Abstract
Amplification of the gene encoding the epidermal growth factor (EGF) receptor (EGFR) occurs commonly in glioblastoma, leading to activation of downstream kinases including phosphatidylinositol 3′-kinase (PI3K), Akt, and mammalian target of rapamycin (mTOR). Here, we show that phosphorylation of mTOR and its downstream substrate rpS6 (ribosomal protein S6) are robust biomarkers for the antiproliferative effect of EGFR inhibitors. Inhibition of EGFR signaling correlated with decreased abundance of phosphorylated mTOR (p-mTOR) and rpS6 (p-rpS6) in cells wild type for the gene encoding PTEN (phosphatase and tensin homolog on chromosome 10), a negative regulator of PI3K. In contrast, inhibition of EGFR signaling failed to affect p-mTOR or p-rpS6 in cells mutant for PTEN, which are resistant to EGFR inhibitors. Although the abundance of phosphorylated Akt (p-Akt) decreased in response to inhibition of EGFR signaling, Akt was dispensable for signaling between EGFR and mTOR. We identified an Akt-independent pathway linking EGFR to mTOR that was critically dependent on protein kinase C (PKC). Consistent with these observations, the abundance of EGFR generally correlated with phosphorylation of rpS6 and PKC in primary human glioblastoma tumors, and correlated poorly with phosphorylation of Akt. Inhibition of PKC led to decreased viability of glioma cells regardless of PTEN or EGFR status, suggesting that PKC inhibitors should be tested in glioma. These findings underline the importance of signaling between EGFR and mTOR in glioma, identify PKCα as essential to this network, and question the necessity of Akt as a critical intermediate coupling EGFR and mTOR in glioma.
INTRODUCTION
Astrocytomas are the most prevalent form of brain tumor. Most patients present at diagnosis with advanced grade 4 (glioblastoma multiforme) tumors. Primary glioblastomas frequently show amplification of the receptor tyrosine kinase (RTK) EGFR and are distinguished from secondary glioblastomas, which arise through further transformation of low-grade tumors, and less frequently show EGFR amplification. Because abnormalities in EGFR signaling feature so prominently in glioblastoma, therapies that target EGFR signaling have been tested extensively in this disease.
EGFR signals through a complex network of intermediates including PI3K, Akt, mitogen-activated protein kinase (MAPK), and phospholipase C-γ (PLC-γ)(1). The kinase mTOR is a critical target of EGFR signaling, linking growth factor abundance to cell growth and proliferation. Signaling pathways linking EGFR, PI3K, and Akt to downstream kinases including mTOR have received scrutiny in various cancers, in part because mutation of the gene encoding the tumor suppressor PTEN (a phosphatase downstream of EGFR) drives activation of PI3K and Akt in an EGFR independent manner and may confer resistance to upstream inhibition of EGFR (2). In particular, with EGFR implicated as a driving oncogene in malignant glioma, it was anticipated that inhibition of EGFR signaling would represent an effective therapeutic strategy. Initial results with EGFR inhibitors in glioblastoma have been disappointing, however, with most patients not responding. Only patients with amplified, mutationally activated EGFR and wild-type PTEN show short-lived responses to EGFR inhibitors (3). However, these patients account for only a minority (~10%) of glioblastoma patients. What about the large number of patients with EGFR-driven tumors that carry PTEN mutations who do not respond to EGFR inhibitor therapy?
To address the apparent inactivity of EGFR inhibitors against EGFR-driven, PTEN-mutant glioma, we have further analyzed signaling between EGFR, Akt, and mTOR in glioma-derived cell lines and in primary tumors from glioma patients. Here, we confirm that p-mTOR is a robust biomarker for the antiproliferative activity of EGFR inhibitors. In contrast, Akt activity correlated poorly with the antiproliferative effects of EGFR blockade. We show that (i) inhibition of EGFR signaling affects mTOR through a pathway that depends on protein kinase C (PKC) and is independent of Akt, (ii) PKC signals downstream of PTEN in glioma, and (iii) PKC inhibitors block proliferation in glioma irrespective of PTEN and EGFR status. These studies suggest PKC as an important signaling intermediate between EGFR and mTOR and as a therapeutic target in malignant glioma.
RESULTS
EGFR-driven glioma cells wild type for PTEN (PTENwt) generally respond to blockade of EGFR, whereas EGFR-driven glioma cells mutant for PTEN (PTENmt) do not (3-5). Consistent with these observations, we found that treatment of PTENwt cell lines with the EGFR inhibitor erlotinib led to their arrest at G1, whereas comparable treatment of PTENmt lines had little effect (Fig. 1A). The amount of p-Akt in EGF-treated cells showed a dose-dependent decrease in all cell lines, although this decrease was attenuated in PTENmt lines compared with PTENwt lines (Fig. 1B). The abundance of p-mTOR and its target p-rpS6 correlated with blockade of proliferation in response to 5 μM erlotinib, consistent with the antiproliferative activity of mTOR inhibitors in glioma (Fig. 1, B and C) (6-8). Thus, p-mTOR and p-rpS6 were robust biomarkers of G1 arrest in response to erlotinib, whereas the ability of erlotinib to inhibit Akt phosphorylation in PTENmt lines did not correlate with proliferation block.
Fig. 1.

Biochemical and antiproliferative effects of EGFR inhibition correlate with blockade of mTOR and the mTOR target rpS6. Glioma cells in 10% FBS were treated with 5 μM erlotinib for 24 hours as indicated. EGF (50 ng/ml) was added 15 min before harvest for immunoblot. (A) Flow cytometric analyses show that erlotinib induced arrest at G0/G1 only in PTENwt cell lines. (B and C) Abundances of p-Akt and p-Erk were reduced by erlotinib in a dose-dependent manner in PTENwt (LN229:EGFR)and PTENmt (U373:EGFR) lines. In contrast, the abundances of p-mTOR (B) and p-rpS6 (B and C) were affected by erlotinib only in PTENwt cells. β-Tubulin is shown as loading control. A blot representative of two independent experiments is shown in (B). Panels in (B) also represent identical exposures and were taken from a single gel.
This discordance between the ability of erlotinib to affect Akt phosphorylation and that of mTOR and rpS6 in PTENmt glioma raised questions about Akt's role in signaling between EGFR and mTOR. To better address this issue, we analyzed LN229:EGFR PTENwt and U373:EGFR PTENmt glioma cells, adding erlotinib for 1 or 24 hours. Erlotinib blocked Akt phosphorylation irrespective of serum concentration [1% versus 10% fetal bovine serum (FBS)] or incubation time (fig. S1A). Although erlotinib treatment led to decreased p-Akt in both cell lines, the abundance of p-rpS6 decreased progressively from 1 to 24 hours only in LN229:EGFR cells (in both low and high serum concentrations), again showing a lack of alignment between phosphorylation of Akt and of rpS6.
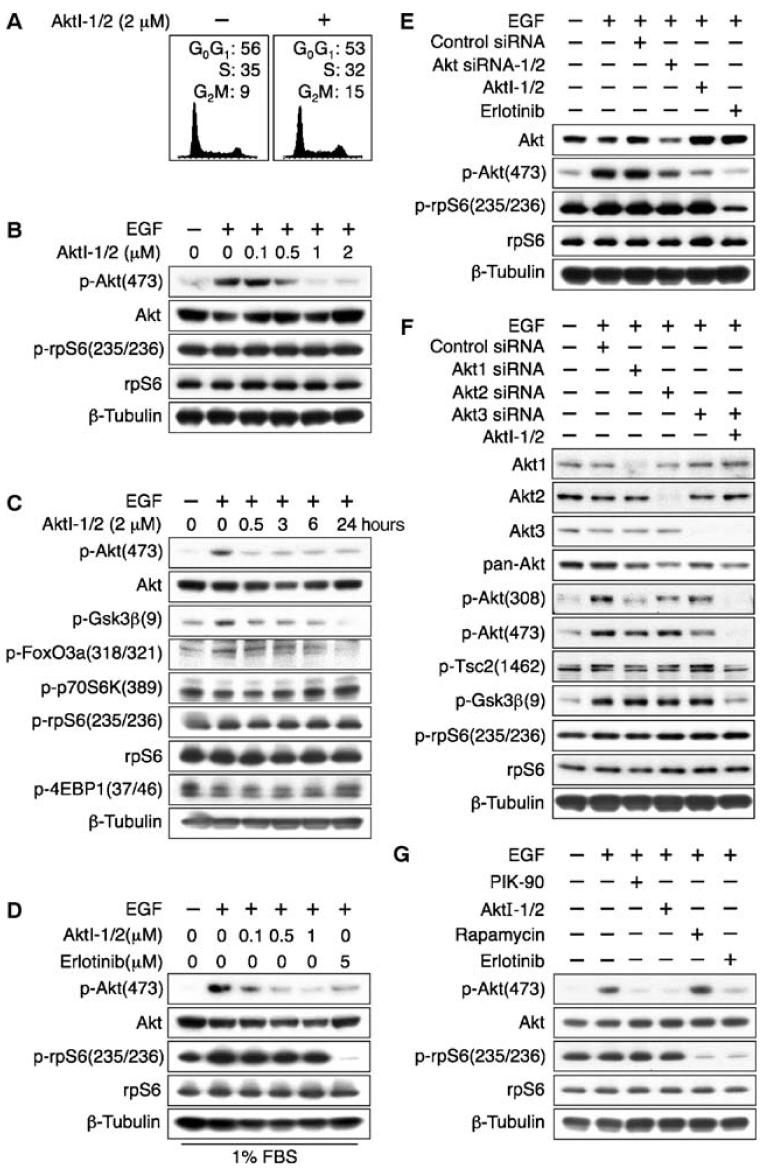
To further investigate the role of Akt as a signaling intermediate between EGFR and mTOR, we treated PTENwt glioma cells with AktI-1/2, a selective inhibitor of Akt1 and 2 (9), and found that proliferation was minimally affected (Fig. 2A and fig. S1B). Phosphorylation of Akt and its targets glycogen synthase kinase 3β (Gsk3β) and FoxO3a was reduced in response to treatment with AktI-1/2. However, neither pharmacological inhibition nor small interfering RNA (siRNA)-mediated knockdown of Akt1 and Akt2 affected concentrations of p-S6K or p-rpS6 (Fig. 2, B to E). These data suggest either that an alternative isozyme of Akt mediates signaling between EGFR and mTOR in glioma (Akt3, for which there is no inhibitor) or that Akt is dispensable in coupling EGFR to mTOR.
Fig. 2.
Small-molecule inhibition or siRNA knockdown of Akt isozymes fails to affect phosphorylation of the mTOR target rpS6 in PTENwt LN229:EGFR cells. LN229:EGFR cells were treated for 24 hours with indicated dosages of the EGFR inhibitor erlotinib, AktI-1/2 [a PH-domain- dependent isozyme selective inhibitor of Akt1/2 (9)], or siRNA directed against Akt1, Akt2, and Akt3. EGF (50 ng/ml) was added 15 min before harvest, and lysates were immunoblotted. (A) AktI-1/2 (2 μM) had no effect on cell cycle distribution.(B and C)AktI-1/2(2 μM) decreased phosphorylation of Akt and that of substrates of Akt, but had no effect on the abundance of p-p70S6K and p-rpS6. (D) Addition of EGF to cells grown in 1% FBS led to increased p-rpS6 abundance. Inhibition of Akt1/2 had little effect on the abundance of p-rpS6, whereas inhibition of EGFR markedly decreased phosphorylation of this mTOR target protein. (E) The effects of siRNA directed against Akt1/2 were consistent with results obtained with inhibitors of AktI1/2, showing knockdown of Akt and p-Akt, without affecting p-rpS6. (F) The combination of siRNA directed against Akt3 with AktI-1/2 led to undetectable p-Akt. The abundance of p-rpS6 was unaffected, whereas phosphorylation of the Akt targets p-Gsk3β and p-Tsc2 was decreased. The experiment was performed in 10% FBS. (G) Phosphorylation of rpS6 in LN229:EGFR cells was blocked by both the mTOR inhibitor rapamycin (100 nM) and the EGFR inhibitor erlotinib (5 μM) and unaffected by the PI3K inhibitor PIK-90 (1 μM) or AktI-1/2 (2 μM).
To distinguish between these possibilities, we combined inhibition or knockdown of Akt1, Akt2, and Akt3, analyzing the canonical Akt targets Gsk3β and tuberous sclerosis complex 2 (Tsc2) (10) and the mTOR target rpS6 (Fig. 2F). Even with combinations of inhibitors and siRNAs that decreased total p-Akt with concomitant reduction in p-Gsk3β and p-Tsc2, we observed no decrease in p-rpS6. This lack of correlation was most marked in experiments combining AktI-1/2 with siRNA against Akt3, which markedly decreased total p-Akt, p-Gskβ, and p-Tsc2, but failed to decrease p-rpS6 (Fig. 2 and fig. S1C).
We next showed that inhibition of mTOR led to decreased abundance of p-rpS6. LN229:EGFR cells were treated with the PI3Kα inhibitor PIK-90, AktI-1/2, the mTOR inhibitor rapamycin, or erlotinib. To avoid the off-target effects of PIK-90 against mTOR observed at doses of 10 μM (6), we used this compound at 1 μM. Phosphorylation of rpS6 was blocked by both rapamycin and erlotinib, and was unaffected by PIK-90 or AktI-1/2 (Fig. 2G). This result was extended to U373:MG and U87:MG cells, both of which showed a decrease in p-rpS6 in response to rapamycin (fig. S1, D and E).
To assess whether activation of Akt could drive proliferation, we transduced LN229:EGFR cells with a constitutively active myristoylated allele of Akt (Myr-Akt). In the presence of EGF, Myr-Akt elicited a modest increase in cell viability, and no increase in proliferation (Fig. 3, A and B). These results do not preclude the possibility that Myr-Akt might phosphorylate rpS6 under serum-free conditions. In the presence of 10% FBS, however, erlotinib treatment of Myr-Akt cells minimally affected the abundance of p-Akt, whereas it decreased p-rpS6 abundance. Further, the ability of erlotinib to block both viability and proliferation was unaffected by Myr-Akt (Fig. 3).
Fig. 3.

Activation of Akt has no effect on proliferation and does not affect response to erlotinib. (A to C) LN229:EGFR cells were transduced with activated myristoylated Akt (Myr-Akt) and treated as indicated. Viability (WST-1 assay) and flow cytometric analyses (A and B) confirmed that Myr-Akt had negligible effects on cell viability and proliferation and did not affect response to erlotinib. (P > 0.05 by Student's t test for vector-transduced cells versus Myr-Akt-transduced cells in response to erlotinib. Data shown are means ± SDs for quadruplicate measurements). (C) Myr-Akt diminished the ability of erlotinib to block Akt phosphorylation at both Ser473 and Ser308, but had no effect on abundance of p-rpS6. A blot representative of two independent experiments is shown. Erlotinib dosage was 5 μM in all experiments.
Data in Fig. 1 suggest that p-mTOR and p-rpS6 are robust biomarkers for the antiproliferative effects of EGFR inhibitors in glioma cells, whereas data in Figs. 2 and 3 indicate that Akt is dispensable for these effects. To identify intermediates critical to signaling between EGFR and mTOR, we treated PTENwt and PTENmt glioma cells with erlotinib and analyzed the phosphorylation status of EGFR and that of various proteins that signal downstream of EGFR. The abundances of p-EGFR and p-PLC-γ were affected similarly by erlotinib regardless of PTEN status, whereas the abundance of p-PKC isozymes was affected differentially (Fig. 4).
Fig. 4.
PKC signals between EGFR and mTOR in PTENwt glioma. (A) LN229:EGFR cells were treated with the MEK inhibitor PD98059, lysed at 24 hours, and immunoblotted as indicated. Dosages of PD98059 sufficient to block MAPK signaling had negligible effects on abundance of p-rpS6. (B) Glioma cell lines shown were treated with erlotinib (5 μM) and analyzed for phosphorylated intermediates differentially affected as a function of PTEN status. Appearance of a phosphorylated PKC isoform in response to EGF (50 ng/ml) in PTENwt cells was blocked by erlotinib. Panels representing LN229:EGFR and U87:EGFR cells were from a single gel, as were panels representing SF767:EGFR and U373:EGFR cells. (C) PTEN dependence was established by treating LN229:EGFR cells with the PTEN inhibitor bpv (11)(3 μM), which blocked the ability of erlotinib (5 μM) to decrease phosphorylation of rpS6. (D and E) Treatment of LN229:EGFR cells with phorbol ester (100 nM) induced a supershifted PKC isozyme, abrogated the activity of erlotinib (5 μM) against p-rpS6 in both low and high serum concentrations (D), and blocked the antiproliferative activity of erlotinib (E). EGF (50 ng/ml) was added 15 min and PMA (100 nM) was added 30 min before harvest for immunoblot. PMA (100 nM) was added 24 hours before harvest for flow cytometry analysis. Collectively, these observations suggest that PKC signals downstream of EGFR and that activation of PKC thus blocks both the biochemical activity of erlotinib against mTOR and the antiproliferative activity of this inhibitor.
The abundance of p-rpS6 was unaffected by treatment with the MAPK kinase (MEK) inhibitor PD98059, excluding a role for MAPK in signaling between EGFR and mTOR (Fig. 4A). These data are consistent with results in Fig. 1B and indicate that erlotinib concentrations sufficient to block MAPK signaling do not decrease the abundance of p-rpS6 in PTENmt glioma U373:EGFR cells. In contrast, erlotinib treatment of PTENwt LN229:EGFR cells inhibited phosphorylation of Erk and rpS6. Thus, erlotinib can block Erk phosphorylation in a PTEN-independent manner, whereas its inhibition of rpS6 phosphorylation is PTEN dependent. We thus conclude that EGFR signaling to mTOR is independent of MAPK signaling.
Baseline abundance of total p-PKC was higher in PTENmt lines compared with that in PTENwt (Fig. 4B). In addition, immunoblotting of PTENmt lines, in contrast to PTENwt lines, with an antibody that recognized the phosphorylated forms of seven PKC isoforms revealed constitutive expression of a broad band, likely a doublet, although we were unable to cleanly resolve two separate bands. EGF treatment led to the appearance in PTENwt cells of a more slowly migrating p-PKC isozyme that was blocked by erlotinib (Fig. 4B). A faint upper band was seen in LN229:EGFR cells in 10% serum. This band was diminished or absent in LN229 parental cells and in primary cultures wild type or amplified for EGFR and wild type for PTEN (fig. S2, A to C). We hypothesized that the shifted PKC isozyme is a candidate p-PKC isozyme that links EGFR signaling to mTOR activation in glioma. To show that differences in PKC phosphorylation between PTENwt and PTENmt cells depended on PTEN, we generated an isogenic set of cell lines that differed only in Pten activity (fig. S3B). Although treatment of PTENwt cells with the Pten inhibitor bisperoxovanadium (bpv) (11) had no effect on abundance or mobility of any PKC isoform, bpv attenuated the ability of erlotinib to block this shifted PKC isoform and abrogated the decrease in p-rpS6 produced by erlotinib (Fig. 4C).
We hypothesized that the shifted form of PKC induced by EGF was a key intermediate linking EGFR to mTOR and that this PKC isoform signals downstream of PTEN. The induction of PKC isozymes by phorbol esters represents one of the earliest observations linking PKC to malignant progression in cancer (12). Glioma cells treated with the phorbol ester phorbol 12-myristate 13-acetate (PMA) showed a supershifted PKC isoform (Fig. 4D and fig. S3B), with PMA increasing the abundance of p-rpS6 in low serum (Fig. 4D). PMA alone had no effect on proliferation in PTENwt cells (Fig. 4E). Erlotinib alone decreased the abundance of p-rpS6. PMA attenuated both the erlotinib-mediated decrease in p-rpS6 and the antiproliferative activity of erlotinib in PTENwt glioma cells (Fig. 4E).
To assess signaling between EGFR, PKC, and mTOR in the absence and presence of EGFR activation, we analyzed LN229 parental cells, GBM43 (cells cultured from a primary glioma xenograft wild type for both PTEN and EGFR) and GBM12 (cells cultured from a primary glioma xenograft wild type for PTEN and amplified for EGFR)(13). In all cases, EGF induced a slowly migrating p-PKCα band that was abrogated in response to erlotinib (fig. S2, A to C). Erlotinib treatment also blocked induction of p-rpS6 by EGF. From these data, we conclude that the pathway linking EGFR to mTOR through PKC is active in glioma regardless of EGFR amplification.
To identify specific PKC isozymes that mediate signaling between EGFR and mTOR, we immunoblotted lysates from PTENwt cells, analyzing candidate isozymes supershifted by both PMA and EGF and supershifted by EGF blocked in response to erlotinib. Supershifted p-PKCδ and p-PKCα met all three criteria (Fig. 5A). PKCδ was excluded by showing that siRNA directed against PKCδ blocked appearance of only the rapidly migrating p-PKC isoform and not the relevant more slowly migrating form (fig. S4A). Although siRNA directed against PKCα decreased the abundance of total PKCα, it did not affect the amount of p-PKCα (fig. S4A). Using cycloheximide pulse-chase analysis, we showed that p-PKCα had a half-life of >24 hours (fig. S4B), precluding the use of siRNA to ablate this isoform. We therefore used short hairpin RNA (shRNA) to stably knock down p-PKCα, showing abrogation of the supershifted PKC isozyme in response to both EGF and PMA (Fig. 5B). Consistent with a role for PKCα in linking EGFR signaling to mTOR in glioma, we showed that phosphorylation of the PKCα substrate MARCKS (myristoylated alaninerich C-kinase substrate) correlated with activation of p-PKCα (Fig.5, A and B) and that overexpression of PKCα led to increased abundance of p-PKCα without affecting the ability of erlotinib to decrease the abundance of p-rpS6 (fig. S4C).
Fig. 5.
PKCα is a signaling intermediate linking EGFR to mTOR in glioma. (A) Analysis of different p-PKC isoforms showed an increase in both p-PKCα and p-PKCδ in response to treatment with either EGF (50 ng/ml) or PMA (100 nM) and inhibition of the increase in phosphorylation of these PKC isoforms by EGF in response to erlotinib (5 μM). (B) Knockdown of PKCα by shRNA led to decreased abundance of total PKCα,p-PKCα, and p-rpS6K by immunoblot, consistent with a pathway linking EGFR, PKCα, and mTOR. (C) In cells transduced with a dominant-active allele of PKCα (PKCα-Cat) (14) erlotinib (5 μM) has reduced ability to block rpS6. Immunoblot indicates that the abundance of p-PKCα-Cat was comparable to that of endogenous p-PKCα. Erlotinib (5 μM) decreased the abundance of p-rpS6 in control cells, but was less effective in cells transduced with PKCα-Cat. The band at ~60 kDa is a nonspecific contaminant. A blot representative of three independent experiments is shown. (D)By flow cytometry, PKCα-Cat abrogated the antiproliferative activity of erlotinib (5 μM). (P < 0.0001 by Student's t test for vector-transduced cells versus PKCα-Cat-transduced cells in response to erlotinib. Data shown are means ± SDs for triplicate measurements). In (A) to (D), LN229:EGFR cells were used.
To confirm the role of PKCα as a signaling intermediate between EGFR and mTOR, we transfected PTENwt cells with a dominant-active construct, PKCα-Cat (14). Decreased phosphorylation of rpS6 by erlotinib was attenuated by expression of PKCα-Cat at abundance below that of endogenous p-PKCα, consistent with a role for PKCα as an intermediate between EGFR and mTOR (Fig. 5C and fig. S2D). PKCα-Cat also abrogated the antiproliferative activity of erlotinib in PTENwt cells (Fig. 5D).
Data in Figs. 1 to 5 indicate that Akt is dispensable for signaling between EGFR and mTOR in glioma cells and instead point to PKCs as critical intermediates linking EGFR signaling to mTOR activation. To validate the relevance to primary astrocytoma, we analyzed primary human glioblastoma specimens obtained by surgical resection before therapy. The abundance of EGFR in these specimens correlated with that of p-rpS6, total p-PKC, and p-PKCα, but showed little correlation with the abundance of p-Akt (Fig. 6 and fig. S5).
Fig. 6.
EGFR abundance correlates better with p-rpS6 and p-PKC than with p-Akt in primary human glioblastoma tumors. Normal brain (autopsy specimen) or primary human glioblastoma tumors obtained from the Brain Tumor Research Center at UCSF were lysed and immunoblotted. In EGFR immunoblot, the top band has mobility of wild-type EGFR, whereas the lower band has mobility consistent with mutationally activated EGFR. Abundances of EGFR, p-PKCα, p-PKC (pan), p-rpS6, and p-Akt in normal brain and primary human glioblastoma tumors are quantified in fig. S4. Samples showing abundant p-PKC in the absence of high concentrations of EGFR may be activated by alternative RTKs (17).
Although the efficacy of erlotinib in patients is dependent on EGFR and PTEN status, we reasoned that inhibition of PKC should be effective even in EGFR diploid, PTENmt glioma, supporting a role for inhibition of PKC as a general therapeutic strategy in glioma. Consistent with this hypothesis, glioma cells treated with the pan-PKC inhibitor bis-indolyl maleimide I (BIM I) (15, 16)) showed decreased viability regardless of EGFR or PTEN status (Fig. 7B and fig. S6). Whereas erlotinib affected the abundance of p-rpS6 only in PTENwt cells (Fig. 1, B and C), BIM I decreased phosphorylation of both the PKCα substrate MARCKS and that of the mTOR substrate rpS6 in PTENwt and PTENmt cells, although the effect of BIM I on p-rpS6 in U373:EGFR cells was modest (P > 0.05 by Student's t test for cells treated with EGF plus BIM I versus vehicle control or versus cells treated with EGF alone) (Fig. 7, A and C). BIM I treatment induced arrest at G1 in PTENwt and at G2 in PTENmt cells, suggesting that the effect of this compound was achieved through inhibition of PKC, rather than through nonspecific toxicity (fig. S6).
Fig. 7.
Inhibition of PKC decreases viability of both PTENwt and PTENmt glioma cells. (A) A PKC inhibitor blocks phosphorylation of rpS6 and not that of Akt. LN229:EGFR and U373:EGFR cells were treated with 5 μM pan-PKC inhibitor BIM I for 24 hours. The abundances of p-PKC (pan) and p-rpS6 was reduced. In contrast, abundance of p-Akt was not changed. In U373:EGFR cells, abundance of p-rpS6 increased by 1.22-fold in response to EGF and decreased by 0.88-fold after treatment with EGF plus BIM I (as compared with vehicle control and normalized to β-tubulin). (B) PKC inhibits glioma cell viability regardless of PTEN and EGFR status. LN229:parental and LN229:EGFR (PTENwt) and U373:parental and U373:EGFR (PTENmt) cells were treated with BIM I at doses indicated for 3 days. Viability was measured by WST-1 assay (8). (C) Immunoblot analysis of cells from (B) indicated that inhibition of PKC led to decreased phosphorylation of the mTOR target rpS6. In U373:EGFR cells, abundance of p-rpS6 decreased by 0.65-fold in response to treatment with BIM I, increased 1.2-fold in response to EGF, and decreased 0.6 fold after treatment with EGF plus BIM I. EGF (50 ng/ml) was added 15 min before harvest for immunoblot.
DISCUSSION
To explore the failure of EGFR inhibitors to block proliferation in PTENmt glioma cells, we looked for signaling intermediates whose activation correlated with the efficacy of EGFR blockade against proliferation in tumor-derived cell lines. These data confirmed that phosphorylation of mTOR and its downstream targets S6K and rpS6 were robust biomarkers for the ability of EGFR inhibitors to block proliferation of glioma cells. The ability of EGFR inhibitors to block Akt phosphorylation, however, correlated poorly with response to therapy. Using both gain- and loss-of-function approaches, we showed that Akt activity did not correlate with activation of mTOR or with proliferation. Rather, we identified PKC as critical to signaling between EGFR and mTOR in two PTENwt glioma cell lines. We also present data from primary tumor specimens that the abundances of EGFR, p-PKC, and p-rpS6 were strongly aligned, but correlated poorly with the abundance of p-Akt. Finally, we show that pharmacological inhibition of PKC blocked proliferation even in PTENmt glioma, where inhibition of EGFR had no effect. Although the BIM I inhibitor used was not specific for PKCα, this agent effectively blocked the PKCα substrate p-MARCKS. In addition, BIM I induced arrest at G1 in PTENwt cells and at G2 in PTENmt cells (fig. S6). If the antiproliferative effects of this compound were nonspecific, then cell cycle arrest induced by BIM I should not vary as a function of PTEN status.
What are the implications of these observations? Amplification of EGFR has a well-known association with advanced glioblastoma multiforme tumors. This observation, combined with the poor outcome in this disease, set high expectations for the potential therapeutic efficacy of EGFR inhibitors in glioma. That EGFR inhibitors are of limited use clinically results both from the failure of these drugs to block PI3K signaling in PTENmt tumors and from activation of multiple RTKs in glioma (17), making it unlikely that blockade of any single RTK would result in a durable clinical response.
The problem presented by frequent PTEN mutation combined with activation of multiple RTKs collectively argues for blockade of downstream signaling pathways into which these signaling inputs converge. The prominence of Akt as a signaling intermediate downstream of EGFR has generated enthusiasm for the clinical development of small-molecule inhibitors of Akt. We were therefore surprised to find that inhibition of Akt activation could be achieved in PTENmt glioma with dosages of erlotinib that failed to affect proliferation. We showed further that neither blockade nor activation of Akt affected proliferation or response to erlotinib in glioma. Collectively, our results suggest that EGFR blockade decreases mTOR activation in an Akt-independent manner. These data do not necessarily argue against Akt blockade as a therapeutic strategy in glioma, although we saw little effect of pharmacological inhibition of Akt or siRNA directed against Akt on the proliferation of glioma cells. Akt signals to effector molecules in addition to mTOR, leaving open the possibility that Akt blockade could affect tumor biology independently of its apparent inability to affect mTOR or to achieve proliferation arrest in vitro.
Although this work introduces a previously underrecognized signaling pathway linking EGFR to mTOR in glioma cells, a number of important questions remain. How does EGFR signal to PKC? PDK1 (pyruvate dehydrogenase kinase, isozyme 1) is an attractive candidate in this regard, because PDK1 phosphorylates both Akt and PKCα in a PI3K-dependent manner (18, 19). Once activated, does PKC signal to mTOR through the Tsc complex? Combined inhibition and knockdown of Akt 1 to 3 failed to block mTOR activation despite inhibiting Tsc2 (Fig. 2), arguing against Tsc2 as a critical intermediate. Does mTOR complex 2 (mTORC2) contribute to this pathway? PKCα is a substrate for mTORC2 (20, 21), raising the possibility that mTORC2 is involved in a pathway including EGFR, PKC, and mTOR complex 1 (mTORC1).
The development of allosteric inhibitors of mTOR such as rapamycin has led to their clinical application in glioma, with early results suggesting some therapeutic efficacy (22, 23). The presence of a loop linking activation of mTOR to blockade of PI3K and Akt, however (24-26), raises the question of whether inhibition of mTOR could lead to the activation of other Akt targets, potentially abrogating to some degree the efficacy of these agents (23). Dual inhibitors of PI3K and mTOR may block mTOR activation without activating PI3K and Akt (6), and such agents are now entering clinical trials (27). Although PKC inhibitors may offer one approach to mTOR blockade independent of that achievable by rapamycin, our studies suggest an additional rationale for the use of PKC inhibitors as an alternative to mTOR inhibitors in malignant glioma. Data in Fig. 7A suggest that PKC inhibitors block mTOR signaling, though less consistently activating Akt. The mechanistic details underlying the apparent ability of mTOR inhibitors to act more potently than PKC inhibitors in activating PI3K and Akt remain uncertain and are the study of ongoing experiments.
Our studies confirm the importance of mTOR blockade as a biomarker of therapeutic efficacy in glioma and thus support the importance of mTOR signaling in the malignant gliomas. In the cell lines tested, we identified PKCα as a key intermediate linking EGFR signaling to mTOR in a pathway independent of canonical Akt signaling. Although signaling from EGFR to mTOR likely involves other proteins besides PKC, our experiments support PKC as a therapeutic target in glioma and argue that inhibitors of PKC, in contrast to inhibitors of EGFR, may show therapeutic efficacy even in PTENmt tumors.
MATERIALS AND METHODS
Cell lines, reagents, proliferation, and flow cytometry
Cell lines LN229, SF763, U373, and U87 cells transduced or not with EGFR (28), GBM12, and GBM43 (15) were grown in 10% FBS unless otherwise specified. Primary tumors were obtained with consent through University of California, San Francisco's (UCSF's) Brain Tumor Research Center and Committee on Human Research. Erlotinib tablets (Genentech) were pulverized and dissolved in HCl, and the aqueous phase was extracted with ethyl acetate. Combined organic extracts were dried over sodium sulfate and concentrated. EGF was from Roche; PMA, cycloheximide, and PD098059 were from Sigma; and Akt inhibitor VIII and BIM I were from EMD Biosciences. PIK-90 was synthesized as described (6). Cells (105) were seeded in 12-well plates in the absence or presence of 2 μM BIM I for 3 days. Viability, determined by WST-1 assay (Roche), and flow cytometry were as previously described (6).
Immunoblotting
Membranes were blotted with antibodies directed against p-Akt (Ser473), p-Akt (Thr308), Akt (pan), Akt1, Akt2, Akt3, p-Erk (Thr202/Tyr204), p-S6 ribosomal protein (Ser235/Ser236), S6 ribosomal protein, p-mTOR (Ser2448), mTOR, p-PLC-γ1 (Tyr783), PLC-γ1, PTEN, p-PKC (pan) (βII Ser660), PKCα, p-PKCδ(Thr505), p-PKCθ (Thr538), p-Gsk3β (Ser9), p-Tsc2 (Thr1462), p-FoxO3a (Ser318/Ser321), p-p70 S6 kinase (Thr389), p-MARCKS (Ser152/Ser156) (Cell Signaling), p-PKCβI (Thr642), p-PKCβII (Ser660), p-PKCη (Thr655), p-FoxO3a (Ser318/321), p-p70 S6 kinase (Thr389), p-MARCKS (Ser152/Ser156) (Cell Signaling), 4G10 for the detection of tyrosine phosphorylation on EGFR, β-tubulin (Upstate Biotechnology); EGFR (1005), p-PKCα (Ser657), PKCα (C-20), p-PKCε (Ser729), Erk (Santa Cruz Biotechnology). Cycloheximide pulse-chase analysis was as described (29). Bound antibodies were detected with horseradish peroxidase-linked antibodies against mouse or rabbit immunoglobulin G (Amersham), followed by ECL (Amersham).
Constructs, siRNA, shRNA, transfections, and transductions
A constitutively active form of PKCα (PKCα-Cat), a gift from J.-W. Soh, was generated by deleting the regulatory N-terminal domain of PKCα (14, 30). pHACE-PKCα-Cat plasmid and pcDNA3 empty vector control were transfected transiently into LN229:EGFR cells with Effectene (Qiagen). Akt siRNA Akt3-1 was purchased from Santa Cruz Biotechnology. Control siRNA and siRNA against Akt1, Akt2, Akt3-2, PKCα, and PKCδ were purchased (Dharmacon), and transfected with Lipofectamine 2000 (Invitrogen). Lentiviral PKCα (TRCN0000001693) and scramble shRNAs were purchased (Sigma) and infected as previously described (8).
Supplementary Materials
Acknowledgments
This work was supported by grants from the Burroughs Wellcome Fund, the Brain Tumor Society, the Howard Hughes Medical Institute, the Samuel Waxman Cancer Research Foundation, and the National Cancer Institute Specialized Program of Research Excellence. We thank R. Messing for advice; M. Grimmer, C. Hackett, T. Nicolaides, and D. Ruggero for critical review; and J.-W. Soh for providing genetic PKC reagents.
REFERENCES AND NOTES
- 1.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: Targets for cancer therapy. Nat. Rev. Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 2.Cheng CK, Fan QW, Weiss WA. PI3K signaling in glioma—Animal models and therapeutic challenges. Brain Pathol. 2009;19:112–120. doi: 10.1111/j.1750-3639.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, Riggs BL, Horvath S, Liau LM, Cavenee WK, Rao PN, Beroukhim R, Peck TC, Lee JC, Sellers WR, Stokoe D, Prados M, Cloughesy TF, Sawyers CL, Mischel PS. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 4.Fan QW, Specht KM, Zhang C, Goldenberg DD, Shokat KM, Weiss WA. Combinatorial efficacy achieved through two-point blockade within a signaling pathway— A chemical genetic approach. Cancer Res. 2003;63:8930–8938. [PubMed] [Google Scholar]
- 5.Haas-Kogan DA, Prados MD, Tihan T, Eberhard DA, Jelluma N, Arvold ND, Baumber R, Lamborn KR, Kapadia A, Malec M, Berger MS, Stokoe D. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J. Natl. Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 6.Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang MY, Lu KV, Zhu S, Dia EQ, Vivanco I, Shackleford GM, Cavenee WK, Mellinghoff IK, Cloughesy TF, Sawyers CL, Mischel PS. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864–7869. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- 8.Fan QW, Cheng CK, Nicolaides TP, Hackett CS, Knight ZA, Shokat KM, Weiss WA. A dual phosphoinositide-3-kinase α/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res. 2007;67:7960–7965. doi: 10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, Duggan ME. Allosteric Akt (PKB) inhibitors: Discovery and SAR of isozyme selective inhibitors. Bioorg. Med. Chem. Lett. 2005;15:761–764. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–38. doi: 10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- 12.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 13.Sarkaria JN, Carlson BL, Schroeder MA, Grogan P, Brown PD, Giannini C, Ballman KV, Kitange GJ, Guha A, Pandita A, James CD. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin. Cancer Res. 2006;12:2264–2271. doi: 10.1158/1078-0432.CCR-05-2510. [DOI] [PubMed] [Google Scholar]
- 14.Soh JW, Lee EH, Prywes R, Weinstein IB. Novel roles of specific isoforms of protein kinase C in activation of the c-fos serum response element. Mol. Cell. Biol. 1999;19:1313–1324. doi: 10.1128/mcb.19.2.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase. C. J. Biol. Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 16.Kiss Z, Phillips H, Anderson WH. The bisindolylmaleimide GF 109203X, a selective inhibitor of protein kinase C, does not inhibit the potentiating effect of phorbol ester on ethanol-induced phospholipase C-mediated hydrolysis of phosphatidylethanolamine. Biochim. Biophys. Acta. 1995;1265:93–95. doi: 10.1016/0167-4889(94)00242-7. [DOI] [PubMed] [Google Scholar]
- 17.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, Stegh AH, Bradner JE, Ligon KL, Brennan C, Chin L, DePinho RA. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 18.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 19.Dutil EM, Toker A, Newton AC. Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1) Curr. Biol. 1998;8:1366–1375. doi: 10.1016/s0960-9822(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 20.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, Sessa WC, Qin J, Zhang P, Su B, Jacinto E. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J, Peralba JM, Jenkins RB, Dakhil SR, Morton RF, Jaeckle KA, Scheithauer BW, Dancey J, Hidalgo M, Walsh DJ. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: A North Central Cancer Treatment Group Study. J. Clin. Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 23.Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, Liau L, Martin N, Becker D, Bergsneider M, Lai A, Green R, Oglesby T, Koleto M, Trent J, Horvath S, Mischel PS, Mellinghoff IK, Sawyers CL. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol. Cancer Ther. 2005;4:1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 25.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 26.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chene P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, Simonen M, Murphy L, Finan P, Sellers W, Garcia-Echeverria C. Identification and characterization of NVPBEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 28.Fan QW, Weiss WA. RNA interference against a glioma-derived allele of EGFR induces blockade at G2M. Oncogene. 2005;24:829–837. doi: 10.1038/sj.onc.1208227. [DOI] [PubMed] [Google Scholar]
- 29.Chesler L, Schlieve C, Goldenberg DD, Kenney A, Kim G, McMillan A, Matthay KK, Rowitch D, Weiss WA. Inhibition of phosphatidylinositol 3-kinase destabilizes Mycn protein and blocks malignant progression in neuroblastoma. Cancer Res. 2006;66:8139–8146. doi: 10.1158/0008-5472.CAN-05-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagawa R, Soh JW, Michie AM. Subversion of protein kinase Ca signaling in hematopoietic progenitor cells results in the generation of a B-cell chronic lymphocytic leukemia-like population in vivo. Cancer Res. 2006;66:527–534. doi: 10.1158/0008-5472.CAN-05-0841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.