Abstract
Substantial evidence has suggested that the activity of the bed nucleus of the stria terminalis (BNST) mediates many forms of anxiety-like behavior in human and non-human animals. These data have led many investigators to suggest that abnormal processing within this nucleus may underlie anxiety disorders in humans, and effective anxiety treatments may restore normal BNST functioning. Currently some of the most effective treatments for anxiety disorders are drugs that modulate serotonin (5-HT) systems, and several decades of research have suggested that the activation of 5-HT can modulate anxiety-like behavior. Despite these facts, relatively few studies have examined how activity within the BNST is modulated by 5-HT. Here we review our own investigations using in vitro whole-cell patch-clamp electrophysiological methods on brain sections containing the BNST to determine the response of BNST neurons to exogenous 5-HT application. Our data suggest that the response of BNST neurons to 5-HT is complex, displaying both inhibitory and excitatory components, which are mediated by 5-HT1A, 5-HT2A, 5-HT2C and 5-HT7 receptors. Moreover, we have shown that the selective activation of the inhibitory response to 5-HT reduces anxiety-like behavior, and we describe data suggesting that the activation of the excitatory response to 5-HT may be anxiogenic. We propose that in the normal state, the function of 5-HT is to dampen activity within the BNST (and consequent anxiety-like behavior) during exposure to threatening stimuli; however, we suggest that changes in the balance of the function of BNST 5-HT receptor subtypes could alter the response of BNST neurons to favor excitation and produce a pathological state of increase anxiety.
Keywords: Stress, Amygdala, Raphe, Fear, Patch Clamp, 5-HT
Anxiety disorders affect more than 40 million Americans annually (DuPont et al., 1996), and changes in serotonin (5-HT) functioning have been linked to both their etiology and treatment. Although there is substantial evidence that changes in 5-HT functioning can modulate fear and anxiety-like states in humans and animals alike, the literature is unclear regarding the valence of this modulation, such that treatments that deplete central 5-HT have been shown to produce both anxiolytic or anxiogenic behavioral changes (see (Handley, 1995) for review). This paradox is highlighted by the observation that selective serotonin reuptake inhibitors (SSRIs), which currently are the most-prescribed medication for the treatment of anxiety disorders, produce therapeutic reductions in anxiety only after several weeks of treatment, and yet the acute effects of SSRI treatment often are associated with increases in anxiety-like behavior in both humans and animals alike (Burghardt et al., 2004; Grillon et al., 2007).
Substantial evidence suggests that the activity of the bed nucleus of the stria terminalis (BNST) mediates many forms of anxiety-like behavior in humans and animals (Straube et al., 2007; Walker et al., 2003; Walker and Davis, 2008), leading investigators to suggest that abnormal processing within this nucleus may underlie anxiety disorders in humans, and effective anxiety treatments may depend, in part, on restoring normal BNST functioning. As mentioned above, the most effective treatments for anxiety disorders are drugs that modulate 5-HT systems, either by blocking the reuptake of 5-HT or by modulating the activity of specific 5-HT receptor subtypes. As we will show, the BNST receives a reasonably dense innervation by serotonergic afferents (Commons et al., 2003; Phelix et al., 1992), and multiple 5-HT receptor subtypes are expressed within this region (see below). Despite these important observations, surprisingly few studies have directly examined how neural activity within the BNST is modulated by 5-HT, or how this modulation may be affected by stress. Here, we review our ongoing behavioral, immunohistochemical, genetic, and whole-cell patch-clamp investigations into the effects of 5-HT on BNST function. Our data suggest that the response of individual BNST neurons to 5-HT is complex, and can display both inhibitory and excitatory components, and that this response itself depends on both cell type and the prior stress history of the animal. Postsynaptic 5-HT responses are mediated primarily by activation of one or more of 5-HT1A, 5-HT2A, 5-HT2C, and 5-HT7 receptors. Specifically, we have shown that the activation of 5HT1A receptors mediates an inhibitory response to 5-HT in the majority of BNST neurons, and reduces anxiety-like behavior (Levita et al., 2004). Conversely, we describe data suggesting that the activation of 5-HT2A/2C/7 receptors mediates an excitatory response to 5-HT, which may be anxiogenic. Moreover, we propose that in the normal state, the net effect of 5-HT release in the BNST is to dampen neural activity via postsynaptic 5-HT1A receptor activation, which would consequently act to reduce anxiety-like behavior. However, during exposure to threatening/stressful stimuli we suggest that changes in the functional expression of 5-HT receptor subtypes in BNST neurons alters their 5-HT response in favor of excitation. This may represent the normal adaptive transient response to changes in environmental pressure. However, prolonged exposure to threatening/stressful stimuli may result in a pathological state of persistent anxiety.
We argue that the complex 5-HT response profile observed in the BNST may 1) help to explain some of the confusion regarding the role of 5-HT in modulating anxiety, and 2) may be altered by stress. Moreover, we argue that changes in the BNST response to 5-HT could represent an important mechanism underlying anxiety disorders in humans. Novel treatment strategies could therefore be designed to reverse stress-induced changes in BNST 5-HT responding and/or make it less likely that these alterations could occur.
The bed nucleus of the stria terminalis and anxiety
Studies investigating the neurobiology of fear- and anxiety-like behavior have utilized fear-learning procedures combined with brain-lesion techniques to determine the brain pathways that ascribe affective behavioral responses to neutral stimuli. Within these pathways, these studies have implicated the amygdala as a critical structure in which neutral stimuli are associated with affective behavioral states, and in particular, the amygdala has been shown to be critical for fear responding (see (Davis et al., 1993; Fanselow and LeDoux, 1999; LeDoux, 1993; Maren and Fanselow, 1996)). Importantly, the amygdala has been divided into several subnuclei, including the basolateral amygdala (BLA), which receives thalamic and cortical input from sensory regions, and the central nucleus of the amygdala (CeA), which projects to brain regions that mediate the individual behaviors associated with fear responding. Sensory information from a stimulus with an affective component (i.e. electric shock or a cue previously paired with shock) has been argued to activate the BLA, which in turn activates the CeA to produce a coordinated fear response (see (Davis et al., 1993) for review). Because many anxiety disorders seem to involve exaggerated or inappropriate fear responding, many studies have suggested that malfunction of BLA and CeA neural circuits underlie these disorders in humans. While it is likely that changes in these regions contribute to the etiology of some anxiety disorders, an increasing body of data has implicated the BNST in the mediation of certain fear and anxiety-like behaviors that are not mediated by the anatomically-related CeA pathway (Davis et al., 1997; Davis, 1998; Davis and Shi, 1999; Walker and Davis, 1997; Walker et al., 2003; Walker and Davis, 2008).
Davis and colleagues (1997) initially found that excitotoxic lesions of the BNST blocked the enhanced startle response (a behavioral measure of anxiety) observed after central administration of the stress hormone, corticotropin-releasing factor (CRF), whereas CeA lesions did not (Lee and Davis, 1997). Other anxiety-like behaviors that have subsequently been shown to be mediated by the BNST (and not the CeA) include an unconditioned enhancement of the startle response by a prolonged bright light (Walker and Davis, 1997), freezing behavior induced by predator odor (Fendt et al., 2003), fear responding to long-duration conditioned stimuli previously paired with shock (Waddell et al., 2006), and the anxiogenic behavioral changes observed after uncontrollable stress (Hammack et al., 2004). Importantly, the BNST and CeA both receive substantial afferent information from the BLA, and both project to many of the same subcortical regions involved in mediating individual fear responses (see (Davis et al., 1997) for review). Based on these data, Davis and colleagues (2003 Davis and colleagues (2008) suggested that the BNST mediates a sluggish fear response system that controls behavioral responding to diffuse long-duration stimuli and continues to influence behavior long after the stimulus has terminated, while the CeA mediates a rapid response system to specific threat that terminates when the threat is removed ((Walker et al., 2003; Walker and Davis, 2008); Also see Walker, Miles and Davis in this volume). Davis and colleagues initially likened the former response system to “anxiety” and have suggested that maladaptive responding of the BNST may underlie some forms of anxiety disorders in humans. Consistent with this argument, Waddell et al. (2006) found that BNST lesions blocked fear responding to a 10-min tone that was previously paired with shock, but not a 1-min tone previously paired with shock. This group argued that an anxiety state was conditioned to the 10-min tone that was dissociable from the fear state conditioned to the shorter tone (Waddell et al., 2006). Importantly, the BNST has also been linked to fear behavior in nonhuman primates, where BNST activity was positively correlated with individual differences in rhesus monkey fear responding (Kalin et al., 2005). More recently, activity within the BNST has been associated with the anticipatory anxiety experienced prior to the presentation of a phobic stimulus in humans (Straube et al., 2007).
Consistent with a role for the BNST in anxiety-like responding, electrical stimulation of the anterolateral region produces many of the endocrine, cardiovascular and respiratory responses that are normally elicited by anxiogenic stimuli (Casada and Dafny, 1991). Moreover, anxiogenic pharmacological agents increase the expression of transcription factors, such as the immediate early gene, c-fos, in the anterolateral BNST (Singewald et al., 2003). Hence, activation of BNST neurons is thought to be associated with the expression of anxiety-like behavior. Significantly, the anterolateral BNST and CeA, are two extrahypothalamic regions that display high levels of CRF-immunoreactivity (Sawchenko and Swanson, 1985; Swanson et al., 1983) and CRF mRNA (Makino et al., 1994). The dense expression of CRF in cell bodies and fibers in these brain regions suggest that CRF plays an important role in the modulation of neural activity within the BNST, CeA and their projection regions. Extrahypothalamic CRF has been implicated heavily in mediating many of the behavioral responses to stressful stimuli, including increases in anxiety-like behavior (see (Davis et al., 1997; Koob et al., 1993; Koob and Heinrichs, 1999; Schulkin et al., 1998)), and CRF1 receptor knockout mice exhibit an anxiolytic behavioral profile (Smith et al., 1998). Significantly, local infusion of CRF receptor antagonists into the BNST acts to reduce the anxiogenic response elicited by intracerebroventricular (ICV) CRF injection (Lee and Davis, 1997). Together, these results suggest that release of CRF and/or activation of CRF receptors in the BNST mediate anxiety-like behavior, and further suggest that the anterolateral BNST may be a particularly important subregion for mediating anxiety-like behavioral states.
Serotonin and anxiety
A substantial body of literature has implicated 5-HT systems in the modulation of fear and anxiety behaviors (see (Graeff et al., 1996; Handley et al., 1993; Handley, 1995; Lowry et al., 2005; Lowry et al., 2008)). However, as mentioned above, the nature of the relationship between 5-HT receptor activation and anxiety-like behavior is still unclear. Hence, 5-HT receptor activation can be anxiogenic or anxiolytic depending on the dosage of the 5-HT agonist used, the brain region targeted, and the method of testing anxiety-like behavior (see reviews listed above). Furthermore, some of the complexity of the behavioral response to 5-HT also can be attributed to the presence of multiple 5-HT receptor subtypes in the CNS (Hoyer et al., 2002; Uphouse, 1997). These receptors have been classified into seven distinct families (5-HT1–7) that mediate both the excitatory and the inhibitory actions of 5-HT. More than one 5-HT receptor subtype may mediate the actions of 5-HT in a single neuron (Araneda and Andrade, 1991; Davies et al., 1987), which by default would allow an even more complex response pattern to local 5-HT release than a single receptor system would allow for (for review, see (Uphouse, 1997)).
Similarly, the complex relationship between the activation of 5-HT systems and anxiety-like behavior could be explained, in part, by a single receptor subtype initiating diverse behavioral responses depending on the anxiety-associated brain region in which it is expressed. Consistent with this hypothesis, 5-HT2 receptor activation has been shown to be anxiogenic in the elevated plus maze (Handley et al., 1993; Kshama et al., 1990), hole-board test (Kshama et al., 1990), social interaction test and self-grooming (Bagdy et al., 2001), and acoustic startle (Davis, 1987). However, in a few instances activation of 5-HT2 receptors has been shown to be anxiolytic (Nic Dhonnchadha et al., 2003); these inconsistencies may be explained by different sites of 5-HT2 receptor activation. Importantly, 5-HT1A receptor activation has been shown to be anxiolytic in many of the same tests (Cheeta et al., 2001; Levita et al., 2004; Sakaue et al., 2003).
The dorsal raphé nucleus (DRN)
Many of the regions that mediate fear- and anxiety-like behavior, such as the BNST and CeA receive much of their 5-HT input from the caudal part of the dorsal raphe nucleus (DRN, (Commons et al., 2003; Phelix et al., 1992)). Indeed, Lowry et al. (2005) have argued that 5-HT neurons in the caudal region of the DRN form a specific, topographically organized mesolimbocortical 5-HT system that regulates anxiety-related behavior (Lowry et al., 2005). Consistent with this hypothesis, several discrete anxiogenic stimuli with non-overlapping modes of action converge at the level of the caudal DRN to increase c-fos expression in 5-HT neurons, including uncontrollable shock, high doses of caffeine, metachlorophenylpiperazine (mCPP), urocortin II (ucn II), and benzodiazepine inverse agonists (Abrams et al., 2005; Grahn et al., 1999; Singewald and Sharp, 2000; Staub et al., 2005). Significantly, the caudal DRN receives afferents originating from the BNST and CeA (Peyron et al., 1998) that contain CRF (see (Gray, 1993)). While the regulation of DRN 5-HT neurons by CRF is complex and involves multiple CRF receptor subtypes expressed by both 5-HT and GABAergic neurons (Day et al., 2004; Hammack et al., 2003a; Kirby et al., 2008; Maier and Watkins, 2005)the caudal DRN is the DRN subregion in which putative serotonergic neurons are excited by CRF application (Kirby et al., 2008; Lowry et al., 2000), suggesting that forebrain CRF neurons may actively regulate 5-HT release in the corticolimbic 5-HT pathway.
Thus, acute anxiogenic stimuli likely activate neurons in the anterolateral BNST, thereby increasing levels of CRF within the caudal DRN. Release of CRF in the caudal DRN could subsequently activate a discrete subpopulation of 5-HT neurons that in turn, lead to increased 5-HT levels in the anterolateral BNST. Based on the data we describe below, we propose that in response to an acute stress increased 5-HT release within the anterolateral BNST would likely reduce anxiety-like behavior. In this way, activation of the caudal DRN 5-HT by the BNST forms part of a negative-feedback loop by which anxiety levels are modulated in the presence of an anxiogenic stimulus (see Figure 1A).
Figure 1. A proposed negative feedback function of 5-HT projections to the BNST, and its potential breakdown during states of pathological anxiety.
A) We suggest that acute anxiogenic stimuli activate neurons in the anterolateral BNST, thereby increasing levels of CRF within the caudal DRN. The release of CRF within the caudal DRN excites a subpopulation of 5-HT neurons projecting to the BNST, and in turn increases levels of 5-HT within the BNST. 5-HT release in the BNST preferentially acts on 5-HT1A receptors to reduce BNST activity. B) In a state of pathological anxiety, we suggest that exposure to stress may downregulate 5-HT1A receptor function and/or upregulate 5-HT2A, 5-HT2C, and/or 5-HT7 function, thereby shifting the balance of these receptors towards excitation. Hence, the normal negative-feedback role of BNST 5-HT release is compromised, increasing anxiety.
However, serotonin receptor levels are themselves under tight control by stress hormones (Harvey et al., 2003; Holmes et al., 1995; Young et al., 1992); hence, as we show below, the net response of the BNST to 5-HT release is critically dependent on the prior stress history of the animal. By extrapolation we further propose that this adaptive feedback loop could break down when the anxiogenic threat or stressor is overwhelming, or chronic.
Serotonin and the BNST
While mounting evidence has implicated both 5-HT systems and the BNST in mediating anxiety-like states, very few studies have directly investigated how the BNST is modulated by 5-HT. In agreement with previous reports (Commons et al., 2003; Phelix et al., 1992), our immunohistochemical studies have shown that 5-HT transporter-immunoreactive fibers target the anterolateral BNST and that 5-HT terminals can be observed surrounding the soma of CRF-containing BNST neurons as well as non-CRF neurons (Figure 2). The anterolateral BNST also receives the highest concentration of 5-HT input in nonhuman primates (Freedman and Shi, 2001), suggesting that 5-HT innervation of the BNST is conserved across species. Hence, release of 5-HT within the anterolateral BNST likely modulates anxiety-like behavior by modulating the activity of multiple neuronal subpopulations, including the CRF-containing subpopulation. For this reason, we have focused our studies on examining the effects of 5-HT modulation of neuronal activity in the anterolateral BNST.
Figure 2. Photomicrograph showing 5-HT transporter (5-HTT) immunoreactive fibers targeting the anterolateral BNST in close association with the soma and proximal dendrites of CRF immunoreactive neurons.
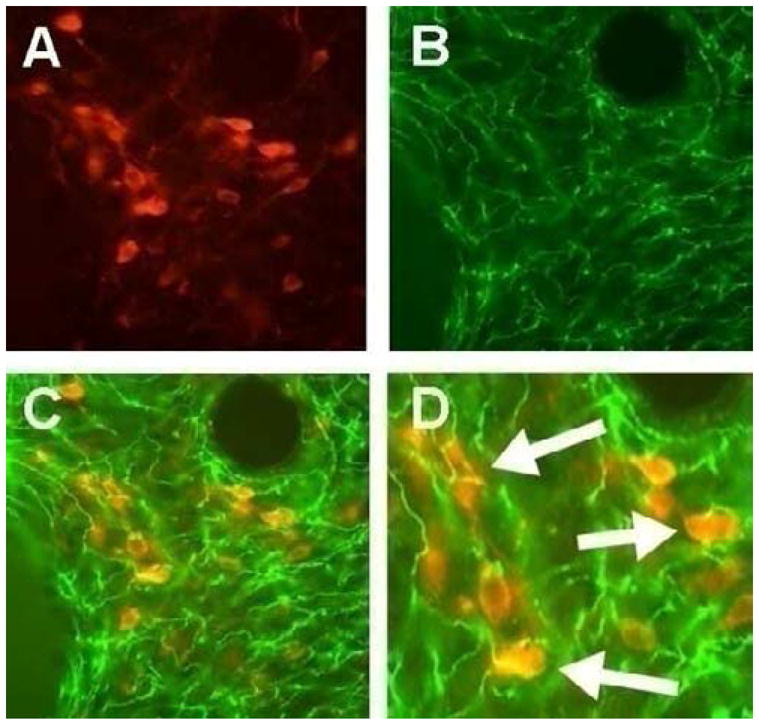
A) Immunohistochemical visualization of CRF-containing neurons (red) within the anterolateral BNST (Rabbit anti-CRF; 1:250 dilution; Abcam). B) Immunohistochemical visualization of 5-HTT immunoreactive fibers (green) in the anterolateral BNST (Mouse anti-5HTT; 1: 500 dilution; Chemicon-Millipore). C) An overlay of A and B shows that 5-HTT positive fibers appear to contact CRF-containing neurons in the anterolateral BNST (20X). D) At higher magnification (40X) 5-HTT terminals are seen to contact the soma of CRF-positive neurons (Arrows).
5-HT receptor subtype expression
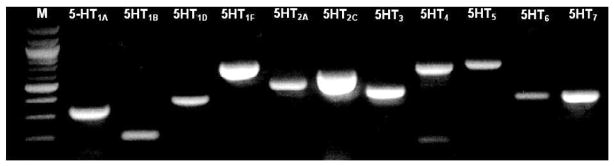
Although previous studies have reported a diverse array of 5-HT receptor expression in the BNST (Cornea-Hebert et al., 1999; Heidmann et al., 1998; Kia et al., 1996; Mengod et al., 1990; Sari et al., 1998; To et al., 1995; Waeber et al., 1994; Waeber and Moskowitz, 1995; Wright et al., 1995), these studies were not designed to examine 5-HT receptor subtypes expression in specific subregions of the BNST, or in single neurons from these subregions. The BNST is a complex structure (Dong et al., 2001) and most probably shows regional variations in 5-HT receptor expression. Hence, we used a micro-dissection technique to isolate the anterolateral BNST, extract whole tissue RNA, and then probe the tissue sample for 5-HT receptor subtype mRNA transcripts using a reverse transcriptase polymerase chain reaction (RT-PCR). As shown in Figure 3, multiple 5-HT receptor subtype mRNAs were expressed in the anterolateral BNST, including the transcripts for 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1F, 5-HT2A, 5-HT2C, 5-HT3, 5-HT4, 5-HT5A, 5-HT6 and 5-HT7 receptors. However, this approach could not distinguish between 5-HT receptor expressions in neuronal versus non-neuronal compartments. Consequently, we next combined our whole-cell patch-clamp recording approach with the RT-PCR technique to analyze mRNA transcript expression in the cytoplasm of physiologically identified BNST neurons. Consistent with the whole tissue mRNA expression, the mRNA transcript for multiple 5-HT receptor receptors was expressed in individual neurons of the anterolateral BNST. Hence, permutations and combinations of the mRNA signal for the following receptors were observed in individual neurons: 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C, 5-HT3, 5-HT4, 5-HT6, and 5-HT7, with the most frequently observed subtypes being 5-HT1A, 5-HT2A, and 5-HT7 (R. Hazra and D.G. Rainnie, unpublished observations). The mRNA expression implies that anterolateral BNST neurons potentially express these 5-HT receptor proteins. The cellular localization of some receptor subtypes are likely somatodendritic, while others, such as the 5-HT1B receptor, may be shuttled downstream to presynaptic axon terminals. The 5-HT receptor subtype expression patterns were consistent with our pharmacological data reported below, and also tended to group according to physiological cell types (see below).
Figure 3. Whole tissue expression of 5-HT receptor mRNA transcripts in the anterolateral BNST.
Total RNA was isolated from the anterolateral BNST and reverse transcribed into cDNA. The cDNA was amplified by PCR with primers specific for each of the 5-HT receptors. The PCR products were electrophoresed on a 1% agarose gel. M denotes a molecular weight marker.
Response of anterolateral BNST neurons to 5-HT receptor activation
Direct postsynaptic response to 5-HT receptor activation
Consistent with our molecular biological data, the response of BNST neurons to exogenous 5-HT application is complex. In our initial in vitro whole-cell patch-clamp study of 38 BNST neurons, we reported that neurons of the anterolateral BNST exhibited a range of responses to exogenous 5-HT application, including an inhibitory membrane hyperpolarization, an excitatory membrane depolarization, or a biphasic response of hyperpolarization followed by depolarization. In addition, a subpopulation of neurons was unaffected by exogenous 5-HT application (Rainnie, 1999). However, in those neurons that did respond to 5-HT, the response was always accompanied by a decrease in membrane input resistance (range: 23 – 40%), suggesting that both the inhibitory- and the excitatory response were mediated by the opening of ion channels. We subsequently confirmed these results in a much larger sample (n = 175; (Levita et al., 2004)). Here, we demonstrated that the hyperpolarizing response was the predominant membrane response to 5-HT, occurring in ~35% of BNST neurons, with an EC50 of ~6 μM and which was associated with an outward current (~ 14pA) that had an apparent reversal potential (E5-HT = −77 mV).
The second most frequently observed response was the mixed response (hyperpolarization followed by depolarization), which occurred in 25% of anterolateral BNST neurons. In these neurons, the hyperpolarizing (inhibitory) response was typically more pronounced than the depolarizing response. Hence, in control conditions the net response of the majority of BNST neurons (~60%) to local 5-HT release was inhibition. Significantly, a closer examination of the 5-HT reversal potential in BNST neurons that responded with “pure” membrane hyperpolarization revealed two subpopulations; one that had an E5-HT = −85 mV, which was near the reversal potential expected for the opening of a potassium channel, and one characterized by a more depolarized reversal potential (E5-HT = −71 mV) that suggested the activation of mixed ionic currents. Moreover, this reversal potential was similar to that observed in BNST neurons showing a biphasic 5-HT response, and suggested that a depolarizing response was masked in some of the neurons that appeared to have a “pure” inhibitory response. Subsequent reanalysis of our data revealed that 49% of BNST neurons display the mixed responses to 5-HT, which represents the majority of BNST neurons that respond to 5-HT (Figure 4A). The significance of this observation should not be overlooked. The presence of two opposing responses to a single neurotransmitter in the same neuron suggests that the response to 5-HT in the majority of BNST neurons is dynamic and that the net action of 5-HT on the output of the anterolateral BNST is critically dependent on factors that regulate the relative expression of the inhibitory versus the excitatory response to 5-HT in these neurons. As noted above, in naïve animals the net response of most BNST neurons to 5-HT is inhibition and hence local 5-HT release would tend to reduce anxiety-like behavior. However, as outlined below chronic activation of stress hormones can significantly alter this response.
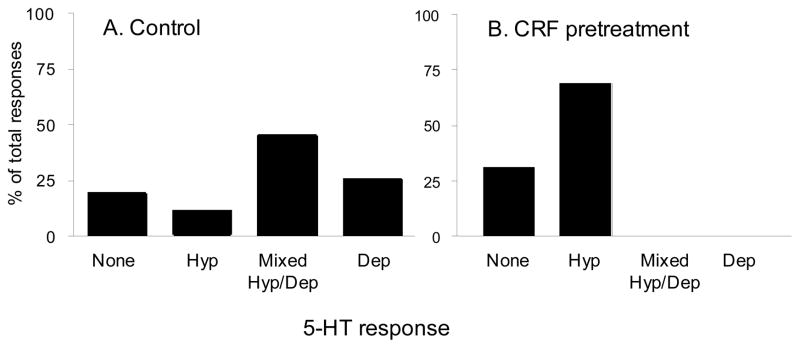
Figure 4. CRF pretreatment increased the percentage of inhibitory responses to 5-HT application in anterolateral BNST neurons.
Using whole-cell patch clamp electrophysiological techniques on anterolateral BNST neurons in vitro, the changes in membrane potential or steady state current to 50 μM 5-HT were determined. A) The percentages of 5-HT responses in neurons obtained from untreated control slices. Neurons responded to 5-HT with one of four responses, pure hyperpolarization (Hyp), pure depolarization (Dep), a mixed response where a hyperpolarization was immediately followed by depolarization (Mixed Hyp/Dep) or no response (None). B) The percentages of 5-HT responses in neurons obtained from slices that were bathed in CRF (1 μM) for 30 min before the establishment of whole cell patch clamp (n=13). Pretreatment with CRF abolished excitatory responding to 5-HT (χ2 = 9.758, p < 0.05).
Consistent with the more negative E5-HT (−85 mV) in a subpopulation of BNST neurons, we demonstrated that the “pure” inhibitory response of BNST neurons to 5-HT was mediated by activation of a G-protein coupled inwardly rectifying potassium current (Levita et al., 2004). Similar properties have been reported elsewhere in the brain following activation of 5-HT1A receptors (Sprouse and Aghajanian, 1988), and as predicted the inhibitory response to 5-HT was fully blocked by prior application of the selective 5-HT1A receptor antagonist, WAY 100635 (200 nM). Furthermore, the mixed 5-HT1/7 receptor agonist, 5-carboxyamidotryptamine (5-CT), mainly mimicked the inhibitory response to 5-HT, and this response was also blocked by WAY 100635. Interestingly, the selective 5-HT1A receptor agonist R(±)8-hydroxydipropylaminotetralin hydrobromide (8-OH-DPAT) failed to mimic the inhibitory response to 5-HT in any BNST neuron tested. Although the lack of effect of 8-OH-DPAT is confounding, it may be consistent with previous reports suggesting that 8-OH-DPAT may have only partial agonist properties at postsynaptic 5-HT1A receptors (Van den Hooff and Galvan, 1992; Williams et al., 1988).
In the course of these experiments it was noted that 5-CT occasionally elicited a membrane depolarization similar to that observed in a subset of BNST neurons in response to 5-HT. As noted above, our single cell RT-PCR studies had indicated that 5-HT7 receptors are expressed at comparatively high levels in anterolateral BNST neurons. Activation of 5-HT7 receptors has been reported to excite neurons in the hippocampus (Tokarski et al., 2003), anterodorsal thalamus (Chapin and Andrade, 2001), and prefrontal cortex (Beique et al., 2004), and this gave us our first indication of a potential substrate for the 5-HT-induced depolarization observed in BNST neurons.
However, our single cell RT-PCR experiments also indicated a high level of expression of 5-HT2A/C receptors in anterolateral BNST neurons, and these receptors have also been reported to mediate excitatory postsynaptic responses in several brain regions (Bonsi et al., 2007; Marek and Aghajanian, 1994; Sheldon and Aghajanian, 1991). As may be expected, our investigation of the receptor substrate/s mediating the depolarizing response to 5-HT in BNST neurons has revealed a more complex profile for excitation than we find for inhibition.
For example, we first tested the effects of prior application of the broad spectrum 5-HT2 receptor antagonist pirenperone (10 μM). Surprisingly, pirenperone fully blocked the depolarizing component of the mixed 5-HT response in every neuron tested suggesting that activation of 5-HT2 receptors mediated the excitatory component of the 5-HT response. However, at the concentration used for these studies, pirenperone can also act as an antagonist at 5-HT7 receptors and, hence, the identity of the receptor subtype mediating the excitatory response was inconclusive. Consequently, we next examined the response of BNST neurons to prior application of more selective 5-HT receptor subtype antagonists.
Here too, however, the response to prior application of selective antagonists was mixed. For example, in a subpopulation (25%) of neurons showing a biphasic response to 5-HT, application of the 5-HT2A receptor antagonist MDL 100907 (10 μM) fully blocked the depolarizing component of the response. However, in another population of neurons (50%) that also showed a biphasic response to 5-HT, the depolarizing component was fully blocked by prior application of the selective 5-HT7 receptor antagonist SB 269970 (1 μM). In yet another population of neurons, the depolarizing component appeared to be mediated by the activation of both 5-HT2A and 5-HT7 receptors, as prior application of both antagonists was required to fully block the depolarizing response to 5-HT. Nevertheless, the physiological data are in agreement with our single cell RT-PCR data and suggest that the most common physiological phenotype, the biphasic response to 5-HT, is mediated by combinations of 5-HT1A receptors together with either 5-HT2A receptors, 5-HT7 receptors, or both.
Finally, a subpopulation of neurons was identified that responded to 5-HT with a pure depolarization, and this response was fully blocked by prior application of the selective 5-HT2C receptor antagonist, RS 102221 (10 μM).
Presynaptic action of 5-HT in the BNST
In addition to a direct postsynaptic action in the BNST, local release of 5-HT also activates presynaptic 5-HT receptors. Hence, stimulation of the primary afferent input into the BNST, the stria terminalis, elicits a monosynaptic excitatory postsynaptic current (EPSC) in neurons of the anterolateral BNST (Egli and Winder, 2003; Rainnie, 1999). Exogenous application of 5-HT (50 μM) consistently reduced the amplitude of stimulus-evoked EPSCs by 36% (J. Guo and D.G. Rainnie, submitted, 2009). Significantly, the effects of 5-HT could be mimicked by the 5-HT1B/D receptor selective agonist, sumatriptan (10 μM), as well as the 5-HT1B receptor selective agonist CP93129 (10 μM), suggesting that 5-HT1B receptor activation may modulate glutamate transmission in the BNST. Consistent with this hypothesis, the 5-HT or sumatriptan effect could be blocked by the 5-HT1B selective antagonist GR55562 (10 μM).
We then tested whether the 5-HT effect was mediated by a presynaptic or postsynaptic mechanism using a paired pulse stimulation paradigm in which two stimuli are separated by an interval of ~ 50 ms. In this paradigm, the amplitude of the second EPSC is thought to be governed by alterations in the relative probability of presynaptic release (Hess et al., 1987; Manabe et al., 1993). Exogenous application of 5-HT (50 μM) or sumatriptan (10 μM) caused a significant increase in the paired pulse ratio, which was associated with a decrease in the amplitude of both EPSCs. Furthermore, sumatriptan application decreased the frequency of miniature EPSCs, but had no effect on the amplitude of miniature EPSCs. Taken together, these results suggest a presynaptic site of action of 5-HT1B receptor activation in the inhibitory modulation of glutamate release in the anterolateral BNST. These data are consistent with the presynaptic 5-HT1B receptor modulation of neurotransmitter release reported in other brain regions (Bouryi and Lewis, 2003; Laurent et al., 2002).
In summary, these electrophysiological studies suggest that the inhibition of BNST neurons by 5-HT is always mediated by activation of 5-HT1A receptors, and that the excitation of BNST neurons by 5-HT is mediated by activation of 5-HT2A, 5-HT2C and/or 5-HT7 receptors, depending on the cell type. If an increase in neural activity in the BNST is correlated with an increase in anxiety-like behavior (ibid), then the selective activation of 5-HT1A receptors would be expected to be anxiolytic, whereas the selective activation of 5-HT2A, 5-HT2C and/or 5-HT7 receptors might be expected to be anxiogenic.
Behavioral response to 5-HT receptor activation
As noted above, bath application of 5-CT produced a response profile that favors inhibition. We used the relative selectivity of the 5-CT response to examine the in vivo effects of 5HT1A receptor activation in the BNST. Here, we used baseline startle behavior as an index of the basal level of anxiety in rats. As predicted bilateral injection of 5-CT directly into the BNST immediately prior to testing produced a pronounced anxiolytic response (Levita et al., 2004). These data are consistent with the hypothesis that 5-CT predominantly inhibits BNST neurons, presumably by activating BNST 5-HT1A receptors. However, as outlined above, 5-CT also activates 5-HT7 receptors on a subset of BNST neurons. If these neurons function as local circuit inhibitory interneurons then 5-HT7 receptor-evoked excitation may also functionally reduce the output of the BNST. Hence, we injected the selective 5-HT1A antagonist, WAY 100635 into the BNST and observed a dose-dependent increase in anxiety-like behavior as measured by social interaction (F(4,24) = 3.027, p < 0.05; K.M. Rhodes and S.E. Hammack, unpublished data). These data confirm that BNST 5-HT1A receptor activation is anxiolytic.
We have yet to find any agonists that can selectively mimick the 5-HT2A- or 5-HT2C, or 5-HT7 receptor-mediated depolarization in BNST neurons. However, one 5-HT agonist meta-chlorophenylpiperazine (mCPP) has been shown to produce anxiety-like behavioral changes in humans and animals (Broocks et al., 1999; Cornelio and Nunes-de-Souza, 2007; Klaassen et al., 2002). mCPP has a higher affinity for 5-HT2C receptors and 5-HT2A receptors than 5-HT1A receptors(Porter et al., 1999), and hence may be more likely to produce a response profile that favors excitation. Significantly, systemic mCPP has been shown to increase the expression of c-fos in the anterolateral BNST(Singewald et al., 2003), and mCPP injection within the BNST of mice increases baseline startle responding (W.A. Falls, personal communication).
Together these data suggest that local 5-HT release 1) inhibits BNST neurons by activating 5-HT1A receptors and the selective activation of BNST 5-HT1A receptors is anxiolytic, and 2) excites BNST neurons by activating 5-HT2A, 5-TH2C, and/or 5-HT7 receptors, and the activation of one or more of these receptors is anxiogenic. Interestingly, while 5-HT2A, 5-HT2C and 5-HT7 receptors excite anterolateral BNST neurons, it is unknown whether these receptor subtypes are differentially expressed by projection neurons and/or local-circuit interneurons; hence, their activation could increase or decrease anxiety-like behavior depending on their relative expression by these different cell types. The anxiogenic action of mCPP suggests that at least one of these receptor subtypes is expressed by BNST projection neurons. Given that the majority of BNST neurons express multiple 5-HT receptors that have opposing physiologic actions in the same cell, we proposed that any treatment that could modulate the relative efficacy of one receptor subtype in relation to another would profoundly impact the response of the BNST to local 5-HT release.
Modulation of the serotonin response in BNST neurons
Modulation of BNST 5-HT responses by acute CRF treatment and isolation
As discussed above, a decrease in 5-HT1A receptor function would be expected to change the 5-HT response profile of BNST neurons to favor excitation and increased anxiety, whereas a decrease in the function of 5-HT2A, 5-HT2C and/or 5-HT7 receptors would be expected to change the 5-HT response profile to favor increased inhibition and reduced anxiety. Several treatments have been shown to alter the functional expression of 5-HT receptor subtypes in other brain regions, including chronic stress (Ferretti et al., 1995; Lanfumey et al., 1999; Lopez et al., 1998; Lopez et al., 1999; McKittrick et al., 1995; Ossowska et al., 2001; Takao et al., 1997), treatment with stress hormones (Crayton et al., 1996; Fernandes et al., 1997; Ferretti et al., 1995; Katagiri et al., 2001; Kuroda et al., 1992; Maines et al., 1999; Takao et al., 1997), antidepressant treatment (Elena Castro et al., 2003; Gurevich et al., 1993; Hanson et al., 1998; Lund et al., 1992; Riad et al., 2004; Strome et al., 2005), and voluntary exercise (Greenwood et al., 2005).
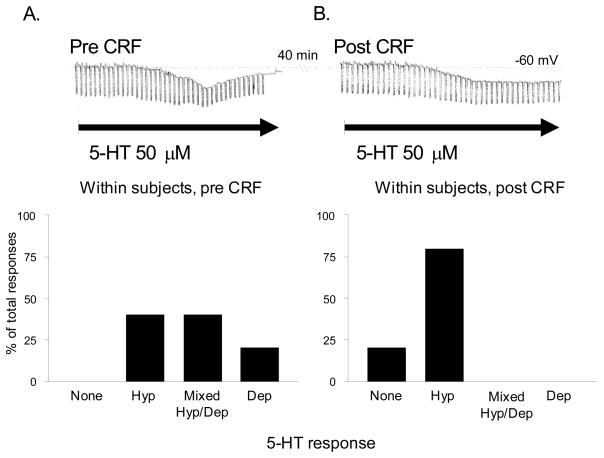
Consistent with these observations, results from our own studies suggest that the electrophysiological response of BNST neurons to 5-HT can be altered either by prior treatment of BNST slices with CRF, or by one week of isolation housing, which is a mild stressor in rats. In our in vitro experiments, slices containing the anterolateral BNST were perfused with a bolus application of 1 μM CRF in 10 ml artificial cerebrospinal fluid (ACSF) approximately 30 minutes prior to the establishment of whole-cell patch clamp recording from BNST neurons. Each neuron was then probed to determine their response to exogenous application of 5-HT (50 μM). Intriguingly, after bolus CRF application, the 5-HT response pattern of BNST neurons shifted from the heterogeneous response pattern described above to one of predominantly pure inhibition (χ2 = 9.758, p < 0.05, see Figure 4B), such that no excitatory response to 5-HT was ever observed after CRF application. We next determined whether CRF could abolish the excitatory response in BNST neurons using a within-subjects design. Here, whole-cell patch clamp was established, and the initial response to 50 μM 5-HT was determined. The slice was then perfused with a bolus application of 200 nM CRF in 10 ml ACSF and the response to a second 5-HT application was determined after a 20–40 min wash-off period. Consistent with the first experiment, application of CRF attenuated the depolarizing response in neurons that had previously responded to 5-HT with pure depolarization or with a biphasic response (see Figure 5). Previous studies had confirmed that the excitatory 5-HT response was reproducible and, hence, these data suggested that acute CRF receptor activation can trigger a cascade of events in BNST neurons that ultimately results in the apparent abolition of the excitatory response. Moreover, these data further suggest that the 5-HT response can be altered for a period of at least 45 minutes after CRF receptor activation. As noted above, CRF expression is dense within this region of the BNST (Chappell et al., 1986; Gray and Magnuson, 1992; Wong et al., 1994), and CRF receptor activation in this region is both necessary and sufficient for the expression of anxiety-like behavior (Lee and Davis, 1997; Sahuque et al., 2006).
Figure 5. Acute CRF treatment (200 nM) potentiates the inhibitory response of individual anterolateral BNST neurons to 5-HT application.
A) Upon the establishment of whole cell patch clamp, an initial response to 5-HT was determined. Upper: A representative mixed Hyp/Dep response in current-clamp mode that contained both an inhibitory and excitatory component. Lower: The percentages of 5-HT responses obtained in anterolateral BNST neurons prior to CRF treatment (n = 10). B) 5-HT responses 40 min after CRF treatment determined by 5-HT reapplication. Upper: The same representative neuron shown in A responded to 5-HT with a pure Hyp response 40 min after CRF treatment. The depolarizing component of the initial 5-HT response was abolished by CRF treatment. Lower: The percentages of 5-HT responses in BNST neurons 40 min after CRF treatment. CRF treatment abolished the depolarization response in all neurons.
Hence, in response to a physical or psychological threat, CRF release is expected to transiently increase in the BNST, and this release would coordinate an anxiety-like response to this threat. Several reports have suggested that the CRF infusion into the BNST is anxiogenic (Erb and Stewart, 1999; Lee and Davis, 1997; Sahuque et al., 2006), and that BNST CRF blockade reduces anxiety-like behavior (Erb and Stewart, 1999; Jasnow et al., 2004; Lee and Davis, 1997; Sahuque et al., 2006). However, the data we presented above suggest that BNST CRF release would also induce a delayed shift in the valence of the 5-HT release to favor inhibition of BNST neurons, and hence BNST 5-HT release in response to threat may mediate a delayed inhibitory response that normally functions to prevent BNST overstimulation by CRF We argue below that the initial increase in BNST CRF activity would trigger the activation of the caudal DRN, which in turn would result in a delayed release of 5-HT into the BNST and inhibit further BNST activity, forming a inhibitory feedback loop designed to maintain homeostasis.
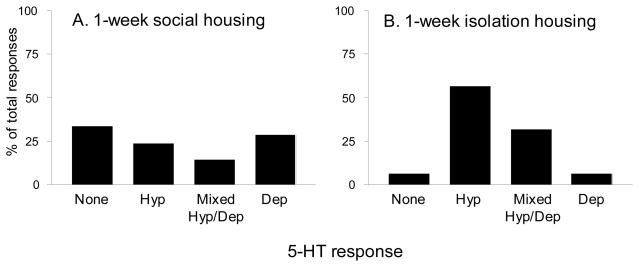
In order to determine whether a behavioral manipulation associated with enhanced anxiety-like behavior might also shift the population of BNST 5-HT responses, we housed rats singly or in groups (2–4 per cage) for one week prior to experimentation. Because rats are social organisms that live in hierarchical communities, long- and short- term isolation housing has been shown to produce a behavioral state associated with increased anxiety (Haller and Halasz, 1999). After one week, rats were sacrificed and their brains prepared for electrophysiological recording. Again whole-cell patch clamp was established in anterolateral BNST neurons, and their response to 50 μM 5-HT was determined. Although isolation housing did not abolish the depolarizing response to 5-HT as effectively as acute CRF application, a chi-square analysis revealed that isolation housing significantly shifted the response profile of BNST 5-HT responses to favor inhibition, consistent with the effects of bolus CRF application (χ2 = 9.207, p < 0.05, see Figure 6).
Figure 6. Responses of anterolateral BNST neurons to 50 μM 5-HT in single- or group-housed rats.
One week of isolation housing is a mild stressor in rats, and can increase anxiety-like behavior. Rats were either single- or group- housed for 1 week prior to sacrifice and the preparation of BNST slices. Patch clamp recordings were performed and 5-HT responses of BNST neurons were examined. A) The percentages of BNST 5-HT responses in group-housed rats (n = 21). B) The percentages of BNST 5-HT responses in single-housed rats (n = 16). Consistent with an enhancement of BNST CRF tone, One week of isolation housing shifted the 5-HT response profile to favor inhibition, reducing the percentage of neurons that were excited by 5-HT (χ2 = 9.207, p < 0.05).
Based on these data, we propose that in the non-pathological state, 5-HT may serve as a feedback inhibitor of BNST function during stressor exposure, so that stressors activate neurons within the anterolateral BNST, which project to and activate the caudal DRN. Caudal DRN projections, in turn, project back to the anterolateral BNST, where the release of 5-HT after recent CRF receptor activation more efficiently dampens neuronal activity in the anterolateral BNST via actions at postsynaptic 5-HT1A receptors (see Figure 1A).
Prolonged/Chronic stress
Compared to most studies that observe pathological behavioral changes after at least 4+ weeks of isolation (Serra et al., 2008), one week of isolation housing is likely a relatively mild stressor, and the physiological responses induced by one week of isolation may not differ substantially from those induced by an acute stressor. Although acute CRF and one week of isolation housing may shift the BNST 5-HT response to favor inhibition, chronic and/or severe stressors, which are often associated with pathological anxiety states, may shift the profile to favor excitation and an anxiogenic response to increased BNST 5-HT release. Hence, the down-regulation of 5-HT1A receptors (or up-regulation of 5-HT2A/2C and/or 5-HT7 receptors) would be expected to favor an excitatory anxiogenic BNST response to 5-HT release. Conversely, the up-regulation of 5-HT1A receptors (or down-regulation of 5-HT2A and/or 5-HT7 receptors) would be expected to favor an inhibitory anxiolytic BNST response to 5-HT release. Because stress has also been shown to activate central 5-HT systems, such as those projecting from the caudal DRN to the BNST (Grahn et al., 1999), an alteration of the BNST 5-HT response to favor anxiety could represent an important mechanism underlying pathological anxiety states.
Stress has been shown to modulate 5-HT receptors in a manner that would be consistent with an anxiogenic response. An increase in cortical 5-HT2 receptor function is observed after chronic variable stress (Ferretti et al., 1995; Ossowska et al., 2001), social stress (McKittrick et al., 1995), and swim stress (Takao et al., 1997), and similar stressors have been shown to decrease 5-HT1A function in the hippocampus (Lopez et al., 1998; Lopez et al., 1999; Takao et al., 1997). 5-HT1A receptors are also down-regulated in the DRN following uncontrollable shock (Short, 2000) and a repeated stress paradigm (Lanfumey et al., 1999). Consistent with these data, the chronic administration of the stress hormone corticosterone (Fernandes et al., 1997; Kuroda et al., 1992; Takao et al., 1997) or the synthetic glucocorticoid dexamethasone (Katagiri et al., 2001; Lanfumey et al., 1999) has been shown to increase the density and function of cortical 5-HT2A receptors, while at the same time decreasing the density of 5-HT1A receptors in frontal cortex (Crayton et al., 1996) and hippocampus (Fernandes et al., 1997; Maines et al., 1999; Takao et al., 1997). Together, these data suggest that chronic stress could shift the balance of BNST 5-HT responses to favor excitation, so that the stress-induced release of 5-HT would produce an anxiogenic response. Significantly, this type of 5-HT receptor modulation was only observed after uncontrollable and/or chronic stressors.
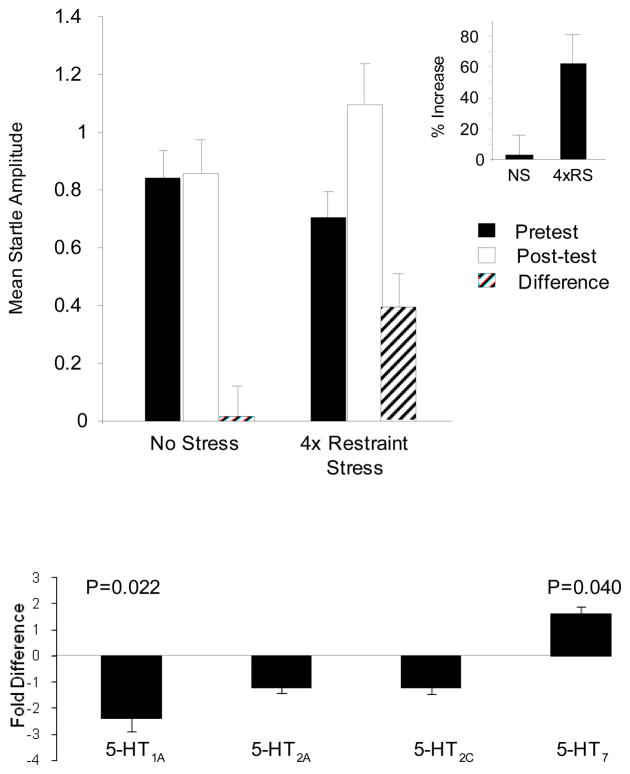
In order to determine whether chronic stress modulates the relative expression of BNST 5-HT receptor subtypes, we subjected rats to a 4-day restraint stress paradigm, measured anxiety-like behavior, and sacrificed and processed brain tissue for the analysis of BNST 5-HT receptor subtype mRNA expression using quantitative RT-PCR. On day 1, baseline startle responding was measured and did not differ between experimental groups (t(14) =1.068; p = 0.304). After baseline measurements, half of the rats received 4 days of stress such that on each day they were restrained in custom-made wire mesh restrainers that kept them almost completely immobilized for one hour. Control rats were not handled during the four day period. On day 5 for control animals and day 10 for stressed animals, rats were administered a second baseline startle test. Baseline startle responding was significantly increased in rats that received chronic stress (see Figure 7A). A 2-way mixed measures ANOVA revealed a significant Group × Test interaction (F(1, 14)=6.019; p=.028). When the data are expressed as difference scores (post-test minus pretest), the group difference was reliable (t(14)=-2.453; p=.028). Likewise, when the data are expressed as percent increase from pretest to posttest (shown in the insert in Figure 7A), the group difference was reliable (t(14)=-2.685; p=.018). Hence, chronic stress significantly increased anxiety-like behavior measured 6 days after the termination of the last stress session.
Figure 7. Chronic restraint stress enhances baseline startle and modulates the relative expression of BNST 5-HT receptor subtypes.
A) On day 1 (pretest) all rats were administered a baseline startle test, and subsequently assigned to a stress or no-stress experimental group such that pretest baseline startle responding did not differ (t(14) =1.068; p = 0.304). Stressed rats then received 4 daily sessions of restraint in custom-made wire mesh restrainers that kept them almost completely immobilized for one hour; no-stress rats were not handled during the 4 day period. On the 5th day for the control group and the 10th day for the stressed group, rats received a second startle test (post-test), and prior chronic stress treatment significantly enhanced startle amplitude, indicative of increased anxiety (inset: % change from pretest to posttest was also significant). B) Immediately after the second startle test, rats were sacrificed and tissue containing the anterolateral BNST was processed for quantitative RT-PCR. Chronic stress reduced BNST 5-HT1A receptor expression, and increased 5-HT7 expression.
In order to determine whether chronic stress altered the relative expression of BNST 5-HT receptor subtypes, following the post-test, rats were sacrificed and tissue containing the anterolateral BNST was processed for quantitative RT-PCR. Here, 500 μm coronal sections containing the BNST were prepared using standard methods (see Hammack et al., 2006). The BNST from each section was then manually isolated using a binocular dissecting microscope and immediately placed in Trizol to extract the total RNA. Total RNA was isolated and reverse-transcribed to cDNA using Multiscribe reverse-transcriptase. The cDNA was then amplified by quantitative RT-PCR using specific primers for 5-HT1A, 5-HT2A, 5-HT2C, 5-HT7 receptors as well as 18S rRNA to act as a control. Each sample was run in triplicate and fold differences of the target gene mRNA expression were calculated relative to the mRNA expression levels of the 18S rRNA in each sample. As shown in Figure 7B, repeated restraint stress caused a significant reduction in 5-HT1A receptor mRNA expression (p = 0.022; n=8). In contrast, 5-HT7 receptor mRNA transcript expression was significantly increased (p = 0.04; n=8). No significant difference was observed in the mRNA expression for either 5-HT2A, or 5-HT2C receptors. These data are consistent with our hypothesis that chronic stress may disrupt the normal adaptive response to stress in such a way as to reduce 5-HT-mediated feedback inhibition (reduced 5-HT1A mRNA) in the anterolateral BNST and favor excitation (increased 5-HT7 mRNA expression).
Serotonin and the BNST: A functional hypothesis
We have shown that the BNST exhibits a complex response pattern to 5-HT that is mediated by multiple 5-HT receptor subtypes. Our data suggest that BNST neurons are capable of responding to 5-HT with both inhibition and excitation, and we propose that this complexity may help explain some of the data describing both anxioytic and anxiogenic roles for central 5-HT activation. We found that 49% of BNST neurons respond to exogenous 5-HT with a mixed inhibitory/excitatory response; however, the magnitude of the inhibitory response was almost always greater than the excitatory response, so that the net response of BNST neurons to 5-HT was predominantly inhibitory. Hence, the majority of BNST neurons were more hyperpolarized in the presence of exogenous 5-HT, suggesting that the release of 5-HT within the BNST may act to dampen BNST activity and anxiety-like behavior in a non-pathological state. Moreover, 5-HT has a slightly higher affinity for 5-HT1A receptors when compared to the other receptor subtypes expressed in the BNST (see (Uphouse, 1997; Zifa and Fillion, 1992)), so that low levels of BNST 5-HT would also favor BNST inhibition. As noted above, the BNST receives 5-HT input from the caudal region of the DRN, and the caudal region is also activated by stressful anxiogenic stimuli. In response to anxiogenic stimuli, BNST 5-HT release would serve to prevent prolonged activation of the stress response circuit and thereby reduce the behavioral impact of these stimuli. Moreover, anxiogenic stimuli would also be expected to activate BNST CRF systems, and as shown above, the release of CRF within the BNST would enhance the inhibitory effects of 5-HT, consistent with a regulatory role for BNST 5-HT in the presence of stressful anxiogenic stimuli.
Notably, high doses of CRF and CRF-analogues have been shown to activate 5-HT neurons in the caudal DRN (Amat et al., 2004; Lowry et al., 2000), and the blockade of DRN CRF receptors attenuates the behavioral consequences of stressor exposure (Hammack et al., 2002; Hammack et al., 2003b). The source of DRN CRF is currently unknown; however, multiple studies have shown that DRN 5-HT neurons respond to CRF (Kirby et al., 2000; Lowry et al., 2000; Price et al., 1998; Price and Lucki, 2001), and have suggested that the source of raphe CRF may come from CRF-rich regions like the BNST (see (Maier and Watkins, 2005) for review). Moreover, some DRN-projecting neurons originate from the CRF-rich oval nucleus of the BNST (S.E. Hammack, unpublished observations). Hence, CRF neurons within the BNST itself may activate caudal DRN 5-HT neurons in response to stressful anxiogenic stimuli, and the subsequent release of 5-HT within the BNST may represent a long forebrain-midbrain negative feedback loop designed to dampen maladaptive anxiety-like activity following exposure to stressful anxiogenic stimuli (see Figure 1A). However, as suggested above, because the BNST also has the capacity to respond to 5-HT with excitation, chronic and/or severe stress states may modulate this circuitry such that feedback inhibition is either attenuated, or in dramatic cases reversed to positive feedback. This type of modulation would be expected to produce a state of pathological anxiety (Figure 1B).
Other considerations
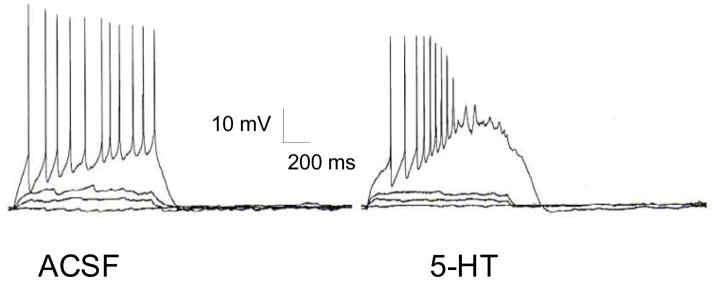
The inhibitory and excitatory actions of 5-HT on BNST neurons described above were based on changes in membrane potential; however, we have shown that several intrinsic membrane currents are expressed by BNST neurons at rest, and that modulation of these currents can substantially alter the excitability and firing properties of BNST neurons (Hammack et al., 2007). Furthermore, we have characterized 3 distinct physiological cell types within the anterolateral BNST, based on the relative expression of these membrane currents. Type I neurons were characterized by a regular firing pattern in response to depolarization and a depolarizing sag in response to hyperpolarizing current injection that was mediated by the hyperpolarization-activated cation current, Ih. Type II neurons were also characterized by the expression of Ih, but also exhibited a burst-firing pattern that was mediated by activation of the low-threshold calcium current, IT. Type III neurons did not exhibit characteristics of Ih or IT expression, but instead exhibited a pronounced fast rectification indicative of the inwardly rectifying potassium current, IK(IR). Most BNST neurons were also found to express the voltage-dependent potassium current IA, as well as the persistent sodium current INaP. Moreover, broadly speaking the 5-HT receptor subtypes tended to group according to physiological cell type. Hence, Type I neurons of the anterolateral BNST show a tendency to co-express 5-HT1A and 5-HT2A receptors, Type II neurons co-express 5-HT1A and 5-HT7 receptors, and Type III neurons co-express 5-HT2A and 5-HT2C receptors, suggesting that local 5HT release would have distinct actions on neurons of the anterolateral BNST. Moreover, we found that modulating these currents in BNST neurons could dramatically alter their excitability and firing properties. For example, we showed that the activation characteristics of IA in BNST neurons were similar to the activation characteristics of IT, and we suggested that these two currents act in concert to regulate burst-firing activity in BNST type II neurons. Consistent with this hypothesis, blockade of IA with 4-aminopyridine (4-AP, 1–10 mM) dramatically enhanced burst firing in type II neurons, whereas blockade of IT with 500 μM nickel chloride blocked burst firing in these same neurons. Importantly, 5-HT has been shown to modulate IA (Farley and Auerbach, 1986) and IT (Fraser and MacVicar, 1991) in other brain regions, and such an action in the BNST could dramatically alter the firing properties of BNST neurons even if membrane potential is not affected. Figure 8 represents a BNST neuron that did not respond to 5-HT with a change in membrane potential or input resistance; however, in response to depolarizing current injection, the firing properties of this neuron were dramatically altered in the presence of 5-HT to reflect a higher frequency, bursting pattern. Moreover, this effect of 5-HT was blocked by the 5-HT antagonist pirenperone. High-frequency burst-firing is thought to promote the release of peptide neurotransmitters from hippocampal GABAergic neurons (Baraban and Tallent, 2004) and BNST GABAergic neurons have been shown to coexpress several neuropeptides, including vasoactive intestinal polypeptide, cholecystokinin, substance P, neurotensin, CRF, and methionine-enkephalin (Woodhams et al., 1983); hence, by modulating burst firing activity in BNST neurons, 5-HT could function to promote neuropeptide release from BNST GABAergic neurons.
Figure 8. An example showing that 5-HT changes the firing pattern of anterolateral BNST neurons without affecting membrane potential.
Although this neuron did not respond to 5-HT (50 μM) with a change in membrane potential (data not shown), the relative regular action potential firing induced by depolarizing current injection was dramatically altered to a higher frequency, burst firing pattern in the presence of 5-HT. The anterolateral BNST expresses many intrinsic membrane ion channels that could be modulated by 5-HT, and a reduction in A-type potassium channel function and/or an enhancement of T-type calcium channel function by 5-HT could enhance the excitability of BNST neurons upon depolarization to produce the phenotype observed here.
5-HT has also been shown to modulate Ih (Cardenas et al., 1999) and INaP (Carr et al., 2002); hence, 5-HT could dramatically alter the excitability of BNST neurons independent of changes in membrane potential by modulating any of these intrinsic membrane currents. It remains to be determined how the modulation of these intrinsic currents might alter anxiety-like behavior.
Conclusions
We have summarized anatomical, physiological, and behavioral data that suggest that 1) changes in anxiety-like behavior following manipulations of central 5-HT activity may be mediated by the BNST, 2) the response of the BNST to 5-HT is complex, such that BNST neurons can be inhibited by 5-HT1A receptor activation and excited by 5-HT2A, 5-HT2C, and/or 5-HT7 receptor activation, 3) most neurons in the BNST respond to 5-HT with concurrent inhibition and excitation, mediated by multiple 5-HT receptor subtypes, 4) BNST 5-HT1A activation is anxiolytic, and it is likely that the activation of BNST 5-HT2A, 5-HT2C and/or 5-HT7 receptors is anxiogenic, 5) pretreatment with the stress hormone, CRF or isolation stress can alter the 5-HT response profile in the BNST to favor pure inhibition, and 6) chronic restraint stress increases anxiety-like behavior and modulates the relative expression of BNST 5-HT receptor subtypes to favor excitation. Based on these data, we argue that 5-HT projections from the caudal DRN form a negative feedback loop with the anterolateral BNST in the face of a stressful anxiogenic stimulus, such that an anxiogenic stimulus would activate CRF neurons in the anterolateral BNST that project to and activated DRN 5-HT neurons. These activated caudal DRN neurons, in turn, release 5-HT within the anterolateral BNST, where 5-HT would have a predominantly inhibitory action due to recent BNST CRF receptor activation. We further argue that when exposure to stressful anxiogenic stimuli becomes chronic and/or extreme, the BNST response to 5-HT might shift to favor excitation, attenuating the negative-feedback function of 5-HT and potentially creating a positive-feedback loop from the same system. This response to chronic or extreme stress would be expected to produce a persistent anxiety-like state. In this way, interactions between the 5-HT neurons in the caudal DRN and neurons in the BNST may represent an important site of action for pharmacological therapies in the treatment of anxiety disorders in humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–97. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–19. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. International Journal of Neuropsychopharmacology. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Tallent MK. Interneuron Diversity series: Interneuronal neuropeptides--endogenous regulators of neuronal excitability. Trends in Neurosciences. 2004;27:135–42. doi: 10.1016/j.tins.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Beique JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. Journal of Neuroscience. 2004;24:4807–17. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, Ding J, Sciamanna G, Ulrich S, Tscherter A, Bernardi G, Surmeier DJ, Pisani A. Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT 2C, 5-HT6, and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology. 2007;32:1840–54. doi: 10.1038/sj.npp.1301294. [DOI] [PubMed] [Google Scholar]
- Bouryi VA, Lewis DI. The modulation by 5-HT of glutamatergic inputs from the raphe pallidus to rat hypoglossal motoneurones, in vitro. Journal of Physiology. 2003;553:1019–31. doi: 10.1113/jphysiol.2003.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broocks A, Meyer T, George A, Hillmer-Vogel U, Meyer D, Bandelow B, Hajak G, Bartmann U, Gleiter CH, Ruther E. Decreased neuroendocrine responses to meta-chlorophenylpiperazine (m-CPP) but normal responses to ipsapirone in marathon runners. Neuropsychopharmacology. 1999;20:150–61. doi: 10.1016/S0893-133X(98)00056-6. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–8. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Mar LP, Vysokanov AV, Arnold PB, Cardenas LM, Surmeier DJ, Scroggs RS. Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. Journal of Physiology. 1999;518:507–23. doi: 10.1111/j.1469-7793.1999.0507p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. Journal of Neuroscience. 2002;22:6846–55. doi: 10.1523/JNEUROSCI.22-16-06846.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull. 1991;27:207–12. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- Chapin EM, Andrade R. A 5-HT(7) receptor-mediated depolarization in the anterodorsal thalamus. I. Pharmacological characterization. Journal of Pharmacology & Experimental Therapeutics. 2001;297:395–402. [PubMed] [Google Scholar]
- Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. Journal of Neuroscience. 1986;6:2908–14. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeta S, Tucci S, Sandhu J, Williams AR, Rupniak NM, File SE. Anxiolytic actions of the substance P (NK1) receptor antagonist L-760735 and the 5-HT1A agonist 8-OH-DPAT in the social interaction test in gerbils. Brain Res. 2001;915:170–5. doi: 10.1016/s0006-8993(01)02846-3. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–15. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cornelio AM, Nunes-de-Souza RL. Anxiogenic-like effects of mCPP microinfusions into the amygdala (but not dorsal or ventral hippocampus) in mice exposed to elevated plus-maze. Behavioural Brain Research. 2007;178:82–9. doi: 10.1016/j.bbr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Crayton JW, Joshi I, Gulati A, Arora RC, Wolf WA. Effect of corticosterone on serotonin and catecholamine receptors and uptake sites in rat frontal cortex. Brain Research. 1996;728:260–2. doi: 10.1016/0006-8993(96)00189-8. [DOI] [PubMed] [Google Scholar]
- Davies MF, Deisz RA, Prince DA, Peroutka SJ. Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Research. 1987;423:347–52. doi: 10.1016/0006-8993(87)90861-4. [DOI] [PubMed] [Google Scholar]
- Davis M. Mescaline: excitatory effects on acoustic startle are blocked by serotonin2 antagonists. Psychopharmacology. 1987;93:286–91. doi: 10.1007/BF00187244. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behavioural Brain Research. 1993;58:175–98. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1997;352:1675–87. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–47. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Annals of the New York Academy of Sciences. 1999;877:281–91. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. Journal of Comparative Neurology. 2004;474:364–78. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- DuPont RL, Rice DP, Miller LS, Shiraki SS, Rowland CR, Harwood HJ. Economic costs of anxiety disorders. Anxiety. 1996;2:167–72. doi: 10.1002/(SICI)1522-7154(1996)2:4<167::AID-ANXI2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Egli RE, Winder DG. Dorsal and ventral distribution of excitable and synaptic properties of neurons of the bed nucleus of the stria terminalis. Journal of Neurophysiology. 2003;90:405–14. doi: 10.1152/jn.00228.2003. [DOI] [PubMed] [Google Scholar]
- Elena Castro M, Diaz A, del Olmo E, Pazos A. Chronic fluoxetine induces opposite changes in G protein coupling at pre and postsynaptic 5-HT1A receptors in rat brain. Neuropharmacology. 2003;44:93–101. doi: 10.1016/s0028-3908(02)00340-4. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. Journal of Neuroscience. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–32. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Farley J, Auerbach S. Protein kinase C activation induces conductance changes in Hermissenda photoreceptors like those seen in associative learning. [erratum appears in Nature 1986 Dec 18–31;324(6098):702] Nature. 1986;319:220–3. doi: 10.1038/319220a0. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–8. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, McKittrick CR, File SE, McEwen BS. Decreased 5-HT1A and increased 5-HT2A receptor binding after chronic corticosterone associated with a behavioural indication of depression but not anxiety. Psychoneuroendocrinology. 1997;22:477–491. doi: 10.1016/s0306-4530(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Ferretti C, Blengio M, Gamalero SR, Ghi P. Biochemical and behavioral changes induced by acute stress in a chronic variate stress model of depressioin: the effect of amitriptyline. Eur J Pharmacol. 1995;280:19–26. doi: 10.1016/0014-2999(95)00172-h. [DOI] [PubMed] [Google Scholar]
- Fraser DD, MacVicar BA. Low-threshold transient calcium current in rat hippocampal lacunosum-moleculare interneurons: kinetics and modulation by neurotransmitters. Journal of Neuroscience. 1991;11:2812–20. doi: 10.1523/JNEUROSCI.11-09-02812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LJ, Shi C. Monoaminergic innervation of the macaque extended amygdala. Neuroscience. 2001;104:1067–84. doi: 10.1016/s0306-4522(01)00157-9. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacology, Biochemistry & Behavior. 1996;54:129–41. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–60. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Annals of the New York Academy of Sciences. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha 1b-adrenergic receptor mRNA in the rat raphe nuclei. Biological Psychiatry. 2005;57:559–68. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: A fear-potentiated startle study. Neuropsychopharmacology. 2007;32:225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Aleksandrova IA, Otmakhova NA, Katkov YA, Nesterova IV, Bobkova NV. Effects of bulbectomy and subsequent antidepressant treatment on brain 5-HT2 and 5-HT1A receptors in mice. Pharmacology, Biochemistry & Behavior. 1993;45:65–70. doi: 10.1016/0091-3057(93)90087-a. [DOI] [PubMed] [Google Scholar]
- Haller J, Halasz J. Mild social stress abolishes the effects of isolation on anxiety and chlordiazepoxide reactivity. Psychopharmacology. 1999;144:311–5. doi: 10.1007/s002130051012. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. Journal of Neuroscience. 2002;22:1020–6. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Pepin JL, DesMarteau JS, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected into the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behavioural Brain Research. 2003a;147:55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003b;23:1019–25. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behavioral Neuroscience. 2004;118:443–8. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol. 2007;98:638–56. doi: 10.1152/jn.00382.2007. [DOI] [PubMed] [Google Scholar]
- Handley SL, McBlane JW, Critchley MA, Njung’e K. Multiple serotonin mechanisms in animal models of anxiety: environmental, emotional and cognitive factors. Behavioural Brain Research. 1993;58:203–10. doi: 10.1016/0166-4328(93)90104-x. [DOI] [PubMed] [Google Scholar]
- Handley SL. 5-Hydroxytryptamine pathways in anxiety and its treatment. Pharmacology & Therapeutics. 1995;66:103–48. doi: 10.1016/0163-7258(95)00004-z. [DOI] [PubMed] [Google Scholar]
- Hanson LA, Gorzalka BB, Brotto LA. The antidepressant, nefazodone, attenuates corticosterone-induced increases in 5-HT2A receptor-mediated behaviors in the female rat. European Journal of Pharmacology. 1998;342:163–5. doi: 10.1016/s0014-2999(97)01574-4. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Naciti C, Brand L, Stein DJ. Endocrine, cognitive and hippocampal/cortical 5HT 1A/2A receptor changes evoked by a time-dependent sensitisation (TDS) stress model in rats. Brain Research. 2003;983:97–107. doi: 10.1016/s0006-8993(03)03033-6. [DOI] [PubMed] [Google Scholar]
- Heidmann DE, Szot P, Kohen R, Hamblin MW. Function and distribution of three rat 5-hydroxytryptamine7 (5-HT7) receptor isoforms produced by alternative splicing. Neuropharmacology. 1998;37:1621–32. doi: 10.1016/s0028-3908(98)00070-7. [DOI] [PubMed] [Google Scholar]
- Hess G, Kuhnt U, Voronin LL. Quantal analysis of paired-pulse facilitation in guinea pig hippocampal slices. Neuroscience Letters. 1987;77:187–92. doi: 10.1016/0304-3940(87)90584-2. [DOI] [PubMed] [Google Scholar]
- Holmes MC, French KL, Seckl JR. Modulation of serotonin and corticosteroid receptor gene expression in the rat hippocampus with circadian rhythm and stress. Brain Research Molecular Brain Research. 1995;28:186–92. doi: 10.1016/0169-328x(94)00207-u. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacology, Biochemistry & Behavior. 2002;71:533–54. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behavioral Neuroscience. 2004;118:1052–61. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Kagaya A, Nakae S, Morinobu S, Yamawaki S. Modulation of serotonin2A receptor function in rats after repeated treatment with dexamethasone and L-type calcium channel antagonist nimodipine. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2001;25:1269–81. doi: 10.1016/s0278-5846(01)00179-8. [DOI] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. [erratum appears in Neuropsychopharmacology 2000 Apr;22(4):449] Neuropsychopharmacology. 2000;22:148–62. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. Journal of Neuroscience. 2008;28:12927–37. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen T, Riedel WJ, van Praag HM, Menheere PP, Griez E. Neuroendocrine response to meta-chlorophenylpiperazine and ipsapirone in relation to anxiety and aggression. Psychiatry Research. 2002;113:29–40. doi: 10.1016/s0165-1781(02)00250-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Foundation Symposium; 1993. pp. 277–89. discussion 290–5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Research. 1999;848:141–52. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Kshama D, Hrishikeshavan HJ, Shanbhogue R, Munonyedi US. Modulation of baseline behavior in rats by putative serotonergic agents in three ethoexperimental paradigms. Behavioral & Neural Biology. 1990;54:234–53. doi: 10.1016/0163-1047(90)90617-f. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Mikuni M, Ogawa T, Takahashi K. Effect of ACTH, adrenalectomy and the combination treatment on the density of 5-HT2 receptor binding sites in neocortex of rat forebrain and 5-HT2 receptor-mediated wet-dog shake behaviors. Psychopharmacology (Berl) 1992;108:27–32. doi: 10.1007/BF02245281. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Pardon MC, Laaris N, Joubert C, Hanoun N, Hamon M, Cohen-Salmon C. 5-HT1A autoreceptor desensitization by chronic ultramild stress in mice. Neuroreport. 1999;10:3369–74. doi: 10.1097/00001756-199911080-00021. [DOI] [PubMed] [Google Scholar]
- Laurent A, Goaillard JM, Cases O, Lebrand C, Gaspar P, Ropert N. Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. Journal of Neuroscience. 2002;22:886–900. doi: 10.1523/JNEUROSCI.22-03-00886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory: in search of systems and synapses. Ann N Y Acad Sci. 1993;702:149–57. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–46. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hammack SE, Mania I, Li XY, Davis M, Rainnie DG. 5-hydroxytryptamine1A-like receptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience. 2004;128:583–96. doi: 10.1016/j.neuroscience.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. Regulation of 5-HT1a receptor, glucocorticoid and mineralocorticoid receptor in rat and human hippocampus: Implications fo the neurobiology of depression. Biol Psychiatry. 1998;43:543–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Liberzon I, Vazquez DM, Young EA, Watson SJ. Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress. Biological Psychiatry. 1999;45:934–7. doi: 10.1016/s0006-3223(98)00224-8. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. Journal of Neuroscience. 2000;20:7728–36. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–46. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Annals of the New York Academy of Sciences. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lund A, Mjellem-Jolly N, Hole K. Desipramine, administered chronically, influences 5-hydroxytryptamine1A-receptors, as measured by behavioral tests and receptor binding in rats. Neuropharmacology. 1992;31:25–32. doi: 10.1016/0028-3908(92)90156-j. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience & Biobehavioral Reviews. 2005;29:829–41. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maines LW, Keck BJ, Smith JE, Lakoski JM. Corticosterone regulation of serotonin transporter and 5-HT1A receptor expression in the aging brain. Synapse. 1999;32:58–66. doi: 10.1002/(SICI)1098-2396(199904)32:1<58::AID-SYN8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Research. 1994;657:141–9. doi: 10.1016/0006-8993(94)90961-x. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. Journal of Neurophysiology. 1993;70:1451–9. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. Excitation of interneurons in piriform cortex by 5-hydroxytryptamine: blockade by MDL 100,907, a highly selective 5-HT2A receptor antagonist. European Journal of Pharmacology. 1994;259:137–41. doi: 10.1016/0014-2999(94)90502-9. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. The amygdala and fear conditioning: has the nut been cracked? Neuron. 1996;16:237–40. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biological Psychiatry. 1995;37:383–93. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- Mengod G, Nguyen H, Le H, Waeber C, Lubbert H, Palacios JM. The distribution and cellular localization of the serotonin 1C receptor mRNA in the rodent brain examined by in situ hybridization histochemistry. Comparison with receptor binding distribution. Neuroscience. 1990;35:577–591. doi: 10.1016/0306-4522(90)90330-7. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Bourin M, Hascoet M. Anxiolytic-like effects of 5-HT2 ligands on three mouse models of anxiety. Behavioural Brain Research. 2003;140:203–14. doi: 10.1016/s0166-4328(02)00311-x. [DOI] [PubMed] [Google Scholar]
- Ossowska G, Nowa G, Kata R, Klenk-Majewska B, Danilczuk Z, Zebrowska-Lupina I. Brain monoamine receptors in a chronic unpredictable stress model in rats. J Neural Transm. 2001;108:311–319. doi: 10.1007/s007020170077. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–68. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Serotonin-CRF interaction in the bed nucleus of the stria terminalis: a light microscopic double-label immunocytochemical analysis. Brain Research Bulletin. 1992;28:943–8. doi: 10.1016/0361-9230(92)90217-l. [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. British Journal of Pharmacology. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. Journal of Neuroscience. 2001;21:2833–41. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG. Neurons of the bed nucleus of the stria terminalis (BNST). Electrophysiological properties and their response to serotonin. Annals of the New York Academy of Sciences. 1999;877:695–9. doi: 10.1111/j.1749-6632.1999.tb09304.x. [DOI] [PubMed] [Google Scholar]
- Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. Journal of Neuroscience. 2004;24:5420–6. doi: 10.1523/JNEUROSCI.0950-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, McGeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology. 2006;186:122–32. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Baba A, Matsuda T. The 5-HT1A receptor agonist MKC-242 reverses isolation rearing-induced deficits of prepulse inhibition in mice. Psychopharmacology. 2003;170:73–9. doi: 10.1007/s00213-003-1515-x. [DOI] [PubMed] [Google Scholar]
- Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Verge D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: Immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1998;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Federation Proceedings. 1985;44:221–7. [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–43. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Mostallino MC, Sanna E, Biggio G. Changes in neuroactive steroid content during social isolation stress modulate GABAA receptor plasticity and function. Brain Research Reviews. 2008;57:520–30. doi: 10.1016/j.brainresrev.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Sheldon PW, Aghajanian GK. Excitatory responses to serotonin (5-HT) in neurons of the rat piriform cortex: evidence for mediation by 5-HT1C receptors in pyramidal cells and 5-HT2 receptors in interneurons. Synapse. 1991;9:208–18. doi: 10.1002/syn.890090307. [DOI] [PubMed] [Google Scholar]
- Short KR. Uncontrollable stress induces both anxiety and downregulation of dorsal raphe 5-HT1A receptors in rats: Both effects follow the same timecourse. Society for Neuroscience. 2000;26:2267. [Google Scholar]
- Singewald N, Sharp T. Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by Fos immunocytochemistry. Neuroscience. 2000;98:759–70. doi: 10.1016/s0306-4522(00)00177-9. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biological Psychiatry. 2003;53:275–83. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Responses of hippocampal pyramidal cells to putative serotonin 5-HT1A and 5-HT1B agonists: a comparative study with dorsal raphe neurons. Neuropharmacology. 1988;27:707–15. doi: 10.1016/0028-3908(88)90079-2. [DOI] [PubMed] [Google Scholar]
- Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Research. 2005;1044:176–89. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–36. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Strome EM, Clark CM, Zis AP, Doudet DJ. Electroconvulsive shock decreases binding to 5-HT2 receptors in nonhuman primates: an in vivo positron emission tomography study with [18F]setoperone. Biological Psychiatry. 2005;57:1004–10. doi: 10.1016/j.biopsych.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Takao K, Nagatani T, Kitamura Y, Yamawaki S. Effects of corticosterone on 5-HT1A and 5-HT2 receptor binding and on the receptor-mediated behavioral responses of rats. Eur J Pharmacol. 1997;333:123–128. doi: 10.1016/s0014-2999(97)01126-6. [DOI] [PubMed] [Google Scholar]
- To ZP, Bonhaus DW, Eglen RM, Jakeman LB. Characterization and distribution of putative 5-HT7 receptors in guinea-pig brain. Br J Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarski K, Zahorodna A, Bobula B, Hess G. 5-HT7 receptors increase the excitability of rat hippocampal CA1 pyramidal neurons. Brain Research. 2003;993:230–4. doi: 10.1016/j.brainres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Uphouse L. Multiple serotonin receptors: too many, not enough, or just the right number? Neurosci Biobehav Rev. 1997;21:679–98. doi: 10.1016/s0149-7634(96)00022-x. [DOI] [PubMed] [Google Scholar]
- Van den Hooff P, Galvan M. Actions of 5-hydroxytryptamine and 5-HT1A receptor ligands on rat dorso-lateral septal neurones in vitro. British Journal of Pharmacology. 1992;106:893–9. doi: 10.1111/j.1476-5381.1992.tb14431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behavioral Neuroscience. 2006;120:324–36. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Nieoullon A, Bockaert J, Dumuis A. Regional distribution and ontogeny of 5-HT4 binding sites in rodent brain. Neuropharmacology. 1994;33:527–541. doi: 10.1016/0028-3908(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Waeber C, Moskowitz MA. [3H]sumatriptan labels both 5-HT1D and 5-HT1F receptor binding sites in the guinea pig brain: an autoradiographic study. Naunyn-Schmiedeberg’s Arch Pharmacol. 1995;352:263–275. doi: 10.1007/BF00168556. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Structure & Function. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Williams JT, Colmers WF, Pan ZZ. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. Journal of Neuroscience. 1988;8:3499–506. doi: 10.1523/JNEUROSCI.08-09-03499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Licinio J, Pasternak KI, Gold PW. Localization of corticotropin-releasing hormone (CRH) receptor mRNA in adult rat brain by in situ hybridization histochemistry. Endocrinology. 1994;135:2275–8. doi: 10.1210/endo.135.5.7956950. [DOI] [PubMed] [Google Scholar]
- Woodhams PL, Roberts GW, Polak JM, Crow TJ. Distribution of neuropeptides in the limbic system of the rat: the bed nucleus of the stria terminalis, septum and preoptic area. Neuroscience. 1983;8:677–703. doi: 10.1016/0306-4522(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Young AH, MacDonald LM, St John H, Dick H, Goodwin GM. The effects of corticosterone on 5-HT receptor function in rodents. Neuropharmacology. 1992;31:433–8. doi: 10.1016/0028-3908(92)90080-9. [DOI] [PubMed] [Google Scholar]
- Zifa E, Fillion G. 5-Hydroxytryptamine receptors. Pharmacological Reviews. 1992;44:401–58. [PubMed] [Google Scholar]