Summary
Modulation of actin dynamics through the N-WASp/Arp2/3 pathway is important in cell locomotion, membrane trafficking, and pathogen infection. Here, we demonstrate that Nck is essential for actin remodeling stimulated by phosphatidylinositol 4,5 bisphosphate (PI(4,5)P2), and conversely that PI(4,5)P2 is necessary for localized actin polymerization induced by Nck in vivo. Nck knockdown or knockout suppressed actin comets induced by phosphatidylinositol 5-kinase (PIP5K), and PIP5K stimulated tyrosine phosphorylation of an Nck SH2 domain binding partner, suggesting that Nck couples phosphotyrosine- and phosphoinositide-dependent signals. We show that PI(4,5)P2 and PIP5K are both enriched at actin comets induced by Nck aggregates, and that formation of actin comets was strongly inhibited by co-clustering with an inositol 5-phosphatase domain to decrease local PI(4,5)P2 levels. The extent of Nck-induced actin polymerization was also modulated by PI(4,5)P2-sensitive N-WASp mutants. This study uncovers a strong reciprocal interdependence between Nck and PI(4,5)P2 in promoting localized N-WASp-mediated actin polymerization in cells.
Introduction
Polarized, spatially restricted cytoskeletal rearrangements underlie many key cellular processes in development and disease. Various aspects of cell motility, including some types of vesicle trafficking, require localized actin polymerization and are intimately involved in the establishment of cell polarity. Changes in tyrosine phosphorylation underlie signaling mechanisms that regulate a host of cellular processes including proliferation, vesicle transport, adhesion and migration (Pawson, 2004). The spatial segregation of inositol phospholipids, on the other hand, contributes to organelle identity and plays a crucial role in intracellular trafficking by directing the assembly of signaling platforms at the membrane-cytosol interface (Di Paolo and De Camilli, 2006). Although signaling mediated by tyrosine phosphorylation and phosphoinositides, particularly PI(4,5)P2, has been widely implicated in the modulation of actin dynamics, very little is known about how actin filament assembly by these distinct signaling mechanisms might be coordinated.
The hematopoietic WASp and the ubiquitously expressed N-WASp physically interact with the Arp2/3 complex and G-actin to promote actin filament nucleation and branching. Their multi-domain molecular structure allows them to integrate signals from the Rho GTPases and other signaling molecules including SH3 domain-containing proteins and PI(4,5)P2 (Takenawa and Suetsugu, 2007). Increased levels of PI(4,5)P2 induced by overexpression of PIP5K promoted the assembly of a complex that includes N-WASp, Nck, Grb2, and WIP, and the formation of actin comets that propel Golgi-derived vesicles (Benesch et al., 2002; Rozelle et al., 2000).
Nck is a two-member family of Src homology (SH) 2 and SH3 domain-containing adaptor proteins that couple tyrosine phosphorylation with downstream effectors that regulate the actin cytoskeleton (Li et al., 2001). Genetic analysis in mouse demonstrated that Nck1 and Nck2 are ubiquitously expressed and share a high degree of functional redundancy; inactivation of both genes caused early embryonic lethality due to profound defects in mesoderm-derived tissues (Bladt et al., 2003). Studies in Drosophila uncovered a critical role for Nck (Dock) in actin rearrangements underlying photoreceptor axon guidance and target recognition in the developing eye (Rao, 2005). In mammalian cells, the analysis of signaling mechanisms during infection by pathogens such as vaccinia virus (Frischknecht et al., 1999b; Moreau et al., 2000) and enteropathogenic E. coli (Gruenheid et al., 2001) disclosed a requirement for Nck in localized actin polymerization through the N-WASp/Arp2/3 pathway. Mechanistic insights were gained by studies in vitro demonstrating that Nck SH3 domains bound to N-WASp and stimulated its actin nucleation promoting activity in the presence of PI(4,5)P2 (Rohatgi et al., 2001). More recently, the induction of increased local concentration of membrane-targeted Nck SH3 domains by clustering with antibodies was shown to be sufficient to recruit N-WASp and induce the formation of actin comets in living cells (Rivera et al., 2004). Likewise, clustering of Nck by a phosphopeptide from Tir, an Enteropathogenic E. coli effector protein, triggered actin tail formation in Xenopus egg extracts (Campellone et al., 2004).
Very little is known about how inputs from diverse signaling molecules influence the targeting and activation of N-WASp in vivo. In the present study, we tested the hypothesis that Nck adaptors provide an essential link that coordinates inputs from tyrosine phosphorylation and PI(4,5)P2 to regulate localized actin polymerization. We show for the first time that Nck adaptors are required for the formation of actin comets induced by PIP5K, and demonstrate that SH3 domains of Nck and PI(4,5)P2 cooperate in N-WASp-stimulated actin polymerization in cells.
Results
Nck adaptors are required for actin polymerization stimulated by PI(4,5)P2 in cells
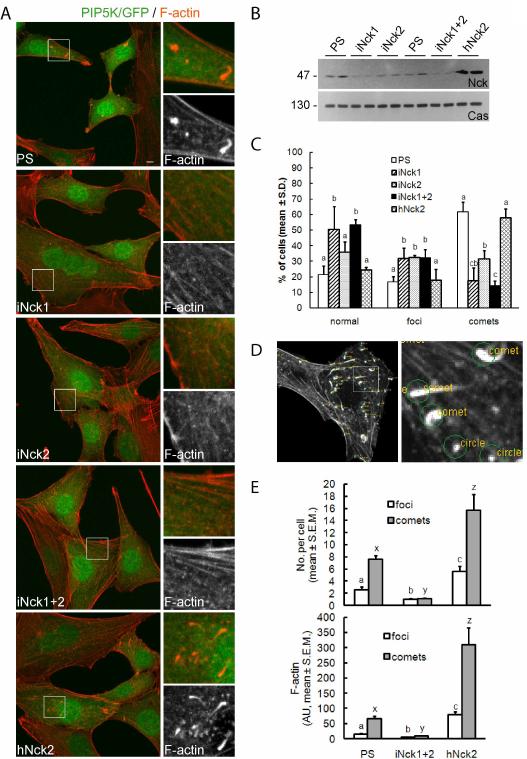
Overexpression of PIP5K has been shown to induce dramatic changes in the actin cytoskeleton, including the formation of actin comets that propel Golgi-derived vesicles and macropinosomes (Guerriero et al., 2006; Orth et al., 2002; Rozelle et al., 2000). Here, we utilized this model to define the role of Nck adaptors in localized actin polymerization induced by elevated cellular levels of PI(4,5)P2. As shown in Fig. 1A, elevated cellular levels of PI(4,5)P2 caused by the expression of PIP5K type I α led to dramatic cytoskeletal changes in control cells (PS) and Nck knockdown cells rescued with hNck2 (hNck2), characterized by the partial disassembly of actin fibers and the formation of prominent actin comets and foci. These phenotypic changes were not observed in cells expressing the catalytically inactive mutant PIP5KD227A (Fig. S3). In contrast, shRNA-mediated depletion of Nck (particularly iNck1 and iNck1+2, Fig. 1A and B) almost completely abolished the formation of actin comets in response to elevated levels of PI(4,5)P2; instead, well defined actin fibers typical of a “normal” cytoskeletal architecture were apparent.
Fig. 1.
Nck is required for the formation of actin comets induced by PI(4,5)P2. A) Confocal images of cells expressing wild type PIP5K type Iα (PIP5K/GFP) and a control vector (PS), a vector expressing shRNA against Nck1 (iNck1), Nck2 (iNck2), both (iNck1+2), or a combination of the two shRNA vectors (iNck1+2) plus a rescuing vector expressing human Nck2 (hNck2). Scale bar represents 5 μm. In this and subsequent figures boxed areas are shown at higher magnification in the right panels. B) Specific Nck protein knockdown by shRNAs demonstrated by western blotting (top, samples from two independent experiments). The same membrane was stripped and reprobed with anti-p130Cas for loading control (bottom). C) Percentage of cells showing normal cytoskeletal appearance, presence of actin foci or actin comets. All cells express PIP5K as in A. Values are mean ± S.D. from observations of 20 randomly selected microscopic fields from each of three independent experiments. Bars with different letters within cytoskeletal phenotype (normal, foci or comets) differ (a,b,c; p<0.05). D) Example of output generated by the algorithm utilized for the computer-assisted analysis of confocal images. E) Quantitative analysis of phenotypic chances induced by PI(4,5)P2. Values represent mean ± S.E.M. from images corresponding to 7–10 cells/treatment analyzed in each of three independent experiments (n=3). Different letters on bars indicate statistical differences (p<0.05, foci: a,b,c; comets: x,y,z).
We quantified the effects of Nck on cytoskeletal changes induced by PI(4,5)P2 by scoring in blinded fashion the percentage of cells with apparently normal cytoskeletal appearance or with clearly identifiable comets and foci. Knockdown of Nck decreased dramatically the percentage of cells forming comets and, in turn, increased the frequency of the normal phenotype (Fig.1C). A slightly higher percentage of Nck knockdown than control cells, however, formed actin foci in response to elevated levels of PI(4,5)P2. It is likely that these foci represent limited localized actin polymerization due to a less stable/active N-Wasp/Arp2/3 complex in the absence of Nck.
To circumvent possible subjectivity in scoring actin phenotypes, we also performed an unbiased, quantitative analysis using a modification of a previously described computational algorithm for the analysis of actin structures from confocal images (Sallee et al., 2008). As shown in Fig.1D, this algorithm can readily discriminate actin fibers, actin comets, and foci (“circles”) based on geometry and size. In addition to cell-based counts of actin comets and foci the algorithm estimates, based on intensity, the amount of F-actin present in the various actin structures. Comparative analysis of images utilizing this algorithm revealed a significant decrease (p<0.05) in the number of comets and foci per cell in response to elevated levels of PI(4,5)P2 in Nck knockdown (iNck1+2) vs. control (PS) cells (Fig. 1E, top panel). In contrast, the number of comets and foci was significantly increased in rescued (hNck2) vs. control (PS) or Nck knockdown (iNck 1+2) cells. Similarly, the total amount of F-actin (Fig.1E, bottom panel) in comets and foci was lower in Nck knockdown (iNck 1+2), intermediate in control (PS), and higher in rescued (hNck2) cells (p<0.05).
Ectopic expression of dominant negative mutants of Nck with inactivation of the SH2 or all three SH3 domains (R308K and KSH3all, respectively) also suppressed the phenotype induced by elevated levels of PI(4,5)P2 in NIH3T3 cells (Fig. S1A). In contrast, overexpression of wild type Nck2 increased significantly the number of actin comets per cell and F-actin levels associated with comets and foci (Fig. S1B).
To further characterize the effects of Nck on PI(4,5)P2-induced cytoskeletal changes, PIP5K was expressed in Nck-deficient (inactivation of both Nck genes) mouse embryonic fibroblasts rescued with an empty vector (EBB), a vector expressing hNck1, hNck2, or both (Fig. S2). Consistent with findings in Nck knockdown/rescue experiments, actin fibers disassembled and prominent actin comets and foci formed in response to elevated PI(4,5)P2 levels only in Nck-deficient mouse embryonic fibroblasts rescued with Nck (Fig.S2A, Nck1, Nck2, Nck1+2, and movie S2). Quantitative analysis showed that a higher (p<0.05) proportion of cells rescued with Nck (Nck1, Nck2, or Nck1+2) vs. the empty vector (EBB) formed actin comets in response to PIP5K overexpression (Fig. S2B).
These observations demonstrate that Nck adaptors are required for PI(4,5)P2-dependent actin remodeling and suggests that Nck adaptors are uniquely positioned to couple extracellular signals that alter phosphoinositide metabolism and tyrosine phosphorylation with actin remodeling.
PIP5K stimulates tyrosine phosphorylation of an Nck SH2 domain binding partner
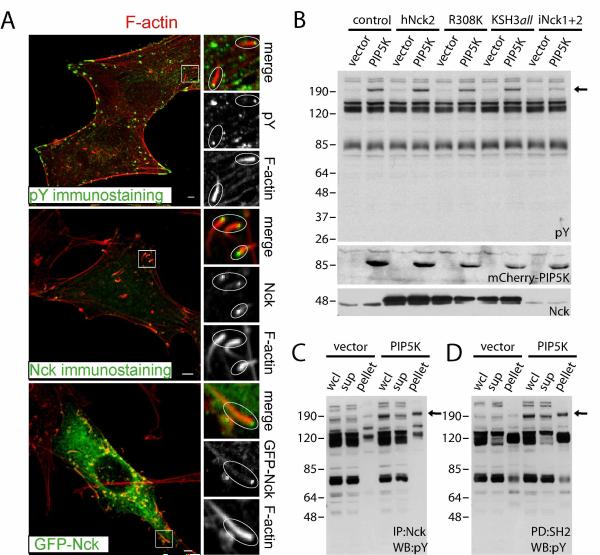
Since we showed that the SH2 domain of Nck is required for PI(4,5)P2-dependent actin remodeling, we therefore sought to analyze the tyrosine phosphorylated Nck SH2 binding partners in cells with elevated PI(4,5)P2. First, we examined the subcellular distribution of tyrosine-phosphorylated proteins and Nck in cells expressing wild type or catalytically inactive PIP5K. In cells expressing catalytically inactive PIP5K, phosphotyrosine accumulated at the tips of actin stress fibers while Nck was distributed diffusely in the cytosol (Fig. S3). However, tyrosine phosphorylated proteins and Nck were enriched at the tips of actin comets in cells expressing wild type PIP5K (Fig. 2A).
Fig. 2.
Expression of PIP5K type Iα induces tyrosine phosphorylation of an Nck SH2 domain binding partner. A) Colocalization of a tyrosine phosphorylated protein(s) and Nck at the tips of actin comets induced by PI(4,5)P2. Cells expressing wild type PIP5K type Iα were subjected to immunostaining with an antibody against phosphotyrosine (top panel), Nck (middle panel), or cotransfected with GFP-Nck (bottom panel). Scale bars represent 5 μm. B ) Perturbation of phosphoinositol metabolism induces changes in phosphotyrosine (phosphotyrosine immunoblot, top panel). The arrow indicates a protein(s) of ~ 190–200 kDa highly tyrosine phosphorylated in cells expressing PIP5K. Middle and bottom panels show expression levels of PIP5K (mCherry-tagged) and Nck, respectively. C and D) The ~ 190–200 kDa protein(s) undergoing PIP5K-induced changes in phosphotyrosine levels is an Nck SH2 domain binding partner. Lysates were subjected to immunoprecipitation with an anti-Nck antibody (C, IP:Nck), or pull-down assay with immobilized SH2 domains from Nck (D, PD:SH2). The whole cell lysate (wcl), proteins remaining in the supernatant (sup), or recovered in the pellets (pellet) were subjected to western blotting with an anti-phosphotyrosine antibody (WB:pY). The arrow indicates the Nck SH2 domain binding partner(s).
Next, we investigated the profile of tyrosine phosphorylation in lysates of cells expressing an empty vector (vector) or wild type PIP5K in conjunction with a control vector (control), wild type (hNck2) or mutant (R308 and KSH3all) Nck, or shRNAs targeting both Nck1 and Nck2 (iNck1+2). Surprisingly, expression of PIP5K, but not the empty vector, induced changes in tyrosine phosphorylation (Fig. 2B). The most prominent change was the increase in tyrosine phosphorylation of a protein(s) co-migrating with the ~190kDa molecular weight marker. One of the consequences of SH2 domain binding in vivo is the “protection” of the phosphotyrosine from the activity of tyrosine phosphatases. The intensity of the ~190kDa band increased (2.4 fold) in lysates from cells overexpressing hNck2 vs. Nck knockdown cells, an observation suggesting that the protein(s) in question is an Nck SH2 domain binding partner. Interestingly, phosphorylation of the ~190kDa protein was not observed in Nck-deficient mouse embryonic fibroblasts expressing PIP5K, but was partially rescued when Nck was re-introduced in these cells (not shown). Immunoprecipitation with an antibody against Nck (Fig. 2C) and pulldown assays with immobilized Nck SH2 domains (Fig. 2D) indicated that the phosphoprotein(s) co-migrating with the ~190kDa marker is indeed an Nck SH2 domain binding partner, which was confirmed by far-western blotting using the SH2 domain from Nck as a probe (not shown).
These findings demonstrate that high intracellular levels of PI(4,5)P2 lead to increased tyrosine phosphorylation of an Nck SH2 binding protein, likely localized to the tips of actin comets. This result, combined with the failure of Nck SH2 mutant to support actin comet formation (R308K, Fig. S1), strongly suggests that modulation of tyrosine phosphorylation by PI(4,5)P2 is critical for the assembly of the Nck/N-WASp signaling complex that directs actin remodeling. We identified candidate proteins of molecular size ~190 kDa known to be tyrosine phosphorylated and likely to interact with the Nck SH2 domain by mining public databases, including Phospho.ELM and PhosphoSite. Immunoprecipitation experiments ruled out p190RhoGap, intersectin-1, and IQGAP-1 as the tyrosine phosphorylated p190 kDa Nck SH2 domain binding partner (data not shown). Experiments are underway to unambiguously identify the protein in question by mass spectrometry.
Recruitment of PIP5K and enrichment of PI(4,5)P2 at sites of actin polymerization induced by clusters of membrane-targeted Nck SH3 domains
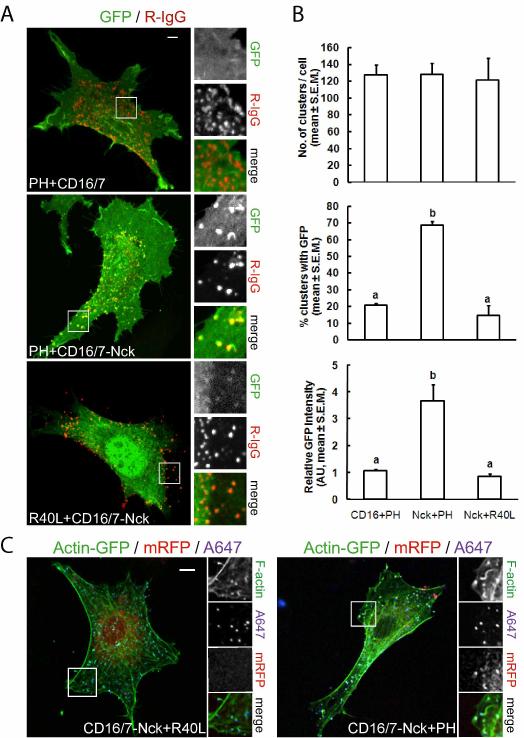
The requirement for Nck in actin rearrangements induced by PI(4,5)P2 led us to consider whether Nck-induced actin remodeling might be dependent on PI(4,5)P2. Previous studies in vitro suggested that Nck SH3 domains and PI(4,5)P2 cooperate, in a synergistic fashion, to stimulate actin polymerization through the N-WASp/Arp2/3 pathway (Rohatgi et al., 2001). To test whether Nck SH3 domains and PI(4,5)P2 also cooperate in vivo to promote N-WASp-dependent actin polymerization, we first analyzed the subcellular distribution of PI(4,5)P2 when membrane-targeted Nck SH3 domains are clustered with antibodies as previously described (Rivera et al., 2004). To assess the localization of PI(4,5)P2, we used the pleckstrin homology (PH) domain from PLCδ1 fused with GFP or mRFP (Balla and Varnai, 2002). Clusters of membrane-targeted Nck SH3 domains, visualized by a fluorescently labeled secondary antibody, colocalized with patches highly enriched in the PI(4,5)P2 reporter (Fig.3 A, middle panel, and animation S1). In contrast, clusters of a control CD16/7 construct (that lacks the Nck SH3 domains) did not induce changes in the subcellular distribution of the PI(4,5)P2 reporter (Fig.3 A, top panel, and animation S2).
Fig. 3.
Enrichment of PI(4,5)P2 at sites of actin polymerization induced by clusters of membrane-targeted Nck SH3 domains. The pleckstrin homology (PH) domain from PLCδ1 fused with GFP was utilized as a reporter of the subcellular distribution of PI(4,5)P2. A fusion of GFP with a mutant PH domain (PHR40L), unable to bind PI(4,5)P2, was utilized as a control of phosphoinositide binding specificity. A) Subcellular localization of PI(4,5)P2 in NIH3T3 cells expressing the fusion of CD16/7 with the three SH3 domains from Nck (CD16/7-Nck) or CD16/7 alone (CD16/7) after clustering with antibodies (R-IgG). B) Quantitative analysis of the localized enrichment of PI(4,5)P2 at clusters of CD16/7-Nck vs. CD16/7 alone (a vs. b, p<0.05). C) Clustering of CD16/7-Nck induces rearrangements of the actin cytoskeleton, including the formation of actin comets and foci. Colocalization of the wild type variant of the PI(4,5)P2 reporter (PH), but not its inactive mutant (R40L), demonstrates enrichment of PI(4,5)P2 at sites of actin polymerization induced by aggregates of Nck SH3 domains. Scale bars represent 5 μm.
The absence of significant colocalization in a number of controls, including the mutant PH domain (R40L, unable to bind PI(4,5)P2) fused to GFP (Fig. 3A bottom panel) and membrane-targeted GFP (myristoylated-GFP, Fig. S4C), demonstrated the specificity of PI(4,5)P2 reporter recruitment. Occasionally, a very dim signal from the mutant sensor R40L could be seen in association with some clusters of CD16/7-Nck. Since clusters of Nck SH3 domains very often induce the formation of finger-like membrane protrusions, it is likely that such weak signals are due to the trapping of cytosolic R40L particles in those protrusive structures. Importantly, clustering of the CD16/7-Nck construct was necessary for the redistribution of the PI(4,5)P2 reporter (Fig. S4A and B). The number of clusters per cell was similar among cells co-expressing CD16/7 or CD16/7-Nck with the wild type PI(4,5)P2 reporter or CD16/7-Nck with the mutant R40L-GFP fusion (Fig. 3B, top panel). However, the percentage of clusters associated with GFP patches (Fig. 3B, middle panel), and the intensity of the GFP patches (Fig. 3B, bottom panel) was significantly higher (p<0.05) in cells expressing the wild type PI(4,5)P2 reporter and CD16/7-Nck vs. CD16/7, or cells expressing CD16/7-Nck with the mutant R40L-GFP fusion.
We also assessed the subcellular distribution of PI(4,5)P2, filamentous actin, and Nck SH3 aggregates in NIH3T3 cells expressing actin-GFP, the PH domain from PLCδ1 fused with mRFP (PH-mRFP), and the CD16/7-Nck construct. As shown in Fig. 3C (right panel) and movie S3, the tip (and in some cases part of the tail) of actin comets induced by clusters of membrane-targeted Nck SH3 domains (labeled with Alexa-fluor 647-IgG, A647) were decorated with patches enriched in PH-mRFP. As expected, the mutant R40L-mRFP, unable to bind PI(4,5)P2, did not colocalize with CD16/7-Nck clusters or decorate actin tails (Fig.3 C, left panel). Similar observations were made in cells co-expressing PH-GFP (but not the mutant R40L-GFP) that were fixed and stained with anti-HA and fluorescent phalloidin to detect CD16/7-Nck and F-actin, respectively (Fig. S4B). Taken together, these results strongly suggest that PI(4,5)P2 is enriched at sites of actin polymerization induced by high local concentration of Nck SH3 domains.
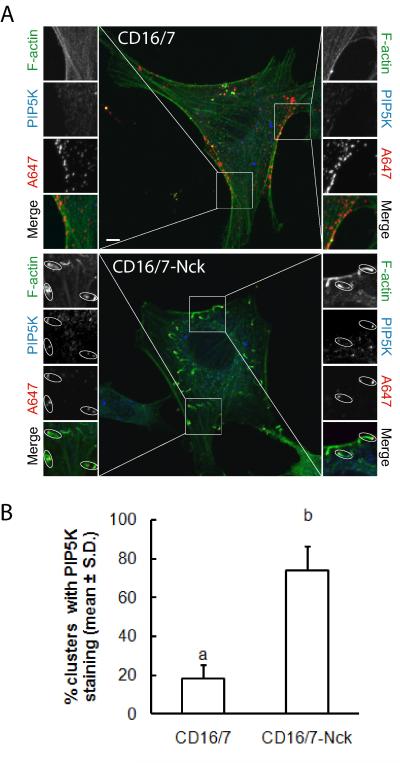
The enrichment of PI(4,5)P2 at sites of actin polymerization induced by clusters of membrane-targeted Nck SH3 domains suggested two potential mechanisms: increased localized synthesis or local sequestration of PI(4,5)P2.To gain insights into the potential mechanism leading to the enrichment of PI(4,5)P2 at clusters of Nck SH3 domains, we performed immunofluorescence staining of endogenous type I PIP5K in cells expressing CD16/7 or CD16/7-Nck subjected to the antibody-mediated clustering protocol. As shown in Fig. 4A, endogenous PIP5K colocalized to clusters of CD16/7-Nck but not to clusters of CD16/7. Of note, PIP5K immunostaining was exclusively observed at the tips, but not the tails, of actin comets induced by CD16/7-Nck clusters. A significantly higher percentage of CD16/7-Nck clusters had positive PIP5K staining compared to clusters of CD16/7 alone (~75% vs. ~18%; Fig. 4B). These results support the notion that the antibody-mediated clustering of Nck leads to the formation of membrane microdomains where increased localized synthesis of PI(4,5)P2 occurs.
Fig. 4.
Recruitment of phosphatidylinositol 5-kinase (PIP5K) to sites of actin polymerization induced by clustering of membrane-targeted Nck SH3 domains. A) NIH3T3 cells co-expressing actin-GFP and the fusion of CD16/7 with the three SH3 domains from Nck (CD16/7-Nck) or CD16/7 alone (CD16/7) were treated with clustering antibodies (A647) before fixation. Corresponding boxed areas are shown at higher magnification. The subcellular distribution of endogenous PIP5K was detected by immunofluorescence using a rabbit polyclonal anti-PIP5K antibody that recognizes type I PIP5K α, β, and γ (H-300, Santa Cruz) followed by a goat anti-rabbit A594-conjugated IgG. Note that PIP5K colocalized to clusters of Nck SH3 domains but not to the tails of actin comets. Scale bar represent 5 μm. B) Computer assisted, quantitative analysis of the colocalization of endogenous PIP5K with clusters of CD16/7 or CD16/7-Nck.
Decreased local concentration of PI(4,5)P2 attenuates Nck SH3 domain-induced actin polymerization
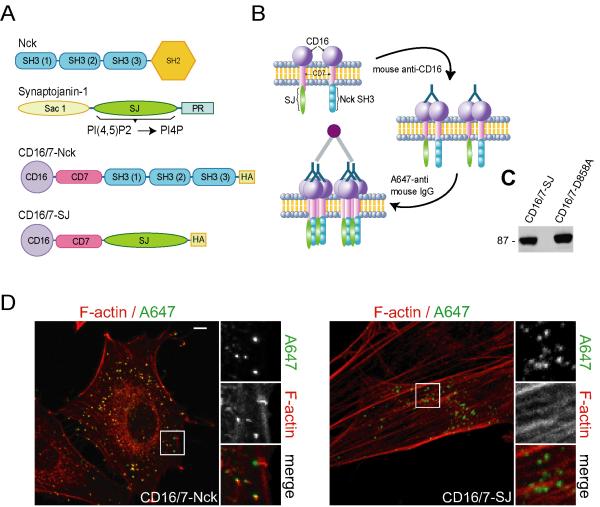
Based on findings showing enrichment of PI(4,5)P2 and PIP5K at sites of actin polymerization induced by aggregates of Nck SH3 domains, we reasoned that the manipulation of PI(4,5)P2 availability at these sites would modulate the extent of localized actin polymerization. We devised a strategy for the simultaneous, localized manipulation of PI(4,5)P2- and Nck-dependent signaling at the plasma membrane (Fig. 5A and B) consisting of co-clustering of a membrane-targeted inositol 5-phosphatase domain of synaptojanin-1, and the fusion of CD16/7 with all three Nck SH3 domains (CD16/7-Nck). The fusion proteins of CD16/7 with the wild type, or mutant (catalytically inactive) PI 5-phosphatase domain of synaptojanin-1(CD16/7-SJ and CD16/7-D858A, respectively) were expressed in NIH3T3 cells (Fig. 5C) and formed membrane clusters (Fig.5D) that were comparable to those of CD16/7-Nck.
Fig. 5.
Strategy for the simultaneous, localized manipulation of PI(4,5)P2- and Nck-dependent signaling at the plasma membrane. A) Diagrams representing the domain structure of Nck, the PI 5-phosphatase synaptojanin-1, and the fusion of CD16/7 with all three Nck SH3 domains (CD16/7-Nck), or the PI 5-phosphatase domain (CD16/7-SJ). The fusion proteins were tagged with the HA epitope. B) Diagram illustrating the antibody-mediated clustering utilized to simultaneously increase the local concentration of membrane-targeted Nck SH3 domains and decrease the local concentration of PI(4,5)P2. C) Expression of CD16/7-SJ (wild type) and CD16/7-D858A (catalytically inactive) in NIH3T3 cells demonstrated by western immunoblotting with anti-HA. D) Confocal images of NIH3T3 cells expressing CD16/7-Nck (CD16/7-Nck, left panel), or a fusion of CD16/7 with the wild type IP 5-phosphatase domain of synaptojanin-1 (CD16/7-SJ) following treatment with clustering antibodies (A647). Scale bar represents 5 μm.
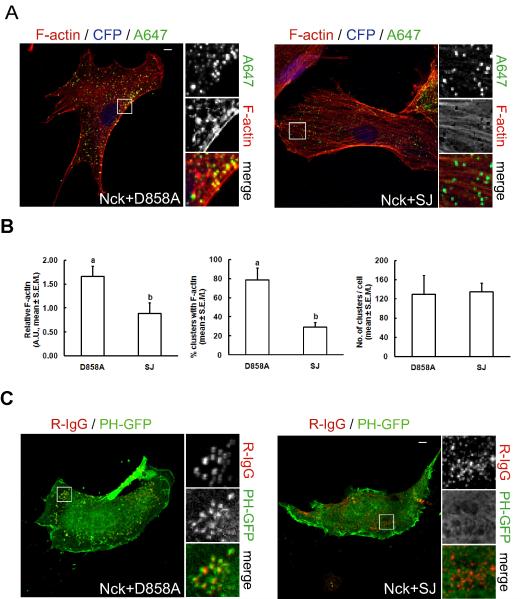
Co-aggregates of CD16/7-Nck and the mutant, catalytically inactive PI 5-phosphatase domain of synaptojanin-1 induced the formation of actin structures (comets and foci) similar to those induced by CD16/7-Nck alone (Fig. 6A, Nck+D858A, and movie S4). However, when CD16/7-Nck was co-clustered with the wild type PI 5-phosphatase domain of synaptojanin-1, localized actin polymerization was greatly decreased or completely absent (Fig.6A, Nck+SJ, and movie S5). The amount of F-actin associated with clusters (Fig. 6B, left panel) and the percentage of clusters with F-actin (Fig. 6B, middle panel) significantly decreased (p<0.05) in co-aggregates with wild type (SJ) vs. mutant (D858A) PI 5-phosphatase domain of synaptojanin-1. The number of clusters per cell, however, remained unchanged (Fig. 6B, right panel). In addition, we performed co-clustering experiments in cells expressing the PI(4,5)P2 biosensor PH-GFP (Fig. 6C). As expected, co-clustering of membrane-targeted Nck SH3 domains with wild type synaptojanin phosphatase domain (Nck+SJ), but not with the mutant (Nck+D858A), abolished the localized enrichment of PI(4,5)P2. These results suggest that locally increased concentrations of both PI(4,5)P2 and Nck, above a threshold level, cooperate in the activation of N-WASp-stimulated actin polymerization in living cells.
Fig. 6.
Decreased local concentration of PI(4,5)P2 attenuates Nck SH3 domain-induced actin polymerization in living cells. A) Co-aggregation (as illustrated in Fig. 4 B) of membrane-targeted Nck SH3 domains with the wild type PI 5-phosphatase domain of synaptojanin-1 (Nck+SJ), but not with its catalytic inactive mutant (Nck+D858A), attenuates localized actin polymerization. The CD16/7-SJ fusion was expressed in conjunction with CFP from a bicistronic transcript. Clusters of membrane-targeted fusions proteins and F-actin were labeled with Alexa Fluor 647-conjugated IgG (A647) and Texas-red phalloidin, respectively. Scale bar represents 5 μm. B) Computer-assisted, quantitative analysis of phenotypic changes induced by co-aggregation of CD16/7-Nck with membrane-targeted wild type (SJ) or catalytically inactive (D858A) IP 5-phosphatase domain from synaptojanin-1 (a vs. b, p<0.05). Values represent mean ± S.E.M. from images corresponding to 5–7 cells/treatment analyzed in each of three independent experiment (n=3). C) Confocal images of cells co-expressing the PI(4,5)P2 biosensor (PH-GFP), and the combination of CD16/7-Nck with CD16/7-SJ or CD16/7-D858A. Clustering of fusion proteins was performed as described in Fig. 4B. Scale bar represents 5 μm.
Modulation of Nck-induced actin polymerization by PI(4,5)P2-sensitive variants of N-WASp
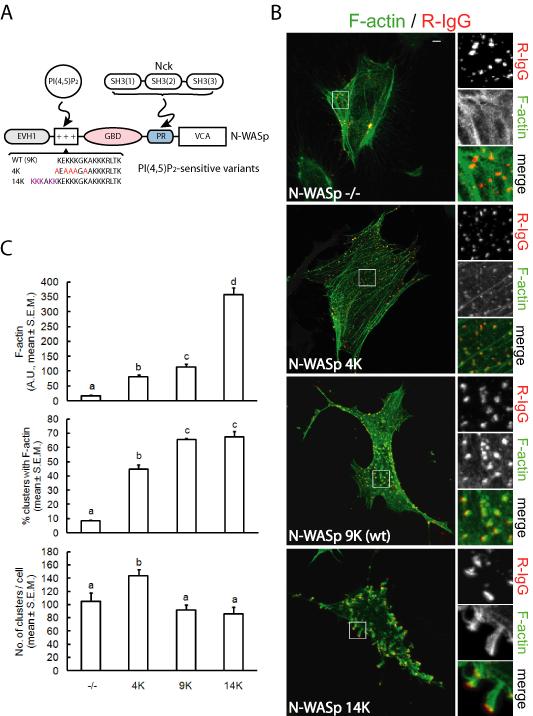
The polybasic motif of N-WASp is a short stretch of nine lysine residues adjacent (N-teminal) to the GTPase binding domain. The engagement of PI(4,5)P2 by the polybasic motif, and the simultaneous binding of GTP-loaded Cdc42 or SH3 domain-containing proteins, releases N-WASp from the autoinhibition by displacing the VCA region and making it available for activation of the Arp2/3 complex. It has been shown that the polybasic motif of N-WASp acts as a sensor of PI(4,5)P2 density and that its sensitivity can be modulated by varying the number of lysine residues (Papayannopoulos et al., 2005). We reasoned that localized actin polymerization in response to clustering of membrane-targeted Nck SH3 domains would be modulated by PI(4,5)P2-sensitive N-WASp variants (Fig. 7A). We performed clustering of membrane-targeted Nck SH3 domains in N-WASp-deficient cells (Snapper et al., 2001), and N-WASp-deficient cells rescued with wild type N-WASp (9K), a PI(4,5)P2 “hypersensitive” N-WASp (14K), or a PI(4,5)P2 “hyposensitive” N-WASp (4K). As previously shown (Rivera et al., 2004), no major actin rearrangements were observed following clustering of Nck SH3 domains in N-WASp-deficient cells (Fig.7B). Remarkably, the extent of localized actin polymerization increased with the length of the polybasic motif of the reconstituted N-WASp (Fig. 7B, and animations S3, S4, and S5). There was a significant (p<0.05) increase in the amount of F-actin associated with SH3 clusters such that 14K>>9K>4K (Fig. 7C, top panel). On the other hand, the percentage of clusters with F-actin did not differ (p>0.05) between cells rescued with 9K or 14K N-WASp variants, though it was significantly higher than in cells rescued with 4K N-WASp or N-WASp-deficient cells (Fig. 7C, middle panel). The total number of clusters per cell was slightly higher in cells expressing 4K N-WASp (Fig. 7C, bottom panel).
Fig. 7.
Modulation of Nck SH3 domain-stimulated F-actin assembly by PI(4,5)P2-sensitive variants of N-WASp. A) Diagram depicting interactions of PI(4,5)P2 and Nck SH3 domains, respectively, with the polybasic region (+++) and the proline-rich segment (PR) of N-WASp. Previously described PI(4,5)P2-sensitive N-WASp variants (Papayannopoulos et al., 2005), with varying number of lysine (K) residues in the polybasic motif, were utilized to rescued N-WASp-deficient mouse embryonic fibroblasts. B) Confocal images of N-WASp-deficient mouse embryonic fibroblasts co-transfected with actin-GFP, CD16/7-Nck, and an empty vector (N-WASp−/−), or N-WASp variants. Clusters of CD16/Nck, induced by aggregation with antibodies, were visualized with rhodamine-IgG (R-IgG). Scale bar represents 5 μm. C) Computer-assisted, quantitative analysis of phenotypic changes induced by aggregation of CD16/7-Nck in N-WASp-deficient cells (−/−),or N-WASp-deficient cells rescued with N-WASp variants. Different letters on bars indicate statistical differences (p<0.05, a,b,c,d). Values represent mean ± S.E.M. from images corresponding to 4–5 cells/treatment analyzed in each of three independent experiments (n=3).
Our data suggest that the polybasic motif of N-WASp plays a critical role in the mechanism of activation of localized actin polymerization induced by Nck SH3 domains in living cells. Interestingly, a previous study showed that a mutant N-WASp with a deletion of the basic region and GBD domain was able to restore PIP5K-induced actin tail formation with the same efficiency as wild type N-WASp (Benesch et al., 2002). The apparent discrepancy with results presented here is likely due to the requirement for the GBD domain for complete inhibition/repression of the VCA output domain of N-WASp (Kim et al., 2000; Prehoda et al., 2000), and to differences in the stimulus being rescued (PIP5K expression vs. Nck aggregation).
Discussion
The present study provides compelling evidence of the existence of a synergistic relationship between Nck and PI(4,5)P2 in promoting N-WASp-dependent actin rearrangements in living cells. For the first time we have shown that the Nck family SH2/SH3 adaptors are essential for the localized actin polymerization stimulated by high levels of PI(4,5)P2, and also that PI(4,5)P2 is required for localized actin polymerization induced by Nck aggregation. Quite unexpectedly, we found that elevation of cellular PI(4,5)P2 levels induces tyrosine phosphorylation of an Nck SH2 domain binding partner(s), and conversely that aggregation of Nck recruits PIP5K and leads to high local concentrations of PI(4,5)P2 at the tips of actin comets. In both cases, high local concentrations of one component induce actin polymerization in a manner that not only requires the other component, but also is able to recruit or generate high local concentrations of the second component. Such cooperative assembly with reciprocal positive feedback is ideally suited for generating localized domains with high N-WASp/Arp2/3 activity needed to promote highly localized actin assembly in the cell.
Our results strongly suggest that Nck links phosphotyrosine- and phosphoinositide-dependent signals converging on the N-WASp/Arp2/3 pathway of actin remodeling. Cooperation between signals mediated by tyrosine phosphorylation and inositol phospholipids likely represents an important, yet underestimated, regulatory mechanism in actin dynamics. Consistent with this notion, it was shown that endogenous membrane vesicles can induce highly motile actin comets when added to Xenopus egg extracts in the presence of inositol phospholipids and sodium orthovanadate, an inhibitor of protein tyrosine phosphatases (Ma et al., 1998). Similarly, treatment with PDGF or pervanadate was shown to increase PI(4,5)P2-stimulated formation of actin tails in fibroblasts (Rozelle et al., 2000). Mechanistic insights into the role of tyrosine phosphorylation were provided by in vitro studies implicating Src-dependent phosphorylation of cortactin in the formation of an activated signaling complex that also includes Nck, WIP and N-WASp (Tehrani et al., 2007).
Although previous investigations have documented a role for PI(4,5)P2 (Benesch et al., 2002; Rozelle et al., 2000) or Nck (Rivera et al., 2004; Rohatgi et al., 2001) in N-WASp/Arp2/3-dependent actin polymerization, a major conceptual advance arising from the present study is the demonstration of a close mutual interdependence between Nck and PI(4,5)P2 in stimulating N-WASp-mediated actin polymerization in living cells. It is particularly striking that, although a number of proteins involved in actin dynamics bind to and are regulated by PI(4,5)P2, we found that Nck adaptors are required for PIP5K-induced cytoskeletal changes. This indicates that Nck plays a surprisingly unique and central role in PI(4,5)P2-mediated actin rearrangements.
The formation of phosphotyrosine-containing actin comets may represent a physiological mechanism utilized by cells for the transport of membrane-bound organelles. For example, Way and colleagues observed the propulsion of Golgi-derived endosomes by spontaneously forming “little actin tails (LATs)” with phosphotyrosine enrichment at their tips in HeLa cells (Frischknecht et al., 1999a). On the other hand, the formation of actin comets stimulated by PI(4,5)P2 has been implicated in the polarized biosynthetic traffic in epithelial cells (Guerriero et al., 2006), and the internalization of macropinosomes forming from membrane ruffles (Orth et al., 2002). Furthermore, degradation of extracellular matrix by Src-transformed fibroblasts (Oikawa et al., 2008) or breast carcinoma cells (Yamaguchi et al., 2005), a process that involves the trafficking and targeted delivery of metalloproteinases, also requires Nck. The above observations, together with our findings demonstrating the requirement of Nck in the formation of actin comets induced by PI(4,5)P2, lend support to the hypothesis that Nck adaptors may play a central role at the crossroads of endocytic and exocytic pathways and/or the sorting of cargo proteins and lipids at the trans-Golgi network during development and disease. The present study is likely to stimulate new hypotheses and prompt new investigations on the regulation of critical processes such as intracellular trafficking and cell locomotion that rely on localized actin polymerization.
Our conclusion that Nck plays a pivotal role in linking phosphotyrosine- and PI(4,5)P2-dependent signals that converge on the N-WASp pathway of actin remodeling is based on the following observations: i) colocalization of tyrosine phosphorylation and Nck at the tips of actin comets induced by PI(4,5)P2, ii) failure of PIP5K overexpression to induce actin comets in cells expressing R308K Nck, a dominant negative mutant with inactivation of the SH2 domain, and iii) the dramatic increase in tyrosine phosphorylation of a ~190 kDa Nck SH2 binding protein in cells with elevated PI(4,5)P2 levels. The latter observation is one of the most surprising and intriguing results from our studies. We assume that this phosphoprotein is localized to the tips of rocketing vesicles, as we have shown that both tyrosine phosphorylated proteins and Nck are concentrated at those sites, and that the 190 kDa protein is a major Nck SH2 binding protein in these cells (Fig. 2). Our findings pose a number of important questions for future investigations: What is the identity and the role of the ~190 kDa Nck SH2 binding partner(s)? What are exactly the mechanisms underlying the PI(4,5)P2-induced increase in tyrosine phosphorylation of Nck SH2 binding partners? Are protein tyrosine kinases activated or protein tyrosine phosphatases inhibited by high cellular levels of PI(4,5)P2? What is the significance of the crosstalk between phosphotyrosine- and phosphoinositide-mediated signaling in development and disease? We plan to address these important questions through a combination of experimentation and computational modeling.
Using an in vitro reconstitution system, we previously demonstrated synergistic activation of N-WASp/Arp2/3-dependent actin polymerization by Nck SH3 domains and PI(4,5)P2 (Rohatgi et al., 2001). In addition, we showed that an increased local concentration of Nck SH3 domains can trigger N-WASp-dependent actin polymerization in living cells (Rivera et al., 2004). The present study provides several lines of evidence supporting the existence of a mechanism of cooperation between Nck SH3 domains and PI(4,5)P2 in living cells: i) the specific recruitment of PIP5K and consequent enrichment of PI(4,5)P2 at sites of actin polymerization induced by clustering of membrane-targeted Nck SH3 domains, ii) significant attenuation of Nck SH3 domain-induced actin polymerization by decreasing the local concentration of PI(4,5)P2, and iii) modulation of Nck-induced actin polymerization by PI(4,5)P2-sensitive variants of N-WASp.
Our results show that the ability of Nck aggregates to generate domains with high local concentration of PI(4,5)P2 is crucial for activation of N-WASp. Two mechanisms, not mutually exclusive, could account for the high PI(4,5)P2 levels at sites of Nck aggregation. First, the recruitment of PIP5K by Nck or Nck-associated proteins as suggested by our results showing that Nck aggregates are associated with high local concentrations of PIP5K(s) (Fig. 4). Consistent with this notion, different PIP5Ks have been shown to be specifically recruited to different areas of the cell, such as membrane ruffles and focal adhesions, via protein-protein interactions (Heck et al., 2007). Second, Nck-associated proteins, in particular N-WASp itself, are likely to be able to concentrate PI(4,5)P2 through interactions with its basic PI(4,5)P2 binding domain. It has been shown that cells utilize raft-associated proteins such as myristoylated alanine-rich protein kinase C substrate (MARCKS), that possess a cluster of basic residues (the “basic effector domain”), to sequester PI(4,5)P2 (Laux et al., 2000). The polybasic motif of N-WASp is a highly sensitive sensor of PI(4,5)P2 density (Papayannopoulos et al., 2005) that may play, in principle, a similar role to the basic effector domain of MARCKS.
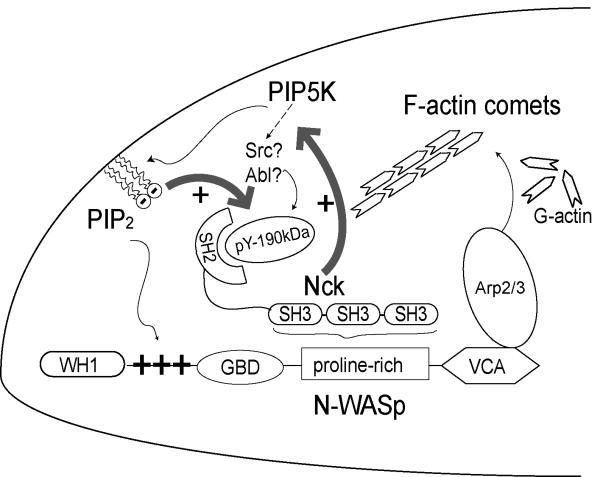
The recently proposed model of “hierarchical” regulation of N-WASp implicates dimerization/oligomerization as an additional level of regulation superimposed on the canonical allosteric relief of autoinhibition (Padrick et al., 2008). Indeed, “dimerizing” ligands such as multiple Nck SH3 domains and high density of PI(4,5)P2 resulted in hyperactivation of N-WASp (Padrick et al., 2008). Therefore, the spatio-temporal convergence of clusters of Nck and PI(4,5)P2 at the plasma membrane may account for the synergistic activation of N-WASp-dependent actin polymerization reported in the present study. Our results favor a model such that aggregates of Nck SH3 domains recruit PIP5K and N-WASp to the plasma membrane, leading to concomitant local enrichment of PI(4,5)P2 (Fig. 8).
Fig. 8.
A Model for the reciprocal interdependence between Nck and PI(4,5)P2 in the generation of localized domains with high N-WASp/Arp2/3 activity. A double positive feedback loop (thick grey arrows) between Nck and PI(4,5)P2 involves the PI(4,5)P2-dependent increase in tyrosine phosphorylation of a ~190kDa Nck SH2 binding partner. High local concentrations of Nck, in turn, induce high focal density of PI(4,5)P2 due to increased synthesis resulting from the Nck-dependent recruitment of PIP5Ks. This mechanism is likely to be reinforced by the clustering of PI(4,5)P2 by the poybasic motif of N-WASp, which is locally enriched by interactions with Nck SH3 domains. N-WASp/Arp2/3-dependent actin dynamics, including the formation of F-actin comets, may play an important role in the trafficking of Golgi-derived vesicles and other membrane-bound organelles. Increased tyrosine phosphorylation of the Nck SH2 binding protein may arise from the selective recruitment/activation of tyrosine kinases or the inactivation/exclusion of a tyrosine phosphatases. We propose that the ~ 190 kDa Nck SH2 binding partner is a critical physical link in the mechanism coordinating phosphotyrosine to PI(4,5)P2 in actin remodeling.
In conclusion, the present study unveils a unique role of the Nck family adaptors in PI(4,5)P2-mediated actin polymerization, and shows that Nck clustering leads to high local concentration of PI(4,5)P2 through a mechanism that involves, at least in part, the recruitment of PIP5K(s). High levels of PI(4,5)P2, in turn, recruit Nck by promoting the tyrosine phosphorylation of Nck SH2 binding sites. The close interdependence between Nck and PI(4,5)P2 in stimulating N-WASp-mediated actin polymerization shown in this study may prove significant in the regulation of membrane trafficking, cell polarity and locomotion.
Experimental Procedures
Cell culture, transfections and viral infections
HEK293T, Nck- (Bladt et al., 2003) and N-WASp-deficient (Snapper et al., 2001) mouse embryonic fibroblasts were cultured in DMEM supplemented with antibiotics and 10% fetal bovine serum. NIH-3T3 cells were cultured in DMEM supplemented with antibiotics and 10% calf serum. Transient transfections were carried out by calcium phosphate precipitation (293T cells) or using the Lipofectamine® reagent (NIH-3T3 cells and mouse embryonic fibroblasts). For viral production, HEK293T cells were transfected with retroviral vector plus packaging plasmids pMD.env and pMD.gag.pol by calcium phosphate precipitation and medium containing virus was harvested within 48 hs of transfection. NIH3T3 were infected with virus in the presence of 2 μg/ml polybrene (Millipore). Cells infected with pMSCV-puro- or p.Super.retro-derived virus were drug selected with 1.0 μg/ml puromycin (Sigma) for 7 days and kept for further experiments for up to 5 weeks.
Microscopy and image analysis
Fluorescent images were collected on a Zeiss LSM 510 confocal microscope with a 63x NA 1.25 Plan-NEOFLUAR oil-immersion objective. Specimens were illuminated with an argon laser with emission at 488 nm and two HeNe lasers with emissions at 543 or 633 nm, respectively. For visualization of actin dynamics in living cells, Nck-deficient cells cotransfected with actin-GFP and EBB, or EBB harboring full-length Nck1 were cultured in Bioptechs Delta T dishes and maintained at 37°C with a stage/objective heater system (Bioptechs). Live images were obtained using a PerkinElmer UltraView spinning microlens confocal system mounted on a Nikon TE2000 inverted microscope equipped with an ORCA-ER Firewire CCD camera and a 60 × 1.4 NA oil-immersion objective.
Quantitative image analysis to identify actin comets and foci (Figs. 3B, 5B, and 6C) was performed with an algorithm previously described (Sallee et al., 2008). The percentage of antibody clusters that have associated GFP/actin clusters and the intensities of these associated GFP/actin clusters were calculated. The relative intensity value is defined as the ratio of the sum of intensities of all associated GFP/actin clusters to the sum of intensities of all antibody clusters. A modified version of the algorithm (see supplemental experimental procedures) was developed to perform quantitative image analysis utilizing a single channel of information (Figs. 1E and S1). Mean number of particles per cell and intensity (F-actin) were calculated for each particle type. Variances among treatments were compared by F-test followed by Scheffe’s multiple comparison procedure.
Pull-down assay, immunoprecipitation, western immunoblotting, and immunofluorescence analysis were performed as previously described (Rivera et al., 2004) and further detailed in supplemental procedures.
Supplementary Material
Acknowledgements
This work was supported by grants CA82258 and RR022232 (B.J.M.) and GM062583 (W.A.L.) from the National Institute of Health. G.M.R. was partly supported by a Scientist Development Grant from the American Heart Association (0735252N). We thank Dr. Ann Cowan for critically reading this manuscript. Vectors containing wild type and mutant synaptojanin-1 were a generous gift from Dr. Pietro De Camilli. Nck-deficient mouse embryonic fibroblasts were kindly provided by Dr. Tony Pawson. N-WASp-deficient cells were a gift of Dr. Scott Snapper. The PI(4,5)P2 biosensor (PHPLCδ1-GFP) was kindly provided by Dr. Leslie Loew. The PIP5K clone was a gift from Dr. C.L. Carpenter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balla T, Varnai P. Visualizing cellular phosphoinositide pools with GFP-fused protein-modules. Sci STKE. 2002;2002:L3. doi: 10.1126/stke.2002.125.pl3. [DOI] [PubMed] [Google Scholar]
- Benesch S, Lommel S, Steffen A, Stradal TE, Scaplehorn N, Way M, Wehland J, Rottner K. Phosphatidylinositol 4,5-biphosphate (PIP2)-induced vesicle movement depends on N-WASP and involves Nck, WIP, and Grb2. J Biol Chem. 2002;277:37771–37776. doi: 10.1074/jbc.M204145200. [DOI] [PubMed] [Google Scholar]
- Bladt F, Aippersbach E, Gelkop S, Strasser GA, Nash P, Tafuri A, Gertler FB, Pawson T. The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol Cell Biol. 2003;23:4586–4597. doi: 10.1128/MCB.23.13.4586-4597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, Rankin S, Pawson T, Kirschner MW, Tipper DJ, Leong JM. Clustering of Nck by a 12-residue Tir phosphopeptide is sufficient to trigger localized actin assembly. J Cell Biol. 2004;164:407–416. doi: 10.1083/jcb.200306032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Frischknecht F, Cudmore S, Moreau V, Reckmann I, Rottger S, Way M. Tyrosine phosphorylation is required for actin-based motility of vaccinia but not Listeria or Shigella. Curr Biol. 1999a;9:89–92. doi: 10.1016/s0960-9822(99)80020-7. [DOI] [PubMed] [Google Scholar]
- Frischknecht F, Moreau V, Rottger S, Gonfloni S, Reckmann I, Superti-Furga G, Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999b;401:926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- Gruenheid S, DeVinney R, Bladt F, Goosney D, Gelkop S, Gish GD, Pawson T, Finlay BB. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat Cell Biol. 2001;3:856–859. doi: 10.1038/ncb0901-856. [DOI] [PubMed] [Google Scholar]
- Guerriero CJ, Weixel KM, Bruns JR, Weisz OA. Phosphatidylinositol 5-kinase stimulates apical biosynthetic delivery via an Arp2/3-dependent mechanism. J Biol Chem. 2006;281:15376–15384. doi: 10.1074/jbc.M601239200. [DOI] [PubMed] [Google Scholar]
- Heck JN, Mellman DL, Ling K, Sun Y, Wagoner MP, Schill NJ, Anderson RA. A conspicuous connection: structure defines function for the phosphatidylinositol-phosphate kinase family. Crit Rev Biochem Mol Biol. 2007;42:15–39. doi: 10.1080/10409230601162752. [DOI] [PubMed] [Google Scholar]
- Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J Cell Biol. 2000;149:1455–1472. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Fan J, Woodley DT. Nck/Dock: an adapter between cell surface receptors and the actin cytoskeleton. Oncogene. 2001;20:6403–6417. doi: 10.1038/sj.onc.1204782. [DOI] [PubMed] [Google Scholar]
- Ma L, Cantley LC, Janmey PA, Kirschner MW. Corequirement of specific phosphoinositides and small GTP-binding protein Cdc42 in inducing actin assembly in Xenopus egg extracts. J Cell Biol. 1998;140:1125–1136. doi: 10.1083/jcb.140.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau V, Frischknecht F, Reckmann I, Vincentelli R, Rabut G, Stewart D, Way M. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat Cell Biol. 2000;2:441–448. doi: 10.1038/35017080. [DOI] [PubMed] [Google Scholar]
- Oikawa T, Itoh T, Takenawa T. Sequential signals toward podosome formation in NIH-src cells. J Cell Biol. 2008;182:157–169. doi: 10.1083/jcb.200801042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JD, Krueger EW, Cao H, McNiven MA. The large GTPase dynamin regulates actin comet formation and movement in living cells. PNAS. 2002;99:167–172. doi: 10.1073/pnas.012607899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrick SB, Cheng HC, Ismail AM, Panchal SC, Doolittle LK, Kim S, Skehan BM, Umetani J, Brautigam CA, Leong JM, Rosen MK. Hierarchical regulation of WASP/WAVE proteins. Mol Cell. 2008;32:426–438. doi: 10.1016/j.molcel.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V, Co C, Prehoda KE, Snapper S, Taunton J, Lim WA. A Polybasic Motif Allows N-WASP to Act as a Sensor of PIP(2) Density. Mol Cell. 2005;17:181–191. doi: 10.1016/j.molcel.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Pawson T. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell. 2004;116:191–203. doi: 10.1016/s0092-8674(03)01077-8. [DOI] [PubMed] [Google Scholar]
- Prehoda KE, Scott JA, Mullins RD, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- Rao Y. Dissecting Nck/Dock Signaling Pathways in Drosophila Visual System. Int J Biol Sci. 2005;1:80–86. doi: 10.7150/ijbs.1.80. Epub 2005 Apr 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera GM, Briceno CA, Takeshima F, Snapper SB, Mayer BJ. Inducible clustering of membrane-targeted SH3 domains of the adaptor protein Nck triggers localized actin polymerization. Curr Biol. 2004;14:11–22. doi: 10.1016/j.cub.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem. 2001;276:26448–26452. doi: 10.1074/jbc.M103856200. [DOI] [PubMed] [Google Scholar]
- Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, Luby-Phelps K, Marriott G, Hall A, Yin HL. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol. 2000;10:311–320. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- Sallee NA, Rivera GM, Dueber JE, Vasilescu D, Mullins RD, Mayer BJ, Lim WA. The pathogen protein EspF(U) hijacks actin polymerization using mimicry and multivalency. Nature. 2008;454:1005–1008. doi: 10.1038/nature07170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper SB, Takeshima F, Anton I, Liu CH, Thomas SM, Nguyen D, Dudley D, Fraser H, Purich D, Lopez-Ilasaca M, et al. N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat Cell Biol. 2001;3:897–904. doi: 10.1038/ncb1001-897. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. PNAS. 2007;104:11933–11938. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.