Abstract
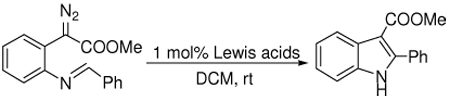
Lewis acids catalyze the cyclization of methyl phenyldiazoacetates with an ortho-imino group, prepared from o-aminophenylacetic acid, to give 2,3-substituted indoles in quantitative yields.
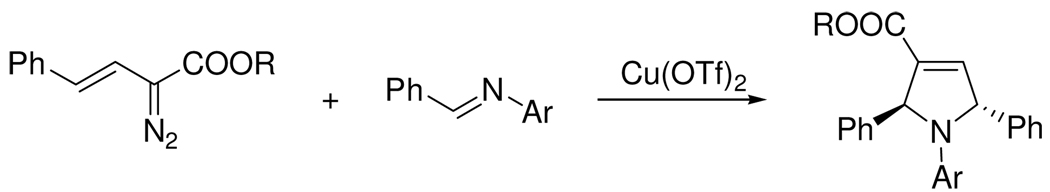
Indoles are referred as “privileged structures” in drug discovery because of their capacity of binding to many receptors with high affinity. The synthesis of indoles has been of considerable interest to organic chemists for more than a century.1 Many powerful methodologies for the synthesis of these heterocycles have been developed, including the Fisherindole synthesis,2 heteroannulation and cyclization of 2-alkynylanilines,3 a metal-catalyzed cascade reaction,4 intramolecular C-H amination of azidoacrylates,5 and a domino reaction of N-aryl amides with ethyl diazoacetate.6 However, efficient methods for the synthesis of indoles from simple precursors continue to be of great value. Diazoacetates and aryldiazoacetates have been widely employed under catalytic conditions for many organic transformations, including cyclopropanation,7 insertion,8 and ylide formation and subsequent reactions.9,10 Aziridination using imine and diazoacetate catalyzed by either Lewis acids11 or Brønsted acids12 is also well-known. In 2003 we reported the synthesis of dihydropyrroles from vinyldiazoacetates and imines, and in that report we provided evidence that copper(II) catalyzed formation of 2,5-dihydropyrroles by activation of the imine towards electrophilic addition to the diazo carbon of the vinyldiazoacetate (Scheme 1).13 We now report an intramolecular analog of the imine-diazoacetate reaction that can be performed with a broad selection of inexpensive Lewis acids with catalyst loadings at/or below 1.0 mol %.
Scheme 1.
Copper(II)-catalyzed Formation of 2,5-Dihydropyrroles
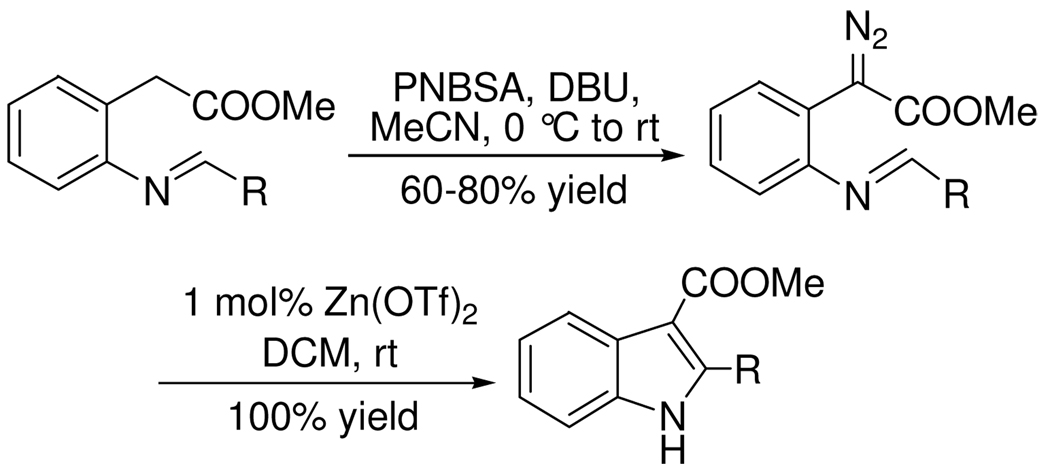
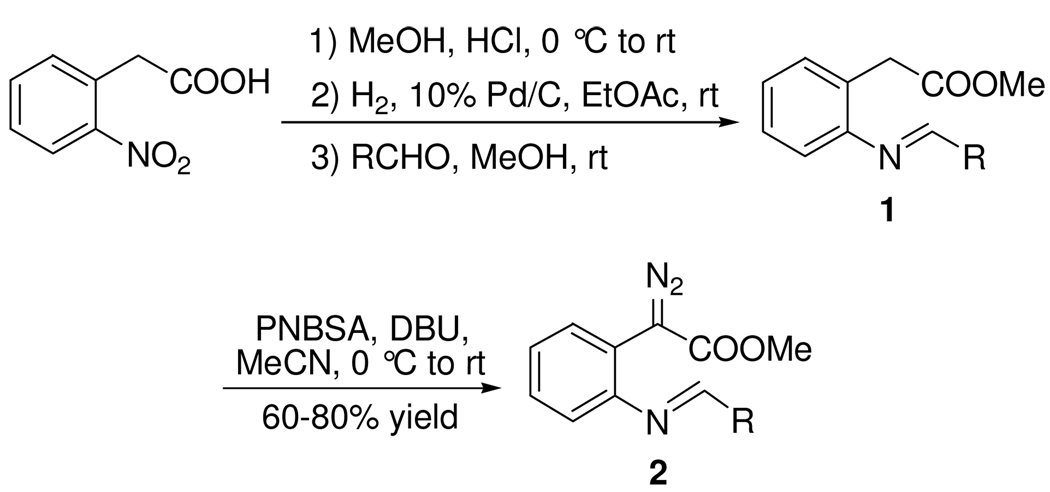
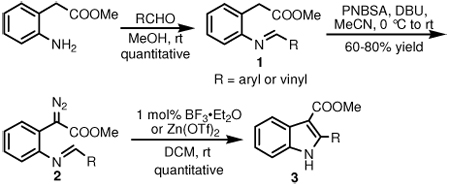
Methyl N-substituted iminophenyldiazoacetates (2) were synthesized efficiently by reactions of p-nitrobenzenesulfonylazide (PNBSA), DBU and methyl 2-arylmethyleneaminophenylacetates (1) that were prepared in quantitative yield from methyl o-aminophenylacetate and aryl and vinyl-substituted aldehydes (Scheme 2). In the simplest case methyl diazo(2-phenylmethyleneaminophenyl)acetate was treated with a broad spectrum of Lewis acids, each at 1.0 mol %. Reaction times and product yields are given in Table 1. Reaction times were determined by monitoring the reaction solution every five minutes. As can be seen from these results, a wide variety of catalysts are suitable, but two were selected for further investigation. No reaction occurred in the absence of acid.
Scheme 2.
Synthesis of Methyl N-Substituted Iminophenyldiazoacetates
Table 1.
Cyclization of Methyl diazo(2-phenylmethyleneaminophenyl)acetate Catalyzed by Lewis Acids
 | |||
|---|---|---|---|
| entry | Lewis acid 1 mol% |
reaction time (min) |
yield (%) |
| 1 | ZnCl2 | 60 | very low conversion |
| 2 | BF3•Et2O | 10 | 100 |
| 3 | TiCl4 | 5 | 100 |
| 4 | SnCl4 | 10 | 100 |
| 5 | Mg(OTf)2 | 60 | no conversion |
| 6 | Sn(OTf)2 | 15 | 100 |
| 7 | In(OTf)3 | 5 | 100 |
| 8a | Zn(OTf)2 | 40 | 100 |
| 9 | Ni(OTf)2 | 15 | 100 |
| 10 | Cu(OTf)2 | 5 | 100 |
| 11 | Sc(OTf)3 | 5 | 100 |
| 12 | La(OTf)3 | 60 | very low conversion |
| 13 | Yb(OTf)3 | 20 | 100 |
Reaction was complete in less than 10 min when 5 mol% Zn(OTf)2 was used; reaction was complete in 10 h when 0.1 mol% Zn(OTf)2 was used.
No attempt was made to rigorously dry the Lewis acids employed, so different times could be suitable for complete reaction dependent on humidity and other relevant conditions. The use of lower amounts of catalyst was investigated. For instance, reaction was complete within 40 min when 1 mol % zinc triflate was used, but was complete at only 10 min when 5 mol % zinc triflate was employed. However, when the reaction is performed in the presence of only 0.1 mol % zinc triflate, for complete reaction 10 h was required.
The scope of the reaction with representative methyl 2-arylmethyleneaminophenylacetates (1) was examined. The reactant diazo compounds (2) were prepared according to Scheme 2 in 60–80% overall yields (Table 2). Cyclization catalyzed by zinc triflate or boron trifluoride etherate furnished indoles 3 in quantitative yields.
Table 2.
Indole Synthesis via Intramolecular Nucleophilic Attack of Imines by Phenyldiazoacetates
 | |||
|---|---|---|---|
| entry | 3 | overall yield (%)a |
|
| BF3·Et2O | Zn(OTf)2 | ||
| a | 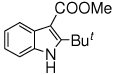 |
77 | 77 |
| b | 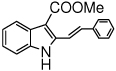 |
71 | 71 |
| c | 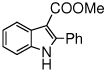 |
80 | 80 |
| d | 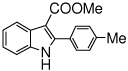 |
72 | 72 |
| e | 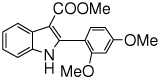 |
73 | 73 |
| f | 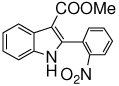 |
65 | 65 |
| g | 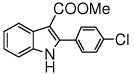 |
65 | 65 |
| h | 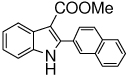 |
70 | 70 |
| i | 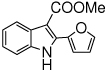 |
60 | 60 |
Calculation of overall yield is based on compound 1.
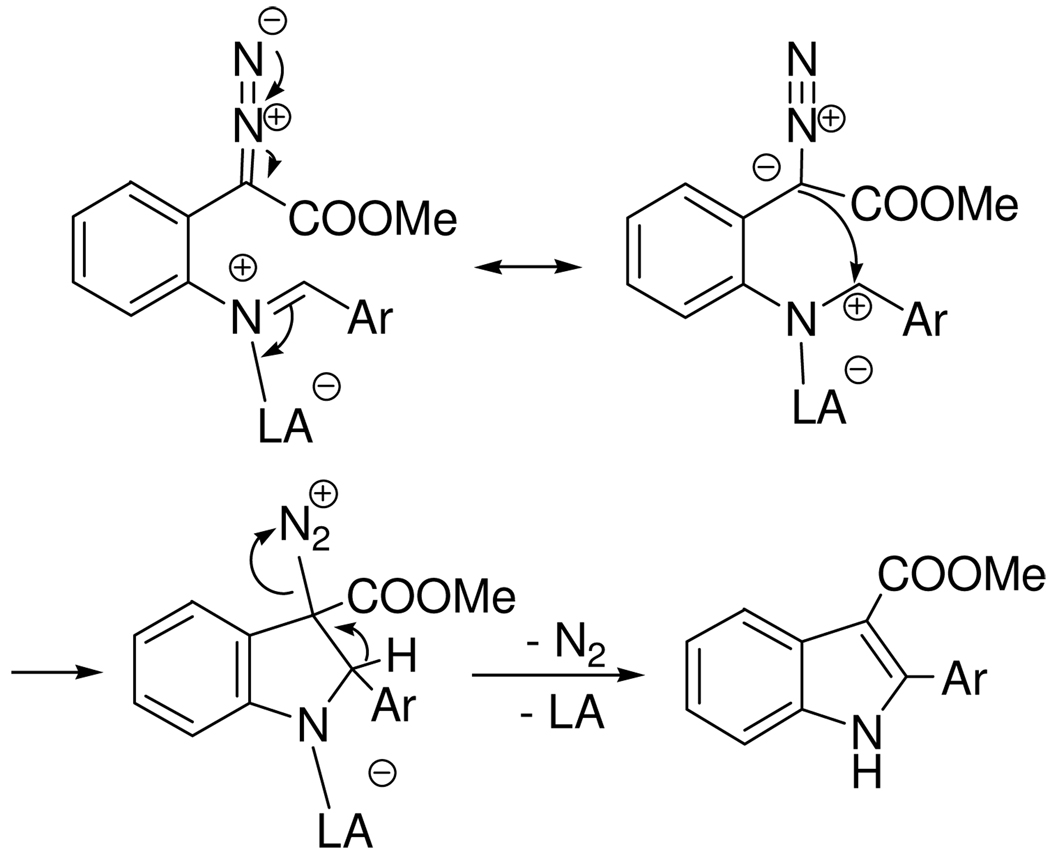
Consistent with these observations, the mechanism of the cyclization involves activation of the imine by the Lewis acid for electrophilic attack on the diazo carbon to form a diazonium ion intermediate. Expulsion of N2 and dissociation of the Lewis acid furnishes the product indole (Scheme 3). The difference in base strength between imine and indole is the driving force for the highly efficient catalytic turnover.
Scheme 3.
Mechanism of Lewis Acid-promoted Indole Synthesis
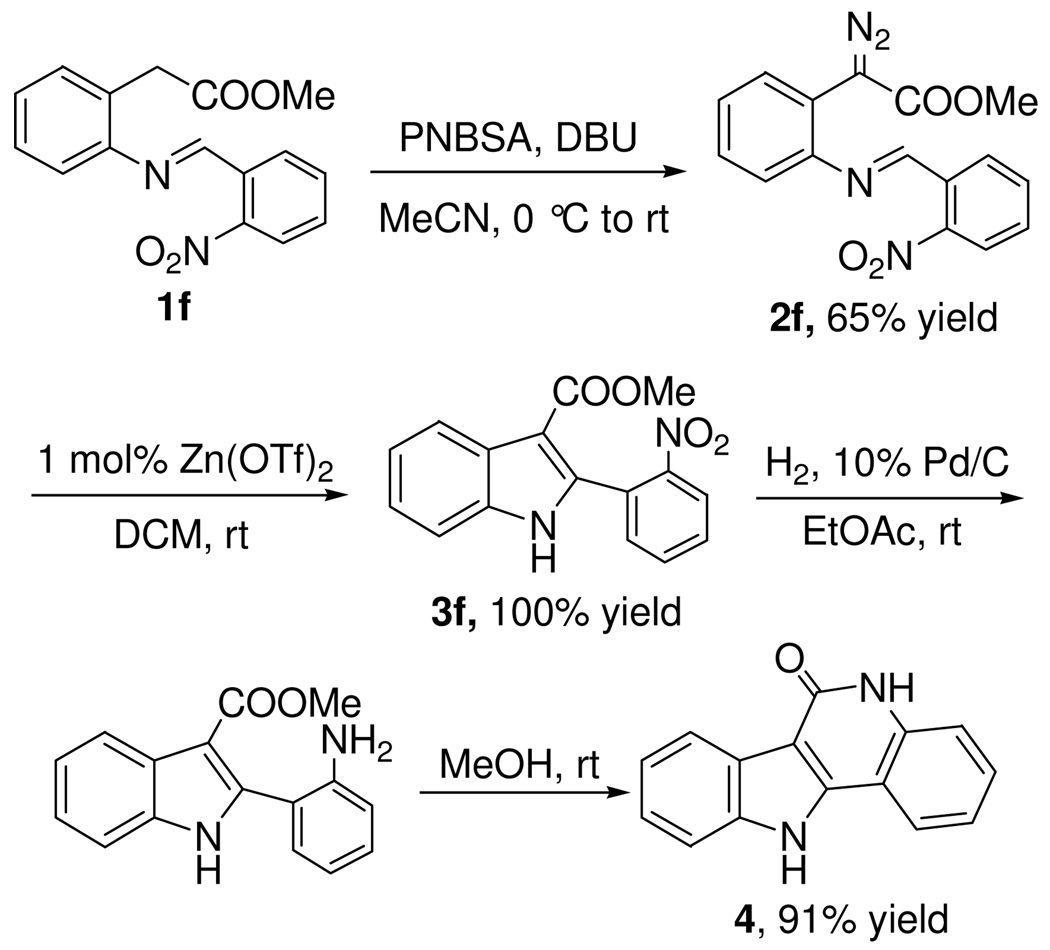
A demonstration of the synthetic utility of this methodology is shown in Scheme 4. Indole 3f was converted to biologically active14 indolo-quinolin-2(1H)-one 4 in high yield following reduction of the nitro group and cyclization in methanol. This synthetic strategy is highly efficient compared with the published method,15 which starts from the reaction of hydrozine with 4-hydroxyquinoline-2(1H)-one, followed by treatment with cyclohexanone, thermal Fischer indolization and dehydrogenation to afford indoloquinoline-2(1H)-one in low yield.
Scheme 4.
Synthesis of Indolo-quinolin-2(1H)-one
In summary, we have developed a general and efficient synthesis of functionalized indoles from Lewis acid-promoted intramolecular nucleophilic attack of imines by phenyldiazoacetates. Reaction conditions are mild, and product yields are good under low catalyst loading with inexpensive, commercially available Lewis acids. The approach proves to be very useful for the synthesis of biologically active and naturally occurring indole derivatives. Efforts are actually directed toward the extension of this methodology to natural products and drug synthesis.
Experimental Section
General Procedure for Diazo compound 2 Synthesis (Table 2)
To a stirred solution of 1 (1 mmol) and PNBSA (2–3 mmol) in MeCN (5 mL) was added DBU (4–6 mmol) at 0°C. The reaction mixture was then allowed to warm to room temperature. After stirring for 12 h, the reaction mixture was quenched with aqueous NH4Cl, extracted with diethyl ether, and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and purified by flash column chromatography on silica gel to give the corresponding diazo compound 2.
General Procedure for Indole 3 Synthesis (Table 2)
To a stirred solution of 2 (0.25 mmol) in DCM (5 mL) was added boron trifluoride etherate, or zinc triflate (1 mol%) at room temperature. The yellow reaction mixture was stirred until it turned pale yellow or colorless within 10–30 min. The mixture was then passed through a silica gel plug to remove the catalyst. After evaporation of the solvent, pure indole 3 was obtained as a light yellow solid in quantitative yield.
Supplementary Material
Acknowledgment
We gratefully acknowledge the financial support provided by the National Institutes of Health (GM46503) and the National Science Foundation.
Footnotes
Supporting Information Available: General experimental procedures and spectroscopic data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Saxton JE. Nat. Prod. Rep. 1997;14:559–590. [Google Scholar]; (b) Robinson B. Chem. Rev. 1963;63:370–401. [Google Scholar]
- 2.(a) Wagaw S, Yang BH, Buchwald SL. J. Am. Chem. Soc. 1999;121:10251–10263. [Google Scholar]; (b) Alex K, Tillack A, Schwarz N, Beller M. Angew. Chem., Int. Ed. 2008;47:2304–2307. doi: 10.1002/anie.200703823. [DOI] [PubMed] [Google Scholar]
- 3.(a) Arcadi A, Cacchi S, Fabrizi G, Marinelli F, Parisi LM. J. Org. Chem. 2005;70:6213–6217. doi: 10.1021/jo050517z. [DOI] [PubMed] [Google Scholar]; (b) Trost BM, McClory A. Angew. Chem., Int. Ed. 2007;46:2074–2077. doi: 10.1002/anie.200604183. [DOI] [PubMed] [Google Scholar]; (c) Li GT, Huang XG, Zhang LM. Angew. Chem., Int. Ed. 2008;47:346–349. doi: 10.1002/anie.200702931. [DOI] [PubMed] [Google Scholar]; (d) Carious K, Ronan B, Mignani S, Fensterbank L, Malacria M. Angew. Chem., Int. Ed. 2007;46:1881–1884. doi: 10.1002/anie.200604026. [DOI] [PubMed] [Google Scholar]; (e) Nakamura I, Yamagishi U, Song D, Konta S, Yamamoto Y. Angew. Chem., Int. Ed. 2007;46:2284–2287. doi: 10.1002/anie.200604038. [DOI] [PubMed] [Google Scholar]; (f) Ohno H, Ohta Y, Oishi S, Fujii N. Angew. Chem., Int. Ed. 2007;46:2295–2298. doi: 10.1002/anie.200604342. [DOI] [PubMed] [Google Scholar]
- 4.(a) Dunetz JR, Danheiser RL. J. Am. Chem. Soc. 2005;127:5776–5777. doi: 10.1021/ja051180l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jensen T, Pedersen H, Bang-Andersen B, Madsen R, Jørgensen M. Angew. Chem., Int. Ed. 2008;47:888–890. doi: 10.1002/anie.200703763. [DOI] [PubMed] [Google Scholar]; (c) Leogane O, Lebel H. Angew. Chem., Int. Ed. 2008;47:350–352. doi: 10.1002/anie.200703671. [DOI] [PubMed] [Google Scholar]; (d) Barluenga J, Jime´nez-Aquino A, Valde´s C, Aznar F. Angew. Chem., Int. Ed. 2007;46:1529–1532. doi: 10.1002/anie.200604407. [DOI] [PubMed] [Google Scholar]; (e) Chen Y, Wang YJ, Sun ZM, Ma DW. Org. Lett. 2008;10:625–628. doi: 10.1021/ol7029382. [DOI] [PubMed] [Google Scholar]; (f) Fayol A, Fang YQ, Lautens M. Org. Lett. 2006;8:4203–4206. doi: 10.1021/ol061374l. [DOI] [PubMed] [Google Scholar]; (g) Coleman CM, O’Shea DF. J. Am. Chem. Soc. 2003;125:4054–4055. doi: 10.1021/ja034283h. [DOI] [PubMed] [Google Scholar]
- 5.Stokes BJ, Dong H-J, Leslie BE, Pumphrey AL, Driver TG. J. Am. Chem. Soc. 2007;129:7500–7501. doi: 10.1021/ja072219k. [DOI] [PubMed] [Google Scholar]
- 6.Cui SL, Wang J, Wang YG. J. Am. Chem. Soc. 2008;130:13526–13527. doi: 10.1021/ja805706r. [DOI] [PubMed] [Google Scholar]
- 7.(a) Davies HML, Venkataramani C. Org. Lett. 2003;5:1403–1406. doi: 10.1021/ol034002a. [DOI] [PubMed] [Google Scholar]; (b) Nagashima T, Davies HML. Org. Lett. 2002;4:1989–1992. doi: 10.1021/ol025758x. [DOI] [PubMed] [Google Scholar]; (c) Nagashima T, Davies HML. J. Am. Chem. Soc. 2001;123:2695–2696. doi: 10.1021/ja005776e. [DOI] [PubMed] [Google Scholar]
- 8.(a) Zhu SF, Chen C, Cai Y, Zhou QL. Angew. Chem., Int. Ed. 2008;47:932–934. doi: 10.1002/anie.200704651. [DOI] [PubMed] [Google Scholar]; (b) Chen C, Zhu SF, Liu B, Wang LX, Zhou QL. J. Am. Chem. Soc. 2007;129:12616–12617. doi: 10.1021/ja074729k. [DOI] [PubMed] [Google Scholar]; (c) Maier TC, Fu GC. J. Am. Chem. Soc. 2006;128:4594–4595. doi: 10.1021/ja0607739. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Liu B, Zhu SF, Zhang W, Chen C, Zhou QL. J. Am. Chem. Soc. 2007;129:5834–5835. doi: 10.1021/ja0711765. [DOI] [PubMed] [Google Scholar]
- 9.(a) Doyle MP, Hu WH, Timmons DJ. Org. Lett. 2001;3:933–935. doi: 10.1021/ol015600x. [DOI] [PubMed] [Google Scholar]; (b) Doyle MP, Hu WH, Timmons DJ. Org. Lett. 2001;3:3741–3744. doi: 10.1021/ol016703i. [DOI] [PubMed] [Google Scholar]; (c) Doyle MP, Forbes DC, Vasbinder MM, Peterson CS. J. Am. Chem. Soc. 1998;120:7653–7654. [Google Scholar]
- 10.(a) Xu XF, Zhou J, Yang LP, Hu WH. Chem. Comm. 2008:6564–6566. doi: 10.1039/b816104f. [DOI] [PubMed] [Google Scholar]; (b) Zhang X, Huang HX, Guo X, Guan XY, Yang LP, Hu WH. Angew. Chem., Int. Ed. 2008;47:6647–6649. doi: 10.1002/anie.200801510. [DOI] [PubMed] [Google Scholar]
- 11.(a) Antilla JC, Wulff WD. J. Am. Chem. Soc. 1999;121:5099–5100. [Google Scholar]; (b) Antilla JC, Wulff WD. Angew. Chem., Int. Ed. 2000;39:4518–4521. [PubMed] [Google Scholar]; (c) Patwardhan AP, Pulgam VR, Zhang Y, Wulff WD. Angew. Chem., Int. Ed. 2005;44:6169–6172. doi: 10.1002/anie.200500923. [DOI] [PubMed] [Google Scholar]; (d) Lu Z, Zhang Y, Wulff WD. J. Am. Chem. Soc. 2007;129:7185–7194. doi: 10.1021/ja069371r. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Uchiyama N, Maruoka K. J. Am. Chem. Soc. 2008;130:14380–14381. doi: 10.1021/ja805635c. [DOI] [PubMed] [Google Scholar]
- 13.Doyle MP, Yan M, Hu WH, Gronenberg LS. J. Am. Chem. Soc. 2003;125:4692–4693. doi: 10.1021/ja029745q. [DOI] [PubMed] [Google Scholar]
- 14.Tominaga M, Tone H, Nakagawa K, Takada K, Hoshino Y, Watanabe K. Chem. Pharm. Bull. 1981;29:2166. doi: 10.1248/cpb.29.2166. [DOI] [PubMed] [Google Scholar]
- 15.(a) Chen YL, Chung CH, Chen IL, Chen PH, Jeng HY. Bioorganic Medicinal Chemistry. 2002;10:2705–2712. doi: 10.1016/s0968-0896(02)00111-6. [DOI] [PubMed] [Google Scholar]; (b) Bergman J, Engqvist R, Stalhandske C, Wallberg Hans. Tetrahedron. 2003;59:1033–1048. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.