Summary
Tetra-acylated lipid As derived from Porphyromonas gingivalis LPS have been synthesized using a key disaccharide intermediate functionalized with levulinate (Lev), allyloxycarbonate (Alloc) and anomeric dimethylthexylsilyl (TDS) as orthogonal protecting groups and 9-fluorenylmethoxycarbamate (Fmoc) and azido as amino protecting groups. Furthermore, an efficient cross metathesis has been employed for the preparation of the unusual branched R-(3)-hydroxy-13-methyltetradecanic acid and (R)-3-hexadecanoyloxy-15-methyl-hexadecanoic acid of P. gingivalis lipid A. Biological studies have shown that the synthetic lipid As can not activate human and mouse TLR2 and TLR4 to produce cytokines. However, it has been found that the compounds are potent antagonist of cytokine secretion by human monocytic cells induced by enteric LPS.
Keywords: lipid A, LPS, antagonist, Toll-like receptor, septic shock
Introduction
Porphyromonas gingivalis is a Gram-negative bacterium implicated in chronic periodontal diseases.1 It releases large amounts of outer membrane vesicles containing lipopolysaccharides (LPS), which can penetrate periodontal tissue. It has been proposed that microbial components such as LPS can induce inflammatory responses resulting in tissue damage and alveolar bone loss.2 Early studies have indicated that P. gingivalis LPS can activate murine macrophages in a TLR2- and TLR4-dependent manner.3 However, it has been suggested that the TLR2 responses maybe due to contaminations with lipoproteins.4, 5 It has also been found that LPS of P. gingivalis can inhibit IL-6 and IL-1β secretion and ICAM expression induced by enteric LPS by U373 and human peripheral mononuclear cells and human gingival fibroblasts, respectively.6 Another study found that a purified tetra-acylated monophosphoryl lipid A structure can antagonize E-selectin expression in human cells exposed to enteric or P. gingivalis LPS.7 It appears that MD-2 represents the principle molecular component used by these LPS derivatives for inhibition.8
Several studies have indicated that compounds that can antagonize cytokine production induced by enteric LPS may have the potential to be developed as therapeutics for the treatment of Gram-negative septicemia.9 Success in this area has been limited and most efforts have been directed towards the synthesis of analogs of lipid A of Rhodobacter sphaeroides10, 11 and derivatives of lipid X.12–15 Analogs of the lipid A moiety of Helicobacter pylori, which have fewer but longer fatty acids and are only phosphorylated at the anomeric center, have also been shown to inhibit IL-6 secretion by human whole blood cells.16 Recently, we reported that synthetic lipid As derived from Rhizobium sin-1, which lacks phosphates but contains an 2-aminogluconolactone and a very long chain fatty acid 27-hydroxyoctacosanoic acid, can prevent the induction of TNF-α by E. coli LPS in human monocytic cells.17–21
The lipid A moiety of the LPS of P. gingivalis displays considerable heterogeneity and the structures of four compounds have been elucidated, which differ in fatty acid substitution pattern (Figure 1).22, 23 A common structural feature of these derivatives is, however, the presence of unusual branched fatty acids such as R-(3)-hydroxy-13-methyltetradecanic acid and R-(3)-hydroxy-15-methyl hexadecanic acid.
Fig. 1.
Structures of E. coli and P. gingivalis lipid A.
The presence of multiple lipid A structures has made it difficult to interpreted innate immune responses elicited by P. gingivalis LPS, which in turn has hindered a thorough understanding of the contributions of P. gingivalis LPS to periodontal diseases. It has also complicated the identification of P. gingivalis lipid A with antagonistic properties, which may have potential therapeutic properties for the treatment or prevention of septic shock. Fortunately, chemical synthesis can afford pure lipid A derivatives for structure activity relationship studies.24, 25 In this respect, the chemical synthesis of a tri- (1) and penta-acylated lipid A (2) has already been reported26 and biological studies have shown that these compounds can activate human and murine cells in TLR4-dependent manner.
Here we describe a highly convergent chemical synthesis of tetra-acylated lipid As 3 and 4 employing levulinate (Lev) and allyloxycarbonate (Alloc) as hydroxyl protecting groups, dimethylthexylsilyl (TDS) as an anomeric protecting group and 9-fluorenylmethoxycarbamate (Fmoc) and azido as amino protecting groups to manipulate each of the critical functionalities in a selective manner. Furthermore, an efficient cross metathesis is employed for the preparation of the branched R-(3)-hydroxy-13-methyltetradecanic acid and (R)-3-hexadecanoyloxy-15-methylhexadecanoic acid. Biological evaluations demonstrate that compound 3 is a potent antagonist of cytokines secretion induces by enteric LPS.
Result and discussion
Chemical synthesis
It was envisaged that lipid As derived from P. gingivalis can easily be obtained from monosaccharide building blocks 5 and 6 and fatty acids 7–10 (Figure 2). Optically pure 3-hydroxy fatty acids such as 7–9, having a terminal isopropyl group, are important constituents and synthetic intermediates of a wide range of biologically interesting natural compounds, including flavolipin,27 N-4909 (a stimulator of apolipoprotein E secretion),28 liposidomycin-B29 and several lipid A derivatives.3 While several chemical and enzymatic approaches have been developed for the preparation of such compounds,30–34 these methods suffer from time-consuming procedures that give low overall yields and may involve harsh and difficult to handle reaction conditions. We envisaged that a cross metathesis35 of a fatty acid terminating in an alkene with 2-methyl-propene or 4-methyl-1-pentene followed by reduction of the double bond of the resulting compound would give easy entry into isopropyl terminating fatty acids. Employing this synthetic strategy, methyl R-(3)-hydroxy-13-methyltetradecanic acid (14) and methyl R-(3)-hydroxy-15-methyl hexadecanic acid (15), which are key intermediates for the chemical synthesis of lipid As derived from P. gingivalis, would be readily available by a cross metatheses of 11 with 2-methyl-propene or 4-methyl-1-pentene followed by asymmetric hydrogenation of the 3-keto function of the resulting product using the asymmetric catalyst RuCl2[(R)-BINAP] and hydrogenation of the alkene (Scheme 1). It was, however, observed that 2-methyl-propene is rather difficult to handle because it is a gas at room temperature and therefore 2-methyl-2-butene was employed, which should provide the same compound.36 Thus, compound 11, which could be easily prepared by a known two-step synthetic procedure,25 was reacted with 2-methyl-2-butene and 4-methyl-1-pentene in the presence of Grubbs 2nd generation catalyst35 to afford 12 and 13, respectively. The ketone of the cross metathesis products 12 and 13 was enantioselectively reduced by catalytic hydrogenation in the presence of (R)-RuCl2(BINAP) to give optically pure 14 and 15 having R-configuration.37 The optical purity of the compounds was established by NMR spectroscopic analysis38 employing the shift reagent Eu(hfmc)3 in CDCl3 (e.e. > 99%). It should be mentioned that the (S)-isomers can be easily prepared using (S)-RuCl2(BINAP)2 as the catalyst. Next, the methyl ester of compounds 14 and 15 were hydrolyzed under standard conditions and the resulting acids were converted into dicyclohexaneamine salts, which were recrystallized from CH3CN. The carboxylates were protected as 2-(4-bromophenyl)-2-oxoethyl esters to give key intermediates 16 and 18.37 The ester protecting group can easily be removed by treatment with zinc in acetic acid without affecting ether or ester groups, and therefore the 3-hydroxyl of 16 and 18 can be protected as a benzyl ether or modified with an acyl group, both of which are important intermediates for the synthesis of the target lipids. Thus, 16 and 18 were treated with benzaldehyde in the presence of TMSOTf and (TMS)2O in THF followed by addition of the reducing agent Et3SiH39 to give benzylated derivatives 17 and 19, respectively. The 2-(4-bromophenyl)-2-oxoethyl esters 17 and 19 were removed by treatment with zinc in acetic acid to give lipids 7 and 8, respectively. Fatty acid 9 was easily obtained by acylation of the hydroxyl of 18 with hexadecanoyl chloride in the presence of pyridine and DMAP to yield 20, which was deprotected using the standard produce.
Fig. 2.
Building blocks for the synthesis of P. gingivalis lipid A.
Scheme 1. Reagents and conditions.
a) 2-methyl-2-butene or 4-methyl-1-pentene, Grubbs 2nd generation catalyst; b) RuCl2[(R)-BINAP], H2 (65 psi), 2 M HCl, CH3OH, 40 °C, then H2 (1 atm), Pd/C, CH3OH; c) LiOH.H2O, THF/H2O, then dicyclohexaneamine, CH3CN, then 2,4’-dibromoacetophenone, Et3N, EtOAc; d) benzaldehyde, (TMS)2O, TMSOTf, THF, Et3SiH; e) Zn/HOAc, 60 °C; f) hexadecanoyl chloride, pyridine, DMAP, DCM.
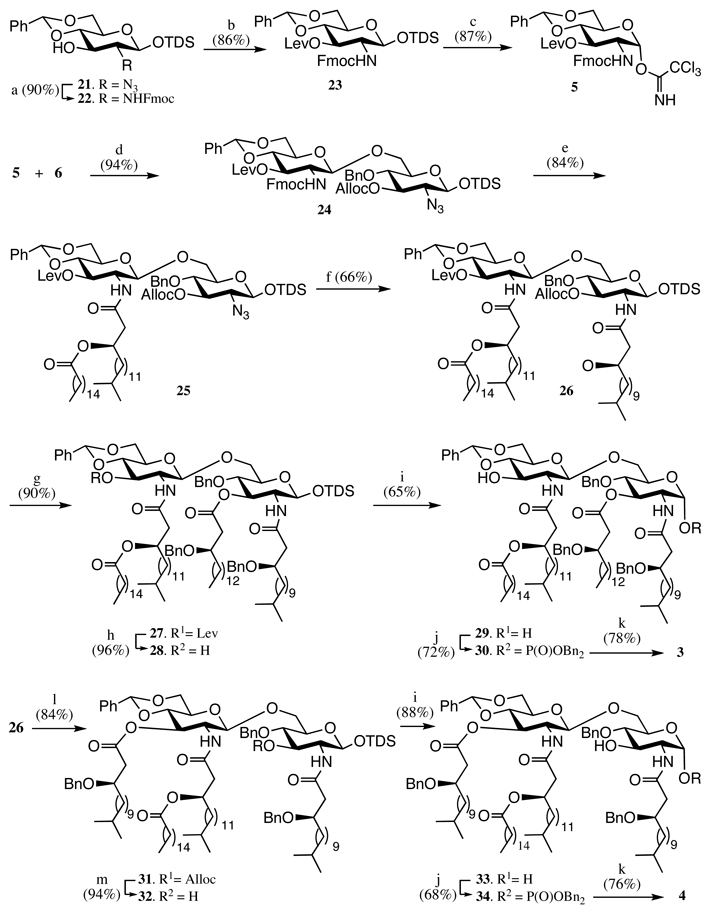
Target compounds 3 and 4 differ in the pattern of O-acylation and compound 3 has an R-(3)-hydroxy-hexadecanoic acid at C-3 of the proximal saccharide moiety whereas compound 4 has a (R)-3-hydroxy-15-methyl-hexadecanoic acid at C-3 of the distal saccharide. To synthesize these structurally similar compounds, we have developed a convergent approach that employs the advanced disaccharide intermediate 24 (Scheme 2), which is protected with Lev, Fmoc, Alloc, azido and anomeric TDS as a set of orthogonal protecting groups and thus disaccharide 24 can selectively be modified with any lipid at C-2, C-3, C-2’ and C-3’. Therefore the strategy provides easy access to a wide range of lipid As for SAR studies. Furthermore, it has been found that 4’-phosphate of lipid As tends to migrate to the 3’-hydroxyl,24 and therefore the phosphate was introduced after installing the fatty acid at the 3’-hydroxyl. The distal 4,6-diol of 24 was protected as a benzylidene acetal, which at a late stage of the synthesis can be regioselectively opened to give a C-4’ hydroxyl which can then be phosphorylated Another attractive feature of the approach is that glycosyl donor 5 and acceptor 6 can be synthesized from the common intermediate 21, which can be easily prepared from glucosamine (Scheme 2).25
Scheme 2. Reagents and conditions.
a) Zn/HOAc, DCM then FmocCl, DIPEA, DCM; b) levulinic acid, DCC, DMAP, DCM; c) Bu4NF/HOAc, THF, then CNCCl3, NaH, THF; d) TfOH, DCM, −50 °C; e) DBU, DCM, then (R)-3-hexadecanoyloxy-15-methyl-hexadecanoic acid 9, DCC, DCM; f) Zn/HOAc, DCM, then (R)-3-benzyloxy-15-methyl-hexadecanoic acid 8, DCC, DCM; g) Pd(PPh3)4, HCO2H, n-BuNH2, THF; then (R)-3-benzyloxy-hexadecanoic acid 10, DCC, DMAP, DCM; h) H2NNH2, HOAc, DCM/CH3OH; i) Bu4NF/HOAc, THF; j) tetrabenzyl diphosphate, LiN(TMS)2, THF, −78 °C; k) H2 (50 psi), Pd black, THF; l) H2NNH2, HOAc, DCM/CH3OH, then (R)-3-benzyloxy-13-methyl-tetradecanoic acid 7, DCC, DMAP, DCM; m) Pd(PPh3)4, HCO2H, n-BuNH2, THF.
Thus, glycosyl acceptor 6 was synthesized from 21 according to the reported procedure.25 For the synthesis of glycosyl donor 5, the azido moiety of 21 could be easily converted to Fmoc carbamate by reduction with zinc in acetic acid followed by reaction of the resulting amine with FmocCl in the presence of DIPEA to give 22 in a yield of 86%. The hydroxyl of compound 22 was protected as a Lev ester using levulinic acid, DCC and DMAP to afford 23. Removal of the anomeric TDS of 23 was easily accomplished by treatment with Bu4NF in the presence of acetic acid to give a lactol, which was immediately reacted with trichloroacetonitrile in the presence of NaH to afford trichloroacetimidate 5.40 A trifluoromethanesulfonic acid (TfOH)-mediated glycosylation of 5 with 6 proceeded in a stereoselective manner to give disaccharide 24 in an excellent yield of 94% (Scheme 2).25
Having the advanced disaccharide 24 and lipids 7–10 at hand, attention focused on the selective acylation of relevant hydroxyls and amines. Thus, removal of the Fmoc protecting group of 24 using 1,8-diazobicyclo[5.4.0]undec-7-ene (DBU) in DCM followed by acylation of the resulting amino group with lipid 9 using dicyclohexylcarbodiimide (DCC) as the activation reagent gave compound 25 (Scheme 2). Next, the azido moiety of 25 was reduced by treatment with zinc and acetic acid in DCM, and the amine of the resulting compound acylated with 7 in the presence of DCC to afford 26 as the common intermediate for the synthesis of target molecules 3 and 4. For the synthesis of 3, the Alloc protecting group of 26 was removed by reaction with Pd(PPh3)4 in the presence of HCOOH and n-BuNH2,41 and the resulting hydroxyl acylated with (R)-3-benzyloxy-hexadecanoic acid 10 using DCC and DMAP as the activation reagent to give 27. Next, removal of the Lev group of 27 was easily accomplished by treatment with hydrazine acetate to give 28, which treated with Bu4NF in the presence of acetic acid to give the desired product 29 in a yield of 72% and a small amount of a side product arising from elimination of the 3-acyloxyl group. The anomeric center of resulting 29 was phosphorylated using tetrabenzyl diphosphate in the presence of lithium bis(trimethyl)silylamide in THF at −78 °C to give 30 as only the α-anomer.42 Global deprotection of 30 by catalytic hydrogenolysis over Pd-black gave requisite lipid A 3.
The synthesis of 4 could easily be accomplished in a similar manner to the synthesis of 3, however, in this case the Lev protecting group of the common intermediate 26 was removed to give an alcohol, which was acylated with lipid 7 using standard conditions to afford 31. Next, subsequent Alloc (→ 32) and anomeric TDS protecting group removal gave 33. As expected no elimination was observed in this reaction, due to the poor leaving group ability of the C-3 hydroxyl. Standard anomeric phosphorylation of 33 and deprotection of the resulting compound 34 gave target lipid A 4.
Biological evaluation of lipid As and LPS
Based on the results of recent studies,43, 44 it is clear that enteric LPS induces cellular activation through TLR4 and it appears that there are two distinct initiation points in the signaling process, one being a specific intracellular adaptor protein called MyD88 and the other an adaptor protein called TRIF, which operates independently of MyD88. It is well established that TNF-α secretion is a prototypical measure for activation of the MyD88-dependent pathway, whereas secretion of IFN-β and IP-10 are commonly used as an indicator of TRIF-dependent cellular activation.
The carbohydrate backbone, degree of phosphorylation and fatty acid acylation patterns differ considerably among lipid As of various bacterial species and there is evidence to support that these structural variations account for significant differences in inflammatory responses.45 Several studies have also indicated that LPS from various bacterial species such as P. gingivalis, Leptospira interrogans, Legionella pneumophila, Bacteroides fragilis NCTC-9343 and Pseudomonas aeruginosa PAC-611 can induce cellular activation in a TLR2-dependent manner.46–49 However, it may be possible that these cellular responses are derived from contamination by lipoproteins.
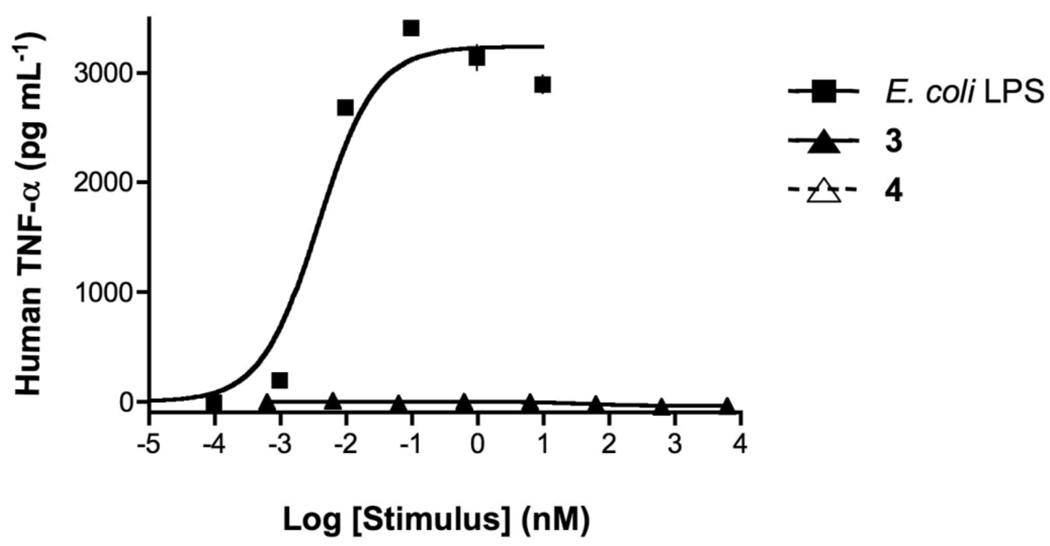
We have chemically synthesized the tetra-acylated lipid As 3 and 4 (Figure 1) to study whether LPS derived from P. gingivalis can induce cellular activation in a TLR2- or TLR4-dependent manner. Furthermore, there are indications that LPS of P. gingivalis can antagonize cytokine production induced by enteric LPS and therefore these properties have also been studied. Thus, a human monocytic cell line (Mono Mac 6 cells) was exposed over a wide range of concentrations to compounds 3 and 4 and E. coli 055:B5 LPS. After 5.5 hours, the supernatants were harvested and examined for human TNF-α using a commercial capture ELISA. Potencies (EC50, concentration producing 50% activity) and efficacies (maximal level of production) were determined by fitting the dose-response curves to a logistic equation using PRISM software. As can be seen in Figure 3, LPS is a potent inducer of TNF-α whereas the synthetic compounds 3 and 4 did not exhibit any activity. A similar experiment using mouse macrophages (RAW 264.7 γNO(−) cells) did not lead to secretion of cytokines (e.a. TNF-α, IL-6, IP-10, IFN-β and IL-1β) when exposed to compounds 3 and 4 (data not shown).
Fig. 3.
Concentration-response curves of E. coli LPS and synthetic compounds 3 and 4 in human monocytic cells. MM6 cells were incubated for 5.5 h at 37 °C with increasing concentrations of E. coli LPS and synthetic compounds 3 and 4 as indicated. TNF-α protein in cell supernatants were measured using ELISA. (Please note that 3 and 4 show background values and therefore overlap in the figure). Treatment with E. coli LPS, 3 and 4 did not affect cell viability, as judged by cellular exclusion of trypan blue.
Synthesis and secretion of the TNF-α protein depends on a complex process involving activation of transcription factors, up-regulation of the genes responsible for production of the cytokine, transcription of the message, and then translation of the mRNA and processing of a protein.50–52 This process is tightly controlled and therefore it may be possible that a compound can activate NF-κB or induce expression of TNF-α mRNA without causing production or secretion of the TNF-α protein.53
To examine the ability of the synthetic compounds to induce activation of NF-κB, HEK 293T cells were employed that were stably transfected with various immune receptors and transiently transfected with a plasmid containing the reporter gene pELAM-Luc (NF-κB-dependent firefly luciferase reporter vector) and a plasmid containing the control gene pRL-TK (Renilla luciferase control reporter vector) (Figure 4). No activation of NF-κB was observed when cells transfected with human TLR4/MD2/CD14 and human or mouse TLR2 were exposed to compounds 3 and 4. As expected, LPS, which is a prototypical activator for TLR4, could activate cells transfected with TLR4/MD2/CD14, and Pam3CysSK4, which is a well-established agonist of TLR2, was able to activate the TLR2-containing cells. However, at high concentrations, compound 4 could induce NF-κB activation in cells transfected with mouse TLR4/MD2/CD14. These results clearly demonstrate that compounds 3 and 4 do not induce cellular activation in a TLR2-dependent manner. Although compound 4 is a weak activator of mouse TLR4, it could not induce the secretion of cytokines.
Fig. 4.
Response of HEK 293T cells expressing human or murine TLRs to 3 and 4. Induction of NF-κB activation was determined in triplicate cultures of HEK 293T cells stably transfected with human or mouse (a) TLR4/MD2/CD14 and (b) TLR2 and transiently transfected with pELAM-Luc and pRL-TK plasmids. Forty-four h post-transfection, cells were treated with (B) E. coli LPS (10 ng mL−1), (C) Pam3CysSK4 (1 µg mL−1), (D, E and F) 3 (0.1, 1 and 10 µg mL−1, respectively), (G, H and I) 4 (0.1, 1 and 10 µg mL−1, respectively) or (A) were left untreated (control). Forty-eight h post-transfection, NF-κB activation was determined by firefly luciferase activity relative to Renilla luciferase activity. n.a. indicates not analyzed.
Compounds that lack proinflammatory properties may still interact with relevant receptors (TLR4/MD2/CD14), and thereby inhibit TNF-α production induced by E. coli LPS. Thus, the human monocytic cells and mouse macrophages (MM6 and RAW cells) were exposed to a combination of E. coli LPS (10 ng/mL) and a wide range of concentrations of lipid As 3 and 4 and, after an incubation time of 5.5 h, the supernatant was examined for human or mouse TNF-α. Only marginal inhibition was observed in the mouse cell line. However, both compounds were able to antagonize TNF production by the human cell line (Figure 5) and it was found that compound 3 was a significantly more potent antagonist than 4 (IC50 concentration producing 50% inhibition for 3 and 4 were 160 nM and 3.2 µM, respectively).
Fig. 5.
Antagonism of E. coli LPS by synthetic compounds 3 and 4 in human monocytic cells. TNF-α concentrations were measured after preincubation of MM6 cells with increasing concentrations of 3 or 4 as indicated for 1 h at 37°C, followed by 5.5 h of incubation with E. coli LPS (1 ng mL−1). Results are expressed as percentage of cytokine concentration of control cells, which are incubated only with E. coli LPS.
It has been reported that P. gingivalis LPS can initiate innate immune responses in a TLR2-and/or TLR4-dependent manner.3 The heterogeneity of LPS and lipid A preparations has limited, however, the identification of specific compounds that are responsible for this unusual mode of activation. It has already been reported that penta-acylated and tri-acylated lipid As 1 and 2 can only activate human and mouse cells in a TLR4-dependent manner.26 Furthermore, we have found no evidence that the tetra-acelyated compounds 3 and 4 can active human or mouse TLR2. It may be possible that a yet to be identified P. gingivalis lipid A may exhibit TLR2-dependent activity, however, it is more likely that lipoprotein contaminants are responsible for the observed activity.
An exciting observation reported here is that the tetra-acylated lipid A 3 is a potent antagonist of TNF-α production induced by enteric LPS. The acylation pattern of 3 is critical for optimal activity because compound 4 exhibits a significantly reduced activity. Antagonists of cell surface receptors that recognize enteric LPS have the potential for being used as therapeutic interventions for patients with Gram-negative septicemia. Success in this area has been limited and most efforts have been directed towards the synthesis of analogs of lipid A of R. sphaeroides 10, 11 These compounds are bis-phophorylated and contain unsaturated and keto containing fatty acids, which complicates the chemical synthesis. Furthermore, the C-4’ phosphate is prone to migration, which results in loss of activity. An attractive feature of compounds 3 and 4 is that they are mono-phosphorylated and can be prepared by a highly convergent synthetic approach. Furthermore, it is to be expected that analog synthesis will provide more potent compounds that have simpler structures.
Experimental
Chemical synthesis
General synthetic methods
Column chromatography was performed on silica gel 60 (EM Science, 70–230 mesh). Reactions were monitored by thin-layer chromatography (TLC) on Kieselgel 60 F254 (EM Science) and compounds were detected by examination under UV light and by charring with 10% sulfuric acid in MeOH. Solvents were removed under reduced pressure at <40 °C. CH2Cl2 was distilled from NaH and stored over molecular sieves (3 Å). Tetrahydrofuran (THF) was distilled from sodium directly prior to the application. MeOH was dried by refluxing with magnesium methoxide and then was distilled and stored under argon. Pyridine was dried by refluxing with CaH2 and then was distilled and stored over molecular sieves (3 Å). Molecular sieves (3 and 4 Å) used for reactions, were crushed and activated in vacuo at 390 °C during 8 h and then for 2–3 h at 390 °C directly prior to application. Optical rotations were measured with a Jasco model P-1020 polarimeter. 1H NMR and 13C NMR spectra were recorded with Varian spectrometers (models Inova500 and Inova600) equipped with Sun workstations. 1H NMR spectra were recorded in CDCl3 and referenced to residual CHCl3 at 7.24 ppm and 13C NMR spectra were referenced to the central peak of CDCl3 at 77.0 ppm. Assignments were made by standard gCOSY and gHSQC. High resolution mass spectra were obtained on a Bruker model Ultraflex MALDI-TOF mass spectrometer. Signals marked with a subscript L symbol belong to the biantennary lipids, whereas signals marked with a subscript L’ symbol belong to their side chain. Signals marked with a subscript S symbol belong to the monoantennary lipids.
Synthesis of 5, 7–9, 12–20, 22 and 23 and 1H and 13C NMR spectra are given in the Supplementary Information.
Dimethylthexylsilyl 6-O-[4,6-O-benzylidene-2-deoxy-2-(9-fluorenylmethoxycarbonyl amino)-3-O-levulinoyl-β-D-glucopyranosyl]-3-O-allyloxycarbonyl-2-azido-4-O-benzyl-2-deoxy-β-D-glucopyranoside (24)
A suspension of trichloroacetimidate 5 (600 mg, 0.82 mmol), acceptor 6 (407 mg, 78 mmol) and molecular sieves (4 Å, 500 mg) in DCM (10 mL) was stirred at room temperature for 1 h. The mixture was cooled (−50 °C) and then trifluoromethanesulfonic acid (TfOH) (10 µL, 0.078 mmol) was added. After stirring the reaction mixture for 15 min, it was allowed to warm up to −20 °C in 1 h, after which it was quenched with solid NaHCO3. The solids were removed by filtration and the filtrate was washed with saturated aqueous NaHCO3 (2 × 50 mL) and brine (2 × 40 mL). The organic phase was dried (MgSO4) and filtered. Next, the filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate, 3/1, v/v) to give 24 as an amporphous solid (840 mg, 94%). Rf = 0.40 (hexane/ethyl acetate, 2/1, v/v); [α]26D = −15.5°(c = 1.0, CHCl3); 1H NMR (300 MHz, CD3COCD3): δ 7.84-7.22 (m, 18H, aromatic), 6.79 (d, 1H, JNH’,2’ = 9.3 Hz, NH’), 5.87 (m, 1H, OCH2CH=CH2), 5.65 (s, 1H, >CHPh), 5.37 (t, 1H, J2’,3’ = J3’,4’ = 9.9 Hz, H-3’), 5.30 (d, 1H, J = 18.3 Hz, OCH2CH=CHH), 5.17 (d, 1H, J = 10.5 Hz, OCH2CH=CHH), 4.94 (d, 1H, J1,2 = 8.7 Hz, H-1), 4.86-4.80 (m, 2H, H-1, H-3), 4.71 (d, 1H, J = 10.8 Hz, CHHPh), 4.59-4.55 (m, 3H, OCH2CH=CH2, CHHPh), 4.32-4.29 (m, 2H, H-6’a, CO2CHH of Fmoc), 4.17-4.08 (m, 3H, H-6a, CO2CHHCH of Fmoc), 3.92-3.67 (m, 6H, H-2’, H-4, H-4’, H-5, H-6b, H-6’b), 3.56 (m, 1H, H-5’), 3.41 (dd, 1H, J1,2 = 7.5 Hz, J2,3 = 10.2 Hz, H-2), 2.61 (t, 2H, J = 6.6 Hz, CH2 of Lev), 2.47 (t, 2H, J = 6.6 Hz, CH2 of Lev), 1.95 (s, 3H, CH3 of Lev), 1.70 (m, 1H, CH of TDS), 0.91 [bs, 12H, SiC(CH3)2CH(CH3)2], 0.26 (s, 3H, SiCH3), 0.25 (s, 3H, SiCH3). 13C NMR (75 MHz, CD3OCD3): δ 172.47 (C=O), 156.64 (C=O), 154.94 (C=O), 144.94-120.54 (aromatic), 132.67 (OCH2CH=CH2), 118.63 (OCH2CH=CH2), 102.42 (C-1’), 101.58 (>CHPh), 97.12 (C-1), 79.49 (C-4’), 79.24 (C-3), 76.68 (C-4), 75.09 (CH2Ph), 74.50 (C-5), 72.64 (C-3’), 68.96-68.92 (m, C-6’, OCH2CH=CH2), 68.57 (C-6), 67.28 (C-2), 67.08 (CH2 of Fmoc), 66.92 (C-5’), 57.26 (C-2’), 47.64 (CH of Fmoc), 38.04 (CH2 of Lev), 34.49 (CH of TDS), 29.33 (CH3 of Lev), 28.44 (CH2 of Lev), −1.77 (SiCH3), −3.22 (SiCH3). HR MS (m/z) calcd for C58H70N4O15Si [M + Na]+, 1113.4499; found, 1113.6394.
Dimethylthexylsilyl 6-O-{4,6-O-benzylidene-2-deoxy-2-[(R)-3-hexadecanoyloxy-15-methyl-hexadecanoylamino]-3-O-levulinoyl-β-D-glucopyranosyl}-3-O-allyloxycarbonyl-2-azido-4-O-benzyl-2-deoxy-β-D-glucopyranoside (25)
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) (60 µl) was added dropwise to a solution of 24 (620 mg, 0.569 mmol) in DCM (8 mL). The reaction mixture was stirred at room temperature for 4 h, after which it was concentrated in vacuo. The residue was purified by silica gel column chromatography (DCM/methanol, 100/1 → 100/3, v/v) to afford an amine as a colorless syrup (454 mg, 92%). Rf = 0.30 (hexane/ethyl acetate, 1/1, v/v); HR MS (m/z) calcd for C43H60N4O13Si [M + Na]+, 891.3818; found, 891.2115. DCC (188 mg, 0.913 mmol) was added to a stirred solution of (R)-3-hexadecanoyl-15-methylhexadecanoic acid 9 (345 mg, 0.659 mmol) in DCM (5 mL). After stirring the reaction mixture for 10 min, the resulting amine (440 mg, 0.507 mmol) in DCM (2 mL) was added and the stirring was continued for another 12 h. The insoluble materials were removed by filtration and the residue was washed with DCM (2 × 2 mL). The combined filtrates were concentrated in vacuo and the residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate, 2/1, v/v) to give 25 as an amorphous solid (634 mg, 91%). Rf = 0.65 (hexane/ethyl acetate, 2/1, v/v); [α]25D = −15.2°(c = 1.0, CHCl3); 1H NMR (300 MHz, CDCl3): δ 7.23-7.03 (m, 10H, aromatic), 5.81 (d, 1H, JNH’,2’ = 8.4 Hz, NH’), 5.69 (m, 1H, OCH2CH=CH2), 5.27 (s, 1H, >CHPh), 5.19 (t, 1H, J2’,3’ = J3’,4’ = 9.6 Hz, H-3’), 5.14 (d, 1H, J = 17.1 Hz, OCH2CH=CHH), 5.04 (d, 1H, J = 10.2 Hz, OCH2CH=CHH), 4.83 (m, 1H, H-3L), 4.72 (d, 1H, J1’,2 = 8.4 Hz, H-1’), 4.54 (t, 1H, J2,3 = J3,4 = 9.9 Hz, H-3), 4.42-4.37 (m, 5H, H-1, CH2Ph, OCH2CH=CH2), 4.08 (dd, 1H, J5’,6’a = 4.8 Hz, J6’a,6’b = 10.2 Hz, H-6’a), 3.74 (d, 1H, J6a,6b = 10.5 Hz, H-6a), 3.63-3.36 (m, 5H, H-2’, H-4, H-4’, H-6b, H-6’b), 3.33-3.26 (m, 2H, H-5, H-5’), 3.11 (dd, 1H, J1,2 = 7.5 Hz, J2,3 = 9.9 Hz, H-2), 2.59-2.30 (m, 4H, CH2 of Lev), 2.17 (dd, 1H, J2La,2Lb = 14.4 Hz, J2La,3L = 6.0 Hz, H-2La), 2.29-2.22(m, 3H, H-2L’, H-2Lb), 1.91 (s, 3H, CH3 of Lev), 1.51-1.28(m, 5H, H-4L, H-3L’, CH of TDS), 1.04 (broad, 44H, 22 × CH2 of lipid), 0.70-0.64 (m, 21H, 4 × CH3 of thexyl, 3 × CH3 of lipid), 0.00 [s, 6H, Si(CH3)2]. 13C NMR (75 MHz, CDCl3): δ 206.45 (C=O), 173.75 (C=O), 172.17 (C=O), 170.05 (C=O), 154.32 (C=O), 137.51-126.19 (aromatic), 131.26(OCH2CH=CH2), 119.21 (OCH2CH=CH2), 101.42 (>CHPh), 100.91 (C-1’), 96.92 (C-1), 78.84 (C-4’), 78.58 (C-3), 76.02 (C-4), 74.63 (CH2Ph), 74.35 (C-5), 71.42 (C-3’), 70.75 (C-3L), 68.90 (OCH2CH=CH2), 68.63 (C-6’), 68.06 (C-6), 66.46 (C-2), 66.19 (C-5’), 55.80 (C-2’), −1.86 (SiCH3), −3.56 (SiCH3). HR MS (m/z) calcd for C76H122N4O16Si [M + Na]+, 1397.8517; found, 1397.7814.
Dimethylthexylsilyl 6-O-{4,6-O-benzylidene-2-deoxy-2-[(R)-3-hexadecanoyloxy-15-methyl-hexadecanoylamino]-3-O-levulinoyl-β-D-glucopyranosyl}-3-O-allyloxycarbonyl-4-O-benzyl-2-[(R)-3-benzyloxy-15-methyl-hexadecanoylamino]-2-deoxy-β-D-glucopyranoside (26)
A suspension of 25 (256 mg, 0.186 mmol), zinc (< 10 micron, 121 mg, 1.86 mmol) and acetic acid (100 µL) in DCM (5 mL) was stirred at room temperature for 2 h, after which it was diluted with ethyl acetate (30 mL). The solids were removed by filtration and washed with ethyl acetate (2 × 4 mL) and the combined filtrates were washed with saturated aqueous NaHCO3 (2 × 20 mL) and brine (2 × 20 mL). The organic phase was dried (MgSO4) and filtered. Next, the filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography (eluent: DCM/methanol, 50/1, v/v) to afford an amine as a pale yellow syrup (188 mg, 75%). Rf = 0.30 (hexane/ethyl acetate, 1/1, v/v); HR MS (m/z) calcd for C76H124N2O16Si [M + Na]+, 1371.8612; found, 1371.9028. DCC (51 mg, 0.246 mmol) was added to a stirred solution of (R)-3-benzyloxy-15-methyl-hexadecanoic acid 8 (69 mg, 0.185 mmol) in DCM (3 mL). After stirring the reaction mixture for 10 min, the amine (166 mg, 0.123 mmol) in DCM (1 mL) was added and the stirring was continued for another 12 h. The insoluble materials were removed by filtration and the residue was washed with DCM (2 × 1 mL). The combined filtrates were concentrated in vacuo and the residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate, 6/1, v/v) to give 26 as an amorphous solid (184 mg, 88%). Rf = 0.55 (hexane/ethyl acetate, 4/1, v/v); [α]25D = −9.5°(c = 1.0, CHCl3); 1H NMR (300 MHz, CDCl3): δ 7.36-7.18 (m, 15H, aromatic), 6.28 (d, 1H, JNH,2 = 8.7 Hz, NH), 5.90 (d, 1H, JNH’,2’ = 8.4 Hz, NH’), 5.77 (m, 1H, OCH2CH=CH2), 5.40 (s, 1H, >CHPh), 5.34 (t, 1H, J2’,3’ = J3’,4’ = 9.6 Hz, H-3’), 5.23 (d, 1H, J = 17.1 Hz, OCH2CH=CHH), 5.13 (d, 1H, J = 9.9 Hz, OCH2CH=CHH), 4.99-4.86 (m, 3H, H-1’, H-3, H-3L), 4.56-4.37 (m, 7H, H-1, 4 × CHHPh, OCH2CH=CH2), 4.25 (dd, 1H, J5’,6’a = 5.1 Hz, J6’a,6’b = 10.8 Hz, H-6’a), 3.88 (d, 1H, J6a,6b = 11.4 Hz, H-6a), 3.75-3.51 (m, 7H, H-2, H-2’, H-4, H-4’, H-6b, H-6’b, H-3S), 3.46-3.38 (m, 2H, H-5, H-5’), 2.73-2.41 (m, 4H, CH2 of Lev), 2.40-2.18 (m, 6H, H-2S, H-2L, H-2L’), 2.05 (s, 3H, CH3 of Lev), 1.53-1.39 (m, 7H, H-4S, H-3L’, H-4L, CH of TDS), 1.17-1.07 (m, 64H, 32 × CH2 of lipid), 0.79-0.73 (m, 27H, 4 × CH3 of TDS, 5 × CH3 of lipid), 0.06 (s, 3H, SiCH3), 0.00 (s, 3H, SiCH3). 13C NMR (75 MHz, CDCl3): δ 206.47 (C=O), 173.75 (C=O), 172.12 (C=O), 170.82 (C=O), 170.03 (C=O), 154.85 (C=O), 138.22-126.22 (aromatic), 131.40 (OCH2CH=CH2), 118.94 (OCH2CH=CH2), 101.41 (>CHPh), 100.92 (C-1’), 95.85 (C-1), 78.90 (C-4’), 78.75 (C-3), 76.37 (C-4), 76.05 (C-3S), 74.46 (CH2Ph), 74.27 (C-5), 71.42 (C-3’), 70.80 (C-3L, CH2Ph), 68.63-68.54 (C-6, C-6’, OCH2CH=CH2), 66.20 (C-5’), 56.04 (C-2, C-2’), −1.52 (SiCH3), −3.28 (SiCH3). HR MS (m/z) calcd for C100H162N2O18Si [M + Na]+, 1730.1484; found, 1730.1412.
Dimethylthexylsilyl 6-O-{4,6-O-benzylidene-2-deoxy-2-[(R)-3-hexadecanoyloxy-15-methyl-hexadecanoylamino]-3-O-levulinoyl-β-D-glucopyranosyl}-4-O-benzyl-3-O-[(R)-3-benzyloxy-hexadecanoyl]-2-[(R)-3-benzyloxy-15-methyl-hexadecanoylamino]-2-deoxy-β-D-glucopyranoside (27)
Tetrakis(triphenylphosphine)palladium (11 mg, 0.01 mmol) was added to a solution of 26 (80 mg, 0.047 mmol), n-BuNH2 (9.4 µL, 0.094 mmol) and HCOOH (3.5 µL, 0.094 mmol) in THF (2 mL). After stirring the reaction mixture at room temperature for 30 min, it was diluted with DCM (15 mL) and washed with water (10 mL), saturated aqueous NaHCO3 (2 × 10 mL) and brine (2 × 10 mL). The organic phase was dried (MgSO4) and filtered. Next, the filtrate was concentrated in vacuo. The residue was purified by preparative silica gel TLC chromatography (eluent: hexane/ethyl acetate, 3/2, v/v) to give an alcohol as a pale yellow syrup (72 mg, 95%). Rf = 0.55 (hexane/ethyl acetate, 3/2, v/v); 1H NMR (500 MHz, CDCl3): δ 7.43-7.24 (m, 15H, aromatic), 6.37 (d, 1H, JNH,2 = 6.0 Hz, NH), 5.90 (d, 1H, JNH’,2’ = 8.5 Hz, NH’), 5.46 (s, 1H, >CHPh), 5.37 (t, 1H, J2’,3’ = J3’,4’ = 9.5 Hz, H-3’), 5.03 (m, H-3L), 4.90 (d, 1H, J = 11.0 Hz, CHHPh), 4.87 (d, 1H, J1’,2’ = 8.0 Hz, H-1’), 4.63 (d, 1H, J = 11.0 Hz, CHHPh), 4.58 (d, 1H, J1,2 = 8.0 Hz, H-1), 4.55 (d, 1H, J = 12.0 Hz, CHHPh), 4.49 (d, 1H, J = 12.0 Hz, CHHPh), 4.28 (dd, 1H, J5’,6’a = 5.0 Hz, J6’a,6’b = 11.0 Hz, H-6’a), 3.98 (d, 1H, J6a,6b = 10.0 Hz, H-6a), 3.80-3.67 (m, 5H, H-2’, H-3, H-6b, H-6’b, H-3S), 3.63 (t, 1H, J3’,4’ = J4’,5’ = 9.5 Hz, H-4’), 3.50-3.36 (m, 4H, H-2, H-4, H-5, H-5’), 2.78-2.48 (m, 4H, CH2 of Lev), 2.43-2.23 (m, 6H, H-2S, H-2L, H-2L’), 2.11 (s, 3H, CH3 of Lev), 1.67-1.45 (m, 7H, H-4S, H-3L’, H-4L, CH of TDS), 1.23-1.12 (m, 64H, 32 × CH2 of lipid), 0.87-0.80 (m, 27H, 4 × CH3 of TDS, 5 × CH3 of lipid), 0.14 (s, 3H, SiCH3), 0.09 (s, 3H, SiCH3). HR MS (m/z) calcd for C96H158N2O16Si [M + Na]+, 1646.1273; found, 1646.1384. A solution of (R)-3-benzyloxy-hexadecanoic acid 10 (15 mg, 0.042 mmol) and DCC (11.5 mg, 0.056 mmol) in DCM (2 mL) was stirred at room temperature for 10 min, after which the alcohol intermediate (45 mg, 0.028 mmol) and DMAP (1 mg, 8 µmol) were added. The reaction mixture was stirred at room temperature for 10 h, after which the solids were removed by filtration and washed with DCM (2 × 1 mL). The combined filtrates were concentrated in vacuo and the residue was purified by preparative silica gel TLC chromatography (eluent: hexane/ethyl acetate, 5/2, v/v) to afford 27 as an amorphous white solid (52 mg, 95%). Rf = 0.45 (hexane/ethyl acetate, 5/2, v/v); [α]26D = −8.8°(c = 1.0, CHCl3); 1H NMR (300 MHz, CDCl3): δ 7.37-7.14 (m, 20H, aromatic), 6.12 (d, 1H, JNH,2 = 9.3 Hz, NH), 5.88 (d, 1H, JNH’,2’ = 8.1 Hz, NH’), 5.39 (s, 1H, >CHPh), 5.34 (t, 1H, J2’,3’ = J3’,4’ = 9.9 Hz, H-3’), 5.34 (t, 1H, J2,3 = J3,4 = 9.9 Hz, H-3), 5.00 (m, 1H, H-3L), 4.85 (d, 1H, J1’,2’ = 8.1 Hz, H-1’), 4.52-4.35 (m, 7H, H-1, 6 × CHHPh), 4.25 (dd, 1H, J5’,6’a = 4.5 Hz, J6’a,6’b = 10.5 Hz, H-6’a), 3.87 (d, 1H, J6a,6b = 10.5 Hz, H-6a), 3.81-3.46 (m, 8H, H-2, H-2’, H-4, H-4’, H-6b, H-6’b, 2 × H-3S), 3.46-3.36 (m, 2H, H-5, H-5’), 2.76-2.61 (m, 2H, CH2 of Lev), 2.52-2.44 (m, 3H, CH2 of Lev, H-2S), 2.35-2.15 (m, 7H, 3 × H-2S, H-2L, H-2L’), 2.06 (s, 3H, CH3 of Lev), 1.54-1.37 (m, 9H, 2 × H-4S, H-3L’, H-4L, CH of TDS), 1.23-1.06 (m, 86H, 43 × CH2 of lipid), 0.83-0.73 (m, 30H, 4 × CH3 of TDS, 6 × CH3 of lipid), 0.07 (s, 3H, SiCH3), 0.00 (s, 3H, SiCH3). 13C NMR (75 MHz, CDCl3): δ 206.43 (C=O), 173.70 (C=O), 172.12 (C=O), 171.38 (C=O), 170.73 (C=O), 169.96 (C=O), 138.59-126.19 (aromatic), 101.36 (>CHPh), 100.88 (C-1), 96.08 (C-1’), 78.86 (C-4’), 75.91 (C-4), 75.76 (C-3S), 75.43 (C-3S), 74.58 (C-3), 74.40 (C-5), 74.08 (CH2Ph), 71.43 (C-3’), 71.33 (CH2Ph), 70.77 (C-3L), 70.54 (CH2Ph), 68.15 (C-6, C-6’), 66. 15 (C-5’), 55.91 (C-2’), 55.80 (C-2), −1.50 (SiCH3), −3.24 (SiCH3).CHR MS (m/z) calcd for C119H194N2O18Si [M + Na]+, 1990.3988; found, 1990.3204.
6-O-{4,6-O-Benzylidene-2-deoxy-2-[(R)-3-hexadecanoyloxy-15-methyl-hexadecanoylamino]-β-D-glucopyranosyl}-4-O-benzyl-3-O-[(R)-3-benzyloxy-hexadecanoyl]-2-[(R)-3-benzyloxy-15-methyl-hexadecanoylamino]-2-deoxy-α-D-glucopyranose (29)
A reaction mixture of 27 (25 mg, 0.013 mmol) and hydrazine acetate (1.3 mg, 0.014 mmol) in a mixture of DCM (2 mL) and methanol (0.2 mL) was stirred at room temperature 6 h, after which it was concentrated in vacuo. The residue was purified by preparative silica gel TLC (eluent: hexane/ethyl acetate, 5/2, v/v) to afford 28 as a pale yellow syrup (23 mg, 96%). Rf = 0.40 (hexane/ethyl acetate, 5/2, v/v); 1H NMR (300 MHz, CDCl3): δ 7.30-7.12 (m, 20H, aromatic), 6.15 (d, 1H, JNH,2 = 9.3 Hz, NH), 5.87 (d, 1H, JNH’,2’ = 5.7 Hz, NH’), 5.47 (s, 1H, >CHPh), 5.08 (t, 1H, J2,3 = J3,4 = 9.9 Hz, H-3), 5.05 (m, 1H, H-3L), 4.73 (d, 1H, J1’,2’ = 8.1 Hz, H-1’), 4.53-4.36 (m, 7H, H-1, 6 × CHHPh), 4.23 (dd, 1H, J5’,6’a = 5.2 Hz, J6’a,6’b = 10.2 Hz, H-6’a), 4.16 (t, 1H, J2’,3’ = J3’,4’ = 9.6 Hz, H-3’), 3.92 (d, 1H, J6a,6b = 10.2 Hz, H-6a), 3.83-3.76 (m, 2H, H-2, H-3S), 3.70-3.58 (m, 3H, H-5’, H-6b, H-3S), 3.52–3.73 (m, 4H, H-4, H-4’, H-5, H-6’b), 3.26 (m, 1H, H-2’), 2.50 (dd, 1H, J2Sa,2Sb = 15.9 Hz, J2Sa,3S = 6.9 Hz, H-2Sa), 2.38-2.18 (m, 7H, 3 × H-2S, H-2L, H-2L’), 1.54-1.38 (m, 9H, 2 × H-4S, H-3L’, H-4L, CH of TDS), 1.26-1.09 (m, 86H, 43 × CH2 of lipid), 0.81-0.73 (m, 30H, 4 × CH3 of thexyl, 6 × CH3 of lipid), 0.06 (s, 3H, SiCH3), 0.00 (s, 3H, SiCH3). MS (m/z) calcd for C114H188N2O16Si [M + Na]+, 1892.3620; found, 1892.4476. Acetic acid (100 µL) was added to a solution of Bu4NF (1 N in THF, 1mL) and then 28 (35 mg, 0.019 mmol) was added. The reaction mixture was stirred at room temperature for 10 h, after which it was diluted with ethyl aetate (10 mL) and washed with saturated aqueous NaHCO3 (2 × 10 mL) and brine (2 × 10 mL). The organic phase was dried (MgSO4) and filtered. Next, the filtrate was concentrated in vacuo. The residue was purified by preparative silica gel TLC chromatography (eluent: DCM/acetone, 6/1, v/v) to afford 29 as a pale yellow syrup (21 mg, 65%). Rf = 0.40 (DCM/acetone, 6/1, v/v); 1H NMR (500 MHz, CDCl3): δ 7.51-7.19 (m, 20H, aromatic), 6.31 (d, 1H, JNH,2 = 9.5 Hz, NH), 6.19 (d, 1H, JNH’,2’ = 5.5 Hz, NH’), 5.55 (s, 1H, >CHPh), 5.43 (t, 1H, J2,3 = J3,4 = 9.5 Hz, H-3), 5.15-5.09 (m, 2H, H-1, H-1’), 5.01 (m, 1H, H-3L), 4.63-4.45 (m, 6H, 6 × CHHPh), 4.36 (m, 1H, H-6’a), 4.22 (m, 1H, H-2), 4.14 (m, 1H, H-3’), 4.02 (d, 1H, J6a,6b = 11.5 Hz, H-6a), 3.85-3.76 (m, 3H, H-6’b, 2 × H-3S), 3.67-3.47 (m, 3H, H-4’, H-5’, H-6b), 3.41 (m, 1H, H-4), 3.30 (m, 1H, H-2’), 2.61-2.24 (m, 8H, 2 × H-2S, H-2L, H-2L’), 1.66-1.49 (m, 8H, 2 × H-4S, H-3L’, H-4L), 1.26-1.17 (m, 86H, 43 × CH2 of lipid), 0.91-0.87 (m, 18H, 6 × CH3 of lipid). MS (m/z) calcd for C106H170N2O16 [M + Na]+ 1750.2443; found, 1750.2439.
6-O-{2-Deoxy-2-[(R)-3-hexadecanoyloxy-15-methyl-hexadecanoylamino]-β-D-glucopyranosyl}-3-O-[(R)-3-hydroxy-hexadecanoyl]-2-deoxy-2-[(R)-3-hydroxy-15-methylhexadecanoylamino]-α-D-glucopyranose 1-phosphate (3)
To a cooled (−78 °C) solution of 29 (10 mg, 0.0058 mmol) and tetrabenzyl diphosphate (12 mg, 0.022 mmol) in THF (1.5 mL) was added dropwise lithium bis(trimethylsilyl)amide in THF (1.0 M, 15 µL, 0.015 mmol). The reaction mixture was stirred for 1 h and then allowed to warm up to −20 °C. After the reaction mixture was stirred at −20 °C for 1 h, it was quenched with saturated aqueous NaHCO3 (10 mL) and extracted with ethyl acetate (10 mL). The organic phase was washed with brine (2 × 10 mL), dried (Na2SO4) and concentrated in vacuo. The residue was purified by Iatro beads column chromatography (hexane/ethyl acetate, 5/1 → 3/1 → 4/3, v/v) to give 30 as a pale yellow oil (8.3 mg, 72%). Rf = 0.55 (hexane/ethyl acetate, 3/2, v/v); 1H NMR (500 MHz, CDCl3): δ 7.44-7.18 (m, 30H, aromatic), 6.30 (d, 1H, JNH,2 = 9.0 Hz, NH), 5.63(bs, 1H, H-1), 5.55 (s, 1H, >CHPh), 5.31 (t, 1H, J2,3 = J3,4 = 10.0 Hz, H-3), 5.23 (m, 1H, H-3L), 5.13-4.91 (m, 5H, H-1, 4 × CHHPh), 4.62 (d, 1H, J = 11.0 Hz, CHHPh), 4.52-4.45 (m, 4H, 4 × CHHPh), 4.39 (d, 1H, J = 12.0 Hz, CHHPh), 4.34-4.26 (m, 2H, H-2, H-6’a), 4.14 (m, 1H, H-5), 3.95-3.91 (m, 2H, H-3’, H-6a), 3.84-3.74 (m, 3H, H-6b, H-6’b, H-3S), 3.70 (m, 1H, H-3S), 3.61 (m, 1H, H-2’), 3.55 (t, 1H, J3’,4’ = J4’,5’ = 9.5 Hz, H-4’), 3.46 (t, 1H, J3,4 = J4,5 = 9.5 Hz, H-4), 3.36 (m, 1H, H-5’), 2.57 (dd, 1H, J2Sa,2Sb = 16.0 Hz, J2Sa,3S = 8.0 Hz, H-2Sa), 2.51-2.40 (m, 3H, H-2S, H-2L), 2.26-2.18 (m, 4H, H-2S, H-2L’), 1.63-1.50 (m, 8H, 2 × H-4S, H-3L’, H-4L), 1.32-1.17 (m, 86H, 43 × CH2 of lipid), 0.90-0.87 (m, 18H, 6 × CH3 of lipid). MS (m/z) calcd for C120H183N2O19P [M + Na]+, 2010.3045; found, 2010.2429. A mixture of 30 (10.5 mg, 0.0053 mmol) and Pd black (15.0 mg) in anhydrous THF (5 mL) was shaken under an atmosphere of H2 (50 psi) at room temperature for 26 h, after which it was neutralized with triethylamine (10 µL). The catalyst was removed by filtration and the residue washed with THF (2 × 1 mL). The combined filtrates were concentrated in vacuo to afford 3 as a colorless film (6.0 mg, 78%). 1H NMR (500 MHz, CDCl3/CD3OD, 1/1, v/v): δ 5.28 (broad, 1H, H-1), 4.96-4.82 (m, 3H, H-1’, H-3, H-3L). HR MS (m/z) (negative) calcd for C78H149N2O19P, 1449.0492; found, 1449.7284.
Dimethylthexylsilyl 6-O-{4,6-O-benzylidene-3-O-[(R)-3-benzyloxy-13-methyl-tetradecanoylamino]-2-deoxy-2-[(R)-3-hexadecanoyloxy-15-methyl-hexadecanoylamino]-β-D-glucopyranosyl}-3-O-allyloxycarbonyl-4-O-benzyl-2-[(R)-3-benzyloxy-15-methyl-hexadecanoylamino]-2-deoxy-β-D-glucopyranoside (31)
A reaction mixture of 26 (80 mg, 0.047 mmol) and hydrazine acetate (4.7 mg, 0.052 mmol) in a mixture of DCM (3 mL) and methanol (0.3 mL) was stirred at room temperature 6 h, after which it was concentrated in vacuo. The residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate, 3/1, v/v) to afford an alcohol as a pale yellow syrup (69 mg, 92%). Rf = 0.40 (hexane/ethyl acetate, 5/2, v/v); 1H NMR (300 MHz, CDCl3): δ 7.46-7.17 (m, 15H, aromatic), 6.35 (d, 1H, JNH,2 = 9.0 Hz, NH), 5.99 (d, 1H, JNH’,2’ = 5.7 Hz, NH’), 5.77 (m, 1H, OCH2CH=CH2), 5.46 (s, 1H, >CHPh), 5.23 (d, 1H, J = 17.1 Hz, OCH2CH=CHH), 5.14 (d, 1H, J = 10.2 Hz, OCH2CH=CHH), 5.02 (m, 1H, H-3L), 4.94 (dd, 1H, J = 8.7 Hz, J = 10.5 Hz, H-3), 4.75 (d, 1H, J1’,2’ = 8.1 Hz, H-1’), 4.58-4.37 (m, 7H, H-1, 4 × CHHPh, OCH2CH=CH2), 4.23 (dd, 1H, J5’,6’a = 4.5 Hz, J6’a,6’b = 10.5 Hz, H-6’a), 4.13(m, 1H, H-3), 3.88 (d, 1H, J6a,6b = 10.5 Hz, H-6a), 3.76-3.31 (m, 8H, H-2, H-4, H-4’, H-5, H-5’, H-6b, H-6’b, H-3S), 3.27 (m, 1H, H-2’), 2.33-2.17 (m, 6H, H-2S, H-2L, H-2L’), 1.55-1.37 (m, 7H, H-4S, H-3L’, H-4L, CH of TDS), 1.17-1.07 (m, 64H, 32 × CH2 of lipid), 0.82-0.73 (m, 27H, 4 × CH3 of TDS, 5 × CH3 of lipid), 0.06 (s, 3H, SiCH3), 0.00 (s, 3H, SiCH3). HR MS (m/z) calcd for C95H156N2O16Si [M + Na]+, 1632.1116; found, 1631.8767. A solution of (R)-3-benzyloxy-13-methyl-tetradecanoic acid 7 (21 mg, 0.061 mmol) and DCC (17 mg, 0.081 mmol) in DCM (2 mL) was stirred at room temperature for 10 min, after which the alcohol intermediate (65 mg, 0.040 mmol) and DMAP (1 mg, 8 µmol) were added. The reaction mixture was stirred at room temperature for 12 h, after which the solids were removed by filtration and washed with DCM (2 × 1 mL). The combined filtrates were concentrated in vacuo and the residue was purified by preparative silica gel TLC chromatography (eluent: hexane/ethyl acetate, 4/1, v/v) to afford 31 as an amorphous solid (71 mg, 91%). Rf = 0.50 (hexane/ethyl acetate, 3/1, v/v); [α]24D = −11.1°(c = 1.0, CHCl3); 1H NMR (600 MHz, CDCl3): δ 7.37-7.21 (m, 20H, aromatic), 6.35 (d, 1H, JNH,2 = 9.0 Hz, NH), 5.84 (m, 1H, OCH2CH=CH2), 5.79 (d, 1H, JNH’,2’ = 9.0 Hz, NH’), 5.41 (s, 1H, >CHPh), 5.41 (t, 1H, J2’,3’ = J3’,4’ = 9.6 Hz, H-3’), 5.29 (d, 1H, J = 17.4 Hz, OCH2CH=CHH), 5.19 (d, 1H, J = 10.2 Hz, OCH2CH=CHH), 5.00-4.96 (m, 2H, H-3, H-3L), 4.87 (d, 1H, J1’2’ = 7.8 Hz, H-1’), 4.61-4.37 (m, 9H, H-1, 6 × CHHPh, OCH2CH=CH2), 4.29 (dd, 1H, J5’,6’a = 5.4 Hz, J6’a,6’b = 10.8 Hz, H-6’a), 3.94 (d, 1H, J6a,6b = 10.2 Hz, H-6a), 3.81-3.78 (m, 3H, H-2, H-6b, H-3S), 3.74-3.67 (m, 3H, H-2’, H-6’b, H-3S), 3.64-3.58 (m, 2H, H-4, H-4’), 3.50-3.45 (m, 2H, H-5, H-5’), 2.64-2.12 (m, 8H, H-2S, H-2L, H-2L’), 1.59-1.46 (m, 9H, H-4S, H-3L’, H-4L, CH of TDS), 1.23-1.13 (m, 80H, 40 × CH2 of lipid), 0.86-0.79 (m, 33H, 4 × CH3 of TDS, 7 × CH3 of lipid), 0.13 (s, 3H, SiCH3), 0.06 (s, 3H, SiCH3). 13C NMR (75 MHz, CDCl3): δ 173.80 (C=O), 171.06 (C=O), 170.82 (C=O), 169.65 (C=O), 154.86 (C=O), 138.41-126.14 (aromatic), 131.40 (OCH2CH=CH2), 118.94 (OCH2CH=CH2), 101.41 (>CHPh), 100.93 (C-1’), 95.86 (C-1), 78.93 (C-4’), 78.75 (C-3), 76.31 (C-4), 76.05 (C-3S), 75.65 (C-3S), 74.45 (CH2Ph), 74.21 (C-5), 71.24 (C-3’), 71.16 (CH2Ph), 70.80 (CH2Ph), 70.74 (C-3L), 68.63 (C-6, OCH2CH=CH2), 68.28 (C-6’), 66.27 (C-5’), 56.03 (C-2), 55.73 (C-2’), −1.52 (SiCH3), −3.27 (SiCH3). HR MS (m/z) calcd for C117H190N2O18Si [M + Na]+, 1962.3675; found, 1962.3035.
6-O-{4,6-O-Benzylidene-3-O-[(R)-3-benzyloxy-13-methyl-tetradecanoylamino]-2-deoxy-2-[(R)-3-hexadecanoyloxy-15-methyl-hexadecanoylamino]-β-D-glucopyranosyl}-4-O-benzyl-2-[(R)-3-benzyloxy-15-methyl-hexadecanoylamino]-2-deoxy-β-D-glucopyranose (33)
Tetrakis(triphenylphosphine)palladium (6.3 mg, 0.0054 mmol) was added to a stirred solution of 31 (35 mg, 0.018 mmol), n-BuNH2 (3.6 µL, 0.036 mmol) and HCOOH (1.4 µL, 0.036 mmol) in THF (2 mL). After stirring the reaction mixture at room temperature for 1 h, it was diluted with DCM (10 mL) and washed with water (10 mL), saturated aqueous NaHCO3 (2 × 10 mL) and brine (2 × 10 mL). The organic phase was dried (MgSO4) and filtered. Next, the filtrate was concentrated in vacuo. The residue was purified by preparative silica gel TLC chromatography (eluent: hexane/ethyl acetate, 5/2, v/v) to give 32 as a pale yellow syrup (31 mg, 94%). Rf = 0.50 (hexane/ethyl acetate, 3/2, v/v); 1H NMR (300 MHz, CDCl3): δ 7.29-7.13 (m, 20H, aromatic), 6.29 (d, 1H, JNH,2 = 5.7 Hz, NH), 5.69 (d, 1H, JNH’,2’ = 8.7 Hz, NH’), 5.31 (s, 1H, >CHPh), 5.28 (t, 1H, J2’,3’ = J3’,4’ = 8.7 Hz, H-3’), 4.89 (m, 1H, H-3L), 4.80 (d, 1H, J = 11.7 Hz, CHHPh), 4.70 (d, 1H, J1’,2’ = 8.4 Hz, H-1’), 4.53-4.26 (m, 6H, H-1, 5 × CHHPh), 4.18 (dd, 1H, J5’,6’a = 4.5 Hz, J6’a,6’b = 10.5 Hz, H-6’a), 3.89 (d, 1H, J6a,6b = 10.8 Hz, H-6a), 3.78-3.58 (m, 5H, H-2’, H-6b, H-6’b, 2 × H-3S), 3.53 (t, 1H, J =8.4 Hz, J = 9.6 Hz, H-4), 3.44-3.25 (m, 4H, H-2, H-4, H-5, H-5’), 2.54 (dd, 1H, J2Sa,2Sb = 15.0 Hz, J2Sa,3S = 6.0 Hz, H-2Sa), 2.33-2.17 (m, 6H, H-2S, H-2L, H-2L’), 2.6 (dd, 1H, J2La,2Lb = 15.0 Hz, J2La,3L = 5.7 Hz, H-2La), 1.49-1.36 (m, 9H, H-4S, H-4L’, H-4L, CH of TDS), 1.14-1.06 (m, 80H, 40 × CH2 of lipid), 0.76-0.71 (m, 33H, 4 × CH3 of TDS, 7 × CH3 of lipid), 0.05 (s, 3H, SiCH3), 0.00 (s, 3H, SiCH3). HR MS (m/z) calcd for C113H186N2O16Si [M + Na]+, 1878.3464; found, 1878.3721. Acetic acid (100 µL) was added to a solution of Bu4NF (1 N in THF, 1 mL) and then 32 (26 mg, 0.014 mmol) was added. The reaction mixture was stirred at room temperature for 20 h, after which it was diluted with ethyl acetate (10 mL) and washed with saturated aqueous NaHCO3 (2 × 10 mL) and brine (2 × 10 mL). The organic phase was dried (MgSO4) and filtered. Next, the filtrate was concentrated in vacuo. The residue was purified by preparative silica gel TLC chromatography (eluent: hexane/ethyl acetate, 1/1, v/v) to afford 33 as a pale yellow syrup (21 mg, 88%). Rf = 0.40 (hexane/ethyl acetate, 1/1, v/v); 1H NMR (300 MHz, CDCl3): δ 7.40-7.25 (m, 20H, aromatic), 6.67 (d, 1H, JNH,2 = 7.8 Hz, NH), 5.91 (d, 1H, JNH’,2’ = 8.1 Hz, NH’), 5.45-5.39 (m, 2H, H-3’, >CHPH), 5.24 (d, 1H, J1’,2’ = 8.4 Hz, H-1’), 5.08 (d, 1H, J1,2 = 2.7 Hz, H-1), 4.96 (m, 1H, H-3L), 4.92 (d, 1H, J = 11.7 Hz, CHHPh), 4.64-4.32 (m, 6H, H-6’a, 5 × CHHPh), 4.05-3.51 (m, 10H, H-2’, H-3, H-4’, H-5, H-5’, H-6a, H-6b, H-6’b, 2 × H-3S), 3.53 (dd, 1H, J =8.4 Hz, J = 9.6 Hz, H-4), 3.44-3.25 (m, 4H, H-2, H-4, H-5, H-5’), 2.63 (dd, 1H, J2Sa,2Sb = 16.4 Hz, J2Sa,3S = 6.0 Hz, H-2Sa), 2.53-2.17 (m, 7H, H-2S, H-2L, H-2L’), 1.64-1.47 (m, 8H, H-4S, H-4L’, H-4L), 1.25-1.15 (m, 80H, 40 × CH2 of lipid), 0.87-0.85 (m, 21H, 7 × CH3 of lipid). HR MS (m/z) calcd for C105H168N2O16Si [M + Na]+, 1736.2286; found, 1736.3901.
6-O-{2-Deoxy-2-[(R)-3-hexadecanoyloxy-15-methyl-hexadecanoylamino]-3-O-[(R)-3-hydroxy-13-methyl-tetradecanoylamino]-β-D-glucopyranosyl}-2-deoxy-2-[(R)-3-hydroxy-15-methyl-hexadecanoylamino]-α-D-glucopyranose 1-phosphate (4)
Compound 33 (15 mg, 0.0088 mmol) was phosphorylated in a manner similar to the synthesis of 29 to afford 34 as a pale yellow syrup (11.8 mg, 68%). Rf = 0.60 (hexane/ethyl acetate, 3/2, v/v); 1H NMR (500 MHz, CDCl3): δ 7.39-7.26 (m, 30H, aromatic), 6.65 (d, 1H, JNH’,2’ = 8.0 Hz, NH’), 6.50 (d, 1H, JNH,2 = 8.5 Hz, NH), 5.65 (bs, 1H, H-1), 5.42 (s, 1H, >CHPh), 5.35 (t, 1H, J2’3’ = J3’,4’ = 10.0 Hz, H-3’), 5.10-4.99 (m, 5H, H-3L, 4 × CHHPh), 4.92 (d, 1H, J1,2 = 9.0 Hz, H-1), 4.81 (d, 1H, J = 10.5 Hz, CHHPh), 4.61 (d, 1H, J = 10.5 Hz, CHHPh), 4.52-4.42 (m, 4H, 4 × CHHPh), 4.32 (m, 1H, H-6’a), 4.13 (m, 1H, H-2), 3.95-3.74 (m, 6H, H-2’, H-6a, H-6b, H-6’b, 2 × H-3S), 3.66-3.60 (m, 2H, H-3, H-4’), 3.43 (m, 1H, H-5’), 3.36 (m, 1H, H-5), 2.69 (dd, 1H, J2Sa,2Sb = 14.5 Hz, J2Sa,3S = 6.0 Hz, H-2Sa), 2.52-2.26 (m, 7H, H-2S, H-2L, H-2L’), 1.59-1.50 (m, 8H, H-4S, H-4L’, H-4L), 1.27-1.17 (m, 80H, 40 × CH2 of lipid), 0.89-0.87 (m, 21H, 7 × CH3 of lipid). HR MS (m/z) calcd for C119H181N2O19P [M + Na]+, 1996.2888; found, 1996.0125. Compound 34 (9.6 mg, 0.0049 mmol) was deprotected in a manner similar to the synthesis of 3 to provide 4 as a colorless film (5.3 mg, 76%). 1H NMR (500 MHz, CDCl3/CD3OD, 1/1, v/v): δ 5.18 (broad, 1H, H-1), 4.80-4.64 (m, 2H, H-3’, H-3L), 4.56 (broad, 1H, H-1’). HR MS (m/z) (negative) calcd for C77H147N2O19P, 1435.0336; found, 1435.5624.
Biological experiments
Reagents for biological experiments
E. coli 055:B5 LPS was obtained from List Biologicals and Pam3CysSK4 was obtained from Calbiochem. All data presented in this study were generated using the same batch of E. coli 055:B5 LPS. Synthetic compounds 3 and 4 were reconstituted in PBS with dry THF (10%) and stored at −80°C.
Cell maintenance details are given in the Supplementary Information.
Cytokine induction and ELISAs
On the day of the exposure assay differentiated MM6 cells were harvested by centrifugation and suspended (106 cells mL−1) in tissue culture tubes and RAW 264.7 γNO(−) cells were plated as 2 × 105 cells/well in 96-well tissue culture plates (Nunc). Cells were then incubated with different combinations of stimuli for 5.5 hours. Culture supernatants were then collected and stored frozen (−80°C) until assayed for cytokine production. All cytokine ELISAs were performed in 96-well MaxiSorp plates (Nalge Nunc International). Concentrations of human TNF-α protein in culture supernatants were determined by a solid phase sandwich ELISA. Plates were coated with purified mouse anti-human TNF-α antibody (Pharmingen). TNF-α in standards and samples was allowed to bind to the immobilized antibody. Biotinylated mouse anti-human TNF-α antibody (Pharmingen) was then added. Next, avidin-horseradish peroxidase conjugate (Pharmingen) and ABTS peroxidase substrate (Kirkegaard & Perry Laboratories) were added. After the reaction was stopped by adding peroxidase stop solution (Kirkegaard & Perry Laboratories), the absorbance was measured at 405 nm using a microplate reader (BMG Labtech). Cytokine DuoSet ELISA Development Kits (R&D Systems) were used for the cytokine quantification of mouse TNF-α, mouse IL-6, mouse IP-10 and mouse IL-1β according to the manufacturer’s instructions. The absorbance was measured at 450 nm with wavelength correction set to 540 nm. Concentrations of mouse IFN-β in culture supernatants were determined as follows. Plates were coated with rabbit polyclonal antibody against mouse IFN-β (PBL Biomedical Laboratories). IFN-β in standards and samples was allowed to bind to the immobilized antibody. Rat anti-mouse IFN-β antibody (USBiological) was then added. Next, horseradish peroxidase (HRP) conjugated goat anti-rat IgG (H+L) antibody (Pierce) and a chromogenic substrate for HRP 3,3’,5,5’-tetramethylbenzidine (TMB; Pierce) were added. After the reaction was stopped, the absorbance was measured at 450 nm with wavelength correction set to 540 nm. All cytokine values are presented as the means ± SD of triplicate measurements, with each experiment being repeated three times.
Transfection and NF-κB activation assay
The day before transfection, HEK 293T wild type cells and HEK 293T cells stably transfected with human and murine TLR4/MD2/CD14 and human and murine TLR2 were plated in 96-well tissue culture plates (16,000 cells/well). The next day, cells were transiently transfected using PolyFect Transfection Reagent (Qiagen) with expression plasmids pELAM-Luc (NF-κB-dependent firefly luciferase reporter plasmid, 50 ng/well)54 and pRL-TK (Renilla luciferase control reporter vector, 1 ng/well; Promega) as an internal control to normalize experimental variations. The empty vector pcDNA3 (Invitrogen) was used as a control and to normalize the DNA concentration for all of the transfection reactions (total DNA 70 ng/well). Forty-four h post-transfection, cells were exposed to the stimuli in the presence of FCS to provide sCD14 for 4 h, after which cell extracts were prepared. The luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions and a combination luminometer/fluorometer microplate reader (BMG Labtech). Expression of the firefly luciferase reporter gene was normalized for transfection efficiency with expression of Renilla luciferase. The data are reported as the means ± SD of triplicate treatments. The transfection experiments were repeated at least twice.
Data analysis
Concentration-response and inhibition data were analyzed using nonlinear least-squares curve fitting in Prism (GraphPad Software, Inc.). Concentration-response data were fit with the following four parameter logistic equation: Y = Emax / (1 + (EC50/X)Hill slope), where Y is the cytokine response, X is logarithm of the concentration of the stimulus, Emax is the maximum response and EC50 is the concentration of the stimulus producing 50% stimulation. Inhibition data were fit with the following logistic equation: Y = Bottom + (Top − Bottom) / (1 + 10(X − Log IC50)), where Y is the cytokine response, X is the logarithm of the concentration of the inhibitor and IC50 is the concentration of the inhibitor that reduces the response by half.
Supplementary Material
Acknowledgements
This research was supported by the Institute of General Medicine of the National Institutes of Health (GM061761).
Footnotes
Electronic supplementary information (ESI) available: NMR spectra of synthesized compounds.
References
- 1.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 2.Darveau RP. In: Oral Bacterial Ecology: The Molecular Basis. Kuramitsu HK, Ellen RP, editors. Wymond Norfolk: Horizon Scientific Press; 2000. pp. 169–218. [Google Scholar]
- 3.Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, Howald WN, Way SS, Hajjar AM. Infect. Immun. 2004;72:5041–5051. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa T, Asai Y, Hashimoto M, Takeuchi O, Kurita T, Yoshikai Y, Miyake K, Akira S. Int. Immunol. 2002;14:1325–1332. doi: 10.1093/intimm/dxf097. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa T, Asai Y, Makimura Y, Tamai R. Front. Biosc. 2007;12:3795–3812. doi: 10.2741/2353. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura A, Kaneko T, Kato Y, Golenbock DT, Hara Y. Infect. Immun. 2002;70:218–225. doi: 10.1128/IAI.70.1.218-225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reife RA, Coats SR, Al-Qutub M, Dixon DM, Braham PA, Billharz RJ, Howald WN, Darveau RP. Cell. Microbiol. 2006;8:857–868. doi: 10.1111/j.1462-5822.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 8.Coats SR, Pham TTT, Bainbridge BW, Reife RA, Darveau RP. J. Immunol. 2005;175:4490–4498. doi: 10.4049/jimmunol.175.7.4490. [DOI] [PubMed] [Google Scholar]
- 9.Rossignol DP, Hawkins LD, Christ WJ, Kobayashi S, Kawata T, Lynn M, Yamatsu I, Kishi Y. In: Endotoxin in Health and Disease. Brade H, Opal SM, Vogel SN, Morrison DC, editors. vol. 1. New York: Marcel Dekker, Inc.; 1999. pp. 699–717. [Google Scholar]
- 10.Christ W, McGuinness P, Asano O, Wang Y, Mullarkey M, Perez M, Hawkins L, Blythe T, Dubuc G, Robidoux A. J. Am. Chem. Soc. 1994;116:3637–3638. [Google Scholar]
- 11.Christ WJ, Asano O, Robidoux AL, Perez M, Wang Y, Dubuc GR, Gavin WE, Hawkins LD, McGuinness PD, Mullarkey MA, Lewis MD, Kishi Y, Kawata T, Bristol JR, Rose JR, Rossignol DP, Kobayashi S, Hishinuma I, Kimura A, Asakawa N, Katayama K, Yamatsu I. Science. 1995;268:80–83. doi: 10.1126/science.7701344. [DOI] [PubMed] [Google Scholar]
- 12.Golenbock DT, Hampton RY, Qureshi N, Takayama K, Raetz CR. J. Biol. Chem. 1991;266:19490–19498. [PubMed] [Google Scholar]
- 13.Lam C, Hildebrandt J, Schutze E, Rosenwirth B, Proctor RA, Liehl E, Stutz P. Infect. Immun. 1991;59:2351–2358. doi: 10.1128/iai.59.7.2351-2358.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawata T, Bristol JR, Rossignol DP, Christ WJ, Asano O, Dubuc GR, Gavin WE, Hawkins LD, Lewis MD, McGuinness PD, Mullarkey MA, Perez M, Robidoux AL, Wang Y, Kishi Y, Kobayashi S, Kimura A, Hishinima I, Katayama K, Yamatsu I. In: Novel Therapeutic Strategies in the Treatment of Sepsis. Morrison DC, Ryan JL, editors. New York: Marcel Dekker; 1995. pp. 171–186. [Google Scholar]
- 15.Peri F, Granucci F, Costa B, Zanoni I, Marinzi C, Nicotra F. Angew. Chem. Int. Ed. 2007;46:3308–3312. doi: 10.1002/anie.200604932. [DOI] [PubMed] [Google Scholar]
- 16.Fujimoto Y, Iwata M, Imakita N, Shimoyama A, Suda Y, Kusumoto S, Fukase K. Tetrahedron Lett. 2007;48:6577–6581. [Google Scholar]
- 17.Demchenko AV, Wolfert MA, Santhanam B, Moore JN, Boons GJ. J. Am. Chem. Soc. 2003;125:6103–6112. doi: 10.1021/ja029316s. [DOI] [PubMed] [Google Scholar]
- 18.Santhanam B, Wolfert MA, Moore JN, Boons GJ. Chem.-Eur. J. 2004;10:4798–4807. doi: 10.1002/chem.200400376. [DOI] [PubMed] [Google Scholar]
- 19.Lee HS, Wolfert MA, Zhang YH, Boons GJ. Chembiochem. 2006;7:140–148. doi: 10.1002/cbic.200500298. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Wolfert MA, Boons GJ. Bioorg. Med. Chem. 2007;15:4800–4812. doi: 10.1016/j.bmc.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasan M, Wolfert MA, Boons GJ. Org. Biomol. Chem. 2007;5:2087–2097. doi: 10.1039/b704427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa T. FEBS Lett. 1993;332:197–201. doi: 10.1016/0014-5793(93)80512-s. [DOI] [PubMed] [Google Scholar]
- 23.Kumada H, Haishima Y, Umemoto T, Tanamoto KI. J. Bacteriol. 1995;177:2098–2106. doi: 10.1128/jb.177.8.2098-2106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Gaekwad J, Wolfert MA, Boons GJ. J. Am. Chem. Soc. 2007;129:5200–5216. doi: 10.1021/ja068922a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Gaekwad J, Wolfert MA, Boons GJ. Chem.-Eur. J. 2008;14:558–569. doi: 10.1002/chem.200701165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawada N, Ogawa T, Asai Y, Makimura Y, Sugiyama A. Clin. Exp. Immunol. 2007;148:529–536. doi: 10.1111/j.1365-2249.2007.03346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai Y, Yano I, Kaneda K. Eur. J. Biochem. 1988;171:73–80. doi: 10.1111/j.1432-1033.1988.tb13760.x. [DOI] [PubMed] [Google Scholar]
- 28.Hiramoto S, Kinoshita N, Hatanaka S, Seto H. J. Antibiot. 1996;49:949–952. doi: 10.7164/antibiotics.49.949. [DOI] [PubMed] [Google Scholar]
- 29.Ubukata M, Kimura K, Isono K, Nelson CC, Gregson JM, Mccloskey JA. J. Org. Chem. 1992;57:6392–6403. [Google Scholar]
- 30.Katoh O, Sugai T, Ohta H. Tetrahedron: Asymmetry. 1994;5:1935–1944. [Google Scholar]
- 31.Shiozaki M, Deguchi N, Mochizuki T, Nishijima M. Tetrahedron Lett. 1996;37:3875–3876. [Google Scholar]
- 32.Shioiri T, Terao Y, Irako N, Aoyama T. Tetrahedron. 1998;54:15701–15710. [Google Scholar]
- 33.Shiozaki M, Deguchi N, Mochizuki T, Wakabayashi T, Ishikawa T, Haruyama H, Kawai Y, Nishijima M. Tetrahedron. 1998;54:11861–11876. [Google Scholar]
- 34.Yanai M, Hiramoto S. J. Antibiot. 1999;52:150–159. doi: 10.7164/antibiotics.52.150. [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J. Am. Chem. Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee AK, Sanders DP, Grubbs RH. Org. Lett. 2002;4:1939–1942. doi: 10.1021/ol0259793. [DOI] [PubMed] [Google Scholar]
- 37.Keegan DS, Hagen SR, Johnson DA. Tetrahedron: Asymmetry. 1996;7:3559–3564. [Google Scholar]
- 38.Nakahata M, Imaida M, Ozaki H, Harada T, Tai A. Bull. Chem. Soc. Jpn. 1982;55:2186–2189. [Google Scholar]
- 39.Fukase K, Fukase Y, Oikawa M, Liu WC, Suda Y, Kusumoto S. Tetrahedron. 1998;54:4033–4050. [Google Scholar]
- 40.Schmidt RR, Stumpp M. Liebigs Ann. Chem. 1983:1249–1256. [Google Scholar]
- 41.Tsukamoto Y, Nakagawa H, Kajino H, Sato K, Tanaka K, Yanai T. Biosc. Biotechnol. Biochem. 1997;61:1650–1657. doi: 10.1271/bbb.61.1650. [DOI] [PubMed] [Google Scholar]
- 42.Oikawa M, Shintaku T, Sekljic H, Fukase K, Kusumoto S. Bull. Chem. Soc. Jpn. 1999;72:1857–1867. [Google Scholar]
- 43.Akira S, Takeda K, Kaisho T. Nat. Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 44.Pasare C, Medzhitov R. Semin. Immunol. 2004;16:23–26. doi: 10.1016/j.smim.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Dixon DR, Darveau RP. J. Dent. Res. 2005;84:584–595. doi: 10.1177/154405910508400702. [DOI] [PubMed] [Google Scholar]
- 46.Werts C, Tapping RI, Mathison JC, Chuang TH, Kravchenko V, Saint Girons I, Haake DA, Godowski PJ, Hayashi F, Ozinsky A, Underhill DM, Kirschning CJ, Wagner H, Aderem A, Tobias PS, Ulevitch RJ. Nat. Immunol. 2001;2:346–352. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- 47.Girard R, Pedron T, Uematsu S, Balloy V, Chignard M, Akira S, Chaby R. J. Cell Sci. 2003;116:293–302. doi: 10.1242/jcs.00212. [DOI] [PubMed] [Google Scholar]
- 48.Erridge C, Pridmore A, Eley A, Stewart J, Poxton IR. J. Med. Microbiol. 2004;53:735–740. doi: 10.1099/jmm.0.45598-0. [DOI] [PubMed] [Google Scholar]
- 49.Que-Gewirth NLS, Ribeiro AA, Kalb SR, Cotter RJ, Bulach DM, Adler B, Saint Girons I, Werts C, Raetz CRH. J. Biol. Chem. 2004;279:25420–25429. doi: 10.1074/jbc.M400598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jongeneel CV. Immunobiology. 1995;193:210–216. doi: 10.1016/s0171-2985(11)80545-0. [DOI] [PubMed] [Google Scholar]
- 51.Mijatovic T, Houzet L, Defrance P, Droogmans L, Huez G, Kruys V. Eur. J. Biochem. 2000;267:6004–6011. doi: 10.1046/j.1432-1327.2000.01676.x. [DOI] [PubMed] [Google Scholar]
- 52.Crawford EK, Ensor JE, Kalvakolanu I, Hasday JD. J. Biol. Chem. 1997;272:21120–21127. doi: 10.1074/jbc.272.34.21120. [DOI] [PubMed] [Google Scholar]
- 53.Wolfert MA, Murray F, Boons GJ, Moore JN. J. Biol. Chem. 2002;277:39179–39186. doi: 10.1074/jbc.M204885200. [DOI] [PubMed] [Google Scholar]
- 54.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. J. Biol. Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.