Abstract
The present study tested the effect of ketamine on the fetal reflex responses of late-gestation sheep to brachiocephalic occlusion (BCO), a stimulus that mimics the reduction in cerebral blood flow that results from severe fetal hypotension. Ketamine, a dissociative anesthetic and known non-competitive antagonist of N-methyl D-aspartate (NMDA) receptors, has previously been shown to impair chemoreceptor responsiveness. Studies from this laboratory suggest that fetal reflex ACTH responses to hypotension are largely mediated by chemoreceptors; therefore we hypothesized that ketamine would inhibit the reflex hormonal response to BCO. Chronically catheterized fetal sheep were subjected to acute cerebral hypoperfusion through occlusion of the brachiocephalic artery. Fetal blood pressure and heart rate were continuously recorded and fetal blood samples drawn during the experiment were analyzed with specific hormone assays. Our results demonstrate that ketamine attenuates hemodynamic responses to cerebral hypoperfusion and is a potent inhibitor of adrenocorticotropin (ACTH) and proopiomelanocortin (POMC) / pro-ACTH release. These data support the hypothesis that fetal reflex responses hypotension are chemoreceptor mediated. Given the potency with which ketamine inhibits ACTH response to fetal hypotension, we suggest that the use of ketamine, or other anesthetic or analgesic drugs that block or otherwise interact with the NMDA-glutamate pathways, in late pregnancy or in pre-term newborns be reconsidered.
Keywords: chemoreceptor, baroreceptor, adrenocorticotropin, proopiomelanocortin, NMDA, glutamate
INTRODUCTION
Fetal sheep respond to hypoxia (7; 17; 18), hypotension (57; 65), and hypovolemia (43; 44) with increases in circulating concentrations of adrenocorticotropin (ACTH), vasopressin, and cortisol via activation of the hypothalamic-pituitary-adrenal (HPA) axis. In both the fetus and adult, neuroendocrine activation to hypotension and hypoxemia is mediated by changes in afferent neural activity of arterial baroreceptors and chemoreceptors (40; 50; 57; 65; 69). Data from our laboratory indicate that hormone responses in the fetus might be mediated more strongly through chemoreceptors than baroreceptors (67).
The apparent dependence of the fetus on peripheral chemoreceptors for generation of reflex responses to central hypotension and hypoxemia suggests that the responses will be vulnerable to drugs that inhibit the fetal chemoreflex. Ketamine, a dissociative anesthetic and known non-competitive inhibitor of glutamatergic N-methyl-d-aspartate (NMDA) receptors, blocks the fetal reflex bradycardic response to maternal ventilatory hypoxia (8). Given that neurotransmission within cardiovascular centers of the brainstem are largely glutamatergic and mediated by both NMDA and non-NMDA receptors (48), we reasoned that ketamine might block the reflex ACTH response to cerebral hypoperfusion in the fetal lamb, similarly to the effect produced by denervation of baroreceptors and chemoreceptors. Cerebral hypoperfusion produced by brachiocephalic occlusion (BCO) is a stimulus that mimics the reduction in cerebral blood flow that results from severe fetal hypotension and potently stimulates the hypothalamic-pituitary-adrenal axis (58). We designed the present study to test the hypothesis that ketamine blocks the reflex ACTH response to BCO.
MATERIALS AND METHODS
These experiments were approved by the University of Florida Animal Care and Use Committee and were performed in accordance with the Guiding Principles for Use of Animals of the American Physiological Society. We studied 18 fetal sheep of known gestational ages. Pregnant ewes of mixed western breeds all had singleton (n = 11) and twin (n = 7) pregnancies of 124–135 days (term = 148) at the time of experimentation.
Fetal surgery
Fetal surgery was performed as previously described (Tong & Wood, 1999). Food was withheld from the pregnant ewe for 24 hours before surgery. Before and during surgery, the ewe was anesthetized using 0.5 – 2% halothane in oxygen. Using aseptic techniques, the uterus was exposed with a midline incision. The fetal hindlimbs were delivered through a small incision in the uterus and a polyvinylchloride catheter (0.030 in i.d., 0.050 in o.d.) was inserted into each tibial artery and the tip advanced to the subdiaphramatic aorta. Catheters (.040 in i.d., .070 in o.d.) were also inserted into the saphenous veins bilaterally. After closure of the skin incision, the amniotic fluid was catheterized using a polyvinylchloride catheter (0.050 in i.d., 0.090 in o.d.) sutured to the exterior of one hindlimb. The hindlimb was returned to the amniotic cavity and the uterine incision was closed. The uterus was next incised near the fetal head. The head was delivered and a single midline incision was made over the trachea at the level of the angle of the jaw. Both lingual arteries were exposed and catheterized (0.030 i.d., 0.050 in o.d.) and the tips advanced retrograde into the common carotid arteries. The incision was closed and the catheters sutured to the skin rostral to the incision. The left forelimb was then delivered and the chest exposed to the level of the third intercostal space. The left side of the fetal chest was incised, the ribs spread between the 2nd and 3rd intercostal space using Weatlander retractors. An extravascular balloon occluder (In Vivo Metric, 8 mm diameter, Healdsburg, CA, USA) was then placed around the brachiocephalic artery. The incision was then closed, the fetus returned to the uterus, and the uterine incision was closed. Ampicillin (750 mg, Polyflex, Ft. Dodge Laboratories, Ft. Dodge, IA, USA) was administered into the amniotic cavity before closure of the maternal linea alba and skin in separate layers. The catheters were routed subcutaneously to an incision in the maternal flank where were protected within a fabric pocket that was kept in place underneath a commercial bandage wrapped around the ewe (Spandage, Medi-Tech International, Brooklyn, NY, USA).
All ewes were treated with ampicillin (750 mg, sq), and rectal temperatures and food consumption were recorded twice a day for five days following the surgery. The ewes were monitored for fever, anorexia, lethargy, and other signs of infection or distress.
In vivo experimental procedures
Thirty minutes prior to the experiment, the pregnant ewe was moved to an experimental cart within the room in which it was housed and was allowed free access to food. Each fetus was subjected to one experiment. One lingual, one aortic, and the amniotic fetal catheter were connected to transducers (Cobe Instruments, Lakewood, CO) for measurement of fetal arterial and amniotic fluid pressures and heart rate from femoral arterial pressure pulse. Lingual arterial pressure was measured in order to verify occlusion. Measurements were transmitted to an on-line data acquisition system (Labview version 6.01, National Instruments, Austin, TX). Lingual, femoral arterial, and amniotic fluid pressures were recorded for a total of 35 minutes for the control group and 50 minutes for ketamine-treated fetuses. One-minute averages of fetal heart rate and vascular and amniotic pressures were recorded. In fetuses that received ketamine, a blood sample was drawn before the drug was administered in order to determine basal hormone values. Ten minutes prior to occlusion a subset of animals received 10 mg ketamine hydrochloride (Ketaject ®, estimated to be approximately 3mg/kg, Phoenix Scientific Inc., St. Joseph MO, USA) infused into the venous catheter (n = 4). A blood sample was drawn from control animals prior to BCO but no drug was administered (n = 14). In both groups, cerebral hypoperfusion was initiated by maximal inflation of the brachiocephalic occluder with 2 mL saline starting at 0 minutes and lasting 10 minutes. The second fetal femoral catheter was used to collect fetal arterial blood samples (5mL) at 0, 5, 10, 20, and 30 minutes. In twin pregnancies only one of the twins underwent occlusion. In the ketamine group, 3 of the 4 fetuses had twins, and in the control group, 4 of the 14 fetuses had twins.
Blood samples were placed in chilled tubes containing K2EDTA (10.8 mg, Vacutainer, Becton Dickinson, Franklin Lakes, NJ, USA). An additional 1.5 mL of blood was drawn anaerobically into syringes coated with heparin for measurement of blood gases using an ABL77 analyzer (Radiometer, Copenhagen, Denmark). Blood samples were kept on ice until centrifuged at 3000 × g for 20 minutes at 4° C (Sorvall RT 6000B, Dupont, Newton, CA, USA). After centrifugation, the plasma was divided into aliquots, transferred to polypropylene tubes, and stored at −20 °C until hormones were assayed.
Hormone assays
Adrenocorticotropin (ACTH)
Plasma ACTH concentrations were measured using a commercially available immunoradiometric assay (Diasorin, Stillwater, MN, USA) according to manufacturer’s instructions. Polystyrene beads coated with a purified polyclonal goat antibody specific for ACTH26–39 was incubated for 24 hours at room temperature with fetal plasma (200µL) and 125I ACTH tracer (50µL) labeled monoclonal antibody specific for ACTH1–17. The unbound radioactivity was then washed with a provided buffer and the bound radioactivity measured with a gamma counter. As characterized by Myers and colleagues, this assay measures only ACTH1–39 (31).
Pro-opiomelanocortin (POMC) / pro-ACTH
Plasma POMC / pro-ACTH concentrations were measured using a commercially available enzyme immunoassay kit (IDS Ltd. Boldon, UK) as per manufacturer’s instructions. This assay recognizes both POMC (31kD) and pro-ACTH (22kD). Fetal plasma samples (100µL) were assayed in duplicate in an anti-mouse POMC monoclonal antibody pre-coated 96 well microplate. The samples were incubated at room temperature overnight in buffer containing BSA and heparin. The plate was then washed with buffer provided by the manufacturer and incubated for 2 hours at room temperature with biotinylated anti-POMC mouse monoclonal antibody. The plate was washed again before incubation for 30 minutes at room temperature with avidin-linked horseradish peroxidase enzyme conjugate. After a final wash, tetramethylbenzidine plus hydrogen peroxide substrate was added for thirty minutes and the reaction was stopped with 0.5M HCl. The plate was then read at 450nM on a microplate reader (Tecan Group Limited, Salsburg, Austria).
Cortisol
Plasma cortisol concentrations were measured using a commercially available enzyme immunoassay (EIA) kit (Oxford Biomedical Research, Oxford, MI, USA, catalog number EA65) according to manufacturer’s instructions. Fetal cortisol was extracted from plasma (10µL) after deproteinization in ethanol (1mL) in borosilicate glass test tubes (12×75 mm). After centrifugation to pellet the precipitated plasma proteins, the ethanol was evaporated in a Jouan evaporative concentrator (Jouan, Inc., Winchester, VA, USA). The extracted steroids were reconstituted in the provided assay buffer (120 µL). Samples (50 µL) were assayed in duplicate in an anti-rabbit antibody pre-coated 96 well microplate using rabbit anti-cortisol horseradish peroxidase concentrate enzyme conjugate for one hour at room temperature. After washing with the provided buffer the plate was developed for 30 minutes with tetramethylbenzidine plus hydrogen peroxide substrate and the reaction was stopped with 1 N HCl. The plate was then read at 450 nM, as described above.
Calculations and Statistics
Data are presented as mean values ± SE. Fetal lingual and femoral arterial blood pressures were corrected by subtraction of amniotic fluid pressure. For analysis of acute fetal heart rate responses to BCO, heart rate averages were calculated in 10-second bins off-line. Acute changes in fetal heart rate were calculated as the difference between the average heart rate in the first ten seconds of the occlusion and the average heart rate in the ten seconds immediately preceding the occlusion. Cortisol values were logarithmically transformed to correct for heteroscedasticity. Unless stated, plasma hormone, blood gas/pH, blood pressure and heart rate data were analyzed by two-way ANOVA corrected for repeated measures in one dimension (time), and if significant, by Bonferroni criterion. All statistics were performed using SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Cardiovascular variables
Fetal arterial blood gases, pH and base excess are reported in Table I. In control fetuses, brachiocephalic occlusion stimulated increases in PaO2 (P< 0.001) and PaCO2 (P< 0.05) at 5 minutes, presumably due to increased perfusion of the placenta. In addition, changes in pH following BCO were statistically significant (P< 0.001). Fetuses receiving ketamine prior to occlusion became progressively hypoxic compared to control fetuses (P< 0.05). Base excess decreased in both groups (p<0.05 for main effect of time in two-way ANOVA), suggesting that BCO produces metabolic acidemia. Interestingly, there was a significant difference between groups (p<0.05 for main effect of group in two-way ANOVA), but no significant interaction term in the ANOVA, indicating that the changes in base excess were similar in the two groups throughout the period of study. Ketamine also modestly increased arterial blood pressure prior to BCO (p<0.05, data not shown).
Table I.
Fetal blood gases, pH and base excess §
| PaO2 (mmHg) | PaCO2 (mmHg) | pH | Base Excess (mEq/L) | |||||
|---|---|---|---|---|---|---|---|---|
| Time (min) |
Control | Ket | Control | Ket | Control | Ket | Control | Ket |
| 0 | 19.92 ± 0.96 | 19.00 ± 0.58 | 56.15 ± 0.93 | 58.25 ± 1.89 | 7.35 ± 0.01 | 7.37 ± 0.01 | 4.43 ± 0.51 | 6.88± 0.38 |
| 5 | 22.85 ± 1.31 | 18.75 ± 1.03 | 53.00 ± 0.75 | 56.25 ±1.11 | 7.35 ± 0.01 | 7.37 ±0.003 | 3.44 ± 0.52 | 5.68 ± 0.58 |
| 10 | 20.23 ± 1.49 | 17.25 ± 0.48 | 57.54 ± 1.30 | 56.50 ± 0.96 | 7.32 ± 0.01 | 7.36 ±0.004 | 2.43 ± 0.53 | 5.40 ± 0.27 |
| 20 | 20.00 ± 0.88 | 16.25 ±0.85 | 56.31 ± 1.03 | 57.25 ± 1.80 | 7.32 ± 0.01 | 7.36 ± 0.01‡ | 2.24 ± 0.64 | 5.37 ± 0.71 |
| 30 | 19.69 ± 1.08 | 15.75 ±0.75 | 55.77 ± 0.94 | 52.50 ± 3.84 | 7.33 ± 0.01 | 7.36 ±0.003 | 2.64 ± 0.56 | 3.03 ± 2.03 |
Data presented as mean ± SE. (n = 13) of control measurements and (n = 4) of ketamine-treated measurements taken at 0, 5, 10, 20, and 30 minutes (brachiocephalic occlusion occurs between 0 and 10 minutes).
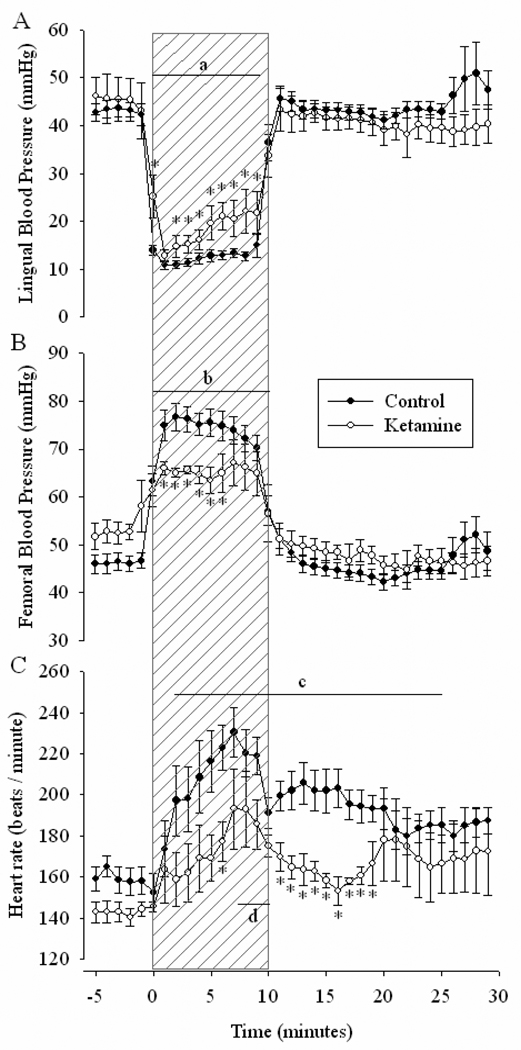
During BCO, a significant decrease in lingual blood pressure (P< 0.001, Figure 1 A) and increase in femoral pressure was observed in the control group (P< 0.001 Figure 1 B). Similar alterations in blood pressure were observed in ketamine-treated fetuses, however, both lingual and femoral responses were attenuated compared to the control group (P< 0.05).
Figure 1. Fetal lingual and femoral blood pressures and heart rate.
The lingual and femoral arterial blood pressures and heart rate of fetal sheep before, during and after a 10-minute period of brachiocephalic occlusion (indicated by hatched areas) with (open circles, n = 14) or without (closed circles, n = 4) pre-treatment with 3mg/kg ketamine. Data are presented as mean values ± SE. Baseline is considered to be at −5 minutes. A. a P < 0.001 During occlusion, lingual pressure significantly decreased from baseline in both groups. * P < 0.05 Significant interaction of time × group. B. b P < 0.001 Femoral pressure increased significantly from baseline during occlusion in both groups. * P < 0.05 Significant interaction of time ×group. C. c P < 0.001 Fetal heart rate significantly increased from baseline during and after occlusion in control fetuses. d P < 0.05 Heart rate increased above baseline in ketamine-treated fetuses. * P < 0.05 Significant interaction of time × group.
Control fetuses experienced a biphasic heart rate response to BCO. An initial transient bradycardia occurred during the first 10 seconds of occlusion (−15±4.2 min−1, P< 0.01 by paired t-test) and continued in 9 of 14 fetuses for up to 3 minutes. This phase was followed by a sustained tachycardia that continued through minute 23 of the experiment (Figure 1 C). By comparison, heart rate in ketamine-treated fetuses did not significantly change during the onset of BCO (0±0.4 min−1, P> 0.05) and significantly increased only during the last minutes of hypotension (P< 0.05). Heart rate in this group was significantly lower than control fetuses for approximately 10 minutes after the occlusion was released.
Endocrine variables
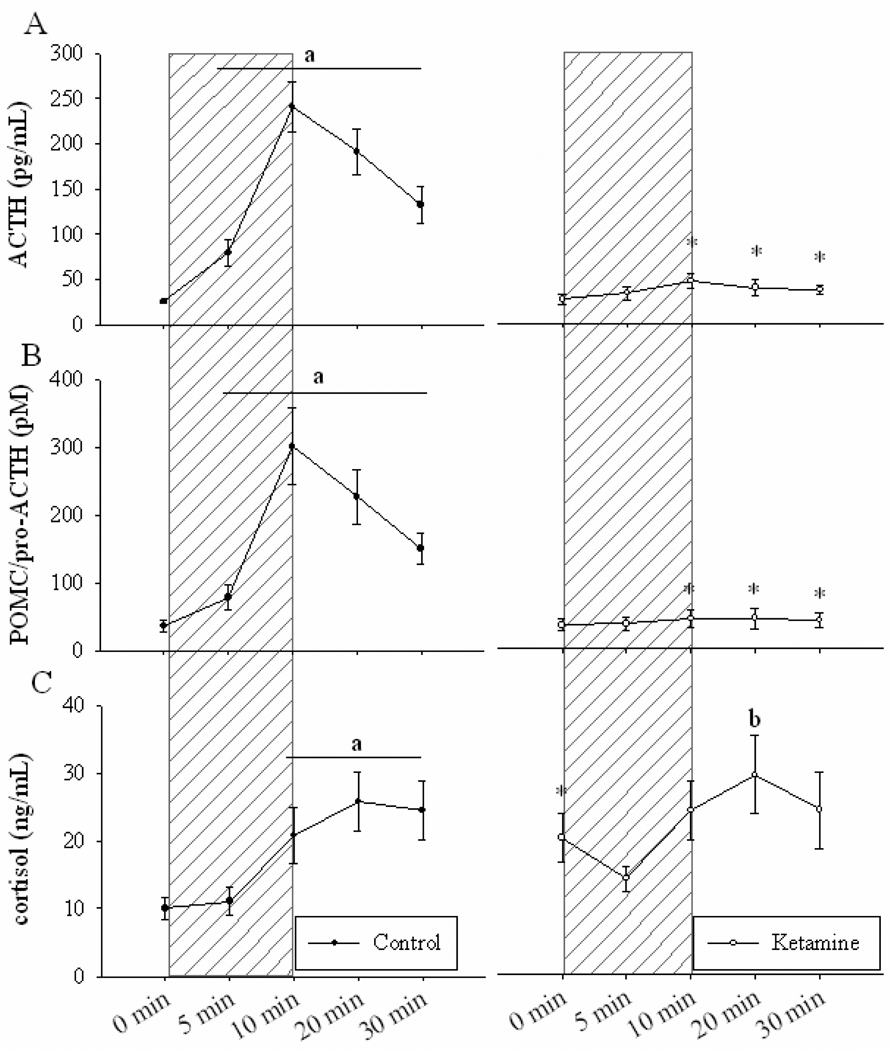
Ketamine did not alter pre-BCO plasma hormone concentrations. ACTH and POMC / pro-ACTH levels prior to drug injection were 28.2 ± 5.5 pg/mL and 26.4 ± 3.6 pM, respectively (mean ± SE). Plasma cortisol concentration before ketamine infusion was 17.8 ± 3.8 ng/mL (mean ± SE). Compared to control fetuses, cortisol levels in ketamine-treated fetuses were significantly higher prior to BCO (0 minutes) (P < 0.001, Figure 2 C).
Figure 2. Fetal plasma concentrations of adrenocorticotropin, proopiomelanocortin, and cortisol.
Fetal plasma ACTH (A), POMC / pro-ACTH (B), and cortisol (C) before, during and after a 10-minute period of brachiocephalic occlusion. Hatched area is period of cerebral hypoperfusion. Mean values of hormones ± SE in control (open circles, n = 14) and 3mg/kg ketamine-treated (closed circles, n = 4) fetuses. a P < 0.001 Significant increase from control 0 minute value. b P < 0.01 Significantly different from ketamine 5 minute value. * P < 0.001 Significant interaction of time × group.
BCO robustly increased plasma concentrations of ACTH, POMC / pro-ACTH and cortisol in the control group (P < 0.001, Figure 2). Pretreatment with ketamine effectively inhibited both the ACTH and POMC / pro-ACTH responses to BCO. Hormone values at 10, 20, and 30 minutes were significantly attenuated in ketamine-treated animals compared to controls (P < 0.001, Figure 2 A, B). Unlike ACTH or POMC / pro-ACTH, cortisol responses to BCO were not attenuated by ketamine. Plasma cortisol concentrations in ketamine-treated fetuses were significantly increased at 20 minutes compared to nadir levels occurring 5 minutes into occlusion (P < 0.01) (Figure 2 C).
DISCUSSION
In the present study, we tested the effect of ketamine (approximately 3mg/kg) on the fetal reflex responses of late-gestation sheep to brachiocephalic occlusion, a stimulus that mimics the reduction in cerebral blood flow that results from severe fetal hypotension (58). The results demonstrate that ketamine blunts the hemodynamic reflex responses to BCO and is a potent inhibitor of the ACTH and POMC / pro-ACTH release.
Fetal sheep defend challenges to cardiovascular homeostasis by neuroendocrine responses that include increases in circulating concentrations of ACTH, vasopressin, and cortisol (7; 43; 44; 57; 65). These responses are dependent on the integrity of afferent neural activity from the carotid sinus (57; 65). While it is known that both fetal baroreceptors and chemoreceptors are active during fetal life (6; 22; 71), fetal arterial blood pressure may be regulated at levels only slightly above the threshold for activation of the carotid sinus baroreceptors (5), suggesting a prominent role for the central and peripheral chemoreceptors. Secretion of ACTH, vasopressin, and renin are stimulated by fetal acidemia (66), hypercapnia (13), and hypoxia (7; 17; 39; 68) and are mediated through both central (39) and peripheral (68) chemoreceptors. In addition, progressive hemorrhage in the fetal sheep stimulates hormonal and hemodynamic responses that are more highly correlated with changes in arterial blood gases than blood pressure (67).
Central neuronal pathways mediating the chemo- and baroreflexes have been well characterized. Afferent signaling from baroreceptors and chemoreceptors is relayed to glutamatergic neurons in the nucleus of the solitary tract (NTS) of the medulla through both N-methyl-D-aspartate (NMDA) and non-NMDA receptors (59; 60) to alter autonomic nervous system responses. HPA activation is coordinated through catecholaminergic projections connecting the medulla with the paraventricular nucleus (PVN) of the hypothalamus (24; 29; 36). ACTH release in the fetus also involves NMDA receptor-mediated glutamatergic synapses (10; 33).
The dependence of the fetus on central and peripheral chemoreceptors for generation of reflex responses to cardiovascular stress suggests that the responses will be vulnerable to drugs that inhibit the fetal chemoreflex. Ketamine, a dissociative anesthetic used in adults and children, acts primarily as a non-competitive antagonist of NMDA receptors (2; 55). Although the anesthetic has reported direct negative inotropic actions on isolated heart (53) and cardiomyocyte preparations (19; 46), substantial evidence suggests that ketamine primarily affects cardiovascular function through changes in central afferent signaling via NMDA receptors within the brainstem (23; 49; 51; 63). For example, in adult rats iv ketamine attenuates blood pressure and heart rate responses to the reduction of cerebral blood flow as a result of traction of the carotid artery or direct NMDA injection into the NTS (35). In the fetal lamb, ketamine attenuates chemoreceptor responsiveness through a reduction of the bradycardic response to maternal ventilatory hypoxia (8) and prevents fetal hypertension and bradycardia produced by partial cord occlusion (54).
Arterial Pressure
BCO produced a significantly reduced arterial pressure within the carotid sinus (as measured by a fall in lingual pressure) and increased femoral pressure in both groups, similarly to previous studies (57; 58). Although the initial fall in lingual pressure of ketamine-treated fetuses was similar to controls, the average lingual pressure within the ketamine group was significantly higher, possibly the result of ketamine-induced cerebral vasodilation. Other investigators have reported that ketamine vasodilates cerebral vessels both in vivo and in vitro (41; 64). With the exception of one fetus, the range of lingual pressure values in the two groups overlapped, and the hormonal responses did not correlate to the magnitude of the drop in pressure. In addition, the reflex femoral arterial pressure response to BCO was attenuated compared to the control group. These data agree with previous studies demonstrating that ketamine suppresses afferent signaling of chemoreceptors and baroreceptors through the antagonism of central NMDA receptors.
Heart rate
The bradycardic component of chemoreflex activation is mediated by central NMDA receptors rather than other excitatory amino acid receptors (27). In this study, control fetuses experienced initial transient bradycardia in response to cerebral hypoperfusion whereas this effect was not seen in ketamine-treated animals. These data agree with other studies demonstrating that ketamine attenuates bradycardic responses to both maternal hypoxemia (8) and partial umbilical cord occlusion (54). Tachycardia during and following BCO was likely a direct effect of cerebral ischemia.
Blood gas/pH
Fetal blood gases and pH are highly dependent on umbilical-placental blood flow and, secondarily, fetal arterial blood pressure (21; 45). We propose that the difference in fetal blood gas responses to BCO in the present study was the result of the attenuation of the increase in fetal blood pressure caused by ketamine. That is, control fetuses responded to BCO with a greater increase in oxygen tension, likely the result of increased umbilical-placental perfusion (poor autoregulation in this vascular bed allows increased perfusion in response to increased arterial pressure). Femoral blood pressure was not increased as dramatically in the ketamine-treated fetuses and perhaps contributed to the observed hypoxemia in this group. Although reports of the effects of ketamine on uterine tone are controversial (16; 28), an increase in uterine tone producing decreased placental blood flow may also have caused fetal hypoxemia in this group. Finally, a slight metabolic acidemia was observed in response to BCO in both groups. This finding is in agreement with previous studies demonstrating that maternal administration of ketamine does not cause or worsen metabolic acidosis under normal (14; 26) or asphyxic (38; 54) fetal conditions.
ACTH and POMC / pro-ACTH
In this study, BCO stimulated robust increases in circulating ACTH1–39 and POMC / pro-ACTH in control fetuses. Ketamine pre-treatment completely inhibited these reflex responses. These results are consistent with previous studies from this laboratory demonstrating reduced ACTH release after interruption of chemoreceptor and baroreceptor afferents by carotid sinus denervation (57; 65) and suggest that fetal ACTH responses to hypotension are, in part, mediated by the chemoreflex or baroreflex. Given the ability of NMDA to stimulate ACTH release in the late gestation fetal sheep (9; 33), it is possible that ketamine inhibited ACTH secretion by blocking NMDA-mediated neurotransmission in the PVN. We know of no other studies investigating the effects of ketamine on HPA activation to hypotension. Nistico et al. demonstrated that ketamine inhibited corticosterone secretion to the stress of handling and laparotomy (34), an effect that could also involve blockade of PVN NMDA receptors.
We believe that the near complete inhibition of ACTH and POMC / pro-ACTH response to BCO in ketamine-treated animals is not simply the result of the attenuated drop in carotid sinus pressure during BCO or to the increase in blood pressure prior to BCO in this group. Fetal vena caval occlusion, for example, decreases mean arterial pressure to an extent similar to that seen ketamine fetuses (approximately 25 mmHg) and produces a robust ACTH release (57). Prior to BCO, the ketamine-induced increase in arterial blood pressure would be expected to inhibit fetal ACTH secretion (by baroreflex); nevertheless, we did not observe inhibition of pre-BCO HPA activity, and we believe that the transient hypertension produced by ketamine would have been an insignificant influence on the response to BCO because of its timing and relatively small magnitude. In other experiments in this laboratory, we have found that intracerebroventricular injection of nimesulide, a cyclooxygenase-2 inhibitor, increases fetal arterial blood pressure by a similar magnitude but does not block the fetal ACTH response to BCO (42). Finally, it is unlikely that the inhibition of stress-induced ACTH release in this study is a consequence of direct ketamine action on the pituitary. Little to no NMDA receptor expression within corticotropes has been observed (4). In addition, the integrity of the PVN is necessary for ACTH and corticosterone release to intravenous NMDA (72).
Previous data from the laboratory suggests that the majority of the radioimmunoassayable ACTH that circulates in fetal plasma during and after hypotension is POMC and/or 22 kDa pro-ACTH. The results of the two-site IRMA analysis of plasma ACTH1–39 concentrations in the present study suggest that the concentrations of the fully processed peptide are low relative to POMC and pro-ACTH. This conclusion is consistent with work previously reported by Rose and colleagues (11; 12) and other investigators (47; 56) who have reported that the biologically-active ACTH in plasma is a small proportion of the total immunoassayable peptide.
Cortisol
Ketamine is known to stimulate adrenocortical activity in adult rats (15; 34) and humans (1; 25). Although higher than the control group, we did not observe an increase in circulating cortisol to ketamine application prior to BCO. The reasons for this are unclear, but might be related to possible postnatal development of specific glutamatergic pathways controlling PVN function or pharmacokinetics of drug action in the fetus relative to postnatal ages. These data also suggest that ketamine does not have a direct effect on the adrenal cortex in the fetal animal. NMDA subunits are present in the adult rat adrenal medulla but not in the adrenal cortex (20). Potential actions of ketamine at other central receptors, however, cannot be ruled out (52; 61).
The significant difference in basal levels (0 minute values) of cortisol between the two groups is an interesting finding that is most likely a result of placental transfer of elevated maternal cortisol. Elevated levels of cortisol can inhibit the release of pituitary hormones through negative feedback (70); however, we do not think that negative feedback explained the suppression of ACTH and POMC / pro-ACTH responses to BCO after ketamine. Retrospective linear regression analysis did not reveal any statistically significant relationship between pre-BCO cortisol concentrations and peak ACTH values after BCO.
Cortisol secretion during BCO in ketamine-treated fetuses was significantly increased only at the 20-minutes time point compared to nadir levels at 5 minutes. This is in contrast to control animals, which demonstrated significantly stimulated cortisol levels at the 10, 20, and 30-minute time points compared to nadir levels at 0 minutes. This may suggest that cortisol levels are attenuated during BCO in ketamine-treated fetuses; however, circulating levels of this hormone were not significantly different between groups. While ACTH and POMC / pro-ACTH release during cerebral hypoperfusion is clearly reduced by ketamine, further study of cortisol release during BCO may be necessary to reach a definitive conclusion about the regulation of this hormone during hypotensive stimuli.
Interestingly, these data are similar to previous studies in this laboratory which demonstrated normal adrenocortical activation to hypotension in sinoaortic denervated fetuses despite the attenuated ACTH response (57; 65) and suggest that other factors may contribute to cortisol release during cardiovascular stress in the fetus. Splanchnic innervation of the adrenal (30; 32) and paracrine interactions of glucocorticoids and catecholamines controlled by vasoactive intestinal peptide (3) have been shown to mediate non-ACTH dependent release of cortisol. Other stress-induced hormones, such as prolactin, may also stimulate fetal adrenocortical activation or participate in the increase of adrenal sensitivity to ACTH (37; 62).
Finally, it is also possible that by changing the distribution of combined ventricular output, BCO decreased the metabolic clearance rate of cortisol or increased the rate of cortisol transfer from the maternal to the fetal circulation. While BCO stimulates reflex cardiovascular compensation, changes in cortisol clearance or transplacental transfer during BCO have not been investigated.
Conclusions
Our results demonstrate that ketamine potently inhibits ACTH and POMC / pro-ACTH release and abolishes bradycardic responses during cerebral hypoperfusion. This blockade is consistent with the known actions of ketamine at central NMDA receptors of the cardiovascular regulatory centers of the medulla and the known role of NMDA-mediated glutamatergic pathways in the control of the hypothalamus-pituitary-adrenal axis. We propose that ketamine, through antagonism of central NMDA receptors, blocks the chemoreflex activation of these afferent pathways thereby preventing hormonal and chronotropic responses to BCO.
Perspectives
The HPA axis is a critical component of the ability to survive stress, both in adult and fetal animals. Our previous work suggests that chemoreceptor pathways are a major influence on fetal HPA responses to cardiovascular stresses such as hypoxia and hypotension, and it seems likely that there is a similar dependency upon chemoreception in preterm infants. Because of the dramatic effect of ketamine on fetal HPA responsiveness to hypotension, it is seems logical to propose that the use of ketamine or other NMDA-antagonists would be ill-advised in late pregnancy or in the neonatal intensive care unit because they will interfere with the reflex responsiveness to cardiovascular stress.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Heath grant HD-42135 and an Florida/Puerto Rico American Heart Association Predoctoral Fellowship awarded to Melanie J. Powers.
Reference List
- 1.Adams HA, Thiel A, Jung A, Fengler G, Hempelmann G. Studies using S-(+)-ketamine on probands. Endocrine and circulatory reactions, recovery and dream experiences. Anaesthesist. 1992;41:588–596. [PubMed] [Google Scholar]
- 2.Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. British Journal of Pharmacology. 1983;79:565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berghorn KA, Li C, Nathanielsz PW, McDonald TJ. VIP innervation: sharp contrast in fetal sheep and baboon adrenal glands suggests differences in developmental regulation. Brain Research. 2000;877:271–280. doi: 10.1016/s0006-8993(00)02683-4. [DOI] [PubMed] [Google Scholar]
- 4.Bhat GK, Mahesh VB, Chu ZW, Chorich LP, Zamorano PL, Brann DW. Localization of the N-methyl-D-aspartate R1 receptor subunit in specific anterior pituitary hormone cell types of the female rat. Neuroendocrinology. 1995;62:178–186. doi: 10.1159/000127003. [DOI] [PubMed] [Google Scholar]
- 5.Blanco CE, Dawes GS, Hanson MA, McCook HB. International Satellite Symposium on Perinatal Physiology and Behaviour. Australia: Melbourne; 1983. Stimulus-response characteristics of chemoreceptors and baroreceptors in fetal and newborn sheep; p. 41. [Google Scholar]
- 6.Blanco CE, Dawes GS, Hanson MA, McCooke HB. Carotid baroreceptors in fetal and newborn sheep. Pediat Res. 1988;24:342–346. doi: 10.1203/00006450-198809000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Boddy K, Jones CT, Mantell C, Ratcliffe JG, Robinson JS. Changes in plasma ACTH and corticosteroid of the maternal and fetal sheep during hypoxia. Endocrinology. 1974;94:588–591. doi: 10.1210/endo-94-2-588. [DOI] [PubMed] [Google Scholar]
- 8.Boekkooi PF, Baan J, Teitel DF, Rudolph AM. Effect of drugs on chemoreceptor responsiveness in fetal sheep. Pediat Res. 1995;38:938–943. doi: 10.1203/00006450-199512000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Brooks AN, Howe DC. Adrenocorticotrophin and luteinizing hormone responses to N-methyl-D-aspartate during fetal development in sheep. J Neuroendocrinol. 1996;8:315–321. doi: 10.1046/j.1365-2826.1996.04639.x. [DOI] [PubMed] [Google Scholar]
- 10.Brooks AN, Howe DC. Adrenocorticotrophin and luteinizing hormone responses to N-methyl-D-aspartate during fetal development in sheep. J Neuroendocrinol. 1996;8:315–321. doi: 10.1046/j.1365-2826.1996.04639.x. [DOI] [PubMed] [Google Scholar]
- 11.Castro MI, Valego NK, Zehnder T, Rose JC. The ratio of plasma bioactive to immunoreactive ACTH-like activity increases with gestational age in the fetal lamb. J Dev Physiol. 1992;18:193–201. [PubMed] [Google Scholar]
- 12.Castro MI, Valego NK, Zehnder TJ, Rose JC. Bioactive-to-immunoreactive ACTH activity changes with severity of stress in late-gestation ovine fetus. Am J Physiol. 1993;265:E68–E73. doi: 10.1152/ajpendo.1993.265.1.E68. [DOI] [PubMed] [Google Scholar]
- 13.Chen HG, Wood CE. The adrenocorticotropic hormone and arginine vasopressin responses to hypercapnia in fetal and maternal sheep. Am J Physiol. 1993;264:R324–R330. doi: 10.1152/ajpregu.1993.264.2.R324. [DOI] [PubMed] [Google Scholar]
- 14.Craft JB, Jr, Coaldrake LA, Yonekura ML, Dao SD, Co EG, Roizen MF, Mazel P, Gilman R, Shokes L, Trevor AJ. Ketamine, catecholamines, and uterine tone in pregnant ewes. Am J Obstet Gynecol. 1983;146:429–434. doi: 10.1016/0002-9378(83)90823-2. [DOI] [PubMed] [Google Scholar]
- 15.Fahringer EE, Foley EL, Redgate ES. Pituitary adrenal response to ketamine and the inhibition of the response by catecholaminergic blockade. Neuroendocrinology. 1974;14:151–164. doi: 10.1159/000122255. [DOI] [PubMed] [Google Scholar]
- 16.Galloon S. Ketamine for obstetric delivery. Anesthesiology. 1976;44:522–524. doi: 10.1097/00000542-197606000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Giussani DA, McGarrigle HH, Moore PJ, Bennet L, Spencer JA, Hanson MA. Carotid sinus nerve section and the increase in plasma cortisol during acute hypoxia in fetal sheep. J Physiol. 1994;477:75–80. doi: 10.1113/jphysiol.1994.sp020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giussani DA, McGarrigle HH, Spencer JA, Moore PJ, Bennet L, Hanson MA. Effect of carotid denervation on plasma vasopressin levels during acute hypoxia in the late-gestation sheep fetus. J Physiol Lond. 1994;477:81–87. doi: 10.1113/jphysiol.1994.sp020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara Y, Chugun A, Nakaya H, Kondo H. Tonic block of the sodium and calcium currents by ketamine in isolated guinea pig ventricular myocytes. The Journal of Veterinary Medical Science. 1998;60:479–483. doi: 10.1292/jvms.60.479. [DOI] [PubMed] [Google Scholar]
- 20.Hinoi E, Fujimori S, Nakamura Y, Balcar VJ, Kubo K, Ogita K, Yoneda Y. Constitutive expression of heterologous N-methyl-D-aspartate receptor subunits in rat adrenal medulla. J Neurosci Res. 2002;68:36–45. doi: 10.1002/jnr.10202. [DOI] [PubMed] [Google Scholar]
- 21.Itskovitz J, Goetzman BW, Rudolph AM. Effects of hemorrhage on umbilical venous return and oxygen delivery in fetal lambs. Am J Physiol. 1982;242:H543–H548. doi: 10.1152/ajpheart.1982.242.4.H543. [DOI] [PubMed] [Google Scholar]
- 22.Itskovitz J, LaGamma EF, Rudolph AM. Baroreflex control of the circulation in chronically instrumented fetal lambs. Circ Res. 1983;52:589–596. doi: 10.1161/01.res.52.5.589. [DOI] [PubMed] [Google Scholar]
- 23.Jin YH, Bailey TW, Doyle MW, Li BY, Chang KS, Schild JH, Mendelowitz D, Andresen MC. Ketamine differentially blocks sensory afferent synaptic transmission in medial nucleus tractus solitarius (mNTS) Anesthesiology. 2003;98:121–132. doi: 10.1097/00000542-200301000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Kannan H, Kasai M, Osaka T, Yamashita H. Neurons in the paraventricular nucleus projecting to the median eminence: a study of their afferent connections from peripheral baroreceptors, and from the A1-catecholaminergic area in the ventrolateral medulla. Brain Research. 1987;409:358–363. doi: 10.1016/0006-8993(87)90722-0. [DOI] [PubMed] [Google Scholar]
- 25.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 26.Levinson G, Shnider SM, Gildea JE, DeLorimier AA. Maternal and foetal cardiovascular and acid-base changes during ketamine anaesthesia in pregnant ewes. British Journal of Anaesthesia. 1973;45:1111–1115. doi: 10.1093/bja/45.11.1111. [DOI] [PubMed] [Google Scholar]
- 27.Machado BH. Neurotransmission of the cardiovascular reflexes in the nucleus tractus solitarii of awake rats. Ann N Y Acad Sci. 2001;940:179–196. doi: 10.1111/j.1749-6632.2001.tb03676.x. [DOI] [PubMed] [Google Scholar]
- 28.Marx GF, Hwang HS, Chandra P. Postpartum uterine pressures with different doses of ketamine. Anesthesiology. 1979;50:163–166. doi: 10.1097/00000542-197902000-00019. [DOI] [PubMed] [Google Scholar]
- 29.McDonald TJ, Le WW, Hoffman GE. Brainstem catecholaminergic neurons activated by hypoxemia express GR and are coordinately activated with fetal sheep hypothalamic paraventricular CRH neurons. Brain Research. 2000;885:70–78. doi: 10.1016/s0006-8993(00)02936-x. [DOI] [PubMed] [Google Scholar]
- 30.McDonald TJ, Nathanielsz PW. The involvement of innervation in the regulation of fetal adrenal steroidogenesis. Horm Meta Res. 1998;30:297–302. doi: 10.1055/s-2007-978888. [DOI] [PubMed] [Google Scholar]
- 31.Myers DA, Bell PA, Hyatt K, Mlynarczyk M, Ducsay CA. Long-term hypoxia enhances proopiomelanocortin processing in the near-term ovine fetus. American Journal of Physiology Endocrinology and Metabolism. 2005;288:R1178–R1184. doi: 10.1152/ajpregu.00697.2004. [DOI] [PubMed] [Google Scholar]
- 32.Myers DA, Robertshaw D, Nathanielsz PW. Effect of bilateral splanchnic nerve section on adrenal function in the ovine fetus. Endocrinology. 1990;127:2328–2335. doi: 10.1210/endo-127-5-2328. [DOI] [PubMed] [Google Scholar]
- 33.Nardo L, Soong Y, Wu D, Young IR, Walker D, Szeto HH. Site and mechanism of action of dynorphin A-(1–13) and N-methyl-D-aspartate on ACTH release in fetal sheep. American Journal of Physiology Endocrinology and Metabolism. 2002;282:E1301–E1307. doi: 10.1152/ajpendo.00527.2001. [DOI] [PubMed] [Google Scholar]
- 34.Nistico G, Pisanti N, Rotiroti D, Preziosi P, Cuocolo R, de Martino G, Nistico GM. Effects of althesin and ketamine on resting and stress stimulated adrenocortical activity in rats. British Journal of Anaesthesia. 1978;50:891–897. doi: 10.1093/bja/50.9.891. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa A, Uemura M, Kataoka Y, Ol K, Inokuchi T. Effects of Ketamine on Cardiovascular-Responses Mediated by N-Methyl-D-Aspartate Receptor in the Rat Nucleus-Tractus-Solitarius. Anesthesiology. 1993;78:163–167. doi: 10.1097/00000542-199301000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Patel KP, Schmid PG. Role of paraventricular nucleus (PVH) in baroreflex-mediated changes in lumbar sympathetic nerve activity and heart rate. Journal of the Autonomic Nervous System. 1988;22:211–219. doi: 10.1016/0165-1838(88)90109-9. [DOI] [PubMed] [Google Scholar]
- 37.Pepe GJ, Waddell BJ, Albrecht ED. The effects of adrenocorticotropin and prolactin on adrenal dehydroepiandrosterone secretion in the baboon fetus. Endocrinology. 1988;122:646–650. doi: 10.1210/endo-122-2-646. [DOI] [PubMed] [Google Scholar]
- 38.Pickering BG, Palahniuk RJ, Cote J, Wade JG, Pash MG. Cerebral vascular responses to ketamine and thiopentone during foetal acidosis. Canadian Anaesthetists' Society Journal. 1982;29:463–467. doi: 10.1007/BF03009409. [DOI] [PubMed] [Google Scholar]
- 39.Raff H, Kane C, Wood CE. Vasopressin responses to hypoxia and hypercapnia in late-gestation fetal sheep. Am J Physiol. 1991;260:R1077–R1081. doi: 10.1152/ajpregu.1991.260.6.R1077. [DOI] [PubMed] [Google Scholar]
- 40.Raff H, Shinsako J, Dallman MF. Renin and ACTH responses to hypercapnia and hypoxia after chronic carotid chemodenervation. Am J Physiol. 1984;247:R412–R417. doi: 10.1152/ajpregu.1984.247.3.R412. [DOI] [PubMed] [Google Scholar]
- 41.Reicher D, Bhalla P, Rubinstein EH. Cholinergic cerebral vasodilator effect of ketamine in rabbits. Stroke. 1987;18:445–449. doi: 10.1161/01.str.18.2.445. [DOI] [PubMed] [Google Scholar]
- 42.Reimsnider S, Wood CE. Differential modulation of ovine fetal ACTH secretion by PGHS-1 and PGHS-2. Neuroendocrinology. 2006;83:4–11. doi: 10.1159/000093177. [DOI] [PubMed] [Google Scholar]
- 43.Robillard JE, Weitzman RE, Fisher DA, Smith FG. The dynamics of vasopressin release and blood volume regulation during fetal hemorrhage in the lamb fetus. Pediat Res. 1979;13:606–610. doi: 10.1203/00006450-197905000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Rose JC, Morris M, Meis PJ. Hemorrhage in newborn lambs: effects on arterial blood pressure, ACTH, cortisol, and vasopressin. Am J Physiol. 1981;240:E585–E590. doi: 10.1152/ajpendo.1981.240.6.E585. [DOI] [PubMed] [Google Scholar]
- 45.Rudolph AM. Congenital diseases of the heart. Chicago: Year Book Medical Publishers; 1974. [Google Scholar]
- 46.Rusy BF, Amuzu JK, Bosscher HA, Redon D, Komai H. Negative inotropic effect of ketamine in rabbit ventricular muscle. Anesthesia and Analgesia. 1990;71:275–278. doi: 10.1213/00000539-199009000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Saoud CJ, Wood CE. Ontogeny of proopiomelanocortin posttranslational processing in the ovine fetal pituitary. Peptides. 1996;17:649–653. doi: 10.1016/0196-9781(96)00054-x. [DOI] [PubMed] [Google Scholar]
- 48.Sapru HN. Glutamate circuits in selected medullo-spinal areas regulating cardiovascular function. Clin Exper Pharm Physiol. 2002;29:491–496. doi: 10.1046/j.1440-1681.2002.03661.x. [DOI] [PubMed] [Google Scholar]
- 49.Sapru HN, Krieger AJ. Cardiovascular and respiratory effects of some anesthetics in the decerebrate rat. European Journal of Pharmacology. 1979;53:151–158. doi: 10.1016/0014-2999(79)90160-2. [DOI] [PubMed] [Google Scholar]
- 50.Share L. Role of peripheral receptors in the increased release of vasopressin in response to hemorrhage. Endocrinology. 1967;81:1140–1146. doi: 10.1210/endo-81-5-1140. [DOI] [PubMed] [Google Scholar]
- 51.Slogoff S, Allen GW. The role of baroreceptors in the cardiovascular response to ketamine. Anesthesia and Analgesia. 1974;53:704–707. [PubMed] [Google Scholar]
- 52.Smith DJ, Pekoe GM, Martin LL, Coalgate B. The interaction of ketamine with the opiate receptor. Life Sciences. 1980;26:789–795. doi: 10.1016/0024-3205(80)90285-4. [DOI] [PubMed] [Google Scholar]
- 53.Stowe DF, Bosnjak ZJ, Kampine JP. Comparison of etomidate, ketamine, midazolam, propofol, and thiopental on function and metabolism of isolated hearts. Anesthesia and Analgesia. 1992;74:547–558. doi: 10.1213/00000539-199204000-00015. [DOI] [PubMed] [Google Scholar]
- 54.Swartz J, Cumming M, Biehl D. The effect of ketamine anaesthesia on the acidotic fetal lamb. Canadian Journal of Anaesthesia. 1987;34:233–237. doi: 10.1007/BF03015158. [DOI] [PubMed] [Google Scholar]
- 55.Thomson AM, West DC, Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985;313:479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- 56.Thorburn GD, Hollingworth SA, Hooper SB. The trigger for parturition in sheep: fetal hypothalamus or placenta? J Dev Physiol. 1991;15:71–79. [PubMed] [Google Scholar]
- 57.Tong H, Lakhdir F, Wood CE. Endogenous prostanoids modulate the ACTH and AVP responses to hypotension in late-gestation fetal sheep. Am J Physiol. 1998;275:R735–R741. doi: 10.1152/ajpregu.1998.275.3.R735. [DOI] [PubMed] [Google Scholar]
- 58.Tong H, Wood CE. Indomethacin attenuates the cerebral blood flow response to hypotension in late-gestation fetal sheep. Am J Physiol. 1999;277:R1268–R1273. doi: 10.1152/ajpregu.1999.277.5.R1268. [DOI] [PubMed] [Google Scholar]
- 59.Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol. 1993;264:R41–R50. doi: 10.1152/ajpregu.1993.264.1.R41. [DOI] [PubMed] [Google Scholar]
- 60.Viard E, Sapru HN. Carotid baroreflex in the rat: role of glutamate receptors in the medial subnucleus of the solitary tract. ne. 2004;126:785–794. doi: 10.1016/j.neuroscience.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 61.Vincent JP, Cavey D, Kamenka JM, Geneste P, Lazdunski M. Interaction of phencyclidines with the muscarinic and opiate receptors in the central nervous system. Brain Research. 1978;152:176–182. doi: 10.1016/0006-8993(78)90145-2. [DOI] [PubMed] [Google Scholar]
- 62.Walker ML, Pepe GJ, Albrecht ED. Regulation of baboon fetal adrenal androgen formation by pituitary peptides at mid- and late gestation. Endocrinology. 1988;122:546–551. doi: 10.1210/endo-122-2-546. [DOI] [PubMed] [Google Scholar]
- 63.Wang WZ, Rong WF, Wang JJ, Yuan WJ. Effect of ketamine on presympathetic neurons in rostral ventrolateral medulla of rats. Acta Pharmacologica Sinica. 2001;22:97–102. [PubMed] [Google Scholar]
- 64.Wendling WW, Daniels FB, Chen D, Harakal C, Carlsson C. Ketamine directly dilates bovine cerebral arteries by acting as a calcium entry blocker. Journal of Neurosurgical Anesthesiology. 1994;6:186–192. doi: 10.1097/00008506-199407000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Wood CE. Sinoaortic denervation attenuates the reflex responses to hypotension in fetal sheep. Am J Physiol. 1989;256:R1103–R1110. doi: 10.1152/ajpregu.1989.256.5.R1103. [DOI] [PubMed] [Google Scholar]
- 66.Wood CE, Chen HG. Acidemia stimulates ACTH, vasopressin, and heart rate responses in fetal sheep. Am J Physiol. 1989;257:R344–R349. doi: 10.1152/ajpregu.1989.257.2.R344. [DOI] [PubMed] [Google Scholar]
- 67.Wood CE, Chen H-G, Bell ME. Role of vagosympathetic fibers in the control of adrenocorticotropic hormone, vasopressin, and renin responses to hemorrhage in fetal sheep. Circ Res. 1989;64:515–523. doi: 10.1161/01.res.64.3.515. [DOI] [PubMed] [Google Scholar]
- 68.Wood CE, Kane C, Raff H. Peripheral chemoreceptor control of fetal renin responses to hypoxia and hypercapnia. Circ Res. 1990;67:722–732. doi: 10.1161/01.res.67.3.722. [DOI] [PubMed] [Google Scholar]
- 69.Wood CE, Rudolph AM. Carotid vascular control of ACTH secretion in lambs. Am J Physiol. 1983;244:E555–E559. doi: 10.1152/ajpendo.1983.244.6.E555. [DOI] [PubMed] [Google Scholar]
- 70.Wood CE, Rudolph AM. Negative feedback regulation of adrenocorticotropin secretion by cortisol in ovine fetuses. Endocrinology. 1983;112:1930–1936. doi: 10.1210/endo-112-6-1930. [DOI] [PubMed] [Google Scholar]
- 71.Yardley RW, Bowes G, Wilkinson M, Cannata JP, Maloney JE, Ritchie BC, Walker AM. Increased arterial pressure variability after arterial baroreceptor devervation in fetal lambs. Circ Res. 1983;52:580–588. doi: 10.1161/01.res.52.5.580. [DOI] [PubMed] [Google Scholar]
- 72.Zelena D, Mergl Z, Makara GB. Glutamate agonists activate the hypothalamic-pituitary-adrenal axis through hypothalamic paraventricular nucleus but not through vasopressinerg neurons. Brain Research. 2005;1031:185–193. doi: 10.1016/j.brainres.2004.10.034. [DOI] [PubMed] [Google Scholar]