Abstract
Osteopontin plays an important role in the development and perpetuation of rheumatoid arthritis (RA). Antibodies targeting osteopontin have shown promising therapeutic benefits against this disease. We have previously reported a novel anti-RA monoclonal antibody, namely, 23C3, and shown it capable of alleviating the symptoms of RA in a murine collagen-induced arthritis model, restoring the cytokine production profile in joint tissues, and reducing T-cell recall responses to collagen type II. We describe here the crystal structure of 23C3 in complex with its epitope peptide. Analyses of the complex structure reveal the molecular mechanism of osteopontin recognition by 23C3. The peptide folds into two tandem β-turns, and two key residues of the peptide are identified to be critical for the recognition by 23C3: TrpP43 is deeply embedded into a hydrophobic pocket formed by AlaL34, TyrL36, LeuL46, TyrL49, PheL91, and MetH102 and therefore has extensive hydrophobic interactions with 23C3, while AspP47 has a network of hydrophilic interactions with residues ArgH50, ArgH52, SerH53, and AsnH56 of the antibody. Besides the complementarity-determining region loops, the framework region L2 of 23C3 is also shown to interact with the epitope peptide, which is not common in the antibody–antigen interactions and thus could be exploited in the engineering of 23C3. These results not only provide valuable information for further improvement of 23C3 such as chimerization or humanization for its therapeutic application, but also reveal the features of this specific epitope of osteopontin that may be useful for the development of new antibody drugs against RA.
Abbreviations: mAb, monoclonal antibody; RA, rheumatoid arthritis; SF, synovial fluid; CIA, collagen-induced arthritis; CDR, complementarity-determining region; BSA, buried surface area; FWR, framework region
Keywords: osteopontin, rheumatoid arthritis, 23C3, epitope, antibody structure
The pathogenesis of rheumatoid arthritis (RA), a chronic autoimmune disease, involves accumulation of T lymphocytes in the synovial compartment and destruction of cartilage and bone.1–3 Several antirheumatic drugs are available in the market including two monoclonal antibody (mAb) drugs against tumor necrosis factor-α, namely, Infliximab (Centocor) and Adalimumab (Abbott). However, about 20–30% of the patients treated do not respond to the antibodies and some drugs lose efficacy or accumulate toxicity over time, demanding development of new strategies for the treatment of RA, probably against other factors involved in the pathogenesis of RA.4–6
One such target is osteopontin, also known as early T-cell activation gene-1. It is one of the major noncollagenous bone matrix proteins and is expressed at elevated level at the inflammation site in various cell types including epithelial cells, mesenchymal cells, and immune cells.7 The protein is usually phosphorylated and can be cleaved by thrombin in vivo. When cleaved, the N-terminal fragment is capable of binding to various cell surface receptors such as integrins αvβ3, αvβ1, αvβ5, and α8β1 via its RGD motif,8–10 and α9 and α4 integrins via a crypt region (162SVVYGLR168 of human osteopontin);11–13 both the N-terminal and C-terminal fragments can bind to CD44 variants with the assistance of β1 integrins independently of the RGD sequence.14 Considering the various interactions between osteopontin and multiple cell receptors, it is not surprising that osteopontin exerts diverse functions in immunity, infection, and cancer (reviewed in the work of Denhardt and Guo15). Specifically, the multifaceted protein is involved in various aspects of immunity: osteopontin is considered an early component of cell-mediated immunity16 and seems to play a complicated role in TH2 effector responses17 and to mediate natural killer T-cell function.18 Moreover, osteopontin prevents monocyte apoptosis19 and seems to regulate the expression of cell-surface CD44 on macrophages and to modulate CD44 activities.20
An important role of osteopontin in amplification and perpetuation of RA is suggested by the confined expression of osteopontin in synovial fluid (SF) and synovial tissue of RA patients21,22 and the co-expression of osteopontin receptors (αv, β1 and CD44) in T cells in SF.21 In addition, when T cells are treated with RA-SF, osteopontin expression is elevated; on the other hand, overexpression of osteopontin induces proinflammatory chemokines and cytokines and activates nuclear factor-kappa B.21 Osteopontin deficiency is consistently shown to prevent the destruction of joint cartilage and joint swelling through suppression of chondrocyte apoptosis and angiogenesis in the RA model.21
Due to the association between osteopontin and RA, several anti-osteopontin antibodies have been developed and studies of these antibodies in animal RA models show promising results.23–25 The antibody M5 recognizes and binds to a cryptic epitope of thrombin-cleaved mouse osteopontin (147SLAYGLR153) and thereby blocks the binding of cleaved osteopontin to α9 and α4 integrins, which result in abrogation of monocyte migration.18 Treatment with M5 leads to inhibition of synovial proliferation, bone resorption, and inflammatory reaction in arthritic joints in the murine RA model induced by mAb/lipopolysaccharides.24 Another mAb, 2K1 or its chimeric form C2K1, recognizes the equivalent epitope region of human osteopontin (162SVVYGLR168) and is shown to ameliorate collagen-induced arthritis (CIA) in cynomolgus monkey.23
Recently, we have developed a novel murine anti-osteopontin mAb, namely, 23C3, which is of great potential in treatment of RA.25 Administration of 23C3 to mice with CIA not only strongly suppresses the development of CIA but also decreases the severity of the existing arthritis. Mice treated with 23C3 showed a cytokine production profile in joint tissues similar to that of the wild-type mice and reduced T-cell recall responses to collagen type II. We have also produced another murine mAb, F8E11, which is also included for the same assay, however, it shows much weaker efficacy for the treatment of the established disease in the murine model.25 As both F8E11 and 23C3 are capable of inhibiting monocyte migration, the robust therapeutic effect of 23C3 may be attributed to its ability to promote the apoptosis of activated T cells by inhibition of nuclear factor-kappa B and alteration of the expression of preapoptotic proteins,25 which may be unique, as neither M5 nor 2K1 is reported to be capable of induction of cell apoptosis. Intriguingly, 23C3 recognizes a distinct epitope motif (43WLNPDP48) of the full-length osteopontin, which may account for the differences.25 In this study, we report the crystal structure of the Fab fragment of 23C3 in complex with its epitope peptide of osteopontin. Our results reveal the structural basis for the 23C3 recognition of osteopontin and provide hints for further development of mAb drugs for the treatment of RA.
The structure of the 23C3 Fab
The Fab fragment of the anti-osteopontin mAb 23C3 was co-crystallized with a 12-mer peptide (40VATWLNPDPSQK51) that contains the previously mapped epitope region of osteopontin recognized by 23C3.25 The structure of 23C3 Fab in complex with the epitope peptide was determined to 2.8 Å resolution with an R−factor of 24.8% and a free R−factor of 29.2% (Table 1, Fig. 1). There is one Fab–peptide complex in an asymmetric unit. Overall, the complex exhibits good geometry and has well-defined electron density even at the conserved loop region between GlyH131 and SerH137, which is disordered in many other Fab structures.
Table 1.
Summary of diffraction data and structure refinement statistics
| Diffraction data | ||
| Crystal | 23C3-epitope peptide | 23C3-W43A peptide |
| Wavelength (Å) | 1.5418 | 1.5418 |
| Space group | P212121 | P212121 |
| Cell parameters | ||
| a/b/c (Å) | 42.3/92.0/122.5 | 41.7/92.1/122.1 |
| Resolution range (Å) | 20.0–2.80 (2.90–2.80)a | 20.0–2.80 (2.90–2.80) |
| Observed reflections | 82,967 (8415) | 66,404 (6296) |
| Unique reflections [I/σ(I) > 0] | 12,316 (1192) | 12,155 (1171) |
| Average redundancy | 6.7 (7.1) | 5.4 (5.3) |
| Average I/σ(I) | 5.0 (2.1) | 6.3 (3.1) |
| Completeness (%) | 98.4 (98.4) | 99.4 (99.4) |
| Wilson B-factor (Å2) | 58.9 | 55.6 |
| Rmerge (%)b | 13.5 (29.3) | 8.4 (22.9) |
| Statistics of refinement and model | ||
| No. of reflections [Fo > 0σ(Fo)] | ||
| Working set | 11,470 (836) | 11,476 (824) |
| Free R set | 613 (32) | 609 (35) |
| R−factor (%)c | 24.8 (37.3) | 24.1 (31.5) |
| Free R−factor (%) | 29.2 (47.7) | 29.7 (39.5) |
| No. of protein residues | 438 | 436 |
| Fab/Peptide | 429/9 | 429/7 |
| No. of sugar residues | 4 | 4 |
| Average B-factor of all atoms (Å2) | 35.4 | 33.5 |
| Fab/Peptide | 34.8/40.7 | 33.0/33.2 |
| Sugar | 64.2 | 62.1 |
| r.m.s.d. bond lengths (Å) | 0.006 | 0.010 |
| r.m.s.d. bond angles (°) | 1.003 | 1.171 |
| Luzzati atomic positional error (Å) | 0.46 | 0.42 |
| Ramachandran plot (%) | ||
| Most favored regions | 84.9 | 86.8 |
| Allowed regions | 14.0 | 11.4 |
| Generously allowed regions | 0.3 | 1.1 |
| Disallowed regions | 0.8 | 0.8 |
Numbers in parentheses refer to the highest-resolution shell.
Rmerge = ∑hkl∑i|Ii(hkl)i − 〈I(hkl)〉|/∑hkl∑iIi(hkl).
R−factor = ∑hkl ||Fo|−|Fc||/∑hkl |Fo|.
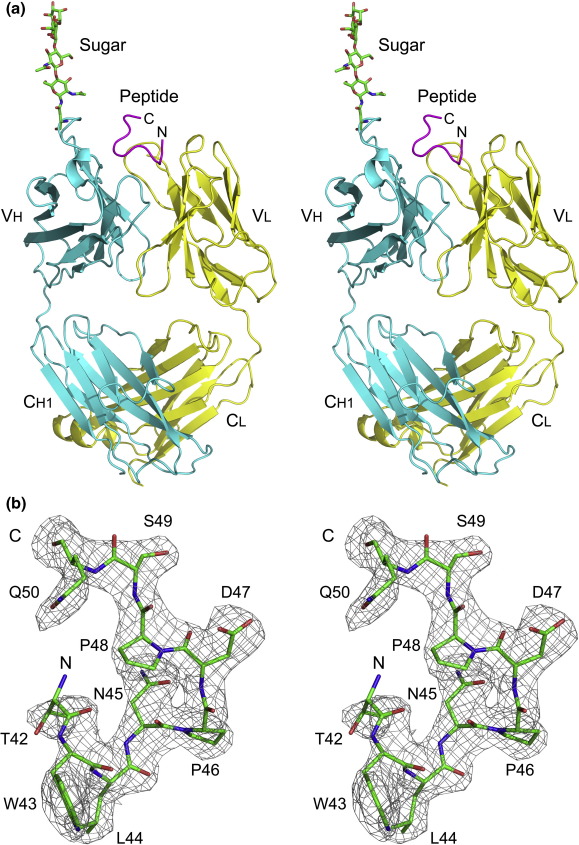
Fig. 1.
Overall structure of the 23C3 Fab–epitope peptide complex. (a) Stereoview of the overall structure of the 23C3 Fab–epitope peptide complex. The 23C3 Fab light chain is colored yellow, the heavy chain is cyan, and the bound peptide is purple. The sugar chain is shown with a ball-and-stick model. (b) A stereo view representation of SIGMAA-weighted 2Fo − Fc map (1σ contour level) for the bound epitope peptide. The peptide is shown with a ball-and-stick model.
The Fab fragment displays a canonical Fab structure with four immunoglobulin domains of VL, CL, VH, and CH1 (Fig. 1a). The elbow angle relating the disposition of VH to VL and CH1 to CL is about 150°, consistent with the fact that 23C3 has a κ light chain.26 The complementarity-determining region (CDR) loops L1, L2, L3, H1, and H2 belong to Chothia canonical classes 2, 1, 1, 1, and 4, respectively.27 The CDR loop H3 of 23C3 contains only five residues, which is significantly shorter than the average length of 8.7 residues for murine mAbs.28 Interestingly, the Fab is glycosylated at AsnH57 of CDR H2 (Fig. 1a). An N-linked core tetrasaccharide (MANα-1,3MANβ-1,4GlcNAcβ-1,4GlcNAcβ-1-AsnH57) was modeled without ambiguity. N-linked glycosylation of mAbs in both framework regions (FWRs)29 and CDR loops30,31 has been reported, and sugar chains on the CDR loops may also participate in the interactions with antigens.31 In our structure, there is no interaction between the sugar chain and the bound peptide. Due to the lack of a structure of the full-length osteopontin, the possibility that the sugar chain might be involved in the recognition of the full-length antigen cannot be excluded.
The structure of the bound peptide
The bound peptide has well-defined electron density (from ThrP42 to GlnP50) except for several terminal residues (Fig. 1b). As shown in Fig. 1, the peptide displays a twisted structure and is composed of the N-terminal two tandem β-turns (β-turn is the most common structural element of antigens in mAb–peptide complexes32) and the two C-terminal extended residues (SerP49 and GlnP50). The first β-turn (ThrP42 to AsnP45) is of type I and the second (AsnP45 to ProP48) is of type VIII. The conformation of the peptide is mainly stabilized by the interactions between the peptide and the Fab (see the discussion later), while a hydrogen bond between the main-chain amide of AspP47 and the side-chain carbonyl of AsnP45 (2.88 Å) also contributes to the stability.
The interactions between the Fab and peptide
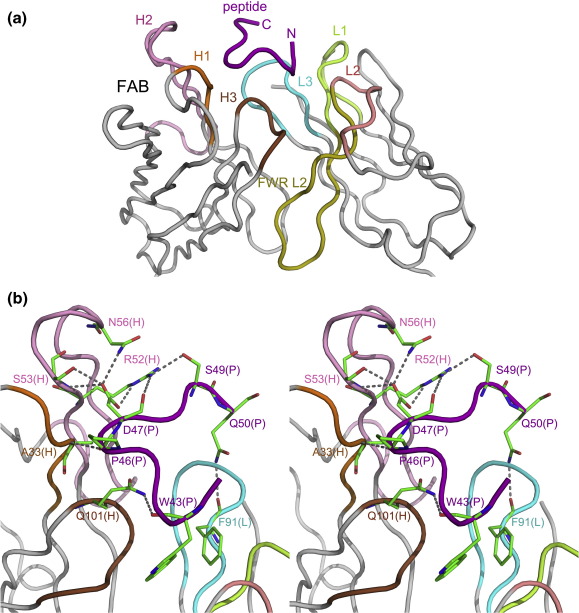
In the complex structure, the peptide is bound in a pocket formed by the CDR loops and the tip of the FWR L2 (defined by Kabat definition). CDR loops L1, L3, H1, H2, and H3 participate in the interactions with the peptide, while loop L2 makes no direct contact with the peptide, as frequently observed in antipeptide or antihapten Fab structures (Fig. 2a).33 Uncommonly, some residues of the FWR L2 (including TyrL36, LeuL46, and TyrL49) have van der Waals contacts with the peptide, probably due to the deep embedment of the side chain of TrpP43 into the Fab (see detailed discussion later). The buried surface area (BSA) of the interaction interface is about 591.3 Å2 within the average range of 400–700 Å2 for mAb–peptide complexes,34 accounting for 47.4% of the total peptide surface area. The shape complementarity value of this complex is 0.80, much higher than the average value (0.64–0.68) for antibody–antigen complexes.35 Between the Fab and the peptide, nine hydrogen bonds, one salt bridge, and 126 van der Waals contacts are formed (Tables 2 and S1 and Fig. 2b and c). The CDR loops L1, L3, H1, H2, and H3 contribute 23.0 Å2 (5.0%), 74.2 Å2 (15.5%), 76.1 Å2 (15.9%), 98.6 Å2 (20.6%), and 132.9 Å2 (27.7%) of the BSA, respectively. Additionally, FWR L2 also has 74.4 Å2 (15.5%) of the BSA. These results are consistent with the notion that the H chain makes more interactions than the L chain when a mAb binds to a small-molecule antigen or a peptide antigen.32
Fig. 2.
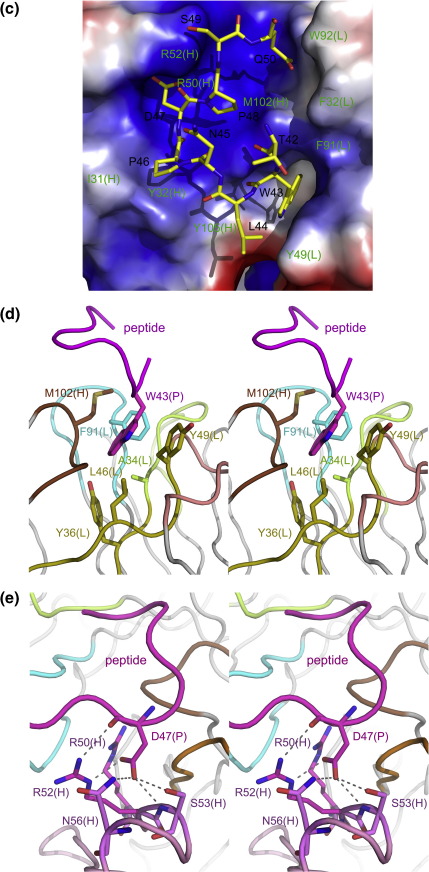
The interactions between the 23C3 Fab and the bound peptide. (a) An enlarged view of the interface surface. The peptide is bound at a pocket formed mainly by CDRs and interacts with CDRs L1, L3, H1, H2, and H3 and FWR L2. The Fab CDR L1 is colored lemon; L2, salmon; L3, cyan; H1, orange; H2, pink; H3, brown; FWR L2, olive; and other FWRs, silver. The peptide is colored purple. (b) A stereo view showing the hydrogen bonds between residues of the 23C3 Fab and residues of the epitope peptide. The color coding is the same as above. The residues that participate in the hydrogen-bonding interactions are shown with ball-and-stick models. (c) An electrostatic potential surface of the 23C3 Fab at the interface. The Fab accommodates the peptide with great structural and chemical complementarity. (d) The extensive hydrophobic interactions between the Fab and the key residue TrpP43 of the epitope peptide. Residues AlaL34 of CDR L1, PheL91 of CDR L3, MetH102 of CDR H3, and TyrL36, LeuL46, and TyrL49 of FWR L2 form a deep hydrophobic pocket to accommodate TrpP43 of the peptide. (e) The hydrophilic recognition of AspP47 by the Fab. AspP47 makes hydrogen-bonding interactions with SerH53, AsnH56, and ArgH52. It also forms electrostatic interactions with residues ArgH50 and ArgH52 of CDR H2. The color coding is the same as in (a).
Table 2.
The hydrophilic interactions between 23C3 Fab and the peptide
| Peptide atoms | Fab atoms | CDR loops | Distance (Å) |
|---|---|---|---|
| TrpP43-O | GlnH101-Nε2 | H3 | 2.9 |
| ProP46-O | AlaH33-N | H1 | 3.0 |
| AspP47-Oδ1 | SerH53-N | H2 | 3.0 |
| AspP47-Oδ1 | SerH53-Oγ | H2 | 2.8 |
| AspP47-Oδ1 | AsnH56-Nδ2 | H2 | 2.9 |
| AspP47-Oδ2 | ArgH52-Nε | H2 | 2.7 |
| AspP47-O | ArgH52-Nη1 | H2 | 3.1 |
| SerP49-Oγ | ArgH52-Nη1 | H2 | 3.0 |
| GlnP50-Nε2 | PheL91-O | L3 | 3.0 |
Analysis of the complex structure shows that all of the detected nine residues of the peptide interact with the 23C3 Fab and, particularly TrpP43 and AspP47, are the key residues recognized by 23C3. Specifically, the aromatic residue TrpP43 has a large hydrophobic indole group buried in a deep hydrophobic cage formed by AlaL34, TyrL36, LeuL46, TyrL49, PheL91, and MetH102, forming 46 van der Waals contacts and contributing a large BSA of 233.0 Å2 (Fig. 2d). This residue alone contributes 36.5% of all the van der Waals contacts between the Fab and the peptide and 33.1% of all the BSA of the peptide. Additionally, the main-chain carbonyl of TrpP43 forms a hydrogen bond with the side-chain amide of GlnH101 of CDR loop H3. The acidic residue AspP47 is embedded into a positively charged cleft to form electrostatic interactions with ArgH50 and ArgH52 (Fig. 2e). The side chain of AspP47 has three hydrogen-bonding interactions with SerH53 and AsnH56, and its main-chain carbonyl forms a hydrogen bond with the side chain of ArgH52 as well. These interactions make AspP47 the most important contributor for the hydrophilic interactions between the peptide and the Fab.
To further validate the critical roles of TrpP43 and AspP47 in the binding of 23C3, we carried out structural studies of the 23C3 Fab in complexes with the mutant peptides containing single mutation W43A or D47A, or double mutations W43A/D47A. The crystal structure of the 23C3 Fab in complex with the W43A mutant peptide was determined at 2.8 Å resolution with an R−factor of 24.1% and a free R−factor of 29.7% (Table 1). The structure of the mutant complex resembles that of the wild-type peptide complex (Fig. S1). Superposition of the two complexes yields an RMSD of 0.4 Å for 436 aligned Cα atoms. In this mutant peptide complex, only seven (LeuP44 to GlnP50) of the nine peptide residues (ThrP42 to GlnP50) observed in the wild-type peptide complex could be defined with clear electron density and the two N-terminal residues ThrP42 and AlaP43 are disordered, indicating that the binding of this region with the Fab is substantially impaired due to the loss of the hydrophobic interactions caused by the W43A mutation. Moreover, crystallization of the 23C3 Fab in complex with the D47A or W43A/D47A mutant peptide was unsuccessful so far. It is most likely that the D47A mutation of the peptide would disrupt the extensive hydrophilic interactions of the peptide with the Fab and lead to the loss of the peptide's ability to bind the Fab. These results support our notion that TrpP43 and AspP47 are essential for the binding of 23C3.
Although less important, the other residues within the mapped epitope 43WLNPDP4825 are also involved in the binding of the Fab (Fig. 2b, Tables 2 and S1). The side chains of residues LeuP44 and AsnP45 stretch out against the Fab–peptide interaction interface and each forms 3 van der Waals contacts. The main-chain carbonyl of ProP46 forms a hydrogen bond with the main-chain amide of AlaH33. The side chain of ProP46 is surrounded by a hydrophobic concave formed by residues LeuH31 and TyrH32 and forms 14 van der Waals contacts. The side chain of ProP48 is covered by AsnP45 and points to the interface forming 7 van der Waals contacts. Besides the 43WLNPDP48 motif, the other three residues (ThrP42, SerP49, and GlnP50) also contribute some van der Waals contacts with the Fab. The side chains of SerP49 and GlnP50 each form a hydrogen bond with the side chain of ArgH52 and the main-chain carbonyl of PheL91, respectively.
It is worth noting that besides CDRs, three conserved κ chain residues of FWR L2 (TyrL36, LeuL46, and TyrL49) are also involved in the interactions between the peptide and the Fab in addition to their typical roles in the interactions between VL and VH domains. In our structure, they all make van der Waals contacts with the peptide due to the deep embedment of TrpP43, which is rare, as residues TyrL36 and LeuL46 generally do not participate in the Fab–antigen interactions and TyrL49 is only occasionally found in interactions between the Fab and nuclear acids or hapten antigens.
In summary, 23C3 is a novel anti-osteopontin mAb with great potential for the treatment of RA. By determination of the crystal structure of 23C3 Fab in complex with its epitope peptide, we have revealed the interactions between the antibody and the peptide and identified the most important determinants for this epitope (TrpP43 and AspP47). We have also showed that FWR L2 plays an important role in the recognition and binding of the epitope by the antibody, suggesting that this region should be exploited in the development of chimeric or humanized antibodies. Besides, the detailed conformation of the epitope peptide may also be useful for the design of peptide vaccines against RA. As an example, a chemically constrained peptide mimicking the structural conformation of the epitope peptide of gp41 shows much stronger binding with the neutralizing HIV antibody 4E10 than the free peptide and therefore would be a better immunogen.36
Protein Data Bank accession code
The coordinates and structural factors of the 23C3 Fab fragment in complexes with its epitope peptide and the W43A mutant peptide have been deposited in the RCSB Protein Data Bank with accession codes 3CXD and 3DSF, respectively.
Acknowledgements
We are grateful to other members of our laboratories for technical assistance and helpful discussion. This work was supported by grants of the Ministry of Science and Technology of China (2004CB720102, 2006AA02Z112, 2006AA02A313, and 2007CB914302) and the National Natural Science Foundation of China (90713046 and 30730028).
Edited by I. Wilson
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jmb.2008.07.075
Contributor Information
Yajun Guo, Email: yjguo@smmu.edu.cn.
Jianping Ding, Email: jpding@sibs.ac.cn.
Appendix A. Supplementary data
References
- 1.Boers M. Rheumatoid arthritis. Treatment of early disease. Rheum. Dis. Clin. North. Am. 2001;27:405–414. doi: 10.1016/s0889-857x(05)70209-x. [DOI] [PubMed] [Google Scholar]
- 2.Holmdahl R., Bockermann R., Backlund J., Yamada H. The molecular pathogenesis of collagen-induced arthritis in mice—a model for rheumatoid arthritis. Ageing Res. Rev. 2002;1:135–147. doi: 10.1016/s0047-6374(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 3.Salmon M., Gaston J.S. The role of T-lymphocytes in rheumatoid arthritis. Br. Med. Bull. 1995;51:332–345. doi: 10.1093/oxfordjournals.bmb.a072964. [DOI] [PubMed] [Google Scholar]
- 4.Genovese M.C., Bathon J.M., Martin R.W., Fleischmann R.M., Tesser J.R., Schiff M.H. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46:1443–1450. doi: 10.1002/art.10308. [DOI] [PubMed] [Google Scholar]
- 5.Lipsky P.E., van der Heijde D.M., St Clair E.W., Furst D.E., Breedveld F.C., Kalden J.R. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N. Engl. J. Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 6.Campion G.V., Lebsack M.E., Lookabaugh J., Gordon G., Catalano M. Dose-range and dose-frequency study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis. The IL-1Ra Arthritis Study Group. Arthritis Rheum. 1996;39:1092–1101. doi: 10.1002/art.1780390704. [DOI] [PubMed] [Google Scholar]
- 7.Denhardt D.T., Noda M., O'Regan A.W., Pavlin D., Berman J.S. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross F.P., Chappel J., Alvarez J.I., Sander D., Butler W.T., Farach-Carson M.C. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin αvβ3 potentiate bone resorption. J. Biol. Chem. 1993;268:9901–9907. [PubMed] [Google Scholar]
- 9.Hu D.D., Lin E.C., Kovach N.L., Hoyer J.R., Smith J.W. A biochemical characterization of the binding of osteopontin to integrins αvβ1 and αvβ5. J. Biol. Chem. 1995;270:26232–26238. doi: 10.1074/jbc.270.44.26232. [DOI] [PubMed] [Google Scholar]
- 10.Denda S., Reichardt L.F., Muller U. Identification of osteopontin as a novel ligand for the integrin α8β1 and potential roles for this integrin–ligand interaction in kidney morphogenesis. Mol. Biol. Cell. 1998;9:1425–1435. doi: 10.1091/mbc.9.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokosaki Y., Matsuura N., Sasaki T., Murakami I., Schneider H., Higashiyama S. The integrin a9b1 binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J. Biol. Chem. 1999;274:36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 12.Barry S.T., Ludbrook S.B., Murrison E., Horgan C.M. Analysis of the α4β1 integrin–osteopontin interaction. Exp. Cell Res. 2000;258:342–351. doi: 10.1006/excr.2000.4941. [DOI] [PubMed] [Google Scholar]
- 13.Green P.M., Ludbrook S.B., Miller D.D., Horgan C.M., Barry S.T. Structural elements of the osteopontin SVVYGLR motif important for the interaction with α4 integrins. FEBS Lett. 2001;503:75–79. doi: 10.1016/s0014-5793(01)02690-4. [DOI] [PubMed] [Google Scholar]
- 14.Katagiri Y.U., Sleeman J., Fujii H., Herrlich P., Hotta H., Tanaka K. CD44 variants but not CD44s cooperate with β1-containing integrins to permit cells to bind to osteopontin independently of arginine–glycine–aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–226. [PubMed] [Google Scholar]
- 15.Denhardt D.T., Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993;7:1475–1482. [PubMed] [Google Scholar]
- 16.Ashkar S., Weber G.F., Panoutsakopoulou V., Sanchirico M.E., Jansson M., Zawaideh S. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 17.Xanthou G., Alissafi T., Semitekolou M., Simoes D.C., Economidou E., Gaga M. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat. Med. 2007;13:570–578. doi: 10.1038/nm1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diao H., Kon S., Iwabuchi K., Kimura C., Morimoto J., Ito D. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity. 2004;21:539–550. doi: 10.1016/j.immuni.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Burdo T.H., Wood M.R., Fox H.S. Osteopontin prevents monocyte recirculation and apoptosis. J. Leukoc. Biol. 2007;81:1504–1511. doi: 10.1189/jlb.1106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu B., Suzuki K., Goldberg H.A., Rittling S.R., Denhardt D.T., McCulloch C.A., Sodek J. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J. Cell. Physiol. 2004;198:155–167. doi: 10.1002/jcp.10394. [DOI] [PubMed] [Google Scholar]
- 21.Xu G., Nie H., Li N., Zheng W., Zhang D., Feng G. Role of osteopontin in amplification and perpetuation of rheumatoid synovitis. J. Clin. Invest. 2005;115:1060–1067. doi: 10.1172/JCI23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrow P.K., Hummel K.M., Schedel J., Franz J.K., Klein C.L., Muller-Ladner U. Expression of osteopontin messenger RNA and protein in rheumatoid arthritis: effects of osteopontin on the release of collagenase 1 from articular chondrocytes and synovial fibroblasts. Arthritis Rheum. 2000;43:1597–1605. doi: 10.1002/1529-0131(200007)43:7<1597::AID-ANR25>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto N., Nakashima T., Torikai M., Naruse T., Morimoto J., Kon S. Successful treatment of collagen-induced arthritis in non-human primates by chimeric anti-osteopontin antibody. Int. Immunopharmacol. 2007;7:1460–1470. doi: 10.1016/j.intimp.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto N., Sakai F., Kon S., Morimoto J., Kimura C., Yamazaki H. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J. Clin. Invest. 2003;112:181–188. doi: 10.1172/JCI17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan K., Dai J., Wang H., Wei H., Cao Z., Hou S. Treatment of collagen-induced arthritis with an anti-osteopontin monoclonal antibody through promotion of apoptosis of both murine and human activated T cells. Arthritis Rheum. 2008;58:2041–2052. doi: 10.1002/art.23490. [DOI] [PubMed] [Google Scholar]
- 26.Stanfield R.L., Zemla A., Wilson I.A., Rupp B. Antibody elbow angles are influenced by their light chain class. J. Mol. Biol. 2006;357:1566–1574. doi: 10.1016/j.jmb.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Al-Lazikani B., Lesk A.M., Chothia C. Standard conformations for the canonical structures of immunoglobulins. J. Mol. Biol. 1997;273:927–948. doi: 10.1006/jmbi.1997.1354. [DOI] [PubMed] [Google Scholar]
- 28.Wu T.T., Johnson G., Kabat E.A. Length distribution of CDRH3 in antibodies. Proteins. 1993;16:1–7. doi: 10.1002/prot.340160102. [DOI] [PubMed] [Google Scholar]
- 29.Bell C.H., Pantophlet R., Schiefner A., Cavacini L.A., Stanfield R.L., Burton D.R., Wilson I.A. Structure of antibody F425-B4e8 in complex with a V3 peptide reveals a new binding mode for HIV-1 neutralization. J. Mol. Biol. 2008;375:969–978. doi: 10.1016/j.jmb.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanfield R.L., Fieser T.M., Lerner R.A., Wilson I.A. Crystal structures of an antibody to a peptide and its complex with peptide antigen at 2.8 Å. Science. 1990;248:712–719. doi: 10.1126/science.2333521. [DOI] [PubMed] [Google Scholar]
- 31.Wallick S.C., Kabat E.A., Morrison S.L. Glycosylation of a VH residue of a monoclonal antibody against alpha (1–6) dextran increases its affinity for antigen. J. Exp. Med. 1988;168:1099–1109. doi: 10.1084/jem.168.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson I.A., Stanfield R.L. Antibody–antigen interactions: new structures and new conformational changes. Curr. Opin. Struct. Biol. 1994;4:857–867. doi: 10.1016/0959-440x(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 33.Wilson I.A., Stanfield R.L., Rini J.M., Arevalo J.H., Schulze-Gahmen U., Fremont D.H., Stura E.A. Structural aspects of antibodies and antibody–antigen complexes. Ciba Found. Symp. 1991;159:13–28. doi: 10.1002/9780470514108.ch3. [DOI] [PubMed] [Google Scholar]
- 34.Stanfield R.L., Wilson I.A. Protein–peptide interactions. Curr. Opin. Struct. Biol. 1995;5:103–113. doi: 10.1016/0959-440x(95)80015-s. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence M.C., Colman P.M. Shape complementarity at protein/protein interfaces. J. Mol. Biol. 1993;234:946–950. doi: 10.1006/jmbi.1993.1648. [DOI] [PubMed] [Google Scholar]
- 36.Cardoso R.M., Brunel F.M., Ferguson S., Zwick M., Burton D.R., Dawson P.E., Wilson I.A. Structural basis of enhanced binding of extended and helically constrained peptide epitopes of the broadly neutralizing HIV-1 antibody 4E10. J. Mol. Biol. 2007;365:1533–1544. doi: 10.1016/j.jmb.2006.10.088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.