Abstract
OBJECTIVES
The purpose of this study was to assess coronary arterial remodeling as a marker of subclinical atherosclerosis using coronary wall MRI in an asymptomatic population-based cohort.
BACKGROUND
In early atherosclerosis, compensatory enlargement of both the outer wall of the vessel as well as the lumen, termed compensatory enlargement or positive remodeling, occurs before luminal narrowing.
METHODS
179 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) were evaluated using black-blood coronary wall MRI. Coronary cross-sectional area (vessel size), lumen area, and mean wall thickness of the proximal coronary arteries were measured.
RESULTS
Men had a greater vessel size, lumen area, and mean wall thickness than women (38.3±11.3 versus 32.6±9.4 mm2, 6.7±3.2 versus 5.3±2.4 mm2, and 2.0±0.3 versus 1.9±0.3 mm, respectively, p<0.05). No significant coronary artery narrowing was present by magnetic resonance angiography. Overall, coronary vessel size increased 25.9 mm2 per millimeter increase in coronary wall thickness, while lumen area increased only slightly at 3.1 mm2 for every millimeter increase in wall thickness (difference in slopes, p<0.0001). Adjusting for age and gender, participants with Agatston score greater than zero were more likely to have wall thickness greater than 2.0 mm (odds ratio 2.0, 95% CI 1.01–3.84).
CONCLUSIONS
Coronary wall MRI detected positive arterial remodeling, in asymptomatic men and women with subclinical atherosclerosis.
Keywords: subclinical atherosclerosis, magnetic resonance imaging, coronary artery disease, plaque
INTRODUCTION
Coronary artery disease (CAD) is currently defined as clinically significant when luminal narrowing is present, typically at the 50% diameter reduction threshold. However in early atherosclerosis, the first arterial changes consist of compensatory enlargement of both the outer wall of the vessel as well as the lumen, termed compensatory enlargement or positive remodeling (1–3). Evidence from histopathologic and clinical studies suggests that positive remodeling is associated with plaque vulnerability and plaque rupture (4–7). Prior studies have indicated that the process of positive remodeling can be prevented (8) and modulated (9) by statin therapy.
While positive remodeling has been shown in patients with symptomatic CAD, it has not been demonstrated in asymptomatic individuals and its relationship to other measures of sub-clinical atherosclerosis has not been established. This knowledge gap stems from the absence of non-invasive methods that allow for quantification of coronary vessel wall size in large asymptomatic populations. The most commonly used imaging technique to identify positive remodeling has been intravascular ultrasound (IVUS). IVUS detects positive remodeling and can characterize plaque composition (2–7). The technique however is not suitable for a screening method due to its invasive nature. Multidetector computed tomography (MDCT) has been used to assess positive remodeling in patients with symptomatic CAD (10). Potential disadvantages of MDCT include ionizing radiation and injection of iodinated contrast.
Black-blood coronary wall magnetic resonance imaging (MRI) can detect increased wall thickness in patients with X-ray angiography documented coronary artery disease with good reproducibility (11–14). While coronary wall MRI is complex and sophisticated, the technology can be applied to asymptomatic population because it is non-invasive and radiation-free. The purpose of this study was to detect and quantify subclinical atherosclerosis in a large asymptomatic population using coronary MR angiography and coronary arterial wall MRI.
METHODS
Study Participants
Study subjects were recruited from participants in the Baltimore and Chicago field centers of the Multi-Ethnic Study of Atherosclerosis (MESA). The study design of MESA has previously been described in detail (15). In brief, participants of four ethnic origins (Caucasian, African American, Hispanic, and Chinese) age 45 to 84 years were enrolled from six US field centers (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern Manhattan, NY; and St. Paul, MN). Individuals with known clinical cardiovascular disease at baseline were excluded.
179 participants from examination 4 of MESA (October 2005 to February 2008) were enrolled in this prospective cohort study (Table 1). The mean age was 61 ± 9 years; 90 were men and 89 women. Sixty four percent were Caucasian (64%) and 36% were African American. We excluded participants with heart rate above 85 beats per minute (due to technical limitations of the MRI technique), claustrophobia, contraindications to MRI, and without sinus cardiac rhythm. Information on demographics, smoking, and medical history were collected and the measurements of covariates, including plasma lipids, fasting glucose, resting blood pressure, Framingham risk score, and common carotid intimal-medial thickness (IMT) were obtained according to the MESA study design and methods (15). Left ventricle (LV) mass was determined by cardiac MRI as described previously (16). The study was approved by the respective institutional review boards. All participants provided written informed consent.
TABLE 1.
Participants Characteristics by Gender
| Characteristic | Men (n=90) Mean (SD) |
Women (n=89) Mean (SD) |
Difference p-value |
|---|---|---|---|
| Age, years | 61(10) | 62(8) | 0.59 |
| Race | 0.92 | ||
| Caucasian | 57 (63%) | 57 (64%) | |
| African American | 33 (37%) | 32 (36%) | |
| Diabetes, N | 10 (11%) | 5 (6%) | 0.28 |
| Dyslipemia*, N | 49 (54%) | 33 (37%) | 0.02 |
| Hypertension, N | 39 (43%) | 36 (40%) | 0.70 |
| Agatston calcium>0, N | 53 (59%) | 29 (33%) | 0.0004 |
| Ever Smoked in life, N | 51 (57%) | 37 (42%) | 0.04 |
| # of images | |||
| All vessels | 192 | 173 | |
| Left main | 60 | 48 | |
| Left anterior descending | 64 | 57 | |
| Right | 68 | 68 | |
| BMI, kg/m2 | 27.7 (4.1) | 27.4 (5.4) | 0.69 |
| Triglycerides, mg/dl | 109.2 (53.7) | 105.1 (44.8) | 0.58 |
| Total cholesterol, mg/dl | 184.8 (33.5) | 200.9 (35.0) | 0.002 |
| LDL - C, mg/dl | 117.5 (31.2) | 115.6 (35.0) | 0.71 |
| HDL - C, mg/dl | 45.5 (9.9) | 64.6 (18.4) | <0.0001 |
| Glucose, mg/dl | 102.7 (27.7) | 94.2 (19.8) | 0.02 |
| SBP, mm Hg | 123.8 (17.5) | 123.2 (21.4) | 0.82 |
| DBP, mm Hg | 73.77 (9.2) | 68.48 (9.6) | 0.0002 |
| Framingham risk score | 11.6 (2.8) | 13.3 (4.0) | 0.002 |
| Agatston calcium score | 163 (382) | 50 (146) | 0.01 |
Notes: Dyslipidemia means total cholesterol>200mg/dl, LDL>160mg/dl, HDL< 40mg/dl, and/or triglycerides>150 mg/dl.
IMT: intimal-medial thickness, SBP: systolic blood pressure, DBP: diastolic blood pressure, BMI: body mass index, LDL-C: low density lipoprotein, HDL: high density lipoprotein, N = number
Coronary MR imaging
All participants were imaged on a 1.5T whole-body magnetic resonance scanner (Avanto, Siemens, Erlangen, Germany) with a gradient strength of 45 mT/m, slew rate of 200T/m/sec, and 12-channel receive coils (6 anterior and 6 posterior). The period of coronary diastasis was determined for each participant based on a four-chamber cine steady-state free precession (SSFP) sequence. Coronary MR angiograms (MRA) were acquired during free breathing using an axial 3D whole-heart, navigator and ECG-gated, fat suppressed, T2-prepared SSFP sequence. Sequence parameters were: TR/TE = 3.8/1.7 ms, bandwidth = 975 Hz/pixel, flip angle = 90°, field of view = 320 × 320 mm2, matrix = 288 × 288 interpolated to 512 × 512, slice thickness = 3.0 mm interpolated to 1.5 mm, navigator acceptance window = ±2 mm.
3D multi-planar reformations (MPR) were performed on coronary MR angiographic images to localize the left main (LM), the proximal left anterior descending (LAD) and the right coronary artery (RCA). Cross-sectional coronary wall MR images were acquired at 5 mm intervals from the origins of the LM (1 image), the proximal portions of the LAD and RCA (3 images for each artery at 5 mm intervals) as previously described (17). Coronary wall MR images were acquired with a double inversion recovery, motion adapted navigator gated, asymmetric adiabatic spectral inversion pulse (AASPIR) for fat suppression, T2 turbo spin-echo (TSE) sequence with the parameters: TR= 2R-R intervals, TE = 33ms, echo-spacing = 6.6 ms, bandwidth = 305 Hz/pixel, matrix = 512 × 512, field of view = 420 × 420 mm2, slice thickness = 5 mm, navigator acceptance window = ±2 mm. The examination time was limited to 60 minutes or less to complete the protocol.
MR image analysis
For coronary MRA analysis, the images were transferred to a 3D image analysis workstation (Leonardo, Siemens). Analysis of 3D multiplanar and maximum intensity projections for LM, the proximal portions of LAD and RCA were performed by two experienced observers to detect any significant luminal narrowing (>50% diameter reduction). Black blood images were graded on a 3-point scale; 1 = nonvisualization, 2 = adequate, 3 = good. Coronary wall images with a score of 2 or 3 were analyzed using VesselMASS software (Division of Image Processing, Radiology Dept, Leiden University Medical Center (18)) by an observer blinded to coronary MRA results and other clinical information. The images were zoomed to 1000%. The outer (adventitial) and inner (luminal) boundaries of coronary wall were traced manually using a region of interest tool. The outer contour area (cross-sectional vessel size), lumen area, and the mean vessel wall thickness (related to the plaque burden) were obtained from the software reports.
Coronary artery calcium measurement
Computed tomography scanning of the chest was performed with a prospectively ECG-triggered scan acquisition at 50% of each RR interval with a multidetector computed tomography system (Volume Zoom, Siemens, Erlangen, Germany) that acquired 4 simultaneous 2.5-mm slices for each cardiac cycle in a sequential axial scan mode. Each participant was scanned twice consecutively. Scans were read centrally at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center to identify and quantify coronary calcification (19). Coronary artery calcium (CAC) measurements between participants were adjusted with a standard calcium phantom scanned simultaneously with each participant. The mean Agatston score was used in all analyses.
Statistical analysis
All of the statistical analyses were performed with the use of statistical software SAS version 9.2 (Statistical Analysis System, SAS Institute, Cary, NC). Continuous variables are presented as means with standard deviations. When multiple measurements of a single coronary artery were available, they were averaged for that vessel to constitute a single data point. Both procedures UNIVARIATE and CAPABILITY in SAS were employed to assess normality of the variables of interest. Unpaired t-test was used to compare to group mean values after assessing for normality of the distributions. Since coronary artery calcium score (CAC) was not normally distributed, log-transformed CAC was used in the comparisons. Chi-square test was used to compare categorical variables. Linear regression analysis was used to investigate the relationship between coronary artery wall thickness and a) outer contour area and b) lumen area before and after adjustment for measures of body and heart size (body mass index, left ventricular mass, respectively). Logistic regression analysis was used to evaluate the relationship between dichotomized CAC and coronary arterial wall thickness. In order to examine whether two correlation confidents were equal, a method proposed by Cohen J and Cohen P (20) was used and SAS procedure CALIS was used to perform the analysis.
RESULTS
Participant characteristics
The characteristics of the study population are summarized in Table 1. The number of participants with dyslipidemia, smoking history or non-zero coronary Agatston calcium score in men was greater than those in women (p<0.05). Agatston calcium score and common carotid IMT were greater in men than in women (p<0.05). Framingham risk score was greater in women than in men (p<0.05). All participants meeting the enrollment criteria completed coronary artery MRA and coronary wall MRI. The image quality of all coronary MR angiograms was adequate for analysis at the sites at which coronary artery wall images were obtained. No significant stenoses (≥50% luminal diameter reduction) in the LM and the proximal portions of RCA and LAD were identified.
Coronary arterial wall remodeling
A total of 365 coronary wall MR images (LM 108, LAD 121, and RCA 136) with grade 2 and 3 of image quality scores in 179 participants were evaluated. The imaging protocol was not completed for 52 arterial locations primarily due to patient tolerance or examination time exceeding 60 minutes. The coronary artery wall could not be visualized at 129 locations. The overall image quality scores of 365 coronary wall images were 2.6±0.24, 2.6±0.24, and 2.7±0.21 for the LM, LAD, and RCA respectively. There was no significant differences in image quality score between the coronary artery segments (p>0.05). Coronary arterial wall MRI measurements are summarized in Table 2. Coronary vessel size, expressed as either outer contour area or lumen area had a normal distribution. Men had a greater mean outer contour area and lumen area than women (38.8 ± 11.3 and 6.7 ± 3.2 mm2 versus 32.6 ± 9.4 and 5.3 ± 2.4 mm2, respectively, p<0.05). Mean wall thickness in men was greater than that of women (2.0±0.3 mm versus 1.9±0.3 mm, respectively, p<0.05). The LM outer contour area and lumen area were greater than that of the LAD or RCA (p<0.0001). There was no significant difference of coronary artery wall thickness between LM and LAD or RCA.
TABLE 2.
Coronary Wall MRI Measurements
| Outer contour area |
Lumen area | Wall area | Mean wall thickness |
|
|---|---|---|---|---|
| Mean (SD) mm2 |
Mean (SD) mm2 |
Mean (SD) mm2 |
Mean (SD) mm |
|
| Men | 38.8 (11.3) | 6.7 (3.2) | 31.6 (8.7) | 2.0 (0.3) |
| Women | 32.6 (9.4) | 5.3 (2.4) | 27.3 (7.7) | 1.9 (0.3) |
| p-value for difference |
0.0003 | 0.002 | 0.0005 | 0.01 |
| Coronary artery: | ||||
| Left main | 40.5(15.4)#$ | 7.87 (4.3) #$ | 32.6(12.0) #$ | 1.97 (0.4) |
| Left anterior descending |
34.2 (10.5)# | 5.74 (3.0)# | 28.5 (8.3)# | 1.94 (0.3) |
| Right | 33.60 (10.9)$ | 5.33 (3.0)$ | 28.5 (8.7)$ | 1.96 (0.3) |
| p-value for difference |
<0.0001 | <0.0001 | 0.0008 | 0.79 |
| All vessels | 35.4 (10.9) | 5.96 (2.9) | 29.5 (8.5) | 1.97 (0.3) |
Notes:
indicates that there is a significant difference between LM and LAD (p-value < 0.05)
indicates that there is a significant difference between LM and RCA (p-value < 0.05)
As coronary artery wall thickness increased, the overall vessel size (outer contour area) increased at a greater rate than the change in the lumen area. Specifically, the outer contour area increased by 25.9 mm2 for every millimeter increase in mean wall thickness (p<0.0001). Lumen area also increased but at a smaller rate of 3.1 mm2 for every millimeter increase in mean wall thickness (p<0.0001). This indicated the coronary vessel size enlarged to compensate for atherosclerotic change, i.e. positive remodeling (examples in Figure 2 and Figure 3).
Figure 2. 84 year old male participant, eccentric coronary plaque with positive remodeling.
(a) Right coronary artery magnetic resonance angiogram (MRA) showing no significant stenosis in the proximal coronary artery. Multiplanar reconstruction (MPR) of the MRA shows a round lumen proximally (b) and oval lumen distally (d). Black blood MRI at the same levels shows (c) a normal wall and eccentric plaque 5 mm distally (e). The lumen area proximally (c) was 7.7 mm2 and was slightly larger distally at 9.4 mm2 (e). The corresponding wall areas were 42.4 and 45.1 mm2.
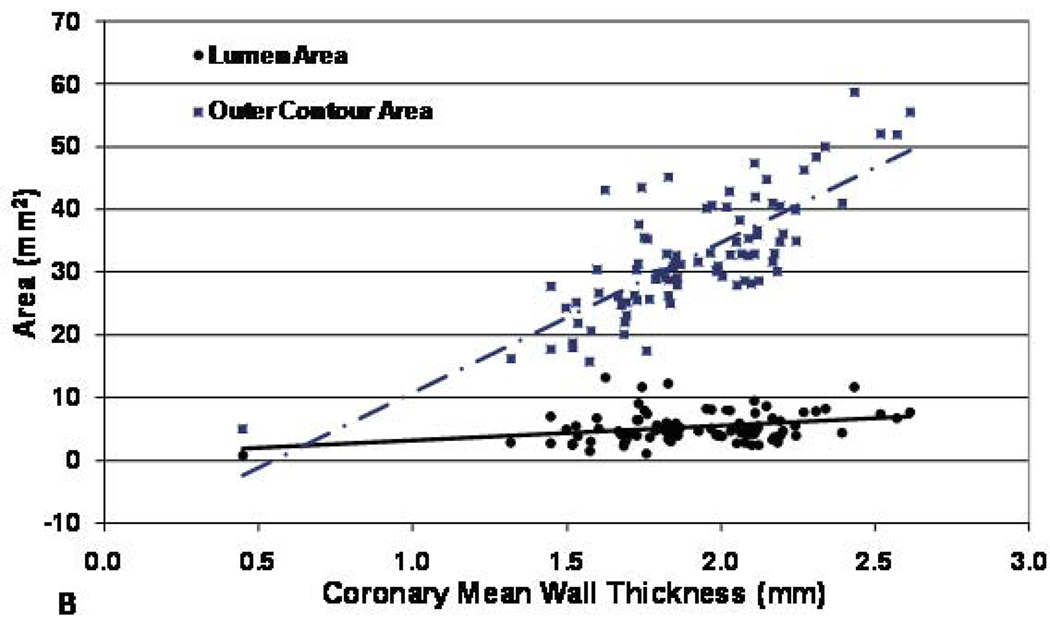
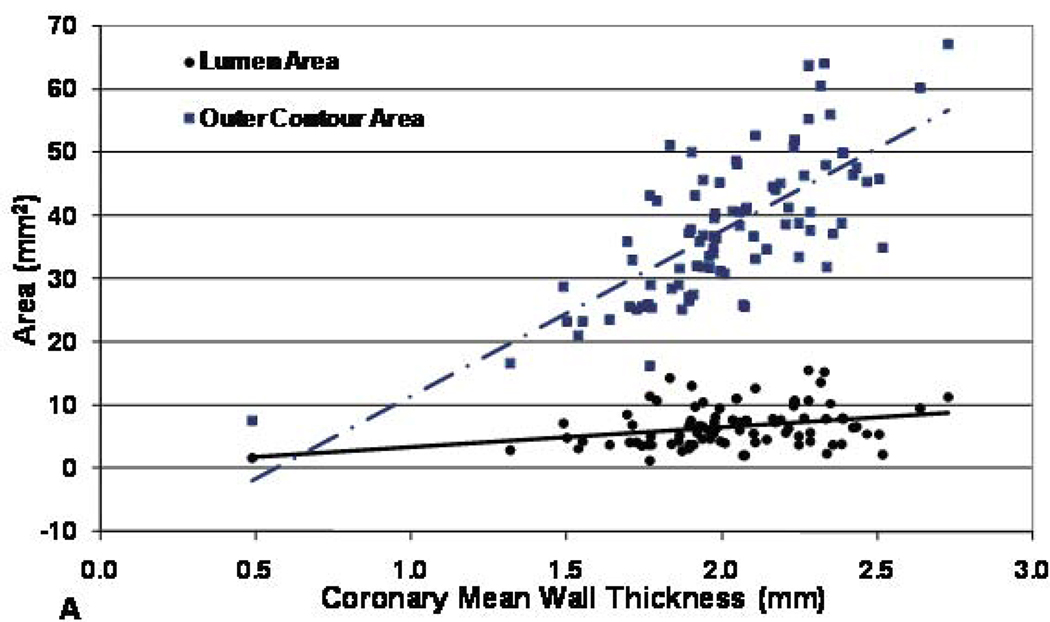
Figure 3. Coronary artery cross-sectional area (outer contour area) and lumen area vs. mean wall thickness for men (A) and women (B) participants.
Both outer contour area and lumen area were positively correlated mean wall thickness (r= 0.53 and 0.09, p< 0.0001 and <0.05 respectively for male and r= 0.62 and 0.09, p< 0.0001 and < 0.05 respectively for female), but the slope of the outer contour area increase was significantly greater than that of the lumen area increase (p<0.0001 for both men and women).
For men, the outer contour area increased 26.2 mm2 for every millimeter increase in wall thickness, while the lumen area increased 3.2 mm2 per millimeter increase in wall thickness (difference between slopes, p< 0.0001, Figure 3, panel A). For women, the relationship was similar: outer contour area increased 23.9 mm2 per millimeter increase in wall thickness, while lumen area increased 2.3 mm2 per millimeter increase in wall thickness (difference between slopes, p< 0.0001, Figure 3, panel B).
In order to consider the possibility that increased vessel size and wall thickness could be explained by body size alone, the regression analyses were repeated after adjustment for body mass index or left ventricular mass. After adjustment, the relationships of mean wall thickness to outer contour area and lumen area for both men and women remained statistically significant. Adjusting for left ventricular size (mass), the outer contour area increased 0.141 mm2/g and the lumen area index increased 0.018 mm2/g per millimeter increase in wall thickness in men (p<0.0001). For women the outer contour area index increased 0.187 mm2/g and the lumen area index increased 0.019 mm2/g per millimeter increase in wall thickness (p<0.0001). When the LM, LAD and RCA were examined individually, the positive correlations between outer contour area and vessel wall thickness remained significant (p<0.0001, all territories).
Coronary calcification and positive remodeling
82 participants (82/179, 46%) had coronary calcification. The mean calcium scores in men and women were 163 and 50 Agatston units, respectively, indicating mild atherosclerosis. The distribution of CAC (or log CAC +1) versus wall thickness was nonlinear due to wide variation in wall thickness for participants with CAC score of zero (i.e., noncalcified plaque). In order to test the hypothesis that CAC was associated with increased wall thickness, logistic regression was used to relate Agatston score to median wall thickness of the study population (1.96 mm). Arterial wall thickness greater than the median was significantly associated with positive CAC score (odds ratio 2.1, 95% CI 1.1–3.8) in univariate logistic regression analysis. There was little change after adjustment for age and gender (odds ratio 2.0, 95% CI 1.01–3.8).
DISCUSSION
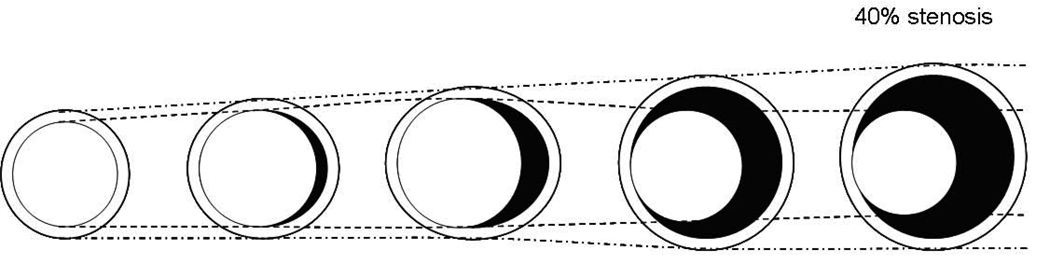
This is the first study to noninvasively demonstrate positive coronary wall remodeling in asymptomatic individuals with no history of CAD as well as the relationship between coronary calcification and arterial wall thickness by black-blood coronary MRI. Positive remodeling was present in the coronary arteries of both men and women. Mean coronary wall thickness was assessed by MRI as a measure of atherosclerosis, and the lumen area on average showed a slight increase as wall thickness increased (Figures 3, panel A,B). However the predominant change in the vessel size was a more substantial increase in the outer contour area of the vessel wall (Figures 3, panel A, B). These relationships persisted after adjusting for body size or heart size. The combination of these findings is consistent with positive arterial remodeling in early atherosclerosis (1–3). Glagov et al. (1) observed that human coronary artery cross-sectional area increased with increasing plaque area to maintain the lumen area until plaque occupied 40% of the potential lumen area. For vessels with less than 20% stenosis, cross-sectional vessel area enlarged at a faster rate than plaque area increased, consequently lumen area also increased at a small amount [Figure 4]. In the present study the outer contour area of the vessel wall increased with increasing mean wall thickness and lumen area also increased, but at a much slower rate than outer contour area. This indicated overcompensation, consistent with the above histopathological studies.
Figure 4. Diagram of positive arterial remodeling.
Coronary cross-sectional vessel size enlarges with increasing plaque area to maintain lumen size until plaque occupies 40% of the potential lumen area. In the early stages of atherosclerosis, vessel size increases at a faster rate than plaque area increases, therefore lumen size also enlarges, but in a smaller amount, indicating overcompensation. Adapted from (1).
Arterial calcium by CT is an established marker of coronary atherosclerosis. Prior studies have shown that CAC is closely correlated with plaque burden(21,22). Although CT CAC measures only a fraction of the total plaque burden, we may expect to see an overall relationship with arterial wall thickness defined by MRI. In the present study, CAC score using the Agatston score was positively associated with increased arterial wall thickness before and after adjustment for age and gender.
MRI presents several advantages as well as limitations for identification of early atherosclerosis. The method is noninvasive without ionizing radiation and a contrast agent is not used. The arterial wall signal is readily identified due to its relatively high signal intensity compared to the low signal of adjacent peri-adventitial fat and lumen. This high level of conspicuity for the arterial wall is offset by several disadvantages. Each image of the arterial wall takes 2–3 minutes to acquire, and we excluded patients with elevated heart rates due short periods of coronary diastasis. Low image quality is present when respiratory patterns are irregular, since imaging is simultaneously gated to both the electrocardiogram and the motion of the diaphragm. Also, our protocol was challenging to implement: two MESA sites in Baltimore and Chicago with multiple technologists were used for a sophisticated protocol that required near real-time reformation of the coronary angiogram, precise placement of perpendicular image slices with avoidance of coronary branches. The spatial resolution of MRI is much worse than intravascular ultrasound although contrast resolution is generally better. Ultrasound methods have defined a remodeling index based on identification of the external elastic membrane, but this structural detail is not present on MRI. Factors such as partial volume effects and insufficient blood flow suppression currently limit this method to determine a “threshold” for normal coronary artery wall thickness. Current MRI methods have not resolved plaque components of the coronary artery in vivo. Despite these issues, evidence of positive coronary arterial remodeling was detected in both men and women without symptomatic coronary arterial disease and no significant (>50%) coronary luminal narrowing as assessed by whole heart MR angiography.
A previous study by Fayad et al used two-dimensional black-blood MRI to evaluate coronary arteries in 8 normal participants and 5 patients with ≥40% stenosis of coronary arteries as assessed by x-ray angiography (12). The results showed the average maximum coronary wall thickness in patients were significantly greater than that in normal participants (4.38 ± 0.71 versus 0.75 ± 0.17mm p<0.0001). In another study, two-dimensional black-blood coronary wall MRI showed both mean vessel wall thickness and mean wall area were greater in coronary artery disease patients than in healthy participants (1.5±0.2 versus 1.0±0.2 mm and 21.2±3.1 versus 13.7±4.2 mm2, p<0.02) (11) as well as in patients with diabetic nephropathy (23).
Worthley et al used serial MRI at baseline and 6 months after aortic balloon denudation in Watanabe heritable hyperlipidemic rabbits model to demonstrate positive arterial remodeling (24). Their results showed the vessel wall area increased significantly after treatment. Outer arterial wall area showed much larger changes compared to proportionately smaller increases in lumen area. These MRI results were confirmed by histopathology. Kim et al. used 3D black-blood coronary wall MRI to detect positive arterial remodeling in the RCA of 6 patients in areas of nonsignificant coronary artery disease (10% to 50% diameter reduction) (14). Both mean coronary wall thickness and wall area were significantly increased in the patients compared to 6 healthy participants, whereas the lumen diameter and lumen area were similar, indicating positive arterial remodeling.
Positive arterial remodeling may be related to accumulation of plaque in the arterial wall, with overcompensation of nondiseased areas due to endothelium-dependent vasodilatation in response to increase of shear stress (25,26). Based on this mechanism, eccentric plaque should contribute more to the positive arterial remodeling than diffuse atherosclerosis. Early coronary artery plaques are usually eccentric(27), therefore positive remodeling mostly occurs in the early stage of atherosclerosis.
Clinical significance
These results suggest progress in using noninvasive imaging for the detection of early atherosclerosis. Currently most noninvasive imaging studies using computed tomography (CT) or magnetic resonance angiography are focused on detection coronary artery narrowing since 50% coronary narrowing is the current threshold for angiographic treatment. If the reliability and spatial resolution of MRI can be improved, early and potentially more treatable atherosclerotic disease could be evaluated and monitored noninvasively.
Conclusions
In conclusion, positive remodeling of the coronary artery wall was detected in asymptomatic men and women using MRI. With further development, MRI has potential as a noninvasive method for assessing plaque burden and coronary artery size.
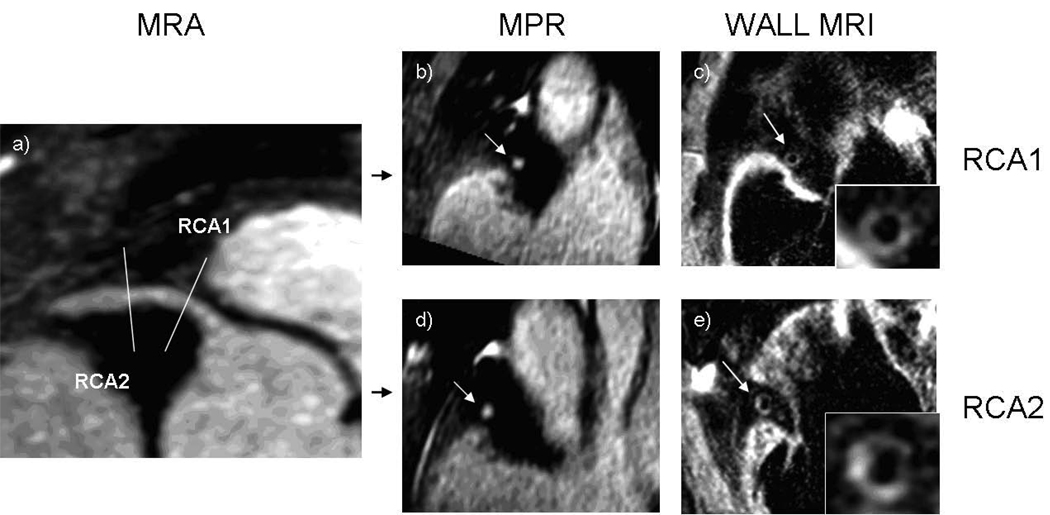
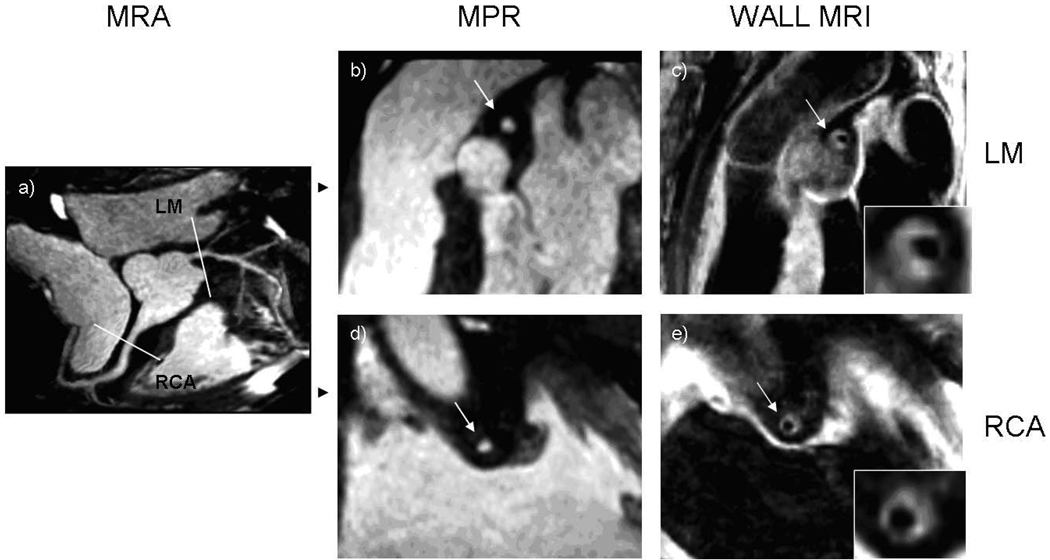
Figure 1. 73 year old male participant with increased wall thickness and preservation of lumen area.
(a) Magnetic resonance angiogram (MRA) shows no significant stenosis in the proximal portions of the coronary arteries. Multiplanar reconstruction (MPR) of the MRA of the left main (b) and the right (d) coronary arteries shows a normal lumen in cross-sections. Coronary wall MRI of the corresponding arterial cross-sections (c, e) shows eccentrically thickened arterial walls. Mean wall thickness: 4.1 mm for the left main, 2.5 mm for right coronary artery. Lumen and outer contour areas 7.1 mm2 and 54.3 mm2 for the left main and 7.0 mm2 and 51.4 mm2 for the right coronary artery.
Acknowledgement
This research was supported by R01 HL78909 and contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
ABBREVIATIONS
- CAD
Coronary artery disease
- IVUS
intravascular ultrasound
- MRI
magnetic resonance imaging
- MRA
Coronary magnetic resonance angiograms
- SSFP
steady-state free precession
- MDCT
multidetector spiral computed tomography
- CAC
Coronary artery calcium
- IMT
intimal-medial thickness
- MESA
Multi-Ethnic Study of Atherosclerosis
- LV
Left ventricle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT-OF-INTEREST: none
FINANCIAL DISCLOSURE STATEMENTS: none
REFERENCES
- 1.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 2.Hermiller JB, Tenaglia AN, Kisslo KB, et al. In vivo validation of compensatory enlargement of atherosclerotic coronary arteries. Am J Cardiol. 1993;71:665–668. doi: 10.1016/0002-9149(93)91007-5. [DOI] [PubMed] [Google Scholar]
- 3.McPherson DD, Sirna SJ, Hiratzka LF, et al. Coronary arterial remodeling studied by high-frequency epicardial echocardiography: an early compensatory mechanism in patients with obstructive coronary atherosclerosis. J Am Coll Cardiol. 1991;17:79–86. doi: 10.1016/0735-1097(91)90707-g. [DOI] [PubMed] [Google Scholar]
- 4.Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 5.Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes : an intravascular ultrasound study. Circulation. 2000;101:598–603. doi: 10.1161/01.cir.101.6.598. [DOI] [PubMed] [Google Scholar]
- 6.Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation. 2002;105:939–943. doi: 10.1161/hc0802.104327. [DOI] [PubMed] [Google Scholar]
- 7.von Birgelen C, Klinkhart W, Mintz GS, et al. Plaque distribution and vascular remodeling of ruptured and nonruptured coronary plaques in the same vessel: an intravascular ultrasound study in vivo. J Am Coll Cardiol. 2001;37:1864–1870. doi: 10.1016/s0735-1097(01)01234-7. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki M, Saito M, Nagai T, Saeki H, Kazatani Y. Prevention of positive coronary artery remodeling with statin therapy in patients with coronary artery diseases. Angiology. 2006;57:259–265. doi: 10.1177/000331970605700301. [DOI] [PubMed] [Google Scholar]
- 9.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. Jama. 2004;291:1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 10.Achenbach S, Ropers D, Hoffmann U, et al. Assessment of coronary remodeling in stenotic and nonstenotic coronary atherosclerotic lesions by multidetector spiral computed tomography. J Am Coll Cardiol. 2004;43:842–847. doi: 10.1016/j.jacc.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 11.Botnar RM, Stuber M, Kissinger KV, Kim WY, Spuentrup E, Manning WJ. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation. 2000;102:2582–2587. doi: 10.1161/01.cir.102.21.2582. [DOI] [PubMed] [Google Scholar]
- 12.Fayad ZA, Fuster V, Fallon JT, et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation. 2000;102:506–510. doi: 10.1161/01.cir.102.5.506. [DOI] [PubMed] [Google Scholar]
- 13.Hazirolan T, Gupta SN, Mohamed MA, Bluemke DA. Reproducibility of black-blood coronary vessel wall MR imaging. J Cardiovasc Magn Reson. 2005;7:409–413. doi: 10.1081/jcmr-200053464. [DOI] [PubMed] [Google Scholar]
- 14.Kim WY, Stuber M, Bornert P, Kissinger KV, Manning WJ, Botnar RM. Three-dimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation. 2002;106:296–299. doi: 10.1161/01.cir.0000025629.85631.1e. [DOI] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 16.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 17.Macedo R, Chen S, Lai S, et al. MRI detects increased coronary wall thickness in asymptomatic individuals: The multi-ethnic study of atherosclerosis (MESA) J Magn Reson Imaging. 2008 doi: 10.1002/jmri.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wasserman BA, Sharrett AR, Lai S, et al. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution MRI: the multi-ethnic study of atherosclerosis (MESA) Stroke. 2008;39:329–335. doi: 10.1161/STROKEAHA.107.498634. [DOI] [PubMed] [Google Scholar]
- 19.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J, Cohen P, editors. Applied Multiple Regression / Correlation Analysis for the Behavioral Sciences End edition ed. New Jersey: IEA; 1983. [Google Scholar]
- 21.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 22.Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 23.Kim WY, Astrup AS, Stuber M, et al. Subclinical coronary and aortic atherosclerosis detected by magnetic resonance imaging in type 1 diabetes with and without diabetic nephropathy. Circulation. 2007;115:228–235. doi: 10.1161/CIRCULATIONAHA.106.633339. [DOI] [PubMed] [Google Scholar]
- 24.Worthley SG, Helft G, Fuster V, et al. Serial in vivo MRI documents arterial remodeling in experimental atherosclerosis. Circulation. 2000;101:586–589. doi: 10.1161/01.cir.101.6.586. [DOI] [PubMed] [Google Scholar]
- 25.Furchgott RF. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983;53:557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- 26.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 27.Bond MGIWJ, Glagov S, Chandler AB, Cornhill JF, editors. Clinical diagnosis of atherosclerosis: quantitative methods of evaluation. New York: Springer-Verlag; 1983. S G. Quantitating atherosclerosis: problems of definition; pp. 11–35. [Google Scholar]