Abstract
Background/Aims
Both prostaglandin E2 (PGE2) and estradiol stimulate fetal ACTH secretion and augment fetal ACTH responses to stress. We have reported that estradiol increases prostaglandin endoperoxide synthase-2 (PGHS-2), and we have proposed that there is a positive feedback relationship between estrogen and fetal Hypothalamus-Pituitary-Adrenal (HPA) axis activity that is dependent upon PGHS activity in the fetal brain. The present study was designed to test the hypothesis that blockade of estrogen receptors in the fetal brain decrease PGHS-2 expression and reduces fetal HPA axis activity.
Methods
In study 1, six time-dated pregnant ewes with chronically-catheterized twin fetuses were used in this study. In each pregnancy, one twin was treated intracerebroventricularly (icv) with the estrogen receptor antagonist ICI 182,780 (25 ug/day; n=6) while the other twin served as an age-matched control. In study 2, plasma samples were drawn from 10 singleton chronically-catheterized fetuses on alternating days until the time of spontaneous parturition.
Results
ICI infusion caused significantly decreased PGHS-2 mRNA abundance in fetal central nervous system and pituitary, with the greatest decreases occurring in hippocampus and pituitary. There were no statistically significant changes in PGHS-1 mRNA. ICI infusion did not significantly change fetal plasma concentrations of proopiomelanocortin (POMC), ACTH, or cortisol in fetuses 130-134 days gestation (study 1) but did decrease the preparturient rise in plasma proopiomelanocortin concentrations in study 2.
Conclusion
We conclude that PGHS-2 expression in the late-gestation fetal brain is in part stimulated by circulating estrogens in fetal plasma. Blockade of CNS estrogen receptors reduces preparturient plasma concentrations of POMC, but does not reduce fetal HPA axis activity in 130-134 day fetal sheep.
Introduction
Previous work from this laboratory has suggested the possibility that there is a positive feedback loop involving placental estrogen biosynthesis and the fetal hypothalamus-pituitary-adrenal (HPA) axis that may contribute to the preparturient increase in fetal HPA axis activity triggering parturition in this species [1]. While we have previously reported that prostaglandin synthase isoforms are expressed in the fetal brain [2-5] and that estradiol influences the expression of prostaglandin biosynthetic enzymes in the fetal brain in response to brachiocephalic occlusion [3], it is not known if the estradiol increases expression of prostaglandin synthase isoforms in unstressed fetuses. The aim of the current study is to answer this question.
In both fetal and adult animals, prostaglandin E2 stimulates ACTH secretion [6-9]. We have previously demonstrated that the endogenous production of cyclooxygenase metabolites modifies and, in some cases, mediates fetal responsiveness to cardiovascular stress [10-12]. Pretreatment of fetal sheep with indomethacin, an inhibitor of prostaglandin biosynthesis, reduced the fetal ACTH response to hypotension [10]. At the same time, there appears to be a physiological influence of estrogen on prostaglandin biosynthesis. Estradiol treatment increases the expression of prostaglandin synthase-2 (PGHS-2) in endometrium and placenta [13], as it does in various brain regions [3]. The upregulation of prostaglandin biosynthetic capacity in the fetal brain by estrogen led us to suggest that there is an interplay among estrogen, fetal CNS prostaglandin biosynthesis, and fetal Hypothalamus-Pituitary-Adrenal (HPA) stress responsiveness [1;3].
Near term in the sheep fetus, estrogen biosynthesis is stimulated by preparturient increases in HPA axis activity secondary to the upregulation of 17α hydroxylase and 17,20 lyase activities in placenta by cortisol [14;15]. We have reported that estradiol, in turn, stimulates the fetal HPA axis [16-18]. Exogenous estradiol treatment augments basal and stimulated fetal plasma ACTH secretion and elevates plasma cortisol [16]. The stimulatory effect of estrogen on the HPA axis has been documented in adult rats as well. Ovariectomized female rats have attenuated adrenal corticosteroid production which is reversed upon estradiol replacement [19;20].
The present study was performed to test the hypothesis that blockade of estrogen receptors in fetal sheep decreases the expression of PGHS-2 and decreases fetal HPA axis activity. Our study focused on both PGHS-1 and PGHS-2 expression as well as other genes that are relevant to the HPA axis or its activity.
Materials and Methods
In study 1, six time-dated ewes with twin pregnancies were used in this study (12 fetuses, 120 to 127 days gestation). One fetus served as the experimental fetus while its twin served as an internal age-matched control. There were two experimental groups: ICI 182,780 intracerebroventricularly (icv, 25 μg/day, n=6, 3 male and 3 female fetuses) versus untreated control (n=6, 2 male and 4 female fetuses). Twin fetuses were randomly assigned to the two groups. In study 2, 10 singleton fetuses were studied (122-128 days gestation at the time of surgery). Two groups of fetuses were studied, an experimental group in which fetuses received ICI 182,780 icv (25 μg/day, n=5) and a second experimental group in which fetuses received infusion of vehicle (n=5). All animals were housed in individual pens located in the Animal Resources Department at the University of Florida and all of these experiments were approved by the University of Florida Institutional Animal Care and Use Committee. The rooms maintained controlled lighting and temperature and sheep were given food and water ad libitum.
Surgical Preparation
Food was withheld from the pregnant ewes for 24 hours before surgery. Ewes were intubated and anesthetized with halothane (0.5 to 2%) in oxygen before and during surgical preparation as previously described [21]. Surgery and arterial catheter placement for all fetuses was performed using aseptic technique as previously described, with femoral arterial and venous catheters as well as amniotic fluid catheters [21]. For placement of the catheter into the lateral cerebral ventricle, the scalp was retracted and a small catheter (outside diameter, 0.05 in.; inside diameter, 0.03 in.) attached to an osmotic mini-pump (size 2mL4Alza Corp., Palo Alto, CA) was inserted through a hole made in the skull. This catheter was held in place using VetBond (3M Corp., St. Paul, MN). The exposed catheter and osmotic mini-pump were placed subcutaneously before closing the incision on the head. All fetuses in study 1 received intracerebroventricular catheters and minipumps. Minipumps in the treated fetuses were filled with ICI 182,780 (Tocris Biosciences, Bristol, UK) in vehicle (dimethylsulfoxide:water::50:50) and minipumps in the control fetuses were filled with vehicle only. The position of the catheters and the function of the pumps was verified by visual inspection at the time of sacrifice and tissue collection. In study 1, twin fetuses were catheterized; one received icv infusion of ICI 182,780 and one received icv infusion of vehicle. In study 2, all of the fetuses were singleton fetuses; 5 received icv infusion of ICI 182,780 and 5 received icv infusion of vehicle. Antibiotics (750 mg ampicillin) were administered into the amniotic cavity via direct injection. Finally, the hindlimb catheters were exteriorized through the flank of the ewe using a trochar, where they were maintained in a cloth pocket. Ewes were given 1 mg/kg flunixin meglumine (Webster Veterinary, Sterling, MA) for analgesia and returned to their pens where they were monitored until they could stand on their own. Twice daily during a 5-day recovery period ewes were treated with antibiotic (ampicillin, 750 mg im: Polyflex®, Fort Dodge Laboratories, Fort Dodge, IA) and rectal temperatures were monitored for indication of post-operative infection.
Blood Collection
Following the recovery period in both studies, fetal blood samples (3 mL) were drawn from the arterial catheter every other morning (between 0800 and 1000) for use in hormone assays. In study 1, fetal blood sampling continued until day 131-134 of gestation. The actual number of blood samples varied from 1-5 pairs of samples. In study 2, the blood sampling was continued until spontaneous parturition. Samples were kept on ice until centrifuged at 3,000 × g for 15 minutes at 4°C to separated red blood cells and plasma. Plasma was stored at -20°C until analysis. Blood gases were measured at the time of blood sampling in a separate 1 mL blood sample using an ABL 77 Radiometer (Radiometer America Inc., Cleveland, OH) blood gas analyzer.
Tissue Collection
Twin fetuses in study 1 (131 to 134 days) were euthanized with an overdose of sodium pentobarbital. These fetuses were euthanized 6, 6, 7, 8, 14, and 14 days after surgery and the start of the icv infusion of ICI 182,780 or vehicle. Brains were rapidly removed, dissected into distinct regions, and snap frozen in liquid nitrogen. The following tissues were collected: brainstem, hippocampus, frontal cortex, cerebellum, hypothalamus, and pituitary. Tissues were stored at -80°C until processed for mRNA or protein. Fetuses in study 2 were not used for tissue collection.
Plasma Hormone Assays
Assays for estradiol, estradiol sulfate, cortisol, DHEAS, progesterone, ACTH, ACTH1-39, and POMC were measured using ELISA or RIA. Estradiol: Plasma estradiol concentration was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Oxford Biomedical Research, cat.no. EA70) after extraction with hexane/ethyl acetate (3:2 vol/vol). Cross reactivity with 17β-estradiol, estriol, and estrone in this kit is 100%, 0.41%, and 0.10% respectively, as reported by the manufacturer. Estradiol sulfate: Plasma estradiol sulfate concentration was measured using the estradiol ELISA (Oxford Biomedical Research, cat.no. EA70) after deproteinization with ethanol, as previously reported [21]. Cortisol: Plasma cortisol concentration was measured using the cortisol ELISA kit from Oxford Biomedical Research (cat.no. EA65) after deproteinization with ethanol, as described previously [22]. Cross reactivity with cortisone, 11-deoxycortisol, and corticosterone in this kit is 15.77%, 15%, and 4.81% respectively, as reported by the manufacturer. Dehydroepiandrosterone sulfate (DHEAS): Plasma DHEAS concentration was measured with the 125I-DHEA-SO4 Coat-A-Count assay from Diagnostic Products Corporation (Los Angeles, CA; cat. no. TKDS5). Percent cross reactivity with DHEA, and Estrone-3-SO4 was 0.57 and 0.25 respectively, as reported by the manufacturer. Progesterone: Plasma progesterone concentration was measured with a 125I-Progesterone Coat-A-Count kit from Diagnostic Products Corporation (Los Angeles, CA; cat. no. TKPG5). Percent cross reactivity with 17α-hydroxyprogesterone, medroxyprogesterone, and pregnenolone was 3.4, 0.3, and 0.1 respectively, as reported by the manufacturer. Adrenocorticotropin hormone (ACTH): ACTH1-39 was measured using a 2-site immunoradiometric assay kit purchased from DiaSorin (Stillwater, MN, cat.no. 27130). This assay has been previously validated for use in fetal sheep plasma by Myers and Ducsay [23]. Pro-opiomelanocortin (POMC): Pro-opiomelanocortin was measured using an ELISA kit from Immunodiagnostic Systems Ltd. (cat. no. AC-71F1) according to the manufacturer's instructions. Cross reactivity with POMC and Pro-ACTH is 100%, as reported by the manufacturer. However, this assay does not recognize ACTH1-39.
RNA Isolation and Real Time RT-PCR
Tissues were individually pulverized using Bio-Pulverizer (Bio-Spec Products, Bartlesville, OK), a trigger-style mortar and pestle device. Total RNA was extracted using Trizol® (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. A high speed Polytron homogenizer (Tekmar, Janke and Kunkel, West Germany) was used for homogenization. RNA pellets were resuspended in 200μl RNAsecure (Ambion, Austin, TX) pre-heated to 60°C. The pellets were incubated in a 60°C water bath for 10 minutes in order to inactivate RNAses. RNA concentration in each sample was quantified by spectrophotometry.
RNA samples were converted into 4 μg stable cDNA by reverse transcription using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Reverse transcription reactions were performed in RNase/DNase free microcentrifuge tubes on a thermocycler (Biometra, Ltd., Kent, ME) using a thermal profile that ran for 10 minutes at 25°C followed by 120 minutes at 37°C. The resulting cDNA samples were stored at -20°C until real-time RT-PCR was performed. Prostaglandin endoperoxide synthase-1 and -2 (PGHS-1, PGHS-2), arginine vasopressin (AVP), corticotrophin releasing hormone (CRH), POMC, serum- and glucocorticoid-regulated kinase-1 (sgk-1), prohormone convertase-1 (PC1) and 18S rRNA gene expression was analyzed using real-time RT-PCR with 100 ng cDNA template. Primer and probe sets were designed using Primer Express version 2.0 (Applied Biosystems) using known ovine sequences. Real-time RT-PCR methodology for AVP, CRH, sgk-1, POMC, PGHS-1 and PGHS-2 have been reported previously [3;24-26]. Primers and probes for ovine luteinizing hormone (oLH), follicle stimulating hormone (oFSH), and prolactin (oPRL) were designed from known ovine mRNA sequences for the beta subunits of oLH (accession NM_001009380) and oFSH (NM_001009798), and for oPRL (NM_001009306.1). oLH and oFSH were designed as a duplex assay reaction, validated according to the Applied Biosystems methodology (Applied Biosystems, South San Francisco, CA). Primers and probe for 18S rRNA were purchased from Applied Biosystems. Reaction concentrations and primer and probe sequences for all mRNA assays (except AVP, which was run with SYBR© green instead of probe) can be found in Table 1. Ribosomal RNA reactions contained 0.1 ng cDNA, 100 nM forward and reverse primer, and 50 nM probe. Total reaction volume was 25 μl, and included pre-mixed reagents (universal master mix, Applied Biosystems). Samples were run in triplicate on an ABI Prism 7000 PCR instrument (Applied Biosystems). With the exception of oFSH and 18S rRNA, all probes were 5′-labeled with 6-FAM (6-carboxyfluoresceine) and 3′-labelled with TAMRA (carboxytetramethyl rhodamine). oFSH and 18S probes were 5′-labelled with VIC and 3′-labelled with TAMRA.
Table 1.
Primer and probe sequences used in real time RT-PCR
| gene | forward primer sequence | FW nM |
reverse primer sequence | RV nM |
Taqman probe sequence | Tqm nM |
|---|---|---|---|---|---|---|
| AVP | TTCCAGAACTGCCCAAGGG | 250 | AGACACTGTCTCAGCTCCAGGTC | 50 | SYBR green | --- |
| CRH | TCCCATTTCCCTGGATCTCA | 300 | GAGCTTGCTGCGCTAACTGA | 300 | TTCCACCTCCTCCGAGAAGTCTTGGAAAT | 250 |
| POMC | CCG GCA ACT GCG ATG AG | 900 | GGA AAT GGC CCA TGA CGT ACT | 900 | AGC CGC TGA CTG AGA ACC CCC G | 250 |
| oLH | CCGCTCCCAGATATCCTCTTC | 150 | GTCTGCTGGCTTTGGGAGTTA | 150 | TCTAAGGATGCCCCACTTCAATCTCCCA | 150 |
| oFSH | CCCAACATCCAGAAAGCATGT | 900 | GCACAGCCAGGCACTTTCA | 900 | TTCAAGGAGCTGGTGTACGAGACG | 150 |
| oPRL | TGAGCTTGATTCTTGGGTTGCT | 300 | CCCCGCACCTCTGTGACTA | 300 | CTCCTGGAATGACCCTCTGTATCAC | 100 |
| PC1 | GCGGGCATCTTCGCTCTAG | 900 | TCCATACAACCAAGTGCTGCAT | 900 | AAGCAAATCCAAATCTCACCTGGCGAG | 250 |
| PGHS1 | GGCACCAACCTCATGTTTGC | 100 | TCTTGCCGAAGTTTTGAAGA | 100 | TTCTTTGCCCAACACTTCACCCATCA | 200 |
| PGHS2 | GCACAAATCTGATGTTTGCATTCT | 100 | CTGGTCCTCGTTCATATCTGCTT | 100 | TGCCCAGCACTTCACCCATCAATTTT | 200 |
| sgk-1 | GACTTTGGACTCTGCAAGGAGAA | 900 | CGGGCGTGCCACAGAA | 900 | TTGAACACAATGGCACGACGTCCAC | 250 |
Statistical Analysis
mRNA was analyzed by two-way analysis of variance (ANOVA), with brain region and ICI treatment as the two main factors. Post hoc analysis was performed using the Bonferroni test. Fetal plasma hormone concentrations were also analyzed by two-way ANOVA with day of blood sampling and ICI treatment as the two main factors. Because not all pairs of twins were sampled for the same number of days, this analysis was not performed as a repeated measures analysis in the time dimension. Because there was no significant effect of sampling day on any of the measured variables, differences between groups were assessed using a paired t-test analysis of the mean plasma concentrations of each hormone or blood gas in treated and untreated twins. Multiple linear regression was used to test for a statistically significant effect of gender and/or ICI treatment on mean plasma hormone concentrations and on PGHS-1 and -2 mRNA expression in brain regions and pituitary. SPSS 14.0 (SPSS Corp., Chicago, IL) was used for data analysis. A significance level of P<0.05 was used to reject the null hypothesis. Values are reported as mean ± SEM.
Results
mRNA Expression of Brain and Pituitary PGHS-1 and PGHS-2 in Study 1
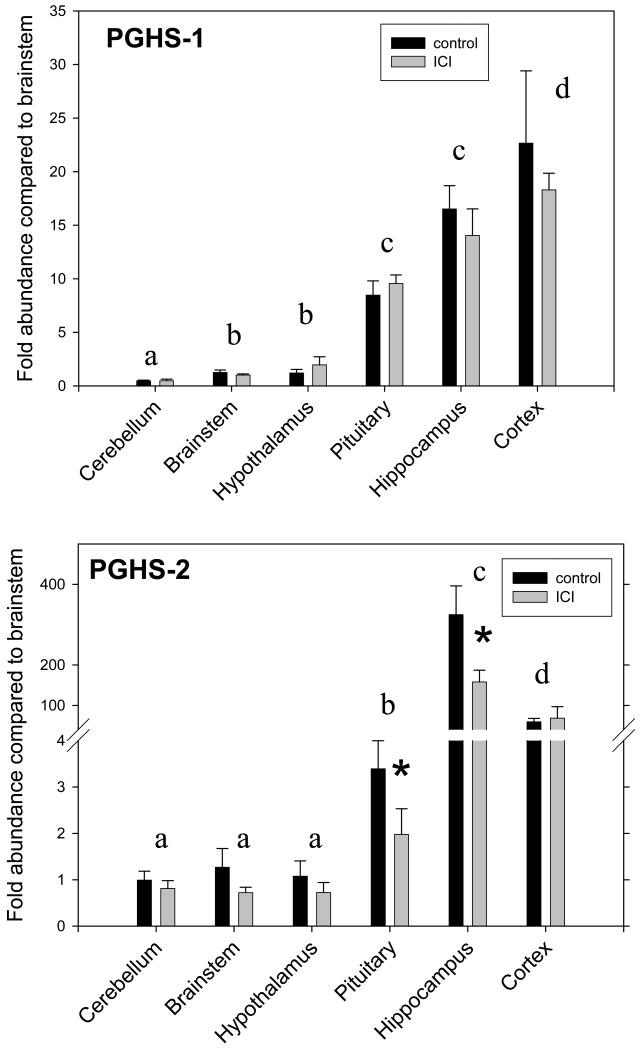
ICI significantly decreased the expression of PGHS-2 (significant main effect of ICI treatment by two-way ANOVA). The expression of PGHS-2 also varied significantly among regions tested (significant main effect of region by two-way ANOVA). As shown in Figure 1, the highest expression of PGHS-2 was in hippocampus, significantly greater than in cerebral cortex, in turn significantly greater than in pituitary, in turn significantly greater than in hypothalamus, brainstem, and cerebellum (as tested by Bonferroni test following two-way ANOVA). Interestingly, the changes in PGHS-2 expression were greatest (significant for pairwise ICI comparison by Bonferroni test) in hippocampus and pituitary, two of the three tissues that express this gene at the highest level (Figure 1). ICI did not significantly alter the expression of PGHS-1, although the expression of PGHS-1 did vary significantly among regions tested (significant main effect of region in two-way ANOVA). The greatest expression of PGHS-1 was in hippocampus and cerebral cortex, which were significantly greater than in pituitary, in turn significantly greater than in brainstem and hypothalamus, in turn significantly greater than in cerebellum (significant as tested by Bonferroni test following two-way ANOVA, Figure 1).
Figure 1.
PGHS-1 (top panel) and PGHS-2 (bottom panel) mRNA abundance in cerebellum, brainstem, hypothalamus, pituitary, hippocampus, and cerebral cortex in twin fetuses treated with 25 μg/day of ICI 182,780 (grey bars, n=6) or vehicle (black bars, n=6). Data are represented as mean±SEM. *, statistically significant difference between groups. Letters appearing over pairs of vertical bars represent significant differences in PGHS-1 and -2 expression among regions. Different letters represent statistically significant differences.
Plasma Hormones and Blood Gases in Study 1
Treatment with ICI did not produce significant changes in any of the measured plasma hormone concentrations except for estradiol (Table 2). The plasma estradiol concentrations were slightly lower in the female fetuses when analyzed using an ANOVA that accounted for both gender and ICI treatment. There were no differences in arterial oxygen tension (18.2±0.9 and 17.7±1.2 mm Hg, in vehicle- and ICI-treated fetuses, respectively), arterial carbon dioxide tension (56.4±0.8 and 56.9±0.9 mm Hg in vehicle- and ICI-treated fetuses, respectively) or pH (7.34±0.01 and 7.35±0.01) between groups.
Table 2.
Plasma Hormone Concentrations in ICI 182,780-treated and untreated twin fetuses. Data are reported as mean values±SEM.
| analyte | control fetus | ICI treated fetus |
|---|---|---|
| ACTH (pg/mL) | 24 ± 6 | 21 ± 2 |
| Cortisol (ng/mL) | 12.3 ± 5.9 | 9.9 ± 3.4 |
| Estradiol (pg/mL) | 35 ± 2 | 31 ± 1* |
| Estradiol-3-Sulfate (pg/mL) | 288 ± 25 | 344 ± 43 |
| DHAS (pg/mL) | 58 ± 8 | 61 ± 13 |
| Progesterone | 7.2 ± 1.1 | 7.1 ± 0.7 |
| POMC | 37.1 ± 10.1 | 30.7 ± 2.9 |
p<0.05 compared to control fetuses
mRNA Expression of Hypothalamic and Pituitary Genes in Study 1
Consistent with the lack of change of plasma concentrations of ACTH and cortisol, there were no differences in expression of CRH or AVP in the hypothalamus, or of POMC or PC1 in the pituitary (Table 3). There were also no statistically significant changes in luteinizing hormone (oLH), follicle stimulating hormone (oFSH), or prolactin (PRL) mRNA, although there was an appearance (not statistically significant) of an increase (0.20>p>0.05) in oFSH mRNA.
Table 3.
Fold changes in mRNA abundance relative to control fetus. There were no statistically significant differences between groups for any of the measured genes. Data are reported as mean values±SEM.
| gene | tissue | control fetus | ICI treated fetus |
|---|---|---|---|
| (fold change from mean control value) | |||
| CRH | Hypothalamus | 1.16 ± 0.32 | 1.91 ± 0.91 |
| AVP | Hypothalamus | 1.27 ± 0.37 | 1.78 ± 0.99 |
| PC1 | Pituitary | 1.04 ± 0.13 | 1.18 ± 0.11 |
| POMC | Pituitary | 1.14 ± 0.11 | 1.17 ± 0.30 |
| oLH | Pituitary | 1.24 ± 0.21 | 1.46 ± 0.47 |
| oFSH | Pituitary | 1.07 ± 0.17 | 2.43 ± 0.54 |
| oPRL | Pituitary | 1.43 ± 0.51 | 1.31 ± 0.27 |
| sgk-1 | Pituitary | 1.03 ± 0.12 | 1.09 ± 0.27 |
| sgk-1 | Hippocampus | 1.01 ± 0.06 | 1.33 ± 0.16 |
Effect of Chronic ICI 182780 icv on Preparturient Plasma HPA axis Hormone concentrations in Study 2
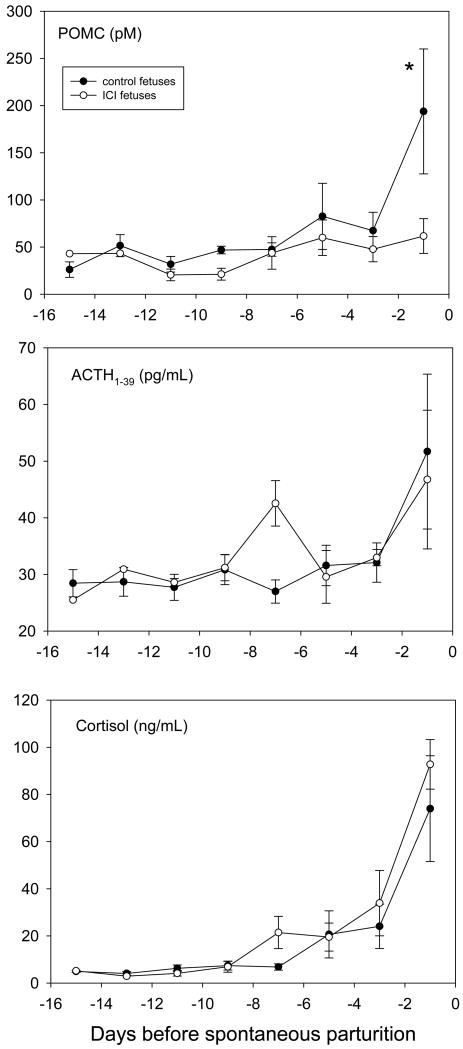
Chronic icv infusion of ICI 182,780 into singleton fetuses throughout the last 2 weeks of gestation produced statistically significant reductions in plasma concentrations of pro-opiomelanocortin (POMC) compared to fetuses receiving chronic infusion of vehicle icv (Figure 3, as tested by two-way ANOVA). The preparturient increase in ACTH1-39 and cortisol were not reduced in ICI 182,780-treated fetuses compared to vehicle-treated fetuses. Spontaneous parturition in ICI 182,780-treated fetuses occurred at 141±3 and in vehicle-treated fetuses at 144±1 days (not significantly different as tested by Student's t-test).
Discussion
We have proposed that a positive feedback loop exists between placental production of estradiol and increased activity of the HPA axis near parturition [1]. The synthesis of estrogen by the placenta near term is stimulated by cortisol, in turn secreted by the adrenal gland. Cortisol induces 17α-hydroxylase and 17,20 lyase activities in the placenta [14;27;28] which enables estrogens and androgens to be synthesized and secreted into the fetal and maternal circulations which, according to our hypothesis, further enhances HPA activity. We and others have demonstrated that PGE2 stimulates ACTH secretion, and that endogenous prostanoids modulate the fetal HPA responsiveness to hypotension stress [6-10]. Exogenous estradiol increases PGHS-2 mRNA abundance in the fetal brain [3] as it does in other tissues [13] and estradiol stimulates concomitant increases in fetal HPA axis activity [3;29]. In a previous study, the increase in PGHS-2 and HPA axis activity was stimulated by estradiol despite the well-known inhibitory effect of glucocorticoid on PGHS-2 gene expression [3;30]. The results of the present study do not specifically link PGHS-2 activity and fetal HPA axis activity, but they do demonstrate that in late gestation at a time before the largest increases in fetal cortisol and estrogen plasma concentrations, there is a tonic stimulatory influence of estrogen on fetal brain and pituitary PGHS-2 expression. The effect of the estrogen receptor blockade on fetal HPA activity was not evident at 131-134 days gestation, but was observed only later in gestation, in the final 1-2 days of fetal life, as an inhibition of circulating proopiomelanocortin (POMC) concentrations. It is unclear whether a higher dose of the inhibitor would have more fully suppressed the fetal HPA axis, possibly reducing fetal ACTH1-39 and cortisol as well as POMC.
The results of the present study are consistent with our previously-stated hypothesis that there is a positive feedback cycle between placental estrogen biosynthesis and fetal HPA axis activity. The site and mechanism of this interaction is unclear: circulating estrogen could possibly act at the pituitary, in the central nervous system, or both. And while the results of this and previous experiments [3] are consistent with the conclusion that there is a tonic effect of estrogen on PGHS-2 expression in brain and pituitary, there is no assurance that the effect of estrogen on fetal HPA axis activity is actually mediated by its effect on PGHS-2. Experiments performed in this laboratory so far suggest that PGHS-2 affects the fetal HPA axis in ways that are time-dependent. Inhibition of this enzyme by injection of nimesulide into the lateral cerebral ventricle increases fetal ACTH secretion [31]. Infusion of nimesulide into the fetal brain for longer periods of time inhibits fetal HPA axis activity (J. Gersting and C.E. Wood, unpublished observations). Nevertheless, experiments will be required to specifically test whether the stimulatory effect of estrogen on fetal HPA axis activity is mediated by PGHS-2 activity.
PGHS-2 expression is upregulated by inflammatory mediators in various tissues, and is often referred to as the inducible form of prostaglandin synthase [32]. We and others, however, have reported expression of PGHS-2 in normal uncompromised brain tissue [4;5;33;34]. While it is logical to speculate that the expression of this enzyme is constitutive in brain [33], the present results suggest that the expression is influenced by circulating estrogens. Interestingly, there appears to be a concordance between the expression of estrogen receptors and PGHS-2 in various brain regions (C.E. Schaub, J. Gersting, and C.E. Wood, unpublished observations), suggesting that the abundance of PGHS-2 might be at least partly influenced by both circulating estrogen concentrations and receptor abundance.
Pituitary expressed high levels of both PGHS-1 and -2 mRNA. The ICI compound decreased the abundance of the PGHS-2 mRNA in this gland, suggesting that the inhibitor might have gained access to the pituitary in meaningful concentrations via the hypothalamo-hypophyseal portal vasculature. It is seems less likely that the expression of the enzyme was reduced secondary to a reduction in the secretion of hypothalamic releasing factors because we did not find any statistically significant effect of the ICI compound on POMC, oLH, oFSH, or oPRL mRNA's. Nevertheless, we cannot be certain that this is not a secondary effect without a more complete investigation of both pituitary and hypothalamic hormone synthesis and secretion. Although we do not fully understand the physiological roles of PGHS-1 and -2 in the pituitary, we do suspect that one or both of these enzymes influences ACTH secretion. PGHS-1 expression is at least partially co-localized in corticotropes, and PGHS-2 expression is often found in cells adjacent to the corticotropes [35]. Brooks and colleagues have previously reported that PGE2 enhances the ACTH response to AVP [36]. Interestingly, estrogen appears to downregulate CRH expression. We have previously reported that physiological increases in fetal plasma estradiol concentrations do not significantly change hypothalamic CRH content [18]. Nevertheless, tamoxifen treatment of fetal sheep increases hypothalamic CRH [18], reminiscent of the stimulatory effect of ICI 182,780 on CRH gene expression in the placenta [21].
The lack of effect of ICI treatment on HPA axis activity in Study 1 combined with the reduction in POMC plasma concentrations in Study 2 suggests that the influence of estrogen on the HPA axis might be more important during the preparturient increase in fetal HPA axis activity than at earlier times. The partial nature of the inhibition of the HPA axis (POMC was reduced, but not ACTH1-39 or cortisol) suggests the possibility that a higher dose of ICI 182,780 might have been more effective. One difference between the two studies is the use of twins in study 1 and singleton fetuses in study 2. A possible drawback in the use of twins is the potential for both twins to be affected by the drug treatment. We do not consider this to be likely in the present study, given the central route of administration, the low dose of ICI 182,780 and the relatively large volume of distribution of both fetuses and ewe.
We conclude that the expression of PGHS-2, but not PGHS-1, in brain and pituitary of late-gestation fetal sheep, is in part influenced by estrogen. While increases in prostaglandin biosynthesis are known to stimulate fetal HPA axis activity, our data suggest that estradiol does not tonically stimulate fetal HPA axis activity between 130 and 134 days gestation but that it does at least partially account for the increase in fetal HPA axis activity in the final days prior to parturition. These results are consistent with previous data from this laboratory demonstrating that estradiol augments PGHS-2 expression [3] and consistent with our previously-stated hypothesis that there is a positive feedback cycle involving placental estrogen production and fetal HPA axis activity [1].
Figure 2.
Fetal plasma hormone concentrations in singleton fetuses in study 2 treated with 25 μg/day of ICI 182,780 (open symbols, n=5) or vehicle (filled symbols, n=5). Proopiomelanocortin is abbreviated POMC in the top panel. Data are represented as mean±SEM. *, statistically significant difference between groups.
Acknowledgments
This work was supported by NIH grant HD42135 to CEW. We thank Ms. Xiaoyang Fang for her outstanding technical contributions.
Reference List
- 1.Wood CE. Estrogen/hypothalamus-pituitary-adrenal axis interactions in the fetus: The interplay between placenta and fetal brain. J Soc Gynecol Investig. 2005;12:67–76. doi: 10.1016/j.jsgi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Deauseault D, Giroux D, Wood CE. Ontogeny of Immunoreactive Prostaglandin Endoperoxide Synthase Isoforms in Ovine Fetal Pituitary, Hypothalamus and Brainstem. Neuroendocrinology. 2000;71:287–291. doi: 10.1159/000054548. [DOI] [PubMed] [Google Scholar]
- 3.Wood CE, Giroux D. Central nervous system prostaglandin endoperoxide synthase-1 and -2 responses to oestradiol and cerebral hypoperfusion in late-gestation fetal sheep. J Physiol. 2003;549:573–581. doi: 10.1113/jphysiol.2002.038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong H, Richards E, Wood CE. Prostaglandin endoperoxide synthase-2 abundance is increased in brain tissues of late-gestation fetal sheep in response to cerebral hypoperfusion. J Soc Gynecol Investig. 1999;6:127–135. doi: 10.1016/s1071-5576(99)00012-x. [DOI] [PubMed] [Google Scholar]
- 5.Tong H, Gridley KE, Wood CE. Induction of immunoreactive prostaglandin H synthases 1 and 2 and fos in response to cerebral hypoperfusion in late-gestation fetal sheep. J Soc Gynecol Investig. 2002;9:342–350. [PubMed] [Google Scholar]
- 6.Cudd TA, Wood CE. Prostaglandin E2 releases ovine fetal ACTH from a site not perfused by the carotid vasculature. Am J Physiol. 1992;263:R136–R140. doi: 10.1152/ajpregu.1992.263.1.R136. [DOI] [PubMed] [Google Scholar]
- 7.Thorburn GD. The placenta, PGE2 and parturition. Early Hum Dev. 1992;29:63–73. doi: 10.1016/0378-3782(92)90059-p. [DOI] [PubMed] [Google Scholar]
- 8.Young IR, Deayton JM, Hollingworth SA, Thorburn GD. Continuous intrafetal infusion of prostaglandin E2 prematurely activates the hypothalamo-pituitary-adrenal axis and induces parturition in sheep. Endocrinology. 1996;137:2424–2431. doi: 10.1210/endo.137.6.8641195. [DOI] [PubMed] [Google Scholar]
- 9.Cudd TA, Wood CE. Does intracarotid PGE2 increase plasma ACTH concentration in concious adult ewes. Am J Physiol. 1991;261:E395–E401. doi: 10.1152/ajpendo.1991.261.3.E395. [DOI] [PubMed] [Google Scholar]
- 10.Tong H, Lakhdir F, Wood CE. Endogenous prostanoids modulate the ACTH and AVP responses to hypotension in late-gestation fetal sheep. Am J Physiol. 1998;275:R735–R741. doi: 10.1152/ajpregu.1998.275.3.R735. [DOI] [PubMed] [Google Scholar]
- 11.Cudd TA, Wood CE. Thromboxane A2 receptor antagonism prevents the hormonal and cardiovascular responses to mineral acid infusion. Am J Physiol. 1994;267:R1235–R1240. doi: 10.1152/ajpregu.1994.267.5.R1235. [DOI] [PubMed] [Google Scholar]
- 12.Cudd TA, Wood CE. Prostanoid cascade inhibition prevents cardiovascular and adrenocorticotropic responses to mineral acid infusion. Am J Physiol. 1993;264:R1235–R1241. doi: 10.1152/ajpregu.1993.264.6.R1235. [DOI] [PubMed] [Google Scholar]
- 13.Wu WX, Wolf R, Chakrabarty K, Collins V, Unno N, Nathanielsz PW, Rose JC. Induction of uterine prostaglandin H synthase 2 by estradiol following fetal adrenalectomy. Endocrine. 2005;26:153–159. doi: 10.1385/ENDO:26:2:153. [DOI] [PubMed] [Google Scholar]
- 14.Steele PA, Flint APF, Turnbull AC. Activity of steroid C-17,20 lyase in the ovine placenta: Effect of exposure to foetal glucocorticoid. J Endocr. 1976;69:239–246. doi: 10.1677/joe.0.0690239. [DOI] [PubMed] [Google Scholar]
- 15.Liggins GC, Fairclough RJ, Grieves SA, Kendall JZ, Knox BS. The mechanism of initiation of parturition in the ewe. Rec Prog Horm Res. 1973;29:111–159. doi: 10.1016/b978-0-12-571129-6.50007-5. [DOI] [PubMed] [Google Scholar]
- 16.Saoud CJ, Wood CE. Modulation of ovine fetal adrenocorticotropin secretion by androstenedione and 17beta-estradiol. Am J Physiol. 1997;272:R1128–R1134. doi: 10.1152/ajpregu.1997.272.4.R1128. [DOI] [PubMed] [Google Scholar]
- 17.Wood CE, Saoud CJ. Influence of estradiol and androstenedione on ACTH and cortisol secretion in the ovine fetus. J Soc Gynecol Investig. 1997;4:279–283. [PubMed] [Google Scholar]
- 18.Wood CE, Saoud CJ, Stoner TA, Keller-Wood M. Estrogen and androgen influence hypothalamic AVP and CRF concentrations in fetal and adult sheep. Regul Pept. 2001;98:63–68. doi: 10.1016/s0167-0115(00)00231-7. [DOI] [PubMed] [Google Scholar]
- 19.Kitay JI. Pituitary-adrenal function in the rat after gonadectomy and gonadal mormone replacement. Endocrinology. 1963;73:253–260. doi: 10.1210/endo-73-2-253. [DOI] [PubMed] [Google Scholar]
- 20.Coyne MD, Kitay JI. Effects of ovariectomy on pituitary secretion of ACTH. Endocrinology. 1969;85:1097–1102. doi: 10.1210/endo-85-6-1097. [DOI] [PubMed] [Google Scholar]
- 21.Wood CE, Gridley KE, Keller-Wood M. Biological activity of 17beta-estradiol-3-sulfate in ovine fetal plasma and uptake in fetal brain. Endocrinology. 2003;144:599–604. doi: 10.1210/en.2002-220764. [DOI] [PubMed] [Google Scholar]
- 22.Wood CE, Cudd TA, Kane C, Engelke K. Fetal ACTH and blood pressure responses to thromboxane mimetic U46619. Am J Physiol. 1993;265:R858–R862. doi: 10.1152/ajpregu.1993.265.4.R858. [DOI] [PubMed] [Google Scholar]
- 23.Myers DA, Bell PA, Hyatt K, Mlynarczyk M, Ducsay CA. Long-term hypoxia enhances proopiomelanocortin processing in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1178–R1184. doi: 10.1152/ajpregu.00697.2004. [DOI] [PubMed] [Google Scholar]
- 24.Ali NS, Keller-Wood M, Wood CE. Ontogenetic changes in the extra-pituitary expression of pro-opiomelanocortin in the developing ovine fetus. Peptides. 2005;26:301–306. doi: 10.1016/j.peptides.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Keller-Wood M, Wood CE, Hua Y, Zhang D. Mineralocorticoid receptor expression in late-gestation ovine fetal lung. J Soc Gynecol Investig. 2005;12:84–91. doi: 10.1016/j.jsgi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Keller-Wood M, Powers MJ, Gersting JA, Ali N, Wood CE. Genomic analysis of neuroendocrine development of fetal brain-pituitary-adrenal axis in late gestation. Physiol Genomics. 2006;24:218–224. doi: 10.1152/physiolgenomics.00176.2005. [DOI] [PubMed] [Google Scholar]
- 27.Anderson ABM, Flint AP, Turnbull AC. Mechanism of activation of glucocorticoids in induction of ovine parturition: Effect on placental steroid metabolism. J Endocr. 1975;66:61–70. [PubMed] [Google Scholar]
- 28.France JT, Magness RR, Murry BA, Rosenfeld CR, Mason JI. The regulation of ovine placental steroid 17 alpha-hydroxylase and aromatase by glucocorticoid. Mol Endocrinol. 1988;2:193–199. doi: 10.1210/mend-2-3-193. [DOI] [PubMed] [Google Scholar]
- 29.Purinton SC, Wood CE. Oestrogen augments the fetal ovine hypothalamus-pituitary-adrenal axis in response to hypotension. J Physiol. 2002;544:919–929. doi: 10.1113/jphysiol.2002.025635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goppelt-Struebe M. Molecular mechanisms involved in the regulation of prostaglandin biosynthesis by glucocorticoids. Biochem Pharmacol. 1997;53:1389–1395. doi: 10.1016/s0006-2952(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 31.Reimsnider S, Wood CE. Differential modulation of ovine fetal ACTH secretion by PGHS-1 and PGHS-2. Neuroendocrinology. 2006;83:4–11. doi: 10.1159/000093177. [DOI] [PubMed] [Google Scholar]
- 32.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 33.Breder CD, DeWitt DL, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong H, Dhillon H, Wood CE. Expression of PGHS-2 mRNA and protein in ovine fetal brain regions in response to cerebral hypoperfusion. Prostaglandins and Other Lipid Mediators. 2000;62:165–172. doi: 10.1016/s0090-6980(00)00064-2. [DOI] [PubMed] [Google Scholar]
- 35.Reimsnider SK, Wood CE. Colocalisation of Prostaglandin Endoperoxide Synthase and Immunoreactive Adrenocorticotropic Hormone in Ovine Foetal Pituitary. J Endocr. 2004;180:303–310. doi: 10.1677/joe.0.1800303. [DOI] [PubMed] [Google Scholar]
- 36.Brooks AN, Gibson F. Prostaglandin E2 enhances AVP-stimulated but not CRF-stimulated ACTH secretion from cultured fetal sheep pituitary cells. J Endocrinol. 1992;132:33–38. doi: 10.1677/joe.0.1320033. [DOI] [PubMed] [Google Scholar]