Summary
Hippocampal (HC) function and morphology have been implicated in the pathophysiology of depression. Reduced HC volume has been observed in depressed humans, although the effect is not always significant. Studies of functional differentiation of the HC have revealed that the anterior portion is associated with emotional and anxiety-related functioning, and the posterior portion with memory processing. As such, measuring whole HC volume may mask differences seen only in the anterior or posterior HC. We used unbiased stereology to measure whole, anterior, and posterior HC volumes in 12 adult female cynomolgus macaques, half of which exhibited spontaneously occurring depressive behavior defined as a slumped/collapsed body posture with open eyes, and a relative lack of responsivity to environmental stimuli. The two groups were otherwise matched on circulating estradiol, progesterone, and cortisol levels, social status, estimated age, and body weight. Frozen postmortem HC tissue from depressed and nondepressed monkeys was serially sectioned and thionin-stained. According to established neuroanatomical guidelines and with the aid of Neurolucida software (MBF Bioscience), every tenth section throughout the extent of the HC was manually traced and used to reconstruct the 3-D models used to determine volumes. Anterior and posterior HC were delineated by the presence or absence of the uncus. No significant differences were found between depressed and nondepressed monkeys for whole or posterior HC volume, although the average HC volume was 4% smaller in depressed than nondepressed monkeys. Anterior HC volumes were significantly smaller (15.4%) in depressed compared to nondepressed monkeys. These results indicate that reduced volume in the anterior HC, an area previously implicated in emotional functioning, may be associated with a depressive phenotype in female cynomolgus macaques.
Keywords: behavioral depression, female, hippocampal volume, monkey, nonhuman primate
Introduction
Depression is the fourth leading cause of disease burden worldwide (World Health Organization, 2001). As less than half of patients respond to standard treatment, depression remains a significant treatment challenge (Nemeroff, 2007), and a greater understanding of the underlying neurobiology is needed to generate novel therapeutic interventions. The results of two meta-analyses suggest that HC volume measured with magnetic resonance imaging (MRI) is reduced bilaterally in depressed patients (Campbell et al., 2004; Videbech and Ravnkilde, 2004). However, clinical studies of the neurobiology of depression are complicated by heterogeneity in age, gender, age at onset of illness, comorbidities, drug and alcohol use, current depression versus remission, prior antidepressant exposure, treatment response, and number, duration, and severity of depressive episodes, and all of these have been shown to differentially affect HC volume (Campbell et al., 2004; Videbech and Ravnkilde, 2004; Neumeister et al., 2005; Ballmaier et al., 2008).
Controlled animal studies support an association between HC volume and mood-related behavior. HC volume reductions have been observed in male rodents in association with anxiety-related behavior and in response to chronic corticosterone exposure, and in male tree shrews in response to chronic psychological stress (Ohl et al., 2000; Kalisch et al., 2006; Murray et al., 2008). While the data from preclinical stress models are compelling, the degree to which stress responses in animal models are relevant to human depression remains controversial. Most animal models of depression are male rodents. However, depression is twice as prevalent in women as in men, and there are sex differences in stress responses in humans and rodents, and perhaps in HC volume in humans (Konkle et al., 2003; Lopez et al., 2006; Maller et al., 2007; Dalla et al., 2008). Moreover, HC structure is affected by ovarian steroids (Singh, 2006; Spencer et al., 2008) and anterior HC volume changes with menstrual cycle phase in women (Protopopescu et al., 2008). These observations implicate female reproductive system function in depression. Investigations of HC volume in an adult female animal model that more closely resembles human neurobiology and behavior may be helpful in understanding human depression.
We have developed an adult female cynomolgus macaque (Macaca fascicularis) model of depression. Depressed female monkeys display behavioral and physiological characteristics associated with human depression and these have been described in detail in a series of publications (Shively et al., 1997; 2005; 2009). Briefly, depressive behavior in this model is defined as a slumped or collapsed body posture with eyes open, accompanied by a relative lack of responsivity to environmental stimuli (Suomi et al., 1975). In these studies, female monkeys live in small social groups and form stable social status hierarchies within each group (Shively and Kaplan, 1991). Subordinates appear stressed behaviorally as they receive more aggression and less grooming, are more vigilant, and spend more time alone than dominants (Shively, 1998). Subordinate females are significantly more likely to display depressive behavior, which may be a consequence of the stress associated with low social status (Shively et al., 1997). Depressed females have higher overnight heart rates, impaired ovarian function, and disturbed hypothalamic-pituitary-adrenal (HPA) function, including reduced sensitivity to glucocorticoid negative feedback in a dexamethasone suppression test (Shively et al., 1997; Shively et al., 2002). Cynomolgus macaques have menstrual cycles like those of women in length and associated hormonal fluctuations. Furthermore, the macaque HC bears greater resemblance to the human HC than does the rat with regard to nuclear organization, projection pathways, and innervation patterns (Amaral and Lavenex, 2007). Thus, this female primate model may further our understanding of the neurobiology of depression in women and provide avenues for new treatment modalities.
The HC is functionally differentiated along its anterior-posterior axis (Colombo et al., 1998; Strange and Dolan, 1999). Human and rodent studies suggest that the anterior HC functions primarily in mood and emotional processing, whereas the posterior HC is implicated more in memory (Moser et al., 1993; Bannerman et al., 2004; Van den Hove et al., 2006). Indeed, volume reductions were recently observed in the anterior HC of depressed patients versus controls (Ballmaier et al., 2008). Using the adult female monkey model of depression, we investigated whole, anterior, and posterior HC volumes postmortem. We hypothesized that HC volume would be smaller in depressed compared to nondepressed monkeys, with the greatest deficits occurring in the anterior HC. The depressed and nondepressed animals were carefully matched on variables known to affect HC structure, including circulating hormone levels (Protopopescu et al., 2008) and social status due to the stress associated with subordination (Shively et al., 1997; Sapolsky, 2000).
Materials and Methods
Animals
Forty-eight adult (6-12 years of age as estimated by dentition) female cynomolgus monkeys (Macaca fascicularis) of reproductive age were obtained from Charles River Research Primates (Port Washington, NY) and housed under a 12/12 light/dark cycle in stable social groups of four animals each for 26 months. As part of a study of behavioral effects on atherosclerosis, the animals were fed a diet designed to mimic a Western diet containing 0.25 mg cholesterol/kcal and 40% of calories as fat ad libitum for 32 months (Shively and Clarkson, 1994). Six animals died during the course of the experiment from causes unrelated to the experiment, resulting in a final sample of 42 animals.
All procedures involving monkeys were conducted in accordance with state and federal laws, standards of the department of Health and Human Services, and guidelines established by the institutional Animal Care and Use Committee.
Social Status and Behavioral Depression
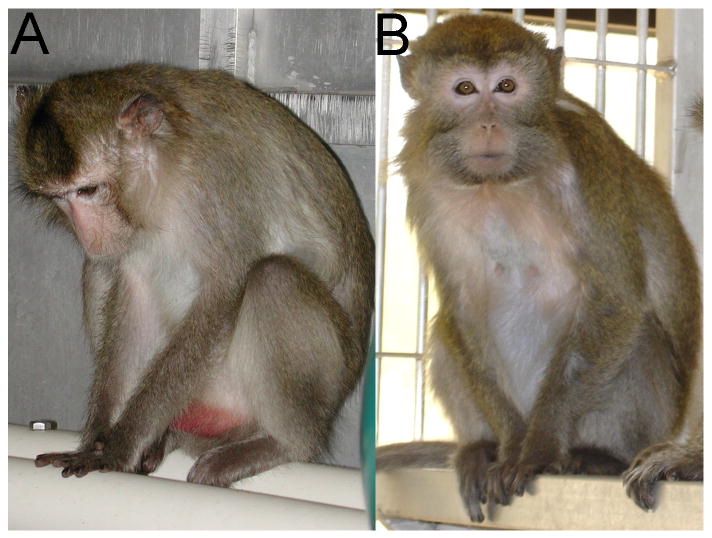
Social status was determined monthly throughout the experiment by recording the outcomes of aggressive interactions between cage mates, as previously described (Shively et al., 1997; Shively et al., 2002). The resulting social status hierarchies for each social group were stable over time, as observed in previous experiments (Shively and Kaplan, 1991). Behavioral depression was defined as a slumped or collapsed body posture, with opened eyes, accompanied by a relative lack of responsiveness to environmental stimuli to which other monkeys are attending (Suomi et al., 1975). This depressed posture is depicted in Figure 1. Cynomolgus macaques in captivity may spontaneously exhibit depressive behavior; behavioral depression in this experiment was not induced by a specific experimental manipulation. Time spent depressed was recorded once a week throughout the 26-month period using a 30-minute group ad libitum observation method, punctuated with scan samples every three minutes (Shively et al., 1997). Characteristics of depressive behavior in these animals have been described in detail elsewhere (Shively et al., 1997; Shively et al., 2002). Animals that never displayed the depressed posture were characterized as nondepressed (N=26), whereas animals that ever exhibited this posture were characterized as depressed (N=16, or 38%). Interobserver reliability was ≥ 92% throughout the study.
Figure 1.
A monkey displaying the depressed posture (A) compared to a nondepressed monkey (B). The nondepressed monkey is alert and attending to the photographer, a potential threat; the monkey displaying depressive behavior appears inattentive to the photographer, and is in a slumped posture, with eyes open and directed downward.
HPA Axis Function
A dexamethasone suppression test (DST) was used to assess the sensitivity of the HPA axis to negative feedback from circulating levels of cortisol and has been described elsewhere (Davidson et al., 1984; Mossman and Somoza, 1989; Shively et al., 1997; Shively et al., 2002). Briefly, a morning blood sample was taken for a baseline measure of cortisol, an evening dose (130 ug/kg body weight, I.M.) of dexamethasone was administered, and another blood sample was taken the following morning for cortisol assay. The difference between the first and second morning cortisol concentrations (percent change from baseline or percent suppression) was used as an indicator of sensitivity to glucocorticoid negative feedback (Kalin and Carnes, 1984). This test was administered one month prior to necropsy, after the monkeys had lived in their social groups for 25 months.
Cortisol, estradiol, and progesterone asssays
Estradiol and progesterone were assayed in blood taken at necropsy. Steroid concentrations were determined by radioimmunoassay in the Yerkes Assay Laboratory of Yerkes Regional Primate Research Center (Emory University, Atlanta, GA).
Tissue Preparation and Subject Selection
At necropsy, brains were rapidly removed, hemisected, and frozen at −80°C. The brains of six depressed animals were randomly chosen for autoradiographic investigation. Six nondepressed animals that most closely matched the six depressed animals in body weight, age estimated from dentition, social status history, basal cortisol levels, and estradiol and progesterone levels at the time of necropsy were selected as a comparison group (Table 1). This matching was done to reduce variance in this relatively small sample and to assure that the only statistically significant difference in characteristics that might affect HC volume was depressive behavior. An equivalent number of left and right brains were used in each group. In an attempt to develop a method to reduce the number of sections and experimenter workload, three randomly selected brains (two from the nondepressed group and one from the depressed group) were sagittally-sectioned at 50 μm from the lateral to medial extent of the HC. The remaining brains (N=9) were coronally-sectioned at 50 μm throughout the anterior-posterior extent of the HC. In preparation for volumetric determinations, approximately every tenth section along the anterior-posterior (coronally-sectioned HC, x̄=29 sections) or medial-lateral (sagittally-sectioned HC, x̄=19 sections) axis was thionin-stained to identify HC cytoarchitecture and differentiate the HC from surrounding grey and white matter.
Table 1.
Group characteristics.
| Depressed N=6 |
Nondepressed N=6 |
t(10) | p≤ | |
|---|---|---|---|---|
| Body weight (kg) | 2.74 ± 0.18 | 3.07 ± 0.23 | 1.14 | 0.28 |
| Age (yrs) | 9.37 ± 0.78 | 9.70 ± 0.69 | 0.31 | 0.76 |
| Progesterone (ng/ml) | 0.71 ± 0.35 | 0.26 ± 0.036 | 1.27 | 0.23 |
| Estradiol (pg/ml) | 68.62 ± 21.87 | 77.11 ± 25.61 | 0.25 | 0.81 |
| Social status (range 0-1, 1=dominant) |
0.47 ± 0.14 | 0.52 ± 0.15 | 0.24 | 0.82 |
| Baseline cortisol (μg/dl) | 34.48 ± 3.86 | 41.52 ± 4.64 | 1.17 | 0.27 |
| Cortisol response to dexamethasone | 8.52 ± 2.47 | 6.09 ± 0.63 | 0.95 | 0.36 |
| Difference in cortisol between baseline and response to dexamethasone | -25.96 ± 2.97 | -35.43 ± 4.22 | 1.84 | 0.10 |
| % Suppression of Cortisol | 76.15 ± 4.68 | 84.97 ± 1.29 | 1.82 | 0.10 |
Values represent means ± SEM.
Hippocampal Volumetry
Using Neurolucida mapping software (MicroBrightField, Inc., Williston, VT) and a Nikon Optiphot (Melville, NY) microscope with motorized stage (Ludl Electronics Products, Hawthorne, NY) and Magnafire digital camera (Optronics, Goletta,CA), volumes were determined from manual tracings of approximately every tenth serial section along the anterior-posterior (coronally-sectioned HC) or medial-lateral (sagittally-sectioned HC) axis of the HC. Structures included in each tracing were the cornu ammonis (CA1- CA3), dentate gyrus, subiculum, fimbria (throughout the HC head and body), and alveus, as depicted in Figure 2. Given the 50 μm section thickness and the distance between each section (approximately 450 μm), Neurolucida's automatic 3-D reconstruction program used the 2-D tracings to reconstruct 3-D models of the HC (Figures 3A & 3B). Using these models, volumes were determined by the Neurolucida software for the whole, anterior, and posterior HC. The anterior HC (head) was delineated from the posterior HC (body and tail) at the presence of the uncus (Figure 3C) (Pruessner et al., 2000).
Figure 2.
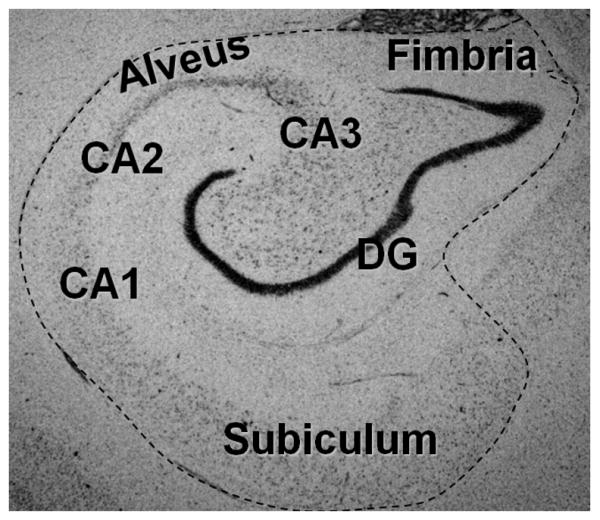
Thionin-stained coronal HC section delineating areas included in volume determinations. Every tenth section throughout the extent of the HC was manually traced and used to determine volumes. Structures included in tracings were the cornu ammonis (CA1- CA3), dentate gyrus, subiculum, alveus, and fimbria.
Figure 3.
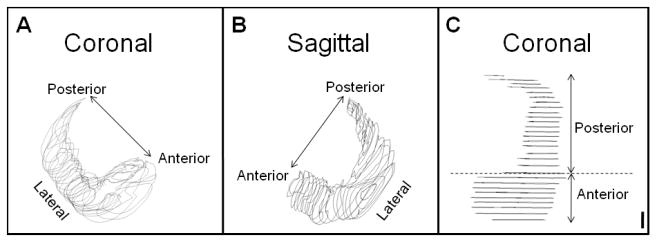
3-Dimensional HC Models. Every tenth serial section throughout the extent of the HC was traced. The tracings were used to create 3-D models, based on section thickness and distance between sections, and volumes were determined from these models. A. Coronally-sectioned right hemisphere HC model, oblique view. B. Sagittally-sectioned left hemisphere HC model, oblique view. C. Top-down view of coronally-sectioned left hemisphere HC model depicting the delineation of the anterior HC from the posterior HC by the presence of the uncus. Scale bar: 1000μm.
Statistical Analysis
Student's t-tests were used to determine group differences for whole, anterior and posterior volumes, and to confirm a lack of statistically significant differences in other characteristics of the depressed and nondepressed groups that might affect HC volume. All p values are the result of two-sided tests, with the level of significance set at p ≤ 0.05.
Results
Group Characteristics
Characteristics of the depressed and nondepressed animals are described in Table 1. There were no differences between the two groups in body weight (t(10) = 1.14, p = 0.28), age (t(10) = 0.31, p = 0.76), social status (t(10) = 0.24, p = 0.82), and estradiol (t(10) = 0.25, p = 0.81) and progesterone (t(10) = 1.27, p = 0.23) concentrations measured at the time of necropsy. Monkeys with behavioral depression were not different from nondepressed monkeys in baseline cortisol levels (t(10) = 1.17, p = 0.27), the cortisol response to dexamethasone in the DST (t(10) = 0.95, p = 0.36), or suppression of cortisol in the DST (percent suppression: t(10) = 1.82, p = 0.10).
Hippocampal Volume
There were no significant differences between depressed and nondepressed animals in whole HC volumes (t(10) = 0.89, p = 0.39; Figure 4). There was, however, an average 4% reduction in whole HC volumes in depressed animals compared to controls. No significant differences were observed in posterior volumes between the two groups (t(10) = 1.13, p = 0.28). Anterior HC volumes were significantly smaller (t(10) = 2.42, p = 0.036) in depressed animals compared to their nondepressed counterparts (Figure 4). On average, the anterior HC of depressed monkeys was 15.4% smaller than nondepressed monkeys. Similar results were obtained between the two groups for whole, anterior, and posterior volumes using only coronal sections (N=5 depressed HC, N=4 nondepressed HC). Moreover, the median and range of volumes from sagittally (258.11, 250.81-276.36) and coronally-sectioned (244.40, 218.09-281.16) HC were not different, suggesting that comparable postmortem measurements can be obtained from either neuroanatomical plane.
Figure 4.
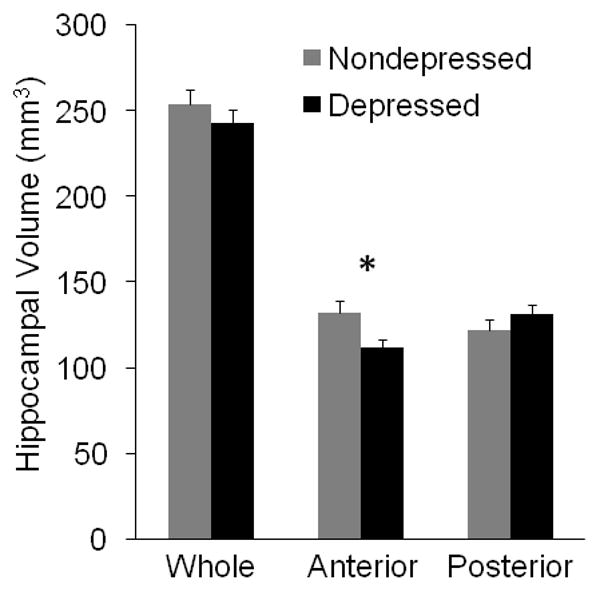
Anterior HC volume is reduced in behavioral depression. Brains were sectioned at 50μm throughout the extent of the HC, and every tenth serial section was traced to determine whole, posterior, and anterior HC volumes. There was no difference in whole or posterior HC volumes, but there was a significant average reduction of about 15.4% in anterior HC volume in depressed compared to nondepressed animals. Data depicted are raw means ± SEMs; *p<0.05.
Discussion
This is the first postmortem assessment of whole, anterior, and posterior HC volumes in either human or nonhuman primates with depression. The results indicate that reduced anterior HC volume is associated with behavioral depression in adult female cynomolgus macaques. This observation is supported by the neuroanatomical relationships that exist between the anterior HC and other primary brain areas known to function in depression. In contrast to the posterior HC, the anterior HC maintains close connectivity to the amygdala and hypothalamus, and projects to and from the prefrontal cortex, areas that are implicated in depression (Bannerman et al., 2004). Human and rodent studies suggest that the anterior HC functions primarily in mood and emotional processing, whereas the posterior HC is associated more with memory (Moser et al. 1993; Bannerman et al., 2004; Van den Hove et al., 2006). The findings reported here support and extend rodent and human observations of functional heterogeneity in the HC to nonhuman primates and further implicate the anterior HC in mood disorders.
HC volume alterations in depression have been investigated in a multitude of human studies using MRI, and meta-analyses demonstrate that despite subject heterogeneity, methodological differences, and incongruent results among human studies, HC volume is consistently reduced bilaterally in depressed persons compared to age and sex-matched controls (Campbell et al., 2004; Videbech and Ravnkilde, 2004), with an average volume reduction in the left and right HC of eight percent and ten percent, respectively (Videbech and Ravnkilde, 2004). Furthermore, the only postmortem human study in which HC volume has been investigated reported this same trend bilaterally in the HC, although the difference did not reach statistical significance (Bielau et al., 2005). In the present study, this trend is further supported and clarified by the non-significant four percent reduction observed postmortem in whole HC volume in depressed versus nondepressed monkeys.
More recently, human studies have begun to evaluate the morphometry of the anterior and posterior HC separately. Reduced anterior HC volume measured with MRI has been recently reported in depressed patients and in response to psychological stress (Szeszko et al., 2003; Ballmaier et al., 2008). Maller et al. (2007) also reported significantly smaller HC tails in depressed subjects. However, the anterior HC head was not evaluated separately from the posterior body, thus their findings are difficult to directly compare to those presented here.
The wide variety of results obtained in human studies of HC volume in depression may to some extent be attributed to subject heterogeneity. In addition, methodological differences among studies, such as those in scanning protocols, segmentation techniques, and neuroanatomical structure delineations, contribute to the heterogeneity of results (Pantel et al., 2000; Pruessner et al., 2000). Given the limited resolution of MRI, several groups have experienced difficulty delineating the HC head from the amygdala and thus included the two structures in the same measurement (Campbell et al., 2004). Such variability has made it difficult to confidently draw conclusions from the results of human MRI investigations of HC volume in depression.
The use of this animal model eliminated much of the variability inherent in clinical studies. Delineation of the HC from surrounding structures is relatively simple using light microscopy, as done in the present study. The animals all lived in the same housing conditions and consumed the same diet, and were not exposed to antidepressant pharmacotherapy, recreational drugs or alcohol. Depressive behavior was objectively documented continuously over the course of two years prior to necropsy, and depressed and nondepressed monkeys were matched on a number of characteristics that might influence the results. At necropsy, the brains were all collected in the same manner with no delay after death, and experienced equivalent freezer time. Given the careful matching and lack of subject heterogeneity, this is the least confounded assessment of HC volume to date.
The exact mechanism or combination of mechanisms underlying volumetric alterations in depression have yet to be fully elucidated. The structure of the HC is highly plastic and reactive to environmental conditions. HPA axis dysfunction, including the inability to suppress glucocorticoid production in response to stress, is a well-established occurrence in depressed patients. The HC contains high levels of glucocorticoid receptors and plays a major role in the modulation of HPA axis function. Insensitivity to negative feedback may lead to chronically elevated glucocorticoid levels, and an extensive literature documents that prolonged stress or glucocorticoid exposure has adverse effects on the rodent hippocampus that may result in HC atrophy (Sapolsky, 2000; McEwen, 2001 and 2005; Czeh and Lucassen, 2007). Likewise, an extensive literature documents the effects of ovarian steroids on HC architecture and function. The recent observation of changes in anterior HC size between phases of the menstrual cycle further supports the role of reproductive system function as a determinant of HC volume (Spencer et al., 2008; Singh, 2006; Protopopescu et al., 2008).
In the parent population from which the sample reported here was derived, depressed animals (N=16) were significantly less sensitive to glucocorticoid negative feedback than their nondepressed counterparts (N=26). Likewise, the ovarian function of depressed females was suppressed relative to their nondepressed counterparts as reflected in luteal phase progesterone concentrations (Shively et al. 1997; 2002). These attributes of the parent population are well to keep in mind since the lack of a statistically significant difference in HPA or reproductive system function in the relatively small sample presented here may be as much a function of small sample size as biological equivalence. Thus, the matching of depressed and nondepressed subjects on physiological characteristics known to influence HC volume in this small sample is not definitive evidence for a lack of involvement of the HPA or reproductive axis as determinants of HC size.
Postmortem histological analyses of human HC in depression have failed to find substantial neuronal and glial loss, suggesting that apoptosis contributes only slightly to volume alterations (Lucassen et al., 2001; Stockmeier et al., 2004). Instead, increases in neuronal and glial packing densities and well as decreased soma size in the HC were observed in a postmortem assessment of three adjacent human HC sections, suggesting that reduced neuropil may be contributing to volume reductions in depression (Stockmeier et al., 2004). By contrast, a more comprehensive study of the entire HC in psychosocially stressed tree shrews demonstrated significantly decreased size and number of astroglia with stress (Czeh et al., 2006). In that study, HC volume correlated significantly with astroglial number, and antidepressants prevented glial number reductions. This suggests the possibility that antidepressant therapy in depressed patients may affect study results. More controlled studies like these in a nonhuman primate model of depression in which the neurobiology more closely resembles that of humans and drug exposure is not a factor may clarify these issues and further elucidate the cellular mechanisms responsible for HC volume reductions in depression.
In conclusion, behavioral depression in female cynomolgus macaques is associated with volume reductions in the anterior HC. This finding supports the observation in human subjects of an anterior HC volume reduction, and further implicates the functioning of the anterior HC in depression. Likewise, this finding suggests that reports of functional differentiation of the HC in other species can be extended to nonhuman primates. A critical question yet to be adequately addressed is whether small hippocampi precede and predispose to depression, or are the result of depression. Because of the ability to control many of the variables that confound human studies, and the accuracy afforded by postmortem investigation of the whole, anterior, and posterior HC, this primate model may provide the means to address this issue in future studies.
Acknowledgments
The authors would like to thank April Davenport and Elizabeth Glover for their technical support. We would also like to thank Drs. Rene Hen, Mark Alter and Bruce McEwen for their kind advice and direction.
Role of Funding Source
This research was supported by NIH MH56881, GM6424, HL39789, The John D. and Catherine T. MacArthur Foundation and Venture Funds from Wake Forest University Health Sciences. These funding agencies had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflicts of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Contributors
Carol Shively designed the study. Stephanie Willard collected the data, managed the literature searches, undertook the statistical analysis, and wrote the first draft of the manuscript. Dr. Friedman provided guidance and expertise in primate neuroanatomy. Dr. Henkel provided guidance and expertise in unbiased stereology. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stephanie L. Willard, Interdisciplinary Graduate Program in Neuroscience, Wake Forest University School of Medicine
David P. Friedman, Department of Physiology & Pharmacology, Wake Forest University School of Medicine
Craig K. Henkel, Department of Neurobiology & Anatomy, Wake Forest University School of Medicine
Carol A. Shively, Department of Pathology (Comparative Medicine), Wake Forest University School of Medicine
References
- Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Anderson P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Oxford UP; New York: 2007. pp. 37–114. [Google Scholar]
- Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, Haroon E, Pham D, Heinz A, Kumar A. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165:229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bielau H, Trubner K, Krell D, Agelink MW, Bernstein HG, Stauch R, Mawrin C, Danos P, Gerhard L, Bogerts B, Baumann B. Volume deficits of subcortical nuclei in mood disorders A postmortem study. Eur Arch Psychiatry Clin Neurosci. 2005;255:401–412. doi: 10.1007/s00406-005-0581-y. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Colombo M, Fernandez T, Nakamura K, Gross CG. Functional differentiation along the anterior-posterior axis of the hippocampus in monkeys. J Neurophysiol. 1998;80:1002–1005. doi: 10.1152/jn.1998.80.2.1002. [DOI] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2008;33:1559–1569. doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- Davidson J, Lipper S, Zung WWK, Strickland R, Krishnan R, Mahomey S. Validation of four definitions of melancholia by the dexamethasone suppression test. Am J Psychiatry. 1984;141:1220–1223. doi: 10.1176/ajp.141.10.1220. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Carnes M. Biological correlates of attachment bond disruption in humans and nonhuman primates. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:459–469. [PubMed] [Google Scholar]
- Kalisch R, Schubert M, Jacob W, Kessler MS, Hemauer R, Wigger A, Landgraf R, Auer DP. Anxiety and hippocampus volume in the rat. Neuropsychopharmacology. 2006;31:925–932. doi: 10.1038/sj.npp.1300910. [DOI] [PubMed] [Google Scholar]
- Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Muller MB, Holsboer F, Bauer J, Holtrop A, Wouda J, Hoogendijk WJ, De Kloet ER, Swaab DF. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JJ, Daskalakis ZJ, Fitzgerald PB. Hippocampal volumetrics in depression: the importance of the posterior tail. Hippocampus. 2007;17:1023–1027. doi: 10.1002/hipo.20339. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann NY Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Anderson P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman D, Somoza E. Maximizing diagnostic information from the dexamethasone suppression test. Arch Gen Psychiatry. 1989;46:653–660. doi: 10.1001/archpsyc.1989.01810070079013. [DOI] [PubMed] [Google Scholar]
- Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur J Pharmacol. 2008;583:115–127. doi: 10.1016/j.ejphar.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The burden of severe depression: a review of diagnostic challenges and treatment alternatives. J Psychiatr Res. 2007;41:189–206. doi: 10.1016/j.jpsychires.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, Bain EE, Charney DS, Drevets WC. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Ohl F, Michaelis T, Vollmann-Honsdorf GK, Kirschbaum C, Fuchs E. Effect of chronic psychosocial stress and long-term cortisol treatment on hippocampus-mediated memory and hippocampal volume: a pilot-study in tree shrews. Psychoneuroendocrinology. 2000;25:357–363. doi: 10.1016/s0306-4530(99)00062-1. [DOI] [PubMed] [Google Scholar]
- Pantel J, O'Leary DS, Cretsinger K, Bockholt HJ, Keefe H, Magnotta VA, Andreasen NC. A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus. 2000;10:752–758. doi: 10.1002/1098-1063(2000)10:6<752::AID-HIPO1012>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Protopopescu X Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen B, Silbersweig D, Stern E. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior and central monoaminergic function in female cynomolgus monkeys. Biol Psychiatry. 1998;44:882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. Social status and coronary artery atherosclerosis in female monkeys. Arterioscler Thromb. 1994;14:721–726. doi: 10.1161/01.atv.14.5.721. [DOI] [PubMed] [Google Scholar]
- Shively CA, Kaplan JR. Stability of social status rankings of female cynomolgus monkeys, of varying reproductive condition, in different social groups. Am J Primatol. 1991;23:239–245. doi: 10.1002/ajp.1350230404. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom Med. 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol Psychol. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neurosci Biobehav Rev. 2009;33:133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. Progesterone-induced neuroprotection. Endocrine. 2006;29:271–274. doi: 10.1385/ENDO:29:2:271. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange B, Dolan R. Functional segregation within the human hippocampus. Mol Psychiatry. 1999;4:508–511. doi: 10.1038/sj.mp.4000593. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Eisele CD, Grady SA, Harlow HF. Depressive behavior in adult monkeys following separation from family environment. J Abnorm Psychol. 1975;84:576–578. doi: 10.1037/h0077066. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Goldberg E, Gunduz-Bruce H, Ashtari M, Robinson D, Malhotra AK, Lencz T, Bates J, Crandall DT, Kane JM, Bilder RM. Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:2190–2197. doi: 10.1176/appi.ajp.160.12.2190. [DOI] [PubMed] [Google Scholar]
- Van den Hove DL, Lauder JM, Scheepens A, Prickaerts J, Blanco CE, Steinbusch HW. Prenatal stress in the rat alters 5-HT1A receptor binding in the ventral hippocampus. Brain Res. 2006;1090:29–34. doi: 10.1016/j.brainres.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The World Health Report 2001 Mental Health: New Understanding, New Hope. Geneva: World Health Organization; 2001. [Google Scholar]