Abstract
Both obesity and aging increase intrahepatic fat (IHF) content, which leads to non-alcoholic fatty liver disease and metabolic abnormalities such as insulin resistance. We evaluated the effects of diet and diet in conjunction with exercise on IHF content and associated metabolic abnormalities in obese older adults. Eighteen obese (BMI ≥30 kg/m2) older (≥65 years old) adults completed a 6-month clinical trial. Participants were randomized to diet (D group; n=9) or diet+exercise (D+E group; n=9). Primary outcome was IHF quantified by magnetic resonance spectroscopy. Secondary outcomes included insulin sensitivity (assessed by oral glucose tolerance), body composition (assessed by DXA), physical function (VO2peak and strength), glucose, lipids, and blood pressure. Body weight (D: −9±1%, D+E: −10±2%, both p<0.05) and fat mass (D: −13±3%, D+E −16±3%, both p<0.05) decreased in both groups but there was no difference between groups. IHF decreased to a similar extent in both groups (D: −46±11%, D+E: −45 ± 8%, both p<0.05), which was accompanied by comparable improvements in insulin sensitivity (D: 66±25%, D+E: 68±28%, both p<0.05). The relative decreases in IHF correlated directly with relative increases in insulin sensitivity index (r=−0.52; p<0.05). Improvements in VO2peak, strength, plasma triglyceride and HDL-cholesterol concentration, and diastolic blood pressure occurred in the D+E group (all p<0.05) but not in the D group. Diet with or without exercise results in significant decreases in IHF content accompanied by considerable improvements in insulin sensitivity in obese older adults. The addition of exercise to diet therapy improves physical function and other obesity- and aging-related metabolic abnormalities.
Keywords: Weight loss, exercise, intrahepatic fat, insulin resistance
INTRODUCTION
The increasing prevalence of obesity among older adults is a major public health problem, because of the increasing number of older adults and the medical complications associated with obesity (1-4). Obese older adults are at particularly high risk for developing nonalcoholic fatty liver disease (NAFLD) because both obesity (5) and aging (6) are associated with increased IHF accumulation. The development of NAFLD in older adults has serious clinical implications, because of the potential progression to severe liver disease and the association of NAFLD with metabolic risk factors for coronary heart disease, including insulin resistance, diabetes, and dyslipidemia (7-9). In addition, data from studies conducted in both animal models (10, 11) and humans (8) suggest that the accumulation of excessive IHF content is not just a marker of metabolic disease but is directly involved in the pathogenesis of insulin resistance and dyslipidemia. Therefore, decreasing IHF content in obese older adults who have excessive IHF content could have important beneficial therapeutic effects.
Weight loss induced by diet and exercise is typically recommended as therapy for obese patients with metabolic disease (12). Although weight loss decreases IHF content and improves metabolic function in young and middle-aged obese adults (13-17), it is not known whether lifestyle intervention affects IHF content, or whether diet and exercise have independent or synergistic effects on IHF content in older adults. Moreover, weight-loss therapy in older adults is controversial, because of the reduction in relative health risks associated with increasing BMI and the concern that weight loss could have potential harmful effects in the older population (4, 18).
The purpose of this study was to determine the effect of diet-induced weight loss and diet-induced weight loss in conjunction with exercise training on IHF content and associated metabolic abnormalities in obese older adults. We hypothesized that a dietary weight loss program would reduce IHF and improve insulin sensitivity and other metabolic abnormalities, and that these positive effects would be augmented by the addition of an exercise program.
METHODS
Subjects
This study was conducted at Washington University School of Medicine, St Louis, MO, and was approved by the institutional review board. Written informed consent was obtained from each subject. Volunteers were recruited from the community through advertisements. All potential subjects underwent a standard screening procedure including medical history, physical examination, blood tests, and electrocardiography.
Eligibility criteria included obese (BMI≥30 kg/m2), older (age 65–82 years) sedentary lifestyle (did not participate in regular exercise more than twice a week), stable body weight (± 2 kg) over the past year, and no changes in medications for at least 6 months before enrolling in the study. Subjects with diabetes, current smoking history, anemia, severe cardiopulmonary disease, renal disease, visual, hearing, or cognitive impairments, history of malignant neoplasm, and recent use of corticosteroid or sex-steroid compounds agents were excluded from the study. Liver disease was not exclusionary.
Study design
Treatment interventions
Eligible volunteers were randomized to receive 6 months of diet therapy (D) or diet and exercise training (D+E), by using a computer-generated block random permutation procedure stratified for sex.
Diet therapy
Subjects randomized to this intervention group were prescribed a balanced diet to provide an energy deficit of 500–1,000 kcal/d from daily energy requirement. daily calorie requirement was determined by estimating resting energy expenditure and multiplying the obtained value by 1.3 (19). The diet contained approximately 30% of energy as fat, 50% as carbohydrate, and 20% as protein. Total calorie intake was adjusted so that the weight loss was regulated at a rate of ~0.4 to 0.9 kg (1–2 lb per week) (4). Once 10% body weight was lost, total caloric intake was again adjusted to maintain a constant body weight and prevent further weight loss. On a weekly basis, the subjects met as a group for ~60 minutes with a dietitian who was experienced in group behavioral education. During each session, subjects were instructed in standard behavior modification with advice on food selection, meal portion, stimulus control, problem-solving skills, relapse prevention, and self-monitoring techniques (12). The participants were instructed to set weekly behavioral goals and to attend weekly weigh-in sessions throughout the intervention, and were encouraged to use food diaries as a means of daily monitoring to enhance adherence. Each subject was given the most recent edition of The Doctors Pocket Guide of Calorie, Fat and Carbohydrate Counter (20) a book with information on the calorie content of foods, a binder to file educational materials distributed during the group sessions and a food diary. Weight was obtained at each follow-up visit, and the food diary was reviewed with the dietitian. New goals were based on diary reports.
Diet and exercise training
Subjects randomized to this group participated in an intervention consisting of a combination of diet and exercise training. The dietary and behavioral intervention was identical to that of the D group; however, because of the calories burned during the exercise, the D+E group was prescribed a slightly higher caloric intake to achieve the same 10% weight loss. The caloric cost of the exercise was ~300 kcal/session (performed three times a week), which resulted in an additional caloric deficit of ~130 kcal/day. The exercise-training program focused on improving endurance, strength, and balance (4, 21). Exercise-training sessions were conducted as a group on three nonconsecutive days each week at our indoor exercise facility and supervised by a physical therapist. Each session lasted ~90 min: 15 min of flexibility exercises, followed by 30 min of aerobic exercise, 30 min of strength training, and 15 min of balance exercises. Initially, subjects exercised at moderate intensity (~70% of peak heart rate), and the intensity of exercise was gradually increased over several weeks to ~85% of peak heart rate. To modify resistance exercises, one-repetition maximums (1-RMs) were used. The initial, weight lifting sessions consisted of 1–2 sets performed at a resistance of ~65% of 1-RM, with 8–12 repetitions. The exercise volume was gradually increased to 2–3 sets at a resistance of ~80% of 1-RM, with 6–8 repetitions.
Body composition and metabolic outcomes
Proton magnetic resonance spectroscopy (1H-MRS)
IHF content was measured by using 1H-MRS. The 1H-MRS was performed at rest and with subjects in the supine position with the use of a 3.0-T whole-body scanner (Siemens Magneton Vision scanner; Siemens, Erlanger, Germany) as previously described (22). Briefly, IHF content was obtained within a voxel size of 15×15×20 mm3 by using a point resolved spectroscopy (PRESS) single-voxel technique. Data were averaged from 10 scans, obtained with a repetition time of 2 s while the subjects held their breath. Spectra were collected at echo times (time delay between the application of the radio frequency excitation pulse and data acquisition) of 34 ms and 64 ms to approximate and correct for the T2 decay of the signals. Analyses were performed by a blinded investigator (AS) using a java-based magnetic resonance user interface quantification package to quantify peak areas (23). Three voxels in the right lobe of the liver were positioned in three different areas (upper, middle, lower) avoiding major blood vessels, adipose tissue and the biliary tree without overlap in the voxel areas in the liver. We averaged the values from the 3 voxels provide an estimate of the percent of total liver volume comprised of fat. Prior to volume localized data acquisition, an imaging protocol was performed which included acquisition of anatomical images and positioning of the spectroscopic voxel in the area of the liver. Ten signal averages were obtained over a 20 s period. Both the anatomical images and the spectroscopic data were obtained while subjects held their breath. The anatomical images from the baseline scans were utilized to assure the same liver locations were collected in the follow-up studies. The coefficient of variation of replicate values of the triplicate determinations for 3 voxels was 1.5% (8, 24). Excessive IHF or NAFLD is defined as IHF content >5% of liver volume or liver weight (22). Subjects fasted for at least four hours prior to the MRS studies.
Body composition
Total body mass, mineral-free lean mass (non-bone fat-free mass), fat mass and trunk fat were measured by using whole body dual-energy X-ray absorptiometry (DXA) (Hologic Delphi 4500/w, Waltham, MA Enhanced Whole Body 11.2 software version Hologic Inc.,). The coefficients of variation for these measurements in our laboratory are < 2% (25).
Oral glucose tolerance test
A 75-g oral-glucose-tolerance test (OGTT) was performed after an overnight fast. OGTT was performed 48–72 hours after the last exercise session. Venous blood samples were obtained in the fasted state and 30, 60, 90, and 120 min after glucose ingestion for the measurement of plasma glucose (glucose oxidase method) and insulin concentrations (26). The area under the curve (AUC) for glucose and insulin were calculated using the trapezoid method (27). The insulin sensitivity index (ISI) was calculated from the OGTT by using the method of Matsuda: ISI = 10,000 / square root of [(mean plasma insulin X mean plasma glucose during OGTT) X (fasting plasma glucose X fasting plasma insulin)] (28). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as [fasting glucose (mg/dl) x fasting insulin (μU/mL)]/22.5 (29).
Serum lipids
Fasted blood samples were also used to determine lipid concentrations. In the D+E group, blood samples were obtained 48–72 h after the last exercise session. Cholesterol and triglycerides (TG) were measured by automated enzymatic commercial kits (Miles-Technicon, Tarrytown, NY). High-density lipoprotein cholesterol (HDL) was measured in plasma after precipitation of apolipoprotein B-containing lipoproteins by dextran sulfate and magnesium. The CVs of these assays were all <10%.
Blood pressure
Subjects remained in the supine position for 15 min with a deflated sphygmomanometer cuff of the appropriate size. Cuff size was determined by measuring the circumference of the arm at half the length of the humerus. Blood pressure was measured manually and recorded to the nearest 2 mm Hg. The measurement was repeated twice, and the values were averaged for the final reading.
Physical function
Peak endurance power (VO2 peak) was assessed during graded treadmill walking as described (25). During a ~5-minute warm-up at 0% grade, the speed was adjusted to identify the fastest comfortable walking speed. Speed was held constant and treadmill incline was increased by 3% every 2 minutes until volitional exhaustion. 1-RM for upper (biceps curl, bench press and seated row) and lower body exercises (knee extension, knee flexion and leg press), were determined to assess strength training. Total body strength was calculated as the sum of all 1-RM values (30).
STATISTICAL ANALYSES
The primary outcome in this study was change in IHF. It was estimated that 8 subjects per group would be needed to detect a clinically meaningful 10 ± 6 % difference in IHF between the two groups with a power of 0.8 and α-level of 0.05 (31). Baseline characteristics between groups were compared by using independent t tests (continuous variables) or Fisher’s exact test (categorical variables). Analysis of covariance was used to determine whether the changes in the outcomes were significantly different between groups while using the baseline values and gender as the covariates. Values for changes in HOMA-IR were not normally distributed and, therefore, were log-transformed prior to using analyses of covariance. Paired t test was performed to determine whether there were significant within-group changes in the outcomes. Pearson’s correlation was performed to assess associations between changes in selected outcomes. Results are reported as mean±SE. SPSS version 15.0 (SPSS Inc, Chicago, IL) was used for all statistical analyses. A P value ≤0.05 was considered to be statistically significant.
RESULTS
Nineteen subjects met the study inclusion criteria and participated in the diet (n=9) and diet + exercise (n=10) interventions. One subject dropped out because of difficulty with compliance. Therefore, eighteen subjects successfully completed the study and are included in this report. Baseline characteristics such as age, body weight, body composition and metabolic parameters did not differ between the two groups (Tables 1-3).
Table 1.
Physical characteristics before and after 6 months of diet-induced weight loss or diet-induced weight loss + exercise training
| Diet (n=9) |
Diet + exercise (n=9) |
Between-group P value |
|
|---|---|---|---|
| Age (year) | 68.6 ± 1.1 | 68.5 ± 1.3 | |
| Females [n (%)] | 6 (67) | 7 (78) | |
| Body weight (kg) | |||
| Baseline | 106.5 ± 6.0 | 95.4 ± 3.4 | .94 |
| Final | 97.3 ± 5.8 | 87.1 ± 4.5 | |
| Absolute change | −9.2 ± 1.6 | −8.3 ± 1.7 | |
| Within-group P value | .001 | .001 | |
| Mineral-free lean mass (kg) | |||
| Baseline | 63.1 ± 4.7 | 54.3 ± 2.6 | 0.13 |
| Final | 59.6 ± 4.5 | 52.6 ± 2.8 | |
| Absolute change | −3.5 ± 1 | −1.7 ± 0.6 | |
| Within-group P value | .006 | .02 | |
| Fat mass (kg) | |||
| Baseline | 43.3 ± 2.6 | 41.1 ± 2.4 | 0.60 |
| Final | 37.7 ± 2.8 | 34.5 ± 2.8 | |
| Absolute change | −5.6 ± 1.2 | −6.6 ± 1.2 | |
| Within-group P value | .002 | .0001 | |
| Trunk fat (kg) | |||
| Baseline | 21.5 ± 1.5 | 21.2 ± 1.2 | 0.38 |
| Final | 18.3 ± 1.6 | 17.2 ± 1.4 | |
| Absolute change | −3.2 ± 0.8 | −4.0 ± 0.4 | |
| Within-group P value | .33 | .09 | |
| VO2 peak (L/min) | |||
| Baseline | 1.79 ± 0.18 | 1.65± 0.07 | .04 |
| Final | 1.78 ± 0.18 | 1.86 ± 0.09 | |
| Absolute change | −0.01 ± 0.02 | −0.21 ± 0.01 | |
| Within-group P value | .93 | .001 | |
| Muscle strength (lb) | |||
| Baseline | 667 ± 130 | 585 ± 44 | .04 |
| Final | 673 ± 124 | 690 ± 62 | |
| Absolute change | −6 ± 23 | 105 ± 20 | |
| Within-group P value | .37 | .02 | |
Values are mean ± SE. VO2 peak, peak oxygen uptake.
Table 3.
Serum glucose, lipids, blood pressure and liver enzymes, before and after 6 months of diet-induced weight loss or diet-induced weight loss plus exercise training
| Diet (n=9) |
Diet + exercise (n=9) |
Between-group P value |
|
|---|---|---|---|
| Glucose (mg/dL) | |||
| Baseline | 98.9 ± 3.5 | 105.7 ± 4.1 | |
| Final | 95.1 ± 3.4 | 98.8 ± 2.6 | |
| Absolute change | −3.8 ± 0.7 | −6.9 ± 2.6 | .08 |
| Within-group P value | .001 | .01 | |
| Total cholesterol (mg/dL) | |||
| Baseline | 178.3 ± 13.5 | 179.4 ± 16.9 | |
| Final | 171.8 ± 16.2 | 161.5 ± 11.5 | |
| Absolute change | −6.6 ± 12.1 | −17.9 ± 8.5 | .04 |
| Within-group P value | .60 | .048 | |
| Triglyceride (mg/dL) | |||
| Baseline | 140.4 ± 17.7 | 122.7 ± 16.6 | |
| Final | 122.8 ± 23.7 | 95.6 ± 13.0 | |
| Absolute change | −17.6 ± 14.3 | −27.1 ± 11.6 | .49 |
| Within-group P value | .24 | .03 | |
| LDL cholesterol (mg/dL) | |||
| Baseline | 91.3 ± 8.3 | 101.4 ± 13.2 | |
| Final | 93.3 ± 14.5 | 84.6 ± 8.2 | |
| Absolute change | 2.0 ± 9.4 | −16.8 ± 7.0 | .19 |
| Within-group P value | .84 | .04 | |
| HDL cholesterol (mg/dL) | |||
| Baseline | 53.2 ± 3.8 | 54.6 ± 4.0 | |
| Final | 54.3 ± 4.1 | 58.9 ± 3.6 | |
| Absolute change | 1.1 ± 1.0 | 4.3 ± 2.2 | .45 |
| Within-group P value | .33 | .09 | |
| Systolic blood pressure (mmHg) | |||
| Baseline | 131.3 ± 6.6 | 138.6 ± 8.6 | |
| Final | 111.4 ± 2.8 | 114.5 ± 6.6 | |
| Absolute change | −19.9 ± 6.3 | −24.4 ± 4.2 | .002 |
| Within-group P value | .01 | .0001 | |
| Diastolic blood pressure (mmHg) | |||
| Baseline | 72.3 ± 4.3 | 75.1 ± 3.3 | |
| Final | 69.4 ± 1.2 | 67.3 ± 3.7 | |
| Absolute change | −3.0 ± 3.9 | −7.8 ± 2.1 | .007 |
| Within-group P value | .47 | .005 | |
| Alanine aminotransferase (U/L) | |||
| Baseline | 32.6 ± 4.1 | 28.4 ± 3.8 | |
| Final | 32.6 ± 4.1 | 25.3 ± 4.5 | |
| Absolute change | 5.2 ± 1.1 | 3.1 ± 1.1 | .80 |
| Within-group P value | .14 | .19 | |
| Aspartate aminotransferase (U/L | |||
| Baseline | 29.8 ± 3.6 | 28.4 ± 3.8 | |
| Final | 27.3 ± 2.1 | 26.1 ± 1.5 | |
| Absolute change | −2.5 ± 1.4 | −1.0 ± 0.8 | |
| Within-group P value | .49 | .60 | .20 |
Values are as mean ± SE. HDL; High density lipoprotein; LDL, Low density lipoprotein To convert glucose to mmol/L, multiply by 0.0555; HDL cholesterol to mmol/L, multiply by 0.0259; and triglyceride to mmol/L, multiply by 0.0113
Mean attendance at the group diet and behavioral therapy in the D group was 80 ± 4% and in the D+E group was 84 ± 5%. Mean attendance at the exercise sessions was 86 ± 3% with an average frequency of 2.6 ± 0.3 days/week. At 6 months, body weight decreased significantly and similarly in the D group (−9 ± 1%) and D+E group (−10 ± 2%) (Table 1). Likewise, fat mass (D: −13 ± 3%, D+E: −16 ± 3%), trunk fat (D −14 ± 4%, D+E: 20 ± 3%), and mineral-free lean mass (D: −6 ± 2%, D+E: −3 ± 1%) decreased significantly and similarly in both groups. Absolute VO2peak and muscle strength increased significantly in the D + E group (VO2peak: 9 ± 2%; strength: 18 ± 3%) but not in the D group (VO2peak: −0 ± 1%; strength: 3 ± 6%).
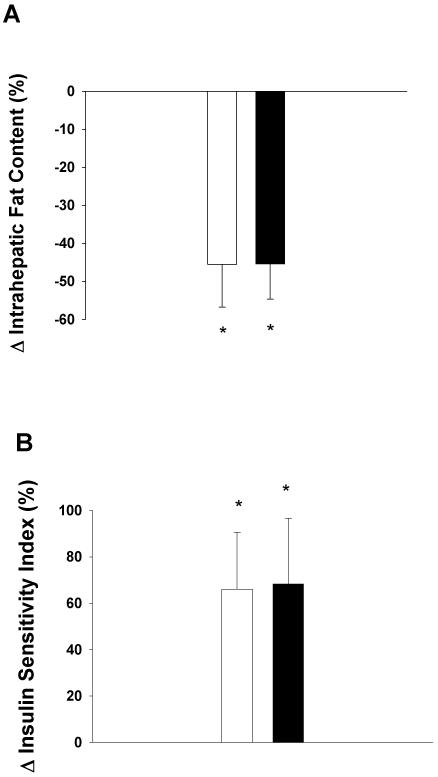
IHF content and insulin sensitivity are shown in Table 2 and Figure 1. IHF content decreased significantly and similarly in response to D (−46 ± 11%) and D+E (−45 ± 8%). The decreases in IHF were accompanied by significant and similar improvements in the insulin sensitivity index (D: 66 ± 25%, D+E: 68 ± 28%) and HOMA-IR value (D: −23 ± 8%, D+E: −33 ± 9%). In addition, glucose AUC (D:−11 ± 3%, D+E:−12 ± 5%) and insulin AUC (D: −19 ± 10%, D+E: −32 ± 7%) significantly decreased to a similar extent in the two groups.
Table 2.
Intrahepatic fat content and oral-glucose-tolerance variables before and after 6 months of diet-induced weight loss or diet-induced weight loss + exercise training
| Diet (n=9) |
Diet + exercise (n=9) |
Between-group P value |
|
|---|---|---|---|
| Intrahepatic Fat (%) | |||
| Baseline | 7.1 ± 1.1 | 8.6 ± 2.2 | |
| Final | 3.4 ± 0.6 | 4.6 ± 1.6 | |
| Absolute change | −3.7 ± 1.1 | −3.8 ± 1.2 | .95 |
| Within-group P value | .01 | .02 | |
| Insulin Sensitivity Index | |||
| Baseline | 2.6 ± 0.6 | 3.5 ± 0.8 | |
| Final | 3.7 ± 0.8 | 5.3 ± 0.9 | |
| Absolute change | 1.1 ± 0.4 | 1.8 ± 1.1 | .84 |
| Within-group P value | .04 | .04 | |
| HOMA-IR | |||
| Baseline | 5.5 ± 1.3 | 4.1 ± 1 | |
| Final | 4.0 ± 1.2 | 2.9 ± 1 | |
| Absolute change | 1.5 ± 0.6 | 1.2 ± 0.4 | .41 |
| With in-group P value | .047 | .02 | |
| Glucose AUC (×103 mg · min/dL) | |||
| Baseline | 17.7 ± 1.0 | 18.0 ± 1.6 | |
| Absolute change | 15.7 ± 0.7 | 15.4 ± 1.3 | |
| Change | −2.0 ± 0.6 | −2.3 ± 1.0 | .20 |
| Within-group P value | .02 | .04 | |
| Insulin AUC (×103 μU · min/mL) | |||
| Bas eline | 13.6 ± 2.5 | 10.2 ± 1.6 | |
| Final | 10.2 ± 1.7 | 6.8 ± 1.1 | |
| Absolute change | −3.4 ± 1.6 | −3.4 ± 1.1 | .32 |
| Within-group P value | .067 | .045 | |
Values are as mean ± SE. ISI, composite insulin sensitivity index; AUC, area under the curve; HOMA-IR, Homeostasis model assessment of insulin resistance.
Figure 1.
Changes in intrahepatic fat content (panel A) and insulin sensitivity index (panel B) in obese older adults randomized to 6 months of diet-induced weight loss (open bars) or diet-induced weight loss + exercise training (closed bars) Values are means ± SE. * Indicates significant difference from baseline within group P<0.05.
Significant improvements in serum triglyceride (−19 ± 7%) and HDL-cholesterol (10 ± 6%) concentrations, and diastolic blood pressure (−8 ± 2%) occurred in the D+E group only (Table 3). Systolic blood pressure (D: −14 ± 4%, D+E: −18 ± 3%) and fasting blood glucose decreased (D: −4 ± 1%, D+E: −6 ± 2%) significantly in both the groups. Compared with the D group, the D+E group had a greater reduction in systolic and diastolic BP and had a trend for a greater reduction in fasting glucose levels. Liver enzymes in both groups were within the normal range and did not change significantly after treatment.
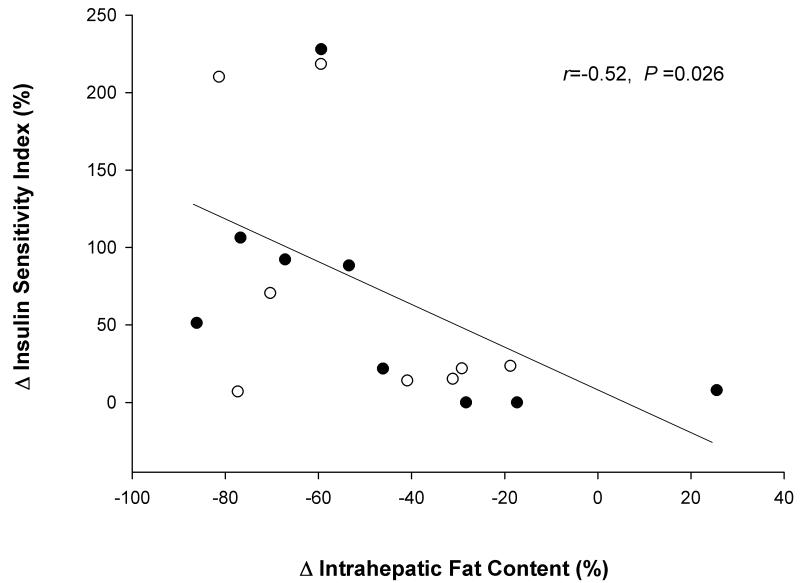
The changes in insulin sensitivity index correlated significantly with the changes in IHF (r=−0.52; P = 0.026) (Figure 2). In addition, there was a trend for the changes in HOMA-IR to strongly correlate with the changes in IHF content (r = 0.32; P = 0.13). On the other hand, the changes in insulin sensitivity index did not correlate with the changes in total body fat (r = −0.17; P = 0.48) or trunk fat mass (r = −0.31; P = 0.19). Similarly the changes in HOMA-IR did not correlate with the changes in total body fat (r = 0.26; P = 0.28) or trunk fat mass (r = 0.17; P = 0.48).
Figure 2.
Relationship between changes in intrahepatic fat content and changes in insulin sensitivity index in obese older adults randomized to 6 months of diet-induced weight loss (open circles) or diet-induced weight loss + exercise training (closed circles).
At baseline, the prevalence of NAFLD was high (22) and similar in both groups: 67 % (6/9) in the D group and 77% (7/9) in the D+E group (P = 1.0). At 6 months, the prevalence of NAFLD decreased significantly and similarly in the D (11% [1/9] and D+E group (33% [3/9]). (P = .56).
None of the subjects experienced any clinical adverse effects related to the diet-induced weight loss or exercise intervention.
The calculated effect size of exercise added to diet on IHF was 0.03 (Cohen’s d = between-group difference in change in IHF/SD of change in IHF).
DISCUSSION
Obesity is closely associated with an increase in IHF (5) and metabolic abnormalities such as Type 2 diabetes (T2D) (4). While aging is similarly linked with an increase in IHF content (6) and metabolic disturbances (4), little is known regarding NAFLD in obese older adults. To our knowledge, the present study is the first randomized trial to examine the effects of lifestyle intervention (D and D+E) on IHF and associated metabolic abnormalities in obese older adults. Our findings demonstrate that weight loss induced by D or D+E are equally effective in reducing IHF content (by ~50%) and improving insulin sensitivity (by ~60%) in obese older adults. Our six-month intervention induced a considerable reduction (by ~70%) in the prevalence of NAFLD in both study groups. Therefore, D and D+E can ameliorate obesity- and aging-associated increases in IHF content and accompanying metabolic abnormalities in high-risk obese older adults.
The findings of our study are consistent with the findings of other clinical trials examining the effects of diet-induced weight loss on IHF in young and middle aged adults (13-17). In particular, our findings of 50% reduction in IHF after 9% weight loss in older subjects are similar to the findings of 36–80% reduction in IHF after 6–8% weight loss in younger subjects (13, 16). These data demonstrate that the liver readily mobilizes intrahepatic triglycerides in response to a negative energy balance and that moderate weight loss has a profound effect on IHF content.
The results of our study show that exercise training did not have an additive effect on diet-induced weight loss in reducing IHF, as indicated by the small calculated effect size (Cohen’s d = .03). It has been suggested that exercise could reduce IHF accumulation through activation of the AMP-activated protein kinase pathway, resulting in stimulation of lipid oxidation and inhibition of lipid synthesis (32). Indeed, cross-sectional studies have shown that levels of habitual physical activity are inversely correlated with IHF content in healthy, young and middle-aged adults (33, 34). However, we are aware of only two interventional studies that have compared the effects of a structured exercise program plus weight loss with a weight loss program alone on IHF content (14, 17). These studies also showed that exercise training did not have an additional effect in reducing IHF, supporting our findings that D and D+E induced similar changes in IHF. Nonetheless, these two studies utilized exercise programs that are different from those in the present intervention. One used an acute program (~2 weeks) (14) while the other used a chronic (6 month) program (17); both involved only aerobic exercise and no resistance training. The discordant findings between these cross-sectional and interventional studies might be resolved by speculating that exercise is a possible preventive approach, but may not be an effective treatment without weight loss for NAFLD.
Data from cross-sectional studies have demonstrated that high levels of IHF content are strongly associated with impaired insulin sensitivity (6, 35). Possible mechanisms that identify IHF as part of the pathogenesis of insulin resistance include 1) release of reactive oxygen species derived from steatosis-stimulated FFA oxidation and 2) imbalance of adipocytokines derived from steatosis, ensuing a highly proinflammatory status closely related to the metabolic syndrome (36, 37). Accordingly, we observed that the reduction in IHF strongly correlated with the improvement in insulin sensitivity, highlighting the potential importance of IHF in the pathogenesis of insulin resistance. Our findings support and extend data from previous weight-loss studies conducted in younger subjects that found an association between decrease in IHF and increase in insulin sensitivity (16).
The results from the present study also demonstrate that exercise is still an important component of lifestyle therapy in older adults because it can have additional beneficial effects on CHD risk factors. In particular, the D+E group had a greater reduction in systolic and diastolic BP than the D group, suggesting that combining diet and exercise had an additive effect on improving BP. To date, previous studies designed to investigate the additive effects of diet and exercise on BP have been conducted in the young and middle aged populations. These studies have yielded mixed results. Some studies (38, 39) but not all (40, 41) have shown that combining diet and exercise has an additive effect on improving BP. Both weight loss (42, 43) and exercise (44, 45) have been shown to reduce arterial stiffness and improve endothelial function, which may underlie their BP-lowering effects. The improvement in endothelial function in response to both weight loss and exercise training are associated with reduced oxidative stress (46, 47) and improved NO availability (45). Exercise training may contribute to further lowering of BP by attenuating the sympathetic nervous activity in the trained state (48). Additionally, we also observed that D+E but not D alone induced significant reductions in serum levels of triglyceride and HDL-cholesterol. Although there were no treatment differences, our findings support previous reports showing the importance of combining diet with exercise to effectively improve serum lipids (49). In addition to the positive effects on CHD risk factors, we found that the addition of exercise to the D group improved endurance power and muscle strength. These findings emphasize the importance of exercise therapy to improve physical function in older adults (21, 50).
In conclusion, the results of this randomized intervention trial provide evidence that lifestyle intervention through D or D+E results in significant and similar decreases (~50%) in IHF in obese older adults. The decrease in IHF is accompanied by considerable improvement in insulin sensitivity (~60%), which could decrease the risk for developing T2D - the risk of which is certainly high in the older obese population. Further studies are needed to determine the clinical significance of the reduction in IHF. The addition of exercise training to a weight-loss program causes further improvement in other metabolic CHD risk factors and physical function. Therefore, diet therapy, plus exercise is recommended to ameliorate obesity- and aging-related derangements in metabolic and physical function in older adults.
ACKNOWLEDGEMENTS
This publication was made possible by Grant Number UL1 RR024992 from the National Center for Research Resources (NCRR), National Institutes of Health grants AG025501, DK 37948, DK 56341 (Clinical Nutrition Research Unit).TH was supported by a fellowship from the Foundation for Physical Therapy. We are grateful to Nichole Wright, for study coordination, Kathleen Obert and Laura Weber for weight loss training, Ellen Frye and Stacie Metzger for exercise training, and the participants for their cooperation in this study.
REFERENCES
- 1.Arterburn DE, Crane PK, Sullivan SD. The coming epidemic of obesity in elderly Americans. J Am Geriatr Soc. 2004;52:1907–12. doi: 10.1111/j.1532-5415.2004.52517.x. [DOI] [PubMed] [Google Scholar]
- 2.van Baak MA, Visscher TL. Public health success in recent decades may be in danger if lifestyles of the elderly are neglected. Am J Clin Nutr. 2006;84:1257–8. doi: 10.1093/ajcn/84.6.1257. [DOI] [PubMed] [Google Scholar]
- 3.Lapane KL, Resnik L. Obesity in nursing homes: an escalating problem. J Am Geriatr Soc. 2005;53:1386–91. doi: 10.1111/j.1532-5415.2005.53420.x. [DOI] [PubMed] [Google Scholar]
- 4.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–63. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 5.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 6.Cree MG, Newcomer BR, Katsanos CS, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–71. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Lymp JF, St SJ, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–31. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–75. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000;275:8456–60. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 11.Nagle CA, An J, Shiota M, et al. Hepatic overexpression of glycerol-sn-3-phosphate acyltransferase 1 in rats causes insulin resistance. J Biol Chem. 2007;282:14807–15. doi: 10.1074/jbc.M611550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology. 2002;123:882–932. doi: 10.1053/gast.2002.35514. [DOI] [PubMed] [Google Scholar]
- 13.Sato F, Tamura Y, Watada H, et al. Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J Clin Endocrinol Metab. 2007;92:3326–9. doi: 10.1210/jc.2006-2384. [DOI] [PubMed] [Google Scholar]
- 14.Tamura Y, Tanaka Y, Sato F, et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90:3191–6. doi: 10.1210/jc.2004-1959. [DOI] [PubMed] [Google Scholar]
- 15.Huang MA, Greenson JK, Chao C, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–81. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 16.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–8. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–44. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc. 2008;9:302–12. doi: 10.1016/j.jamda.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Harris JA, Benedict FF. A Biometric Study of Basal Metabolism in Man. Carnegie Institution of Washington; Washington, DC: 2005. [Google Scholar]
- 20.Borushek A. The Calorie King’s 2006 Calorie, Fat & Carbohydrate Counter 2007. Family health Publications; Costa Mesa, Calif: [Google Scholar]
- 21.American College of Sports Medicine Position Stand Exercise and physical activity for older adults. Med Sci Sports Exerc. 1998;30:992–1008. [PubMed] [Google Scholar]
- 22.Frimel TN, Deivanayagam S, Bashir A, O’Connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–8. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 23.Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–52. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 24.Schonfeld G, Patterson BW, Yablonskiy DA, et al. Fatty liver in familial hypobetalipoproteinemia: triglyceride assembly into VLDL particles is affected by the extent of hepatic steatosis. J Lipid Res. 2003;44:470–8. doi: 10.1194/jlr.M200342-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical Frailty and Body Composition in Obese Elderly Men and Women. Obes Res. 2004;12:913–20. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 26.Morgan DR, Lazarow A. Immunoassay of insulin: two antibody system. Diabetes. 1963;12:115–26. [Google Scholar]
- 27.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–50. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Blackman MR, Sorkin JD, Munzer T, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–92. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 31.Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 32.Lavoie JM, Gauthier MS. Regulation of fat metabolism in the liver: link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell Mol Life Sci. 2006;63:1393–409. doi: 10.1007/s00018-006-6600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perseghin G, Lattuada G, De CF, et al. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30:683–8. doi: 10.2337/dc06-2032. [DOI] [PubMed] [Google Scholar]
- 34.Krasnoff JB, Painter PL, Wallace JP, Bass NM, Merriman RB. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2008;47:1158–66. doi: 10.1002/hep.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koska J, Stefan N, Permana PA, et al. Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am J Clin Nutr. 2008;87:295–302. doi: 10.1093/ajcn/87.2.295. [DOI] [PubMed] [Google Scholar]
- 36.Abdelmalek MF, Diehl AM. Nonalcoholic fatty liver disease as a complication of insulin resistance. Med Clin North Am. 2007;91:1125–49. ix. doi: 10.1016/j.mcna.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–40. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Blumenthal JA, Sherwood A, Gullette EC, et al. Exercise and weight loss reduce blood pressure in men and women with mild hypertension: effects on cardiovascular, metabolic, and hemodynamic functioning. Arch Intern Med. 2000;160:1947–58. doi: 10.1001/archinte.160.13.1947. [DOI] [PubMed] [Google Scholar]
- 39.Watkins LL, Sherwood A, Feinglos M, et al. Effects of exercise and weight loss on cardiac risk factors associated with syndrome X. Arch Intern Med. 2003;163:1889–95. doi: 10.1001/archinte.163.16.1889. [DOI] [PubMed] [Google Scholar]
- 40.Dengel DR, Galecki AT, Hagberg JM, Pratley RE. The independent and combined effects of weight loss and aerobic exercise on blood pressure and oral glucose tolerance in older men. Am J Hypertens. 1998;11:1405–12. doi: 10.1016/s0895-7061(98)00185-x. [DOI] [PubMed] [Google Scholar]
- 41.Gordon NF, Scott CB, Levine BD. Comparison of single versus multiple lifestyle interventions: are the antihypertensive effects of exercise training and diet-induced weight loss additive? Am J Cardiol. 1997;79:763–7. doi: 10.1016/s0002-9149(96)00864-8. [DOI] [PubMed] [Google Scholar]
- 42.Pierce GL, Beske SD, Lawson BR, et al. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–9. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dengel DR, Kelly AS, Olson TP, Kaiser DR, Dengel JL, Bank AJ. Effects of weight loss on insulin sensitivity and arterial stiffness in overweight adults. Metabolism. 2006;55:907–11. doi: 10.1016/j.metabol.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 44.Sugawara J, Otsuki T, Tanabe T, Hayashi K, Maeda S, Matsuda M. Physical activity duration, intensity, and arterial stiffening in postmenopausal women. Am J Hypertens. 2006;19:1032–6. doi: 10.1016/j.amjhyper.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Hambrecht R, Wolf A, Gielen S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–60. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 46.Ozcelik O, Ozkan Y, Karatas F, Kelestimur H. Exercise training as an adjunct to orlistat therapy reduces oxidative stress in obese subjects. Tohoku J Exp Med. 2005;206:313–8. doi: 10.1620/tjem.206.313. [DOI] [PubMed] [Google Scholar]
- 47.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30:400–18. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 48.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA, American College of Sports Medicine position stand Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–53. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 49.Stefanick ML, Mackey S, Sheehan M, Ellsworth N, Haskell WL, Wood PD. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N Engl J Med. 1998;339:12–20. doi: 10.1056/NEJM199807023390103. [DOI] [PubMed] [Google Scholar]
- 50.Evans WJ. Exercise as the standard of care for elderly people. J Gerontol A Biol Sci Med Sci. 2002;57:M260–M261. doi: 10.1093/gerona/57.5.m260. [DOI] [PubMed] [Google Scholar]