Abstract
Background
Enhanced Recovery After Surgery (ERAS) programs are associated with reduced hospital morbidity and mortality. The aim of the present study was to evaluate whether the introduction of ERAS care improved the adverse events in colorectal surgery. In a cohort study, mortality, morbidity, and length of stay were compared between ERAS patients and carefully matched historical controls.
Methods
Patients were matched for their type of disease, the type of surgery, P-Possum (Portsmouth-Possum), CR-Possum (Colorectal-Possum) Physiological and Operative Score for Enumeration of Mortality and Morbidity (POSSUM), gender, and American Society of Anesthesiologists (ASA) grade. The primary outcome measures of this study were mortality and morbidity. Secondary outcome measures were fluid intake, length of hospital stay, the number of relaparotomies, and the number of readmissions within 30 days. Data on the ERAS patients were collected prospectively.
Results
Sixty-one patients treated according to the ERAS program were compared with 122 patients who received conventional postoperative care. The two groups were comparable with respect to age, ASA grade, P-Possum (Portsmouth-Possum), CR-Possum (Colorectal-Possum) score, type of surgery, stoma formation, type of disease, and gender. Morbidity was lower in the ERAS group compared to the control group (14.8% versus 33.6% respectively; P = <0.01). Patients in the ERAS group received significantly less fluid and spent fewer days in the hospital (median 6 days, range 3–50 vs. median 9 days, range 3–138; P = 0.032). There was no difference between the ERAS and the control group for mortality (0% vs. 1.6%; P = 0.55) and readmission rate (3.3% vs. 1.6%; P = 0.60).
Conclusion
Enhanced Recovery After Surgery program reduces morbidity and the length of hospital stay for patients undergoing elective colonic or rectal surgery.
Keywords: Enhanced recovery, Colorectal surgery, Abdominal surgery, Fast track, Mortality, Morbidity
Introduction
Colorectal resections are associated with an in-hospital stay of 6 to 11 days and a complication rate of 15% to 20%. “Fast-track” or enhanced recovery programs are developed to improve perioperative care in these patients.1–3
Enhanced Recovery After Surgery (ERAS) protocols aim at reducing the surgical stress response and optimizing recovery, thus reducing the length of hospital stay. All elements in ERAS separately have been shown to improve patient outcome. Preoperative education about the ERAS program diminishes anxiety and is associated with an earlier return of gastrointestinal motility after surgery.4 Preoperative carbohydrate loading is associated with earlier return of gastrointestinal motility and a significantly shorter hospital stay.5 Colonic lavages are associated with patient discomfort and electrolyte disturbances and can safely be avoided in elective colonic surgery.6–10 Epidural analgesia provides better treatment of postoperative pain and leads to an earlier gastrointestinal motility.11,12 Hypotension, a common physiologic side effect of epidural analgesia, can be treated safely with a vasopressor.13 Postoperative pain relief is best managed without opioid analgesia because of the adverse effects it has on the central nervous system, respiratory function, and gastrointestinal function.14
Intraoperative fluid management aiming at a zero balance reduces the number of patients who experience morbidity and shortens the time to the recovery of gastrointestinal motility and reduces hospital stay.15,16 Early postoperative enteral feeding shows a reduction in the risk of postoperative complications, hospital stay, and mortality.17 Bed rest after surgery is undesirable because it impairs pulmonary function and tissue oxygenation and predisposes to pulmonary complications.18 To avoid this, mobilizing patients as soon as possible is an important factor in improving postoperative care.
The aim of the present study was to compare mortality, morbidity, and in-hospital stay in a cohort of carefully matched patients receiving conventional postoperative care and the ERAS program to evaluate the clinical relevance of the improved perioperative care.
Methods
Identification of Patients
A cohort of consecutive patients that underwent elective open colonic or rectal resection following the ERAS regime was compared with a matched historical cohort who underwent colonic or rectal resection with conventional perioperative care. Between May 2006 and July 2008, patients who were above 18 years of age and were scheduled for any colonic or rectal resection and had an American Society of Anesthesiologists (ASA) grade of 1–3 were treated according to an ERAS program. In all patients, a colorectal resection was performed, with or without primary anastomosis. A loop ileostomy was created in any low rectal anastomosis and in patients with a high estimated risk to develop anastomotic leakage.
Running two protocols of postoperative care in one surgical ward would be prone to bias in a randomized trial. For this reason, a matched cohort study was performed. Since all eligible patients operated in the time span mentioned above received ERAS, a historical control group was used, composed of patients that would have been eligible for ERAS in the successive period. Patients in the control group were operated from January 2003 to May 2006. The latter group was obtained from a surgical database. All procedures were performed by the same team of surgeons.
Each patient from the ERAS group was matched with two patients from the control group on age, gender, P-Possum (Portsmouth-Possum), CR-Possum (Colorectal-Possum) Physiological and Operative Score for Enumeration of Mortality and Morbidity (POSSUM), American Society of Anesthesiologists grade, type of disease, and surgical procedure.
Criteria of Exclusion
Patients with an ASA grade 4–5 and younger than 18 years were excluded from analysis.
ERAS Protocol
In the outpatient clinic, patients who were treated according to the ERAS protocol were informed about the operative procedure and rehabilitation program. Before surgery, patients were consulted by an anesthesiologist and if necessary by a dietitian. All patients were admitted the day before surgery and could eat until midnight, including four drinks of carbohydrate (PreOP®, Nutricia; Numico, Zoetermeer, the Netherlands). Patients could drink water freely until 2 h before surgery. Two hours before surgery, patients received two drinks of PreOP®.
In the case of a planned left-sided resection, a phosphate enema was given the evening before and on the day of surgery. Thrombotic prophylaxis (nadroparin 2850 IE) was started the day before surgery. Antibiotic prophylaxis (cefazolin 2 g and metronidazole 500 mg intravenously) was given 30 min before incision. A transverse incision was preferred, except in Crohn’s disease and rectal surgery. In order to maintain a normothermic body temperature, the temperature in the operating theatre was increased to 22°C, and a Bair hugger and warmed intravenous fluids were applied. Anesthesia consisted of a combination of epidural analgesia and general anesthesia. Before the induction of anesthesia, an epidural catheter was inserted at level Th7/8. After the confirmation of proper placement by a test dose (Lignocaine 2% 3 ml), bolus infusion of 4 ml sufentanil produced sufficient analgesia for the first 30 min of surgery. Afterwards, repeated bolus infusion of 2–3 ml bupivacaine 0.5% maintained the operative analgesia. No additional opioids were given intravenously. At the end of surgery, continuous epidural infusion of 6 ml/h of ropivacain 0.2% with 1 μg/ml sufentanil was started for postoperative analgesia. This infusion lasted for 2 days postoperatively.
During and after surgery, hypotension was preferably treated with a vasopressor agent (ephedrine 5 mg or phenylefrine 0,1 mg) instead of intravenous fluid bolus in order to maintain a neutral fluid balance throughout the perioperative period. No drains were used except in rectal surgery, and the nasogastric tubes were removed immediately after surgery. To prevent postoperative nausea and vomiting, 4 mg ondansetron was administered intravenously at the end of surgery. After surgery, the patient was allowed to drink water, and, if tolerated, patients received two drinks of PreOP®. On postoperative day 1, patients were offered a normal diet. Intravenous fluid administration aimed at a urine production of at least 0.5 ml/kg and the total fluid intake should not exceed 2 l/24 h. Fluid balances were recorded daily. A structured mobilization program was also included in the ERAS protocol. Patients were encouraged to sit out of bed on the day of surgery and to walk the length of the ward on the first postoperative day. The inserted urinary catheter was removed at the same time as the thoracic epidural catheter. Subsequently, pain was managed with paracetamol and nonsteroidal anti-inflammatory drugs. The use of oral opioid analgesics was limited to relieve breakthrough pain.
Each protocol item and any deviation from the protocol was noted on a bedside checklist. Discharge criteria were: adequate pain relief on non-opioid oral analgesia, normal food intake, and return to preoperative mobility level.
Conventional Postoperative Care Protocol
The perioperative care, before the ERAS program was implemented, was according to the surgeon’s preference. Thrombotic and antibiotic prophylaxis was given and the practice of bowel preparation was largely abandoned. Discharge criteria were identical to the ERAS.
Data Extraction
After retrieving all reports and information from paper and electronic patient files, the following data were extracted: sex, age, indication for surgery, type of surgery, ASA grade, POSSUM score, P-POSSUM score, CR-POSSUM score, stoma formation, type of medication, oral and intravenous fluid intake, urinary output, stoma production, nasogastric tube production, length of stay in the hospital, number of readmissions, complication, and mortality rate.
In the ERAS group, additional data were prospectively collected: first day of defecation, length of epidural analgesia, first day of mobilization, and the number of days that oral analgesia was used.
Outcome Measures
The primary outcome measures were mortality and morbidity. Mortality was defined as death within 30 days after surgery. A complication was defined as an unfavorable postoperative course with the need for an intervention to prevent further harm, according to the definition of the Dutch Association of Surgeons. Individual complications were defined as stated in Table 1. Secondary outcome measures were fluid intake, reinsertion of nasogastric tubes, number of relaparotomies, length of hospital stay, and number of readmissions within 30 days.
Table 1.
Definitions of Separate Complications
| Surgical complications | |
| Wound hemorrhage | Local hematoma requiring evacuation |
| Deep hemorrhage | Postoperative bleeding requiring re-exploration |
| Burst abdomen | Deep wound breakdown, requiring surgical closure of the abdominal wall |
| Deep infection | The presence of an intra-abdominal collection confirmed clinically or radiologically |
| Anastomotic leak | Discharge of bowel content via the drain, wound, or abnormal orifice |
| Wound infection | Wound cellulitis or the discharge of purulent exudate and the necessity of opening the wound |
| Medical complications | |
| Chest infection | Production of purulent sputum with positive bacteriological cultures, with or without chest radiography changes or pyrexia or consolidation seen on chest radiograph |
| Urinary infection | The presence of >105 bacteria/ml with the presence of white cells in the urine in previously clear urine |
| Septicemia | Positive blood culture |
| Pyrexia of unknown origin | Any temperature above 37°C for more than 24 h occurring after the original pyrexia following surgery (if present) had settled, for which no obvious cause could be found |
| Deep venous thrombosis and pulmonary embolus | When suspected, confirmed radiologically by venography or ventilation/perfusion scanning or diagnosed at post mortem |
| Cardiac failure | Symptoms or signs of left ventricular or congestive cardiac failure (alteration from preoperative measures) |
| Impaired renal function | Arbitrarily defined as an increase in blood urea of >5 mmol/l from preoperative levels |
| Hypotension | A fall in systolic blood pressure below 90 mmHg for more than 2 h as determined by sphygmomanometry or arterial pressure transducer measurement |
| Respiratory failure | Respiratory difficulty requiring emergency ventilation |
Complications had to occur within 30 days after surgery
Analysis
The analysis was by intention-to-treat principles. No patients were excluded for reasons of protocol violations.
Statistical analyses were performed with SPSS® version 16.0(SPSS, Inc., Chicago IL) for Windows® and STATS direct® (Altrinchem, UK). Medians and ranges or means and standard deviations are presented for all continuous outcome measures. Comparisons between the ERAS and conventional postoperative care group were made using the chi-square test for binary outcomes, and the Student’s t test was used for continuous outcomes. Nonparametric tests were carried out to calculate statistical differences in POSSUM scores.
Results
Sixty-one patients, treated according to the ERAS program, were matched with 122 historical controls who had conventional postoperative care.
The two groups were similar with respect to age, ASA grade, P-Possum (Portsmouth-Possum), CR-Possum (Colorectal-Possum) score, type of surgery, stoma formation, and type of disease (Table 2). Women were slightly overrepresented in the ERAS population (63.9% vs. 36.1%; P = 0.06). Fifty-seven patients (93%) who were treated in the ERAS group had an epidural catheter until the second postoperative day (median; range, 1–4). Four patients in whom placing the epidural catheter could not be realized received a patient-controlled analgesia pump. Patients were mobilized out of bed on the first postoperative day (median; range, 0–3). The stools were passed on day 3 (median; range, 0–11) versus 4 days (median; range, 1–8) in the control group. Nonsteroidal anti-inflammatory drugs were used until day 4 (median; range, 0–15). Paracetamol was used until day 6 (median; range, 0–40). In the control group, 77 patients had epidural anesthesia (63%).
Table 2.
Patient Characteristics and Types of Surgery
| ERAS (%) (n = 61) | Control (%) (n = 122) | P value | |
|---|---|---|---|
| Characteristic | |||
| Malea | 36.1 (n = 22) | 50.8 (n = 62) | 0.06 |
| Femalea | 63.9 (n = 39) | 49.2 (n = 60) | |
| Age (years)b | 57 (17.6) | 60 (17.4) | 0.39 |
| POSSUMb | 7.50 (6.1) | 8.37 (6.7) | 0.37 |
| P-POSSUMb | 2.59 (2.9) | 2.57 (2.8) | 0.92 |
| CR-POSSUMb | 2.75 (3.2) | 2.79 (3.2) | 0.93 |
| Stoma formationa | 11.5 (n = 7) | 9.0 (n = 11) | 0.60 |
| Type of surgerya | 0.95c | ||
| Ileocecal resection | 21.3 (n = 13) | 19.7 (n = 24) | |
| Right hemicolectomy | 37.7 (n = 23) | 39.3 (n = 48) | |
| Left hemicolectomy/resection of sigmoid | 3.3 (n = 2) | 3.3 (n = 4) | |
| (Low) anterior resection | 24.6 (n = 15) | 24.6 (n = 30) | |
| Subtotal colectomy | 13.1 (n = 8) | 13.1 (n = 16) | |
| Type of diseasea | 0.83c | ||
| Cancer | 75.4 (n = 46) | 77.1 (n = 94) | |
| Inflammatory bowel disease | 23.0 (n = 14) | 21.3 (n = 26) | |
| Diverticulitis | 1.6 (n = 1) | 1.6 (n = 2) | |
| ASA gradea | 0.1c | ||
| 1 | 29.5 (n = 18) | 25.4 (n = 31) | |
| 2 | 59.0 (n = 36) | 53.3 (n = 65) | |
| 3 | 11.5 (n = 7) | 21.3 (n = 26) | |
aThe first number is the percentage, and the number in between the brackets is the absolute number
bThe first number is the mean, and the number in between brackets is the standard deviation
cThese P values represent the overall similarity of the two groups in these characteristics
The morbidity rate was higher in the control group than in the ERAS group (33.6% vs. 14.8%; P < 0.01). Total number of complications amounted 63 in the control group versus 12 in the ERAS group (P = <0.01). Corrected for gender, the control group had a 3.4 times higher risk to develop an unfavorable postoperative course than the ERAS group. Individual complications were similar in both groups, except for urinary tract infections. None of the patients in the ERAS group developed a urinary tract infection versus 6.6% of the patients in the control group (P = 0.05). Septicemia occurred in none of the patients in the ERAS group; the incidence was 3.3% in the control group (P = 0.30). Of the patients in the ERAS group, 4.9% developed a wound infection versus 11.5% of the patients in the control group (P = 0.18). In the control group, 6.6% of the patients developed a deep surgical site infection. For ERAS, this amounted 1.6% (P = 0.28). Anastomotic leakage occurred more often in patients who had conventional postoperative care (7.4% vs. 3.3%; P = 0.34). A dehiscence of all layers of the abdominal wall was seen in 1.6% in the ERAS group and in 4.1% of the patients in the control group (P = 0.67; Table 3).
Table 3.
Morbidity Rates in the ERAS and Control Group
| ERAS%;(n) | Standard care%; (n) | P value | |
|---|---|---|---|
| Surgical complicationsa | |||
| Wound hemorrhage | 0 | 0 | |
| Deep hemorrhage | 4.9 (3) | 0.8 (1) | 0.11 |
| Anastomotic leak | 3.3 (2) | 7.4 (9) | 0.34 |
| Wound infection | 4.9 (3) | 11.5 (14) | 0.18 |
| Deep infection | 1.6 (1) | 6.6 (8) | 0.28 |
| Burst abdomen | 1.6 (1) | 4.1 (5) | 0.67 |
| Medical complicationsa | |||
| DVT/embolus | 0 | 0 | |
| Chest infection | 1.6 (1) | 4.1 (5) | 0.67 |
| Cardiac failure | 0 (0) | 2.5 (3) | 0.55 |
| Urinary infection | 0 (0) | 6.6 (8) | 0.05 |
| Septicemia | 0 (0) | 3.3 (4) | 0.30 |
| Pyrexia of unknown origin | 0 (0) | 0 (0) | |
| Impaired renal function | 0 (0) | 2.5 (3) | 0.55 |
| Hypotension | 0 (0) | 0 (0) | |
| Respiratory failure | 1.6 (1) | 2.5 (3) | 0.99 |
| Total number of complicationsb | 12 | 63 | 0.0001 |
| Patients with complication(s) | 14.8 (9) | 33.6 (41) | 0.008 |
aFirst number is percentage, and the number in brackets is absolute number
bOnly the absolute number is shown
No patient died in the ERAS group within 30 days after surgery. Two patients in the control group died (1.6%; P = 0.55). One patient developed congestive heart failure after fluid resuscitation for hypotension. Eight days later, she became septicemic, a laparotomy was carried out, and bowel ischemia was found. The other patient also received an excess of fluid because of her low urine output and low fluid intake. Nevertheless, her renal function deteriorated. Four days later, she also developed fatal heart failure.
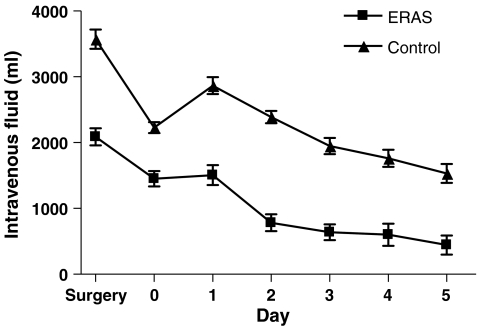
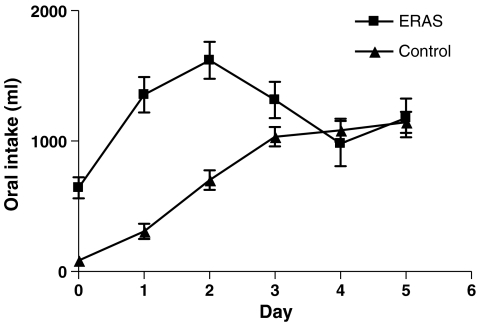
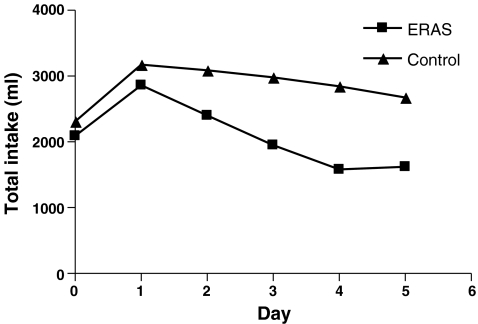
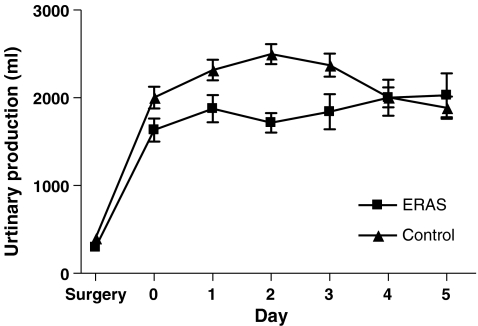
Patients receiving ERAS postoperative care were administered significantly less intravenous fluid during (day of) surgery and postoperative day 1 till 5 (P < 0.001). Oral intake was higher than in the control group on day of, first, and second postoperative day (P < 0.001). This led to a larger urinary production on the first three postoperative days in the control group (P < 0.05). Total fluid intake was higher in the second and third postoperative days (P < 0.05; Figs. 1, 2, 3, and 4).
Figure 1.
Intravenous fluid intake (ml/day).
Figure 2.
Oral fluid intake (ml/day).
Figure 3.
Total fluid intake (ml/day).
Figure 4.
Urinary output (ml/day).
Reinsertion of nasogastric tubes were similar in both populations (P = 0.85; Table 4). Patients treated according to the ERAS regime spent significantly fewer days in the hospital (median 6; range 3–50) than the control group (median 9; range 3–138 ; P = 0.032). The number of readmissions was similar in both groups (3.3% ERAS vs. 1.6% control; P = 0.60; Table 4). Two patients in the ERAS group were readmitted with surgical site infections. One developed a presacral abscess which was drained transrectally. The other patient developed a wound abscess which was incised and drained. One patient in the control group developed an intra-abdominal abscess which was treated conservatively. The other patient had successful conservative treatment for a gastro paresis.
Table 4.
Mortality and Secondary Outcomes of the Patients in the ERAS and Control Group
| ERAS % (n) | P value % (n) | Control | |
|---|---|---|---|
| Mortalitya | 0 (0) | 1.6 (2) | 0.55 |
| Number of reinserted nasogastric tubesa | 19.7 (12) | 21.3 (26) | 0.85 |
| Time to first defecation (days)b | 3 (0–11) | 4 (1–8) | |
| Length of hospital stay (days)b | 6 (3–50) | 9 (3–138) | 0.021 |
| Number of readmissionsa | 3.3 (2) | 1.6 (2) | 0.60 |
| Number of relaparotomiesa | 14.8 (9) | 17.2 (21) | 0.83 |
aFirst number is percentage, and the number in brackets is absolute number
bFirst number is median, and the number in brackets is range
Discussion
The results of this study suggest that the Enhanced Recovery After Surgery program is superior to conventional postoperative care for patients undergoing elective colonic or rectal resection. Patients treated according to an ERAS program develop significantly less complications and have shorter hospital stay.
This study is a historic cohort study with carefully matched controls. The control group was chosen from years prior to the introduction of the ERAS program. Because the discharge criteria were identical in both groups, further reduction of bias was achieved. Observer bias was avoided, though awareness about early recovery may have influenced decisions on early discharge. On the other hand, data in the ERAS group were collected prospectively. The historic nature of the control group is likely to have caused the underreporting of complications, thus leading to an overestimation of the beneficial effect of ERAS. Since patients in both groups were operated by the same team of surgeons, selection bias is thought to be small. A randomized trial on ERAS is difficult to perform because running traditional and ERAS care simultaneously carry the risk of mixing elements of both regimens. Blinding of nursing and medical staff would be impossible. To overcome these flaws, the design of such a study is challenging. In our study, patients were carefully matched. Women were slightly overrepresented in the ERAS group (P = 0.06). Literature states male gender predisposes to an increased incidence of anastomotic leakage after colorectal surgery. One of the main theories is the higher levels of estrogens in women and anatomical differences of the pelvis.19 Further analysis of the data excluded gender as a risk factor for the development of complications. There were less ASA 3 in the ERAS population (not significant). After excluding ASA 3 patients from analysis, significant differences in total number of complications and number of patients with one or more adverse events persisted.
In this study, the targets of ERAS were obtained. All ERAS patients were informed in a standardized way in the outpatient clinic. They received a daily perioperative schedule. Patients knew what was expected and allowed. In the conventional group, it is likely information was not uniform due to variance in information between the individual surgeons. Second, all patients of ERAS received preoperative carbohydrate loading where none of the conventional treated patients had Pre-Op. Since it was policy not to apply colonic lavages before the ERAS era, there was no difference between both groups. Epidural use was good practice in the conventional group; however, in the ERAS protocol it was one of the key elements. This led to a higher epidural use in the ERAS population (93% vs. 63%, respectively; P < 0.001). Epidural analgesia, one of the main issues in fast track protocols, has been suggested to provide an optimal pain relief, thus reducing surgical stress response, and may reduce postoperative morbidity and mortality.3,20–22 Rodgers et al.23 found a significant reduction in deep vein thrombosis (DVT), pulmonary embolism, transfusion requirements, pneumonia, other infections, and respiratory depression in patients with neuroaxial blockade. It is likely that this difference contributes to a reduced complication rate in ERAS. Patients in the ERAS group received less fluid intravenously and started drinking sooner after surgery. Total fluid intake and urinary production was higher in the control group. In our findings, morbidity was higher in the control group. Excessive fluid administration is thought to contribute to an increased complication rate.24–27 It is important to realize more elements than mentioned above may contribute to improved outcome: the use of short-acting and oral anesthetics and prokinetics, lack of premedication and nasogastric tubes, early removal of catheters and drains, minimal length incisions, early mobilization, and the preservation of normothermia.20
It is likely that the combination of elements in ERAS favored uncomplicated outcome after colorectal surgery. Mortality did not differ between both groups. Two patients (83 and 85 years old) in the control group died because of cardiac complications. Patients in the control group had an almost threefold risk to develop one or more complications. Individual complications failed to reach significance. Since data collection in the historic group could lead to underreporting of minor complications, this is less likely for major complications, e.g., anastomotic leakage, surgical site infections, and burst abdomen failed significance. All, however, tend to be more frequent in the conventional care group.
Although this ERAS program is evidence-based, some improvements can be made. Recent evidence suggests that perioperative supplemental oxygen administration reduces the incidence of surgical wound infections.28 It exposes the patient to little or no risks, has little associated costs, while it reduces the incidence of wound infections by half.29 The addition of specialized nutritional products to the standard carbohydrate drinks, offered to patients in the used ERAS program, also shows promising results towards reducing complications after gastrointestinal surgical procedures. The specialized nutritional products are the amino acids arginine and glutamine, omega-3 fatty acids, and nucleotides in the form of RNA. Wound infections, anastomotic leakage, abdominal abscesses, and pneumonia were significantly reduced.30
Patients who were treated according to the ERAS program spent significantly less time in the hospital. This did not result in more readmissions which reflects early recovery, probably due to a more favorable postoperative course. Besides, this implies benefit for the hospital resources because with the implementation of the ERAS program a higher level of cost-effectiveness can be reached.
This study demonstrates that the program as a whole is clearly beneficial and not flawed with unexpected negative effects. Epidural analgesia and a restricted fluid administration are thought to be the main contributing factors to a favorable outcome. More research is necessary to optimize perioperative care.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Zargar-Shoshtari K, Hill AG. Optimization of perioperative care for colonic surgery: a review of the evidence. ANZ J Surg. 2008;78(1–2):13–23. doi: 10.1111/j.1445-2197.2007.04350.x. [DOI] [PubMed] [Google Scholar]
- 2.Wind J, Polle SW, Fung Kon Jin PH, et al. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93(7):800–809. doi: 10.1002/bjs.5384. [DOI] [PubMed] [Google Scholar]
- 3.Fearon KC, Ljungqvist O, Von MM, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24(3):466–477. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Disbrow EA, Bennett HL, Owings JT. Effect of preoperative suggestion on postoperative gastrointestinal motility. West J Med. 1993;158(5):488–492. [PMC free article] [PubMed] [Google Scholar]
- 5.Noblett SE, Watson DS, Huong H, et al. Pre-operative oral carbohydrate loading in colorectal surgery: a randomized controlled trial. Colorectal Dis. 2006;8(7):563–569. doi: 10.1111/j.1463-1318.2006.00965.x. [DOI] [PubMed] [Google Scholar]
- 6.Gravante G, Caruso R, Andreani SM, et al. Mechanical bowel preparation for colorectal surgery: a meta-analysis on abdominal and systemic complications on almost 5,000 patients. Int J Colorectal Dis. 2008;23(12):1145–1150. doi: 10.1007/s00384-008-0592-z. [DOI] [PubMed] [Google Scholar]
- 7.Wille-Jorgensen P, Guenaga KF, Matos D, et al. Pre-operative mechanical bowel cleansing or not? an updated meta-analysis. Colorectal Dis. 2005;7(4):304–310. doi: 10.1111/j.1463-1318.2005.00804.x. [DOI] [PubMed] [Google Scholar]
- 8.Pineda CE, Shelton AA, Hernandez-Boussard T, et al. Mechanical bowel preparation in intestinal surgery: a meta-analysis and review of the literature. J Gastrointest Surg. 2008;12(11):2037–2044. doi: 10.1007/s11605-008-0594-8. [DOI] [PubMed] [Google Scholar]
- 9.Bucher P, Gervaz P, Morel P. Should preoperative mechanical bowel preparation be abandoned? Ann Surg. 2007;245(4):662. doi: 10.1097/01.sla.0000259047.43665.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beloosesky Y, Grinblat J, Weiss A, et al. Electrolyte disorders following oral sodium phosphate administration for bowel cleansing in elderly patients. Arch Intern Med. 2003;163(7):803–808. doi: 10.1001/archinte.163.7.803. [DOI] [PubMed] [Google Scholar]
- 11.Block BM, Liu SS, Rowlingson AJ, et al. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA. 2003;290(18):2455–2463. doi: 10.1001/jama.290.18.2455. [DOI] [PubMed] [Google Scholar]
- 12.Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359(9314):1276–1282. doi: 10.1016/S0140-6736(02)08266-1. [DOI] [PubMed] [Google Scholar]
- 13.Holte K, Foss NB, Svensen C, et al. Epidural anesthesia, hypotension, and changes in intravascular volume. Anesthesiology. 2004;100(2):281–286. doi: 10.1097/00000542-200402000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Kehlet H, Rung GW, Callesen T. Postoperative opioid analgesia: time for a reconsideration? J Clin Anesth. 1996;8(6):441–445. doi: 10.1016/0952-8180(96)00131-6. [DOI] [PubMed] [Google Scholar]
- 15.Nisanevich V, Felsenstein I, Almogy G, et al. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 2005;103(1):25–32. doi: 10.1097/00000542-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89(4):622–632. doi: 10.1093/bja/aef220. [DOI] [PubMed] [Google Scholar]
- 17.Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointest Surg. 2009;13(3):569–575. doi: 10.1007/s11605-008-0592-x. [DOI] [PubMed] [Google Scholar]
- 18.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183(6):630–641. doi: 10.1016/S0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 19.Lipska MA, Bissett IP, Parry BR, et al. Anastomotic leakage after lower gastrointestinal anastomosis: men are at a higher risk. ANZ J Surg. 2006;76(7):579–585. doi: 10.1111/j.1445-2197.2006.03780.x. [DOI] [PubMed] [Google Scholar]
- 20.Fearon KC, Ljungqvist O, von MM, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24(3):466–477. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321(7275):1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359(9314):1276–1282. doi: 10.1016/S0140-6736(02)08266-1. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321(7275):1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holte K, Kehlet H. Fluid therapy and surgical outcomes in elective surgery: a need for reassessment in fast-track surgery. J Am Coll Surg. 2006;202(6):971–989. doi: 10.1016/j.jamcollsurg.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89(4):622–632. doi: 10.1093/bja/aef220. [DOI] [PubMed] [Google Scholar]
- 26.Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshi GP. Intraoperative fluid restriction improves outcome after major elective gastrointestinal surgery. Anesth Analg. 2005;101(2):601–605. doi: 10.1213/01.ANE.0000159171.26521.31. [DOI] [PubMed] [Google Scholar]
- 28.Belda FJ, Aguilera L, Garcia de la AJ, et al. Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. JAMA. 2005;294(16):2035–2042. doi: 10.1001/jama.294.16.2035. [DOI] [PubMed] [Google Scholar]
- 29.Greif R, Akca O, Horn EP, et al. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. Outcomes Research Group. N Engl J Med. 2000;342(3):161–167. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 30.Waitzberg DL, Saito H, Plank LD, et al. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006;30(8):1592–1604. doi: 10.1007/s00268-005-0657-x. [DOI] [PubMed] [Google Scholar]