Abstract
The development of biomaterials for drug delivery, tissue engineering and medical diagnostics has traditionally been based on new chemistries. However, there is growing recognition that the physical as well as the chemical properties of materials can regulate biological responses. Here, we review this transition with regard to selected physical properties including size, shape, mechanical properties, surface texture and compartmentalization. In each case, we present examples demonstrating the significance of these properties in biology. We also discuss synthesis methods and biological applications for designer biomaterials, which offer unique physical properties.
Today, biomaterials are routinely used in medical applications, such as drug delivery, tissue engineering, device-based therapies and medical imaging1. Many organic and inorganic materials, some of which are already available in the marketplace, have been specifically designed for promoting tissue growth and delivery of drugs. It has long been recognized that the material properties affect biological outcomes including the half-life of drugs, biocompatibility of implanted devices, and release rates and toxicity of drug carriers2,3. Similarly, properties of biomaterials can have a profound impact on cell proliferation and remodelling of tissues4. The central question that has fascinated biomedical researchers from the beginning has therefore been how to design and control material properties to achieve a specific biological response. Researchers have traditionally sought help from chemistry in answering this question. For example, release rates of drugs have been controlled through synthesis of new polymers that degrade in predictable ways2, and particle half-lives in the body can be prolonged by coating them with polyethylene glycol (PEG)5. PEG influences several other biological processes including endocytosis, protein adsorption, cell adhesion and activation of the complement system6. A range of biological targeting moieties, including antibodies, targeting peptides, aptamers and vitamins, have been conjugated to particles to modulate their biodistribution and to increase local therapeutic concentrations7. The availability of new materials has spurred the development of immunotherapies that use vaccines against diseases including certain cancers8 and Alzheimer's disease9. In yet another example, short peptides derived from common extracellular matrix components, such as the Arg-Gly-Asp (RGD) tripeptide10, have been shown to mitigate a series of cellular functions11-13. Surface-immobilized ligands have also been discovered and used for controlling differentiation of stem cells14,15. Such extraordinary emphasis on chemistry is consistent with the notion that molecular recognition forms the basis of many biological functions16.
Recently, however, research suggests that the field of biomaterials might be witnessing the emergence of a powerful set of new design parameters. Increasingly, scientists are seeking physics-derived solutions for controlling biological responses. For example, methods have been developed for fabricating polymeric particles with controlled shape17-19, mechanical properties20,21, surface topology and compartmentalization22. Particles with unique physical properties have been shown to influence many vital interactions with the body including phagocytosis17, circulation21, targeting and adhesion23. The local geometry of the microenvironment is now known to influence processes critical in tissue engineering, such as differentiation of stem cells24, proliferation of fibroblasts25, gene delivery26 and cell death27. Mechanical properties of the substrate have been demonstrated to produce a profound impact on cell and tissue behaviour28.
The motivation to use physical properties to control biological function comes from biology itself (Fig. 1). In nature, numerous examples can be found in which physical attributes such as shape, mechanical properties and compartmentalization are crucial to biological function. For example, bacteria come in a broad range of unique shapes including rods, spirals and ellipsoids29. The diversity of bacterial shapes highlights the significance of physical properties in their function. At the same time, macrophages, whose primary function is bacteria clearance, must be able to recognize such enormous diversity in shapes. The human body's own cells including platelets and erythrocytes also possess unique shapes and mechanical properties that make their specific functions possible. For example, erythrocytes are able to avoid filtration in the spleen because of their discoidal shape and mechanical flexibility. Platelets, on the other hand, use their disc-like shape to assist their function of adhesion and rolling on vascular endothelium30. The mechanical properties of the cellular microenvironment have an important role in morphogenesis of tissues such as bone, cartilage and cornea31,32. In addition, mechanical properties deeply affect the functioning of the immune system, where macrophages are unable to phagocytose softer targets33. It is now well known that cells use compartmentalization to control various biochemical reactions in space and time34. Recent research further suggests that the surface texture of cells may also be important in cellular function. In particular, surface roughness of macrophages may be important in their ability to recognize the size of objects35.
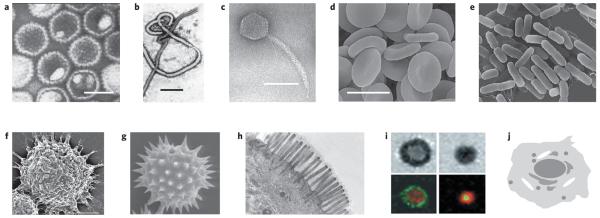
Figure 1. Examples of natural biological objects that have diverse physical properties.
a, Human herpesvirus 3; scale bar, 100 nm (image: Frank Fenner). b, Ebola virus; scale bar, 500 nm (image: Frederick A. Murphy). c, Enterobacteria phage λ; scale bar, 50 nm (image: University of Wisconsin-Madison). d, Human erythrocytes; scale bar approximately 10 μm. e, Escherichia coli; image size approximately 7 μm × 6 μm. (Panels d and e © Dennis Kunkel Microscopy.) f, Surface texture in alveolar macrophages73; scale bar, 5 μm (© 2008 STM). g, Pollen; image size approximately 50 μm × 45 μm (Dartmouth Electron Microscope Facility). h, Intestinal villi; approximate magnification μ5,950 (© Dennis Kunkel Microscopy). i, The immunological synapse; T cell forming a synapse with a supported membrane containing GPI-linked pMHC and ICAM (ref. 134); © 1999 AAAS). j, A schematic of cellular compartmentalization showing several organelles surrounding the nucleus. The images clearly establish that nature uses physical parameters such as size, shape, texture and compartmentalization in designing life.
A clearer picture is emerging that physical attributes such as size, shape and mechanical properties form essential building blocks of biology, just as chemistry and molecular recognition do. This revelation establishes the basis of new design concepts for biomaterials. Here, we describe the origin of this transition, its current status and its future prospects.
Mechanical properties
Tissues of the human body have selective mechanical properties ranging from soft (brain, about 0.5 kPa), to moderately stiff (skin and muscles, around 10 kPa) and stiff (precalcified bone, >30 kPa)28. The narrow specifications of elastic moduli create mechanically defined microenvironments that effectively support the development of cellular architecture36,37. For example, mesenchymal stem cells (MSCs) showed strikingly different morphologies when cultured on polyacrylamide (PAAm) substrates with varying elasticities38. MSCs cultured on a soft substrate (1 kPa) expressed neuronal cytoskeleton markers, such as β-3-tubulin, whereas cells grown on 11-kPa and 34-kPa substrates showed expression of early myogenic and osteogenic transcription factors MyoD and CBFα-1, respectively. But yields were moderate unless advantage was taken of cooperative effects of matrix elasticity and solute differentiation factors, suggesting that both mechanical and chemical cues are essential for cell differentiation. Moreover, further follow-up work with long-term cell culture is warranted to demonstrate that fully differentiated cell populations can reliably be generated in this way. Nevertheless, this work has important implications for regenerative stem-cell therapies, because it may provide important clues on how to obtain appropriate differentiation of MSCs in vivo by mitigating unfavourable elasticity of an already stiffened fibrotic infarct area39.
To transduce mechanical stimuli into biochemical signals, cells use a palette of localized elements including mechanosensitive ion channels, forced unfolding of proteins40 and remodelling of focal adhesion sites41. Details of the mechanisms involved in force sensing, mechanotransduction and mechanoresponsive pathways have been elucidated over the past decade and are discussed elsewhere26,27,42. Many committed cell types have developed mechanosensory machineries43,44, and an increasing number of cellular processes, such as adhesion, cell spreading or cytoskeletal remodelling, are found to depend on mechanical cues originating from the cellular microenvironment43. Beningo and Wang have successfully used these principles to reduce phagocytosis of PAAm particles by making them soft33. However, microinjection of lysophosphatidic acid overrides these mechanical cues, resulting in particle uptake irrespective of the softness of particles33. As is true for many studies in such a complex field, physical and chemical factors seem to influence the biological outcome simultaneously. It is useful to note, though, that differences in mechanical properties may be accompanied by inadvertent changes in the chemical properties. Soft and hard PAAm microparticles were made under slightly different polymerization conditions, with four times as much crosslinker being used for the hard particles: at least in principle, this could not only create differences in the particle elasticity, but also change the particles' zeta-potential, sizes and size distributions. Because all of these factors are important for phagocytosis, only rigorous particle characterization can ensure that the observed biological effects are attributable to a single factor, and not the result of a subtle interplay of multiple, convoluted factors.
In another example, the stiffness of a cell substrate was optimized to achieve optimal growth of muscle cells45. Specifically, very soft and stiff gels produced myosin patterns different from those observed under normal physiological conditions. Fibroblasts, epithelial cells and endothelial cells all showed increased proliferation on stiffer substrates. In contrast, neurons showed greater proliferation on softer substrates, which correlates well with their natural environment in the brain39. In a related study, Mooney and co-workers found that tuning the elasticity of a substrate was a possible way to improve non-viral gene delivery in murine pre-osteoblasts46. In this case, increasing the stiffness of the substrate from 20 to 110 kPa resulted in greater cell proliferation, which was identified as a prerequisite for plasmid DNA uptake. This example shows that the elasticity of the substrate can be a critical factor for non-viral gene delivery, having a role similar to that of the surface density of adhesion ligands.
Particles and substrates of different stiffness can be prepared by various methods, but are most frequently realized by control of crosslinking densities in hydrogels31. For commonly used hydrogels, such as PAAm or agarose, varying the amount of chemical crosslinking agents is a simple and often appropriate means to control mechanical properties over a substantial range, but care is needed to ensure that no other changes are inadvertently induced in substrate properties such as chemistry, size, shape or surface charges31. This creates a need for new materials systems that can be crosslinked on demand while minimizing overall changes to the chemical properties of the hydrogel. In principle, several different chemical reactions, such as Diels–Alder or Huisgen cycloadditions, are suitable for such cell-matrix modifications47,48. Alternatively, microfabricated arrays made of elastomeric polymers can be used to control mechanical stiffness and mechanical anisotropy of substrates by varying the shape of individual posts within an array49. Variants of established photolithographic and soft lithographic methods are typically used to fabricate these micro- and nanoarrays50. Such precisely engineered mechanical substrates are powerful tools for quantitative studies of cell interactions with the extracellular matrix51.
The above examples of materials and material–tissue interactions suggest that precise tailoring of mechanical properties will be essential for future progress in delivery of drugs and genes as well as in regenerative medicine. Substrate elasticity may be used to guide differentiation of stem cells into specific types, for example cardiomyocytes, even though the local microenvironment present in patients who have suffered myocardial infarction is not conducive to this. In the field of drug delivery, softer, flexible particles are expected to minimize phagocytosis and have a longer lifetime in the body. Literature studies have already indicated that flexible, worm-like micelles circulate for prolonged periods in the blood, and this is in part due to their flexible nature21.
Size
Particle size has been much studied in the context of development of biomaterials for drug delivery. Numerous methods have been used to fabricate micro- and nanoparticles of various sizes, including emulsion polymerization52, microfabrication53,54, self-assembly55 and jet breaking56. Particle diameter can often be controlled through physical properties of the materials, such as polymer and surfactant concentration, or through the experimental parameters of the fabrication method, for example the mixing method (vortexing, sonication, stirring), nozzle/capillary diameter and material flow rate. A detailed review of fabrication methods is beyond the scope of this review.
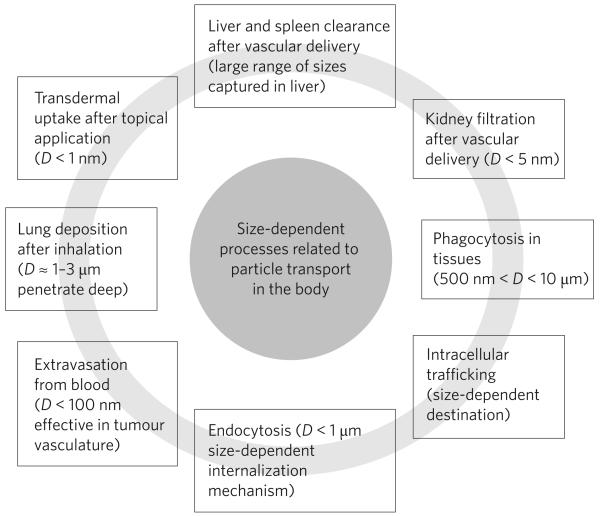
Particles with sizes ranging from less than a nanometre to a few tens of micrometres have been fabricated and tested for biomedical applications57,58. Several important in vivo functions of particles (drug carriers) depend on particle size: these include circulation times, extravasation, targeting, immunogenicity, internalization, intracellular trafficking, degradation, flow properties, clearance and uptake mechanisms (Fig. 2)58-61. Particle diameter dictates their transport and adhesion in blood vessels, airways or gastro-intestinal tract62-64. There exists a variety of size-based clearance mechanisms in blood65. Microparticles are captured by Kupffer cells in liver or physically trapped in the capillary beds66,67, whereas nanoparticles smaller than 100 nm leave the blood vessels through fenestrations in the endothelial lining66,68. In tumours, where the vasculature is leaky, particles can preferentially leave the blood. Regardless of the method of administration, particles larger than 500 nm can be phagocytosed by macrophages, and smaller particles can be endocytosed by professionally phagocytic or non-phagocytic cells69,70. Internalization by targeted non-phagocytic cells is desirable for localized delivery, but phagocytosis or uptake by macrophages clears particles from the body and is usually undesirable. Size, along with surface chemistry, is also believed to affect opsonization (that is, antibody-mediated cell uptake), through the relationship between particle size and curvature of spheres71. Comparing gold nanoparticles with diameters of 14, 50 and 74 nm, Chan et al. found significantly higher uptake for 50-nm particles. Although this study examined only a single material, gold, the identified optimal diameter corresponds well with a study that found highest uptake rates for glycoviruses with a diameter of about 50 nm (ref. 72). In a related study, which investigated the size-dependent immunogenicity of polystyrene particles with diameters between 20 nm and 2 μm, it was found that particles of intermediate size (40 nm) activated dendritic cells in the lymph node. On a similar note, phagocytosis of polystyrene particles also depends strongly on particle size, with maximum phagocytosis occurring at a size range between 2 and 3 μm (ref. 73). Interestingly, this range matches the general size range of bacteria, the most common targets of macrophages. Along with particle diameter, size distribution may be another important factor when evaluating biological function of a specific particle formulation74.
Figure 2. Size-dependent processes of particle transport in the human body.
Particles can pass through biological barriers by a number of different processes. These include passive (diffusive) and active processes ranging from extravasation to transdermal uptake. Most of these processes affect distribution and clearance of micro- and nanoparticles in the human body and they strongly depend on particle size.
There is no doubt that particle size and size distributions have implications for a number of biomedical applications, such as drug delivery or medical imaging. For example, it is widely recognized that particles designed for extravasation into tissues should have sizes below 100 nm (ref. 57). Micrometre-scale particles (diameters 1–10 μm) are generally not considered suitable for intravascular injections because they are cleared rapidly from circulation. Such guidelines, although generally useful, must be viewed in the context of other relevant parameters, because there are well-documented exceptions to such general rules. For example, particles with diameters between 1 and 3 μm are suitable for pulmonary delivery, because they can travel deep into the lungs. However, particles in this size range also happen to be most susceptible to phagocytosis, thus limiting their residence time. Edwards et al. showed that by controlling particle density as an independent design parameter, this paradox can be resolved. They created large (∼10 μm) but light particles (density <0.1 g cm−3), which travelled deep into lungs owing to their low density, but avoided phagocytosis owing to their large size75.
Shape
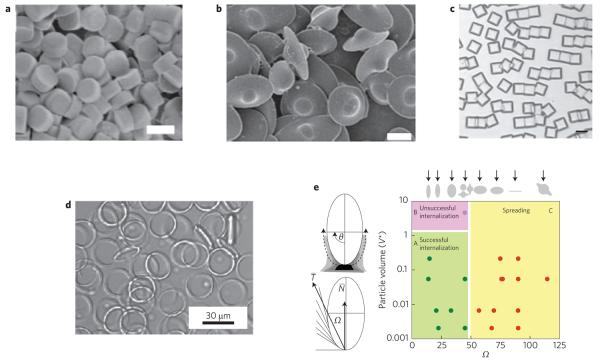
Shape is another essential property of a particle (Fig. 3) and has an important role in mitigating cellular responses and related applications in biotechnology76,77. Theoretical models have already pointed to the benefits of using non-spherical particles for drug delivery based on their influence on cellular internalization and vascular dynamics78-80. Experimental studies have confirmed the role of particle shape in drug delivery. For example, phagocytosis by macrophages, a key step in drug delivery, strongly depends on shape17. In fact, the local geometry of the particle at the point of cell attachment, not the overall particle shape, can dictate whether macrophages initiate internalization17. A macrophage encountering an elliptical disc at the pointed end fully internalized the particle in a few minutes, whereas a macrophage attached to a flat region of the elliptical disc did not succeed for over 12 h. Studies that focused on evaluating the interplay between shape and size revealed that these two parameters have very different influence on phagocytosis (Fig. 3). The local shape of the particle, characterized by an angle Ω (Fig. 3e), fundamentally determined whether phagocytosis was initiated, as judged by formation of an actin ring underneath the macrophage membrane. In a more recent study, Champion and Mitragotri reported on the interplay of size and shape in phagocytosis81. At a much smaller length scale, Gratton et al.82 demonstrated that internalization of cylindrical particles depends strongly on their aspect ratio. Particles with an aspect ratio of three were internalized about four times as fast as their spherical counterparts of the same volume. In contrast, some studies have found that uptake of gold nano-objects by receptor-mediated endocytosis significantly decreased with increased aspect ratios. For instance, gold nanospheres with diameters of 14 nm or 74 nm were taken up by HeLa cells more than three times as often as 74 × 14 nm rods83. Moreover, Muro et al.84 compared targeted accumulation in tissues of spheres of various diameters (ranging from 100 nm to 10 μm) and elliptical discs of microscale dimensions (1 × 3 μm) and found that targeting efficiency of micrometre-scale discs is better than any sphere, even those of nanometre dimensions.
Figure 3. Examples of designer particles with different shapes.
a, Cylindrical particles prepared using PRINT method82; scale bar, 5 μm (© 2008 PNAS). b, UFO-shaped particles prepared by film-stretching18; scale bar, 5 μm (© 2007 PNAS). c, Rectangular discs of SU-8 (ref. 53); scale bar, 150 μm (© 2006 NPG). d, Polymer rings90 (© 2006 NPG). e, Phagocytosis of particles depends on shape17 (© 2006 PNAS).
In a separate study, long cylindrical micelles showed prolonged circulation that might be attributable to their shape21. Specifically, they found that worm-shaped polymeric particles show negligible phagocytosis compared with spheres of the same volume. In this case, however, additional factors such as surface chemistry and the mechanical elasticity of the micelles may have similarly important roles in prolonging circulation times. Several studies also report on the use of carbon nanotubes or gold nanorods, in combination with an appropriate surface ligand, for targeted delivery to tumours in vivo85,86.
Various methods continue to be developed for fabricating particles of diverse shapes ranging from a few nanometres to the micrometre scale. Nanocrystals with a wide range of shapes have already been prepared and current trends have been reviewed elsewhere87. Other methods to control the shape of nano- and microparticles include mechanical stretching18,88,89, soft lithography19, microfluidics90 or self-assembly91. Template-assisted self-assembly can also be used to form clusters and chains of polymer spheres by trapping them in moulded cavities of various shapes and sizes92,93. Often, these methods can be combined to obtain even more complex structures.
An important criterion when selecting fabrication techniques for shape-controlled particles is that the process is versatile enough to accommodate a range of biologically relevant materials. In addition, payloads for biomedical applications are typically biomolecules that severely constrain the types of solvents, pressures and temperatures that can be used for particle fabrication. Ultimately, a successful technology will be able to produce a wide range of shapes through versatile processes that maintain the activity of the entrapped factors and allow for controllable release, if needed.
In some selected examples, new methods have already made it possible to investigate the role of particle shape in biological function. For example, Rolland et al. used soft lithographic moulding methods, but replaced polydimethylsiloxane (PDMS) with non-wetting perfluoropolyether (PFPE) moulds. Using this process, they were able to create isolated particles of PEG, poly(lactic acid) and poly(pyrrole), among others, of various shapes, and thus to study the impact of shape on particle internalization82. Similarly, Champion and Mitragotri et al. have reported methods for making particles of various shapes by liquefaction and stretching18. These particles have allowed identification of the role of shape in phagocytosis and vascular targeting. With new fabrication tools available, studies on how shape influences essentially every major cellular process can be expected. Such examples show that the role of particle size might have been incorrectly interpreted in the literature and should be assessed in the broader context of overall particle geometry.
It is likely that initial applications based on particle shape will arise in the areas of imaging agents, and long-circulating and targeted drug delivery. In many instances, studies have already shown that particles of unique shapes bring benefits that are difficult to achieve through spherical ones. Furthermore, transport of particles in the body, which strongly influences their effectiveness as drug carriers, is affected by particle shape76,78. Spherical particles must be less than 200 nm in diameter to pass through the spleen, but disc-shaped, flexible red blood cells with diameters of some 10 μm routinely pass through. Theoretical models have already brought out the benefits of using non-spherical particles for drug delivery. For example, they predict that oblate particles will adhere more efficiently to the vascular endothelium than would spherical particles of the same volume79, a feature that is critical for targeted delivery to endothelium. These predictions are in part confirmed by experimental studies84. Non-spherical particles may also align or tumble in the presence of flow, which may reduce their clearance in the liver or spleen71, a feature essential to prolong their half-life in the body.
Surface microstructure, texture and porosity
The nano- and microstructure of surfaces has been established as a decisive factor affecting cell morphology, adhesion or motility94. Vast evidence has been gathered for two-dimensional substrates, where nanopatterning methods are available for creating a wealth of surface structures with dimensions in the approximate size range of focal adhesion sites95,96. In comparison, more conventional, soft-lithographic micropatterning methods or aligned microfibres can be used to create surface patterns in the size range of individual cells97,98.
Using surfaces with features between 50 and 500 nm, recent studies found pronounced differences in cell spreading and focal adhesion dynamics, which depended not only on feature size, but also on the spacing between cell-recognizable features27. For example, spacings as small as 13 nm were found to result in increased cell-spreading velocity99 and non-viral gene delivery was found to be strongly increased on substrates with RGD islands arranged at a spacing of 36 nm, but not 85 or 120 nm (ref. 100). Again, the underpinning mechanism leading to higher uptake may be related to increased cell proliferation on the substrates with the highest ligand densities, a phenomenon that mirrors cellular responses to mechanical effects. Similarly, Bastmeyer et al. systematically varied size and spacing of the extracellular matrix patterns and found that the shape of murine melanoma cells was governed by the pattern of spacings larger than 5 μm. For spacings smaller than 2 μm, cells behaved as if they were seeded on homogeneous substrates101. Sanders et al. studied the influence of fibre diameter on fibrous capsule formation, a typical response of the body to isolate foreign bodies. Fibres with diameters above 5.9 μm supported fibrous capsule formation, whereas smaller diameter fibres did not. Interestingly, this trend seemed to be less dependent on the actual fibre material than on the inter-fibre spacing, suggesting that the local geometry might be more important in this context than the materials chemistry102. On the other hand, a recent study with PDMS nanoarrays, which explored larger post diameters between 750 and 1,500 nm and spacings between 1,750 and 4,500 nm, found no significant differences in cell spreading between nanoarrays and unstructured surfaces103, and a study evaluating the effects of nanotopography of titanium found no significant differences in cell adhesion for substrates with different nanostructures104. In contrast, aligned nanofibres have been shown to influence important cell functions, such as extracellular matrix production105. Still, caution must be taken when interpreting these and many other data, because the introduction of surface patterns may inadvertently result in the creation of nanotopological features overlaid on the actual micro- or nanopatterns.
The effects of nano- and microstructure on cells may extend well beyond spreading. Cells can undergo apoptosis27 or directed cell differentiation106, depending on the feature sizes of patterned substrates. MSCs selectively differentiated into either adipocytes or osteocytes when cultured on substrates that presented adhesive islands of various sizes. In this case, a change in cell shape, which depended on the total accessible area, was responsible for the switch in cell-lineage commitment. A deeper understanding of the underlying mechanisms will require more comprehensive studies that systematically delineate the influence of pattern shape from the available surface area. Beyond cell differentiation, a recent study by Curtis et al. suggests that nanotopologies not only guide focal adhesion clustering of human fibroblasts, but may also stimulate frustrated endocytosis, as shown by the highly disrupted cytoskeletons observed in these cells107. The latter may imply that micro- and nanoscale surface patterns are not only important for flat substrates, but could also have a profound impact on endocytosis of particles. Nevertheless, mechanisms of cellular interactions with micro- and nanostructured particles are still largely unexplored, partly because of the absence of adequate patterning methods for particles.
A series of particle technologies are poised to overcome the limited availability of appropriate synthetic strategies for surface-structured particles, such as biphasic particles108 (Fig. 4). Initial approaches included a series of low-throughput methods for Janus particles including partial masking109, selective deposition110 and microcontact printing of particles111. Alternative synthetic approaches, which often lead to higher throughputs, use selective metal deposition through membranes to create multifunctional nanorods. The fact that two metals, such as nickel and gold, are combined in the same rod allows for regional surface modifications that can be selective for either gold or nickel areas. This bipolar architecture allows selective modification with targeting moieties and has resulted in transfection rates that were 830 times as high as background without nanorods112. Nevertheless, overall plasmid loadings are intrinsically low, because only the surface area of the nickel region is available for plasmid binding. In what may turn out to be a more scalable approach, Doyle and co-workers used a photolithographic method to create encoded microparticles for diagnostics applications113. Moreover, spatially controlled immobilization of proteins was achieved by photoreactive coatings based on chemical vapour deposition polymerization114. In contrast to these fully synthetic approaches, DNA-based self-assembly methods have recently been promoted as particularly promising methods for creation of complex nanoscale protein patterns, because they are applicable to both flat and curved surfaces115.
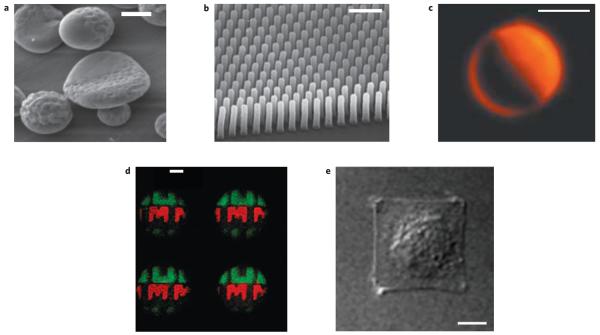
Figure 4. Examples of surfaces with micro- or nanoscale heterogeneity.
a, Biphasic discs with smooth and rough surface; scale bar, 1 μm (image: Joerg Lahann). b, Pillar arrays for mapping force of epithelial cell migration134; scale bar, 5 μm (© 2005 PNAS). c, Asymmetrically coated particles136; scale bar, 5 μm (© 2003 RSC). d, Micropatterned particles114; scale bar, 100 μm (© 2007 PNAS). e, Control of spreading of an endothelial cell through a micropatterned substrate27; scale bar, 10 μm (© 1997 AAAS).
Particles with micro- and nanostructured surfaces may prove useful in important biomedical applications including molecular imaging116, or as carriers for high-throughput screening113. Gold nanoparticles with unique surface patterns were found to access the cytosol of murine dendritic cells directly through membrane penetration, rather than endosomal uptake117. A striking difference between surface-patterned and non-patterned particles of the same size and overall surface composition was found118. Although the underlying mechanism is far from elucidated, this work shows that precise tailoring of surface patterns may provide access to fundamentally different ways of transfering payloads, such as molecular imaging probes, drugs or genes, into the cytosol. As in this example, materials innovations will challenge the way we think about cellular particle uptake and will profoundly impact targeted drug delivery or intracellular imaging.
Compartmentalization
Compartmentalization is one of the key architectural principles of mammalian cells that distinguishes them from less evolved forms of life. The interior of eukaryotic cells is compartmentalized into organelles of various sizes ranging from 10–25 nm (ribosomes, for example) to 100–500 nm (endosomes, lysosomes). These compartments exchange nutrients or metabolites through a variety of processes including vesicle budding, motor-assisted movements, membrane fusion and membrane permeation. Proper functioning of cells requires precise control over metabolic reactions, which is achieved by segregation of biomolecules in compartments and exchange through boundaries by means of selective transport processes. For example, transcription factors, which can induce gene expression, are generally localized in the cytoplasm and can enter the nucleus only on activation. Many other examples of intracellular compartmentalization can be observed during cellular metabolism. In contrast, only a limited number of synthetic mimicries of this abundant natural concept have been realized in the form of synthetic particles, and may find applications as fully integrated multifunctional vehicles for drug delivery, in vivo sensing, or synthetic cell mimicry (Fig. 5).
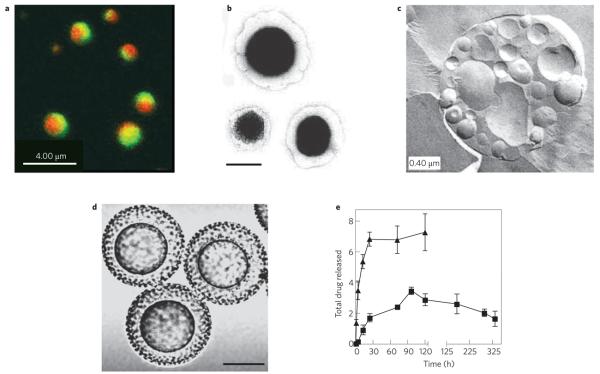
Figure 5. Examples of multifunctional particles based on compartmentalization.
a, Biphasic particles22 (© 2005 NPG). b, Composite liposome-nanoparticle carriers119; nanocells, scale bar 100 nm (© 2005 NPG). c, Vesosomes137 (© 2002 ACS). d, Core–shell microparticles138; scale bar 25 μm (© 2007 STM). e, Differential release of two drugs from two compartments of nanocell particles119 (© 2005 NPG).
In its simplest form, multicompartmental particles can be capsules consisting of a shell compartment surrounding a core element. A number of processes have been established for synthesis of core–shell particles119,120 and many of these particles have generated great interest because of their potential use for drug delivery121. Synthesis methods include self-assembly122, emulsion polymerization123, layer-by-layer adsorption onto solid core particles124, and templated polymerization125-127. All of these methods have their strengths and weaknesses, but emulsion polymerization and self-assembly into liposomes and polymersomes are currently the most successful technologies for preparation of core–shell particles for biomedical applications.
Although synthetic particles with multiple compartments may provide a series of interesting biological properties, such as controlled anisotropy, loading with multiple drugs or dyes, or controlled orientation and hierarchical self-assembly, widely applicable synthetic routes have only recently begun to emerge. Existing methods rely on self-assembly128,129, including fusion of pre-existing particles to create multicompartmental particles and lipid vesicles130. However, particle technologies with the potential to create custom-tailored multicompartmental particles are on the horizon. At the heart of many of these processes is the idea of using relatively simple manipulations of liquids followed by rapid phase transition to create non-equilibrium structures in solid particles. As long as the liquid–solid transitions occur fast enough to minimize mixing, they can be prepared by a number of different solvent-drying processes or chemical reactions. Recent examples include the fabrication of microparticles using hydrodynamic jets131 and electrohydrodynamic co-jetting22. It is a unique property of multicompartmental particles that each compartment can be independently loaded with a different biological payload, such as a growth factor, protein, siRNA or antibacterial material132. At the same time, compartments can be made of different matrix materials, which enable independent surface modification, as well as independent control of extremely diverse functionalities, such as release rate, environmental responsiveness or antibacterial properties of particles. Multicompartmental particles can be expected to find a wide range of applications in fundamental studies of cell architecture as well as in biomedical application, such as drug delivery or molecular imaging. In principle, the design feature of compartmentalization can be multiplexed with all the other physico-chemical design features, as well as with the particle chemistry, to create truly new particle architectures, where one compartment is smooth and the other rough (Fig. 4a), both compartments are made of different polymers (Fig. 5a), or small and large compartments may be combined in a single particle.
Conclusions and future perspectives
Although considerable advances have already been made in understanding how the physical properties of materials affect biological functions, the field is still in its infancy. At present, it is clear that physical properties, such as size, shape, mechanical properties, surface texture and compartmentalization, profoundly impact the function of a biomaterial, once it is placed into a biological environment. Many other physical parameters including density and porosity will also significantly impact a material's function. This greatly widens the design parameter space for the next generation of biomaterials but, at the same time, raises important questions. Substantial work remains to map the dependence of biological response to physical properties and to categorize the relative weight of different physical and chemical factors. For each biomedical application, detailed mechanisms of how physical properties affect biological performance, as well as the interplay between various physico-chemical properties, may have to be elucidated case by case.
Over the past decade, a number of materials fabrication processes have already been devised to control the physical properties of particles. But to ensure biomedical applicability, it is critical that the underpinning processing conditions are compatible with the specific requirements of biological molecules, such as sensitivity to elevated temperatures, organic solvents or extreme pH changes. In addition, materials must be biocompatible within the context of the intended biomedical use. This requires technologies that are versatile enough to accommodate a range of different materials, while ensuring gentle process conditions. Moreover, future applications may require physical and chemical design parameters to be multiplexed to ensure optimal performance133. Again, compatibility among different materials and processes will be a key requirement. For instance, compartmentalization can be used in combination with other physico-chemical design features, such as size, shape or surface structure, to generate particle architectures that ultimately will lead to truly biomimetic materials. Early benefits of such combination strategies are already evident. For instance, a combination of shape, size and surface chemistry has been used to target particles to vascular endothelium for drug-delivery applications84. Similarly, combination of shape and mechanical flexibility has been used to target drugs to tumours more efficiently21. In this case, the mechanical flexibility of the nanoparticles allows them to remain in circulation for longer21. The need to explore the multi-parameter space is particularly exciting for applications in drug delivery, where the number of challenges that the carrier needs to overcome before reaching the target has limited their success. A versatile toolbox of parameters will help carriers to navigate through these hurdles.
Finally, advances in our knowledge base and emerging biomedical applications have to be supported by new synthesis and manufacturing capabilities. Research will need to be directed towards fabrication methods, which must be able not only to produce materials with various physical properties, but also to offer independent control over them. The latter constraint is particularly important, as the biological response to a given biomaterial can show interdependence on multiple parameters. The rapidly emerging design space of biomaterials with controlled physical, chemical and biological properties represents a great opportunity for future research.
Acknowledgements
We acknowledge support from the National Institute of Health Program of Excellence in Nanotechnology (1VO1 HL080718), the Department of Defense (Idea award) and the NSF (Career award DMR-0449462). We thank A. Arora and N. Doshi for help in preparing the manuscript.
References
- 1.Peppas NA, Langer R. New challenges in biomaterials. Science. 1994;263:1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 3.Karp JM, Langer R. Development and therapeutic applications of advanced biomaterials. Curr. Opin. Biotechnol. 2007;18:454–459. doi: 10.1016/j.copbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Mooney DJ, Mikos AG. Growing new organs. Sci. Am. 1999;280:60–65. doi: 10.1038/scientificamerican0499-60. [DOI] [PubMed] [Google Scholar]
- 5.Gref R, et al. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 6.Owens DE, III, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Wang MD, Shin DM, Simons JW, Nie S. Nanotechnology for targeted cancer therapy. Expert Rev. Anticancer Ther. 2007;7:833–837. doi: 10.1586/14737140.7.6.833. [DOI] [PubMed] [Google Scholar]
- 8.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 9.Monsonego A, Weiner HL. Immunotherapeutic approaches to Alzheimer's disease. Science. 2003;302:834–838. doi: 10.1126/science.1088469. [DOI] [PubMed] [Google Scholar]
- 10.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 11.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 12.Beningo KA, Dembo M, Wang Y-L. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc. Natl Acad. Sci. USA. 2004;101:18024–18029. doi: 10.1073/pnas.0405747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva GA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 14.Makino H, Hasuda H, Ito Y. Immobilization of leukemia inhibitory factor to culture murine embryonic stem cells. J. Biosci. Bioeng. 2004;98:374–379. doi: 10.1016/S1389-1723(04)00298-1. [DOI] [PubMed] [Google Scholar]
- 15.Yang SH, Hsu CK, Wang KC, Hou SM, Lin FH. Tricalcium phosphate and glutaraldehyde crosslinked gelatin incorporating bone morphogenetic protein-viable scaffold for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2005;74:468–475. doi: 10.1002/jbm.b.30200. [DOI] [PubMed] [Google Scholar]
- 16.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nature Rev. Mol. Cell. Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 17.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc. Natl Acad. Sci. USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Champion JA, Katare YK, Mitragotri S. Making polymeric micro- and nanoparticles of complex shapes. Proc. Natl Acad. Sci. USA. 2007;104:11901–11904. doi: 10.1073/pnas.0705326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolland JP, et al. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 20.Euliss LE, DuPont JA, Gratton S, DeSimone J. Imparting size, shape, and composition control of materials for nanomedicine. Chem. Soc. Rev. 2006;35:1095–1104. doi: 10.1039/b600913c. [DOI] [PubMed] [Google Scholar]
- 21.Geng Y, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nature Nanotech. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roh KH, Martin DC, Lahann J. Biphasic Janus particles with nanoscale anisotropy. Nature Mater. 2005;4:759–763. doi: 10.1038/nmat1486. [DOI] [PubMed] [Google Scholar]
- 23.Tao SL, Desai TA. Micromachined devices: the impact of controlled geometry from cell-targeting to bioavailability. J. Control. Release. 2005;109:127–138. doi: 10.1016/j.jconrel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Graziano A, et al. Scaffold's surface geometry significantly affects human stem cell bone tissue engineering. J. Cell Physiol. 2008;214:166–172. doi: 10.1002/jcp.21175. [DOI] [PubMed] [Google Scholar]
- 25.Milner KR, Siedlecki CA. Submicron poly(l-lactic acid) pillars affect fibroblast adhesion and proliferation. J. Biomed. Mater. Res. A. 2007;82:80–91. doi: 10.1002/jbm.a.31049. [DOI] [PubMed] [Google Scholar]
- 26.Shen H, Tan J, Saltzman WM. Surface-mediated gene transfer from nanocomposites of controlled texture. Nature Mater. 2004;3:569–574. doi: 10.1038/nmat1179. [DOI] [PubMed] [Google Scholar]
- 27.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 28.Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment: Implications for regenerative medicine and drug delivery. Adv. Drug Deliv. Rev. 2007;59:1329–1339. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young KD. The selective value of bacterial shape. Microbiol. Mol. Biol. Rev. 2006;70:660–703. doi: 10.1128/MMBR.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frojmovic MM, Milton JG. Human platelet size, shape, and related functions in health and disease. Physiol. Rev. 1982;62:185–261. doi: 10.1152/physrev.1982.62.1.185. [DOI] [PubMed] [Google Scholar]
- 31.Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment- Implications for regenerative medicine and drug delivery. Adv. Drug Deliv. Rev. 2007;59:1329–1339. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engler A, Richert L, Wong JY, Picart C, Discher D. Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: correlations between substrate and cell adhesion. Surf. Sci. 2004;570:142–154. [Google Scholar]
- 33.Beningo KA, Wang YL. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J. Cell Sci. 2002;115:849–856. doi: 10.1242/jcs.115.4.849. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama K. Membrane traffic: editorial overview. J. Biochem. 2004;136:751–753. doi: 10.1093/jb/mvh183. [DOI] [PubMed] [Google Scholar]
- 35.Champion JA, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res. 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 37.Bershadsky A, et al. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur. J. Cell Biol. 2006;85:165–173. doi: 10.1016/j.ejcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 39.Engler A, et al. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson CP, Tang H-Y, Carag C, Speicher DW, Discher D. Forced unfolding of proteins within cells. Science. 2007;317:663–664. doi: 10.1126/science.1139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nature Rev. Mol. Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 42.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–27. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 43.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Zeíev A. Virus replication in infected epithelial cells is coupled to cell shape-responsive metabolic controls. J. Cell Physiol. 1983;114:145–152. doi: 10.1002/jcp.1041140202. [DOI] [PubMed] [Google Scholar]
- 45.Griffin MA, Sen S, Sweeney HL, Discher DE. Adhesion-contractile balance in myocyte differentiation. J. Cell Sci. 2004;117:5855–5863. doi: 10.1242/jcs.01496. [DOI] [PubMed] [Google Scholar]
- 46.Kong HJ, et al. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nature Mater. 2005;4:460–464. doi: 10.1038/nmat1392. [DOI] [PubMed] [Google Scholar]
- 47.Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 48.Nandivada H, Chen HY, Bondarenko L, Lahann J. Reactive polymer coatings that click. Angew. Chem. Int. Ed. 2006;45:3360–3363. doi: 10.1002/anie.200600357. [DOI] [PubMed] [Google Scholar]
- 49.Ghosh K, Ingber DE. Micromechanical control of cell and tissue development: implications for tissue engineering. Adv. Drug Deliv. Rev. 2007;59:1306–1318. doi: 10.1016/j.addr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Xia YN, Whitesides GM. Soft lithography. Angew. Chem. Int. Ed. 1998;37:551–575. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 51.Beningo KA, Wang YL. Flexible substrata for the detection of cellular traction forces. Trends Cell Biol. 2002;12:79–84. doi: 10.1016/s0962-8924(01)02205-x. [DOI] [PubMed] [Google Scholar]
- 52.Clark HA, Hoyer M, Philbert MA, Kopelman R. Optical nanosensors for chemical analysis inside single living cells. 1. Fabrication, characterization, and methods for intracellular delivery of pebble sensors. Anal. Chem. 1999;71:4831–4836. doi: 10.1021/ac990629o. [DOI] [PubMed] [Google Scholar]
- 53.Tao SL, Popat K, Desai TA. Off-water fabrication and surface modification of asymmetric 3D SU-8 microparticles. Nature Protocols. 2006;1:3153–3158. doi: 10.1038/nprot.2006.451. [DOI] [PubMed] [Google Scholar]
- 54.Napier ME, DeSimone JM. Nanoparticle drug delivery platform. Polym. Rev. 2007;47:321–327. [Google Scholar]
- 55.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 56.Berkland C, Kim K, Pack DW. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J Control. Release. 2001;73:59–74. doi: 10.1016/s0168-3659(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 57.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharmacol. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kohane DS. Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng. 2007;96:203–209. doi: 10.1002/bit.21301. [DOI] [PubMed] [Google Scholar]
- 59.Singh M, Chakrapani A, O'Hagan D. Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev. Vaccines. 2007;6:797–808. doi: 10.1586/14760584.6.5.797. [DOI] [PubMed] [Google Scholar]
- 60.Ravi Kumar MN. Nano and microparticles as controlled drug delivery devices. J. Pharm. Pharm. Sci. 2000;3:234–258. [PubMed] [Google Scholar]
- 61.Gao H, Shi W, Freund LB. Mechanics of receptor-mediated endocytosis. Proc. Natl Acad. Sci USA. 2005;102:9469–9474. doi: 10.1073/pnas.0503879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldsmith HL, Turitto VT. Rheological aspects of thrombosis and hemostasis: basic principles and applications. ICTH Report: Subcommittee on rheology of the international committee on thrombosis and hemostasis. Thromb. Haemost. 1986;55:415–435. [PubMed] [Google Scholar]
- 63.Patil VRS, Campbell CJ, Yun YH, Slack SM, Goetz DJ. Particle diameter influences adhesion under flow. Biophys. J. 2001;80:1733–1743. doi: 10.1016/s0006-3495(01)76144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lamprecht A, Schafer U, Lehr CM. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001;18:788–793. doi: 10.1023/a:1011032328064. [DOI] [PubMed] [Google Scholar]
- 65.Kwiatkowska K, Sobota A. Signaling pathyways in phagocytosis. BioEssays. 1999;21:422–431. doi: 10.1002/(SICI)1521-1878(199905)21:5<422::AID-BIES9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 66.Tabata Y, Ikada Y. Phagocytosis of polymer microspheres by macrophages. Adv. Polym. Sci. 1990;94:107–141. [Google Scholar]
- 67.Illum L, et al. Blood clearance and organ deposition of intravenously administered colloidal particles: the effects of particle-size, nature and shape. Int. J. Pharm. 1982;12:135–146. [Google Scholar]
- 68.Stolnik S, Illum L, Davis SS. Long circulating microparticulate drug carriers. Adv. Drug Delivery Rev. 1995;16:195–214. [Google Scholar]
- 69.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. J. Cell Sci. 2001;114:1061–1077. doi: 10.1242/jcs.114.6.1061. [DOI] [PubMed] [Google Scholar]
- 71.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 72.Osaki F, Kanamori T, Sando S, Sera T, Aoyama Y. A quantum dot conjugated sugar ball and its cellular uptake. On the size effects of endocytosis in the subviral region. J. Am. Chem. Soc. 2004;126:6520–6521. doi: 10.1021/ja048792a. [DOI] [PubMed] [Google Scholar]
- 73.Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res. 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gratton SEA, et al. Nanofabricated particles for engineering drug therapies: A preliminary biodistribution study of PRINT nanoparticles. J. Control. Rel. 2007;121:10–18. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edwards DA, et al. Large porous particles for pulmonary drug delivery. Science. 1997;276:1868–1871. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 76.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Rel. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitragotri S. In drug delivery, shape does matter. Pharm. Res. 2008 doi: 10.1007/s11095-008-9740-y. doi:10.1007/s11095-008-9740-y. [DOI] [PubMed] [Google Scholar]
- 78.Decuzzi P, Ferrari M. The receptor-mediated endocytosis of non-spherical particles. Biophys. J. 2008;94:3790–3797. doi: 10.1529/biophysj.107.120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Decuzzi P, Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomater. 2006;27:5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 80.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm. Res. 2008 doi: 10.1007/s11095-008-9697-x. doi:10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 81.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm. Res. 2008 doi: 10.1007/s11095-008-9626-z. doi:10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gratton SE, et al. The effect of particle design on cellular internalization pathways. Proc. Natl Acad. Sci. USA. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 84.Muro S, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther. 2008;16:1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dickerson EB, et al. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269:57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zavaleta C, et al. Noninvasive Raman spectroscopy in living mice for evaluation of tumor targeting with carbon nanotubes. Nano Lett. 2008;8:2800–2805. doi: 10.1021/nl801362a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Glotzer SC, Solomon MJ. Anisotropy of building blocks and their assembly into complex structures. Nature Mater. 2007;6:557–562. doi: 10.1038/nmat1949. [DOI] [PubMed] [Google Scholar]
- 88.Mohraz A, Solomon MJ. Direct visualization of colloidal rod assembly by confocal microscopy. Langmuir. 2005;21:5298–5306. doi: 10.1021/la046908a. [DOI] [PubMed] [Google Scholar]
- 89.Ho CC, Keller A, Odell JA, Ottewill RH. Preparation of monodisperse ellipsoidal polystyrene particles. Colloid Polym. Sci. 1993;271:469–479. [Google Scholar]
- 90.Dendukuri D, Pregibon DC, Collins J, Hatton TA, Doyle PS. Continuous-flow lithography for high-throughput microparticle synthesis. Nature Mater. 2006;5:365–369. doi: 10.1038/nmat1617. [DOI] [PubMed] [Google Scholar]
- 91.Manoharan VN, Elsesser MT, Pine DJ. Dense packing and symmetry in small clusters of microspheres. Science. 2003;301:483–487. doi: 10.1126/science.1086189. [DOI] [PubMed] [Google Scholar]
- 92.Yin YD, Xia YN. Self-assembly of monodispersed spherical colloids into complex aggregates with well-defined sizes, shapes, and structures. Adv. Mater. 2001;13:267–271. doi: 10.1021/ja011048v. [DOI] [PubMed] [Google Scholar]
- 93.Sung KE, et al. Programmable fluidic production of microparticles with configurable anisotropy. J. Am. Chem. Soc. 2008;130:1335–1340. doi: 10.1021/ja0762700. [DOI] [PubMed] [Google Scholar]
- 94.Dalby M, Riehle MO, Sutherland DS, Agheli H, Curtis AS. Use of nanotopography to study mechanotransduction in fibroblasts—methods and perspectives. Eur. J. Cell Biol. 2004;83:159–169. doi: 10.1078/0171-9335-00369. [DOI] [PubMed] [Google Scholar]
- 95.Liu WF, Chen CS. Cellular and multicellular form and function. Adv. Drug Deliv. Rev. 2007;59:1319–1328. doi: 10.1016/j.addr.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cao L, Mooney D. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv. Drug Deliv. Rev. 2007;59:1340–1350. doi: 10.1016/j.addr.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Patterning proteins and cells using soft lithography. Biomater. 1999;20:2363–2376. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 98.Senaratne W, Andruzzi L, Ober CK. Self-assembled monolayers and polymer brushes in biotechnology, current applications and future perspectives. Biomacromol. 2005;6:2427–2448. doi: 10.1021/bm050180a. [DOI] [PubMed] [Google Scholar]
- 99.Dalby MJ, et al. Fibroblast reaction to island topography: changes in cytoskeleton and morphology with time. Biomater. 2003;24:927–935. doi: 10.1016/s0142-9612(02)00427-1. [DOI] [PubMed] [Google Scholar]
- 100.Kong HJ, Hsiong S, Mooney DJ. Nanoscale cell adhesion ligand presentation regulates nonviral gene delivery and expression. Nano Lett. 2007;7:161–166. doi: 10.1021/nl062485g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lehnert D, et al. Cell behaviour on micropatterned substrata: limits of extracellular matrix geometry for spreading and adhesion. J. Cell Sci. 2004;117:41–52. doi: 10.1242/jcs.00836. [DOI] [PubMed] [Google Scholar]
- 102.Sanders JE, Bale SD, Neumann T. Tissue response to microfibers of different polymers: polyester, polyethylene, polylactic acid, and polyurethane. J. Biomed. Mater. Res. 2002;62:222–227. doi: 10.1002/jbm.10285. [DOI] [PubMed] [Google Scholar]
- 103.Yang MT, Sniadecki NJ, Chen CS. Geometric considerations of micro- to nanoscale elastomeric postarrays to study cellular traction forces. Adv. Mater. 2007;19:3119–3123. [Google Scholar]
- 104.Rice JM, et al. Quantitative assessment of the response of primary derived human osteoblasts and macrophages to a range of nanotopography surfaces in a single culture model in vitro. Biomater. 2003;24:4799–4818. doi: 10.1016/s0142-9612(03)00381-8. [DOI] [PubMed] [Google Scholar]
- 105.Lee HL, et al. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomater. 2005;26:1261–1270. doi: 10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 106.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 107.Dalby MJ, et al. Attempted endocytosis of nano-environment produced by colloidal lithography by human fibroblasts. Exp. Cell Res. 2004;295:387–394. doi: 10.1016/j.yexcr.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 108.Perro A, et al. Towards large amounts of Janus nanoparticles through a protection-deprotection route. Chem. Commun. 2005;44:5542–5543. doi: 10.1039/b507486j. [DOI] [PubMed] [Google Scholar]
- 109.Bao ZN, et al. Toward controllable self-assembly of microstructures: Selective functionalization and fabrication of patterned spheres. Chem. Mater. 2002;14:24–26. [Google Scholar]
- 110.Paunov VN, Cayre OJ. Supraparticles and “Janus” particles fabricated by replication of particle monolayers at liquid surfaces using a gel trapping technique. Adv. Mater. 2004;16:788–791. [Google Scholar]
- 111.Cayre O, Paunov VN, Velev OD. Fabrication of dipolar colloid particles by microcontact printing. Chem. Commun. 2003;18:2296–2297. doi: 10.1039/b307296g. [DOI] [PubMed] [Google Scholar]
- 112.Salem AK, Searson PC, Leong KW. Multifunctional nanorods for gene delivery. Nature Mater. 2003;2:668–671. doi: 10.1038/nmat974. [DOI] [PubMed] [Google Scholar]
- 113.Pregibon DC, Toner M, Doyle PS. Multifunctional encoded particles for high-throughput biomolecule analysis. Science. 2007;315:1393–1396. doi: 10.1126/science.1134929. [DOI] [PubMed] [Google Scholar]
- 114.Chen HY, Rouillard JM, Gulari E, Lahann J. Colloids with high-definition surface structures. Proc. Natl Acad. Sci. USA. 2007;104:11173–11178. doi: 10.1073/pnas.0702749104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chhabra R, et al. Spatially addressable multiprotein nanoarrays templated by aptamer-tagged DNA nanoarchitectures. J. Am. Chem. Soc. 2007;129:10304–10305. doi: 10.1021/ja072410u. [DOI] [PubMed] [Google Scholar]
- 116.Yoshida M, Roh KH, Lahann J. Short-term biocompatibility of biphasic nanocolloids with potential use as anisotropic imaging probes. Biomater. 2007;28:2446–2456. doi: 10.1016/j.biomaterials.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 117.Jackson AM, Myerson JW, Stellacci F. Spontaneous assembly of subnanometre-ordered domains in the ligand shell of monolayer-protected nanoparticles. Nature Mater. 2004;3:330–336. doi: 10.1038/nmat1116. [DOI] [PubMed] [Google Scholar]
- 118.Verma A, et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nature Mater. 2008;7:588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sengupta S, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 120.Loscertales IG, et al. Micro/nano encapsulation via electrified coaxial liquid jets. Science. 2002;295:1695–1698. doi: 10.1126/science.1067595. [DOI] [PubMed] [Google Scholar]
- 121.Picart C, Discher D. Materials science: Embedded shells decalcified. Nature. 2007;448:879–880. doi: 10.1038/448879a. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y, Gao S, Ye WH, Yoon HS, Yang YY. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nature Mater. 2006;5:791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 123.Basinska T, Slomkowski S, Delamar M. Synthesis and characterization of polystyrene core/polycation shell latexes. J. Bioact. Compat. Polym. 1993;8:205–219. [Google Scholar]
- 124.Caruso F, Caruso RA, MÖhwald H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science. 1998;282:1111–1114. doi: 10.1126/science.282.5391.1111. [DOI] [PubMed] [Google Scholar]
- 125.Carter SR, Rimmer S. Surface molecularly imprinted polymer core-shell particles. Adv. Funct. Mater. 2004;14:553–561. [Google Scholar]
- 126.Zhang YW, Wang ZX, Wang YS, Zhao JX, Wu CX. Facile preparation of pH-responsive gelatin-based core-shell polymeric nanoparticles at high concentrations via template polymerization. Polymer. 2007;48:5639–5645. [Google Scholar]
- 127.Luo XL, Killard AJ, Morrin A, Smyth MR. Electrochemical preparation of distinct polyaniline nanostructures by surface charge control of polystyrene nanoparticle templates. Chem. Commun. 2007;30:3207–3209. doi: 10.1039/b702488f. [DOI] [PubMed] [Google Scholar]
- 128.Kubowicz S, et al. Multicompartment micelles formed by self-assembly of linear ABC triblock copolymers in aqueous medium. Angew. Chem. Int. Ed. 2005;44:5262–5265. doi: 10.1002/anie.200500584. [DOI] [PubMed] [Google Scholar]
- 129.Li Z, Kesselman E, Talmon Y, Hillmyer MA, Lodge TP. Multicompartment micelles from ABC miktoarm stars in water. Science. 2004;306:98–101. doi: 10.1126/science.1103350. [DOI] [PubMed] [Google Scholar]
- 130.Kisak ET, Coldren B, Evans CA, Boyer C, Zasadzinski JA. The vesosome-multicompartment drug delivery vehicle. Curr. Med. Chem. 2004;11:199–219. doi: 10.2174/0929867043456197. [DOI] [PubMed] [Google Scholar]
- 131.Utada AS, et al. Monodisperse double emulsions generated from a microcapillary device. Science. 2005;308:537–541. doi: 10.1126/science.1109164. [DOI] [PubMed] [Google Scholar]
- 132.Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nature Rev. Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 133.Yoshida M, Lahann J. Smart nanomaterials. ACS Nano. 2008;6:1101–1107. doi: 10.1021/nn800332g. [DOI] [PubMed] [Google Scholar]
- 134.Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 135.du Roure O, et al. Force mapping in epithelial cell migration. Proc. Natl Acad. Sci. USA. 2005;102:2390–2395. doi: 10.1073/pnas.0408482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cayre O, Paunov VN, Velev OD. Fabrication of asymmetrically coated colloid particles by microcontact printing techniques. J. Mater. Chem. 2003;13:2445–2450. doi: 10.1039/b307296g. [DOI] [PubMed] [Google Scholar]
- 137.Kisak ET, Coldren B, Zasadzinski JA. Nanocompartments enclosing vesicles colloids, and macromolecules via interdigitated lipid bilayers. Langmuir. 2002;18:284–288. [Google Scholar]
- 138.Berkland C, Pollauf E, Varde N, Pack DW, Kim KK. Monodisperse liquid-filled biodegradable microcapsules. Pharm. Res. 2007;24:1007–1013. doi: 10.1007/s11095-006-9197-9. [DOI] [PubMed] [Google Scholar]