:64–67. doi: 10.1038/385064a0. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chen Y. Q., Bonacci T. M., Bredt D. S., Li S., Bensch W. R., Moller D. E., Kowala M., Konrad R. J., Cao G. Identification and characterization of a major liver lysophosphatidylcholine acyltransferase. J. Biol. Chem. 2008;283:8258–8265. doi: 10.1074/jbc.M710422200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.