Abstract
Purpose: To evaluate national outcomes after endovascular and open surgical repair of ruptured abdominal aortic aneurysms (rAAA).
Methods: The Nationwide Inpatient Sample was interrogated to identify all repairs between 2000 and 2005 for rAAA based on ICD-9 codes. In the study period, 2323 patients (1794 men; median age 75 years, range 45–98) with rAAAs had endovascular repair, while 26,106 patients (20,311 men; median age 73 years, range 22–99) had an open procedure. Outcomes included in-hospital mortality, length of stay (LOS), complications, and hospitalization charge. A secondary analysis was performed to compare outcomes from low-, medium-, and high-volume institutions based on annual rAAA repair volume.
Results: Patients in the endovascular group were significantly older (p<0.05). Mortality was 41% overall: 33% and 41% for endovascular versus open repair, respectively (p<0.001). Mortality after endovascular repair was lower than open surgery for patients ≥70 years (36% versus 47%, p<0.001), but not for those <70 years (24% versus 30%, p = 0.15). LOS was shorter after endovascular repair (7 versus 9 days, p<0.001). Respiratory complications (8% versus 4%, p<0.05) and acute renal failure were more common following open repair (30% versus 23%, p<0.01). Costs were similar (endo $73,590 versus open $67,287, p = 0.15). Mortality decreased as hospital surgical volume increased (low 44%, medium 39%, high 38%; p<0.001). Over time, endovascular repair utilization increased more rapidly at high-volume centers, and a lower mortality was seen with endovascular repair at high-volume compared to low-volume hospitals (22% versus 44%, p<0.001). Multivariate predictors of mortality were age, female gender, lower hospital surgical volume, open repair, and year of surgery.
Conclusion: This population-based study found that mortality associated with rAAAs may be improved by the performance of endovascular repair, especially in older patients. Mortality after rAAA for both endovascular and open repairs was also lower at high-volume institutions.
Keywords: abdominal aortic aneurysm, ruptured aneurysm, endovascular aneurysm repair, open surgery, mortality, outcome analysis, population-based outcomes
Hospital mortality rates for ruptured abdominal aortic aneurysms (rAAA) underestimate overall death rates since a considerable number of patients die of free rupture before presenting to a hospital.1 Among patients (See commentary page 565) presenting acutely to hospitals, mortality remains quite high despite rapid surgical intervention. For the past 4 decades, there seems to have been little progress in the outcomes of emergently repaired rAAAs, with inpatient mortality remaining at 40% and 60%.2 The gold standard for this repair has long been an open surgical approach. However, recently, the less invasive technique of stent-graft repair has been gaining favor with surgeons worldwide.
Endovascular aneurysm repair (EVAR) for intact AAA was brought to worldwide notice by Parodi et al.3 in the early 1990s. The procedure, initially held to be best utilized for poor surgical candidates, has now achieved widespread use, reducing hospital and intensive care unit (ICU) stays, early complications, and early mortality.4–8 Randomized control trials have documented a reduction in perioperative mortality compared with conventional open repair.4–6 In follow-up over 1 to 4 years, other studies have shown that late survival is similar, however.5,8,9
The earliest endovascular repair for ruptured AAA (rEVAR), which was reported in 1994,10 demonstrated the feasibility of the procedure; 5 years later, Ohki et al.11 published a series of 12 patients. The growth of the rEVAR technique, however, has lagged far behind that of its non-emergent counterpart given the vast requirements for technical expertise, facility specialization in endovascular interventions, large inventory, device specifications, anatomical requirements, and the need for reasonable hemodynamic stability for appropriate preoperative imaging. Retrospective series revealed that there could be an advantage to rEVAR,12,13 with early mortality rates of 8% to 40%. Single-center prospective trials and one multicenter trial have shown a potential benefit for rEVAR14–21; however, due to small patient numbers, statistical significance has rarely been shown. Additionally, because of the technical requirements of the procedure, the impact of annual volume on outcomes of rAAA repairs is an important factor that cannot be assessed by single institutional series. It has been shown that there is a significant relationship between higher surgeon and hospital volume and improved patient outcomes after open surgical repair for rAAA.22,23 Holt et al.,24 however, found that there was no significant relationship between volume and outcome for rAAA repair in the UK. Existing data, which includes rEVAR as well as open repair, shows conflicting results and is limited by early experience, small numbers, and variation in the volume criteria used.24–26 To further expand on this work, the current study utilized the Nationwide Inpatient Sample (NIS) in order to analyze national outcomes for in-hospital mortality rates after repair of rAAAs and to assess the impact of procedural volume specifically in the setting of aneurysm rupture.
METHODS
The NIS is a database maintained through the Healthcare Cost and Utilization Project that captures ∼20% of non-federal hospitalizations from 38 states in a stratified sample that reflects ∼90% of all hospitalizations within the US. Data from the NIS has been used extensively in medical research to provide population outcome analyses in a variety of healthcare topics; as it represents an all-payer sample, the NIS is one of the largest and most comprehensive datasets available. Contributing hospitals provide 100% of their discharges, which allows the NIS to be used for volume-outcome calculations as well as population comparisons.27
Clinical data were extracted from the NIS database using diagnosis and procedure codes from the International Classification of Diseases (ICD), 9th Revision, Clinical Modification system codes. Codes 441.3 and 441.5 (ruptured abdominal aneurysm) were merged with procedure codes for aneurysm repair to identify a sample of patients who had undergone either open (codes 38.44/39.25) or endovascular (code 39.71) repair within the admission. As the ICD-9 code for endovascular repair was introduced in the year 2000, the data extraction began there and continued through 2005. Exclusion criteria included age <18 years or a concomitant diagnosis of intact abdominal aneurysms (441.4, 441.9), thoracic or thoracoabdominal aortic aneurysms (441.1/2, 441.6/7), or aortic dissections (441.0). Sampling weights from the NIS were applied to provide population estimates for rAAA within the US. Using these parameters, the database interrogation identified 28,429 rAAA patients (22,105 men; median age 73 years) who underwent either an endovascular [2323 [8.2%]; 1794 men; median age 75 years, range 45–98) or an open [26,106 [91.8%]; 20,311 men; median age 73 years, range 22–99) repair between 2000 and 2005.
After identification of the sample, diagnosis codes were revisited to identify comorbid conditions and complications experienced by ICD-9 coding (Appendix). A Charlson comorbidity score28 based upon the Romano modification29 was calculated for each patient from comorbidity data. Age, gender, and race were also recorded as demographic variables. Outcomes assessed included in-hospital mortality, complications, other procedures, length of stay, and hospitalization cost.
Hospital volume for performance of rAAA repair was determined by identifying each respective hospital associated with a repair. All rAAA repairs were accordingly assigned to a hospital volume tertile: low (LVH; 1 to 3 procedures per annum), medium (MVH; 4 to 6 per annum), or high (HVH; 7 to 25). Comparisons among hospital volume tertiles were then made for repair type, patient demographics, and patient outcomes.
Statistical Analysis
Database queries were conducted with SAS (version 9.1; SAS Institute, Cary, NC, USA), and all statistical analyses were performed using STATA statistical software (Release 8.2; StataCorp LP, College Station, TX, USA). Comparisons between repair types were tested using Wilcoxon rank-sum tests for continuous measures and chi-square tests for categorical variables. Comparisons among hospital procedure volumes were performed using Kruskal-Wallis rank and chi-square tests. A chi-square test of trends in odds was performed to analyze change over time. Univariate logistic regression was used to assess significance of predictive variables for mortality. Multivariate logistic regression analysis was performed by backwards selection of variables obtaining significance at the p<0.1 level on univariate analysis. Statistical significance was determined by p<0.05.
RESULTS
Endovascular Versus Open rAAA Repair
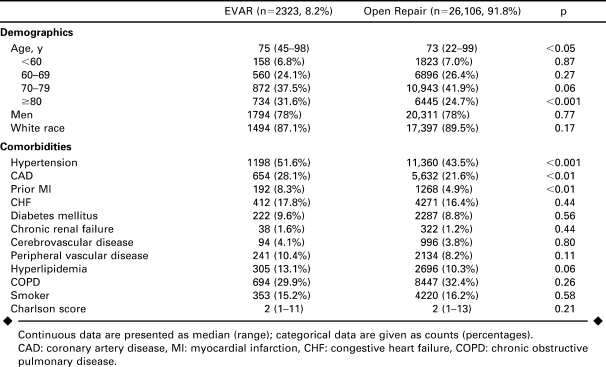
The endovascular group was older than the open group by a median of 2 years (p<0.05), with a higher proportion of octogenarians (Table 1). Gender and race were equivalent. There were significantly higher proportions of patients with hypertension, coronary artery disease, and prior myocardial infarction in the endovascular repair group, while all other comorbidities were similar. The Romano/Charlson comorbidity index score was similar for both methods as well.
TABLE 1.
Demographics and Comorbidities of Patients Undergoing Endovascular (EVAR) Versus Open Repair of Ruptured Abdominal Aortic Aneurysms 2000–2005
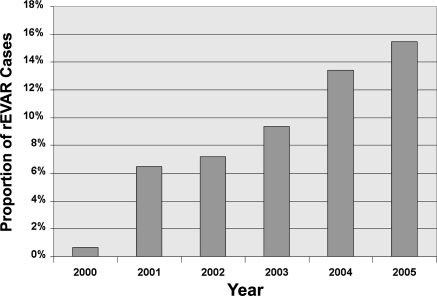
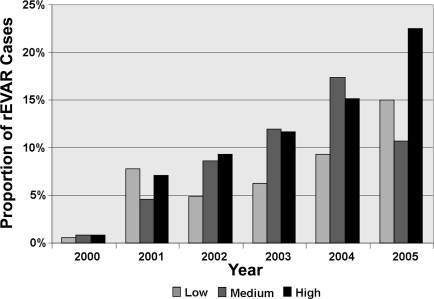
Endovascular repair for rAAA increased over time. In 2000, <1% of repairs were done with the endovascular technique; by 2005, 15.5% of cases were performed with an endovascular method (p<0.001; Fig. 1).
Figure 1.
♦ Proportion of ruptured AAA repairs performed by endovascular techniques (rEVAR) annually. P<0.001 between proportions in 2000 and in 2005.
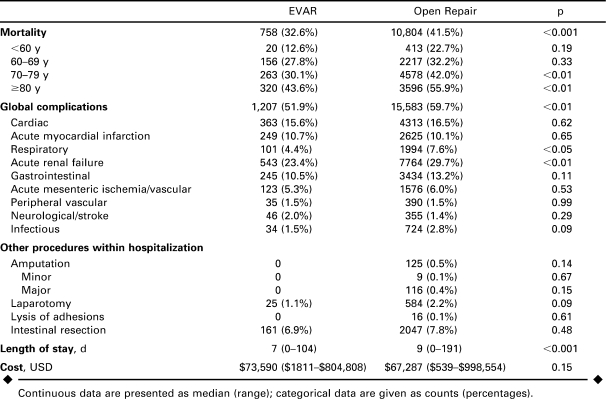
Overall mortality after rAAA repair was 40.8%. Over the entire time period, mortality after endovascular repair was 32.6% and 41.5% after open repair (p<0.001; Table 2). Mortality after open repair changed little over the observation period, while mortality after endovascular repair decreased steadily from 50% to 28% (p<0.05; Fig. 2).
TABLE 2.
Mortality and Secondary Outcomes of Patients Undergoing Endovascular (EVAR) Versus Open Repair of Ruptured Abdominal Aortic Aneurysms 2000–2005
Figure 2.
♦ Mortality rates after open and endovascular repair (rEVAR) of ruptured AAA from 2000 to 2005. P<0.05 between rates in 2000 and in 2005.
Mortality increased with age for both endovascular and open repair (Table 2). For patients 70 years and older, overall mortality was 46.2% and was significantly lower with endovascular (36.3%) versus open repair (47%, p<0.001). For patients under 70 years, the difference was not significant (24% versus 30%, p = 0.15).
Endovascular repair was associated with a shorter LOS (7 versus 9 days, p<0.001; Table 2). Among the survivors, median LOS was also shorter after endovascular repair (9 versus 12 days, p<0.001) and a significantly greater proportion of patients were discharged to home rather than a rehabilitation or short-term care facility (71% versus 56%, p<0.001). Patients undergoing endovascular repair had fewer overall complications (52% versus 60%, p<0.001); specifically, they had fewer respiratory complications (4% versus 8%, p<0.01) and episodes of acute renal failure (23% versus 30%, p<0.05). Cardiac, gastrointestinal, mesenteric ischemic, peripheral vascular, and neurological complications were similar. The incidences of concurrent or subsequent procedures within the hospitalization were similar between repair methods. Median hospital charges were similar ($73,590 rEVAR versus $67,287 open; p = 0.15).
Hospital rAAA Surgical Volume
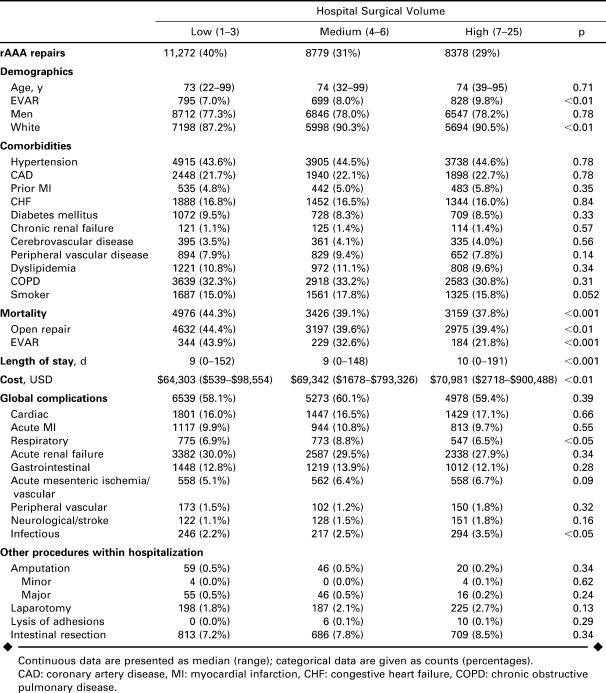
As can be seen in Table 3, there was a slightly higher overall proportion of endovascular repairs performed at higher-volume centers (LVH 7.0%, MVH 8.0%, HVH 9.8%; p = 0.11). Over time, the proportion of endovascular repairs increased more at high-volume centers than at low-volume centers (p<0.001; Fig. 3). In 2005, 22.5% of rAAA repairs at high-volume centers were done by endovascular means versus 10.5% at medium- and 15.3% at low-volume centers (p = 0.08). Age, gender, and race were comparable across volume categories, as were comorbid conditions.
TABLE 3.
Demographics and Subgroup Analyses by Hospital Volume (Tertiles) of Patients Undergoing Endovascular (EVAR) or Open Repair of Ruptured Abdominal Aortic Aneurysms (rAAA) 2000–2005
Figure 3.
♦ Proportion of endovascular repair for ruptured AAA (rEVAR) being performed at low-, medium-, and high-volume hospitals from 2000 to 2005. P<0.001 between high-volume and low-volume centers.
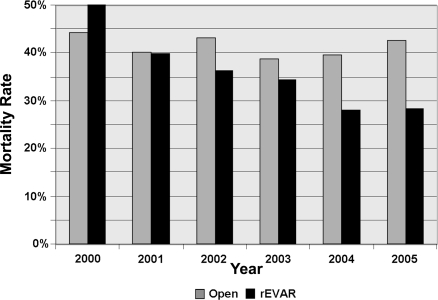
Mortality decreased as rAAA surgical volume increased (Table 3), which was true for overall mortality (HVH 38% versus LVH 44%, p<0.001) as well as for both endovascular and open repair mortality rates. The difference was most prominent among endovascular repairs: high-volume centers had half the mortality of low-volume centers (22% versus 44%, p<0.001), but the difference was also significant for open repair (39% versus 44%, p<0.01).
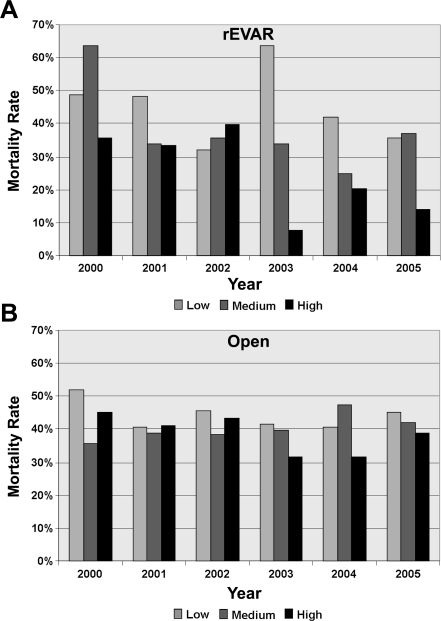
Additionally, endovascular repair mortality from 2000 to 2005 within high-volume centers decreased significantly from 35% to 14% (p<0.05; Fig. 4A). No significant endovascular mortality declines over time were seen at medium- (67% in year 2000 to 37% in 2005, p = 0.64) or low-volume centers (50% to 37%, p = 0.20). Open repair mortality also decreased over time at high-volume centers (45% to 39%, p<0.01) as well as in low-volume centers (52% to 45%, p<0.05), but was stable at medium-volume centers (36% to 42%, p = 0.17; Fig. 4B). Complications overall were similar regardless of volume. LOS and hospitalization costs both increased as rAAA surgical volume increased.
Figure 4.
♦ Mortality of ruptured AAA repair within low-, medium-, and high-volume hospitals for (A) rEVAR and (B) open repair. P<0.05 for difference in rEVAR mortality rates at high-volume centers between 2000 and 2005.
Predictors of Mortality
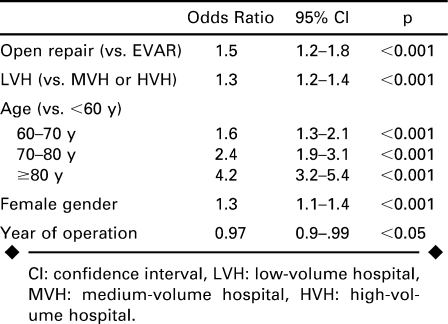
Multivariate predictors of mortality were open surgical repair, low surgical volume hospitals, older age, female gender, and earlier year of procedure (Table 4). Open repair conferred a 50% increased risk of mortality, while treatment at a low-volume center (versus medium or high) was associated with a 30% increased risk. The largest mortality predictor was older age, with octogenarians having a 4-fold increased risk, while septuagenarians had a >2-fold increased risk.
TABLE 4.
Predictors of Mortality After Endovascular (EVAR) or Open Repair for Ruptured Abdominal Aortic Aneurysms 2000–2005
DISCUSSION
This analysis shows that in the US, endovascular repair for rAAA is associated with a lower mortality compared to open repair. In addition, the rEVAR mortality rate has been decreasing over time, while open repair mortality has remained stable. The use of rEVAR has increased over time in centers that see a high volume of rupture cases, adopting the repair method more rapidly than lower volume centers. These higher volume centers have a significantly improved mortality for all repairs and, notably, half of the rEVAR mortality of low-volume centers. High-volume centers additionally have shown significant decreases in mortality over time from 2000 to 2005 for both methods of repair.
Institutional studies have demonstrated the feasibility of emergent endovascular repair programs for rAAA, with mortality rates ranging from 8% to 40% for rEVAR and 12% to 46% for all rAAA repairs after program initiation (compared to the current study of 33% rEVAR and 41% overall).14–17,19–21 In these programs, the proportion of rEVAR versus open repair range from 27% to 75%, with most around 50%. Success rates for rEVAR completion were all cited as above 90%. Mehta et al.20 treated 85 patients with rAAA after establishment of an emergency protocol in 2002; they successfully performed rEVAR in 40 patients with a mortality of 18%. These excellent results are supported by our finding of a difference in mortality at high- versus low-volume centers and a 22% rEVAR mortality at the high-volume centers in 2005. It is significant to note that mortality with rEVAR showed continued improvement over time only in high-volume centers. Interestingly, open repair mortality is decreasing as well in this group. Development of rEVAR protocols may benefit all types of repairs as awareness and response to the arrival of a ruptured aneurysm patient is streamlined. Although experience of the higher volume hospitals may be increasing, leading to improved outcomes with both procedures, it could be that more regionalization of services would increase transit times, resulting in more deaths prior to operation.
Recent meta-analyses have cited overall lower mortality with rEVAR, but they also point to the pitfalls of publication bias.30–32 Rayt et al.31 reviewed studies with cohorts ranging from 5 to 56, with one study of 290 patients. A 25% overall mortality for rEVAR was found; however, an assessment for publication bias proved that only rEVAR studies with good and exceptionally good outcomes are reported.31 Sadat et al.32 analyzed 730 patients from various studies and showed a decreased odds ratio of 0.6 for mortality after rEVAR, with lower ICU and hospital stays.
Administrative databases have also been used to examine the differences between open and endovascular repair for rAAA.22,25 Greco et al.25 examined the databases of 4 large states from 2000 to 2003 and found, even in those earlier years, that mortality in 290 rEVAR patients was lower than in 5508 open repair patients (39% versus 48%, p<0.005). As rEVAR is more widely utilized, it is likely that more complex aneurysms and hemodynamically unstable patients are being repaired, thus the current finding that mortality is still decreasing for rEVAR is even more notable.
Similar to our data, Greco et al.25 also found a hospital volume trend when both elective and ruptured aneurysm repairs were included in the calculations; they documented a 20% difference in absolute mortality for rEVAR between high- and low-volume hospitals (cutoff >100 elective and ruptured EVARs annually).
Procedural volume has been shown previously to have an impact on outcome for open elective AAA and rAAA repair. Dimick et al.22 found improved mortality in high-volume centers after open intact and ruptured AAA repair in the NIS population from 1996 and 1997, while Dardik et al.33 demonstrated improved open rAAA repair mortality in Maryland for surgeons performing a high volume of rAAA repairs but not elective procedures. The meta-analysis by Holt et al.26 also indicated that patients with both nonruptured and ruptured aneurysms had improved mortality in high-volume centers; however, the studies differed with respect to volume definitions. These findings have been used to argue for the regionalization of AAA repair; the current study lends more evidence in support of this for endovascular as well as open repairs for rAAA.
Differences in hemodynamic stability cannot be assessed using the current dataset, and this is something that must be considered in the decision to transfer to higher volume centers and may limit the ability to perform EVAR in centers with limited experience. In a recent retrospective institutional review, Lee et al.34 identified a selection bias in that hemodynamically unstable patients tended to undergo open repair even when potentially anatomically suitable for rEVAR; however, their use of rEVAR (32%) was lower than in most other reports from programs with emergency protocols. As experience increases, hemodynamic stability may not factor into repair method as much or else may push the choice toward rEVAR for unstable patients as access and balloon occlusion are rapidly attained to stop hemorrhage. Given prior volume-outcome relationships for elective AAA repair, high-volume centers may be the first line for elective and semi-elective (symptomatic) AAAs, which may influence the distribution of rAAA admissions among medical centers as well.
Limitations
Length of stay, hospital cost, and complications are likely influenced by a greater survival within high-volume centers; therefore, conclusions from these outcomes should be considered within this context.
Other limitations of this study are those inherent to studies using administrative datasets. There could be coding variability among institutions, limiting the ability to accurately identify comorbid conditions and complications. The standardization of the ICD-9 coding system helps to minimize this; however, the dataset represents only what is recorded in discharge information. The ICD-9 coding system does not differentiate between ruptured and symptomatic AAAs. Likewise, the proportions of free versus contained ruptures are unknown. Related to this and mentioned above is the fact that differences in patient hemodynamic stability, as well as any potential differences in anatomical characteristics, are unknown.
We also are limited by the fact that we cannot longitudinally follow the sample and assess longitudinal outcome. As we have learned from the data on intact AAA repairs, the long-term durability of endovascular repairs may be different than open repair and result in more late AAA-related complications, although open repair is associated with more laparotomy-related complications.5,8,9 It will be important to follow patients undergoing rEVAR to assess long-term outcomes as compared to open repair.
Conclusion
This study lends support for the use of EVAR for ruptured aneurysms, showing that in a national population, overall mortality is lower than with open repair. Additionally, because of lower mortality rates in higher volume hospitals for both types of repairs and a very large difference in endovascular outcomes, regionalization of referrals of ruptured AAA patients to high-volume centers preferentially may improve overall national outcomes. Based upon prior institutional studies and further supported by large database analyses such as this, it is reasonable for hospitals with adequate rEVAR experience to adopt a rEVAR-first strategy for ruptured aneurysms when conditions allow.
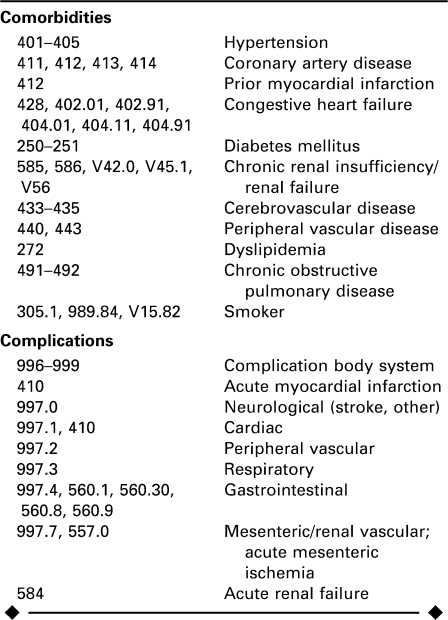
APPENDIX
ICD-9 Code Definitions for Comorbidities and Complications
Footnotes
This research was supported by Grant HL007734 (T32 Harvard-Longwood Research Training in Vascular Surgery) from the National Institutes of Health (NIH). In accordance with the NIH Public Access Policy, this article is available for open access at PubMed Central.
Marc L. Schermerhorn receives an unrestricted educational grant from W.L. Gore & Associates, is a consultant to Boston Scientific Corporation, and serves on the Data and Safety Monitoring Board for Endologix. The other authors have no commercial, proprietary, or financial interest in any products or companies described in this article.
REFERENCES
- Bengtsson H., Berqqvist D. Ruptured abdominal aortic aneurysms: a population-based study. J Vasc Surg. 1993;18:74–80. doi: 10.1067/mva.1993.42107. [DOI] [PubMed] [Google Scholar]
- Bown M.J., Sutton A.J., Bell P.R. et al. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg. 2002;89:714–730. doi: 10.1046/j.1365-2168.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- Parodi J.C., Palmaz J.C., Barone H.D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–499. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- Greenhalgh R.M., Brown L.C., Kwong G.P. et al. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364:843–848. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179–2186. doi: 10.1016/S0140-6736(05)66627-5. [DOI] [PubMed] [Google Scholar]
- Prinssen M., Verhoeven E.L., Buth J. et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607–1618. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- Angle N., Dorafshar A.H., Moore W.S. et al. Open versus endovascular repair of abdominal aortic aneurysms: what does each really cost? Ann Vasc Surg. 2004;18:612–618. doi: 10.1007/s10016-004-0089-3. [DOI] [PubMed] [Google Scholar]
- Schermerhorn M., O'Malley J., Jhaveri A. et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464–474. doi: 10.1056/NEJMoa0707348. [DOI] [PubMed] [Google Scholar]
- Blankensteijn J.D., de Jong S.E., Prinssen M. et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352:2398–2405. doi: 10.1056/NEJMoa051255. [DOI] [PubMed] [Google Scholar]
- Yusuf S.W., Whitaker S.C., Chuter T.A. et al. Emergency endovascular repair of leaking aortic aneurysm [letter] Lancet. 1994;344:1645. doi: 10.1016/s0140-6736(94)90443-x. [DOI] [PubMed] [Google Scholar]
- Ohki T., Veith F.J., Sanchez L.A. et al. Endovascular graft repair of ruptured aortoiliac aneurysms. J Am Coll Surg. 1999;189:102–113. doi: 10.1016/s1072-7515(99)00051-4. [DOI] [PubMed] [Google Scholar]
- Lee W.A., Hirneise C.M., Tayyarah M. et al. Impact of endovascular repair on early outcomes of ruptured abdominal aortic aneurysms. J Vasc Surg. 2004;40:211–215. doi: 10.1016/j.jvs.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Brandt M., Walluscheck K.P., Jahnke T. et al. Endovascular repair of ruptured abdominal aortic aneurysm: feasibility and impact on early outcome. J Vasc Interv Radiol. 2005;16:1309–1312. doi: 10.1097/01.RVI.0000175332.44635.49. [DOI] [PubMed] [Google Scholar]
- Yilmaz N., Peppelenbosch N., Cuypers P.W.M. et al. Emergency treatment of symptomatic or ruptured abdominal aortic aneurysms: the role of endovascular repair. J Endovasc Ther. 2002;9:449–457. doi: 10.1177/152660280200900411. [DOI] [PubMed] [Google Scholar]
- Alsac J.M., Desgranges P., Kobeiter H. et al. Emergency endovascular repair for ruptured abdominal aortic aneurysms: feasibility and comparison of early results with conventional open repair. Eur J Vasc Endovasc Surg. 2005;30:632–639. doi: 10.1016/j.ejvs.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Dalainas I., Nano G., Bianchi P. et al. Endovascular techniques for the treatment of ruptured abdominal aortic aneurysms: 7-year intention-to-treat results. World J Surg. 2006;30:1809–1814. doi: 10.1007/s00268-005-0667-8. [DOI] [PubMed] [Google Scholar]
- Arya N., Makar R.R., Lau L.L. et al. An intention-to-treat by endovascular repair policy may reduce overall mortality in ruptured abdominal aortic aneurysm. J Vasc Surg. 2006;44:467–471. doi: 10.1016/j.jvs.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Peppelenbosch N., Geelkerken R.H., Soong C. et al. Endograft treatment of ruptured abdominal aortic aneurysm using the Talent aortouniiliac system: an international multicenter study. J Vasc Surg. 2006;43:1111–1122. doi: 10.1016/j.jvs.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Coppi G., Silingardi R., Gennai S. et al. A single-center experience in open and endovascular treatment of hemodynamically unstable and stable patients with ruptured abdominal aortic aneurysms. J Vasc Surg. 2006;44:1140–1147. doi: 10.1016/j.jvs.2006.08.070. [DOI] [PubMed] [Google Scholar]
- Mehta M., Taggert J., Darling R.C. et al. Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysms: outcomes of a prospective analysis. J Vasc Surg. 2006;44:1–8. doi: 10.1016/j.jvs.2006.02.057. [DOI] [PubMed] [Google Scholar]
- Anain P.M., Anain J.M., Tiso M. et al. Early and mid-term results of ruptured abdominal aortic aneurysms in the endovascular era in a community hospital. J Vasc Surg. 2007;46:898–905. doi: 10.1016/j.jvs.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Dimick J.B., Stanley J.C., Axelrod D.A. et al. Variation in death rate after abdominal aortic aneurysmectomy in the United States: impact of hospital volume, gender, and age. Ann Surg. 2002;235:579–585. doi: 10.1097/00000658-200204000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueck A.D., Kucey D.S., Johnston K.W. et al. Survival after ruptured abdominal aortic aneurysm: effect of patient, surgeon, and hospital factors. J Vasc Surg. 2004;39:253–260. doi: 10.1016/j.jvs.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Holt P.J., Poloniecki J.D., Loftus I.M. et al. Epidemiological study of the relationship between volume and outcome after abdominal aortic aneurysm surgery in the UK from 2000 to 2005. Br J Surg. 2007;94:441–448. doi: 10.1002/bjs.5725. [DOI] [PubMed] [Google Scholar]
- Greco G., Egorova N., Anderson P.L. et al. Outcomes of endovascular treatment of ruptured abdominal aortic aneurysms. J Vasc Surg. 2006;43:453–459. doi: 10.1016/j.jvs.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Holt P.J., Poloniecke J.D., Gerrard D. et al. Meta-analysis and systematic review of the relationship between volume and outcome in abdominal aortic aneurysm surgery. Br J Surg. 2007;94:395–403. doi: 10.1002/bjs.5710. [DOI] [PubMed] [Google Scholar]
- HCUP Tools and Software. Healthcare Cost and Utilization Project (HCUP) April 2007. Agency for Healthcare Research and Quality, Rockville, MD, USA. Available at: http://www.hcup-us.ahrq.gov/tools_software.jsp. [Google Scholar]
- Charlson M.E., Pompei P., Ales K.L. et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Romano P.S., Roos L.L., Jollis J.G. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- Visser J.J., van Sambeek M.R., Hamza T.H. et al. Ruptured abdominal aortic aneurysms: endovascular repair versus open surgery: systematic review. Radiology. 2007;245:122–129. doi: 10.1148/radiol.2451061204. [DOI] [PubMed] [Google Scholar]
- Rayt H.S., Sutton A.J., London N.J. et al. A systematic review and meta-analysis of endovascular repair (EVAR) for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2008;36:536–544. doi: 10.1016/j.ejvs.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Sadat U., Boyle J.R., Walsh S.R. et al. Endovascular vs open repair of acute abdominal aortic aneurysms—a systematic review and meta-analysis. J Vasc Surg. 2008;48:227–236. doi: 10.1016/j.jvs.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Dardik A., Burleyson G.P., Bowman H. et al. Surgical repair of ruptured abdominal aortic aneurysms in the state of Maryland: actors influencing outcome among 527 recent cases. J Vasc Surg. 1998;28:413–421. doi: 10.1016/s0741-5214(98)70126-0. [DOI] [PubMed] [Google Scholar]
- Lee R.W., Rhodes J.M., Singh M.J. et al. Is there a selection bias in applying endovascular aneurysm repair for rupture? Ann Vasc Surg. 2008;22:215–220. doi: 10.1016/j.avsg.2007.12.006. [DOI] [PubMed] [Google Scholar]