Abstract
Background
Male gender confers enhanced susceptibility to development of age-dependent kidney damage. In other models of progressive renal disease, development of injury is linked to declines in renal nitric oxide synthase (NOS) capacity.
Methods
We investigated the in vitro characteristics of the renal NOS system in young (3 to 5 months), middle-aged (11 to 13 months) and old (18 to 22 months) male and female Sprague-Dawley rats.
Results
NOS activity (pmol [3H]-arginine converted to [3H]-citrulline/mg protein/minute) is reduced in the soluble fraction of renal cortex from old versus young males but not females. In contrast, NOS activity in the soluble fraction of cerebellum is not altered by age or gender. The abundance of endothelial NOS (eNOS) and neuronal (nNOS) is reduced in renal cortex of old versus young males but is unchanged in female cortex. In renal medulla, eNOS protein is reduced with age in both males and females. We found no difference in abundance of either eNOS or nNOS protein in the cortex of young male and female rats. The incidence and severity of glomerular damage increases markedly with age in the male and only slightly in the female.
Conclusion
These findings indicate that a relative reduction occurs in renal NOS in the male kidney with advancing age, whereas NOS protein and activity is maintained during aging in females. This, together with the marked age-dependent kidney damage seen in the male, suggests that the renal NO deficiency in the aging male rat may contribute to the age-dependent kidney damage.
Keywords: endothelial nitric oxide synthase, neuronal nitric oxide synthase, glomerulosclerosis, kidney cortex, kidney medulla, nitric oxide synthase activity
At middle age, many kidney functions begin a slow deterioration [1-3]. In men, a decline in GFR often begins after the fourth decade [4], but this is delayed and attenuated in women [1, 5]. Similar patterns are seen for renal plasma flow [5]. The rat also exhibits age-dependent declines in glomerular filtration rate (GFR) and, as in man [4], the rate of fall is highly variable and is influenced by strain [6]. A marked sexual dimorphism also occurs in rats, with the female of a most strains showing an attenuated rate of decline in GFR versus the male [6, 7]. Renal vasoconstriction contributes to the age-dependent falls in GFR, and in addition structural damage develops, which is much more severe in the male of both species [1-7]. The aging male kidney, therefore, represents a slowly evolving form of chronic renal disease (CRD), which in man makes the kidney vulnerable to failure when other diseases are present (e.g., hypertension), and is a significant cause of death in some rats strains where age-dependent damage progresses rapidly [1-3, 6].
There is now increasing evidence that progression of CRD secondary to a primary renal disease is associated with a deficiency of nitric oxide (NO), both based on clinical studies in CRD and end-stage renal disease (ESRD) patients as well as in animals [8]. Furthermore, experimentally induced NO deficiency produces renal disease [9], suggesting that the NO deficiency due to loss of renal function may contribute to the further progression of the disease (i.e., form part of the “vicious cycle”) [8]. There is also animal data showing that total NO production (determined from the stable NO oxidation products, NO2 and NO3 = NOX) falls significantly in the old male rat, co-incident with the development of kidney damage [10-12].
Accordingly, the present study was conducted to measure indices of NO production in aging male and female Sprague-Dawley rats, a strain in which the male develops significant renal pathology [6]. Our hypothesis was that if NO deficiency contributed importantly to age-dependent injury, we would anticipate greater NO deficiency in aging males versus females. In this study, we measured total NOX excretion, tissue NO synthase (NOS) activities, and NOS protein abundance, as well as histologic evaluation of renal pathology in male and female Sprague-Dawley rats of different ages.
METHODS
Animals
Studies were conducted on 26 female and 32 male Sprague-Dawley rats, purchased from Harlan-Sprague-Dawley (Indianapolis, IN, USA) at age 10 to 12 weeks. Rats were then aged at West Virginia University Animal Facility, maintained throughout their lives under barrier conditions and allowed ad libitum access to standard rat chow (~20% protein; 0.4% sodium) and tap water until sacrifice at ages 3 to5 months (young), 11 to 13 months (middle-aged), and 18 to 22 months (old). One kidney was fixed in 10% buffered formalin and the remaining kidney cortex and medulla were separated and harvested on dry ice (along with the cerebellum), flash frozen in liquid nitrogen, and stored at −80°C. All protocols have been reviewed and approved by the West Virginia University Animal Care and Use Committee. A 24-hour urine collection was made within 1 week prior to sacrifice to measure total NO production represented as NOx (NO3 + NO2).
NOS activity was measured from the conversion of [3H]-arginine to [3H]-citrulline in the soluble fraction of kidney cortex, as described by us previously [13]. NOS protein abundance was measured by Western blot [13]. The neuronal NOS (nNOS) was detected with a rabbit monoclonal antibody [gift of Dr. Kim Lau, 1:5000 dilution, 1-hour incubation; secondary antibody, goat, antirabbit immunoglobulin (IgG)-horseradish peroxide (HRP) (Bio-Rad, Hercules, CA, USA), 1:3000 dilution, 1 hour]. Membranes were then stripped and reprobed for endothelial NOS (eNOS) using a mouse, monoclonal antibody (Transduction Labs., Lexington, KY, USA), 1:250 dilution, 1 hour; secondary antibody goat, antimouse IgG-HRP conjugate (Transduction Labs.) 1:2000 dilution, 1 hour. All steps were performed at room temperature. Bands of interest were visualized using an enhanced chemiluminescence (ECL) reagent and quantitated by densitometry, as integrated optical density after subtraction of background (Optimas 6.2, Bothell, WA, USA). The integrated optical density was factored for ponceau red staining to correct for any variations in total protein loading, and for an internal standard (eNOS = 10 μg bovine aortic endothelial cell lysate; nNOS = 1 μg cerebellar lysate) to allow comparison between different membranes.
For pathology, the formalin-fixed kidney was dehydrated in alcohol, blocked in paraffin wax, and 3 to 5 μ sections were cut and stained with periodic acid-Schiff (PAS) + hematoxylin and eosin counterstain. The level of injury was assessed by histology on a blinded basis by assessing the sclerotic damage to glomeruli (N = 100) using the 0 to 4+ scale and by calculating the total injury score [14].
Statistics were by unpaired t test, one-way analysis of variance (ANOVA), Wilcoxon rank sum analysis, and Kruskal-Wallis test. All data are expressed as mean ± SE.
RESULTS
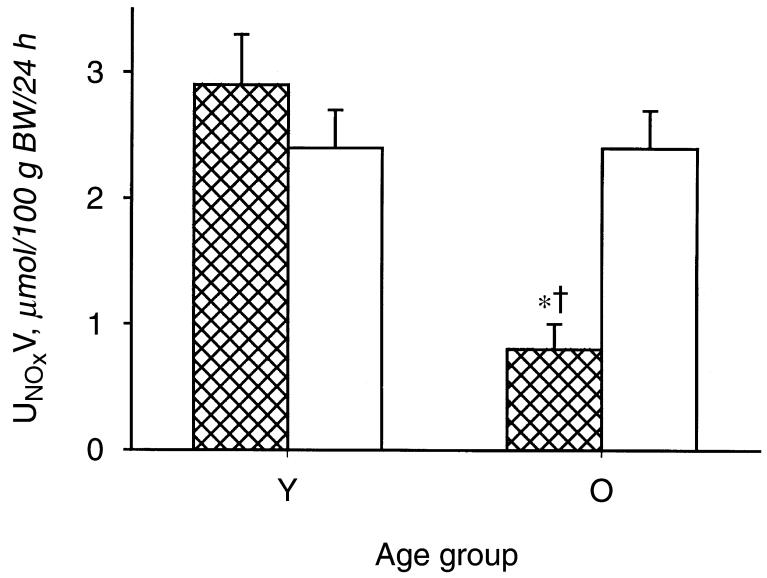
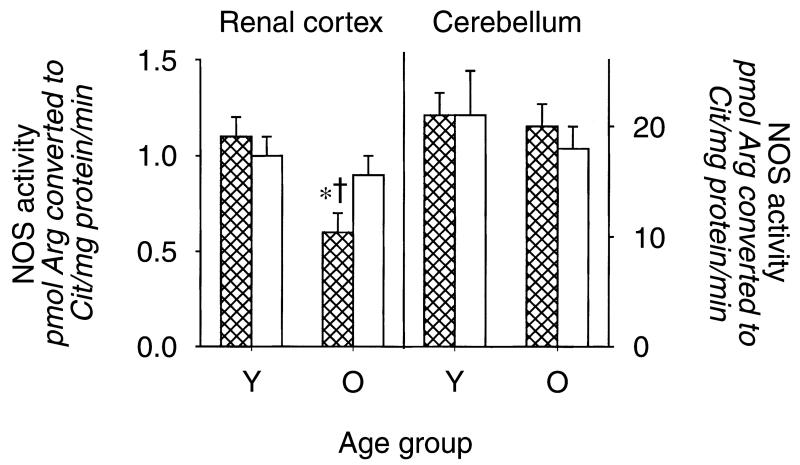
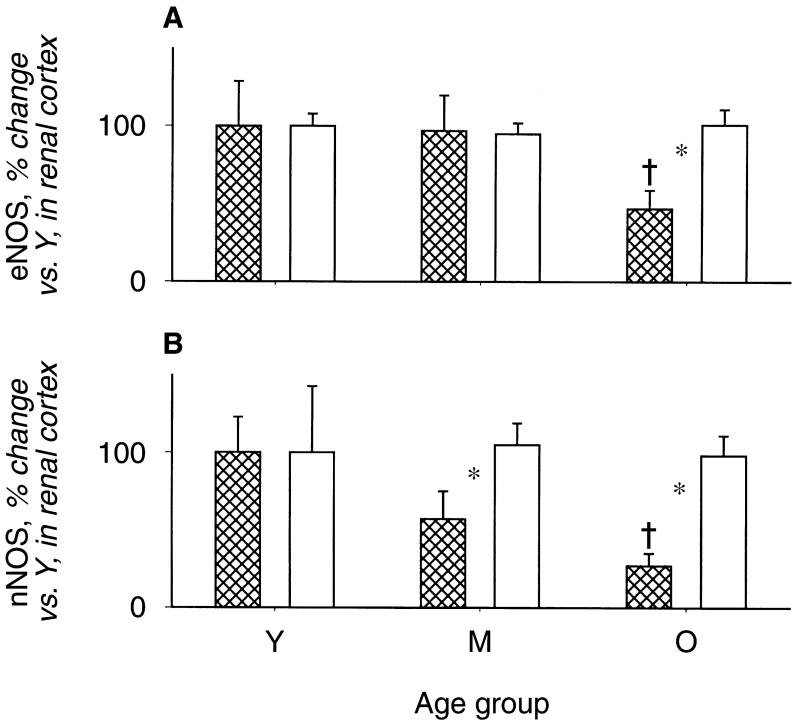
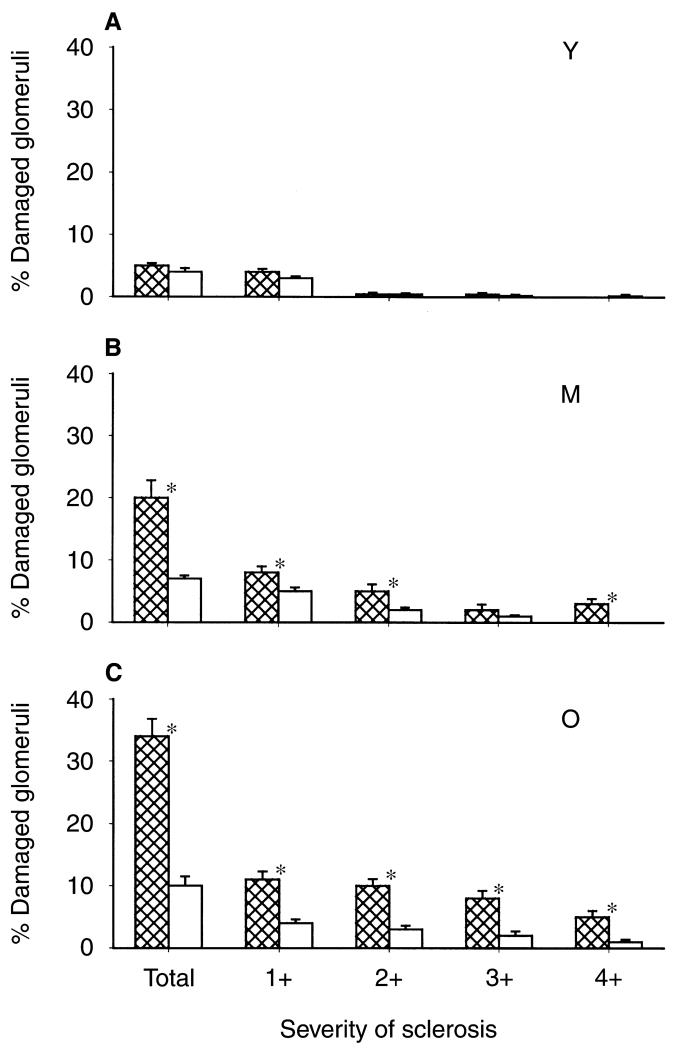
As shown in Figure 1, the old male rats exhibited a marked fall in total NO production, indicated by 24-hour urinary NOx (UNOXV), as reported previously [10-12]. The in vitro NOS activity in renal cortex declined in old versus young males, but age had no impact on cerebellar NOS activity (Fig. 2). The protein abundance of both eNOS and nNOS was significantly reduced in renal cortex of old relative to young males (N = 6 and N = 4, respectively) (Fig. 3). The eNOS and nNOS protein abundance was also measured in middle-aged males (N = 6) and nNOS, but not eNOS, protein had declined by 11 to 13 months of age. Renal medullary eNOS was also reduced in old males, whereas medullary nNOS abundance was unaffected (Fig. 4). As shown in Figure 5, the number of damaged glomeruli and the severity of glomerulosclerosis increased with age in the old male, as reported by us earlier [6, 7, 10]. There was also tubulo-interstitial damage that increased with age and some of the old male kidneys contained many casts. A similar pattern of increasing injury was seen in the subgroups on which Western blot analysis was performed, with an overall glomerular injury score [14] of 6 ± 1 in young males, 45 ± 13 in middle-aged, and 74 ± 8 in old rats.
Fig. 1. Total nitric oxide (NO) production, indexed by 24-hour urinary excretion of NO2 + NO3 = NOX (the stable oxidation products of NO) in young (Y) and old (O) male and female rats. Males represented by the hatched columns.
†Represents a significant difference between old vs. young. *Represents a significant difference between male and female.
Fig. 2. The nitric oxide synthase (NOS) activity (measured from the conversion of arginine to citrulline) in homogenates of renal cortex and cerebellum from young (Y) and old (O), male and female rats.
Males represented by the hatched columns. †Represents a significant difference between old vs. young. * Represents a significant difference between male and female.
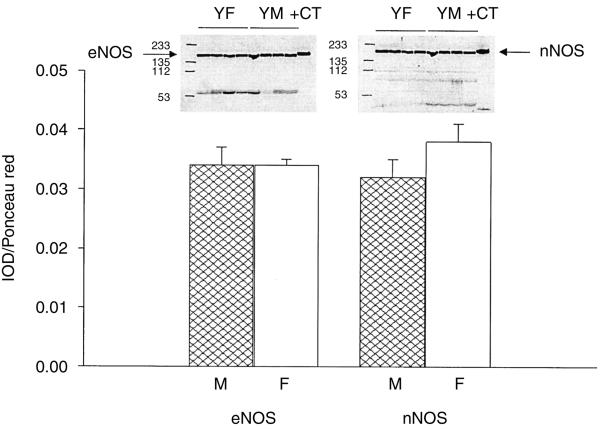
Fig. 3. Relative abundance of the endothelial nitric oxide synthase (eNOS) (A) and neuronal nitric oxice synthase (nNOS) (B) protein in the renal cortex from young (Y), middle-aged (M), and old (O) male and female rats.
Males represented by the hatched columns. Data are given as the integrated optical density/total protein loaded measured from Ponceau red density and expressed as % change from young animals. †Represents a significant difference between old vs. young. *Represents a significant difference between male and female.
Fig. 4. Relative abundance of endothelial nitric oxide synthase (eNOS) (A) and neuronal nitric oxide synthase (nNOS) (B) protein in the renal medulla from young (Y), middle-aged (M) and old (O) male and female rats.
Males represented by the hatched columns. Data are given as the integrated optical density/total protein loaded measured from Ponceau red density and expressed as % change from young animals. †Represents a significant difference between old vs. young.
Fig. 5. Glomerular injury in young (Y) (A), middle-aged (M) (B), and old (O) (C) male and female rats.
Males represented by the hatched columns. The first columns give the total % of glomeruli damaged and the subsequent columns give severity of injury, with 1+ = <5%, 2+ = 25% to 50%, 3+ =51% to 75%, 4+ = 76% to 100% damage to the individual glomerulus. *Represents a significant difference between male and female.
In contrast to males, female rats did not exhibit changes in total body NO production with age (Fig. 1). Furthermore, there were no age-dependent reductions in renal cortex NOS activity or eNOS or nNOS protein abundance (NS for Western blots = 4, 4, and 5 for young, middle-aged, and old, respectively) and, as seen in males, cerebellar NOS activity remained constant with age (Figs. 2 and 3). In the renal medulla the profiles for protein abundance were similar to males, with eNOS, but not nNOS, expression falling in old versus young females (Fig. 4). In contrast to males, the rate of development of age-dependent glomerular damage was markedly attenuated in all rats (Fig. 5) and in the subgroup studied by Western blot (injury score in young females = 6 ± 1, middle-aged = 10 ± 1, and old = 23 ± 5).
In separate groups of young male and female rats (each, N = 4), the absolute abundance of both the eNOS and nNOS proteins in renal cortex were similar (Fig. 6).
Fig. 6. Abundance of the endothelial nitric oxide synthase (eNOS) and neuronal nitric oxide synthase (nNOS) protein in the renal cortex from young male and female rats.
The gels are given above and densitometry values, expressed as integrated optical density/Ponceau red staining to factor for total protein loading, given below.
DISCUSSION
The main, novel findings in this study are that NOS activity and NOS protein abundance decline with age in the male renal cortex as renal injury develops. In contrast, females showed no age-dependent decline in renal cortical NOS activity or NOS protein abundance and only mild renal damage.
There is a substantial literature indicating a progressive endothelial dysfunction with aging, which includes a reduction in NO-dependent responses [15-17]. This reduced peripheral NO activity could reflect a fall in NO synthesis, perhaps secondary to lack of NOS or essential cofactors and/or could involve increased breakdown of NO by oxidants [12, 15-17]. Despite these reports, we were previously unable to document decreases in total NOX production in aging man [18] based on 24-hour UNOXV, probably because the cardiovascular contribution to overall NO generation is such a small percentage of the total [19].
There is less information on NO in the aging kidney, although renal vasoconstriction is a feature of aging [1-3, 5-7, 10] which is consistent with reductions in renal NOS activity. However, in apparent contradiction, both acute and chronic NOS inhibition induce an enhanced renal vasoconstrictor response in old rats [10, 20, 21], suggesting increased overall renal NO activity with aging. Also, the vasodilatory effects of l-arginine and acetylcholine (partly mediated by NO) are preserved in the aging rat, although diminished in man [10, 22]. The present study was conducted to specifically measure the impact of aging on abundance of the constitutive NOS and the overall NOS activity in the kidney.
Our data clearly demonstrate that the abundance of both of the constitutive NOS isoforms decline with age in the male rat kidney. The only other study to address this issue reported a decreased peritubular capillary eNOS distribution (by immunohistochemistry), which appeared to be associated with areas of tubulointerstitial injury [23]. In contrast, these investigators also reported focal increases in eNOS abundance in tubulointerstium [23]. Our findings are novel in that they demonstrate marked overall declines in eNOS in both cortex and medulla and also report that the primary change is in the nNOS (see below). Our in vitro NOS activity data suggest that this decreased abundance is linked to a reduction in renal cortical NO production since the in vitro NOS activity assay is conducted in the presence of added (excess) substrate and cofactors. Of note, we observe a decreased in vitro NOS activity in aging male rat kidney, despite an earlier report of marked increases in the expression of inducible NOS (iNOS) in the old male rat kidney [24]. Perhaps the iNOS is not functioning as a NO generator in this setting? In contrast to the male, we find that the abundance and activity of renal cortical NOS is preserved in the old female kidney. Overall, these observations are in accord with our earlier report in the aging Munich Wistar rat, where renal vasoconstriction was seen in aging males, while mild renal vasodilation occurred in the female [7].
How can a reduction in renal NO-generating capacity in the old male kidney be reconciled with the observation of increased NO dependence in the aging male renal cir-culation [10, 20, 21]? In fact, this is analogous to the impact of aging on the renal prostaglandin system. While production of the vasodilatory prostaglandins falls [25, 26], renal perfusion can become dependent on vasodilatory cyclooxygenase (COX) products in the old kidney, to the point where COX inhibitors can severely compromise renal function [27]. The most likely explanation is that despite a decline in absolute production of endothelial vasodilators with age, the vasodilators present become of increased importance in maintaining renal perfusion in view of the increased renal vasoconstrictor tone due to angiotensin II and the renal nerves [28, 29].
Is the gender difference in the abundance of the renal cortical NOS proteins directly related to the sex hormones? Estrogen is a known stimulus to both nNOS and eNOS activity [30], but in the present study we found that nNOS and eNOS protein abundance is similar in renal cortex of young adult males and females, as also reported by Neugarten et al [31]. We recognize, however, that higher renal medullary eNOS protein abundance is seen in young adult females [31], which is presumably responsible for the findings of Reckelhoff et al [32] who observed more eNOS in whole kidney homogenates from young adult female versus male rats. Since estrogen levels tend to decline with advanced age in females [33], it seems unlikely that estrogens are directly responsible for the maintenance of the constitutive NOS enzymes in the old female kidney. We suggest an alternative mechanism that may explain the gender differences in renal NOS expression in the aging kidney, based on the development of CRD as the initiating factor in reduced renal NOS expression, see below.
There is increasing evidence that multiple mechanisms lead to NO deficiency in CRD, including a reduction in the abundance of the renal NOS enzymes [8]. Although we do not yet understand the cause of the reduced renal NOS expression, a reduction in renal nNOS abundance occurs as early as 2 to 3 weeks after 5/6 ablation/infarction of renal mass [34] and persists at 11 weeks (Erdely, Wagner, and Baylis, unpublished observations). We have also observed reduced renal cortical nNOS abundance in a model of chronic postglomerulonephritis CRD [35]. In the present study, first the nNOS and later the eNOS protein abundance in renal cortex fall as CRD progresses with advancing age in the male Sprague-Dawley rat. In this rat strain, age-dependent CRD develops rapidly in the male and renal failure is a significant cause of death [6].
It is possible that the developing CRD causes the reduction in NOS protein expression, possibly by an angiotensin II-mediated action since angiotensin II type 1 receptor (AT1) blockade attenuates the early fall in nNOS after 5/6 renal ablation [34]. Also, NOS stimulation with excess substrate (l-arginine) and/or angiotensin-converting enzyme (ACE) inhibition similarly attenuated the injury in rats with 5/6 ablation/infarction of renal mass [36]. Since the aging male Sprague-Dawley rat develops a severe CRD, the decline in renal NO-generating capacity observed here could also contribute to further renal injury and could accelerate progression of CRD. Indeed, chronic NO stimulation with excess l-arginine protects the aging male rat kidney from functional and structural damage [37].
As shown in the present study, in contrast to the male, the female Sprague-Dawley develops minimal age-dependent kidney damage and shows no tendency for renal cortical NOS protein abundance to decline. In other words, preservation of structure in the aging female is associated with preservation of renal NO production. A similar correlation is seen in Wistar Furth (male) rats who do not develop CRD after 5/6 ablation/infarction of renal mass [38] and in whom renal cortical NOS protein abundance and activity is preserved (Abstract; Erdely A et al, JAm Soc Nephrol 11:617A, 2000). In contrast, the male Sprague-Dawley rats develop rapidly progressing CRD and decline in renal NO-generating capacity after 5/6 renal ablation/infarction (Abstract; Wagner L, et al, J Am Soc Nephrol 12:828A, 2001). Low level NOS inhibition when combined with 5/6 ablation/infarction causes progressive CRD in Wistar Furth rats while having little additional impact on Sprague-Dawley rats (Abstract; Erdely A, et al J Am Soc Nephrol 11:617A, 2000). These associations strengthen the suggestion that renal NO deficiency plays a key role in progression of renal disease.
CONCLUSION
In conclusion, while declines in renal function and appearance of structural damage are not inevitable [4, 6], the majority of aging individuals will develop progressive structural and functional declines [1-3, 5]. In man, these are rarely so marked as to cause renal failure in the absence of other diseases, but do render the aging kidney more vulnerable to failure when superimposed disease occurs. The present study suggests that renal NO deficiency may contribute to age-dependent kidney damage and may be a factor in the gender difference in the rate of declines in function. Based on observations in other models of CRD and in man with renal disease, NO deficiency may be an important contributor to progressive CRD, irrespective of the primary cause of the injury.
ACKNOWLEDGMENTS
These studies were funded by NIH grant #RO1 DK45517. The excellent technical assistance of Lennie Samsell, Kevin Engels, and Gary Freshour is gratefully acknowledged.
REFERENCES
- 1.Macias-Nunez JF, Cameron JS. Renal function in the elderly. In: Cameron S, Davison AM, Grunfeld JP, et al., editors. Oxford Textbook of Clinical Nephrology. Oxford University Press; Oxford: 1992. pp. 56–70. [Google Scholar]
- 2.Levi M, Rowe JW. Renal function and dysfunction in aging. In: Seldin DW, Giebisch G, editors. The Kidney. Raven Press; New York: 1992. pp. 3433–3456. [Google Scholar]
- 3.Chou SY, Lindeman RD. Structural and functional changes in the aging kidney. In: Jacobson HR, Striker GE, Klahr S, editors. The Principles and Practice of Nephrology. 2nd ed. Mosby; St. Louis: 1995. pp. 510–514. [Google Scholar]
- 4.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 5.Wesson LG., Jr . Physiology of the Human Kidney. Grune and Stratton; New York: 1969. Renal hemodynamics in physiological states; pp. 96–108. [Google Scholar]
- 6.Baylis C, Corman B. The aging kidney: Insights from experimental studies. J Am Soc Nephrol. 1998;9:699–709. doi: 10.1681/ASN.V94699. [DOI] [PubMed] [Google Scholar]
- 7.Baylis C. Age-dependent glomerular damage in the rat: Dissociation between glomerular injury and both glomerular hypertension and hypertrophy. Male gender as a primary risk factor. J Clin Invest. 1994;94:1823–1829. doi: 10.1172/JCI117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baylis C. Nitric oxide deficiency; both consequence and cause of chronic renal disease (CRD) Hypertens Nephrol. 2001;5:193–201. [Google Scholar]
- 9.Zatz R, Baylis C. Chronic nitric oxide inhibition model six years on. Hypertension. 1998;32:958–964. doi: 10.1161/01.hyp.32.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill C, Lateef AM, Engels K, et al. Basal and stimulated nitricoxide in control of kidney function in the aging rat. Am J Physiol. 1997;272:R1747–R1753. doi: 10.1152/ajpregu.1997.272.6.R1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonaka I, Futami Y, Maki T. l-Arginine-nitric oxide pathway and chronic nephropathy in aged rats. J Gerontol Biol Sci. 1990;49:B157–B161. doi: 10.1093/geronj/49.4.b157. [DOI] [PubMed] [Google Scholar]
- 12.Reckelhoff JF, Kellum JA, Blanchard EJ, et al. Changes in nitric oxide precursor, l-arginine and metabolites, nitrate and nitrite, with aging. Life Sci. 1994;55:1895–1902. doi: 10.1016/0024-3205(94)00521-4. [DOI] [PubMed] [Google Scholar]
- 13.Xiao S, Erdely A, Wagner L, Baylis C. Uremic levels of BUN do not cause nitric oxide deficiency in rats with normal renal function. Am J Physiol Renal. 2001;49:F996–F1000. doi: 10.1152/ajprenal.2001.280.6.F996. [DOI] [PubMed] [Google Scholar]
- 14.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension and progressive glomerular damage in Dahl rats. Kidney Int. 1984;26:137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 15.Matz RL, Schott C, Stoclet JC, Andriantsitohaina R. Age-related endothelial dysfunction with respect to nitric oxide, endothelium-derived hyperpolarizing factor and cyclooxygenase products. Physiol Res. 2000;49:11–18. [PubMed] [Google Scholar]
- 16.Dohi Y, Thiel MA, Buhler FR, et al. Activation of endothelial l-arginine pathway in resistance arteries. Effect of age and hypertension. Hypertension. 1990;15:170–179. doi: 10.1161/01.hyp.16.2.170. [DOI] [PubMed] [Google Scholar]
- 17.Docherty JR. Cardiovascular responses in ageing: A review. Pharmacological Reviews. 1992;42:103–125. [PubMed] [Google Scholar]
- 18.Schmidt RJ, Beierwaltes WH, Baylis C. Aging and alterations in dietary sodium intake on total nitric oxide production in normal man. Am J Kidney Dis. 2001;37:900–908. doi: 10.1016/s0272-6386(05)80004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baylis C, Vallance P. Editorial review: Measurement of nitrite and nitrate (NOx) levels in plasma and urine; What does this measure tell us about the activity of the endogenous nitric oxide. Curr Opin Nephrol Hyper. 1998;7:1–4. doi: 10.1097/00041552-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Tank JE, Vera JP, Houghton DC, Anderson S. Altered renal vascular responses in the aging rat kidney. Am J Physiol. 1994;266:F942–F948. doi: 10.1152/ajprenal.1994.266.6.F942. [DOI] [PubMed] [Google Scholar]
- 21.Reckelhoff JF, Manning RD., Jr Role of endothelial-derived nitric oxide in the control of the renal microvasculature in aging male rats. Am J Physiol. 1993;265:R1126–R1131. doi: 10.1152/ajpregu.1993.265.5.R1126. [DOI] [PubMed] [Google Scholar]
- 22.Hollenberg NK, Adams DF, Solomon H, et al. Senescence and the renal vasculature in normal man. Circ Res. 1974;34:309–316. doi: 10.1161/01.res.34.3.309. [DOI] [PubMed] [Google Scholar]
- 23.Thomas SE, Anderson S, Gordon KL, et al. Tubulointerstitial disease in aging: Evidence for underlying peritubular capillary damage, a potential role for renal ischemia. J Am Soc Nephrol. 1998;9:231–242. doi: 10.1681/ASN.V92231. [DOI] [PubMed] [Google Scholar]
- 24.Reckelhoff RF, Hennington BS, Kanji V, et al. Chronic aminoguanidine attenuates renal dysfunction and injury in aging rats. Am J Hypertens. 1999;12:492–498. doi: 10.1016/s0895-7061(98)00264-7. [DOI] [PubMed] [Google Scholar]
- 25.Hornych A, Forette F, Bariety J, et al. The influence of age on renal prostaglandin synthesis in man. Prostaglandins Leukot Essent Fatty Acids. 1991;43:191–195. doi: 10.1016/0952-3278(91)90168-5. [DOI] [PubMed] [Google Scholar]
- 26.Rathaus M, Greenfeld Z, Podjarny E, et al. Sodium loading and renal prostaglandins in old rats. Prostaglandins Leukot Essent Fatty Acids. 1993;49:815–819. doi: 10.1016/0952-3278(93)90031-q. [DOI] [PubMed] [Google Scholar]
- 27.Johnson AG, Day RO. The problems and pitfalls of NSAID therapy in the elderly (Part 1) Drugs Aging. 1991;1:130–143. doi: 10.2165/00002512-199101020-00005. [DOI] [PubMed] [Google Scholar]
- 28.Hajduczok G, Chapleau MW. Increase in sympathetic activity with age I. Role of impairment of arterial baroreflexes. Am J Physiol. 1991;260:H1113–H1120. doi: 10.1152/ajpheart.1991.260.4.H1113. [DOI] [PubMed] [Google Scholar]
- 29.Baylis C. Renal responses to acute angiotensin II inhibition and administered angiotensin II in the aging, conscious, chronically catheterized rat. Am J Kidney Dis. 1993;22:842–850. doi: 10.1016/s0272-6386(12)70344-x. [DOI] [PubMed] [Google Scholar]
- 30.Forstermann U, Boissel JP, Kleinert H. Expressional control of the constitutive isoforms of nitric oxide synthase (NOS I and NOS III) FASEB J. 1998;12:773–790. [PubMed] [Google Scholar]
- 31.Neugarten J, Ding Q, Friedman A, et al. Sex hormones andrenal nitric oxide synthases. J Am Soc Nephrol. 1997;8:1240–1246. doi: 10.1681/ASN.V881240. [DOI] [PubMed] [Google Scholar]
- 32.Reckelhoff JF, Hennington BS, Moore AG, et al. Gender differences in the renal nitric oxide (NO) system: dissociation between expression of endothelial NO synthase and renal hemodynamic response to NO synthase inhibition. Am J Hypertens. 1998;11:97–104. doi: 10.1016/s0895-7061(97)00360-9. [DOI] [PubMed] [Google Scholar]
- 33.Nelson JF. The potential role of selected endocrine systems in aging processes. In: Masoro EJ, editor. Handbook of Physiology. Oxford University Press; New York: 1995. pp. 377–394. [Google Scholar]
- 34.Roczniak A, Fryer JN, Levine DZ, Burns KD. Downregulation of neuronal nitric oxide synthase in the rat remnant kidney. J Am Soc Nephrol. 1999;10:704–713. doi: 10.1681/ASN.V104704. [DOI] [PubMed] [Google Scholar]
- 35.Wagner L, Riggleman A, Erdely A, et al. Reduced NOS activity in rats with chronic renal disease due to glomerulonephritis. Kidney Int. 2002;62:532–536. doi: 10.1046/j.1523-1755.2002.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashab I, Peer G, Blum M, et al. Oral administration of l-arginine and captopril to rats prevents chronic renal failure by nitric oxide production. Kidney Int. 1995;47:1515–1521. doi: 10.1038/ki.1995.214. [DOI] [PubMed] [Google Scholar]
- 37.Reckelhoff JF, Kellum JA, Racusen LC, Hildebrandt DA. Long term dietarysupplementation with l-arginine prevents age-related reduction in renal function. Am J Physiol Regulatory. 1997;272:R1768–1774. doi: 10.1152/ajpregu.1997.272.6.R1768. [DOI] [PubMed] [Google Scholar]
- 38.Fitzgibbon WR, Greene EL, Grewal JS, et al. Resistance to remnant nephropathy in the Wistar-Furth rat. J Am Soc Nephrol. 1999;10:814–821. doi: 10.1681/ASN.V104814. [DOI] [PubMed] [Google Scholar]