Figure 2. CCR5-CD4 association in the ER.
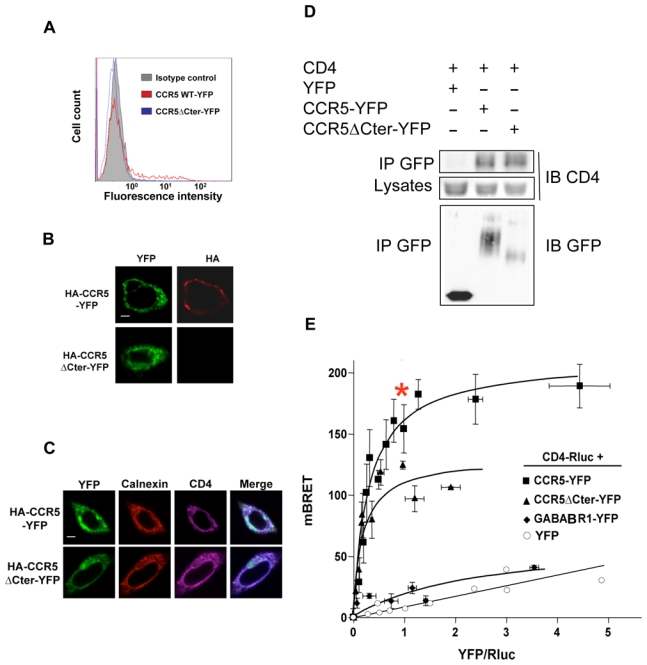
(A) CHO-K1 cells transfected with HA-tagged CCR5-YFP or CCR5ΔCter-YFP were analyzed for surface expression by FACS using the 1/85a Alexa-fluorR-647-conjugated anti-human CCR5, or (B) processed for immunofluorescence using the 3F10 anti-HA antibody. (C) Subcellular colocalization of CD4 with wild-type HA-CCR5-YFP and HA-CCR5ΔCter-YFP. CHO-K1 cells were co-transfected with CD4 and HA-CCR5-YFP or HA-CCR5ΔCter-YFP, permeabilized and stained for CD4 and calnexin using a monoclonal mouse anti-CD4 (OKT4) and goat anti-calnexin polyclonal antibody, respectively. After incubation with the appropriate Cy5 or Cy3-labeled secondary antibody, cells were examined with a confocal microscope. Note the substantial amount of intracellular CD4, likely due to the absence in CHO-K1 cells of the tyrosine kinase lck, which is known to stabilize CD4 at the cell surface of leukocytes. Scale bar: 10μM. (D) Co-immunoprecipitation experiments of CD4 with both wild-type and mutant CCR5ΔCter. CHO-K1 cells were co-transfected with plasmids coding for CD4 and HA-CCR5-YFP or HA-CCR5ΔCter or free YFP. After receptor precipitation with a monoclonal anti-GFP antibody, the presence of co-precipitated CD4 was revealed by immunoblot. (E) BRET analysis of CD4 interaction with wild-type and mutant CCR5. CHO-K1 cells were co-transfected with plasmids coding for CD4-Rluc (the BRET donor) and increasing concentrations of CCR5-YFP, CCR5ΔCter-YFP (the BRET acceptors) or GABABR1-YFP or free YFP (negative controls). Energy transfer was measured after addition of the membrane permeable luciferase substrate coelenterazine h. The BRET signal was determined by calculating the ratio of light emitted at 530nm over the light emitted at 485 nm as described in Material and Methods. BRET specificity was further controlled by showing that the BRET signal remained stable upon increasing the amounts of BRET pairs at a fixed ratio of donor: acceptor (data not shown). Maximal BRET signals were significantly different in cells expressing HA-CCR5ΔCter-YFP and HA-CCR5-YFP, likely because the variable length of the two C-terminal tails, to which the BRET acceptor was fused, affected the energy transfer. In contrast, the value of the YFP: Luc ratio, for which half-maximal BRET obtained, was comparable, indicating that these two forms of CCR5 display the same propensity to associate with CD4. The asterisk indicates the experimental conditions used for quantification of CD4 and CCR5 (see Sup. Figure 3A).
