Abstract
Purpose
Most chemotherapy (CT) administration occurs in routine care settings, yet little is known about treatment-related toxicity outside of clinical trials. To examine trends in toxicity, modify practice, and establish benchmarks for severe toxicity in a community cancer center we created a prospective registry of all treatment-related hospitalizations at the North Shore Medical Center Cancer Center, a community-based cancer facility in Peabody, MA.
Methods
Eligible population consisted of all adult cancer patients admitted to the hospital within 30 days of their last CT administration. Each admission was reviewed by a panel of hospital staff to determine whether admission was treatment-related. Information on admission was collected using a standard form.
Results
Between October 2001 and December 2003, there were 365 hospitalizations among patients receiving CT, 117 (32%) of which were deemed treatment-related. The median age of the cohort with treatment-related toxicity was 67 years, and 41% were male. Most frequent diagnoses were non-Hodgkin's lymphoma (23%) and colorectal cancer (21%), with 49% of the patients receiving treatment with palliative intent. The most common reasons for admission were gastrointestinal toxicity or infection. The mean length of stay was 7.1 days. Seven patients (6%) died during hospitalization. When the registry was reviewed to identify areas where care may be improved, several admissions for decadron-related hyperglycemia in nondiabetic patients with myeloma were noted. This led to introduction of glucose monitoring guidelines with no subsequent admissions for this toxicity since then.
Conclusions
About one third of hospital admissions in patients receiving CT are treatment-related and most occur in patients with advanced disease. Collection of data on toxicity in the routine care setting is feasible and may facilitate quality improvement.
Introduction
Each year more than 1 million Americans are diagnosed with cancer,1many of whom receive chemotherapy as part of their treatment at some point during their illness. It is well appreciated that chemotherapeutic drugs have a narrow therapeutic index with a close trade-off between benefit and toxicity, especially when cure is not the primary treatment goal. To date, the majority of data on this trade-off, including the risks of treatment-related toxicity, have come from clinical trials, but only a minority of cancer patients receives care in such settings. Furthermore, given the high level of patient selection that occurs in clinical trials, coupled with potentially more extensive follow-up often mandated by trial protocols relative to regular care, toxicity outcomes observed in clinical trials may not be a true reflection of the risk associated with therapy in routine care settings.
In addition to the potential negative impact on quantity and/or quality of life, treatment-related toxicity can have significant resource implications. In a study of Medicare beneficiaries with metastatic colorectal cancer, both the incidence and costs of hospitalizations were higher among patients who received fluorouracil-based chemotherapy than in patients who did not.2In a Canadian study of severe chemotherapy-induced diarrhea in patients with colorectal cancer, one third of patients with ≥ grade 3 diarrhea required hospital admission with a median length of stay of 8 days and the mean cost per patient of over $2,500.3Data on treatment-related toxicity in the community is important in order to determine benchmarks for toxicity and to identify ways in which practice may be modified to minimize toxicity. This data can also inform treatment decision-making by giving physicians and patients more realistic expectations regarding the risks of therapy.
The current study was designed in part to help fill the gap on chemotherapy-related toxicity in routine care settings. Our specific goals were to create an easy-to-utilize mechanism for identifying all patients hospitalized with chemotherapy-related toxicity, to create a registry of toxicity cases that can be periodically monitored for trends and that can be utilized to modify practice patterns, and to establish benchmarks for severe toxicity in a community cancer center.
Methods
Setting
The North Shore Medical Center Cancer Center (NSMCCC) is a community-based, freestanding cancer facility located in Peabody, Massachusetts. It is an outpatient facility, which provides on-site chemotherapy and radiation therapy services principally to patients residing in Peabody, MA and eight surrounding towns. This primary service area includes 216,817 adults. It is the main such facility in the area, providing approximately 51% of cancer services to patients in this geographic region. Each year, approximately 1,000 to 1,200 new cancer cases are evaluated in the clinic and about 3,000 chemotherapy administrations are given. The NSMC-CC is affiliated with Salem Hospital (Salem, MA), Union Hospital (Peabody, MA), and with Dana-Farber Partners Cancer Care (Boston, MA). More than 95% of in-patient care required by patients being treated at the NSMC-CC occurs at either the Salem or Union Hospitals. A small proportion may also be admitted to the Beverly Hospital in Beverly, MA.
Case Identification and Data Collection
Prospective data collection on chemotherapy-related hospital admissions began October 1, 2001, as part of a quality improvement project. Eligible population included all adult (age ≥18 years) cancer patients receiving chemotherapy at the NSMC-CC, who were admitted to hospital within 30 days of their last chemotherapy administration. Nurses involved in each patient's care were responsible for case identification. All patients receiving treatment at the NSMC-CC have two nurses involved in their care, one of whom would complete an admission form each time a patient was hospitalized. Most admissions were identified as a result of a cancelled appointment or contact from the patient or family member. In addition, each day, one of the nurses would also track all admissions to the two main hospitals where admissions occurred. We estimate that this resulted in case capture of over 95%.
For each admission, a standard data collection form developed by the investigators was completed using the hospital and clinic charts as the primary data sources. The form was completed at the time of admission and then updated at the time of the review by the team. Information that was collected included patient demographic data, characteristics of the cancer diagnosis, comorbidity data, treatment details and indication, reason for admission, length of hospital stay, and outcome of admission (resolved, ongoing, fatal). Patients were considered to have history of comorbid illness if either the admission note or the original consult note from the Center made note of a prior comorbidity. Only diagnoses that make up the Charlson comorbidity score were considered.4These include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease, diabetes, hemiplegia, renal disease, or AIDS. An actual Charlson comorbidity score was not calculated, as there was not enough data in charts to determine severity of comorbid illness.
All admissions were reviewed on a monthly basis by a team made up of physicians, nurses, and pharmacists to decide whether an admission was treatment- as opposed to disease-related. In general, the peer group chose to be overinclusive when defining treatment-related toxicity. If a patient was admitted with an illness potentially related to chemotherapy, then the team usually elected to consider the process treatment-related even if other potential explanations existed. For example, a thromboembolic event in a patient receiving thalidomide and decadron was considered treatment-related even if other risk factors for thrombosis were present.
Throughout the study period, the NSMC-CC Pharmacy Department kept track of the number of patients treated with intravenous chemotherapy each month. A patient was counted once per month regardless of the number of actual infusions. Starting January 1, 2003, the Pharmacy Department also began to track all patients initiating a new chemotherapy regimen, including patient identification number, diagnosis, and type of regimen.
The study was approved by the North Shore Medical Center Institutional Review Board.
Quality Improvement Component
The data was periodically reviewed by the study team to identify trends that may have required modification of practice at the Center. The initial review occurred in March 2003, with subsequent reviews every 4 months. A trend was defined as an unexpected increase in admissions related to a specific cancer diagnosis or treatment regimen. All potential trends were discussed by the team to determine possible quality improvement initiatives.
Analytical Considerations
We used summary statistics to describe characteristics of patients admitted with chemotherapy-related toxicity and to calculate toxicity rates. To check accuracy of the data collection process, a second data collection form was completed by one of the investigators (J.T.) for 10 randomly chosen admissions, and the form was compared with the original form completed at the time of admission by calculating percent agreement for individual items. Because this exercise revealed a moderate level of disagreement (defined as percent agreement < 70%) for several items, all of the admissions that were deemed treatment-related were reviewed a second time to ensure data accuracy. The second review was performed by two of the investigators (J.O.J., J.T.).
Results
Characteristics of the Cohort Admitted for Toxicity
Between October 1, 2001 and December 31, 2003, a total of 365 cancer patients receiving chemotherapy at the NSMC-CC were admitted to the hospital. Of these admissions, 117 (32%) were deemed as treatment-related by the team. The median time from last chemotherapy administration to admission was 8 days, and the mean length of hospital stay was 7.1 days (range, 0 to 84 days). Seven of the patients (6%) died during the admission.
Characteristics of the cohort are presented in Table 1. The median age was 67 years and more than 50% of the cohort was female. Approximately one third of the patients had history of comorbid illness. The most frequent comorbidities were diabetes and chronic pulmonary disease. The two most common cancer diagnoses among admitted patients were colorectal cancer and nonHodgkin's lymphoma and approximately 50% of patients were receiving treatment with palliative intent. Gastrointestinal or infectious complications were the most frequent reasons for admission. Among patients admitted with gastrointestinal toxicity, the most frequent presenting symptoms were either diarrhea or severe nausea and vomiting, which accounted for approximately three quarters of admissions. Almost three quarters of the patients admitted with toxicity were receiving either fluorouracil or platinum-based therapy or CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone).
Table 1.
Characteristics of Patients Hospitalized with Treatment-related Toxicity (N=117)
| Characteristic | No. of Patients | (%) |
|---|---|---|
| Age, years | ||
| Median | 67 | |
| Range | 21–93 | |
| No. of women | 69 | 59 |
| History of comorbidity | 44 | 38 |
| Cancer type | ||
| NHL | 27 | 23 |
| Colorectal cancer | 24 | 21 |
| Lung | 15 | 13 |
| Breast | 9 | 8 |
| Multiple myeloma | 8 | 7 |
| Esophagus | 6 | 5 |
| Other | 28 | 24 |
| Toxicity responsible for admission | ||
| Gastrointestinal | 44 | 38 |
| Infectious | 42 | 36 |
| Hematologic | 12 | 10 |
| Cardiac | 7 | 6 |
| Metabolic | 6 | 5 |
| Other | 6 | 5 |
| Treatment intent | ||
| Curative | 36 | 31 |
| Adjuvant | 24 | 30 |
| Palliative | 57 | 49 |
| Type of regimen | ||
| FU-based | 30 | 26 |
| Platinum-based* | 30 | 26 |
| CHOP | 24 | 21 |
| Oral | 8 | 7 |
| Other | 25 | 21 |
| Concurrent radiation | 10 | 9 |
Abbreviations: NHL, non-Hodgkin's lymphoma; FU,fluorouracil; CHOP, cyclophosphamide, adriamycin, vincristine, prednisone.
Includes 5 patients who received both FU and cisplatin.
Admission Rates
Figure 1 shows the number of patients treated with chemotherapy each month versus the number of patients admitted with treatment-related toxicity. Each month, between 226 and 400 patients received at least one dose of parenteral chemotherapy, of whom less than 4% were admitted with treatment-related toxicity. Overall, during the study period, approximately 8,258 patient-months of parenteral chemotherapy were administered (defined as the number of patients receiving intravenous chemotherapy per month summed over the 27 months of the study). This translates into a risk of hospitalization as a result of treatment-related toxicity of 0.16 hospitalizations per patient-year of treatment, and risk of mortality from treatment of 0.01 deaths per patient-year of treatment.
Figure 1.
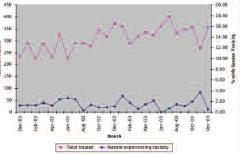
Monthly Treatment and Toxicity Rates
The five most common cancer diagnoses to be treated with chemotherapy at the NSMC-CC during 2003 were lung, breast, colorectal, and esophageal cancers and nonHodgkin's lymphoma. Among these five cancer diagnoses, the proportion of patients admitted with treatment-related toxicity during the same time period ranged from 4% to 20% (Table 2). The proportion of patients with one of these diagnoses that were admitted to hospital with treatment-related toxicity was highest for colorectal cancer (20%).
Table 2.
Annual Admission Rates by Diagnosis During 2003 for the Five Most Common Cancers
| Diagnosis | No. of Patients Who Received at Least One Dose of Chemotherapy in 2003 | Patients Admitted with Treatment-Related Toxicity During 2003 |
|
|---|---|---|---|
| No. of Patients | % | ||
| Lung | 131 | 7 | 5 |
| Breast | 91 | 4 | 4 |
| Colorectal | 65 | 13 | 20 |
| NHL | 39 | 7 | 18 |
| Esophagus* | 26 | 4 | 15 |
NHL = non-Hodgkin's lymphoma
Includes patients with gastroesophageal junction tumors
Quality Improvement Initiative
The data were first reviewed for trends in March 2003 and then every 4 months thereafter to identify ways in which patient care may potentially be improved. During the first review of the data, several admissions for decadron-related hyperglycemia in nondiabetic patients with multiple myeloma receiving high-dose decadron as part of their treatment were identified. This led to the development and institution of glucose monitoring guidelines for all patients on treatment regimens that included high-dose steroids. Subsequently, no further admissions for this complication occurred.
During a subsequent review of the data in July 2003, a significant number of admissions for treatment-related diarrhea among patients with colorectal cancer were noted. This led the team to develop a diarrhea flow-sheet, a copy of which is presented in Figure 2. As this is a fairly recent initiative, the impact of this practice change is still being evaluated.
Figure 2.
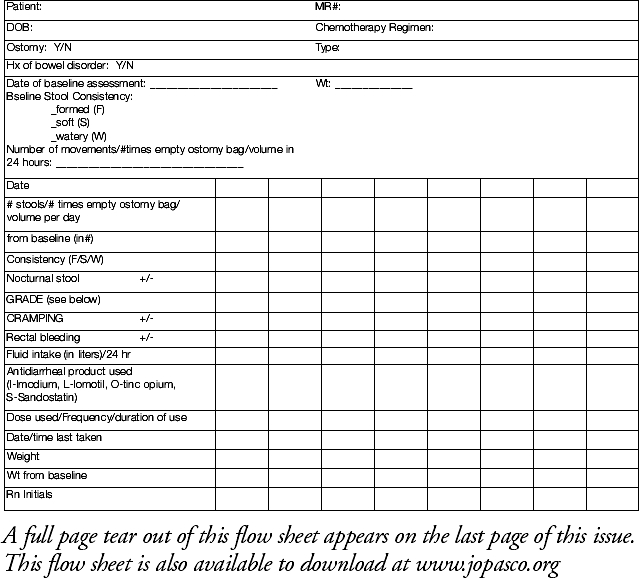
Diarrhea Flow Sheet
Discussion
Our study illustrates that collection of toxicity data in a community cancer center is feasible, can facilitate quality improvement initiatives, and may potentially improve patient outcomes. In our cohort, approximately one third of all admissions among patients receiving chemotherapy were deemed to be treatment-related, and almost half of the patients admitted with toxicity were receiving treatment with palliative intent. While the overall risk of serious toxicity (defined as toxicity resulting in hospitalization) relative to volume of chemotherapy administered was small, the mortality rate associated with hospitalization for treatment-related toxicity was 6%. Interestingly, the risk of admission varied depending on diagnosis, with approximately one in five patients treated for colorectal cancer during 1 year of the study being admitted with treatment-related toxicity compared with one in 20 breast cancer patients.
Many specialties, especially the surgical ones, routinely report adverse outcomes and use the data for credentialing purposes and to modify practice. To date, this has not been standard practice among medical oncologists or other nonsurgical specialties where the majority of treatment is medication-based. However, with the increasing awareness of both the human and fiscal costs of medication-related adverse events5and increasing overall interest in quality of cancer care,6,7this may be changing. Our study is one of the first to focus on the issue of chemotherapy-related toxicity in the routine care setting. It demonstrates that tracking of adverse events in the routine care setting is feasible and can improve outcomes. The NSMC-CC is a good environment to study this issue, as most of the patients served by the Center receive care in a limited number of places, assuring a high ascertainment rate. The few prior studies that have looked at the issue of treatment-related toxicity in chemotherapy patients have tended to use large administrative databases.2,8These databases have the advantage of a large volume of patients and have proven useful for looking at complication rates following surgical interventions; however, they have not been found to be a reliable source of data on complications of nonsurgical cancer treatments such as systemic therapy.9Another unique aspect of our study is that we looked at different types of cancer, whereas previous investigators have generally focused on a single tumor type.2,3,8While we were not able to generate rates of specific toxicities per regimen as is done in clinical trials, we were able to provide insight into overall rates of toxicity for regimens based on certain drugs or for certain diagnoses.
One of the most interesting findings in our study was the high frequency of hospital admissions among patients with colorectal cancer, the majority of which were related to treatment-related diarrhea. Recently, several studies have indicated that the incidence of this complication among patients with colon cancer may be higher than previously appreciated and associated with significant morbidity and cost.2,3This is cause for concern, given that half of the hospitalizations in our study occurred among patients receiving treatment with palliative intent. While patients may be quite willing to accept toxic treatments for rather small benefits, especially when cure or improved survival are at stake,10many patients are likely not aware of the actual magnitude of risk involved with therapy.
One of the main goals of our study was to determine whether collecting data on treatment-related toxicity could facilitate quality improvement. During the study period, we identified two major trends in toxicity. The first related to treatment-related admissions for hyperglycemia among nondiabetic patients with multiple myeloma, and the second to treatment-induced diarrhea among patients with colorectal cancer. What we found in the case of treatment-induced hyperglycemia is that a simple change in our practice, namely awareness of the problem coupled with routine checking of glucose in all patients receiving high-dose corticosteroids as part of their therapy, was all that was necessary to prevent any further serious toxicity of this kind. In the case of treatment-induced diarrhea, it is yet too early to determine whether our efforts thus far have made a substantial impact on patient outcomes. Regardless, our findings regarding chemotherapy-induced diarrhea in patients with colorectal cancer, along with similar findings by a Canadian group,3indicate that this may be a very common but underappreciated toxicity among cancer patients and one that most cancer centers should probably have a closer look at. It may be that lack of awareness by the medical team of the frequency and seriousness of this toxicity that results in late recognition at the stage where admission is required for management.
Several limitations of our study should be noted. Firstly, we may have potentially missed a small number of patients who either died without admission but had treatment-related toxicity or those admitted to hospitals outside of the system. However, given the provision of health services to patients in this part of Massachusetts, this is unlikely to be a significant problem. Secondly, we only collected information on patients receiving chemotherapy who were admitted with toxicity and not on all patients receiving chemotherapy, thus our data do not allow us to comment on risk factors for admission. This is an important issue, and we are in the process of expanding the data collection process to include all patients receiving treatment.
Moreover, our data is limited to a single center providing cancer care in the community and with limited data on this issue from other centers, it is difficult to comment on how our rates compare to other institutions. Lastly, our data is limited to serious toxicity, which we defined as that requiring hospital admission for management, thus we are likely underestimating the overall extent of treatment-related toxicity. However, we felt that toxicity requiring in-patient management was the most important to address initially, as it is likely to have the greatest impact on patient outcomes and resource use.
In conclusion, about one third of hospital admissions among patients receiving chemotherapy in the routine care setting are treatment-related. This study demonstrates that the tracking of chemotherapy-related toxicity in a community cancer center is feasible, can facilitate quality improvement initiatives, as well as potentially improve patient care. It is time that adverse event monitoring among cancer patients receiving chemotherapy outside of clinical trials becomes the standard of care.
Figure 3.

Footnotes
Authors' Disclosures of Potential Conflicts of Interest
The following authors or their immediate family members have indicated a financial interest. No conflict exists for drugs or devices used in a study if they are not being evaluated as part of the investigation. Consultant or Advisory Role: Jean Treacy, Ortho Biotech. Honoraria: Jean Treacy, Biogen Idec.
References
- 1.This study has been presented in part at the 40th Annual Meeting of the American Society of Clinical Oncology, New Orleans, LA, June 6, 2004
- 2.Jemal A, Tiwari RC, Murray T, et al: Cancer statistics, 2004. CA Cancer J Clin 54:8-29, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Delea TE, Vera-Llonch M, Edelsberg JS, et al: The incidence and cost of hospitalization for 5-FU toxicity among Medicare beneficiaries with metastatic colorectal cancer. Value Health 5:35-43, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Dranitsaris G, Maroun J, Shah A: Severe chemotherapy-induced diarrhea in patients with colorectal cancer: A cost of illness analysis. Proc Am Soc Clin Oncol 23:14S, 2004. (abstr 6087) [DOI] [PubMed] [Google Scholar]
- 5.Charlson ME, Pompei P, Ales KL, et al: A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373-383, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Juurlink DN, Mamdani MM, Lee DS, et al: Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 351:543-551, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Schneider EC, Epstein AM, Malin JL, et al: Developing a system to assess the quality of cancer care: ASCO's national initiative on cancer care quality. J Clin Oncol 22:2985-2991, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Ayanian JZ, Chrischilles EA, Wallace RB, et al: Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol 22:2992-2996, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Du XL, Osborne C, Goodwin JS: Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J Clin Oncol 20:4636-4642, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potosky AL, Warren JL, Riedel ER, et al: Measuring complications of cancer treatment using the SEER-Medicare data. Med Care 40:62-68, 2002. ((8 suppl)) [DOI] [PubMed] [Google Scholar]
- 11.Slevin ML, Stubbs L, Plant HJ, et al: Attitudes to chemotherapy: Comparing views of patients with cancer with those of doctors, nurses, and general public. BMJ 300:1458-1460, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]


