Abstract
Purpose: To compare endovascular (EVAR) and open surgical repair (OSR) for ruptured abdominal aortic aneurysms (RAAA) in terms of preoperative hemodynamic status and comorbidities.
Methods: The 2005 to 2007 American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database was interrogated to find all patients undergoing repair for RAAA. Of the 567 RAAA repairs identified, 121 (21%) were endovascular and 446 (79%) were open. Demographics, comorbidities, and preoperative hemodynamic status were compared by repair method.
Results: Age, sex, and race were similar between repair cohorts. EVAR patients had greater incidences of recent myocardial infarction (7% versus 2%, p<0.05), revascularization or amputation for peripheral vascular disease (8% versus 3%, p<0.05), and cerebrovascular disease (22% versus 11%, p<0.01). Preoperative hemodynamic status was similar based on need for >4 units of blood (3% versus 6%, p = 0.31), intubation (12% versus 17%, p = 0.18), impaired sensorium (7% versus 11%, p = 0.25), coma (4% versus 5%, p = 0.65), acute renal failure (2% versus 2%, p = 0.60), and ASA class 5 (29% versus 34%, p = 0.29). Open repair was associated with greater operative time (3.3 versus 2.6 hours, p<0.01) and intraoperative blood transfusions (8 versus 2 units, p<0.001). Overall mortality was 33.5% (EVAR 24% versus OSR 36%; OR 1.8, 95% CI 1.1 to 2.8, p<0.05). After adjusting for preoperative comorbidities and all preoperative hemodynamic variables, mortality after open repair was greater than after EVAR (OR 1.9, 95% CI 1.1 to 3.2, p<0.05). Overall postoperative complications were greater after open repair (62% versus 47%, p<0.01). Graft failure requiring reintervention was higher after EVAR (4% versus 1%, p<0.05), while rates of return to the operating room for a major operation were similar (21% versus 24%, p = 0.43).
Conclusion: For RAAA within NSQIP hospitals in recent years, preoperative hemodynamic status was similar between EVAR and OSR, but EVAR patients had greater comorbidities. Despite this and after accounting for minor differences in hemodynamic status, EVAR mortality was lower than OSR mortality. Institutions with adequate experience and resources should attempt endovascular repair for RAAA when anatomy allows.
Keywords: ruptured abdominal aortic aneurysm, endovascular aneurysm repair, open repair, National Surgical Quality Improvement Program
The adoption of endovascular aneurysm repair (EVAR) for ruptured abdominal aortic aneurysms (RAAA) has lagged behind the technique's application for intact, elective abdominal aortic aneurysms (AAA).1 With emerging data from centers of excellence and community hospitals, it is apparent that EVAR for RAAA has shown a steady increase.2–8 Comparison of this method of repair to its traditional open repair counterpart is difficult, however, as preoperative differences in hemodynamic stability are likely to play a large role in the decision for one type of repair over the other.
Early reports have been especially favorable in centers where a standardized treatment protocol has been established for EVAR as a first-line therapy for RAAA.2–5 Describing the results from one such program, Mehta et al.2 were able to show an 18% mortality rate following EVAR with a 95% success rate for the procedure, which compares favorably to prior repairs in which mortality for RAAA has hovered at 40% to 50% for the past 4 decades.9 Moreover, this and other small institutional studies may not be representative of the population at large. We therefore looked at the current outcomes of EVAR and open repair for RAAA within the National Surgical Quality Improvement Program (NSQIP), which is representative of community as well as academic medical centers. The NSQIP, developed for general and vascular surgical outcomes quality assessment, is a large database with contributions by over 200 hospitals nationwide. It contains a comprehensive list of preoperative comorbidities, preoperative variables reflecting hemodynamic stability, intraoperative variables, 30-day postoperative complications, and 30-day mortality, which makes it well suited for the comparison of RAAA repair methods.
METHODS
Database
The NSQIP is a multi-institutional, risk-adjusted, national, prospectively collected database created to facilitate quality control review of outcomes. It originally began in Veterans Administration centers and was expanded to the private sector in a pilot study of 14 academic medical centers. Since the pilot study concluded in 2004, enrollment has steadily grown to include 221 participating community and academic medical centers across the US. Dependent upon its overall volume, each center contributes all or a portion of operative cases performed, with selection occurring in a rotating manner to ensure random representation of procedures and outcomes. Clinical nurse reviewers are specially trained for data review and recording patient information. A comprehensive array of preoperative comorbidity data is collected along with operative and perioperative information. The 30-day postoperative outcomes, including post-hospitalization information, is collected from hospital records, clinic visits, and/or follow-up phone contact.10
Data Retrieval
The NSQIP database from 2005 to 2007, the most recent years available, was queried using SAS statistical software (version 9.2; SAS Institute Inc., Cary NC, USA). Patients with RAAA were identified by the primary ICD-9 (International Classification of Diseases, 9th ed.) diagnosis codes (441.3, 441.5) and by CPT-4 (Current Procedural Terminology, 4th ed.) procedure codes for EVAR (0078T-81T, 34800, 34802-05, 34812-13, and 34825-26) or open surgical repair (35081-82, 35091-92, and 35102-03). Patients with tandem codes for thoracic and thoracoabdominal aneurysm repairs (33875, 33880-81, and 33877) were excluded. Patients undergoing open conversion after an initial EVAR attempt (34830-32) were included as EVAR patients as an intention-to-treat analysis.
Preoperative demographic and comorbidity variables were recorded for each patient. Preoperative status variables were time dependent and included blood transfusion >4 units within 72 hours, preoperative intubation within 48 hours, altered sensorium within 48 hours, coma entering surgery, acute renal failure within 24 hours, and American Society of Anesthesiology (ASA) classification. Intraoperative variables included blood transfusion volume, operative time, and intraoperative complications (cardiac arrest, myocardial infarction, or unplanned intubation). Thirty-day postoperative events were wound infection (superficial, deep, or organ space infection), wound dehiscence, graft failure, return to the operating room, and 13 other NSQIP-defined complications. Graft failure was defined by NSQIP as “mechanical graft failure requiring return to the operating room, interventional radiology, or balloon angioplasty.” Other postoperative outcomes included perioperative mortality (30-day) and hospital length of stay.
Patient Sample
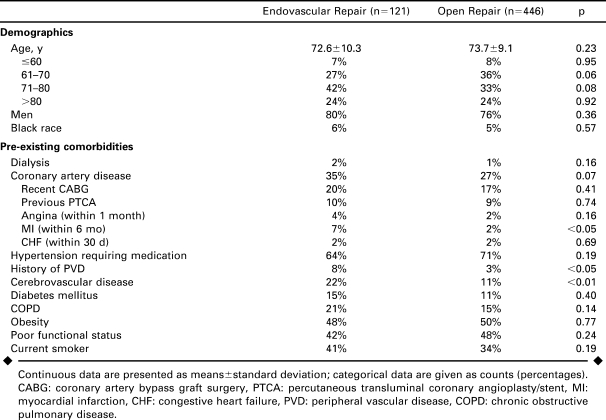
Of the 567 RAAA repairs identified, there were 121 (21%) patients who underwent EVAR (97 men; mean age 72.6±10.3 years) and 446 (79%) patients (339 men; mean age 73.7±9.1 years) who had open repairs during the time period (Table 1). Five patients underwent EVAR with immediate conversion to open repair.
TABLE 1.
Demographics and Comorbidities of Patients Undergoing Endovascular or Open Repair of Ruptured AAAs Within the ACS-NSQIP Population 2005–2007
Statistical Analysis
Demographics, comorbidities, and perioperative events and outcomes were compared between open and EVAR cohorts. Categorical variables were analyzed using chi-square or Fisher exact tests; continuous variables were compared using Student t tests for parametric data or the Wilcoxon rank sum test for nonparametric data. Preoperative predictors of morbidity and mortality were analyzed by univariate and multivariate logistic regression methods. Statistical significance was defined as p<0.05. Statistical analysis was performed using STATA statistical software (StataCorp LP, College Station, TX, USA).
RESULTS
In terms of demographics (Table 1), the 2 groups were evenly matched for age, gender, and race; White males made up more than 3 quarters of the overall group. Prior myocardial infarction, history of a prior revascularization or amputation for peripheral vascular disease, and cerebrovascular disease were all significantly more common in EVAR patients. The only comorbid conditions that showed a greater frequency in open repair patients were hypertension and poor functional status, while the remaining conditions were all slightly greater in EVAR patients.
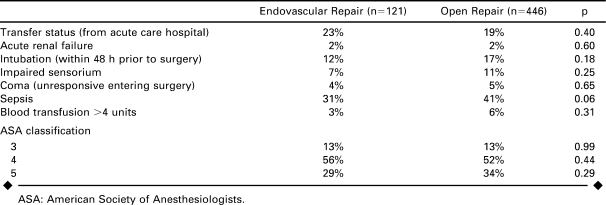
Markers of immediate preoperative status showed that patients were similar going into surgery (Table 2). Seventeen percent of open repair patients were intubated within 48 hours prior to surgery versus 12% of EVAR (p = 0.18) and 5% of open repair patients were unresponsive entering surgery compared to 4% of EVAR (p = 0.65). ASA classification was similar for both types of repair.
TABLE 2.
Preoperative Status Characteristics of Patients Undergoing Endovascular and Open Repair of Ruptured AAAs Within the ACS-NSQIP Population 2005–2007
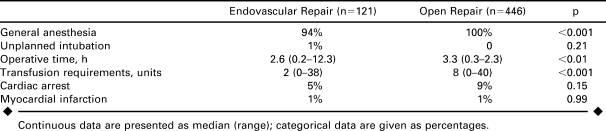
All open repair patients were operated on under general surgery, while 6% of EVAR patients had epidural or monitored anesthesia (p<0.001; Table 3). One EVAR patient required an unplanned intraoperative intubation. The median time for operation was 3.3 hours for open versus 2.6 hours for EVAR (p<0.01). Intraoperative blood transfusion was 4 times greater in open cases. Conventionally treated patients had a 9% incidence of intraoperative cardiac arrest, while EVAR patients had a 5% incidence (OR 2.1, 95% CI 0.8 to 4.6, p = 0.15).
TABLE 3.
Intraoperative Outcomes for Endovascular and Open Repair of Ruptured AAAs Within the ACS-NSQIP Population 2005–2007
Less than 4% of all RAAA repairs were performed by general surgeons as compared to vascular specialists, with only 1 (0.9%) EVAR performed by a general surgeon. In contrast, a review of intact AAA repairs within the database showed that general surgeons performed 2.5% of EVAR cases for intact AAA versus 2.7% of open repairs.
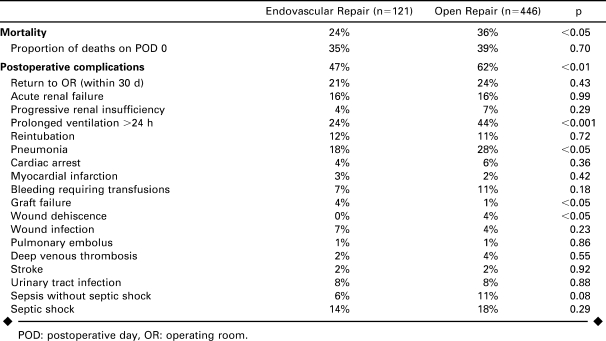
Overall mortality (Table 4) was 33.5%: 24% after EVAR compared to 36% after open surgery (p<0.05). Among patients who died, approximately one third died on the day of operation. Three of the 5 patients who underwent unsuccessful EVAR attempts and required immediate open conversions also died. Mortality of successful EVAR was 22%. Overall morbidity was greater after open repair (62% versus 47% for EVAR, p<0.05). Twenty percent of EVAR patients and 24% of open patients (p = 0.31) required a subsequent major operation within 30 days. The procedure performed and its relation to the aneurysm repair could not be determined from available data; however, 4% of EVAR patients and 1% of open patients were separately reported as having graft failure requiring reoperation or endovascular reintervention (p<0.05).
TABLE 4.
Mortality and Morbidity After Endovascular and Open Repair of Ruptured AAAs Within the ACS-NSQIP Population 2005–2007
Wound dehiscence (4%) and infections (4%; Table 4) occurred more frequently in open patients versus EVAR [0% (p<0.05) and 7% (p = 0.23), respectively]. Respiratory complications, including prolonged ventilation and pneumonia, were more common after open surgery as well.
Median length of stay was 10 days (range 0–99) after open repair and 7 days (range 0–76) after EVAR (p = 0.10). Among survivors, length of stay was 14 days (range 0–99) after open repair and 8 days (range 0–76) after EVAR (p<0.001).
Predictors of Morbidity and Mortality
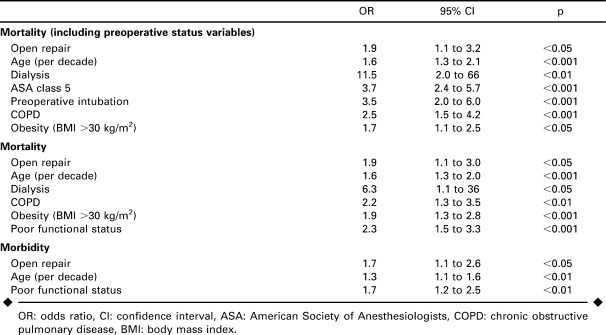
Predictors of mortality (Table 5) on multivariate logistic regression included open repair, age, dialysis, ASA class 5, preoperative intubation, chronic obstructive pulmonary disease, and obesity (body mass index >30 kg/m2). Open repair was associated with a 2-fold increase in mortality risk, while ASA class 5 and intubation within 48 hours prior to surgery were associated with a 3- to 4-fold increase. When markers of preoperative status were not included in the multivariate analysis, functional status became significant. Open repair still had a 2-fold increased mortality risk compared to EVAR. Predictors of morbidity included open repair, age, and poor functional status. None of the preoperative status variables were independently predictive of morbidity.
TABLE 5.
Multivariate Predictors of Mortality and Morbidity After Endovascular or Open Repair of Ruptured AAAs Within the ACS-NSQIP Population 2005–2007
DISCUSSION
This study provides a broad assessment of national outcomes of EVAR and open repair for RAAA. The most important finding was that the rate of mortality for patients undergoing EVAR was reduced by 33% compared to patients undergoing open repair in spite of greater comorbidities in EVAR patients and similar preoperative status. The rate of perioperative complications was also lower in the EVAR cohort. In multivariate analysis, open repair was associated with a 2-fold increased risk of mortality independent of demographics, comorbidities, and preoperative status.
In spite of reduced mortality, complications occur as frequently for patients undergoing EVAR as for open repair. The most frequent complication was prolonged intubation, followed by reoperation, pneumonia, shock, and acute renal failure. The rate of graft failure requiring reintervention (4%) was reasonable, especially considering the urgent nature under which stent-grafts were implanted and the limited time for preoperative planning.
The 24% EVAR mortality compares well to the 18% mortality rate reported by Mehta et al.2 Considering that the NSQIP includes a broad mix of community and academic medical centers, this result is impressive. As NSQIP is a quality improvement tool, however, most participating institutions likely represent high volume centers with an interest in improving their own outcomes. Other institutional studies have reported mortality rates of 8% to 40%, but the number of patients within institutional studies, however, is still quite small.2–8 Two recent meta-analyses reported pooled outcomes on EVAR for RAAA procedures. Rayt et al.12 found a mortality of 24% for 982 patients, while Azizzadeh et al.13 reported a mortality of 30% in 531 patients.
Comparisons are also favorable for EVAR within administrative database studies in the US population, which have included the Nationwide Inpatient Sample, state inpatient databases, and Medicare.14–16 These lack the ability to assess any preoperative status variability, however, so this limitation must be recognized. A recent analysis of RAAA repair within the Medicare population showed a mortality benefit for EVAR compared to open repair within a propensity-matched sample and demonstrated a significant surgeon and hospital volume effect, with higher volume physicians/centers having improved results.16 This further strengthens the argument that when centers have the resources and experience for EVAR in emergency situations, this should be the operation of choice when anatomical factors allow.
We did not identify significant differences in preoperative status that could bias against open repair. This is somewhat surprising given the common assumption that unstable patients are suitable only for open repair.8 It may be that as centers become more experienced with emergency EVAR and develop a standardized treatment protocol that includes an on-call team and decision algorithms to bypass imaging in favor of direct transfer to an endovascular operating room with percutaneous balloon occlusion of the suprarenal aorta, EVAR becomes a viable option despite instability. In our current analysis, which spans recent years and captures a variety of institutional types, we have found that all immediate markers of patient stability, such as intubation, patient unresponsiveness, and ASA class 5, were similar between the cohorts. Nonsignificant trends did exist, however, with a slightly higher percentage of open repair patients having unfavorable status compared to EVAR. It is possible that with a larger sample size, statistically significant differences may have been found. However, adjusting for all preoperative status variables within multivariate analysis did not change the outcome of increased mortality with open surgery.
Limitations
While the variables assessed with the NSQIP database are superior to those within purely administrative databases, there still are omissions and deficiencies that make it difficult to definitively state that all hemodynamic parameters were similar prior to EVAR versus open repair. The markers of preoperative intubation, acute renal failure, coma, impaired sensorium, and preoperative blood transfusion certainly have significant value; however, more specific information on blood pressure parameters, tachycardia, and pressor dependence was not available. The variable of preoperative sepsis was coded in one third of the patients and likely is a reliable marker of hypotension or pressor support as part of the definition of this variable is organ system and/or circulatory system dysfunction. This showed no significant difference between repair groups and also did not prove to be an independent predictor of mortality within this study, however.
The presence of endoleaks, along with other specific complications such as distal ischemia, was unavailable within the NSQIP. We also could not investigate which procedure was performed when a patient underwent re-operation, and conversions were limited to those that occur within the first operation. Length of stay in the intensive care unit was also not included.
We also could not assess anatomical differences between patients undergoing either method of repair or whether preoperative imaging was available for patients. In a 10-year retrospective review of 47 patients presenting with RAAA, Slater et al.17 found that 49% would have been anatomically suitable for EVAR. Comparatively, Lee et al.6 were able to perform EVAR in 76% of patients presenting with RAAA after a treatment algorithm was introduced.
Related to this, the long-term durability of EVAR for RAAA also has yet to be proven. While perioperative mortality results seem promising, future studies should assess long-term mortality and reintervention rates.
Conclusion
Despite greater comorbidities and similar preoperative status, mortality after EVAR was lower than after open repair for ruptured AAAs. Even when adjusting for minor differences in preoperative hemodynamics, EVAR mortality was lower than open repair. Institutions with adequate capabilities should consider implementing EVAR-first programs for cases where anatomical conditions allow. Future randomized trials would be useful, as would more studies from single institutions demonstrating significantly lower total RAAA mortality by instituting EVAR-first programs. Long-term data to assure durability are necessary within future studies as well.
Footnotes
The annual ISES Endovascular Research Competition held on February 9, 2009, at International Congress XXII on Endovascular Interventions (Scottsdale, Arizona, USA) evaluated participants on both their oral and written presentations. ISES congratulates the 2009 winners.
This project was supported by NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734 from the National Institutes of Health (NIH). The contents of this article are the responsibility of the authors and do not necessarily represent the official views of the NIH. In accordance with the NIH Public Access Policy, this article is available for open access at PubMed Central.
Marc L. Schermerhorn receives an unrestricted educational grant from W.L. Gore & Associates, is a consultant to Boston Scientific Corporation, and serves on the Data Safety Monitoring Board for Endologix. The other authors have no commercial, proprietary, or financial interest in any products or companies described in this article.
REFERENCES
- Yusuf S.W., Whitaker S.C., Chuter T.A. et al. Emergency endovascular repair of leaking aortic aneurysm [letter] Lancet. 1994;344:1645. doi: 10.1016/s0140-6736(94)90443-x. [DOI] [PubMed] [Google Scholar]
- Mehta M., Taggert J., Darling R.C. et al. Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysms: outcomes of a prospective analysis. J Vasc Surg. 2006;44:1–8. doi: 10.1016/j.jvs.2006.02.057. [DOI] [PubMed] [Google Scholar]
- Arya N., Makar R.R., Lau L.L. et al. An intention-to-treat by endovascular repair policy may reduce overall mortality in ruptured abdominal aortic aneurysm. J Vasc Surg. 2006;44:467–471. doi: 10.1016/j.jvs.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Moore R., Nutley M., Cina C.S. et al. Improved survival after introduction of an emergency endovascular therapy protocol for ruptured abdominal aortic aneurysms. J Vasc Surg. 2007;45:443–450. doi: 10.1016/j.jvs.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Wibmer A., Schoder M., Wolff K.S. et al. Improved survival after abdominal aortic aneurysm rupture by offering both open and endovascular repair. Arch Surg. 2008;143:544–549. doi: 10.1001/archsurg.143.6.544. [DOI] [PubMed] [Google Scholar]
- Lee W.A., Hirneise C.M., Tayyarah M. et al. Impact of endovascular repair on early outcomes of ruptured abdominal aortic aneurysms. J Vasc Surg. 2004;40:211–215. doi: 10.1016/j.jvs.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Visser J.J., Bosch J.L., Hunink M.G. et al. Endovascular repair versus open surgery in patients with ruptured abdominal aortic aneurysms: clinical outcomes with 1-year follow-up. J Vasc Surg. 2006;44:1148–1155. doi: 10.1016/j.jvs.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Verhoeven E.L., Kapma M.R., Groen H. et al. Mortality of ruptured abdominal aortic aneurysm treated with open or endovascular repair. J Vasc Surg. 2008;48:1396–1400. doi: 10.1016/j.jvs.2008.07.054. [DOI] [PubMed] [Google Scholar]
- Bown M.J., Sutton A.J., Bell P.R. et al. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Brit J Surg. 2002;89:714–730. doi: 10.1046/j.1365-2168.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- American College of Surgeons, National Quality Improvement Program. Available at: http://acsnsqip.org. Last accessed April 21, 2009 [Google Scholar]
- Peppelenbosch N., Geelkerken R.H., Soong C. et al. Endograft treatment of ruptured abdominal aortic aneurysm using the Talent aortouniiliac system: an international multicenter study. J Vasc Surg. 2006;43:1111–1122. doi: 10.1016/j.jvs.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Rayt H.S., Sutton A.J., London N.J. et al. A systematic review and meta-analysis of endovascular repair (EVAR) for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2008;36:536–544. doi: 10.1016/j.ejvs.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Azizzadeh A., Villa M.A., Miller C.C. et al. Endovascular repair of ruptured abdominal aortic aneurysms: systematic literature review. Vascular. 2008;16:219–224. doi: 10.2310/6670.2008.00039. [DOI] [PubMed] [Google Scholar]
- Lesperance K., Andersen C., Singh N. et al. Expanding use of emergency endovascular repair for ruptured abdominal aortic aneurysms: disparities in outcomes from a nationwide perspective. J Vasc Surg. 2008;47:1165–1171. doi: 10.1016/j.jvs.2008.01.055. [DOI] [PubMed] [Google Scholar]
- Greco G., Egorova N., Anderson P.L. Outcomes of endovascular treatment of ruptured abdominal aneurysms. J Vasc Surg. 2006;43:453–459. doi: 10.1016/j.jvs.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Egorova N., Giacovelli J., Greco G. et al. National outcomes for the treatment of ruptured abdominal aortic aneurysm: comparison of open versus endovascular repairs. J Vasc Surg. 2008;48:1092–1100. doi: 10.1016/j.jvs.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater B.J., Harris E.J., Lee J.T. Anatomic suitability of ruptured abdominal aortic aneurysms for endovascular repair. Ann Vasc Surg. 2008;22:716–722. doi: 10.1016/j.avsg.2008.06.001. [DOI] [PubMed] [Google Scholar]