Abstract
Context
Previous magnetic resonance imaging (MRI) findings have demonstrated psychopathological symptom–related smaller gray matter volumes in various cingulate gyrus subregions in schizophrenia and bipolar disorder. However, it is unclear whether these gray matter abnormalities show a subregional specificity to either disorder and whether they show postonset progression.
Objective
To determine whether there are initial and progressive gray matter volume deficits in cingulate gyrus subregions in patients with first-episode schizophrenia (FESZ) and patients with first-episode affective psychosis (FEAFF, mainly manic) and their specificity to FESZ or FEAFF.
Design
A naturalistic cross-sectional study at first hospitalization for psychosis and a longitudinal follow-up approximately 1½ years later.
Setting and Participants
Patients were from a private psychiatric hospital. Thirty-nine patients with FESZ and 41 with FEAFF at first hospitalization for psychosis and 40 healthy control subjects (HCs) recruited from the community underwent high-spatial-resolution MRI, with follow-up scans in 17 FESZ patients, 18 FEAFF patients, and 18 HCs. Individual subjects were matched for age, sex, parental socioeconomic status, and handedness.
Main Outcome Measures
Cingulate gyrus gray matter volumes in 3 anterior subregions (subgenual, affective, and cognitive) and 1 posterior subregion, and whether there was a paracingulate sulcus.
Results
At first hospitalization, patients with FESZ showed significantly smaller left subgenual (P=.03), left (P=.03) and right (P=.005) affective, right cognitive (P=.04), and right posterior (P=.003) cingulate gyrus gray matter sub-regions compared with HCs. Moreover, at the 1½-year follow-up, patients with FESZ showed progressive gray matter volume decreases in the subgenual (P=.002), affective (P<.001), cognitive (P<.001), and posterior (P=.02) cingulate subregions compared with HCs. In contrast, patients with FEAFF showed only initial (left, P<.001; right, P=.002) and progressive subgenual subregion abnormalities (P<.001). Finally, patients with FESZ showed a less asymmetric paracingulate pattern than HCs (P=.02).
Conclusions
Patients with FEAFF and FESZ showed differences in initial gray matter volumes and in their progression. Initial and progressive changes in patients with FEAFF were confined to the subgenual cingulate, a region strongly associated with affective disorder, whereas patients with FESZ evinced widespread initial and progressively smaller volumes.
THE CINGULATE GYRUS IS A cortical area of mixed cytoarchitectonics that links to the limbic system and neocortex.1 The subcomponents of the cingulate gyrus serve a range of functions, including emotional, cognitive and attentional, nociceptive, and motor processing.2-4 Grossly, the anterior cingulate cortex (ACC) is differentiated from the posterior cingulate cortex on the basis of cytoarchitecture, projection patterns, and functions.5-7 For example, the anterior cingulate gyrus is activated by emotional stimuli, whereas the posterior cingulate gyrus is activated by both emotional and nonemotional stimuli and plays an important role in memory access and visuospatial orientation.5,7 Within the ACC, further parcellation can be made on the basis of functional and anatomical studies.8 The rostral area of the ACC (the affective subregion) is connected to the nucleus accumbens, amygdala, insula, hippocampus, and orbitofrontal cortex and assesses the salience of emotional and motivational information regulating emotional responses.8 The caudal (dorsal) area of the ACC (the cognitive subregion) has strong reciprocal interconnections with the lateral prefrontal cortex, parietal cortex, and premotor and supplementary motor areas9 and modulates attention and executive functions, error detection, and working memory.7,10-12 The portion of the ACC located inferior to the genu of the corpus callosum (subgenual subregion) has extensive connections to structures implicated in emotional behavior, mood, and autonomic responses to stressors13 and is the region used for deep-brain stimulation for treatment-resistant depression.14
Abnormalities in these structures play a crucial role in the dysfunction of cognitive and emotional processing in patients with schizophrenia.15 Magnetic resonance imaging (MRI) studies have demonstrated that anterior16-21 and posterior17,22 cingulate gyrus volumes in schizophrenic patients are smaller than in control subjects, although findings have been controversial,23-25 and may differ by sex.18,19 Voxel-based morphometry (VBM) studies have reported decreased cingulate gray matter signal density in subjects at high risk for schizophrenia26 or first-episode schizophrenia (FESZ).27,28 Studies have also reported decreased gray matter in subjects at high risk of schizophrenia (optimized VBM)29 and associated with the allele containing the Val158Met polymorphism of the catechol O-methyltransferase gene in chronic schizophrenia (deformation-based study).30
Decreased volume of the ACC was associated with impaired executive function in schizophrenic patients,31 which is compatible with results of positron emission tomography studies showing abnormal ACC activity for tasks examining the effects of interference32 and attention33,34 in schizophrenia. These studies divided the cingulate gyrus into anterior and posterior parts, without the finer parcellation of the ACC as described in the preceding paragraphs.8-10,13
Abnormalities in the cingulate gyrus have also been reported in affective disorder (mostly bipolar disorder), including smaller subgenual volume35-40 and decreased functional activity.2,39 Postmortem studies have reported more prominent nonpyramidal neuronal loss in layer II in the ACC in affective psychosis compared with schizophrenia, although pyramidal (particularly in layer IV) and nonpyramidal neuron loss were present in schizophrenia.41 However, it remains to be determined whether cingulate gyrus gray matter volumetric abnormalities occur preferentially in schizophrenia or affective disorder, and whether there are specific subregional differences. Patients in their first hospitalization (first-episode patients) present an excellent population in which to examine these issues because they are free of the long-term direct and indirect consequences of the disease, including long-term pharmaceutical treatment.
In addition, longitudinal examination of brain structure in first-episode patients may help determine whether the time of psychotic symptom onset is the critical period for this volume change, and whether this change may be progressive.42 Unfortunately, there are few longitudinal studies of cingulate gyrus.16 A longitudinal study using VBM but without a healthy comparison group showed that left ACC gray matter density decreased over time in FESZ, whereas patients with bipolar disorder showed a bilateral decrease in ACC gray matter density.43 Right ACC gray matter density loss was reported in high-risk subjects developing schizophrenia.44
Abnormalities in the paracingulate sulcus (PCS) pattern may provide a robust marker of the contribution of neurodevelopmental factors to schizophrenia,45,46 because cerebral folding occurs during the second and third trimester47,48 and is stable thereafter, unlike volumes, which may change with disease progression.
Herein, we report cross-sectional and longitudinal gray matter volume findings for cingulate gyrus subregions in patients with FESZ or first-episode affective psychosis (FEAFF, mainly bipolar in a manic phase), compared with healthy control subjects (HCs). Paracingu-late sulcus patterns45 were also examined to investigate the association of gyrification with diagnosis.
METHODS
PARTICIPANTS
Eighty patients with first-episode psychosis (39 patients with FESZ and 41 with FEAFF [of whom 38 had bipolar disorder in a manic phase]) and 40 HCs participated in the cross-sectional study (Table 1). Patients were recruited at McLean Hospital, a Harvard Medical School affiliate. The HCs were recruited through newspaper advertisement. Consistent with the literature and our previous studies, a first episode was operationally defined as the first hospitalization for psychosis.49-51 Subjects had not been previously hospitalized for any psychiatric reason. The inclusion and exclusion criteria for subjects have been described in detail elsewhere.51 Briefly, patients and HCs met criteria for age (18-55 years), IQ (>75), right-handedness, and a history that was negative for seizures, head trauma with loss of consciousness, neurologic disorder, and any lifetime dependence on alcohol or another drug. Patient diagnosis was based on the Structured Clinical Interview for DSM-III-R–Patient Version52 for DSM-III-R or DSM-IV criteria. The HCs had no Axis I or Axis II disorder according to the Structured Clinical Interview for DSM-III-R–Non-Patient Version53 and Structured Clinical Interview for DSM-IV Personality Disorders,54 and no Axis I disorder in their first-degree relatives per self-report.
Table 1.
Demographic and Clinical Characteristics of Cross-sectional Study Subjectsa
| FESZ Group (n=39) | FEAFF Group (n=41) | HC Group (n=40) | Statistical Analysis |
|||
|---|---|---|---|---|---|---|
| F or t Testb | dfc | P Value | ||||
| Age, mean (SD) [range], y | 23.9 (5.5) [18-40] | 22.8 (4.5) [18-41] | 23.0 (3.2) [18-30] | 0.8 | 2, 117 | .47 |
| Sex, No. M/Fd | 30/9 | 32/9 | 31/9 | |||
| Handednesse | 0.75 (0.3) | 0.76 (0.2) | 0.80 (0.2) | 0.8 | 2, 113 | .46 |
| SESf | 3.3 (1.5) | 2.9 (1.3) | 2.3 (1.0) | 6.5 | 2, 117 | .002g |
| Parental SES | 1.9 (0.9) | 1.6 (0.9) | 1.6 (1.0) | 1.1 | 2, 117 | .32 |
| Years of educationh | 13.6 (2.2) | 13.7 (1.9) | 14.9 (2.1) | 5.3 | 2, 115 | .006g |
| WAIS-R Information, scaled baselinei | 11.5 (3.2) | 12.6 (2.7) | 13.3 (2.2) | 4.3 | 2, 113 | .02j |
| WAIS-R Digit Span, scaled baselinek | 10.4 (2.9) | 9.8 (2.5) | 11.3 (2.4) | 3.6 | 2, 113 | .03j |
| Duration of illness, wk | 11.2 (16.3) | 8.2 (13.2) | NA | 0.9 | 78 | .36 |
| Medication dosage at baseline, CPZ equivl | 251.5 (196.1) | 244.2 (188.1) | NA | 0.03 | 66 | .87 |
| Duration of antipsychotic medication before baseline scan, median (range), wk | 3 (0-24) | 1 (0-26) | NA | NA | NA | NA |
| MMSE | 28.4 (2.2) | 28.9 (1.5) | 29.0 (1.0) | 1.5 | 2, 114 | .24 |
| BPRSl | 38.8 (10.8) | 34.2 (9.2) | NA | 1.8 | 75 | .06 |
| GASl | 35.1 (7.7) | 37.7 (11.0) | NA | 1.3 | 74 | .25 |
| No. with/without family history of first-degree relatives with psychosis | 20/17 (among 37) | 30/10 (among 40) | ||||
Abbreviations: BPRS, Brief Psychiatric Rating Scale; CPZ equiv, chlorpromazine equivalent; FEAFF, first-episode affective psychosis; FESZ, first-episode schizophrenia; GAS, Global Assessment Scale; HC, healthy control; MMSE, Mini-Mental State Examination; NA, data not applicable; SES, socioeconomic status; WAIS-R, Wechsler Adult Intelligence Scale–Revised.
Of 41 patients with FEAFF, 38 had bipolar disorder and were in a manic phase, 1 had bipolar disorder and was in a mixed episode, and 2 had major depression with psychotic features. Unless otherwise indicated, data are expressed as mean (SD).
The F tests (1-way analysis of variance) were performed among FESZ, FEAFF, and HC groups for age, handedness, SES, parental SES, WAIS-R Information and Digit Span scaled scores, and MMSE scores. The t tests were performed between FESZ and FEAFF groups for duration of illness, medication dosage, BPRS scores, and GAS scores.
The degrees of freedom differ among variables owing to unavailability of data in some participants.
χ2 test (F2=0.02; P=.99) showed no difference in sex ratio among the 3 groups.
Evaluated using the Edinburgh Handedness Inventory as ([right hand-left hand]×100)/(right hand+left hand); scores >0 indicate right-handedness.
Higher numbers represent lower SES, based on the Hollingshead 2-factor index of SES. The FESZ group showed a significantly lower SES than the HC group (Tukey Honestly Significant Difference [HSD] test, P=.002).
P<.01.
The FESZ (P=.01) and FEAFF (P=.02) groups showed significantly fewer years of education than the HC group in Tukey HSD tests.
The FESZ group (P=.001) showed significantly lower scores than the HC group in Tukey HSD tests.
P<.05.
The FEAFF group (P=.002) showed significantly lower scores than the HC group in Tukey HSD tests.
The t tests were performed between 2 groups. Before magnetic resonance imaging scanning, 5 patients with FESZ and 16 with FEAFF were neuroleptic naive. Duration of neuroleptic therapy was less than 4 weeks in 13 patients with FESZ (including typical neuroleptics [TYP] in 8, atypical neuroleptics [ATYP] in 6, and overlap in 1) and 13 patients with FEAFF (including TYP in 7, ATYP in 8, and overlap in 2) and 4 to 245 weeks in 14 patients with FESZ (TYP in 5, ATYP in 12, and overlap in 3) and 6 patients with FEAFF (TYP in 1 and ATYP in 5). The remaining 7 patients with FESZ (TYP in 2 and ATYP in 5 at testing) and 6 with FEAFF (TYP in 2 and ATYP in 4 at testing) were unable to provide exact information about duration of neuroleptic therapy before hospitalization. For mood stabilizers (MS), including lithium carbonate and valproic acid, 9 of 39 patients with FESZ (23.1%) and 31 of 41 with FEAFF (75.6%) were treated at their first-episode hospitalization. Five of these MS-treated patients with FEAFF received MS for more than 4 weeks before the magnetic resonance imaging.
Eighteen HCs and 35 patients with first-episode psychosis (17 patients with FESZ and 18 with FEAFF [of whom 17 had bipolar disorder in a manic phase]) were longitudinally re-scanned approximately 1½ years later (Table 2). Using only subjects with bipolar disorder did not change the statistical results in the cross-sectional or the longitudinal sample. The cross-sectional and longitudinal groups were matched for age, sex, handedness,55 and parental socioeconomic status. Medication history before and during the first hospitalization, between scans, and during any second hospitalization, if present, was assessed by patient report and through medical chart review. Dosage (Tables 1 and 2) of antipsychotics56 did not correlate with any initial volume or volume change. Subjects’ serum levels of mood stabilizers were monitored by their treating psychiatrist. Two patients with FEAFF self-reported they had not adhered to their treatment regimen before the second scan. This study was approved by the McLean Hospital, Veterans Affairs Boston Healthcare System, and Harvard Medical School institutional review boards. Written informed consent was obtained from all subjects before study participation.
Table 2.
Demographic and Clinical Characteristics of Longitudinal Study Subjectsa
| FESZ Group (n=17) | FEAFF Group (n=18) | HC Group (n=18) | Statistical Analysis |
|||
|---|---|---|---|---|---|---|
| F or t Testb | dfc | P Value | ||||
| Age, mean (SD [range]), y | 24.6 (8.5) [18-40] | 22.4 (3.9) [18-30] | 23.4 (5.7) [18-34] | 1.2 | 2, 50 | .31 |
| Time between scans, mo | 17.8 (11.6) | 18.3 (9.3) | 21.2 (16.1) | 0.5 | 2, 50 | .59 |
| Sex, No. M/Fd | 14/3 | 15/3 | 15/3 | |||
| Handednesse | 0.83 (0.1) | 0.73 (0.2) | 0.81 (0.2) | 2.1 | 2, 49 | .14 |
| SESf | 3.2 (1.4) | 3.0 (1.5) | 2.1 (1.2) | 3.7 | 2, 49 | .03g |
| Parental SESf | 2.0 (0.6) | 1.7 (0.9) | 1.6 (0.9) | 1.4 | 2, 49 | .25 |
| Years of education | 13.7 (2.1) | 13.9 (2.4) | 15.3 (1.8) | 3.1 | 2, 49 | .05 |
| WAIS-R Information, scaled baseline | 11.8 (3.0) | 13.1 (2.3) | 13.1 (2.2) | 1.4 | 2, 49 | .27 |
| WAIS-R Digit Span, scaled baseline | 9.7 (2.0) | 10.5 (2.3) | 11.7 (3.1) | 3.0 | 2, 49 | .06 |
| MMSE | ||||||
| Baseline scan | 27.7 (2.7) | 28.7 (1.5) | 28.9 (0.9) | 2.5 | 2, 49 | .10 |
| Second scan | 27.9 (1.0) | 28.7 (1.2) | 27.8 (2.6) | 2.0 | 2, 49 | .15 |
| BPRSh | ||||||
| Baseline scan | 41.2 (13.6) | 34.5 (8.6) | NA | 1.7 | 32 | .10 |
| Second scan | 34.7 (14.0) | 29.8 (15.0) | NA | 1.0 | 32 | .34 |
| GASh | ||||||
| Baseline scan | 36.9 (7.8) | 41.1 (8.1) | NA | 1.5 | 32 | .14 |
| Second scan | 49.0 (16.2) | 49.4 (18.6) | NA | 0.1 | 31 | .96 |
| Medication dosage at baseline, CPZ equivh | 259.1 (204.5) | 197.4 (133.8) | NA | 1.1 | 33 | .31 |
| Duration of antipsychotic medication before baseline scan, median (range), wk | 3 (0-24) | 2 (0-13) | NA | NA | NA | NA |
| Medication use, No. of patients | ||||||
| Neuroleptics, TYP/ATYP/overlap | ||||||
| At baseline scan | 8/12/5i | 7/11/1 | NA | |||
| At second scan | 2/13/2 | 1/9/1 | NA | |||
| Mood stabilizer, lithiumj/VPA/overlap | ||||||
| At baseline scan | 1/2/0 | 6/7/1 | NA | |||
| At second scan | 3/3/0 | 5/6/0 | NA | |||
Abbreviations: ATYP, atypical neuroleptics; BPRS, Brief Psychiatric Rating Scale; CPZ equiv, chlorpromazine equivalent; FEAFF, first-episode affective psychosis; FESZ, first-episode schizophrenia; GAS, Global Assessment Scale; HC, healthy control; MMSE, Mini-Mental State Examination; NA, data not applicable; SES, socioeconomic status; TYP, typical neuroleptics; VPA, valproic acid; WAIS-R, Wechsler Adult Intelligence Scale–Revised.
Of 18 patients with FEAFF, 17 had bipolar disorder and were in a manic phase, and 1 had major depression. Unless otherwise indicated, data are expressed as mean (SD).
The F tests (1-way analysis of variance) were performed among FESZ, FEAFF, and HC groups for age, time between magnetic resonance imaging scans, handedness, SES, parental SES, WAIS-R Information and Digit Span scaled scores, and MMSE scores. The t tests were performed between FESZ and FEAFF groups for BPRS scores, GAS, duration of illness, and medication dosage.
The degrees of freedom differ among variables owing to unavailability of data in some participants.
χ2 Test (F2=0.01; P=.99) showed no difference in sex ratio among the 3 groups.
Evaluated using the Edinburgh Handedness Inventory as ([right hand-left hand]×100)/(right hand+left hand); scores >0 indicate right-handedness.
Higher numbers represent lower SES, based on the Hollingshead 2-factor index of SES. The FESZ group showed a significantly lower SES than the HC group (Tukey Honestly Significant Difference [HSD] test, P=.037).
P<.05.
The t tests were performed between 2 groups.
Neuroleptic type was unknown for 1 patient with FESZ owing to inclusion in an independent double-blind medication trial.
Indicates lithium carbonate.
Clinical evaluations at times 1 and 2 included the Brief Psychiatric Rating Scale (BPRS),57,58 the Mini-Mental State Examination,59 the Wechsler Adult Intelligence Scale–Revised (WAIS-R),60 and the Global Assessment Scale.61
REGIONS OF INTEREST
The neuroanatomical regions of interest (ROIs) were outlined manually on a workstation (Figure 1). The cingulate gyrus was bounded superiorly by the cingulate sulcus and inferiorly by the callosal sulcus on each of the coronal image sections (slices). The anatomical landmark for dividing the cingulate gyrus into anterior and posterior cingulate regions was a vertical line11 passing through the anterior commissure point in the midsagittal section. Within the anterior cingulate gyrus, further parcellations were made forming subgenual,39 affective (anterorostral),8,62 and cognitive (anterodorsal)8 subregion ROIs.
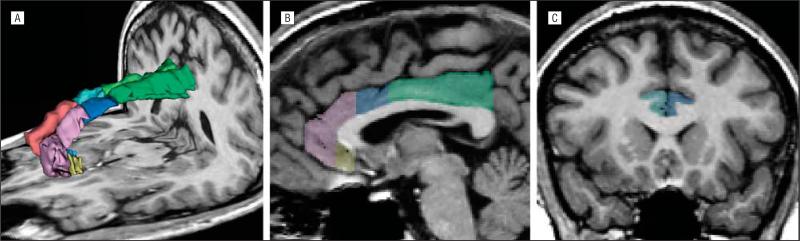
Figure 1.
Cingulate gyrus subregions. Three-dimensional reconstruction (A) of the cingulate gyrus gray matter according to subregions (subgenual, affective [anterorostral], cognitive [anterodorsal], and posterior divisions), seen in sagittal (B) and coronal (C) views. On the sagittal view of the left cingulate gyrus (B), the subgenual subdivision is yellow; the affective subdivision, pink; the cognitive subdivision, blue; and the posterior division, green.
The subgenual subregion39 was defined as the cingulate area under the corpus callosum bounded anteriorly by the line passing through the anterior margin of the genu of corpus callosum and posteriorly 1 section anterior to the internal capsule that divides the striatum. We note that the anatomical boundaries of the area designated as subgenual have varied considerably across studies, and a study38 has shown that findings may depend on whether the anterior or posterior portion of the overall subgenual area is used. The affective subregion (anterorostral)8 was bounded anteriorly by the cingulate sulcus and posteriorly above the corpus callosum by a line62 passing through the most anterior point of the inner surface of the genu of the corpus callosum, and anterior to the subgenual division below the corpus callosum. The cognitive subregion (anterodorsal)8 was defined as the remaining ACC between the affective subregion and posterior cingulate gyrus. The posterior cingu-late subregion extended to the line passing through the most posterior end of the corpus callosum.63 We did not include the most posterior part of the posterior cingulate division, often termed the retrosplenial cortex,64,65 because there were no clear MRI boundaries to define it.
Some, but not all, brains contained a PCS, parallel to the cingulate sulcus. The PCS was classified as prominent if the sulcus extended at least 40 mm and exhibited no more than 20 mm of interruptions between its origin and a coronal plane passing through the anterior commissure. If interruptions exceeded 20 mm and the length was at least 20 mm, the PCS was classified as present. Finally, when the horizontal sulcus was less than 20 mm in length, it was classified as absent.66,67 When the PCS was present, the paracingulate gyrus, which is mainly Brodmann area 32, was excluded from the cingulate gyrus measurement. To determine association with diagnosis, the number of cases with a PCS present were compared among groups.
MRI PROCESSING
The MRI protocol used 2 pulse sequences on a 1.5-T MRI system (GE Medical Systems, Milwaukee, Wisconsin), as described by Kasai et al50 and in our supplemental text (available at http://www.archgenpsychiatry.com). All manual ROI parcellations were performed by investigators blinded to diagnoses and the date of scan. To assess interrater reliability, 3 raters (including M.-S.K. and M.N.) who were blinded to the diagnoses independently delineated the ROIs for 5 random cases and examined the PCS patterns for 30 random cases. Intraclass correlation coefficients were 0.97 for the subgenual, 0.98 for the affective, 0.97 for the cognitive, and 0.96 for the posterior subregions. Interrater reliability for the PCS pattern was also high (κ=0.89 for the left side and κ=0.77 for the right side). The voxel volumes of gray and white matter and cerebrospinal fluid were summed, yielding the total intracranial content (ICC).
STATISTICAL ANALYSIS
Group differences in cingulate gyrus gray matter volumes were first assessed using repeated-measures analysis of variance (ANOVA), with the diagnostic group as the between-subjects factor and the hemisphere (left or right) and region (anterior and posterior) as the within-subjects factors. On finding significant results, we then subdivided the anterior region into subgenual, affective, and cognitive subregions for a second repeated-measures ANOVA. Relative volume (given as a percentage and calculated as [absolute volume/ICC]×100) was used to control for individual head size. Groups did not differ significantly in ICC at time 1 (P=.18) or in their ICC volume changes between imaging (P=.51). Of note, the statistical conclusions reported herein remained the same when we analyzed absolute volumes using the ICC as a covariate. For the longitudinal volume comparison, the percentage of volume change was calculated with the following formula:
We further examined relative volumes of each subregion using 1-way ANOVA, with follow-up post hoc Tukey Honestly Significant Difference tests. In addition, to evaluate which subregion showed differences between times 1 and 2 in gray matter volumes among groups, we examined the percentage of differences of relative volumes for each subregion using 1-way ANOVA with follow-up post hoc Tukey Honestly Significant Difference tests.
To examine the PCS pattern, we assessed intragroup asymmetry using the McNemar test for symmetry. Between-group differences for rightward and leftward asymmetry were assessed using χ2. Asymmetry was measured by an asymmetry index assigned to each individual in terms of a leftward (where left is greater than right), symmetric (where left equals right), or rightward (where left is less than right) prominence of PCS.
To examine the associations between volume change and clinical outcome or cognitive test score (eg, WAIS-R score), Spearman correlation coefficients (ρ) were used. Clinical outcome was evaluated by the percentage of change in factor scores in BPRS using the following equation:
RESULTS
There were no significant group differences in age, sex, handedness, parental socioeconomic status, or Mini-Mental State Examination scores among groups. The patient groups showed lower socioeconomic status, less education, and lower WAIS-R performance, consistent with reduced functioning due to the disorder. There were no significant differences in medication dosage, BPRS scores, or Global Assessment Scale scores between the patients with FESZ and FEAFF in either the cross-sectional or the longitudinal studies (Tables 1 and 2).
INITIAL VOLUMES (CROSS-SECTIONAL STUDY)
Total Cingulate Gyrus Gray Matter Volume
A repeated-measures ANOVA of cingulate gyrus gray matter relative volume with group (FESZ, FEAFF, or HC) as the between-subjects factor and hemisphere (left vs right) and region (anterior vs posterior cingulate region) as the within-subjects factors revealed that groups differed in whole cingulate gray matter volumes (F2,117=7.7 [P=.001]). All groups showed larger cingulate volumes in the right hemisphere than in the left (main effect, F1,117=4.4; P=.037; with no significant interactions of hemisphere × group [F2,117=0.2; P=.83] or region × hemisphere × group [F2,117=0.2; P=.79]). Although the posterior cingulate showed larger volumes than the anterior cingulate (main effect for region, F1,117=5.9; P=.02), group differences were not the same in the anterior and posterior cingulate (interactions of region × group, F2,117=3.2; P=.04). The main effects of group were further analyzed by 1-way ANOVA of the entire cingulate volume and follow-up Tukey tests, with smaller volumes in the FESZ vs HC group (P<.001), equal volumes in the FEAFF vs HC group (P=.12), and equal volumes in the FEAFF vs FESZ group (P=.07). Moreover, the right was larger than the left hemisphere gray matter volume (t119=2.4; P=.02), accounting for the main effect of hemisphere. Follow-up tests indicated highly significant group differences in the anterior cingulate (F2,117=6.9; P=.001) but only trend-level differences in the posterior cingulate (F2,117=2.3; P=.06).
Based on our hypothesis of differences derived from previous studies, we further divided the anterior cingu-late gyrus into the subgenual, affective, and cognitive sub-regions. Groups differed in whole anterior cingulate gray matter volumes (F2,117=6.9; P=.001). All groups showed larger anterior cingulate volumes in the right than in the left hemisphere (main effect, F1,117=7.2; P=.008; with no significant interactions of hemisphere × group [F2,117=0.4; P=.67] or region × hemisphere [F2,234=2.9; P=.06]). Of note, the subregional differences were different among groups (main effect for subregion, F2,234=2479.1; P<.001), and there was a significant interaction between groups and subregion (F4,234=3.7; P=.01) in the anterior cingu-late (Table 3).
Table 3.
Absolute and Relative Volumes of Cingulate Gyrus Gray Matter in the FESZ, FEAFF, and HC Groupsa
| FESZ Group (n=39) |
FEAFF Group (n=41) |
HC Group, Mean (SD) (n=40) | 1-Factor ANOVA |
Post Hoc Tukey HSD Test | ||||
|---|---|---|---|---|---|---|---|---|
| Region and Volumeb | Mean (SD) | Effect Sizec | Mean (SD) | Effect Sizec | F Testd | P Value | ||
| ICC | 1465.1 (111.5) | 1443.0 (137.5) | 1509.0 (149.2) | 2.05 | .183 | FESZ=FEAFF=HC | ||
| Whole cingulate gyrus | ||||||||
| Total | ||||||||
| Absolute, mL | 18.617 (2.599) | 19.415 (2.454) | 21.132 (2.673) | 9.88 | <.001 | |||
| Relative, % | 1.254 (0.196) | 0.93 | 1.347 (0.176) | 0.46 | 1.428 (0.178) | 8.90 | <.001 | FESZ<HC, FESZ=FEAFF, FEAFF=HCe |
| Leftf | ||||||||
| Absolute, mL | 9.288 (1.328) | 9.496 (1.384) | 10.185 (1.449) | 4.55 | .01 | |||
| Relative, % | 0.628 (0.113) | 0.56 | 0.656 (0.095) | 0.33 | 0.690 (0.108) | 3.38 | .04 | FESZ<HC, FESZ= FEAFF, FEAFF=HCg |
| Rightf | ||||||||
| Absolute, mL | 9.329 (1.617) | 9.919 (1.486) | 10.947(1.714) | 10.28 | <.001 | |||
| Relative, % | 0.626 (0.115) | 0.97 | 0.691 (0.112) | 0.42 | 0.739 (0.117) | 9.57 | <.001 | FESZ<HC, FEAFF<HC, FEAFF=FESZh |
| Anterior cingulate gyrus | ||||||||
| Left | ||||||||
| Absolute, mL | 4.588 (0.816) | 4.730 (0.882) | 5.182 (0.950) | 4.89 | .009 | |||
| Relative, % | 0.314 (0.053) | 0.55 | 0.329 (0.059) | 0.27 | 0.345 (0.060) | 3.21 | .04 | FESZ<HC, FESZ=FEAFF, FEAFF=HCi |
| Right | ||||||||
| Absolute, mL | 4.737 (0.874) | 4.950 (0.884) | 5.551 (0.836) | 9.45 | <.001 | |||
| Relative, % | 0.324 (0.057) | 0.85 | 0.345 (0.064) | 0.41 | 0.369 (0.049) | 6.17 | .003 | FESZ<HC, FESZ=FEAFF, FEAFF=HCj |
| Posterior cingulate gyrus | ||||||||
| Left | ||||||||
| Absolute, mL | 4.700 (0.823) | 4.766 (0.827) | 5.003 (1.028) | |||||
| Relative, % | 0.322 (0.060) | 0.19 | 0.330 (0.047) | 0.06 | 0.333 (0.068) | 0.37 | .69 | FESZ=HC, FEAFF=HC, FEAFF=FESZk |
| Right | ||||||||
| Absolute, mL | 4.591 (0.985) | 4.969 (0.974) | 5.396 (1.083) | |||||
| Relative, % | 0.313 (0.060) | 0.73 | 0.344 (0.058) | 0.23 | 0.358 (0.064) | 5.82 | .004 | FESZ<HC, FESZ=FEAFF, FEAFF=HCl |
| Anterior cingulate subdivisions | ||||||||
| Subgenual cingulate gyrus | ||||||||
| Left | ||||||||
| Absolute, mL | 0.449 (0.103) | 0.398 (0.095) | 0.532 (0.169) | |||||
| Relative, % | 0.031 (0.006) | 0.48 | 0.028 (0.007) | 0.81 | 0.035 (0.011) | 9.12 | <.001 | FEAFF<HC, FESZ<HC, FEAFF=FESZm |
| Right | ||||||||
| Absolute, mL | 0.482 (0.098) | 0.425 (0.093) | 0.532 (0.127) | |||||
| Relative, % | 0.033 (0.007) | 0.27 | 0.030 (0.007) | 0.67 | 0.035 (0.008) | 6.01 | .003 | FEAFF<HC, FESZ=HC, FESZ=FEAFFn |
| Affective (anterorostral) cingulate gyrus | ||||||||
| Left | ||||||||
| Absolute, mL | 2.743 (0.583) | 2.898 (0.573) | 3.142 (0.579) | |||||
| Relative, % | 0.188 (0.038) | 0.56 | 0.201 (0.038) | 0.21 | 0.209 (0.037) | 3.32 | .04 | FESZ<HC, FESZ=FEAFF, FEAFF=HCo |
| Right | ||||||||
| Absolute, mL | 2.859 (0.523) | 3.045 (0.597) | 3.359 (0.633) | |||||
| Relative, % | 0.196 (0.035) | 0.75 | 0.212 (0.040) | 0.28 | 0.223 (0.037) | 5.25 | .003 | FESZ<HC, FESZ=FEAFF, FEAFF=HCp |
| Cognitive (anterodorsal) cingulate gyrus | ||||||||
| Left | ||||||||
| Absolute, mL | 1.396 (0.385) | 1.435 (0.420) | 1.507 (0.443) | |||||
| Relative, % | 0.096 (0.026) | 0.15 | 0.099 (0.028) | 0.04 | 0.100 (0.028) | 0.37 | .69 | FESZ=HC, FEAFF=HC, FEAFF=FESZq |
| Right | ||||||||
| Absolute, mL | 1.396 (0.483) | 1.480 (0.395) | 1.659 (0.413) | |||||
| Relative, % | 0.095 (0.031) | 0.51 | 0.103 (0.029) | 0.25 | 0.110 (0.027) | 2.78 | .048 | FESZ<HC, FESZ=FEAFF, FEAFF=HCr |
Abbreviations: ANOVA, analysis of variance; FEAFF, first-episode affective psychosis; FESZ, first-episode schizophrenia; HCs, healthy control subjects; HSD, Honestly Significant Difference; ICC, intracranial contents.
Expressed as cross-sectional data at time 1 (first magnetic resonance imaging). Whole cingulate gyrus equals anterior plus posterior cingulate gyrus; anterior cingulate gyrus, subgenual plus affective plus cognitive cingulate subregions.
Repeated-measures ANOVA of relative volume with group (FESZ, FEAFF, and HC) as the between-subjects factor, and hemisphere (left vs right) and region (anterior vs posterior cingulate regions) as the within-subjects factors revealed a main effect for group (F2,117=7.7; P=.001), hemisphere (F1,117=4.4; P=.04), and region (F1,117=5.9; P=.02). There was a significant interaction of region×group (F2,117=3.2; P=.04). However, there was no significant interaction of hemisphere×group (F2,117=0.2; P=.83) or region×hemisphere×group (F2,117=0.2; P=.79). Within the anterior cingulate gyrus alone, there was a significant interaction of group×subregion (F4,234=3.7; P=.01). Within-subjects factor revealed a main effect for group (F2,117=6.9; P=.001), hemisphere (F1,117=7.2; P=.008), and subregion (F2,234=2479.1; P<.001). There were no significant interactions of hemisphere×group (F2,117=0.4; P=.67), region×hemisphere (F2,234=2.9; P=.06), or region×hemisphere×group (F4,234=0.5; P=.75) (Hyunh-Feldt ε, 0.853 with region, 1.0 with hemisphere, and 0.943 with region×hemisphere).
Calculated as the difference between the patient and HC groups, based on relative volumes ([absolute volume/ICC in cubic centimeters]×100) (in percentages).
df=2,117.
P<.001, P=.07, and P=.12, respectively.
A paired t test showed a significant difference between the left and right hemisphere (t119=2.4; P=.02). Calculation based on relative volumes.
P=.03, P=.47, and P=.33, respectively.
P<.001, P=.04, and P=.15, respectively.
P=.04, P=.46, and P=.41, respectively.
P=.002, P=.22, and P=.15, respectively.
P=.68, P=.97, and P=.82, respectively.
P=.003, P=.055, and P=.56, respectively.
P<.001, P=.03, and P=.27, respectively.
P=.002, P=.34, and P=.11, respectively.
P=.03, P=.23, and P=.62, respectively.
P=.005, P=.14, and P=.38, respectively.
P=.71, P=.99, and P=.78, respectively.
P=.04, P=.40, and P=.53, respectively.
Subregions of Cingulate Gyrus Gray Matter Volume
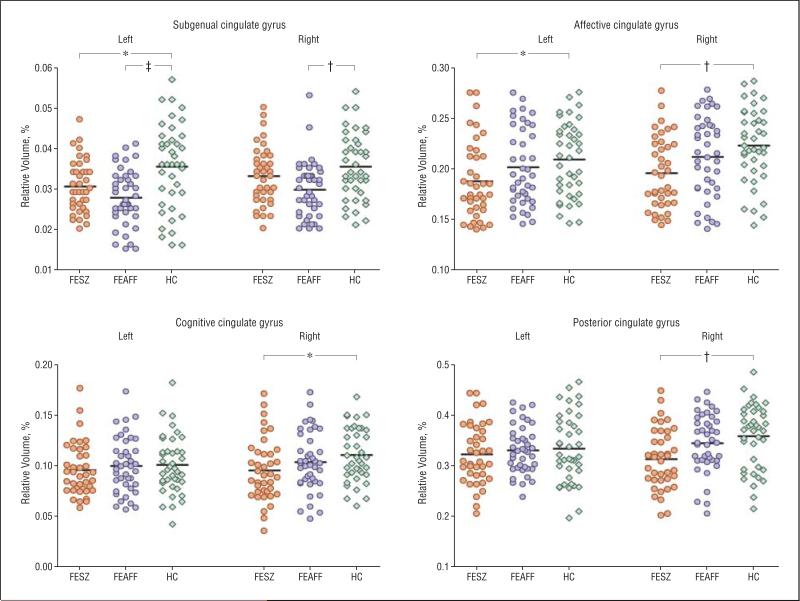
In the subgenual subregion, the FEAFF group showed significantly smaller gray matter volumes than HCs in the left (P<.001; effect size [d]=0.81]) and right sides (P=.002; d=0.67). Also, the left subgenual subregion gray matter volumes of the FESZ group were significantly smaller than those of the HCs (P=.026; d=0.48). In the affective (anterorostral) subregion, the FESZ group showed bilaterally significantly smaller gray matter volumes than the HCs (left, P=.03 [d=0.56]; right, P=.005 [d=0.75]). There were no statistical differences on either side between the FEAFF and HC groups. In the cognitive (anterodorsal) subregion on the right (P=.04; d=0.51) and the posterior subregion on the right (P=.003; d=0.73), the FESZ group showed smaller GM volumes than the HCs (Figure 2 and Table 3).
Figure 2.
Relative cross-sectional volumes of the cingulate gyrus subregions by hemisphere in patients with first-episode schizophrenia (FESZ) (n=39) or first-episode affective psychosis (FEAFF) (n=41) and healthy control subjects (HCs) (n=40). *P<.05; †P<.01; ‡P<.001, by analysis of variance.
The relative subgenual volumes of patients with FEAFF who had a positive family history for a mood disorder were significantly smaller than those of patients with FEAFF who had no family history for both left (t38=3.4; P=.002) and right (t38=3.0; P=.005) sides.
Clinical Correlations With Cingulate Gyrus Volume at Time 1 (Initial Scan)
In patients with FESZ, the BPRS withdrawal factor scores were negatively correlated with relative volumes of the right affective (ρ=−0.47; d=0.93; P=.008) and the right cognitive subregions (ρ=−0.40; d=0.88; trend-level P=.09). In patients with FEAFF, hostility-suspicious factor scores were negatively correlated with left subgenual subregion relative volumes (ρ = −0.35; d = 0.75; P = .03). Examination of volumes and WAIS-R scores revealed, although only in the FESZ group, that relative volumes of the cognitive subregion (right side) were significantly and positively correlated with Digit Span (ρ = 0.36; d = 0.76; P = .03 [n = 38]) and Information (ρ = 0.37; d = 0.77; P = .02 [n = 38]) scaled scores. If the Bonferroni correction was applied with the clinical BPRS factors and the WAIS-R scores, the α threshold would be 0.0031 (0.05/16) and 0.0063 (0.05/8), respectively, and none of these correlation results would be statistically significant. Of note, however, Fisher z transformation and the subsequent comparison tests showed significant differences in FESZ vs FEAFF correlations in the right affective subregion's correlations with BPRS withdrawal factor scores (z = 2.1; P = .02) and also in the left subgenual subregions correlations with BPRS hostility-suspicious factor scores (z=1.7; P=.04).
PCS Patterns
Within-group comparisons showed that HCs had a significant PCS asymmetry (McNemar test χ23=10.9; P=.01). However, in patients with FESZ (McNemar test ; P=.38) as well as in patients with FEAFF (McNemar test ; P=.11), no significant asymmetry was detected. Follow-up between-group comparisons revealed a significant difference for asymmetric index between the FESZ and HC groups (; P=.02) (Table 4). The PCS patterns did not change longitudinally in any group.
Table 4.
Hemispheric Distribution of the Morphological Patterns of the PCS in the FESZ, FEAFF, and HC Groups
| Pattern of Morphology in Left Hemisphere, No. of Cases |
||||||
|---|---|---|---|---|---|---|
| Pattern of Morphology in Right Hemisphere | Prominenta | Presentb | Absentc | Total | Subtotald | e |
| FESZ groupf | 3.1 (.38) | |||||
| Prominent |  |
1 | 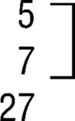 |
12 | ||
| Present | 5 | |||||
| Absent | 21 | |||||
| Total | 27 | |||||
| Subtotald | ||||||
| FEAFF groupg | 6.1 (.11) | |||||
| Prominent |  |
4 | 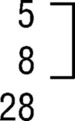 |
13 | ||
| Present | 1 | |||||
| Absent | 17 | |||||
| Total | 22 | |||||
| Subtotald | ||||||
| HC group | 10.9 (.01) | |||||
| Prominent |  |
0 | 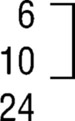 |
16 | ||
| Present | 3 | |||||
| Absent | 14 | |||||
| Total | 17 | |||||
| Subtotald | ||||||
Abbreviations: FEAFF, first-episode affective psychosis; FESZ, first-episode schizophrenia; HCs, healthy control subjects; PCS, paracingulate sulcus.
Indicates the sulcus extended at least 40 mm and exhibited no more than 20 mm of interruptions between its origin and a coronal plane passing through the anterior commissure.
Indicates interruptions exceeded 20 mm and the length was at least 20 mm.
Indicates no clearly horizontal sulcus parallel to the cingulated sulcus could be found or it was less than 20 mm in length.
Indicates subtotal sum of the prominent and present case numbers.
Intragroup asymmetry was assessed using the McNemar test for symmetry.
A χ2 test (; P = .02) showed a significant difference for the asymmetric index between the FESZ and HC groups.
A χ2 test showed no significant differences for the asymmetric index between the FESZ and FEAFF groups (; P = .09) or between the FEAFF and HC groups (; P = .85).
LONGITUDINAL VOLUME CHANGES
Cingulate Gyrus Gray Matter Volume Changes Over Time
Repeated-measures ANOVA of relative volume difference (percentage of change) with group (FESZ, FEAFF, and HC) as the between-subjects factor and hemisphere (left vs right) and region (anterior vs posterior) as the within-subjects factors revealed that the groups differed in percentages of volume change in cingulate gyrus gray matter (F2,50=27.0; P<.001). However, regional group differences in percentages of change were different in the anterior and posterior cingulate (main effect for region, F1,50=25.4; P<.001; significant interaction between region and group, F2,50=7.5; P=.001). There was no left-right hemispheric difference in percentages of change (main effect for hemisphere, F1,50=0.1; P=.783) and no interaction of hemisphere × group (F2,50=0.2; P=.86), region × hemisphere ( F1,50 = 0.2; P = .64), or region × hemisphere × group (F2,50 = 0.8; P = .43) (Table 5). Further analysis using 1-way ANOVA comparisons for anterior and posterior cingulate components showed significant between-group differences for both the anterior component (F2,50=27.5; P<.001) and the posterior component (F2,50=4.4; P=.02).
Table 5.
Absolute and Relative Volumes of Cingulate Gray Matter at Baseline (Time 1) and 1½ Years Later (Time 2) and Percentage of Change in the FESZ, FEAFF, and HC Groups
| FESZ Group (n=17) |
FEAFF Group (n=18) |
HC Group (n=18) |
Overall 3-Group Comparison of Percentages of Change (1-Factor ANOVA by Group)b |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time 1, Mean (SD) |
Time 2, Mean (SD) |
Change, % (SED)a |
Time 1, Mean (SD) |
Time 2, Mean (SD) |
Change, % (SED)a |
Time 1, Mean (SD) |
Time 2, Mean (SD) |
Change, % (SED)a |
2-Group Comparison Tukey HSD Post Hoc Tests |
|||
| Region | F2,50 | P Value | ||||||||||
| ICC, mL | 1487.7 (98.2) | 1487.4 (97.9) | –0.0002 (0.002) | 1480.7 (116.9) | 1476.3 (121.7) | –0.003 (0.015) | 1492.1 (132.0) | 1492.8 (131.9) | 0.0005 (0.006) | 0.7 | .51 | FESZ=FEAFF=HC |
| Anterior cingulate gyrusc | ||||||||||||
| Left+right | ||||||||||||
| Absolute, mL | 9.204 (1.226) | 8.690 (1.181) | –5.57 (0.62) | 9.744 (1.512) | 9.517 (1.433) | –2.23 (0.47) | 10.780 (1.747) | 10.739 (1.781) | –0.42 (0.39) | 27.2 | <.001 | FESZ<HC, FESZ<FEAFF, FEAFF<HCd |
| Relative, % | 0.619 (0.072) | 0.585 (0.072) | 0.660 (0.099) | 0.644 (0.094) | 0.724 (0.107) | 0.721 (0.110) | ||||||
| Left | ||||||||||||
| Absolute, mL | 4.536 (0.656) | 4.294 (0.629) | –5.29 (0.91) | 4.761 (0.955) | 4.637 (0.969) | –2.72 (0.54) | 5.247 (0.922) | 5.230 (0.945) | –0.38 (0.43) | 14.2 | <.001 | FESZ<HC, FESZ<FEAFF, FEAFF<HC |
| Relative, % | 0.305 (0.038) | 0.289 (0.038) | 0.322 (0.059) | 0.313 (0.060) | 0.353 (0.056) | 0.351 (0.059) | ||||||
| Right | ||||||||||||
| Absolute, mL | 4.668 (0.718) | 4.396 (0.724) | –5.92 (0.84) | 4.983 (0.804) | 4.881 (0.747) | –1.89 (0.81) | 5.533 (1.042) | 5.509 (1.052) | –0.46 (0.47) | 15.1 | <.001 | FESZ<HC, FESZ<FEAFF, FEAFF=HC |
| Relative, % | 0.314 (0.046) | 0.296 (0.047) | 0.338 (0.058) | 0.331 (0.055) | 0.371 (0.065) | 0.370 (0.066) | ||||||
| Posterior cingulate gyrus | ||||||||||||
| Left+right | ||||||||||||
| Absolute, mL | 10.008 (1.544) | 9.791 (1.386) | –1.98 (0.57) | 10.046 (1.660) | 9.993 (1.666) | –0.52 (0.45) | 10.597 (2.289) | 10.569 (2.319) | –0.30 (0.21) | 4.4 | .02 | FESZ<HC, FESZ=FEAFF, FEAFF=HCe |
| Relative, % | 0.675 (0.106) | 0.660 (0.094) | 0.677 (0.085) | 0.673 (0.084) | 0.709 (0.132) | 0.707 (0.132) | ||||||
| Left | ||||||||||||
| Absolute, mL | 5.155 (0.796) | 5.046 (0.734) | –1.95 (0.73) | 4.866 (0.865) | 4.854 (0.865) | –0.24 (0.52) | 5.205 (1.137) | 5.196 (1.379) | –0.24 (0.39) | 3.1 | .055 | FESZ=FEAFF=HC |
| Relative, % | 0.348 (0.057) | 0.340 (0.052) | 0.328 (0.427) | 0.327 (0.042) | 0.348 (0.080) | 0.347 (0.080) | ||||||
| Right | ||||||||||||
| Absolute, mL | 4.853 (0.965) | 4.745 (0.907) | –2.05 (0.62) | 5.180 (0.948) | 5.140 (0.946) | –0.78 (0.67) | 5.392 (1.006) | 5.374 (1.017) | –0.35 (0.31) | 2.5 | .09 | FESZ=FEAFF=HC |
| Relative, % | 0.327 (0.063) | 0.319 (0.059) | 0.349 (0.055) | 0.347 (0.054) | 0.361 (0.058) | 0.360 (0.058) | ||||||
| Anterior cingulate subdivisions | ||||||||||||
| Subgenual cingulate gyrus | ||||||||||||
| Left+right | ||||||||||||
| Absolute, mL | 0.914 (0.136) | 0.875 (0.130) | –4.21 (0.89) | 0.799 (0.152) | 0.756 (0.154) | –5.55 (0.92) | 1.004 (0.264) | 1.002 (0.266) | –0.29 (0.34) | 13.1 | <.001 | FEAFF=FESZ<HCf |
| Relative, % | 0.062 (0.007) | 0.059 (0.007) | 0.054 (0.011) | 0.051 (0.011) | 0.067 (0.015) | 0.067 (0.015) | ||||||
| Left | ||||||||||||
| Absolute, mL | 0.444 (0.099) | 0.428 (0.092) | –3.45 (1.11) | 0.390 (0.095) | 0.368 (0.091) | –5.67 (0.89) | 0.488 (0.186) | 0.487 (0.186) | –0.36 (0.78) | 8.3 | .001 | FEAFF<HC, FEAFF=FESZ, FESZ=HC |
| Relative, % | 0.030 (0.006) | 0.029 (0.005) | 0.027 (0.007) | 0.025 (0.007) | 0.033 (0.011) | 0.033 (0.012) | ||||||
| Right | ||||||||||||
| Absolute, mL | 0.469 (0.083) | 0.447 (0.075) | –4.58 (1.03) | 0.409 (0.078) | 0.388 (0.081) | –5.29 (1.19) | 0.515 (0.141) | 0.514 (0.143) | –0.27 (0.48) | 8.3 | .001 | FEAFF=FESZ<HC |
| Relative, % | 0.032 (0.006) | 0.030 (0.005) | 0.028 (0.005) | 0.026 (0.006) | 0.034 (0.008) | 0.034 (0.008) | ||||||
| Affective (anterorostral) cingulate gyrus | ||||||||||||
| Left+right | ||||||||||||
| Absolute, mL | 5.530 (0.984) | 5.199 (1.006) | –6.13 (0.84) | 5.880 (0.989) | 5.750 (0.984) | –2.22 (0.67) | 6.500 (0.761) | 6.480 (0.808) | –0.41 (0.50) | 18.3 | <.001 | FESZ<FEAFF=HCg |
| Relative, % | 0.372 (0.059) | 0.349 (0.061) | 0.397 (0.058) | 0.388 (0.057) | 0.438 (0.056) | 0.437 (0.061) | ||||||
| Left | ||||||||||||
| Absolute, mL | 2.696 (0.554) | 2.537 (0.568) | –6.11 (1.12) | 2.864 (0.644) | 2.779 (0.670) | –3.21 (0.98) | 3.187 (0.387) | 3.178 (0.425) | –0.40 (0.66) | 9.2 | <.001 | FESZ<HC, FESZ=FEAFF, FEAFF=HC |
| Relative, % | 0.181 (0.032) | 0.170 (0.034) | 0.193 (0.040) | 0.187 (0.041) | 0.215 (0.032) | 0.215 (0.339) | ||||||
| Right | ||||||||||||
| Absolute, mL | 2.834 (0.542) | 2.662 (0.575) | –6.36 (1.25) | 3.016 (0.561) | 2.971 (0.543) | –1.40 (0.93) | 3.312 (0.515) | 3.301 (0.569) | –0.44 (0.58) | 11.0 | <.001 | FESZ<FEAFF=HC |
| Relative, % | 0.191 (0.036) | 0.179 (0.039) | 0.204 (0.036) | 0.201 (0.035) | 0.223 (0.356) | 0.222 (0.038) | ||||||
| Cognitive (anterodorsal) cingulate gyrus | ||||||||||||
| Left+right | ||||||||||||
| Absolute, mL | 2.760 (0.523) | 2.616 (0.467) | –4.93 (0.98) | 3.064 (0.756) | 3.011 (0.691) | –1.38 (0.65) | 3.276 (0.928) | 3.254 (0.933) | –0.46 (0.46) | 10.3 | <.001 | FESZ<FEAFF=HCh |
| Relative, % | 0.186 (0.037) | 0.177 (0.034) | 0.208 (0.053) | 0.205 (0.050) | 0.219 (0.055) | 0.218 (0.054) | ||||||
| Left | ||||||||||||
| Absolute, mL | 1.396 (0.291) | 1.329 (0.230) | –4.09 (1.26) | 1.507 (0.461) | 1.490 (0.470) | –1.34 (0.70) | 1.571 (0.530) | 1.561 (0.541) | –0.44 (0.56) | 4.6 | .02 | FESZ<HC, FESZ=FEAFF, FEAFF=HC |
| Relative, % | 0.094 (0.021) | 0.090 (0.018) | 0.102 (0.031) | 0.101 (0.031) | 0.105 (0.030) | 0.104 (0.031) | ||||||
| Right | ||||||||||||
| Absolute, mL | 1.365 (0.337) | 1.287 (0.312) | –5.32 (1.29) | 1.558 (0.402) | 1.521 (0.353) | –1.75 (1.12) | 1.705 (0.511) | 1.693 (0.500) | –0.49 (0.71) | 5.5 | .007 | FESZ<HC, FESZ=FEAFF, FEAFF=HC |
| Relative, % | 0.092 (0.023) | 0.087 (0.022) | 0.106 (0.029) | 0.104 (0.027) | 0.114 (0.033) | 0.114 (0.032) | ||||||
Abbreviations: ANOVA, analysis of variance; FEAFF, first-episode affective psychosis; FESZ, first-episode schizophrenia; HCs, healthy control subjects; HSD, Honestly Significant Difference; ICC, intracranial contents; SED, standard error of the difference.
Percentage of change is calculated as (volume at second scan–volume at baseline scan)/(volume at baseline scan×100).
Repeated-measures ANOVA of relative volume difference (percentage of change) with group (FESZ, FEAFF, and HC) as the between-subjects factor and hemisphere (left vs right) and region (anterior vs posterior regions) as the within-subjects factors revealed a significant main effect for group (F2,50=27.0; P<.001) and region (F1,50=25.4; P<.001). There was no significant effect for hemisphere (F1,50=0.1; P=.78). There was a significant interaction of region×group (F2,50=7.5; P=.001). However, there was no significant interaction of hemisphere×group (F2,50=0.2; P=.86), region×hemisphere (F1,50=0.2; P=.64), or region×hemisphere×group (F2,50=0.8; P=.43) (Hyunh-Feldt ε: 1.0 with region, 1.0 with hemisphere, and 1.0 with region-by-hemisphere). Within the anterior cingulate gyrus alone, groups differed in percentages of volume change in cingulate gyrus gray matter (F2,50=27.0; P<.001), and there was a significant interaction of subregion×group (F4,100=4.8; P=.002). There was no significant effect for hemisphere (F1,50=0.04; P=.84), interaction of hemisphere×group (F2,50=0.9; P=.40), subregion×hemisphere (F2,100=0.6; P=.52), or subregion×hemisphere×group (F4,100=0.2; P=.91) (Hyunh-Feldt ε: 0.976 with region, 1.0 with hemisphere, and 1.0 with region×hemisphere).
Indicates subgenual plus affective plus cognitive cingulate subregions.
Post hoc Tukey HSD tests indicated that entire (left plus right) anterior cingulate volumes percentages of change of the FESZ group were significantly bigger than those of the HC (P<.001) and FEAFF (P<.001) groups. The entire anterior cingulate volume percentages of change of the FEAFF group were also significantly different from those of the HC group (P=.032).
Entire posterior cingulate volumes percentages of change of the FESZ group were significantly bigger than those of the HC group (P=.02). However, entire posterior cingulate volumes percentages of change of the FEAFF group were not significantly different from those of the HC (P=.93) or the FESZ (P=.055) groups.
Both entire subgenual cingulate volumes percentages of change of the FEAFF (P<.001) and FESZ (P=.002) groups were significantly bigger than those in the HC group, whereas the percentages of change of the FEAFF group were not significantly different from those of the FESZ group (P=.44).
Entire affective cingulate volumes percentages of change of the FESZ group were significantly bigger than those of the HC (P<.001) and FEAFF (P=.001) groups. However, entire affective cingulate volumes percentages of change in the FEAFF group were not significantly different from those of the HC group (P=.14).
Entire cognitive cingulate volumes percentages of change of the FESZ group were significantly bigger than those of the HC (P<.001) and FEAFF (P=.003) groups. However, entire cognitive cingulate volumes percentages of change of the FEAFF group were not significantly different from those of the HC group (P=.70).
An analysis of the subregions of the anterior cingulate gyrus (subgenual, affective, and cognitive) (Table 5) showed that groups differed in percentage of volume changes in anterior cingulate gyrus gray matter (F2,50=27.0; P < .001), and there was a significant interaction between subregion and group (F4,100=4.76; P=.002), indicating that the pattern of anterior cingulate gyrus regional change differed among groups. There was no significant effect for hemisphere (F1,50=0.04; P=.84) and no significant interaction of hemisphere × group (F2,50=0.9; P=.40), subregion × hemisphere (F2,100=0.65; P=.52), or subregion × hemisphere × group (F4,100=0.2; P=.91).
Analyzing the volumetric data at times 1 and 2 using time as a within-subjects factor, instead of an analysis with the percentage of change, did not change our conclusions of statistical significance.
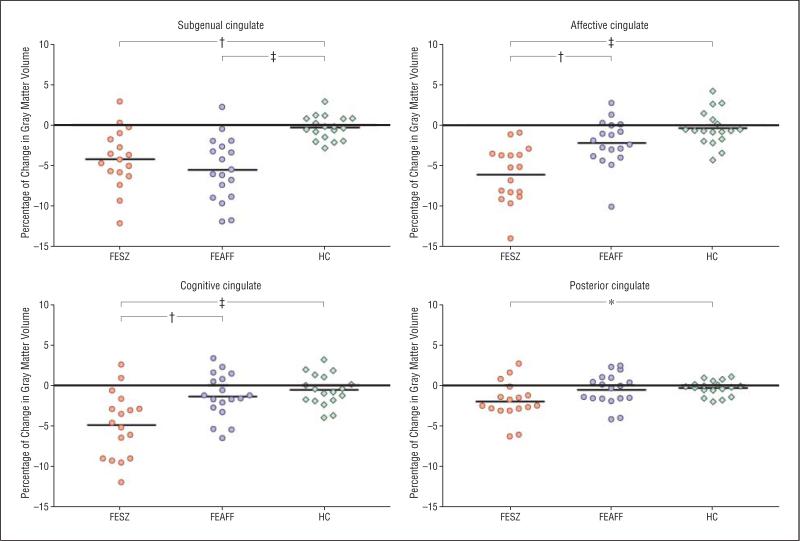
Compared with the HC group, the percentage of change in the FEAFF group over time was significantly different only in the subgenual subregion (P<.001). In contrast, the percentage of volume reduction seen in the FESZ group showed significant differences from those in the HC group for the cingulate gyrus (Table 5, Figure 3, and Figure 4).
Figure 3.
Scattergram of percentage of change for 1½ years in bilateral (left and right) volumes of the cingulate gyrus gray matter in patients with first-episode schizophrenia (FESZ) (n=17) or first-episode affective psychosis (FEAFF) (n=18) and healthy control subjects (HCs) (n=18). Horizontal lines indicate mean. *P<.05; †P<.01; ‡P<.001, by analysis of variance.
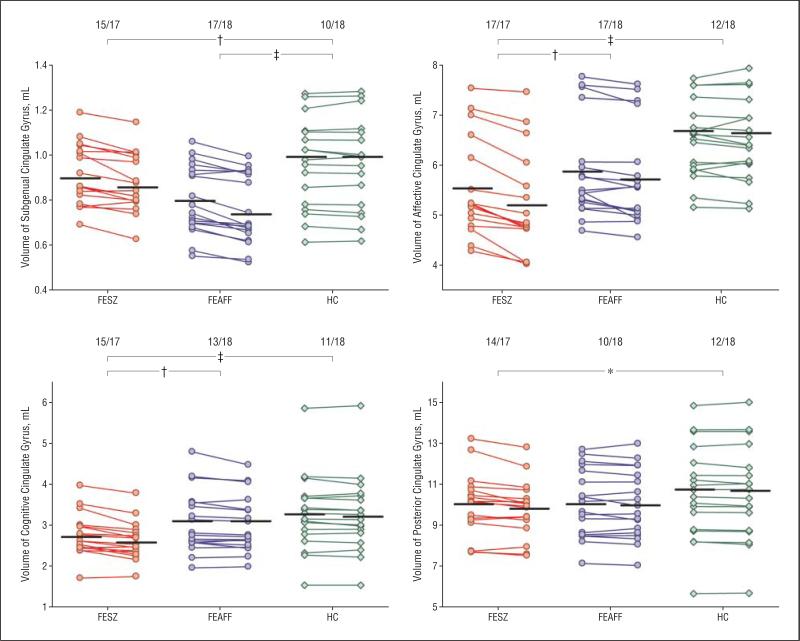
Figure 4.
Volume changes in 1½ years in absolute bilateral (left and right combined) volumes of the cingulate gyrus gray matter at baseline and the second scan in subjects with first-episode schizophrenia (FESZ) (n=17) or first-episode affective psychosis (FEAFF) (n=18) and healthy control subjects (HCs) (n=18). Values of the baseline and second scan in each subject are connected by lines. The numbers at the top of the graphs indicate the proportion of subjects who showed volume reduction over time (number of subjects/total number of subjects). Horizontal lines indicate the mean at the baseline and second scan. *P<.05; †P<.01; ‡P<.001, in comparison of percentages of change between each of the 2 groups by analysis of variance.
Comparison of Percentages of Change of Volumes Among Patient Subgroups by Medication History
There were no statistically significant effects of medication (typical or atypical antipsychotics, or presence or absence of mood stabilizers) on the cingulate volumes in the FESZ or the FEAFF group (eTable 1; available at http://www.archgenpsychiatry.com).
Correlations Between Percentage of Change of Cingulate Gyrus Relative Volumes and Interscan Intervals
Interscan interval did not differ among groups (Table 2), but there were different correlations of volume reduction with interscan interval in the groups. All were negative, with longer interscan intervals (a positive number) resulting in greater volume reductions (a negative number). In the FESZ group, there was an association between the interscan interval and the relative volume change in the left (ρ=−0.58; P=.002) and right (ρ=−0.49; P=.04) anterior cingulate gyrus, particularly in the left (ρ=−0.54; P=.03) and right (ρ=−0.65; P=.005) affective subregions. Fisher z transformation showed significant differences in correlations between the FESZ and HC groups in the left (z=−2.0; P=.048) and right affective subregions(z=−2.4;P=.02).Incontrast,intheFEAFFgroup, thereweresignificantnegativecorrelationsonlybetweenthe interscanintervalandtheleftsubgenualcingulategyrusvolume reduction (ρ=−0.50; P=.03), which was significantly different from the HC correlation (z=−1.6; P=.05).
Clinical Correlations With Volume Change Over Time and Comparison Between Good and Poor Responders
In the groups with good and poor responses, greater volume reductions resulted in worse withdrawal symptom factor scores over time, with the relevant volume differing by group. This correlation was negative because more reduction in volume (a negative number) was associated with larger BPRS factor scores (worsening). In the FESZ group, changes in withdrawal factor scores were negatively correlated with changes of right affective cingulate subregion relative volumes (ρ=−0.60; P=.02) (eTable 2).
We also analyzed the FEAFF and FESZ groups according to treatment response as measured by a BPRS score decrease of more than 20 (eTable 3). The percentage of decrease in volume of the poor-response FESZ subgroup was significantly greater in the affective subregions than those of the good-response FESZ subgroup (Mann-Whitney tests, z=−3.4; P=.001). For the FEAFF group, the percentage of change in volume decrease in the left subgenual subregion in the poor-responder subgroup was greater than that of the good-responder subgroup (z=−2.6; P=.009).
COMMENT
There were 3 major findings. Cross-sectionally, groups were differentiated by regions showing abnormalities. The cingulate gyrus gray matter volume in the FESZ group was significantly smaller than that of the HC group in a number of subregions, including the left subgenual subregion, the left and right affective (anterorostral) sub-regions, and the right cognitive (anterodorsal) and posterior subregions. However, in patients with FEAFF psychoses, smaller gray matter volume was confined to bilateral reductions in the subgenual subregion.
Longitudinally, the FESZ group also demonstrated more widespread gray matter volume reduction over time, with progressive volume reductions in subgenual (4.2%), affective (6.1%), cognitive (4.9%), and posterior (2.0%) subregions. In contrast, in the FEAFF group, progressive volume change was confined to reductions (5.6%) in the subgenual subregion.
Finally, the FESZ group showed less PCS fissurization and less leftward asymmetry than HCs.
CROSS-SECTIONAL FINDINGS
With regard to findings in the FESZ group, these cross-sectional findings are consistent with VBM studies reporting smaller gray matter signal density of anterior cingu-late bilaterally27,29 or in the right hemisphere28 in FESZ, bilaterally in subjects at high risk of schizophrenia,26 and bilaterally in the posterior cingulate in chronic schizophrenia.22 The findings are also compatible with a volu-metric study17 using geometric parcellation showing smaller relative volume in the anterior and posterior cingulate gyrus in patients with chronic schizophrenia. Although these previous reports had shown cingulate structural abnormalities in schizophrenia, there had remained uncertainty regarding the specific subregion or hemisphere for cingulate gyrus deficits in this disorder. This present study directly addressed the issue of subregional localization and showed that the degree and the laterality of the deficits in FESZ differed in specific cingulate subregions.
In the FEAFF group, only the subgenual subregion was significantly smaller bilaterally at time 1 than in the HC group. This finding is consistent with previous reports,38,39,68 showing significantly smaller cingulate volumes in the left subgenual cingulate, but also adds the right subgenual cingulate, which was of trend level significance in a previous report from our group using a smaller and different sample.40 Previous VBM studies showed bilateral gray matter density deficits in the anterior cingulate,35,36 but did not localize to the subcallosal-subgenual region.
LONGITUDINAL FINDINGS
Findings in the present longitudinal study with regard to FESZ were consistent with but are also more ROI-specific than recent longitudinal VBM studies of gray matter density,43 which demonstrated decreases over time in the left anterior cingulate gyrus in the FESZ group.
Our longitudinal findings of bilateral subgenual volume reduction in the FEAFF group compared with the HC group specify which subregion is affected, thus providing a more exact specification of abnormality than a recent VBM study43 reporting decreased bilateral anterior cingulate gyrus gray matter density over time in this disorder. We think it of importance that the specific sub-region implicated in the initial scan and longitudinal data in the FEAFF group is suggested by the literature to have a particularly strong relationship to affective disorder, and is the target of deep brain stimulation14 (Mayberg et al14 also reviewed the literature links between this region and affective disorder). There is thus a strong congruence of structural and functional findings. However, a longitudinal study of 7 patients with psychotic depression by Coryell et al38 demonstrated increases in posterior subgenual volumes; these patients were diagnostically different from those in the present study, had longer durations of illness (4.7 years), and possibly differed in mood stabilizer medication duration, which may lead to volume increases.69
To our knowledge, our FESZ and FEAFF findings, taken together, constitute the first report to demonstrate that the gray matter volume deficits in the cingulate gyrus (cross-sectionally) and their progressive reductions (longitudinally) differ according to both subregion specificity and the type of psychosis. The FESZ group showed more extensive cross-sectional deficits and more extensive progressive reduction over time of cingulate subregions, in contrast to the subgenual subregion of the anterior cingulate gyrus in the FEAFF group.
Our group's previous studies found commonalities and differences in regional gray matter volumes between samples of patients with FESZ and patients with FEAFF. For instance, the FESZ group showed volume deficits in the posterior superior temporal gyrus49 and its subdivisions of the Heschl gyrus and planum temporale,51 as well as in the pre-frontal cortex70 and in fusiform gyrus gray matter.71 Commonalities of these 2 psychoses at first episode included smaller gray matter volumes of the left posterior amygdalahippocampal complex (mostly hippocampus)49 and in the subgenual cingulate cortex.40 These reports suggested that the pathologic regions may show specificity between the 2 psychoses except in the limbic or limbic-linked regions (hippocampus, temporal pole, or subgenual cingulate cortex), where pathological findings overlap.
One important question is when in the course of postonset schizophrenia are volume reductions most prominent. The data by Kasai et al50 suggest that much occurs within the first 10 to 12 months, whereas the present data suggest a more protracted course, although the smaller percentage of reduction in the present study makes direct comparison difficult and regions may differ in time course.
Our third major finding that patients with FESZ showed less PCS fissurization than HCs is similar to a previous report by Yücel et al.45 In contrast, there was no difference between the patients with FEAFF and HCs. Sulcal pattern likely indexes developmental factors (genetic and gestational environment) and may be a vulnerability indicator.72 Taken with the postonset progressive volume decrements, these findings suggest that schizophrenia has both neurodevelopmental and postonset progressive components.
In the present study, we compared the cingulate volume differences between the FESZ subgroups treated with typical and atypical neuroleptics, and between the FEAFF subgroups with and without mood stabilizer treatments. These comparisons did not show significant subgroup differences. Our findings suggestive of medication effects in a study69 of entire neocortical gray matter in patients with FESZ and FEAFF may be related to the greater detection sensitivity of large regions of gray matter compared with the relatively small volumes in the present study.
In terms of association between volume change and clinical measures, we found that worsening withdrawal symptoms were associated with decreased cingulate gray matter volumes, particularly in the right affective cingulate subregion volumes in patients with FESZ (ρ=−0.60; P=.02). These were supported further by the good- vs poor-response group comparisons. However, we emphasize that these symptom correlation analyses were exploratory in nature and therefore confirmation will be needed in future planned studies.
We believe a strength of this study is that it represents, to our knowledge, the largest cross-sectional MRI study sample of cingulate gyrus in patients with FEAFF and FESZ. Furthermore, possible confounding variables that could affect the size of the gray matter, including age, sex, handedness, and other demographic factors (eg, parental socioeconomic status), were carefully controlled, strengthening the validity of our findings of smaller cingulate gray matter volume in patients with FESZ and FEAFF, with regional specificity according to the type of psychosis.
Longitudinally, we found that the cingulate gray matter volume deficits progress during the early stage of the psychotic illness: in the subgenual cingulate in patients with FEAFF and patients with FESZ, and in the cognitive and affective anterior cingulate only in patients with FESZ. This is the first demonstration of progression of gray matter volume deficits in subgenual, affective, and cognitive subregions in patients with FESZ, and of progression of gray matter volume deficits in the subgenual cingulate in patients with FEAFF compared with HCs. In general, our findings support a hypothesis of differential progression in patients with FESZ and FEAFF, as do our previous studies.50,69
Supplementary Material
Funding/Support
This study was supported by merit awards (Drs Shenton and McCarley), a Veterans Affairs Schizophrenia Center Award (Drs Shenton and McCarley), and a Middleton Award (Dr McCarley) from the Department of Veterans Affairs; and by grants K02 MH 01110 and R01 MH 50747 (Dr Shenton), R01 MH 40799 and R01 MH 052807 (Dr McCarley), CIDAR P50MH080272 (Drs McCarley and Shenton), and R01 MH58704 (Dr Salisbury) from the National Institute of Mental Health and grants from the MIND (Mental Illness and Neuroscience Discovery) Foundation (Dr McCarley) and NARSAD (Dr Salisbury).
Footnotes
Financial Disclosure: None reported.
Additional Information: The supplemental text and eTables are available at http://www.archgenpsychiatry.com.
Additional Contributions: Lillian Hsu, BA, provided technical support; Kiyoto Kasai, MD, provided comments and suggestions; and Marie Fairbanks provided administrative support.
REFERENCES
- 1.Mesulam MM. Principles of Behavioral and Cognitive Neurology. Oxford University Press; New York, NY: 2000. [Google Scholar]
- 2.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48(8):813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 3.Kim YH, Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Mesulam MM. The large-scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. Neuroimage. 1999;9(3):269–277. doi: 10.1006/nimg.1999.0408. [DOI] [PubMed] [Google Scholar]
- 4.Mesulam MM, Nobre AC, Kim YH, Parrish TB, Gitelman DR. Heterogeneity of cingulate contributions to spatial attention. Neuroimage. 2001;13(6 pt 1):1065–1072. doi: 10.1006/nimg.2001.0768. [DOI] [PubMed] [Google Scholar]
- 5.Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29(2):452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2(6):435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 8.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 9.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 10.Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10(1):49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45(12):1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 12.Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44(12):1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 13.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363(4):615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 14.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Yücel M, Brewer WJ, Harrison BJ, Fornito A, O'Keefe GJ, Olver J, Scott AM, Egan GF, Velakoulis D, McGorry PD, Pantelis C. Anterior cingulate activation in antipsychotic-naive first-episode schizophrenia. Acta Psychiatr Scand. 2007;115(2):155–158. doi: 10.1111/j.1600-0447.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 16.Baiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P. Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophr Res. 2007;93(13):1–12. doi: 10.1016/j.schres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophr Res. 2005;72(23):91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T, Suzuki M, Kawasaki Y, Hagino H, Yamashita I, Nohara S, Nakamura K, Seto H, Kurachi M. Perigenual cingulate gyrus volume in patients with schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 2003;53(7):593–600. doi: 10.1016/s0006-3223(02)01483-x. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki M, Nohara S, Hagino H, Kurokawa K, Yotsutsuji T, Kawasaki Y, Takahashi T, Matsui M, Watanabe N, Seto H, Kurachi M. Regional changes in brain gray and white matter in patients with schizophrenia demonstrated with voxel-based analysis of MRI. Schizophr Res. 2002;55(12):41–54. doi: 10.1016/s0920-9964(01)00224-9. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tour-ville J, Caviness VS, Jr, Faraone SV, Tsuang MT. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56(6):537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- 21.Choi JS, Kang DH, Kim JJ, Ha TH, Roh KS, Youn T, Kwon JS. Decreased caudal anterior cingulate gyrus volume and positive symptoms in schizophrenia. Psychiatry Res. 2005;139(3):239–247. doi: 10.1016/j.pscychresns.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Hulshoff Pol HE, Schnack HG, Mandl RC, van Haren NE, Koning H, Collins DL, Evans AC, Kahn RS. Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001;58(12):1118–1125. doi: 10.1001/archpsyc.58.12.1118. [DOI] [PubMed] [Google Scholar]
- 23.Szeszko PR, Bilder RM, Lencz T, Pollack S, Alvir JM, Ashtari M, Wu H, Lieberman JA. Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Res. 1999;90(1):1–15. doi: 10.1016/s0925-4927(99)00002-5. [DOI] [PubMed] [Google Scholar]
- 24.Convit A, Wolf OT, de Leon MJ, Patalinjug M, Kandil E, Caraos C, Scherer A, Saint Louis LA, Cancro R. Volumetric analysis of the pre-frontal regions: findings in aging and schizophrenia. Psychiatry Res. 2001;107(2):61–73. doi: 10.1016/s0925-4927(01)00097-x. [DOI] [PubMed] [Google Scholar]
- 25.Kopelman A, Andreasen NC, Nopoulos P. Morphology of the anterior cingulate gyrus in patients with schizophrenia: relationship to typical neuroleptic exposure. Am J Psychiatry. 2005;162(10):1872–1878. doi: 10.1176/appi.ajp.162.10.1872. [DOI] [PubMed] [Google Scholar]
- 26.Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Voxel-based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophr Res. 2003;64(1):1–13. doi: 10.1016/s0920-9964(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 27.Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17(4):1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage. 2002;17(2):880–889. [PubMed] [Google Scholar]
- 29.McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Whalley HC, Johnstone EC, Lawrie SM. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet. 2006;141(1):76–83. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- 30.Ohnishi T, Hashimoto R, Mori T, Nemoto K, Moriguchi Y, Iida H, Noguchi H, Nakabayashi T, Hori H, Ohmori M, Tsukue R, Anami K, Hirabayashi N, Harada S, Arima K, Saitoh O, Kunugi H. The association between the Val158Met polymorphism of the catechol-O-methyl transferase gene and morphological abnormalities of the brain in chronic schizophrenia. Brain. 2006;129(pt 2):399–410. doi: 10.1093/brain/awh702. [DOI] [PubMed] [Google Scholar]
- 31.Szeszko PR, Bilder RM, Lencz T, Ashtari M, Goldman RS, Reiter G, Wu H, Lieberman JA. Reduced anterior cingulate gyrus volume correlates with executive dysfunction in men with first-episode schizophrenia. Schizophr Res. 2000;43(23):97–108. doi: 10.1016/s0920-9964(99)00155-3. [DOI] [PubMed] [Google Scholar]
- 32.Heckers S, Weiss AP, Deckersbach T, Goff DC, Morecraft RJ, Bush G. Anterior cingulate cortex activation during cognitive interference in schizophrenia. Am J Psychiatry. 2004;161(4):707–715. doi: 10.1176/appi.ajp.161.4.707. [DOI] [PubMed] [Google Scholar]
- 33.Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154(12):1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- 34.Yücel M, Pantelis C, Stuart GW, Wood SJ, Maruff P, Velakoulis D, Pipingas A, Crowe SF, Tochon-Danguy HJ, Egan GF. Anterior cingulate activation during Stroop task performance: a PET to MRI coregistration study of individual patients with schizophrenia. Am J Psychiatry. 2002;159(2):251–254. doi: 10.1176/appi.ajp.159.2.251. [DOI] [PubMed] [Google Scholar]
- 35.Lochhead RA, Parsey RV, Oquendo MA, Mann JJ. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biol Psychiatry. 2004;55(12):1154–1162. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 2004;131(1):57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Sharma V, Menon R, Carr TJ, Densmore M, Mazmanian D, Williamson PC. An MRI study of subgenual prefrontal cortex in patients with familial and non-familial bipolar I disorder. J Affect Disord. 2003;77(2):167–171. doi: 10.1016/s0165-0327(02)00109-x. [DOI] [PubMed] [Google Scholar]
- 38.Coryell W, Nopoulos P, Drevets W, Wilson T, Andreasen NC. Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. Am J Psychiatry. 2005;162(9):1706–1712. doi: 10.1176/appi.ajp.162.9.1706. [DOI] [PubMed] [Google Scholar]
- 39.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 40.Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, Yurgelun-Todd D, Zarate C, Kikinis R, Jolesz FA, McCarley RW. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry. 1999;156(7):1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Todtenkopf MS, Vincent SL, Benes FM. A cross-study meta-analysis and three-dimensional comparison of cell counting in the anterior cingulate cortex of schizophrenic and bipolar brain. Schizophr Res. 2005;73(1):79–89. doi: 10.1016/j.schres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M, HGDH Study Group Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62(4):361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- 43.Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58(9):713–723. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 44.Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25(4):1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Yücel M, Stuart GW, Maruff P, Wood SJ, Savage GR, Smith DJ, Crowe SF, Copolov DL, Velakoulis D, Pantelis C. Paracingulate morphologic differences in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biol Psychiatry. 2002;52(1):15–23. doi: 10.1016/s0006-3223(02)01312-4. [DOI] [PubMed] [Google Scholar]
- 46.Le Provost JB, Bartres-Faz D, Paillere-Martinot ML, Artiges E, Pappata S, Recasens C, Perez-Gomez M, Bernardo M, Baeza I, Bayle F, Martinot JL. Paracingulate sulcus morphology in men with early-onset schizophrenia. Br J Psychiatry. 2003;182:228–232. doi: 10.1192/bjp.182.3.228. [DOI] [PubMed] [Google Scholar]
- 47.Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;5(1):56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- 48.Magnotta VA, Andreasen NC, Schultz SK, Harris G, Cizadlo T, Heckel D, Nopoulos P, Flaum M. Quantitative in vivo measurement of gyrification in the human brain: changes associated with aging. Cereb Cortex. 1999;9(2):151–160. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- 49.Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, Kisler T, Arakaki H, Kwon JS, Anderson JE, Yurgelun-Todd D, Tohen M, McCarley RW. Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry. 1998;155(10):1384–1391. doi: 10.1176/ajp.155.10.1384. [DOI] [PubMed] [Google Scholar]
- 50.Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, Yurgelun-Todd DA, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60(8):766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyder-man D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57(7):692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spitzer RL, Williams JBW, Gibbson M, First M. The Structured Clinical Interview for DSM-III-R (SCID). American Psychiatric Association; Washington, DC: 1990. [Google Scholar]
- 53.Spitzer RL, Williams JBW, Gibbson M, First M. The Structured Clinical Interview for DSM-III-R–Non-Patient Edition (SCID-NP). American Psychiatric Association; Washington, DC: 1990. [Google Scholar]
- 54.First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin L. Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II): Interview and Questionnaire. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- 55.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 56.Narr KL, Sharma T, Woods RP, Thompson PM, Sowell ER, Rex D, Kim S, Asuncion D, Jang S, Mazziotta J, Toga AW. Increases in regional subarachnoid CSF without apparent cortical gray matter deficits in schizophrenia: modulating effects of sex and age. Am J Psychiatry. 2003;160(12):2169–2180. doi: 10.1176/appi.ajp.160.12.2169. [DOI] [PubMed] [Google Scholar]
- 57.Overall JE, Beller SA. The Brief Psychiatric Rating Scale (BPRS) in geropsychiatric research, I: factor structure on an inpatient unit. J Gerontol. 1984;39(2):187–193. doi: 10.1093/geronj/39.2.187. [DOI] [PubMed] [Google Scholar]
- 58.Overall JE, Hollister LE, Pichot P. Major psychiatric disorders: a four-dimensional model. Arch Gen Psychiatry. 1967;16(2):146–151. doi: 10.1001/archpsyc.1967.01730200014003. [DOI] [PubMed] [Google Scholar]
- 59.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 60.Wechsler D. Wechsler Adult Intelligence Scale–Revised. Harcourt Brace Jovanovich Inc; New York, NY: 1981. [Google Scholar]
- 61.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 62.Crespo-Facorro B, Kim JJ, Andreasen NC, O'Leary DS, Wiser AK, Bailey JM, Harris G, Magnotta VA. Human frontal cortex: an MRI-based parcellation method. Neuroimage. 1999;10(5):500–519. doi: 10.1006/nimg.1999.0489. [DOI] [PubMed] [Google Scholar]
- 63.Noga JT, Aylward E, Barta PE, Pearlson GD. Cingulate gyrus in schizophrenic patients and normal volunteers. Psychiatry Res. 1995;61(4):201–208. doi: 10.1016/0925-4927(95)02612-2. [DOI] [PubMed] [Google Scholar]
- 64.Vogt BA, Absher JR, Bush G. Human retrosplenial cortex: where is it and is it involved in emotion? Trends Neurosci. 2000;23(5):195–197. doi: 10.1016/s0166-2236(00)01579-4. [DOI] [PubMed] [Google Scholar]
- 65.Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22(7):310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- 66.Fornito A, Yucel M, Wood S, Stuart GW, Buchanan JA, Proffitt T, Anderson V, Velakoulis D, Pantelis C. Individual differences in anterior cingulate/paracingulate morphology are related to executive functions in healthy males. Cereb Cortex. 2004;14(4):424–431. doi: 10.1093/cercor/bhh004. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi T, Kawasaki Y, Kurokawa K, Hagino H, Nohara S, Yamashita I, Nakamura K, Murata M, Matsui M, Suzuki M, Seto H, Kurachi M. Lack of normal structural asymmetry of the anterior cingulate gyrus in female patients with schizophrenia: a volumetric magnetic resonance imaging study. Schizophr Res. 2002;55(12):69–81. doi: 10.1016/s0920-9964(01)00200-6. [DOI] [PubMed] [Google Scholar]
- 68.Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51(4):342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- 69.Nakamura M, Salisbury DF, Hirayasu Y, Bouix S, Pohl KM, Yoshida T, Koo MS, Shenton ME, McCarley RW. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol Psychiatry. 2007;62(7):773–783. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirayasu Y, Tanaka S, Shenton ME, Salisbury DF, DeSantis MA, Levitt JJ, Wible C, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb Cortex. 2001;11(4):374–381. doi: 10.1093/cercor/11.4.374. [DOI] [PubMed] [Google Scholar]
- 71.Lee CU, Shenton ME, Salisbury DF, Kasai K, Onitsuka T, Dickey CC, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59(9):775–781. doi: 10.1001/archpsyc.59.9.775. [DOI] [PubMed] [Google Scholar]
- 72.Huang CC. Sonographic cerebral sulcal development in premature newborns. Brain Dev. 1991;13(1):27–31. doi: 10.1016/s0387-7604(12)80293-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.