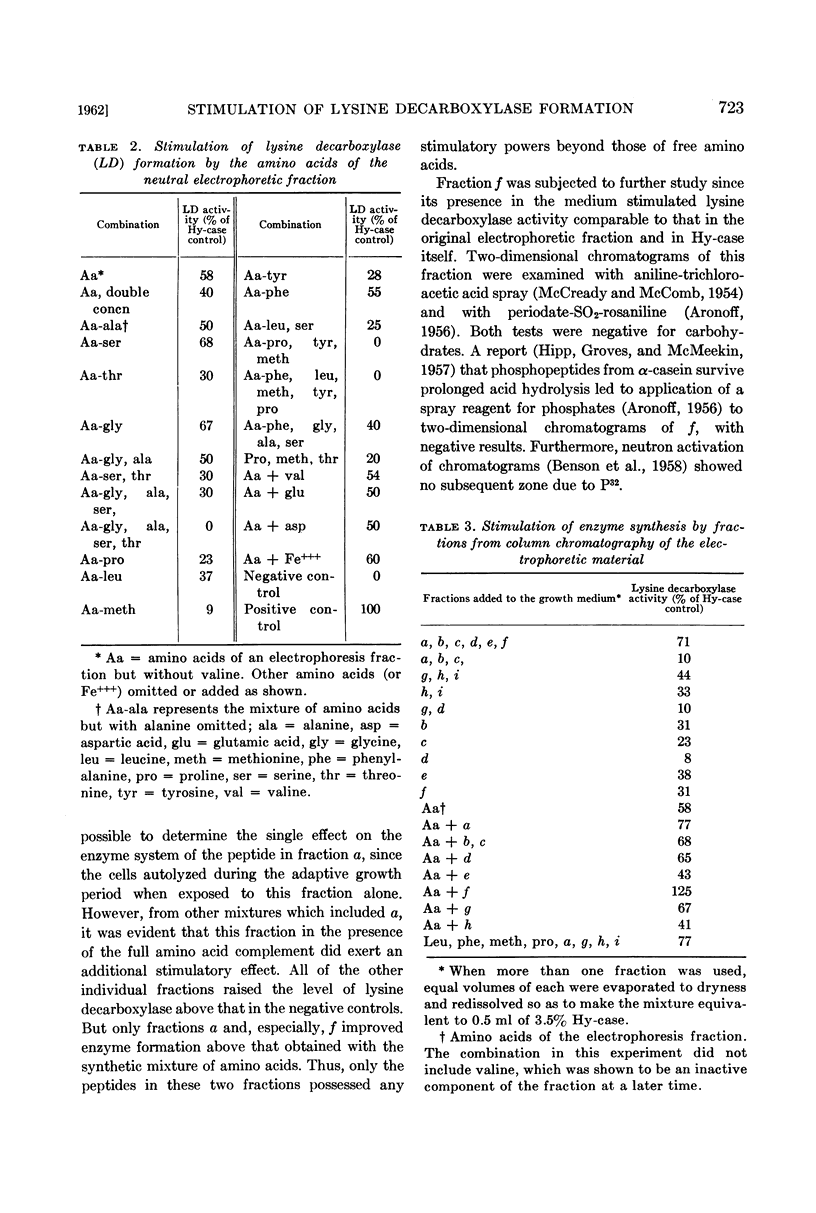
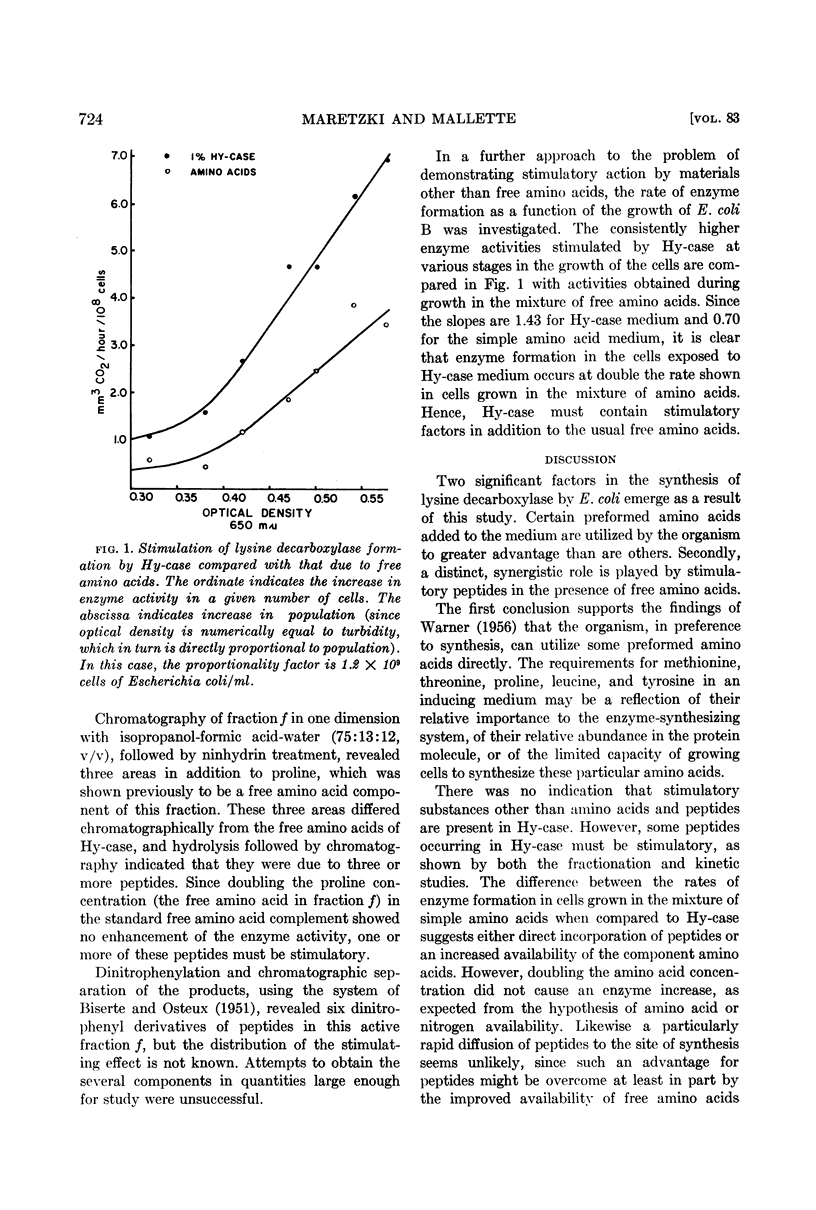
Abstract
Maretzki, Andrew (Pennsylvania State University, University Park) and M. F. Mallette. Nutritional factors stimulating the formation of lysine decarboxylase in Escherichia coli. J. Bacteriol. 83:720–726. 1962 — Inclusion of complex nitrogen sources in the induction medium was shown to be necessary for the synthesis of appreciable amounts of l-lysine decarboxylase by Escherichia coli B. Hy-case, a commercial acid hydrolyzate of casein, was especially effective in enzyme production, which was assayed manometrically after lysis of the bacteria from without by bacteriophage. Partial fractionation of the Hy-case, identification of the free amino acids, and addition of these amino acids to test media revealed stimulatory effects by methionine, threonine, proline, leucine, and tyrosine. A full complement of amino acids did not match the enzyme levels reached in the presence of Hy-case. Certain peptide fractions obtained from this mixture supplemented the effects of the amino acids in such a way as to suggest direct incorporation of peptide rather than transport or protective roles. Added purines, pyrimidines, iron, and water-soluble vitamins were without effect. Neither carbohydrates nor phosphorylated materials could be detected in the stimulatory fractions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISERTE G., OSTEUX R. La chromatographie de partage sur papier des dinitrophényl-aminoacides. Bull Soc Chim Biol (Paris) 1951;33(1-2):50–63. [PubMed] [Google Scholar]
- BROWN H., BROWN J. Hemoglobin peptides used in hemoglobin synthesis. Metabolism. 1960 Jun;9:587–593. [PubMed] [Google Scholar]
- Bishop J., Leahy J., Schweet R. FORMATION OF THE PEPTIDE CHAIN OF HEMOGLOBIN. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1030–1038. doi: 10.1073/pnas.46.8.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX E. N. Peptide requirements for the synthesis of streptococcal proteins. J Biol Chem. 1961 Jan;236:166–171. [PubMed] [Google Scholar]
- Gale E. F., Epps H. M. Studies on bacterial amino-acid decarboxylases: 1. l(+)-lysine decarboxylase. Biochem J. 1944;38(3):232–242. doi: 10.1042/bj0380232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMAN R. E., ZIMMERMAN L. N. Effect of the arginine dihydrolase enzyme system on proteinase biosynthesis by Streptococcus faecalis var. liquefaciens. J Bacteriol. 1960 Dec;80:753–761. doi: 10.1128/jb.80.6.753-761.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIHARA H., IKAWA M., SNELL E. E. Peptides and bacterial growth. X. Relation of uptake and hydrolysis to utilization of D-alanine peptides for growth of Streptococcus faecalis. J Biol Chem. 1961 Jan;236:172–176. [PubMed] [Google Scholar]
- KONINGSBERGER V. V., VAN DER GRINTEN C. O., OVERBEEK J. T. Possible intermediates in the biosynthesis of proteins. I. Evidence for the presence of nucleotide-bound carboxyl-activated peptides in baker's yeast. Biochim Biophys Acta. 1957 Dec;26(3):483–490. doi: 10.1016/0006-3002(57)90094-x. [DOI] [PubMed] [Google Scholar]
- MELNYKOVYCH G., SNELL E. E. Nutritional requirements for the formation of arginine decarboxylase in Escherichia coli. J Bacteriol. 1958 Nov;76(5):518–523. doi: 10.1128/jb.76.5.518-523.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAACKE I. D. Protein synthesis in ripening pea seeds. I. Analysis of whole seeds. Biochem J. 1957 May;66(1):101–110. doi: 10.1042/bj0660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHER I. H., MALLETTE M. F. Purification and study of L-lysine decarboxylase from Escherichia coli B. Arch Biochem Biophys. 1954 Dec;53(2):354–369. doi: 10.1016/0003-9861(54)90417-8. [DOI] [PubMed] [Google Scholar]
- SHER I. H., MALLETTE M. F. The use of bacteriophage in releasing two decarboxylases from Escherichia coli B. J Biol Chem. 1953 Jan;200(1):257–262. [PubMed] [Google Scholar]
- TUBOI S., HUZINO A. Enzymic activation of peptide. Arch Biochem Biophys. 1960 Feb;86:309–310. doi: 10.1016/0003-9861(60)90423-9. [DOI] [PubMed] [Google Scholar]
- TURBA F., LEISMANN A., KLEINHENZ G. Zeitlicher Verlauf des Einbaues von C14-markierten Aminosäuren in die Proteine von Hefezellen. Biochem Z. 1957;329(2):97–103. [PubMed] [Google Scholar]
- TURBA F., PELZER H., SCHUSTER H. Trennung von Nucleinsäure-Abkömmlingen durch Ionenaustausch, Papierchromatographie und Hochspannungs-Ionophorese in Filtrierpapier. Hoppe Seylers Z Physiol Chem. 1954;296(3-4):97–108. [PubMed] [Google Scholar]
- WARNER A. C. The actual nitrogen sources for growth of heterotrophic bacteria in non-limiting media. Biochem J. 1956 Sep;64(1):1–6. doi: 10.1042/bj0640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINBAUM G., MALLETTE M. F. Enzyme biosynthesis in Escherichia coli. J Gen Physiol. 1959 Jul 20;42(6):1207–1218. doi: 10.1085/jgp.42.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]



