Abstract
An abundance of evidence indicates that action selection is guided, at least in certain contexts, by anticipation of action outcomes. In one particularly clear demonstration of this principle, Bechara and colleagues, studying a gambling task, observed phasic skin conductance responses just prior to actions associated with a relatively high risk of monetary loss (Bechara, Damasio, Damasio, & Lee, 1999; Bechara, Damasio, Tranel, & Damasio, 1997; Bechara, Tranel, Damasio, & Damasio, 1996). In the present work, we tested for the same effect in a paradigm where choices resulted not in differential monetary outcomes, but in differential requirements for subsequent mental effort. In two experiments, we observed an anticipatory skin conductance response prior to actions resulting in a high level of cognitive demand. This finding indicates that requirements for effortful cognitive control are anticipated during action selection. We argue, based on convergent evidence, that such anticipation may not only trigger preparation; it may also play a direct role in effort-based decision-making.
Adaptive decision-making requires that the prospective rewards of any action be weighed against its attendant costs. Among the many varieties of action costs, those that spring most immediately to mind tend to involve action outcomes: pain, monetary loss, negative social feedback, and so forth. However, equally important — and more ubiquitous — are the intrinsic costs of action, and in particular the cost associated with effort. Effort has long been recognized to play a central role in decision-making. Indeed by the time of Hull and his contemporaries, its role was codified as a general law (Hull, 1943). The law of least effort, as it was named, stipulated that given two lines of action leading to equal rewards, the least effortful will typically be chosen (Solomon, 1948).
Although the empirical work underpinning the law of least effort focuses almost exclusively on physical effort, the principle has often been assumed to extend equivalently to mental effort. Across a wide range of research settings, it has been either implied or directly asserted that people display a preference for activities or strategies that minimize cognitive demand. For example, Allport (1954) proposed that prejudicial attitudes are adopted because this “takes less effort, and effort…is disagreeable.” Similarly, Baroody and Ginsburg (1986) accounted for strategy selection in arithmetic by invoking a “drive for cognitive economy” (see also Rosch, 1999; Zipf, 1949), and data on the processing of political messages prompted McGuire (1969) to describe humans as “lazy organisms.” According to Camerer and Hogarth (1999) economists, too, “instinctively assume thinking is a costly activity.”
Such statements imply the existence of a ‘law of least mental effort,’ (Balle, 2002) reflected in a bias against tasks and strategies carrying high levels of cognitive demand. In recent work, we reported a straightforward test of this frequently presumed principle (Botvinick, 2007; Botvinick & Rosen, 2007; Botvinick, Rosen, & McGuire, submitted). In a series of experiments, normal subjects were given a recurrent choice between two decks of cards. Each card, once chosen, displayed a numeral, and based on this numeral’s color the subject performed either a magnitude judgment (indicating whether the numeral was less than or greater than five) or a parity judgment (indicating odd or even). Numerals of both colors occurred in each deck. However, unannounced to subjects, in one deck stimulus color tended to alternate across cards, while in the other deck it tended to remain constant. Selection from the former deck resulted in a higher level of cognitive demand, due to the requirement for frequent task-switching (Monsell, 2003). In all experiments, subjects were found to gradually develop a bias toward the low-demand deck, consistent with a law of least mental effort.
The demand-selection paradigm used in the foregoing experiments was deliberately designed to resemble the task used in a series of well-known studies of reward-based decision-making by Bechara and colleagues (Bechara et al., 1999; Bechara et al., 1997; Bechara et al., 1996). In those studies, subjects selected from a set of decks containing cards that signaled monetary wins and losses, and gradually developed a preference for the most advantageous deck (often before they could report the basis for their decisions, a finding also obtained in our demand-selection task; Botvinick & Rosen, 2007; Botvinick et al., submitted). An important aspect of the Bechara “gambling task” studies was the finding that, after a bit of experience with the decks, subjects displayed a phasic skin conductance response (SCR) just prior to selecting from a disadvantageous deck. This observation suggested that the eventual tendency to favor the advantageous deck involved more than a simple response bias. Instead, it appeared to involve an anticipation of the consequences associated with selection from specific decks, or at least their motivational valence.
One can interpret the behavior of subjects in the demand-selection task in the same way, attributing their preference for the low-demand deck to an active anticipation of the consequences of selecting from each deck, in terms of cognitive demand. If this interpretation is valid, then, by further analogy to the Bechara gambling task experiments, subjects performing the demand-selection task should display an SCR prior to selection from the high-demand deck.
A sustained SCR is known to occur during active performance of cognitively demanding tasks (Andreassi, 2000; Gendolla & Richter, 2005; Pecchinenda & Smith, 1996). Moreover, changes in systolic blood pressure and heart rate — signs, like the SCR, of an autonomic response — have been reported in situations where subjects were directly instructed to prepare for a specific cognitively demanding activity (Wright, 1996). With these findings in mind, we conducted two experiments designed to test for similar anticipatory effects in a behavioral setting involving a decision-making component. In both experiments, skin conductance was recorded as normal subjects performed a modified version of the demand-selection task. We predicted, by analogy to results from the Bechara gambling task, that a phasic SCR would be observed just before selections from the high-demand deck.
Experiment 1
Methods
Participants
Twelve native English speakers, between the ages of 18 and 30, were recruited from the University of Pennsylvania psychology and neuroscience communities, and received course credit or a nominal payment for participation. Subjects provided informed consent, following procedures approved by the University of Pennsylvania Institutional Review Board.
Materials and procedure
The behavioral task was computer-based, programmed using E-Prime (Psychology Software Tools, Inc.). On each trial, the monitor displayed the backs of two decks of cards, one orange, the other green, positioned left and right of center. Subjects selected a deck by key-press (f = left, j = right), using the dominant hand. A face-up card then appeared above the chosen deck, displaying a single numeral (1–9, excluding 5). If the numeral appeared in blue, subjects were to say “yes” when the number was less than five, and otherwise “no” (magnitude task). If the number appeared in purple, subjects were to say “yes” when it was even, and otherwise “no” (parity task). A voice key registered verbal responses and immediately restored the original display, initiating the next trial. Subjects were not informed of the critical difference between the decks. In one deck (low-demand) the color of each numeral matched the previous trial on 90% of occasions. In the other (high-demand) deck, a match occurred on only 10% of occasions. The latter deck thus required more frequent switching from one task to the other. The positions and back-colors of the decks were counterbalanced across subjects.
Performance of the task was broken into 34 ten-trial blocks. Instructions indicated that once subjects had performed the first trial in each block, they were to select from the same deck for the succeeding nine trials. Across blocks, subjects were asked to choose “randomly” between the two decks, “avoiding a simple pattern of choices” such as alternation, but attempting to divide their choices roughly evenly between the two decks over the course of the experiment. Following completion of each block, subjects were asked immediately to prepare their first response for the next block by directing their gaze to the appropriate deck. However, they were not permitted to select a card until ten seconds following the previous block’s final verbal response, at which point a 1 mm width white border appeared around each deck. This border remained present until the final verbal response of the new block.
As this procedure makes clear, the goal of the present experiment was not to study deck preference. The instruction to choose evenly between decks was provided to assure an adequate sample of trials involving selections from each deck, while still requiring the subject to choose between the decks on each trial.
The subject’s deck choice was recorded on each trial, as were verbal responses and response times (RT). Skin conductance responses were sampled at 500 Hz using the Biopac (Goleta, CA) MP100MWS/GSR100C system (constant voltage, low-pass filtered at 1 Hz), with Ag-AgCl electrode leads positioned over the thenar and hypothenar eminences of the non-dominant hand. The data included time-stamps to indicate points at which deck choices occurred.
Analysis
The proportion of selections from each deck was noted, in order to confirm compliance with instructions. Verbal reaction times were subjected to a two-way ANOVA with deck and trial-type (task switch vs. task repetition) as factors.
Analysis of skin conductance data was conducted offline using the Biopac Systems AcqKnowledge 3.0 software package. SCR time-series were segmented into performance intervals and choice intervals. Performance intervals began with the first card selection in a block and ended four seconds after the final card selection. Choice intervals began four seconds after the last card selection in each block and ended at the first card selection of the subsequent block. SCR time-series occupying the two interval types were labeled performance SCRs and choice SCRs, respectively. In both cases, individual SCR magnitude was quantified by a base-to-peak subtraction, i.e., subtraction of the lowest conductance value within the choice or decision period from the highest. (A secondary analysis measuring SCR magnitude as area under the curve yielded comparable results, not further reported here.) Individual SCRs with a base-to-peak magnitude greater than 2 μS were considered likely to reflect artifact and were excluded from analysis.
For each subject, average magnitudes were calculated for performance SCRs during high-demand (blocks where the subject selected from the high-demand deck) and low-demand blocks. Means were then compared across the subject sample using a paired t-test. Subject-specific average magnitudes for choice SCRs were computed for SCRs occurring prior to high-demand blocks and prior to low-demand blocks. In order to compensate for potential baseline differences arising from the preceding block-type, each average was first computed separately for choice intervals following high-demand and low-demand block-types. The mean of the two resulting means was then taken, yielding a single mean value for choice SCRs preceding high-demand blocks and a single value for SCRs preceding low-demand blocks. These were compared across subjects by paired t-test.
For visualization, choice-SCR time-courses were computed by averaging across the mean time-courses for individual subjects. Prior to computing this average, data within each decision period were coded relative to the first sample in the time-series from that period. The resulting mean time-courses were smoothed using a moving average spanning 100 samples or 200 msec.
Results
Deck choice
Subjects complied well with the instruction to select evenly between the two decks. The mean proportion of selections from the high-demand deck was 0.49 (range 0.37–0.55)
Verbal RT
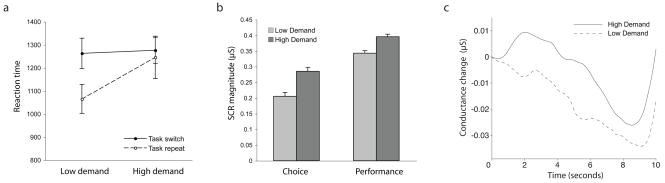
Verbal RT for each deck, according to trial-type (task repetition vs. switch) is shown in Figure 1a. A two-way ANOVA with factors for deck and trial type indicated a significant main effect of deck, F(1, 11) = 5.538, p = 0.038; a significant main effect of trial type, F(1,11) = 8.726, p = 0.013; and a significant interaction, F(1,11) = 7.759, p = 0.018.
Figure 1.
Results from Experiment 1. a. Verbal RTs. Bars indicate standard error. b. SCR magnitude. Bars indicate standard error, computed so as to partial out between-subject variance (Loftus & Masson, 1994). c. Average time-courses for choice SCRs.
FIGURE 1 AROUND HERE
Skin conductance
The median number of measurements excluded per subject, based on the amplitude criterion, was 1 in the choice period (range 0–9) and 0.5 in the decision period (range 0–9). Mean SCR magnitudes for the remaining dataset are shown in Figure 1b. Performance SCRs were larger for the high-demand deck than the low-demand deck, t(11) = 3.332, p = 0.007. A t-test indicated that choice SCRs were larger prior to selection from the high-demand deck than from the low-demand deck, t(11) = 3.289, p = 0.007. As explained under methods, this difference could not be explained by a difference in events preceding each selection-type, since SCR means were computed by first averaging separately over choice intervals preceded by high- and low-demand performance, and then averaging over these two means. Average time-courses for choice SCRs are shown in Figure 1c. Note that the diminished amplitude seen in these time-courses, relative to that reflected in the base-to-peak data shown in Figure 1b, is a natural consequence of the averaging procedure, given that peak values of skin conductance occurred at different latencies across trials.
Discussion
In the economic decision-making task studied by Bechara and colleagues (1999; 1997; 1996), the selection of a costly or disadvantageous course of action is accompanied by a phasic SCR. In the present experiment, we obtained an analogous SCR in a decision-making setting where action costs took the form of cognitive demand rather than monetary loss. Subjects selected between two decks of cards, one of which was associated with greater cognitive demands, due to its requirement for more frequent task-switching. Performance of the tasks associated with this high-demand deck was accompanied by elevated skin conductance, consistent with earlier research. More importantly, selections from the high-demand deck were also preceded by a larger SCR, suggesting that, like economic outcomes, consequences for cognitive demand are spontaneously anticipated during the selection of behavior.
One problem with Experiment 1 derives from a detail of the protocol. Recall that the probability of a task-switch in the high-demand deck, relative to the task occurring on the preceding trial, was 90%, regardless of the last deck chosen (and for the low-demand deck, 10%). This contingency held across blocks, meaning that the classification task occurring on the first trial in each block could, in principle, be predicted with 90% accuracy. If subjects did anticipate the upcoming task during the inter-block interval, this might provide an alternative explanation for the anticipatory SCR observed, since it would mean that subjects were, effectively, shifting task-sets in preparation for high-demand blocks but not for low-demand blocks. In order to exclude this possibility, we repeated the experiment with a slightly modified set of sequential contingencies.
Experiment 2
Experiment 2 followed precisely the same procedure as Experiment 1, with one exception: In this experiment, the task occurring on the first trial of every block was always the same as the task occurring on the last trial of the preceding block.
Methods
Subjects
Twelve native English speakers, between the ages of 18 and 30, were recruited from the University of Pennsylvania psychology and neuroscience communities, and received course credit or a nominal payment for participation. Subjects provided informed consent, following procedures approved by the University of Pennsylvania Institutional Review Board.
Materials and procedure
The task and procedure were identical to those employed in Experiment 1, except that the color of the numeral appearing on the first card of each block was identical to the color of the numeral on the last card of the preceding block.
Analysis
The analyses were identical to those performed in Experiment 1, except that verbal RT data for the first card in each block were omitted from analysis.
Results
Deck choice
Subjects complied well with the instruction to select evenly between the two decks. The mean proportion of selections from the high-demand deck was 0.51 (range 0.35 – 0.60).
Verbal RT
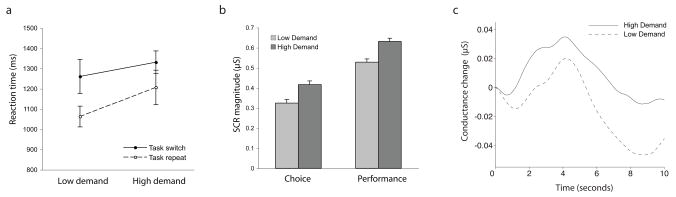
Verbal RTs for low-and high-demand decks, and for task-repetition and task-switch trials, are shown in Figure 1a. A two-way ANOVA with factors for deck and for trial type indicated a significant main effect of deck, F(1,11) = 4.99, p = 0.047; a significant main effect of trial type, F(1,11) = 6.25, p = 0.030; and no significant interaction, F(1,11) = 0.89, p = 0.365.
Skin conductance
SCR data for one subject were excluded from analysis due to the presence of severe artifacts. Among the remaining subjects, the median number of measurements excluded, based on the amplitude criterion, was 0 in the choice period (range 0–6) and 0 in the decision period (range 0–6). Mean SCR magnitudes from the remaining dataset are shown in Figure 2b. Performance SCRs were larger for the high-demand deck than the low-demand deck, t(10) = 3.181, p = 0.010. A t-test indicated that decision SCRs were larger prior to selection from the high-demand deck than from the low-demand deck, t(10) = 2.854, p = 0.017. Average time-courses are shown in Figure 1c.
Figure 2.
Results from Experiment 2. a. Verbal RTs. Bars indicate standard error. b. SCR magnitude. Bars indicate standard error, computed so as to partial out between-subject variance (Loftus & Masson, 1994). c. Average time-courses for choice SCRs.
Discussion
Experiment 2 closely replicated the results of Experiment 1, while eliminating a minor flaw in design. As in Experiment 1, elevated skin conductance was observed during performance blocks involving the high-demand deck. And also as in the previous experiment, an anticipatory SCR was observed prior to selections from the high-demand deck.
General Discussion
A variety of findings in research on judgment and decision-making (Allport, 1954; McGuire, 1969), language (Rosch, 1999; Zipf, 1949), and other topics (Baroody & Ginsburg, 1986; Christenfeld, 1995) have been presumed to reflect what might be called a ‘law of least mental effort’ (Balle, 2002): a general bias against tasks and strategies resulting in high levels of cognitive demand. In recent work, we conducted a simple test of this principle by facing subjects with a recurrent choice between two decks of cards associated with differentially demanding cognitive tasks (Botvinick, 2007; Botvinick & Rosen, 2007; Botvinick et al., submitted). Consistent with the presumed bias, subjects gradually developed a preference for the less demanding deck, a finding that held even among a subgroup of subjects who denied awareness of the difference in demand between the two decks. As noted earlier, the demand-selection task used in those experiments was deliberately designed to resemble the gambling task used in decision-making research by Bechara and colleagues (1999; 1997; 1996). The present study tested for a further parallel. In their gambling task, Bechara and colleagues observed anticipatory SCRs prior to actions associated with a relatively high probability of monetary loss. By analogy, we predicted in our paradigm an anticipatory SCR prior to selections from the high-demand deck. This prediction was confirmed in two separate experiments.
The results obtained clearly indicate that cognitive demand, or a close correlate such as error likelihood (Brown & Braver, 2007), is anticipated during decision-making. As such, the results are consistent with the idea that predictions of cognitive demand form an integral part of the decision-making process, as implied by the law of least mental effort. Interpreted in this way, the anticipatory SCRs obtained in our experiments would play a role comparable to the one played by the SCRs observed in the Bechara gambling task, as characterized by its creators. According to Damasio et al. (1996), “the SCR is part of a very early and automated alarm signal,” which affects further processing “by marking a particular option-outcome pair with a negative bias.” This interpretation of the SCR is also broadly consistent with the observation of anticipatory SCRs in aversive conditioning paradigms (Buchel, Morris, Dolan, & Friston, 1998), and with the proposal that the SCR reflects engagement of a behavioral inhibition system (Fowles, 1980, 1988).
It should be noted that SCRs occurring during the performance of cognitively demanding tasks have often been interpreted in different terms, not as correlates of negative valuation, but rather as a reflection of “task engagement” (Gendolla & Richter, 2005; Pecchinenda & Smith, 1996). Along related lines, changes in blood pressure and heart rate variability in anticipation of cognitive demand have been interpreted as reflecting a preparatory “energization” (Wright & Brehm, 1989) which “presumably occurs to support behavior” (Wright, 1996; Wright & Kirby, 2001). Although the present work is the first to report SCRs in anticipation of cognitive demand, it is admittedly possible to interpret these responses in similar terms, without assuming a link with valuation, bias or any other aspect of decision-making.
However, while the data do not exclude such an interpretation, adopting it would require the anticipatory SCR obtained in our experiments to be attributed to entirely different causes than the anticipatory SCR obtained in experiments with the Bechara gambling task. Another alternative, which we favor, is to consider that the anticipatory SCR obtained in our experiments may reflect both a modulation in the level of task engagement and an evaluative response to anticipated demand, integral to decision-making. A compelling motivation for this dual view can be drawn from research looking into the neural generators of the SCR, as we now consider.
Among several cortical regions implicated in generating galvanic skin responses, a central one appears to be the dorsal anterior cingulate cortex (ACC). ACC activity, as measured through functional neuroimaging, has been observed to correlate with SCR amplitude in a variety of settings (Fredrikson et al., 1998; Nagai, Critchley, Featherstone, Trimble, & Dolan, 2004), and lesions of the same region can reduce or eliminate SCRs (Tranel & Damasio, 1994), including both those induced by cognitively demanding tasks and those occurring in the Bechara gambling task (Naccache et al., 2005). In fact, ACC lesions also eliminate systolic blood pressure and heart rate responses to the exertion of mental effort (Mathias et al., 2003).
In addition to its apparent role in the regulation of autonomic function, the ACC has been widely implicated in the monitoring of cognitive demand and the recruitment of cognitive control (Botvinick, Cohen, & Carter, 2004; Carter & Van Veen, 2007). The anatomical intersection of these functions with that of autonomic regulation appears consistent with the idea that the SCR triggered by cognitive demand reflects the intensity of task engagement, or an “energization” process that bolsters this. However, there is also strong evidence linking the ACC with a role in representing action outcomes and in using these to guide decision-making (Rushworth, Walton, Kennerley, & Bannerman, 2004). Such evidence points, in particular, to ACC involvement in representing action costs, including effort costs (Rudebeck, Walton, Smyth, Bannerman, & Rushworth, 2006; Walton, Kennerley, Bannerman, Phillips, & Rushworth, 2006), and also to its involvement in avoidance learning (Johansen & Fields, 2004; Kim, Shimojo, & O’Doherty, 2006). These points lend weight to the view that the SCR triggered by cognitive demand may reflect the operation of mechanisms for cost evaluation and avoidance.
Taken together, existing data on the ACC, a key generator of the SCR, support the view that the anticipatory SCR observed in our experiments may be connected with both (1) a preparatory intensification of arousal, attention and task engagement, based on a prediction of upcoming cognitive demand, and (2) the registration of expected cognitive demand as an impending cost, relevant to value-based decision-making.
Several testable predictions follow from the second component of this interpretation (see Botvinick, 2007 for related discussion). First, it should be possible to observe ACC activation in anticipation of high levels of cognitive demand. This seems broadly compatible with evidence suggesting that the ACC is activated by anticipation of impending losses and errors (Brown & Braver, 2007) and by anticipation of pain (Koyama, Tanaka, & Mikami, 1998). Second, if analogous responses are observed in anticipation of cognitive demand, they should correlate with the magnitude of contemporaneous SCRs. Third, and most decisively, the magnitude of both ACC activity and anticipatory SCRs should show a direct relation, across individuals, with the strength of the behavioral bias against actions incurring high levels of cognitive demand, as measurable in the demand-selection paradigm described earlier. Finally, ACC lesions should eliminate both the demand-anticipatory SCR and the pattern of demand avoidance observed in the demand-selection paradigm.
Acknowledgments
Thank you to Lesley Fellows for access to galvanometric equipment and to Alisa Padon for technical assistance. The present work was completed with support from the National Institute of Mental Health (P50 MH062196).
Contributor Information
Matthew M. Botvinick, Princeton Neuroscience Institute and Department of Psychology, Princeton University
Zev B. Rosen, Department of Psychology, University of Pennsylvania
References
- Allport GW. The Nature of Prejudice. New York: Addison Wesley; 1954. [Google Scholar]
- Andreassi JL. Psychophysiology: Human Behavior and Physiological Response. New Jersey: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- Balle M. La loi du moindre effort mental: Les representations mentales. Sciences humaines. 2002;128:36–39. [Google Scholar]
- Baroody AJ, Ginsburg HP. The relationship between initial meaningful and mechanical knowledge of arithmetic. In: Hiebert J, editor. Conceptual and Procedural Knowledge: The Case of Mathematics. Hillsdale, NJ: Lawrence Erlbaum Associates; 1986. pp. 75–112. [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Botvinick M. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cognitive, Affective and Behavioral Neuroscience. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Rosen Z. Is mental effort aversive? Some behavioral and psychophysiological evidence; Paper presented at the Cognitive Neuroscience Society Annual Meeting.2007. [Google Scholar]
- Botvinick M, Rosen Z, McGuire JC. Action selection based on anticipated cognitive demand: a test of “the law of least mental effort” submitted. [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cognitive, Affective and Behavioral Neuroscience. 2007;7:266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Camerer CF, Hogarth RM. The effects of financial incentives in experiments: a review and capital-labor-production framework. Journal of Risk and Uncertainty. 1999;19:7–42. [Google Scholar]
- Carter CS, Van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective and Behavioral Neuroscience. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Christenfeld N. Choices from identical options. Psychological Science. 1995;6:50–55. [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions: Biological Sciences. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Fowles DC. The three arousal model: implications of Gray’s two-factor learning theory for heart rate, electrodermal activity and psychopathy. Psychophysiology. 1980;17:87–104. doi: 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Psychophysiology and psychopathology: A motivational approach. Psychophysiology. 1988;25:373–391. doi: 10.1111/j.1469-8986.1988.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Furmark T, Olsson T, Fischer H, Andersson J, Langstrom B. Functional neuroanatomical correlates of electrodermal activity: a positron emission tomographic study. Psychophysiology. 1998;35:179–185. [PubMed] [Google Scholar]
- Gendolla GH, Richter M. Ego involvement and effort: cardiovascular, electrodermal, and performance effects. Psychphysiology. 2005;42:595–603. doi: 10.1111/j.1469-8986.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- Hull CL. Principles of Behavior. New York: Appleton-Century; 1943. [Google Scholar]
- Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nature Neuroscience. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O’Doherty J. Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLOS Biology. 2006;4:1453–1461. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Tanaka Y, Mikami A. Nociceptive neurons in the macaque anterior cingulate activate during anticipation of pain. Neuroreport. 1998;9:2663–2667. doi: 10.1097/00001756-199808030-00044. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin and Review. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolatti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;125:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- McGuire WJ. The nature of attitudes and attitude change. In: Lindzey G, Aronson E, editors. The Handbook of Social Psychology. Vol. 3. Reading, MA: Addision-Wesley; 1969. pp. 136–314. [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Sciences. 2003;7(3):134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Naccache L, Dehaene S, Cohen L, Habert MO, Guichart-Gomez E, Galanaude D, Willera JC. Effortless control: executive attention and conscious feeling of mental effort are dissociable. Neuropsychologia. 2005;43(9):1318–1328. doi: 10.1016/j.neuropsychologia.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: physiological account of a “default mode” of brain function. NeuroImage. 2004;22 doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Pecchinenda A, Smith CA. The affective significance of skin conductance activity during a difficult problem-solving task. Cognition and Emotion. 1996;10:481–503. [Google Scholar]
- Rosch E. Principles of categorization. In: Margolis E, Laurence S, editors. Concepts: Core Readings. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS. Separate neural pathways process different decision costs. Nature Neuroscience. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends in Cognitive Sciences. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Solomon RL. The influence of work on behavior. Psychological Bulletin. 1948;45:1–40. doi: 10.1037/h0055527. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H. Neuroanatomical correlates of electrodermal skin conductance responses. Psychophysiology. 1994;31:427–438. doi: 10.1111/j.1469-8986.1994.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PEM, Rushworth MF. Weighing up the benefits of work: behavioral and neural analyses of effort-related decision making. Neural Networks. 2006;19:1302–1314. doi: 10.1016/j.neunet.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RA. Brehm’s theory of motivation asa model of effort and cardiovascular response. In: Gollwitzer PM, Bargh JA, editors. The Psychology of Action. New York: Guilford Press; 1996. pp. 424–453. [Google Scholar]
- Wright RA, Brehm JW. Energization and goal attractiveness. In: Pervin L, editor. Goal concepts in personality and social psychology. Hillsdale, N.J.: Lawrence Erlbaum Associates; 1989. pp. 169–210. [Google Scholar]
- Wright RA, Kirby LD. Effort determination of cardiovascular response: An integrative analysis with applications in social psychology. In: Zanna MP, editor. Advances in Experimental Social Psychology. San Diego, CA: Academic Press, Inc; 2001. pp. 255–307. [Google Scholar]
- Zipf GK. Human Behavior and the Principle of Least Effort. Cambridge, MA: Addison-Wesley Press; 1949. [Google Scholar]