Abstract
A key consideration in assessing impacts of climate change is the possibility of synergistic effects with other human-induced stressors. In the ocean realm, climate change and overfishing pose two of the greatest challenges to the structure and functioning of marine ecosystems. In eastern Tasmania, temperate coastal waters are warming at approximately four times the global ocean warming average, representing the fastest rate of warming in the Southern Hemisphere. This has driven range extension of the ecologically important long-spined sea urchin (Centrostephanus rodgersii), which has now commenced catastrophic overgrazing of productive Tasmanian kelp beds leading to loss of biodiversity and important rocky reef ecosystem services. Coincident with the overgrazing is heavy fishing of reef-based predators including the spiny lobster Jasus edwardsii. By conducting experiments inside and outside Marine Protected Areas we show that fishing, by removing large predatory lobsters, has reduced the resilience of kelp beds against the climate-driven threat of the sea urchin and thus increased risk of catastrophic shift to widespread sea urchin barrens. This shows that interactions between multiple human-induced stressors can exacerbate nonlinear responses of ecosystems to climate change and limit the adaptive capacity of these systems. Management actions focused on reducing the risk of catastrophic phase shift in ecosystems are particularly urgent in the face of ongoing warming and unprecedented levels of predator removal from the world's oceans.
Keywords: climate change, overgrazing, sea urchin, temperate reefs, trophic interactions
Globally, ecosystems are being increasingly perturbed by human activity (1). While ecosystems appear able to absorb some level of stress, “catastrophic shifts” in structure and function can occur once a critical stress-threshold is passed, with a return to former states unlikely (2, 3). Importantly, ecosystems are rarely perturbed by a single stressor but by multiple stressors simultaneously, the effects of which may act synergistically (4). The modern context for marine ecosystems involves changing climate, overfishing, habitat loss, invasive species, and pollutants (5, 6). With increasing intensity and frequency of multiple stressors, there is an urgent need to understand how this influences ecosystem dynamics to curb trends of major ecosystem change and loss of important ecosystem services (3, 7, 8).
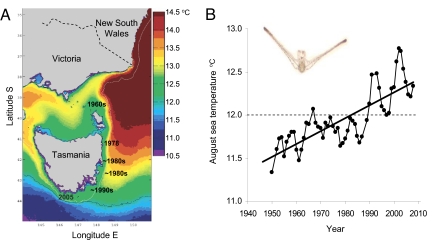
One of the most commonly observed shifts in shallow subtidal temperate marine systems is the transition from productive kelp beds to sea urchin “barrens” habitat, as a result of overgrazing by sea urchins (9). In Australia, no other benthic herbivore has had as large a role in determining the state of shallow reef communities as the long-spined diadematid sea urchin Centrostephanus rodgersii (10). Such is the ecological importance of this sea urchin that in central and southern New South Wales (NSW, Fig. 1A) this species maintains barrens on approximately 50% of near-shore rocky reefs (10). Driven by a changing regional climate, C. rodgersii has recently undergone southward range extension to eastern Tasmania (Fig. 1A) where it has commenced overgrazing of kelp beds leading to an impoverished and unproductive barrens state (11, 12). Consistent with the fingerprint of climate change (13), long-term change in the East Australian Current (EAC) has resulted in greater poleward (southward) penetration of warm water leading to warming of coastal waters in eastern Tasmania (14). Importantly, the sea urchin displays high reproductive potential in Tasmania and coastal warming has led to a regime exceeding the lower thermal limit (12 °C) for the sea urchins' larval development (15) (Fig. 1B). Given predictions of continued warming for this coast (16), the likelihood of further population expansion of C. rodgersii and associated ecosystem impacts appears considerable (11, 15, 17).
Fig. 1.
Recent climate-driven range extension of the long-spined sea urchin to eastern Tasmania. (A) Sea surface temperature (SST) map of south eastern Australia showing influence of the warm East Australian Current in eastern Tasmania during Austral winter; data are mean SST (Pathfinder, 4 × 4 km pixels) for June–August 1993–2007. Dates show year of first observations of Centrostephanus rodgersii at sites on the Tasmanian coast. (B) Long-term winter warming trend of coastal waters in eastern Tasmania 1950–2008; data are 4 year running means (see Materials and Methods) for August, the month of major C. rodgersii spawning (15); dashed line indicates the lower temperature limit for the development of C. rodgersii larvae (15); inset shows 21-day-old C. rodgersii echinopluteus.
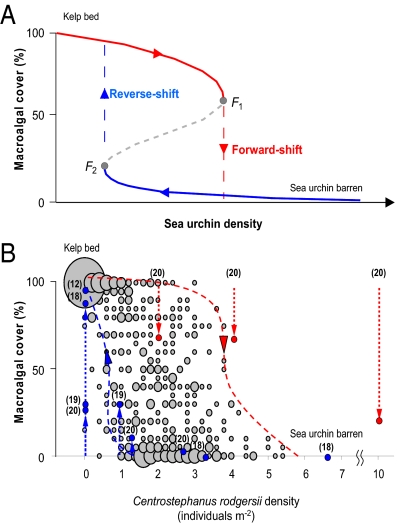
The transition from kelp beds to Centrostephanus rodgersii barrens on rocky reefs represents a catastrophic phase shift between alternative reef states because this shift demonstrates hysteresis (Fig. 2 A and B). The hysteresis effect is evident because return to the kelp-dominated state requires reducing sea urchin densities to much lower levels than the threshold at which destructive overgrazing occurs in the first place (Fig. 2B). That is, overgrazing causes the underlying ecosystem dynamic to shift to an alternative domain of attraction characterized by the sea urchin barrens state with a return to former kelp bed habitat difficult once a critical grazing threshold is passed. Given strong negative effects of the C. rodgersii barrens state on local biodiversity (12) and lucrative reef-based fisheries for abalone and rock lobster (combined value in Tasmania of approximately AUS $150 M per year before processing) (11, 21), the threat that barrens may spread throughout eastern Tasmania to reflect patterns in NSW (10) and north eastern Tasmania (11) is a major concern for biodiversity and important fisheries dependent on kelp-dominated reefs.
Fig. 2.
Catastrophic shift between kelp beds and sea urchin barrens. (A) Conceptual schematic of discontinuous phase shift (redrawn from ref. 3). If the reef system occurs in the kelp state on the upper path (red) but close to the threshold F1, a slight increase in sea urchin density may induce a catastrophic “forward-shift” to the alternative and stable sea urchin barrens state. Once barrens have formed, reverting back to the kelp state is difficult because the system demonstrates hysteresis (18), and the “reverse-shift” (blue path) occurs only if sea urchin density is reduced below the return threshold at F2. The broken gray line indicates the region of instability between the alternative stable states. (B) Macroalgal cover versus Centrostephanus rodgersii density in eastern Tasmania. Bubble size represents relative frequency of particular urchin density and macroalgal cover combinations as measured in 575 individual 5 m2 plots at 13 sites spanning the east coast (11). Overlaid arrows and numbers in parentheses indicate magnitude and direction of ecosystem response to removals and additions of C. rodgersii. Removals of C. rodgersii from barrens (blue arrows) in: NSW after 18 months (18, 19) where starting sea urchin densities were 10 and 6 m−2 respectively; after approximately 5 months (20), with a starting sea urchin density of 4 m−2; in Tasmania after 18 months (12) with a starting sea urchin density of 2 m−2. Additions of C. rodgersii to kelp beds (red arrows) in NSW after approximately 5 months (20), starting sea urchin density 0 m−2. Dashed lines with arrows represent the theoretical “forward-shift” and “reverse-shift” paths as explained in (A).
Isolating the exact mechanism(s) determining the shift from kelp beds to sea urchin barrens has long engaged ecologists. While few generalities can be made across systems, and despite lack of critical evidence for particular systems, a consistent theme is that barrens-habitat arises in areas where sea urchin predators are heavily fished (9, 22, 23). Given this global perspective, we assessed evidence for “top-down” predatory effects on the long-spined sea urchin within its extended range in eastern Tasmania. Explicitly, we examined whether fishing has reduced kelp bed resilience and thus increased the risk of catastrophic overgrazing by the range-extending C. rodgersii (i.e., the “forward-shift” in Fig. 2). Indeed, superimposed on the climate-driven range extension of C. rodgersii is heavy fishing on rocky reefs in Tasmania. Long-term changes to reef species inside marine protected areas (MPAs) relative to adjacent fished sites show that fishing has a major impact on the abundance and size-structure of major target species (24). Most striking is the recovery of the palnulirid spiny lobster Jasus edwardsii inside MPAs, as evidenced by rapid increases in individual size and population abundance following protection from fishing (24). Importantly, large individuals of this lobster (> minimum legal-size limit) are known to prey upon native sea urchins in eastern Tasmania (25). However, it was unknown whether J. edwardsii, or any other reef predators in Tasmania, were capable of preying on the range-extending C. rodgersii, which is considerably larger and has much longer spines than native sea urchin species.
To examine the potential for predation on Centrostephanus rodgersii, we used remote video cameras inside MPAs to monitor tethered and nontethered, but partially caged, sea urchins (see Materials and Methods). Furthermore, because of the generally large size and considerable spiny canopy of C. rodgersii, which may deter predators, the potential size-specific nature of predation was also assessed (see Materials and Methods). To explicitly examine whether fishing of sea urchin predators has reduced kelp bed resilience, we simulated invasion of Tasmanian reefs by C. rodgersii by translocating large numbers of tagged sea urchins to reefs inside no-take MPAs with a high abundance of predators and to adjacent reefs that are open to fishing and support relatively few predators (see Fig. S1).
Results
Remote video surveillance of tethered and partially caged Centrostephanus rodgersii inside no-take MPAs revealed that lobsters (Jasus edwardsii) were the principal predators capable of consuming this sea urchin in eastern Tasmania (Movie S1 and Fig. S2). Lobsters accounted for 92% of predation events observed on tethered urchins (n = 26 video observations) and 100% of partially caged but nontethered sea urchins (n = 4 video observations). Importantly, lobster predation occurred exclusively at night when the nocturnally active sea urchin emerges from shelter. The only other predator of tethered urchins that we observed, the wrasse Notolabrus tetricus (labridae), is diurnally active, and it only preyed on small sea urchins when unnaturally exposed on tethers during daylight hours (a total of two events observed).
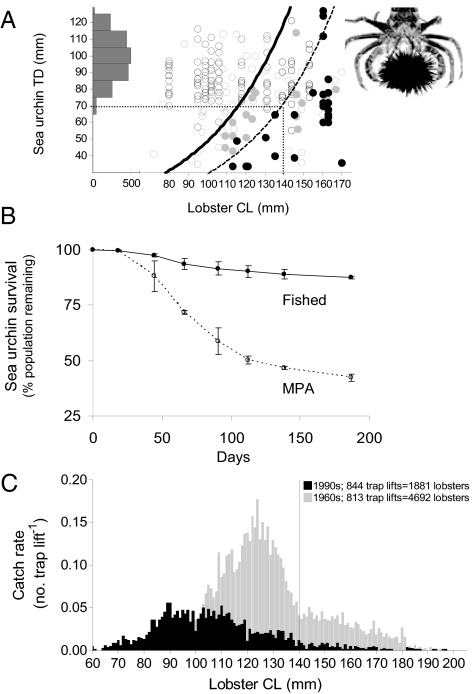
Calibrated video footage of lobster attacks revealed that only very large lobsters were successful predators of Centrostephanus rodgersii. We also observed that lobsters attacked in a consistent fashion whereby the lobster straddles the sea urchin and uses its massive first pair of walking legs to pry it from the substratum, overturn the sea urchin, and consume it through its vulnerable oral surface (see Movie S1). From these observations, we developed a size-specific physical model of predation assuming that the arc of a lobsters' first pair of legs must be sufficiently large to fit over the sea urchins' spine canopy for the lobster to be capable of grappling and successfully overturning the sea urchin (see illustration in Fig. 3Aand Fig. S2) (also see Materials and Methods). We found that the maximum size of C. rodgersii graspable by lobsters increased exponentially with increasing lobster carapace size (solid line Fig. 3A). Overlaying the physical model with observed predation events (from laboratory and in situ caging experiments controlling lobster size, see Materials and Methods) revealed that the upper theoretical limit predicted by the model is in close agreement with the ceiling of observed successful predation events (Fig. 3A). However, the majority of observed predation events near this physical upper “limit,” particularly for smaller lobsters [carapace length (CL) <120 mm], were observed within aquaria. Direct field observations (filled black dots Fig. 3A), indicated that indeed only very large lobsters (CL <140 mm) were capable of preying on C. rodgersii. Importantly, only C. rodgersii >60 mm test diameter (TD) are observed to exist exposed on the reef surface and are vulnerable to lobster predation, i.e., in eastern Tasmania juveniles <60 mm TD are generally located cryptically within the reef matrix and are largely inaccessible to lobsters. Given the size distribution of emergent C. rodgersii in eastern Tasmania (size-frequency histogram, Fig. 3A), and the size-specific predation curve as derived from field observations (i.e., dashed curve, Fig. 3A), we estimate the minimum sized lobster capable of preying on C. rodgersii under natural conditions to be approximately 140 mm CL (see interception of dotted lines Fig. 3A).
Fig. 3.
(A) Size-specific predation by lobsters [Carapace Length (CL)] on long-spined sea urchins [Test Diameter (TD)] in eastern Tasmania. Successful predation events are indicated by filled circles obtained by in situ video monitoring (black circles) and aquarium trials (gray circles), open circles indicate lobster-urchin encounters but with no predation. The solid curve is the theoretical physical limit of predation (y = 5.12e0.023x) determined by the capacity of a lobster to straddle a sea urchin and prise it from the substratum using its first pair of walking legs; LHS of plot is size-frequency of emergent Centrostephanus rodgersii on eastern Tasmanian reefs (n = 1972). As evidenced by the upper limit of observed predation events in situ (i.e., the ceiling of filled black circles, which here is given by the dashed curve fitted as 60% of upper theoretical limit), lobsters must be approximately >140 mm CL to be effective predators of emergent sea urchins in the wild. The minimum legal size-limits for this lobster in Tasmania are 105 mm CL for females and 110 mm CL for males, note that there is no maximum harvestable size-limit. (B) Population trajectories of tagged C. rodgersii on reefs inside and outside MPAs; data are mean percentages (± SEM) of populations surviving (initial population size was 96 individuals at each of two MPA and two fished sites). (C) Change in size-frequency of lobsters pre- (1960s) and post-intense fishing (1990s) in north eastern Tasmania showing pronounced fish-down of the size class CL greater than or equal to140 mm as revealed by fishery independent trap lifts, redrawn from (9).
In the presence of large lobsters (CL ≥140 mm) within MPAs, the percentage of tagged urchins resighted alive declined rapidly (stabilizing at 22.75% ± 0.25 SEM of released populations) compared with adjacent fished reefs (stabilizing at 66.25% ± 4.75 SEM). After factoring for possible differences in resighting probabilities (see Materials and Methods), survival estimates of C. rodgersii showed strong divergence between MPAs and fished reefs (Fig. 3B). Modeling of annual population projections using the apparent survival rates revealed significantly reduced survival for C. rodgersii inside MPAs relative to the fished reefs (mean proportion of population surviving in MPA = 0.094 pop.annum−1 ± 0.010 SEM; Fished = 0.613 pop.annum−1 ± 0.030 SEM; one-way ANOVA, F1,3 = 278.58, P = 0.004).
Discussion
Our experiments demonstrated that lobsters (Jasus edwardsii) are the principal species capable of preying on Centrostephanus rodgersii within the sea urchin's recently extended Tasmanian range. Furthermore, we observed strong overlap between the nocturnal foraging behavior of lobsters and the nocturnal accessibility of C. rodgersii, as predation occurred only at night when large individuals of the sea urchin leave daytime shelters to graze on open rock surfaces where they are vulnerable to attack (Movie S1). Importantly, by describing the size-specific nature of predatory interactions, our experiments demonstrated that only large lobsters (CL ≥140 mm) are capable of grasping and ultimately preying on the sea urchin in the field.
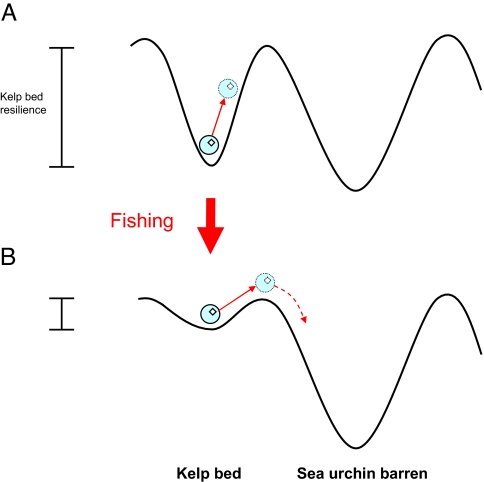
Intensive fishing for well over a century drastically reduced the stock of legal-sized lobsters on eastern Tasmanian reefs to approximately 2–8% of prefished biomass by the 1990s (26). Fishing has shifted the size-distribution of lobsters toward smaller size classes and thus dramatically reduced the abundance of large lobsters capable of preying on the range-extending sea urchin (Fig. 3C). Our experiments inside and outside MPAs clearly showed that survival of C. rodgersii in the presence of large lobsters inside MPAs is considerably reduced relative to that at fished reefs outside MPAs, where large lobsters were absent (Fig. 3B and Fig. S1). Given the overwhelming contribution of spiny lobsters to predation of C. rodgersii and the marked reduction in survival rates of C. rodgersii inside MPAs, our results strongly indicate that heavy fishing of lobsters effectively removes lobsters large enough to be functional predators on sea urchins and thus increases the risk of sea urchin populations achieving densities sufficient to effect widespread overgrazing of kelp bed habitat (Fig. 4).
Fig. 4.
Conceptualization of loss of kelp bed resilience because of fishing and associated increase in risk of catastrophic phase shift to the Centrostephanus rodgersii barrens state. Alternative basins of attraction represent kelp bed and sea urchin barrens states and the position of the ball represents ecosystem status. To shift to barrens habitat the kelp system must be perturbed sufficiently for the ball to roll from one basin to another (dashed arrow). (A) Prefished kelp bed with high abundance of large predatory lobsters and high resilience (indicated by basin depth). (B) Heavily fished kelp beds with shallow ‘basin’ and thus lower resilience. Solid arrows represent perturbation of the kelp bed state in the form of climate-driven incursion of C. rodgersii. The likelihood of catastrophic shift to sea urchin barrens depends on the size of the perturbation, which is the same in both (A) and (B), and the basin depth, i.e., “resilience stability” of the kelp-dominated state.
Of crucial importance to the issue of overgrazing by C. rodgersii in eastern Tasmania is that while the sea urchin has initiated catastrophic overgrazing of kelp beds at many sites (11), the majority of rocky reefs in the region remain in the desirable kelp-dominated state, albeit with low resilience because effective predators are relatively rare. Management must therefore aim to prevent further phase shifts to sea urchin barrens because the strong hysteresis effect makes rehabilitation of existing barrens to kelp beds exceedingly difficult (see “reverse-shift” Fig. 2). Rebuilding the size and abundance of reef predators will increase the resilience of kelp beds and thus reduce the likelihood of widespread sea urchin overgrazing in the first instance (“forward-shift” Fig. 2).
In conclusion, our findings provide a strong empirical basis to shift from traditional equilibrium-based thinking; that is, “top-down” predator-driven vs. “bottom-up” environmentally driven control, toward adopting more integrated resilience-based ecosystem management approaches (7, 8). This shift in conceptual basis, to focus on reducing risk of major ecosystem change, is particularly urgent in the face of rapidly warming climate and unprecedented levels of predator removal from the world's oceans. Finally, interactions between multiple human-induced stressors will continue to exacerbate nonlinear responses of ecosystems and will progressively limit the adaptive capacity of natural systems to cope with rapid climate change.
Materials and Methods
Environmental Signal.
Sea surface temperature data were taken from a coastal monitoring station located adjacent to Maria Island on the 50 m isobath (148.23°E; 42.60°S), courtesy of Commonwealth Scientific and Industrial Research Organization Marine and Atmospheric Research. Note that in situ data were unavailable for years 1996–1998, 2002–2004, 2006, and 2008. For these years satellite-derived SST estimates (Pathfinder, 4 × 4 km interpolated pixels) were obtained. Remotely sensed SSTs are consistent with in situ sea surface measurements in this region (14).
Study Sites.
Experiments were performed in two regions of eastern Tasmania where predator biomass has shown strong recovery inside MPAs relative to adjacent reefs subject to exploitation (24). The Maria Island Marine Reserve (MIMR, 42° 35.26′S, 148° 3.03′E) was established in 1992, with approximately 12 years of protection at the time of experimentation in 2004–2005; the Crayfish Point Research Reserve (CPRR, 42° 57.37′S, 147° 21.30′E) was established in 1971, with approximately 33 years of protection at the time of experimentation in 2005. Experimental reefs were of high relief, experience moderate wave exposure and support kelp communities.
Remote Video Identifying Sea Urchin Predators.
Given that the long-spined sea urchin (Centrostephanus rodgersii) is nocturnal, a remotely operating and continuously recording video system equipped with infrared lighting (27) was used to detect predatory interactions throughout the diel cycle, while eliminating potential effects of visible light on animal behavior. To maximize the ability to observe naturally occurring predators, the video system was set up inside the two no-take MPAs in eastern Tasmania (MIMR and CPRR) where the size and abundance of potential predators has recovered following protection from fishing (24). Given relatively high nocturnal movement in C. rodgersii and a limited camera view-field, particularly at night under infrared illumination, sea urchins were either tethered in front of cameras on open rock surfaces or partially restrained (but untethered) within a partial cage (open sides and open roof) that allowed access and identification of predators.
Tethered C. rodgersii were exposed on open rock surfaces and monitored individually by a series of six tripod-mounted video cameras. Tethering involved drilling two small holes through the test with a hypodermic needle (100 mm long by 1.25 mm diameter), threading a 150-mm length of monofilament line (0.45 mm diameter) through the needle, and threading a size 1 swivel-clip (8-mm clip gape), and numbered spaghetti tag over the monofilament before the line ends were crimped together with a leader sleeve (size 3). This method results in low mortality (< 5%), and no signs of disease or necrosis was observed around the entry and exit points in the test. Because any mortality usually occurs within 2 days of the operation, tagged animals were monitored for several days and only healthy individuals were used in experiments. Three tagged individuals of various sizes (35–127 mm test diameter) were tethered to 2 m lengths of 6 mm diameter galvanised steel chain. Two chains were anchored across rock platforms devoid of crevice refuges for urchins and cleared of macroalgae. Once the sea urchins were in place, continuous recording commenced with battery changes and checks performed daily, and sea urchins replaced as necessary. The partially caged untethered sea urchins were monitored by video cameras (with accompanying infrared lights) focused on the cage openings (roof and sides) and the interior. The partial cage encompassed a reef area of 2 m2, was constructed of 38 mm mesh with a wall height of 1.5 m, weighted with chain at the bottom and buoyed with floats at the top.
Size-Specific Predation.
Video monitoring of tethered C. rodgersii also allowed estimation of predator size using a calibrated view field. In addition, we examined size-specific interactions between lobsters and sea urchins in aquarium trials. To induce attack by lobsters in aquarium experiments, the peristomial membrane of urchins was punctured with a 10-mm hole to release coelomic fluid. Sea urchins wounded in this manner were still able to defend themselves with their spines, and resisted attack by sucking onto the smooth aquarium surface via tube feet attachment. Control urchins (50–115 mm TD) treated in this way (n = 6) were held in aquaria without predators and were all alive 2 months after treatment. For each trial an individual lobster and sea urchin were drawn randomly from holding tanks and put together in a 1,600 L aquarium for a trial period of 2 days and nights, after which it was recorded whether the sea urchin had been successfully captured and eaten. A total of 72 individual trials were conducted between December 2005 and February 2006.
Physical Model Defining Maximum Size-Limit of Predatory Interaction.
Video footage of spiny lobster (Jasus edwardsii) attacks on C. rodgersii in the field revealed a highly consistent method of predation whereby the lobster would straddle the urchin and use the massive first pair of walking legs to prise it from the substratum, and then manipulate it to penetrate through the peristomial membrane on the oral surface (Movie S1). The size of first pair of legs appeared important in initiating the attack (Fig. S2). On this basis we developed a model assuming that J. edwardsii could only predate on C. rodgersii if the span (inside circumference) of this pair of appendages could extend right around the spine canopy (outside circumference) of the sea urchin such that the urchin could be grasped and dislodged from the benthos. The spine canopy dimension was defined as the distance around the semicircle formed when the sea urchin is attached to the benthos, i.e., from where the spines touch the benthos on the left hand side over the sea urchin to where the spines touched the benthos on the right hand side. The span-width of the first thoracic appendages of lobsters was determined by summing the lengths of each leg segment and the inter-leg distance on the underside of the thorax. Equivalence in the span-width of the first thoracic appendages and urchin spine canopy circumference was used to derive a theoretical upper limit of predation capability on C. rodgersii by lobsters of a given carapace length (solid curve Fig. 3A).
Survival of Sea Urchins Inside and Outside No-Take MPAs.
To test the hypothesis that protected reefs with high predator abundance conferred greater resilience against sea urchin grazing, we assessed predation rates on individually tagged C. rodgersii (nontethered and capable of normal behavior) in the two MPAs and on adjacent fished reefs. At each experimental reef, the size and abundance of large mobile predatory invertebrates was assessed with six belt transects (50 × 4 m), while demersal fishes were surveyed by visual size-graded counts from standardised swims along six belt transects (50 × 10 m). Macroinvertebrates were measured in situ using vernier calipers. Spiny lobsters inside MPAs are far more abundant and are much larger than those on nearby reefs open to lobster fishing (Fig. S1A). In contrast, the overall size distribution and abundance of the most abundant wrasse in the area, the protogynous hermaphrodite N. tetricus (Labridae), was similar on reefs inside and outside MPAs, although there was some evidence that terminal phase males were larger inside MPAs (Fig. S1B).
Tagged C. rodgersii were placed within rocky crevice shelters on reefs inside and outside MPAs at both sites and resurveyed through time. Individuals were held in aquaria for at least 2 days after tagging, and only urchins that were healthy after this time were used in the experiment. Tagged individuals displayed normal behavior and remained localized on the experimental reefs. Importantly, all individuals retained their tags unless preyed upon, and pilot trials revealed that readable tags are retained by sea urchins for >2 years in the wild. Multiple resurvey of tagged individuals yielded individual encounter histories for each tagged sea urchin, enabling maximum likelihood estimation of apparent daily survival and resighting probabilities using a Cormack-Jolly-Seber (CJS) mark-recapture technique (28–30). At each site, on reef both inside and outside MPAs, 48 C. rodgersii in each of two size classes (small 40–70 mm TD; large 80–120 mm TD) were placed within crevices (total of 192 tagged sea urchins per site) inside a 60 × 10 m census zone and monitored through time. Searches for tagged sea urchins were performed across the sites on eight resampling occasions (at 17, 37, 60, 86, 106, 131, and 182 days postrelease) within the census zone at each site. Because C. rodgersii typically shows high fidelity for resident crevices, we assumed zero emigration of individuals from the census area. This was supported by a lack of observations of any tagged animals outside the 600 m2 census zone over the duration of the experiment. Inside MPAs an average of 60% of the C. rodgersii population was sighted during each resampling occasion, whereas outside MPAs an average of 77% of the C. rodgersii population was sighted on each resampling occasion. The design enabled modeling of the contributions of “group” (i.e., MPA1, MPA2, Fished1, Fished2) and “time” (i.e., sampling occasions, n = 8) to apparent daily survival probabilities.
Data were analyzed using the CJS routine in the MARK software (31), which identifies the most parsimonious CJS model, while excluding parameters that cannot be justified by the data. Model fit was examined using 1,000 bootstraps within the Goodness-Of-Fit (GOF) routine within MARK. A goodness-of-fit test of the saturated size-specific CJS model indicated satisfactory fit (P = 0.12), with the model reduction process indicating that the most parsimonious model contained survival and resighting probabilities as a function of “group” only. Thus, the model could be used to estimate mean survival rates for C. rodgersii in MPAs and at fished sites.
Supplementary Material
Acknowledgments.
We thank three anonymous reviewers for constructive comments. This work was supported by the University of Tasmania, Tasmanian Aquaculture and Fisheries Institute (TAFI), Tasmanian Abalone Council, and Fisheries Research and Development Corporation #2001/044 (to C.R.J.); S.D.L. received scholarship support from TAFI and was a member of the Commonwealth Scientific and Industrial Research Organization Joint PhD Program in Quantitative Marine Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907529106/DCSupplemental.
References
- 1.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth's ecosystems. Science. 1997;277:494–499. [Google Scholar]
- 2.Holling CS. Resilience and stability of ecological systems. Annu Rev Ecol Syst. 1973;4:1–23. [Google Scholar]
- 3.Scheffer M, Carpenter S, Foley J, Folke C, Walker N. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 4.Beisner BE, Haydon DT, Cuddington K. Alternative stable states in ecology. Front Ecol Environ. 2003;1:376–382. [Google Scholar]
- 5.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 6.Meehl GA, et al. in Climate Change 2007: The Physical Science Basis. In: Solomon S QD, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. United Kingdom and New York, NY: Cambridge Univ Press; 2007. pp. 747–845. [Google Scholar]
- 7.Folke C, et al. Regime shifts, resilience, and biodiversity in ecosystem management. Annu Rev Ecol Evol Syst. 2004;35:557–581. [Google Scholar]
- 8.Hughes TP, Bellwood DR, Folke C, Steneck RS, Wilson J. New paradigms for supporting the resilience of marine ecosystems. Trends Ecol Evol. 2005;20:380–386. doi: 10.1016/j.tree.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Steneck RS, et al. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ Conserv. 2002;29:436–459. [Google Scholar]
- 10.Andrew NL, Byrne M. The ecology of Centrostephanus rodgersii. In: Lawrence JM, editor. Edible Sea Urchins: Biology and Ecology. Elsevier Science; 2001. pp. 149–160. [Google Scholar]
- 11.Johnson CR, Ling SD, Ross J, Shepherd S, Miller K. FRDC Final Report. 2005. Establishment of the long-spined sea urchin (Centrostephanus rodgersii) in Tasmania: First assessment of potential threats to fisheries. ed. 2001/044 PN. [Google Scholar]
- 12.Ling SD. Range expansion of a habitat-modifying species leads to loss of taxonomic diversity: A new and impoverished reef state. Oecologia. 2008;156:883–894. doi: 10.1007/s00442-008-1043-9. [DOI] [PubMed] [Google Scholar]
- 13.Parmesan C, Yohe G. A globally coherent fingerprint of climate change. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 14.Ridgway KR. Long-term trend and decadal variability of the southward penetration of the East Australian Current. Geophys Res Lett. 2007;34:L13613. doi: 13610.11029/12007GL030393. [Google Scholar]
- 15.Ling SD, Johnson CR, Frusher S, King C. Reproductive potential of a marine ecosystem engineer at the edge of a newly expanded range. Glob Chang Biol. 2008;14:907–915. [Google Scholar]
- 16.Poloczanska ES, et al. Climate change and Australian marine life. Oceanogr Mar Biol, Annu Rev. 2007;45:409–480. [Google Scholar]
- 17.Ling SD, Johnson CR, Ridgway K, Hobday AJ, Haddon M. Climate driven range extension of a sea urchin: Inferring future trends by analysis of recent population dynamics. Glob Chang Biol. 2009;15:719–731. [Google Scholar]
- 18.Andrew NL, Underwood AJ. Density-dependent foraging in the sea urchin Centrostephanus rodgersii on shallow subtidal reefs in New South Wales, Australia. Mar Ecol Prog Ser. 1993;99:89–98. [Google Scholar]
- 19.Andrew NL, et al. FRDC Final Report. 1998. Interactions between the abalone fishery and sea urchins in New South Wales. ed. 1993/102 PN. [Google Scholar]
- 20.Hill NA, et al. Grazing effects of the sea urchin Centrostephanus rodgersii in two contrasting rocky reef habitats: Effects of urchin density and its implications for the fishery. Mar Freshw Res. 2003;54:691–700. [Google Scholar]
- 21.Andrew NL, Underwood AJ. Associations and abundance of sea urchins and abalone on shallow subtidal reefs in southern New South Wales. Aust J Mar Freshw Res. 1992;43:1547–1559. [Google Scholar]
- 22.Shears NT, Babcock RC. Continuing trophic cascade effects after 25 years of no take marine reserve protection. Mar Ecol Prog Ser. 2003;246:1–16. [Google Scholar]
- 23.Tegner MJ, Dayton PK. Ecosystem effects of fishing in kelp forest communities. ICES J Mar Sci. 2000;57:579–589. [Google Scholar]
- 24.Barrett NS, Buxton CD, Edgar GJ. Changes in invertebrate and macroalgal populations in Tasmanian marine reserves in the decade following protection. J Exp Mar Biol Ecol. 2009;370 [Google Scholar]
- 25.Pederson HG, Johnson CR. Predation of the sea urchin Heliocidaris erythrogramma by rock lobsters (Jasus edwardsii) in no-take marine reserves. J Exp Mar Biol Ecol. 2006;336:120–134. [Google Scholar]
- 26.Frusher SD. Stock assessment report: Rock lobster. Government of Tasmania, Australia, Internal report No. 35. Tasmanian Department of Primary Industry and Fisheries, Hobart. 1997 [Google Scholar]
- 27.Mills DJ, Verdouw G, Frusher SD. Remote multi-camera system for in situ observations of behaviour and predator/prey interactions of marine benthic macrofauna. N Z J Mar Freshw Res. 2005;39:347–352. [Google Scholar]
- 28.Cormack RM. Estimates of survival from the sighting of marked animals. Biometricka. 1964;51:429–438. [Google Scholar]
- 29.Jolly GM. Explicit estimates from capture-recapture data with both death and immigration - stochastic model. Biometricka. 1965;52:225–247. [PubMed] [Google Scholar]
- 30.Seber GAF. A note on the multiple recapture census Biometrika. 1965;52:249–259. [PubMed] [Google Scholar]
- 31.White GC, Burnham KP. Program MARK: Survival estimation from populations of marked animals. Bird Study. 1999;46:120–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.