Abstract
VH1/BRL2 is a receptor-like kinase of the BRI1 family with a role in vascular development. In developing Arabidopsis leaves, it is expressed first in ground cells and then becomes restricted to provascular and procambial cells as venation forms. We isolated proteins interacting with the activated (phosphorylated) cytoplasmic domain of VH1/BRL2, and found that most belong to three processes: proteasome activity, vesicle traffic and intracellular signal transduction. Included are two adaptor proteins, that we named VIT (VH1 interacting TPR containing protein) and VIK (VH1 interacting kinase), which are co-expressed in the same cells as VH1/BRL2 at two distinct time points in vein differentiation. Mutation of either adaptor or of VH1 results in vein pattern defects, and in alterations in response to auxin and brassinosteroids. We propose that these two adaptors facilitate the diversification and amplification of a ligand signal perceived by VH1/BRL2 in multiple downstream pathways affecting venation.
Introduction
The receptor-like kinase (RLK) class of the Pelle family forms the largest family of membrane-bound receptors in plants, with more than 600 members in Arabidopsis thaliana and over 1100 in rice (Shiu et al., 2004). RLKs are characterized by an extracellular portion devoted to ligand-binding, a single spanning transmembrane domain and a cytoplasmic kinase. The specific ligands that activate individual RLKs are diverse in structure and include peptides (e.g., bacterial flagellin and systemin), steroids (e.g., brassinosteroids), small secreted proteins (e.g., CLV3), and membrane-bound proteins (Torii, 2004; Escobar-Restrepo et al., 2007). Downstream partners are diverse, often including a kinase-associated protein phosphatase (KAPP) (Johnson and Ingram, 2005), other RLKs (e.g., Karlova et al., 2006; Li et al., 2002), calmodulins (Johnson and Ingram, 2005), and transcription factors (e.g., Fujita et al., 2003, Rienties et al., 2005).
The large size of the plant RLK gene family appears to reflect diversity, rather than redundancy. Individual RLKs have roles in processes as diverse as carpel development, epidermis differentiation, embryogenic competence, shoot apical meristem activity, and resistance to pathogens (Torii, 2004; Morillo and Tax, 2006). Even the relatively similar members within RLK subfamilies generally play specialized and not overlapping roles (Morillo and Tax, 2006).
The subfamily of four Brassinosteroid Insensitive (BRI) leucine-rich repeat (LRR) RLKs is a striking example of this functional diversity. BRI1 was isolated in a genetic screen for Arabidopsis plants insensitive to brassinosteroids (Clouse et al., 1996). Three genes are homologous to BRI: BRL1, VH1/BRL2 and BRL3, each characterized by the presence of a ~ 70 amino acid island that interrupts the extracellular LRR (Li and Chory, 1997; Clay and Nelson, 2002; Nakamura et al., 2006a). In the case of BRI1, the island has been shown to be essential for ligand-binding (Li, 2005). The VH1/BRL2 gene differs from other subfamily members in rice and Arabidopsis in the sequence of its island (Nakamura et al., 2006) and by its inability to bind brassinosteroids (Caño-Delgado et al., 2004). Although the BRL genes exhibit similar expression patterns, BRL1 and BRL3 influence xylem/phloem ratio in vascular bundles and rescue the bri1 mutant when expressed under the BRI1 promoter (Caño-Delgado et al., 2004; Zhou et al., 2004), while VH1/BRL2 affects the development of provascular cells and cannot substitute for other family members (Clay and Nelson, 2002; Caño-Delgado et al., 2004).
Two partners of the BRI1 kinase domain were identified by yeast two-hybrid interactions: TTL (a transthyretin-like protein) (Nam and Li, 2004; Wang and Chory, 2006) and BIK (Wang and Chory, 2006). TTL interacts with the active form of the receptor and acts as a negative regulator of brassinosteroid-related plant growth (Nam and Li, 2004; Wang and Chory, 2006); BIK associates with the inactive form of BRI1 and represses the signaling pathway activated by this RLK (Wang and Chory, 2006). Additional BRI1 downstream partners were identified via genetic interactions including BIN2 (a glycogen synthase kinase 3 or Shaggy like kinase), BES1 and BZR1 (two homologous transcription factors), and BSU1 (a threonine phosphatase). Active BRI1 forms a complex with another RLK, BAK1 (Li, 2005). All of these are likely members of the BR response pathway.
We investigated the apparently distinct downstream pathways activated by VH1/BRL2 by identifying interaction partners of its intracellular domain, in which the kinase was activated in vitro. Based on the annotated identities of 33 of the 66 isolated, VH1/BRL2 RLK appears to be linked to targeted protein degradation, vesicle trafficking and signal transduction.
We focused on two of the candidate partners likely to function in downstream signal transduction: a TPR-containing protein, VIT (for VH1-Interacting TPR-containing protein) and a MAP kinase kinase kinase, VIK (for VH1-Interacting Kinase). The predicted structures of both VIT and VIK suggest that they are modular proteins working as adaptors joining VH1/BRL2 to other signal partners. The expression patterns of VIT and VIK and the altered sensitivity of vit and vik insertion mutants to auxin and brassinosteroids are consistent with roles as adaptors. Based on mutant phenotypes, the receptor VH1/BRL2 and its partner VIT appear to be required first during the initiation of continuous vascular strands, while VIK appears to be required at a later stage of vascular development. Thus, the VH1/BRL2 adaptors appear to link the unknown ligand signal bound by the VH1 receptor to several downstream intracellular pathways, including a MAPK cascade, proteasome activity, and regulation of metabolism and transcription.
Results
Isolation of interaction candidates for the VH1/BRL2 kinase domain
The intracellular portion of the VH1/BRL2 protein includes canonical kinase amino acid motifs joined at the carboxyl end to a unique region likely to mediate specific interactions (Clay and Nelson, 2002). We used a 998-amino acid C-terminal fragment fused to a maltose binding protein (MBP) tag as probe against phage libraries expressing Arabidopsis seedling proteins. We chose this in vitro approach to enable us to control kinase activation state, which may influence significant interactions. When the VH1/BRL2kin domain was used as probe in its activated (phosphorylated) state, 66 interaction candidates were recovered, including the VH1/BRL2 protein itself, which were categorized as strong, medium and weak interactors (Table I, Supplementary Tables SI and SII).
Table I.
VHI/BRL2kin interaction candidates with strong signal strength. Column I: gene number; II: annotation from TAIR and NCBI web sites (http://www.arabidopsis.org/Blast/ and http://www.ncbi.nlm.nih.gov/BLAST/); III: presence of signal peptide (sp), transmembrane domain (tmd), or posttranslational modification (ptm) which are predicted to target the protein to membranes; IV: number of serine (S), threonine (T) and tyrosine (Y) embedded in phosphorylation target sequences from the DIPHOSPHO database (http://core.ist.temple.edu/pred/pred.html) (Diella et al., 2004); V: prediction of kinase recognition a: basophilic serine/threonine kinase group; b: kinase binding site group; c: acidophilic serine/threonine kinase group; d: phosphoserine/threonine binding group. H: high stringency (0.2 top percent); M: medium stringency (1 top percent).
| I | II | III | IV | V | |||||
|---|---|---|---|---|---|---|---|---|---|
| S | T | Y | a | b | c | d | |||
| At1g12440 | AN1-like/ubiquitin-like | 7 | M | ||||||
| At1g13750 | Calcineurin-like phosphatase | tmd | 1 | M | H | M | |||
| At1g14000 | VIK/MAPKKK | 4 | M | M | |||||
| At1g61740 | Unknown | sp, tmd | 1 | M | M | M | |||
| At1g68220 | Unknown | sp, tmd | H | M | |||||
| At2g01950 | VH1/BRL2 | sp, tmd | 8 | H | H | H | |||
| At2g28840 | Ankyrin protein | ptm | 3 | H | H | M | |||
| At2g42580 | VIT/TTL3/TPR and THL containing protein | 24 | 1 | 1 | H | H | H | H | |
| At2g47930 | Predicted GPI-anchored protein | sp, tmd | 16 | M | H | ||||
| At3g14220 | Myrosinase-associated protein like | sp, tmd | H | H | H | ||||
| At3g16370 | Putative AGP protein | sp, tmd | M | H | M | ||||
| At3g16640 | TCTP | H | |||||||
| At3g23050 | IAA7 | 1 | M | H | M | ||||
| At3g55520 | Immunophilin, putative | 3 | M | M | M | ||||
| At3g59920 | GDI1 | 1 | H | M | M | ||||
| At3g62290 | ARF-like protein | ptm | M | M | M | ||||
| At4g17170 | RAB2A/RABB1b | ptm | 3 | M | M | ||||
| At4g30150 | WD-containing protein | sp | 1 | H | H | M | M | ||
| At5g14240 | Gibberellin regulated protein | 4 | M | H | M | M | |||
| At5g14920 | Similar to proline-rich family protein | tmd | 9 | 6 | 4 | M | H | M | |
| At5g17190 | Expressed protein | sp, tmd | H | M | |||||
| At5g15230 | GASA4 | sp, tmd | M | ||||||
| At5g43190 | FBx6 | tmd | 1 | H | H | M | |||
| At5g65440 | Expressed protein | H | H | M | H | ||||
The homodimerization of VH1/BRL2 is consistent with its ability to autophosphorylate in vitro (Clay and Nelson, 2002); dimerization and transphosphorylation are properties shared by many RLKs (e.g., Karlova et al., 2006; Li et al., 2002). In VH1/BRL2, this relies on the intracellular portion of the protein: the last 990 nucleotides, encoding a juxtamembrane region, the kinase domain, and the carboxy tail.
The interaction candidates display features characteristic of partners of a membrane-localized kinase
Membrane-localized proteins usually contain features such as a signal peptide and a transmembrane domain, or motifs for membrane-anchoring posttranslational modifications (e.g., prenylation) or for interaction with membrane components. Of the strong signal strength candidates, 11 display a predicted transmembrane domain, 8 of which are associated with a signal peptide, and 3 are predicted targets of post-translational modification for membrane localization (Table I, column III).
Phospho-dependent protein interaction domains typically recognize peptide motifs on their binding partners, including a phosphorylated tyrosine or serine/threonine. Sixteen of the strong interaction candidates display at least one serine embedded in a phosphorylation target sequence (Table I, column IV). All but four of the interaction candidates are predicted to be able to bind a basophilic kinase or belong to the kinase-binding group (Tables I, SI and SII, column V).
Insertion mutants in several of VH1/BRL2kin interaction candidates exhibit cotyledon vein pattern defects
We compared the morphology and leaf venation pattern of plants with homozygous T-DNA insertions in 13 of the VH1/BRL2kin interaction candidates (Table SIII) to a vh1 insertion mutant in the same background. None, including vh1, showed an alteration in gross plant morphology or growth rate. The vh1 mutant and insertion mutants of several of its partner candidates exhibited a variety of defects in the pattern of veins in cotyledons and leaves, compared to wild-type Columbia ecotype (Col-0, Figure 1) or to wild-type siblings of each mutant grown under the same conditions (data not shown).
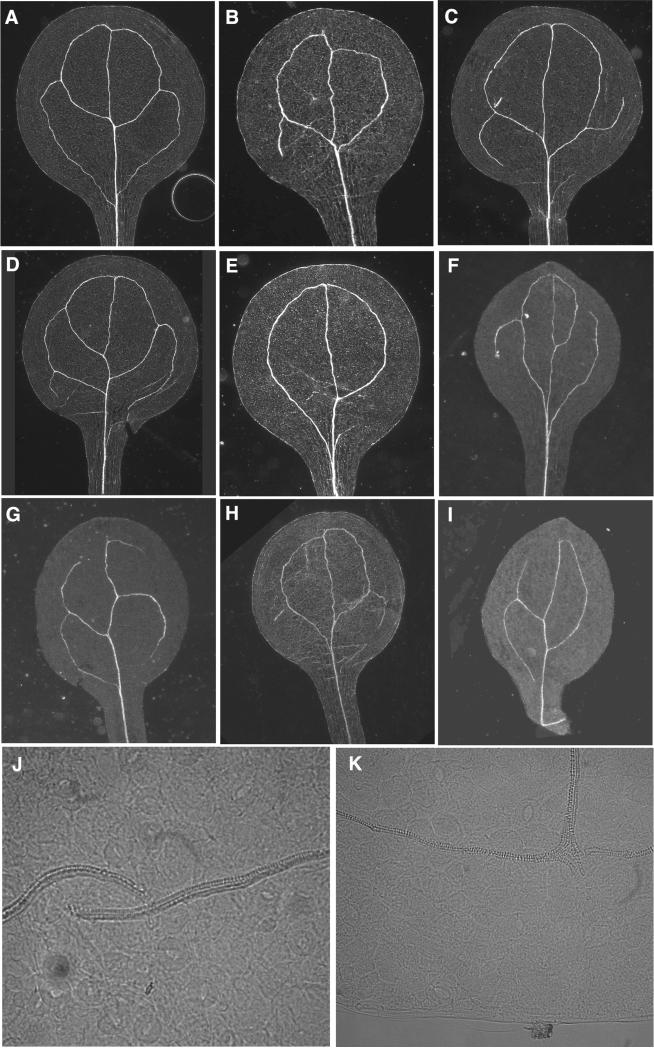
Figure 1.
Cotyledon vein patterns are altered in insertion mutants of 11 of the isolated candidates for interaction with the VH1/BRL2 catalytic domain. Columns 3-8: percentage of cotyledons displaying the venation pattern depicted at top; “gap” = percentage of cotyledons presenting one or more loops unconnected apically to the midvein or interrupted by a gap; n = total number of scored cotyledons. Patterns of wild-type siblings of each mutant were indistinguishable from the Col-0 wild-type depicted.
In wild-type Arabidopsis cotyledons, secondary veins branch from the midvein to form four loops without gaps (Figure 1). Occasionally, the number of loops is reduced when proximal secondary veins do not connect to the base of the midvein or are absent. Gaps in veins or fewer than two secondary veins are observed in the Col-0 ecotype only in mutants (e.g., auxin resistant2, axr2, Figure 1; Seiburth and Deyholos, 2006). T-DNA insertion mutants of 11 of the strong and 1 of the medium interaction candidates display a reduced venation, with fewer cotyledon secondary veins or a higher number of unconnected secondary veins, and more discontinuous venation with gaps in secondary veins (Figure 1). The Col-0 wild-type and an unrelated control insertion line (SALK_031993 in At4g38410) did not exhibit these abnormalities when grown under the same conditions (Figure 1). Each insertion mutant exhibited a unique and constant distribution of pattern defects, although not all of the mutants were confirmed as RNA nulls.
VH1/BRL2 signal transduction involves adaptor proteins
The identity of VH1/BRL2 partners implies an influence on at least three cellular processes: targeted protein degradation, vesicle trafficking and signal transduction (Figure 2). Seven of the interaction candidates bear interaction domains characteristic of adaptor proteins (Figure 2), which link a receptor to alternative outputs and thereby amplify and diversify a signal (Pawson and Scott, 1997). These have a modular structure, including a protein-protein interaction domain, such as ankyrin or tetratricopeptide repeats, for association with the receptor, plus additional domains mediating interactions with downstream proteins or having enzymatic activity that amplifies and transduces a signal.
Figure 2.
VH1/BRL2 direct interactions imply three signaling branches. Listed below each branch are the interaction partners supporting that process.
We tested the interaction of the VH1/BRL2kin with two candidates having adaptor-like protein-protein interaction domains: VIT (VH1-Interacting TPR-containing protein, At2g42580) and VIK (VH1-Interacting Kinase, At1g14000). For both, the in vitro interaction with VH1/BRL2kin was phosphorylation-dependent and mediated by the protein-protein interaction domain of the putative adaptor: the TPR domain of VIT and the ankyrin domain of VIK (Table II and Supplementary Figure S1).
Table II.
Coimmunoprecipitation of VH1-partner complexes. Interactions were detected using the indicated antibodies to pull down complexes and to detect individual partners. VH1-kin, VH1-kin* = VH1 with unphosphorylated or phosphorylated kinase domain; VIT-TPR = tetratricorepeat domain of VIT; VIT-TrxL = thioredoxin-like domain of VIT; VIK-kin = kinase domain of VIK; VIK-Ank = ankyrin domain of VIK. Corresponding gel bands are shown in Supplemental Figure S1
| Combination |
IP Antibody |
Detection Ab |
Signal |
|---|---|---|---|
| VIT + VH1-kin | VIT | VIT | + |
| VH1 | VH1 | + | |
| VH1 | VIT | − | |
| |
VIT |
VH1 |
−
|
| VIT + VH1-kin* | VIT | VIT | + |
| VH1 | VH1 | + | |
| VH1 | VIT | + | |
| |
VIT |
VH1 |
+ |
| VIT-TPR + VH1-kin* | VIT | VIT | + |
| |
VIT |
VH1 |
+ |
| VIT-TrxL + VH1-kin* | VIT | VIT | + |
| |
VIT |
VH1 |
−
|
| VIK + VH1-kin | VIK | VIK | + |
| VH1 | VH1 | + | |
| VH1 | VIK | − | |
| |
VIK |
VH1 |
−
|
| VIK + VH1-kin* | VIK | VIK | + |
| VH1 | VH1 | + | |
| VH1 | VIK | + | |
| |
VIK |
VH1 |
+ |
| VIK-kin + VH1-kin* | VIK | VIK | + |
| |
VIK |
VH1 |
−
|
| VIK-Ank + VH1-kin* | VIK | VIK | + |
| VIK | VH1 | + |
VIK bears a conserved serine-threonine kinase domain downstream of the ankyrin repeat and belongs to the C1 subgroup of MAP kinase kinase kinase (MAPKKK) (Ichimura et al., 2002), based on domain structure. It is possible that this kinase activity is used to transduce the signal perceived by VH1/BRL2 through a MAPK cascade. We were able to detect kinase activity from in vitro-expressed VIK, using a standard myelinic basic protein substrate assay (data not shown); its true substrate is unknown.
VIT bears a thioredoxin-like domain downstream of three tandem TPRs and belongs to the Arabidopsis four-member TTL family (TPR Thioredoxin-Like; Rosado et al., 2006). The thioredoxin-like domain of VIT does not appear to possess a reducing enzymatic activity (Figure 3, and data not shown), in agreement with its lack of the conserved cysteine pairs found in active thioredoxins of the h class (Buchanan and Balmer, 2005). When we screened with VIT as probe, we recovered four interactors in common with VH1/BRL2 (Table III, SIV and SV), suggesting it may serve as an adaptor for multiple complexes, including that for VH1/BRL2.
Figure 3.
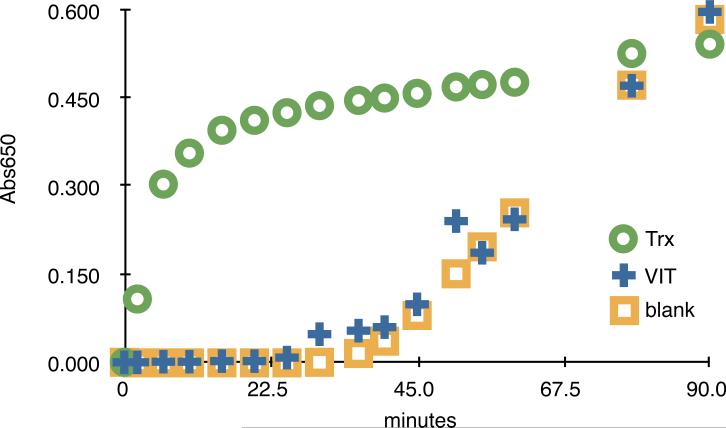
Thioredoxin-like domain of VIT is inactive. Standard insulin reduction assay of VIT and thioredoxin (Trx), as described in Materials and Methods. VIT is inactive. Standard error bars from three independent experiments were smaller than the point symbols plotted.
Table III.
List of VIT interaction candidates with strong signal strength. In bold are highlighted the four interaction candidates shared by VH1/BRL2kin and VIT.
| Annotation | |
|---|---|
| At1g09310 | Unknown |
| At1g12440 | Zinc finger (AN1-like) family |
| At1g62380 | ACO2 (ACC oxidase 2) |
| At1g76920 | F-box protein family (FBX3) |
| At2g20670 | Unknown |
| At2g41410 | Calmodulin, putative |
| At3g14400 | UBP25 (Ubiquitin-specific protease 25) |
| At3g16640 | TCTP |
| At4g14440 | Enoyl-CoA hydratase/isomerase family |
| At5g63190 | MA3 domain containing protein |
VH1/BRL2, VIT and VIK are involved in the establishment of vein pattern in foliar organs
To investigate whether VIT and VIK mutant phenotypes overlap with those of VH1/BRL2, suggesting participation in the same downstream branch of signal transduction, we evaluated plants with likely null mutations in the VH1/BRL2 and VIT (SALK_016024 and SALK_029904, respectively), or producing a truncated RNA for VIK (SALK_002267) with respect to vein pattern, hormone sensitivity, and expression pattern (Figure S2). vh1 and vit mutants show alteration in the positioning, continuity, and complexity of secondary veins in cotyledons (Figures 1 and 4); the vik mutant does not (Figure 1).
Figure 4.
Vein pattern is altered in cotyledons of vh1 and vit mutants. A: Col-0 wild-type. B-E: Pattern defects exhibited by vh1 mutants, including gap (E), mis-positioning of top (B) or bottom (C) secondary loop and the formation of extra veins (D). F-I: Defects in vit mutants, including gaps (F-I), reduced pattern (G,I), islands (H) and alteration in the overall cotyledon shape associated with a simplified and discontinuous pattern (F,I). J-K: Higher magnification of vit mutant cotyledons reveal discontinuities in midvein (J) as well as in the top loops (K). A-I: dark field; J-K: DIC images. Note that Col-0 wild-type cotyledons exhibit some variation from the 4-loop pattern in (A), but at far lower frequencies and without the occurrence of gaps (see Figure 1). Patterns of wild-type siblings of each mutant were indistinguishable from the Col-0 wild-type depicted
For the vh1 mutant, in 57.5% of the gap-containing cotyledons (n=25) the defect is observed in only a single secondary vein (Figure 4C), while in 37.5% (n=25) the vein pattern is extremely reduced (Figure 4E), resembling the one produced in auxin mutants. In 1% (n=180) of the observed cotyledons, one of the two apical loops is misslocalized (Figure 4B), absent or discontinuous. Another 1% (n=180) of the vh1 cotyledons exhibits an extra secondary vein (Figure 4D).
For the vit null mutant, 16.6% (n=25) of the gaps are associated with extremely reduced venation pattern (Figure 4I). In 1% (n=177) of the vit cotyledons, a vascular island was observed (Figure 4H). In a small subset of cotyledons the pattern is highly reduced, with two gaps present (Figure 4G). A small number of vit cotyledons (5/177) display an altered shape associated with a defect in vein patterning (Figure 4F,I). vit cotyledons exhibit rare defects, including misalignment in both the midvein (Figure 4J, 2% (n=177) of the cases) and distal loops (Figure 4K; 5%, n=177), a subset (3%) of which have smaller gaps visible only at high magnification.
Vein pattern defects are present in adult organs of all three mutants. To assess the role of VH1/BRL2, VIT and VIK in post-embryonic vegetative development, veins were scored in two week old primary leaves (n≥50), when a wild-type leaf has a central midvein and three secondary veins on each side. The vh1/brl2 and vit mutations exhibit an increase in the number of veins (Figure 5A). The vik mutant exhibits an increase in veins ending freely, rather than closing in a loop, and therefore a more open pattern than wild-type (Figure 5B-C). In contrast, vh1 and vit mutations lead to a more closed pattern, with a decrease of the ratio of free ending veins to closed loops (Figure 5B). For vit, this includes an increase in branching points in the midvein and a decrease in the tertiary veins (Figure 5C).
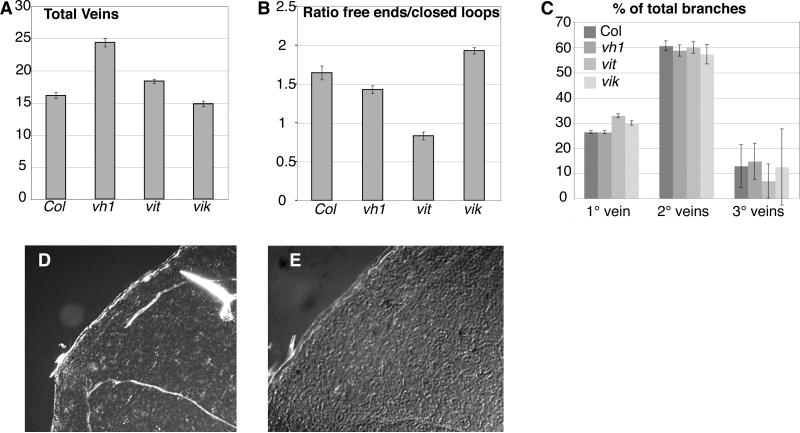
Figure 5.
Vein pattern is altered in primary leaves of vh1, vit and vik mutants. A. Total number of veins per leaf in Col-0 (col) and the three mutants. B. Ratio of free ending veins to closed loops in Col-0 (col) and the three mutant lines. C. Percentage of branches emanating from the primary (1°), secondary (2°) or tertiary (3°) veins in Col-0 (col) and the three mutant lines. D. Dark field and E. DIC images of the top secondary loop of a vit primary leaf exhibiting a gap (D) and absence of cell elongation (E), indicating lack of procambial differentiation.
The distal secondary loops in leaves of the vit mutant exhibit small gaps (10% of vit leaves, 2% of wt) (Figure 5D) which are not spanned by elongated cells, suggesting that procambial differentiation is absent in the gaps (Figure 5E). None of the other insertion lines shows this defect.
VH1/BRL2, VIT and VIK influence in auxin and brassinosteroid signaling
The determination of cells to become vein elements requires the participation of several hormones (Fukuda, 2004). We tested whether hormone responses were affected in vh1, vit, and vik, using root or hypocotyl elongation bioassays. Root growth inhibition was compared in response to exogenous auxin (2,4-dichlorophenoxyacetic acid, 2,4D), auxin transport inhibition by NPA, and brassinosteroids (epibrassinolide, eBL) (Figure 6A,B,C). Root length is reduced in vit, compared to vh1, vik and wild-type (Figure S3). vh1 and vit were hypersensitive and vik was hyposensitive to low concentrations of 2,4-D (25 and 50 nM) and NPA (1μM); all responded as wild-type at higher concentration (Figure 6A,B). vh1 and vik were hyposensitive and vit was hypersensitive to low eBL concentrations (0.02 and 0.2 μM); all responded as wild-type at higher eBL concentration (Figure 6C).
Figure 6.
Responses of root and hypocotyl elongation to four major hormones and to NPA are altered in vh1, vit and vik mutants. The graphs represent the mean of three independent experiments in which 3 day old seedlings were transferred to media containing increasing concentrations of 2,4D, NPA, eBL, ABA or GA3 as indicated. Inhibition of root growth or induction of hypocotyls growth is expressed as percentage of the mean growth without hormone. Error bars are standard errors.
Although it is possible that the defective responses of the mutants at low but not high concentration treatments are a consequence of defects in uptake, the effects were specific to these compounds. Responses to other compounds, including ABA and GAs (in the form of GA3), were normal (Figure 6D,E, and data not shown).
Auxin and brassinosteroids affect the transcription of VH1/BRL2, VIT and VIK
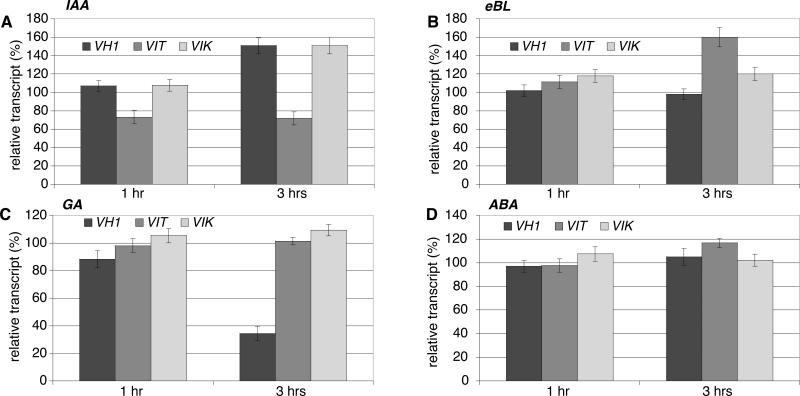
The altered sensitivity observed in the insertion mutants to auxin and brassinosteroid could be due to effects of an altered signaling pathway, if VH1/BRL2, VIT, or VIK are part of it, or to an altered hormone response, if VH1/BRL2, VIT, or VIK are part of the target hormone transcriptome. We measured the effect of auxin, eBL, ABA, and GA3 on the expression levels of VH1/BRL2, VIT and VIK in liquid culture. In wild-type seedlings, both VH1/BRL2 and VIK transcripts were induced after 3 hours of IAA supplementation (1.51 ± 0.08 and 1.50 ± 0.09-fold, respectively), while VIT transcription was repressed (0.72 ± 0.04-fold) (Figure 7A). In contrast, eBL had little effect on VIK and VH1/BRL2 transcripts (0.98 ± 0.06 and 1.19 ± 0.07-fold, respectively), but induced VIT transcription (1.6 ± 0.1-fold) (Figure 7B). GA3 did not affect transcription of the three genes, but VH1/BRL2 was repressed by ABA (Figure 7C-D).
Figure 7.
Transcript levels for VH1, VIT and VIK are hormone-responsive in Col-0 wild-type plants. Plant were treated with IAA (A) (20 μM), eBL (B) (1 μM), GA3 (C) (10 μM), or ABA (D) (100 μM), for 1 or 3 hours, and the indicated mRNAs measured by semiquantitative RT-PCR. Amount of each transcript after hormone treatment is expressed as percentage of the mean level without treatment (100%). Error bars are standard errors of three independent experiments on two biological replicates.
VIT affects the regulation of auxin- and brassinosteroid-responsive genes
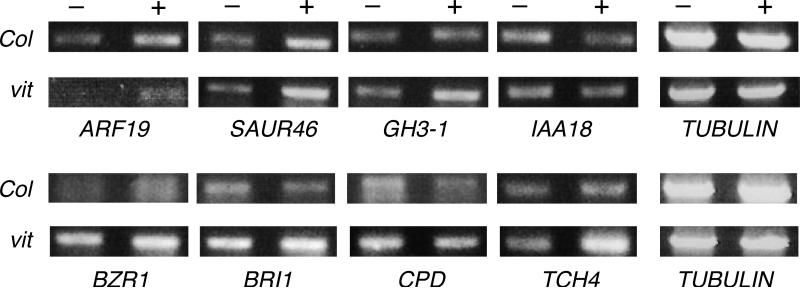
To test whether defects in VH1/BRL2, VIT and VIK cause alterations in auxin- and brassinosteroid-regulated gene expression, we measured the hormone-induced transcripts of several previously characterized responsive genes (Nemhauser et al., 2006; Schmid et al., 2005), all of which are expressed in developing or mature veins, based on cell-specific transcriptome data (N. Gandotra, unpublished results) The auxin responses of the auxin-regulated genes ARF19, SAUR46, GH3-1 and IAA18 are altered only in the vit mutant. In wild-type, vh1, and vik mutants ARF19, SAUR46 and GH3-1 are up-regulated upon IAA treatment, while IAA18 is down-regulated, in agreement with reported data for Col-0 wild-type (data not shown and Nemhauser et al., 2006; Schmid et al., 2005). In the vit mutant ARF19, SAUR46 and GH3-1 are more up-regulated, while IAA18 is not down-regulated as much as in Col-0 (Figure 8), suggesting that VIT normally attenuates their regulation by auxin.
Figure 8.
Levels of a subset of BR-responsive and auxin-responsive transcripts are altered in vit mutants. 7 day old Col-0 (Col) wild-type or vit mutant seedlings were treated with 20 μM IAA (top panel) or 1 μM eBL (bottom panel). Three independent RTPCR measurements on two biological replicas were made of the indicated transcripts with similar results. – and + indicate untreated and treated seedlings, respectively.
BR-regulated genes are similarly affected. In the vh1 and vik mutant backgrounds, the BR-responsive genes BZR1, BRI1, CPD and TCH4 react normally to eBL treatment (data not shown and Nemhauser et al., 2006; Schmid et al., 2005). In vit, however, uninduced levels of BZR1, BRI1 and CPD are higher than in wild-type, eBL inhibits CPD as in wild-type, and eBL causes greater induction of BZR1 and TCH4. Also in vit, BRI1 is up- instead of down-regulated (Figure 8).
VH1/BRL2, VIT and VIK are expressed in overlapping patterns
To determine whether VIT and VIK are co-expressed with VH1/BRL2 in patterns that would permit their interaction during development, we characterized the expression patterns of all three genes, using a combination of GUS reporter expression, RT-PCR, laser microdissection and microarray data-mining. Consistent with previous studies (Clay and Nelson, 2002) VH1/BRL2 was expressed uniformly in early stage embryos and in organ primordia in a pattern progressively restricted to provascular and procambial cells. In leaves, expression ends with the differentiation of mature vascular elements (Figure S3 and S4). VIT exhibits a comparable highly dynamic expression pattern that overlaps VH1/BRL2 in all organs tested (Figure 9, S4, S5). Transcripts for both are present in early stage embryos and in organ primordia in the shoot and in the root (Figure 9A-B, GI, Q, Clay and Nelson, 2002). In the embryo, in the root and in emerging secondary roots, expression of VIT is broad and more diffuse than VH1/BRL2 (Figure 9C-F, R-S, S4). Although initially diffuse in leaf primordia, the two genes are co-expressed in a progressively restricted subset of leaf cells. In emerging and expanding primary leaves, VIT is expressed in hydathodes (Figure 9J-L, S5C), guard cells (Figure 9L), certain petiole cells (Figure 9K-L, S5C), and isolated cells or groups of cells associated with differentiating vascular bundles, apparently immature tracheary elements or xylem parenchyma (Figure 9K, S5C). The same pattern is observed in cauline leaves (Figure 9M) and petals (Figure 9N). The xylem-associated cells are elongated and flank more mature xylem elements (Figure S5E), and are particularly evident at branching points in the vascular network (Figure 9L), although we were unable to identify the cell type unambiguously. VH1/BRL2 is present in the same cells (compare Figure S5E and F). In some cases multiple parallel aligned elongated cells expressed VIT. The VH1/BRL2-VIT-positive cells, individual or multiple, can be above, below or on the flank of differentiated xylem cells. A similar pattern has been reported for the ZINNIA CYSTEINE PROTEASE4 (ZCP4), identified by its transient induction during tracheary element differentiation (Pyo et al., 2004), and confirmed in vivo (Demura et al., 2002). GUS reporter analysis suggested that ZCP4 is expressed exclusively in elongated cells flanking differentiated xylem elements, similar to the pattern for VIT. Unlike VIT, for which this pattern is only observed in adult foliar organs, ZCP4 shows a similar pattern in all organs tested, and the authors concluded that it is a marker for immature tracheary elements (Pyo et al., 2004).
Figure 9.
pVIT::GUS expression is highly dynamic and specific. A-S: GUS-reporter activity from expression directed by VIT promoter. A-C: embryo globular (A), late heart (B), and bent cotyledon stage (C). D-F: seedling 75 hours (D), 84 hours (E) and 120 hours (F) post-imbibition. Note high expression in the shoot apical meristem, in the border between radicle and hypocotyl and in the transition zone after. G-I: leaf primordia. J-L: developing leaves. Note expression in stipule (J), hydathodes and fragmented pattern along the venation (K-L), in the petiole and stomata (K-L). M: cauline leaves; N: petals. O-P: flower. Note expression from early stages in the carpel and stamens (O) that becomes restricted to pollen grains, ovules and funiculi (P). Q-S: secondary root. Note expression throughout from early stages (Q-R), subsequent restriction to more internal tissue (S).
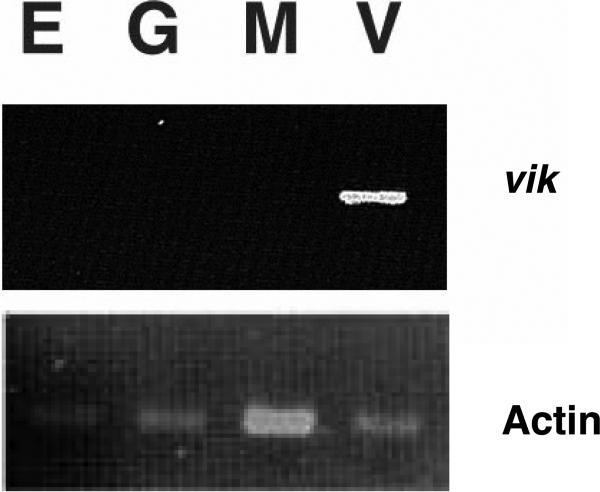
VIK is also expressed in vascular cells, but first appears at a distinctly later stage than VIT. We captured leaf mesophyll, epidermal, vascular (combined xylem, phloem, and parenchymal), and guard cells from 1 month old expanding Arabidopsis leaves, using laser microdissection, as part of a separate study of leaf cell transcriptomes (unpublished data). VIK transcripts are present in the vascular cells, not in epidermal, mesophyll or guard cells, when assayed by RT-PCR (Figure 10). The overlapping patterns and specificity of expression of VIK and VIT revealed by RT-PCR and by GUS reporter expression are confirmed by the mining of data from cell-specific transcriptomes obtained by microarray profiling. In expanding leaves, VIT is present already in the transcriptomes of provascular/procambial cells (early stage vascular) and more mature vascular cells, while VIK is found only in the more mature vascular cells (data not shown). It was not possible to resolve the contributions of subsets of vascular cells.
Figure 10.
Vascular cell-specific expression of VIK. VIK transcripts were assayed by RTPCR in RNA obtained by laser microdissection of leaf epidermal (E), guard (G), mesophyll (M) and vascular bundle (V) cells. VIK RNA is present only in the V lane (upper panel). Lower panel: actin control.
Discussion
Three cellular processes—targeted protein degradation, vesicle trafficking and signal transduction—are likely associated with the VH1/BRL2 receptor-like kinase based on the identified interactions of its cytoplasmic domain. Half of the 66 identified interaction partners of the phosphorylated (active) VH1/BRL2 kinase domain are associated with one of these cellular processes (Figure 2). As expected for a potentially homodimerizing receptor, VH1/BRL2 exhibits strong affinity for its own intracellular domain.
A subset of the interaction candidates is associated with gibberellin (GA) signaling (Table I, SI, SII), previously associated with vascular differentiation (Fukuda, 2004). The absence of an apparent effect of vh1 mutation on other well characterized GA responses, such as seed germination and shoot elongation (Swain and Singh, 2005), may be due either to insufficient sensitivity of the GA3 bioassay, or to the involvement of the GASA4 and/or GA-regulated partners of VH1 in pathways other than GA signaling. Other VH1/BRL2 partners imply a link to regulation of metabolism (e.g., At5g63570, Table SI) and membrane composition (e.g., At3g16370, Table I), and transduction of the VH1-mediated signal into other downstream partners through adaptor proteins (e.g., At1g14000 and At2g42580, Table I).
VH1 and adaptor proteins
Adaptor proteins permit a single receptor to associate with a variety of downstream outputs by means of modular protein-protein interactions (Pawson and Nash, 2003). The VH1/BRL2 kinase domain exhibits several such adaptor interactions (e.g., via ankyrin and TPR regions, Figure 2 and 3). We demonstrated that the interaction of the adaptor proteins VIT and VIK with the VH1/BRL2 catalytic domain is phosphorylation-dependent and mediated by defined protein-protein interaction domains (Figure 3).
We propose that VH1/BRL2 forms distinct VIT and VIK complexes in different stages of vascular cell differentiation, each complex with distinct outputs. The VH1/BRL2-VIT complex appears to be involved in the formation of continuous vascular strands in cotyledons. Mutation of either VH1/BRL2 or VIT causes the venation pattern of cotyledons to be reduced and gapped (Figure 1 and 4). In leaves the two genes are co-expressed in differentiating xylem elements and affect the determination of higher order veins and branching point (Figure 5, S5, 9). Consistent with its proposed role as adaptor for VH1/BRL2, VIT independently binds a subset of other identified VH1/BRL2 interaction partners (Table III).
In contrast, the VH1/BRL2-VIK complex appears to be involved in a later stage of vascular differentiation, perhaps influencing the extension versus termination of a vascular strand (Figure 5 and 10). VIK appears to work as a scaffold that links the VH1/BRL2 kinase activity to downstream activity, probably to a MAPK cascade.
The phenotypes of vh1, vit, and vik mutants in response to hormone treatment (Figure 6) and their distinct effects on auxin- and BR-related genes (Figures 7 and 8 and data not shown) are consistent with the existence of distinct complexes of VH1/BRL2 with VIT and VIK. VIK appears to be a positive transducer of the signal perceived by VH1/BRL2 and VIT a negative one. The provascular specific VH1/BRL2 receptor kinase may serve as an integrator of the hormonal regulation of vein pattern in leaves. The outputs moderated by VIT and VIK appear to be influenced by the perception of auxin and BRs, hormones known to play a primary role in establishing and modulating the position of veins in leaves (Fukuda, 2004).
Protein degradation and vascular development
The degradation of regulatory proteins targeted by specific SCF complexes plays a role in multiple processes in plant development and growth (Lechner et al., 2006; Smalle and Vierstra, 2004). The resetting of metabolism and transcription through turnover of transcription factors and enzyme inhibitors/activators permits the onset of specific cell differentiation fates, like the production of xylem or phloem cells. Since it interacts with SCF components, VH1/BRL2 may regulate one or more of the SCF complexes or VH1/BRL2 could itself be a target for degradation, as a part of its turnover dynamics. Its interaction candidates include two ubiquitins, UBQ3 and UBQ10, and a protein bearing similarity with a RING domain involved in polyubiquination reactions (Table SI and SII). Alternatively, VH1/BRL2 could use this pathway to target specific proteins, like transcription factors, for degradation.
IAA7, one of the 24 members of the AUX/IAA family of auxin-induced transcriptional regulators, also interacts strongly with VH1/BRL2 in vitro (Table I). The function of AUX/IAA proteins as transcription repressors is dependent on their rapid degradation by the SCFTIR1 complex upon auxin perception (Parry and Estelle, 2006). Since TIR1 directly binds both auxin and IAA7 (Tan et al., 2007), VH1/BRL2, by interacting with IAA7, could influence the composition of the TIR1-auxin-IAA complexes.
Vesicle traffic, vascular development and auxin transport
Vesicle trafficking is particularly important in vein formation, which requires the elongation and proliferation of polarized differentiating cells (Berleth and Mattsson, 2000; Seiburth and Deyholos, 2006). Membrane growth and polar auxin transport both rely on accurately regulated vesicle trafficking (Vieten et al. 2007) and many specific molecules are likely to be localized in a polar fashion along the path of the cells aligned as vascular elements. The interaction of RAB2 with VH1/BRL2 suggests that subcellular localization of the receptor may vary depending on its functional state, since a role of such small GTPases is to interact with cargo proteins. GTP Dissociation Inhibitors (GDI) and ADP Ribosylation Factors (ARFs), both isolated as strong interaction partners of VH1 (Table I), are regulators of this process. GDI regulates RAB activity; it is able to extract the small GTPase from the membrane, keep it in an inactive GDP-bound state and prevent its interaction with effector proteins (Olofsson, 1999; Zaleman et al., 1999). Phosphorylation of GDI increases its affinity for RAB (DeMardirossian and Bokoch, 2005). On the other hand, ARFs are involved in the regulation of budding of coated vesicles (Molendijk et al., 2004). The possibility that VH1/BRL2 regulates some part of this process is intriguing.
Vesicle trafficking is essential for the polarized localization of auxin efflux carriers in the plasma membrane of cells determined to become vascular elements (Delbarre et al., 1998; Morris, 2000). The vascular phenotypes of mutations in PIN1 and in GNOM/EMB30, an ARF-GEF involved in trafficking the vesicles transporting PIN1, are consistent with this requirement (Gälweiler et al., 1998; Koizumi et al., 2000; Muday et al., 2003). Other likely participants in the correct positioning of specific molecules inside the cell are VASCULAR ALTERED NETWORK3 (VAN3)/SCARFACE (SCR) and COTYLEDON VASCULAR PATTERN 2 (CVP2). The first encodes an ARF-GAP that could work in antagonism with GNOM, the second a phosphoinositol-5-phosphatase, whose connection to vesicle trafficking is more indirect. CVP2 appears to regulate the availability of specific phosphoinositides for binding to the PH domain of VAN3/SCR to regulate its localization (Carland and Nelson, 2004; Koizumi et al., 2005; Sieburth et al., 2006). Mutations of both these genes result in an altered vascular pattern (Fukuda, 2004; Sieburth and Deyholos, 2006).
Another process affected by vesicle traffic is the distribution of membrane-bound receptors among compartments in the cell. Examples are SERK1 (Rienties et al., 2005), FSL2 (Robatzek et al., 2006), and BRI1 (Geldner et al., 2007), all RLKs.
VH1/BRL2 is present in cells undergoing differentiation in a dynamic environment, possibly integrating numerous extracellular inputs and intracellular outputs. The interaction with adaptors such as VIT and VIK would explain how the activation of RLKs such as BRI1 and VH1/BRL2 can diversify outputs. This is supported by the recent identification of another TPR protein interacting with BRI1 (Tang et al., 2007).
Materials and Methods
DNA constructs and fusion protein expression and purification
PCR reactions were performed using High Fidelity Taq (Roche, www.roche-applied-science.com) and constructs were confirmed by sequencing. Primer sequences are given in Table SVI. For fusion of MBP with VH1, the intercellular domain (positions 2355 to 3352) of VH1kin, was amplified by PCR with BamHI sites at both ends. The pMALcR1 expression vector (New England Biolabs, NEB, www.neb.com) was modified by introducing the sequence CGTCGTGCATCTGTT, encoding a target of kinase A (RRASV) between the EcoRI and BamHI sites creating the MBP* tag for labeling fusion protein probes. The amplified VH1kin digested product was cloned into the BamHI site.
To create a carboxyl-terminal fusion of GST with VIT, the VIT cDNA was obtained from the ABRC (http://www.arabidopsis.org/abrc/), amplified by PCR, inserted in the pDONR201 vector and in the pDEST15 vector (Invitrogen, www.invitrogen.com) by recombination following the manufacturer's instruction.
To create a GST::VIK fusion, the VIK cDNA was isolated using RT-PCR from RNA extracts of one week old plants, amplified by PCR with BamHI and XhoI sites at either ends, and cloned into BamHI-XhoI digested pGEX-4T-1 expression vector (Novagen, www.novagen.com), downstream of the GST tag.
To create deletion constructs of VIT and VIK, the VITTPR (position 603 to 1678), VITTXL (positions 1692 to 2073), VIKANK (positions 1 to 307), and VIKKIN (positions 484 to 1315) cDNA fragments were amplified by PCR with EcoRI and XhoI sites on either ends. After purification and digestion, they were cloned in the EcoRI-XhoI digested pGEX-4T1 vector.
To obtain a fusion of MBP* with VIT, the VIT cDNA was amplified by PCR with SalI and PstI sites at the ends and cloned in the modified pMalcR1 vector containing a kinase A site.
Constructs were transformed in Escherichia coli strain BL21(DE3) codon plus (Stratagene, www.stratagene.com).
MBP*::VH1 and MBP*::VIT were isolated following the procedure described in Clay and Nelson (2002). GST::VIT and GST::VIK were batch-purified according to Novagen after 2 hours 0.1 mM IPTG induction. Purified fusion proteins were quantified by BioRad protein assay (Biorad, www.bio-rad.com) and checked by Western blot analysis using VH1 (Clay and Nelson, 2002), MBP (NEB) or GST (Sigma Aldrich, www.sigma-aldrich.com) antisera.
Interaction cloning
1 μg of purified MBP*::VH1 was phosphorylated as described in Clay and Nelson (2002). 1 μg of purified MBP* or MBP*::VIT were phosphorylated using 5 units of bovine kinase A (Sigma Aldrich) and 10 μCi [γ32P] ATP (Amersham Biosciences, www.amersham.com). Before use, the proteins were purified using MicroBioSpin chromatography columns (BioRad).
The phage expression CD4-13, CD4-14 and CD4-15 A. thaliana cDNA libraries from 3 day old seedlings were obtained from the ABRC. Approximately 5 × 106 plaques were screened for each library, according to Stone et al. (1994) with the following variations: probe filters washed three times 30 minutes, buffer B; dried filters exposed to x-ray film for 3 to 5 days. Positive phage were plaque-purified through 3 screens. Libraries probed with the labeled MBP* alone resulted in no signal after exposure up to 9 days. For MBP*::VH1, screens of the three libraries resulted in the recovery of 66 clones, and for MBP*::VIT, the screen of the CD4-13 library resulted in 43 positive clones.
Based on the primary screen intensities, the clones were classified as strong, medium or weak. The positive clones were isolated and the cDNA excised using the ExAssist helper phage (Stratagene) according to the manufacturer. The resulting bacterial colonies were grown and the plasmid DNA was isolated using Qiagen Spin Miniprep kit (Qiagen, www1.qiagen.com) and sequenced.
Bioinformatic analysis
The following web resources were used for the bioinformatics analysis of the VH1 interaction candidates: NCBI (http://www.ncbi.nlm.nih.gov/BLAST/) and TAIR (http://www. arabidopsis.org/Blast/) for general protein characteristics and annotation; SignalIP (http://www.cbs.dtu.dk/service/SignalIP) (Bendtsen et al., 2004) for presence of a signal peptide; Myristoylator (http://expasy.org/tools/myristorylator) (Bologna et al., 2004), PrePS-prenylation (http://mendel.imp.ac.at/sat/PrePS/index/html) (Mauer-Stroh and Eisenhaben, 2005) and Terminator (http://www.isv.cnrs-gif-fr.terminator2/index.html) (Frottin et al., 2006) for post translational modification; DIPHOSPHO (http://core.ist.temple.edu/pred/pred.html) (Diella et al., 2004) for phosphorylation target sites; Scansite (http://scansite.mit.edu/) (Obenauer et al., 2003) and Prosite (http://ca.expasy.org/tools/scanproposite) (de Castro et al., 2006) for the presence of protein motifs and domains.
Plant growth conditions, identification and verification of T-DNA insertion lines
Plants were grown as described in Clay and Nelson (2002). For comparative studies, wild-type siblings from the same seed stocks were grown on the same plates as mutants. The SALK T-DNA database (http://signal.salk.edu/cgi-bin/tdnaexpress) (Alonso et al. 2003) was searched for insertion lines in VH1 and in its interaction candidates and seeds were obtained from the ABRC stock center. For each insertion line, the presence of a single T-DNA insertion, its location within the gene, and its cosegregation with the phenotype was confirmed. Plants homozygous for the T-DNA insertion were identified by PCR genotyping. For vh1, vit and vik mutant lines, RNA was extracted from plant material using TRIzol (Invitrogen), according to the manufacturer. RT-PCR was performed using 1 μg of extracted RNA and oligo-dT primers (Ambion, Austin, Texas), followed by PCR with the appropriate primers (Table SVI) and the Red Taq Ready Mix (Sigma Aldrich).
Vein pattern characterization
Two week old seedlings were fixed and cleared as described in Carland et al. (1999). After dissection, cotyledons and primary leaves were mounted on slides in 50% glycerol and observed under dark field illumination or in DIC mode to view lignified tracheary elements within the xylem. Col-0 wild-type plants are presented in figures as representative wild-type, since vein patterns of wild-type siblings of each mutants were indistinguishable from Col-0.
In vitro co-immunoprecipitation
3 μg of purified GST::VIT or GST::VIK and 5 μg of MBP::VH1kin were incubated for 2 hrs at 30°C in MBP column buffer (Clay and Nelson, 2002), washed twice, incubated with amylose (NEB) or GST resin (Novagen), washed three times with the appropriate column buffer, resuspended in 1X SDS-sample buffer, and boiled for 3 minutes. After separation on SDS-PAGE, the precipitated proteins were subjected to Western Blot analysis using either GST, MBP or VH1 antisera.
The deletion constructs bound to the GST beads (Novagen) were incubated with 1 μg of phosphorylated MBP*::VH1 at RT for 2 hours, washed three times with GST column buffer, resuspended in 1X SDS-sample buffer and boiled for 4 minutes. The proteins were separated by SDS-PAGE followed by Western blot analysis using GST, MBP or VH1 antisera.
Thioredoxin and kinase activity assays
Reducing activity of MBP*::VIT or GST::VIT (not shown) and a human thioredoxin (Sigma Aldrich) positive control was measured as described in Holmgren (1979). VIK kinase activity was measured as described in Miao et al. (2007).
Hormone bioassays and treatment
Seeds were surface-sterilized in 20% bleach and plated on basal media plus the indicated amount of hormone. After 3 days at 4°C in the dark, plates were transferred to 22°C under constant light (~300 μE m−2sec−1) in a vertical position. After 3 days, seedlings were transferred to plates containing the indicated concentration of 2,4D, NPA, eBL, ABA, or GA3. Measurements of roots or hypocotyls were taken after 7 days. Experiments were repeated three times.
Because root elongation is reduced in the vit mutant compared to Col-0 wild-type (Figure S3), comparisons were made between plants of the same genotype, with and without hormone treatment.
For hormone induction, seedlings were grown in basal liquid media under constant light at RT. After 7 days, IAA (20 μM), eBL (1 μM), ABA (100 μM), and GA3 (10 μM) were added to the media. Whole seedlings were collected 1 or 3 hours later for RNA isolation, dried on Kimwipes, frozen in liquid nitrogen, and stored at −80°C.
The RT reactions were performed as described above. The semiquantitative RT-PCR reactions were performed using the RedTaq Ready Mix (Sigma Aldrich) on 1 μl of cDNA and gene-specific primers (Table SVI) at a final concentration of 0.25 μM. No genomic DNA was ever detected. Scanned gel bands were quantitated with the Image J program (http://rsb.info.nih.gov/nih-image/). The value of the area obtained for each band was normalized to a tubulin (At3g18780) control.
Laser microdissection (LM)
LM of leaf cell types using the Arcturus PixCell IIe system (Molecular Devices, www.moleculardevices.com), isolation of cell-specific RNA, and RT-PCR were performed as described in Kerk et al. (2003). Primers pairs are given in Table SVI.
Supplementary Material
Supplementary Material
Table SI. VHI/BRL2kin interaction candidates with medium signal strength. Column contents as in Table I.
Table SII. VHI/BRL2kin interaction candidates with weak signal strength. Column contents as in Table I.
Table SIII. Characteristics of T-DNA insertion lines in notable interaction candidates.
Table SIV. VIT interaction candidates with medium signal strength.
Table SV. VIT interaction candidates with weak signal strength.
Table SVI. Primer pairs used in this work.
Figure S1. Immunoprecipitations showing interactions of VH1/BRL2kin with the adaptors VIT and VIK.
Figure S2. T-DNA mutant lines in VH1, VIT and VIK
Figure S3. Root length of Col-0 (col) wild-type and vh1, vit, and vik insertion lines after a week of vertical growth on basal media plates.
Figure S4. Comparison of VIT and VH1 reporter lines staining during imbibition
Figure S5. Comparison of VIT and VH1 reporter lines staining during leaf development from 5 to 7 days after germination.
Acknowledgments
We are grateful to Cloe Mara for providing a control Arabidopsis insertion line, to S. Lori Tausta for help with microdissection, and to Francine Carland for helpful comments on the manuscript. This work was supported by NSF awards IBN-0114648 and IBN-0416731 to TN.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Jensen LJ, Blom N, Von Heijne G, Brunak S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng. Des. Sel. 2004;17:349–356. doi: 10.1093/protein/gzh037. [DOI] [PubMed] [Google Scholar]
- Berleth T, Mattsson J. Vascular development: tracing signals along veins. Curr. Opin. Plant Bio. 2000;3:406–411. doi: 10.1016/s1369-5266(00)00104-7. [DOI] [PubMed] [Google Scholar]
- Bologna G, Yvon C, Duvaud S, Veuthey AL. N-terminal myristoylation predictions by ensembles of neural networks. Proteomics. 2004;4:1626–1632. doi: 10.1002/pmic.200300783. [DOI] [PubMed] [Google Scholar]
- Buchanan BB, Balmer Y. Redox regulation: a broadening horizon. Annu. Rev. Plant Biol. 2005;56:187–220. doi: 10.1146/annurev.arplant.56.032604.144246. [DOI] [PubMed] [Google Scholar]
- Caño-Delgado A, Yin Y, Yu C, Vadeados D, Mora-Garcia S, Cheng JC, Nam KH, Li J, Chory J. BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development. 2004;131:5341–5351. doi: 10.1242/dev.01403. [DOI] [PubMed] [Google Scholar]
- Carland FM, Berg BL, FitzGerald JN, Jinamornphongs S, Nelson T, Keith B. Genetic regulation of vascular tissue patterning in Arabidopsis. Plant Cell. 1999;11:2123–2137. doi: 10.1105/tpc.11.11.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland FM, Nelson T. Cotyledon vascular pattern 2 - mediated inositol (1,4,5) triphosphate signal transduction is essential for closed venation pattern of Arabidopsis foliar organs. Plant Cell. 2004;16:1263–1275. doi: 10.1105/tpc.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay NK, Nelson T. VH1, a provascular cell-specific receptor kinase that influences leaf cell patterns in Arabidopsis. Plant Cell. 2002;14:2707–2722. doi: 10.1105/tpc.005884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Langford M, McMorris TC. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–678. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro E, Sigrist CJA, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A, Hulo N. ScanProsite: a detection of Prosite signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34:W362–W365. doi: 10.1093/nar/gkl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre A, Muller P, Guern J. Short-lived and phosphorylated proteins contribute to carrier-mediated efflux, but not to influx of auxin in suspension-cultured tobacco cells. Plant Physiol. 1998;116:833–844. doi: 10.1104/pp.116.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demura T, Tashiro G, Horiguchi G, Kishimoto N, Kubo M, Matsuoka N, Minami A, Nagata-Hiwatashi M, Nakamura K, Okamura Y, Sassa N, Suzuki S, Yazaki J, Kikuchi S, Fukuda H. Visualization by comprehensive microarray analysis of gene expression programs during transdifferentiation of mesophyll cells into xylem cells. Proc. Natl. Acad. Sci. USA. 2002;99:15794–15799. doi: 10.1073/pnas.232590499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in RhoGTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Diella F, Cameron S, Gemund C, Linding R, Via A, Kuster B, Sicheritz-Ponten T, Blom N, Gibson TJ. Phospho.EML: a database of experimentally verified phosphorylation sites in eukaryotic proteins. BMC Bioinformatica. 2004;22:79. doi: 10.1186/1471-2105-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:606–607. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- Frottin F, Martinez A, Peynot P, Mitra S, Holz RC, Giglione C, Meinnel T. The proteomics of N-terminal methionine cleavage. Mol. Cell Proteomics. 2006;5:2336–2349. doi: 10.1074/mcp.M600225-MCP200. [DOI] [PubMed] [Google Scholar]
- Fujita H, Takemura M, Tani E, Remoto K, Yokota A, Kohchi T. An Arabidopsis MADS-Box protein, AGL24, is specifically bound and phosphorylated by meristematic receptor-like kinase (MRLK). Plant Cell Physiol. 2003;44:735–742. doi: 10.1093/pcp/pcg092. [DOI] [PubMed] [Google Scholar]
- Fukuda H. Signals that control plant vascular differentiation. Nat. Rev. Mol. Cell. Biol. 2004;5:379–391. doi: 10.1038/nrm1364. [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- Geldner N, Hyman DL, Wang X, Schumacher K, Chory J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes and Dev. 2007;21:1598–1602. doi: 10.1101/gad.1561307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J. Biol. Chem. 1979;254:9627–9632. [PubMed] [Google Scholar]
- Ichimura K, Tena G, Henry Y, Zhang S, Hirt H, Ellis BE, Morris PC, Wilson C, Champion A, Innes RW, Sheen J, Ecker JR, Scheel D, Klessig DF, Machida Y, Mundy J, Ohashi Y, Kreis M, Heberle-Bors E, Walker JC, Shinozaki K. Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci. 2002;7:301–308. doi: 10.1016/s1360-1385(02)02302-6. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Ingram GC. Sending the right signals: regulating receptor kinase activity. Curr. Op. Plant Bio. 2005;8:648–656. doi: 10.1016/j.pbi.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Karlova R, Boeren S, Russinova E, Aker J, Veervort J, de Vries SC. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell. 2006;18:626–638. doi: 10.1105/tpc.105.039412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerk NM, Ceserani T, Tausta SL, Sussex IM, Nelson TM. Laser capture microdissection of cells from plant tissues. Plant Physiol. 2003;132:27–35. doi: 10.1104/pp.102.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K, Naramoto S, Sawa S, Yahare N, Ueda T, Nakano A, Sugiyama M, Fukuda H. VAN3 ARF-GAP-mediated vesicle transport involved in leaf vascular network formation. Development. 2005;132:1699–1711. doi: 10.1242/dev.01716. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Sugiyama M, Fukuda H. A series of novel mutants of Arabidopsis thaliana that are defective in the formation of continuous vascular network: calling the auxin signal flow canalization hypothesis into question. Development. 2000;127:3197–3204. doi: 10.1242/dev.127.15.3197. [DOI] [PubMed] [Google Scholar]
- Lechner E, Achard P, Vansiri A, Potuschak T, Genschik P. F-box everywhere. Curr. Opin. Plant Biol. 2006;9:631–638. doi: 10.1016/j.pbi.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Li J. Brassinosteroids signaling: from receptor kinases to transcription factors. Curr. Opin. Plant Biol. 2005;8:526–531. doi: 10.1016/j.pbi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Li J, Chory J. A putative leucine–rich repeat receptor kinase gene involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Li J, Nam KH, Vafeados D, Chory J. BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol. 2001;127:14–22. doi: 10.1104/pp.127.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. BAK1, an Arabidopsis LRR-receptor like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/s0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- Mauer-Stroh S, Eisenhaber F. Refinement and prediction of prenylation motifs. Genome Biology. 2005;6:R55. doi: 10.1186/gb-2005-6-6-r55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Laun TM, Smykowski A, Zentgraf U. Arabidopsis MEKK1 can take a short cut: it can directly interact with senescence-related WRKY53 transcription factor on the protein level and can bind to its promoter. Plant Mol Biol. 2007;65:63–76. doi: 10.1007/s11103-007-9198-z. [DOI] [PubMed] [Google Scholar]
- Molendijk AJ, Ruperti B, Palme K. Small GTPases in vesicle trafficking. Curr. Op. Plant Biol. 2004;7:694–700. doi: 10.1016/j.pbi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Morillo SA, Tax FE. Functional analysis of receptor-like kinases in monocots and dicots. Curr. Opin. Plant Biol. 2006;9:460–469. doi: 10.1016/j.pbi.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Morris DA. Transmembrane auxin carrier-systems—dynamic regulators of auxin transport. Plant Growth Regul. 2000;32:161–172. doi: 10.1023/a:1010701527848. [DOI] [PubMed] [Google Scholar]
- Muday GK, Peer WA, Murphy AS. Vesicular cycling mechanisms that control auxin transport polarity. Trends Plant Sci. 2003;8:301–304. doi: 10.1016/S1360-1385(03)00132-8. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Fujioka S, Sunohara H, Kamiya N, Hong Z, Inukai Y, Miura K, Takatsuto S, Yoshida S, Ueguchi-Tanaka M, Hasegawa Y, Kitano H, Matsuoka M. The role of OsBRI1 and its homologous genes, OsBRL1 and OSBRL3, in rice. Plant Physiol. 2006;140:580–590. doi: 10.1104/pp.105.072330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KH, Li J. The Arabidopsis transthyretin-like protein is a potential substrate of Brassinosteroid-Insensitive 1. Plant Cell. 2004;16:2406–2417. doi: 10.1105/tpc.104.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser JL, Hong F, Chory J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell. 2006;126:467–475. doi: 10.1016/j.cell.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson B. Rho guanine dissociation inhibitors: Pivotal molecules in cellular signalling. Cell Signal. 1999;11:545–554. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Parry G, Estelle M. Auxin receptors: a new role for F-box proteins. Curr. Opin. Cell Biol. 2006;18:152–156. doi: 10.1016/j.ceb.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- Pawson T, Nash P. Assembly of cell regulatory systems through protein interaction domains. Science. 2003;300:445–452. doi: 10.1126/science.1083653. [DOI] [PubMed] [Google Scholar]
- Pyo H, Demura T, Fukuda H. Spatial and temporal tracing of vessel differentiation in young Arabidopsis seedlings by the expression of an immature tracheary element-specific promoter. Plant Cell Physiol. 2004;45:1529–1536. doi: 10.1093/pcp/pch175. [DOI] [PubMed] [Google Scholar]
- Rienties IM, Vink J, Borst JW, Russinova E, de Vries SC. The Arabidopsis SERK1 protein interacts with the AAA-ATPase AtCDC48, the 14-3-3-protein GF14lamba and the PP2C phosphatase KAPP. Planta. 2005;221:394–405. doi: 10.1007/s00425-004-1447-7. [DOI] [PubMed] [Google Scholar]
- Robatzek S, Chinchilla D, Boller T. Ligand-induced endocytosis of the pattern recognition receptor FSL2 in Arabidopsis. Genes and Dev. 2006;20:537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado A, Schapire AL, Bressan RA, Harfouche AL, Hasegawa PM, Valpuesta V, Botella MA. The Arabidopsis tetratricopeptide repeat-containing protein TTL1 is required for osmotic stress responses and abscisic acid sensitivity. Plant Physiol. 2006;142:1113–1126. doi: 10.1104/pp.106.085191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann JU. A gene expression map of Arabidopsis thaliana development. Nat. Genet. 2005;37:501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004;16:1220–1234. doi: 10.1105/tpc.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth LE, Deyholos MK. Vascular development: the long and winding road. Curr. Opin. Plant Biol. 2006;9:48–54. doi: 10.1016/j.pbi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Sieburth LE, Muday GK, King EJ, Benton G, Kim S, Metcalf KE, Meyers L, Seaman E, Van Norman JM. SCARFACE encodes an ARF-GAP that is required for normal auxin efflux and vein patterning in Arabidopsis. Plant Cell. 2006;18:1396–1411. doi: 10.1105/tpc.105.039008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Vierstra RD. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004;55:555–590. doi: 10.1146/annurev.arplant.55.031903.141801. [DOI] [PubMed] [Google Scholar]
- Stone JM, Collinge MA, Smith RD, Horn MA, Walker JC. Interaction of a protein phosphatase with an Arabidopsis serine-threonine receptor kinase. Science. 1994;266:793–795. doi: 10.1126/science.7973632. [DOI] [PubMed] [Google Scholar]
- Swain SM, Singh DP. Tall tales from sly dwarves: novel functions of gibberellins in plant development. Trends Plant Sci. 2005;10:123–129. doi: 10.1016/j.tplants.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- Tang W, Deng Z, Oses-Prieto JA, Suzuki N, Zhu S, Zhang X, Burlingame AL, Wang ZY. Proteomic studies of brassinosteroid signal transduction using prefractionation and 2-D DIGE. Mol. Cell Proteomics. 2007;6:2058–2071. doi: 10.1074/mcp.M700358-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU. Leucine-rich repeat receptor kinases in plants: structure, function and signal transduction pathways. Int. Rev. Cyt. 2004;234:1–46. doi: 10.1016/S0074-7696(04)34001-5. [DOI] [PubMed] [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Wang X, Chory J. Brassinosteroids regulate dissociation of BIK1, a negative regulator of BRI1 signaling, from the plasma membrane. Science. 2006;313:1118–1122. doi: 10.1126/science.1127593. [DOI] [PubMed] [Google Scholar]
- Zaleman G, Dorsevil O, Garcia-Ranea JA, Gacon G, Camonis J. RhoGAPs and RhoGDIs, (His)stories of two families. Prog. Mol. Subcell. Biol. 1999;22:85–113. doi: 10.1007/978-3-642-58591-3_5. [DOI] [PubMed] [Google Scholar]
- Zhou A, Wang H, Walker JC, Li J. BRL1, a leucine-rich repeat receptor-like protein kinase, is functionally redundant with BRI1 in regulating Arabidopsis brassinosteroid signaling. Plant J. 2004;40:399–409. doi: 10.1111/j.1365-313X.2004.02214.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Table SI. VHI/BRL2kin interaction candidates with medium signal strength. Column contents as in Table I.
Table SII. VHI/BRL2kin interaction candidates with weak signal strength. Column contents as in Table I.
Table SIII. Characteristics of T-DNA insertion lines in notable interaction candidates.
Table SIV. VIT interaction candidates with medium signal strength.
Table SV. VIT interaction candidates with weak signal strength.
Table SVI. Primer pairs used in this work.
Figure S1. Immunoprecipitations showing interactions of VH1/BRL2kin with the adaptors VIT and VIK.
Figure S2. T-DNA mutant lines in VH1, VIT and VIK
Figure S3. Root length of Col-0 (col) wild-type and vh1, vit, and vik insertion lines after a week of vertical growth on basal media plates.
Figure S4. Comparison of VIT and VH1 reporter lines staining during imbibition
Figure S5. Comparison of VIT and VH1 reporter lines staining during leaf development from 5 to 7 days after germination.