Abstract
Background
The protease inhibitors lopinavir and atazanavir are both recommended for treatment of HIV-infected patients. Considerable inter-individual variability in plasma concentration has been observed for both drugs. The aim of this study was to evaluate which demographic factors and concomitant drugs are associated with lopinavir and atazanavir plasma concentration.
Methods
Data from the Liverpool TDM (therapeutic drug monitoring) Registry were linked with the UK Collaborative HIV Cohort (CHIC) study. For each patient, the first measurement of lopinavir (twice daily) or atazanavir [once daily, ritonavir boosted (/r) or unboosted] plasma concentration was included. Linear regression was used to evaluate the association of dose, gender, age, weight, ethnicity and concomitant antiretroviral drugs or rifabutin with log-transformed drug concentration, adjusted for time since last intake.
Results
Data from 439 patients on lopinavir (69% 400 mg/r, 31% 533 mg/r; 3% concomitant rifabutin) and 313 on atazanavir (60% 300 mg/r, 32% 400 mg/r, 8% 400 mg) were included. Multivariable models revealed the following predictors for lopinavir concentration: weight (11% decrease per additional 10 kg; P = 0.001); dose (25% increase for 533 mg/r; P = 0.024); and rifabutin (116% increase; P < 0.001). For atazanavir the predictors were dose (compared with 300 mg/r: 40% increase for 400 mg/r, 67% decrease for 400 mg; overall P < 0.001) and efavirenz (32% decrease; P = 0.016) but not tenofovir (P = 0.54).
Conclusions
This analysis confirms that efavirenz decreases atazanavir concentrations, and there was a negative association of weight and lopinavir concentrations. The strong impact of rifabutin on lopinavir concentration should be studied further.
Keywords: pharmacokinetics, rifabutin, drug interactions
Introduction
The protease inhibitors (PIs) lopinavir and atazanavir are both recommended for treatment of HIV-infected patients.1–3 A recent trial suggests that both drugs have similar clinical efficacy in treatment-naive patients.3
Considerable inter-individual variability has been observed in plasma concentrations of lopinavir and atazanavir after standard dosing.4,5 A number of factors have been shown to influence plasma exposure including body weight6 and concomitant medications such as non-nucleoside reverse transcriptase inhibitors (NNRTIs),4,7,8 although the effect was not consistent across all studies. For example, conflicting results have been reported regarding a negative impact of concomitant tenofovir on atazanavir concentrations that has been seen in some9,10 but not all studies.8,11,12 Because lopinavir and atazanavir are metabolized almost exclusively by the cytochrome P450 CYP3A isoform, drugs that induce CYP3A may decrease their plasma concentrations and reduce the therapeutic effect, whereas co-administration of drugs that inhibit CYP3A (over and above inhibition by ritonavir) may increase plasma concentrations of lopinavir or atazanavir with the potential of side effects.
The pharmacokinetic profile of both PIs is improved by concomitant administration of ritonavir, which is co-formulated into one drug with lopinavir and also routinely given along with atazanavir. At present, the standard regimens are 400 mg of lopinavir plus 100 mg of ritonavir twice daily, and 300 mg of atazanavir once daily plus 100 mg of ritonavir. To compensate for interactions between antiretroviral drugs, increased PI doses are recommended in some cases, e.g. when given with efavirenz or nevirapine.13,14
Rifampicin is a standard part of multiple drug regimens for the treatment of tuberculosis (TB) in HIV-infected patients, but substantial drug interactions limit its use in antiretroviral regimens containing PIs. In these cases, rifabutin is regarded a reasonable alternative because it is considered a much weaker inducer of CYP3A4 than rifampicin and consequently less prone to drug interactions. However, data on its effect on lopinavir or atazanavir are limited.
In the present study, we used data from the Liverpool HIV TDM (therapeutic drug monitoring) Registry to evaluate the association of plasma exposure of lopinavir and atazanavir with demographic and concomitant antiretroviral and TB drugs among participants in the UK Collaborative HIV Cohort (UK CHIC) study.
Patients and methods
Study cohort and participants
The Liverpool TDM Registry contains data on ∼18 000 samples from HIV-positive patients in whom TDM was requested between 1999 and 2006. For each sample, details of age, gender, weight and medication history (including dose, dosing regimen, time between sampling and last ingestion of HIV medication, concomitant medications) and reason for asking for drug monitoring were routinely requested.
The UK CHIC study is a collaboration of some of the largest centres for the care of HIV-infected individuals in the UK.15 The criteria for inclusion of an individual in the UK CHIC study were that a person was HIV positive, aged over 16 years and had attended one of the collaborating centres for care at any time after 1 January 1996. The data set used for the present analysis contains information on 25 274 patients seen for care at 10 centres (see the Acknowledgements section). Each centre provided electronic data in a standardized format on demographic characteristics, AIDS diagnoses and mortality, laboratory data (CD4 or CD8 counts, viral loads, markers of drug toxicity) and antiretroviral treatment (ART). Both the UK CHIC study and the Liverpool TDM Registry have received ethics approval from Multiregional Research Ethics Committees.
For this cross-sectional study, Liverpool TDM Registry records were linked to demographic (ethnicity) and clinical data (antiretroviral drugs) from UK CHIC. All records were pseudonymized. Linkage was successfully achieved for >90% records from the TDM Registry. The current analysis is based on the first TDM measurement of lopinavir or atazanavir per patient and includes samples up to the end of 2005. Of note, this pre-dates the introduction of the newer tablet formulation of lopinavir/ritonavir. The following inclusion criteria were applied: (i) sample >4 h after drug intake (to reduce absorption-related variation of drug serum concentrations); (ii) patient aged ≥18 years; (iii) white or black African ethnicity (there were small numbers in other categories); and (iv) the following regimens: lopinavir/ritonavir (twice daily 400/100 mg or 533/133 mg); or atazanavir [once daily 300 or 400 mg boosted with 100 mg of ritonavir (/r), or once-daily 400 mg unboosted]. Samples with undetectable drug concentration (potential poor adherence) were not considered.
Laboratory measurements
Plasma atazanavir and lopinavir concentrations were measured by validated HPLC–mass spectrometry as previously described.16 The lower limit of quantification was taken as the lowest point on the standard curve and was 47 ng/mL for atazanavir and 95 ng/mL for lopinavir. The lower limit of detection for each analyte was considerably lower. Inter- and intra-assay coefficients of variation, stability and recovery were as reported. The laboratory participates in an external quality assurance programme (KKGT, the Netherlands).
Statistical analysis
We examined the effect of the following factors, which may influence the plasma concentration of lopinavir/atazanavir, using multivariable linear regression: sex; age; ethnicity (white, black); weight; lopinavir/atazanavir dose; time since last lopinavir/atazanavir intake; time since initiating treatment with lopinavir/atazanavir; time on current ART regimen; concomitant rifabutin; and concomitant antiretroviral drugs (if given in at least 5% of the patients). Ritonavir plasma concentration was measured only in 1% and 0.3% of the patients in the lopinavir and in the atazanavir data set, respectively, and, therefore, it could not be analysed as a possible influence factor (none of these patients was on rifabutin). As weight was missing in 7% (lopinavir) and 11% (atazanavir) of patients, it was imputed using multiple imputation methods.17 Briefly, weight was first predicted 10 times from the same factors plus PI plasma concentration in linear regression models. The analysis of the plasma concentration of lopinavir/atazanavir was then carried out on each of the imputed data sets, and the multiple analyses were finally combined to yield a single set of results, which were adjusted for variability between the results of each imputation. Dose was not available for concomitant antiretroviral drugs so in all models they were classified as given versus not given. Year of measurement and centre showed no significant association with lopinavir/atazanavir plasma concentration and had only a minor effect on other predictors and, therefore, were excluded from the final model. Data on hepatitis B and C co-infection as well as alanine transaminase (ALT) values were only available for 64%–74% of patients (these measurements have only been performed routinely in relatively recent years) and, therefore, were considered in sensitivity analyses only. The indication for drug monitoring was ignored as a possible predictor because this information was often missing on the request form and was generally regarded as unreliable. For all models, drug levels were log transformed to improve the approximation to normality. For continuous variables, the presence of a non-linear association with drug concentration (e.g. for time since last lopinavir/atazanavir intake) was examined using fractional polynomial regression18 and considered appropriately in the model if necessary.
Although definitive data are lacking, a minimum effective concentration has been proposed for lopinavir of 1000 ng/mL, and for atazanavir of 150 ng/mL.3,19 Several studies have reported an association between atazanavir and bilirubin plasma concentrations,20–22 and patients who had atazanavir plasma concentrations >850 ng/mL were significantly more likely to develop hyperbilirubinaemia.23 For lopinavir, no toxicity cut-off has been defined. However, in treatment-experienced patients, a trough concentration of >4000 ng/mL was associated with improved virological response.24 Based on the statistical information from the multivariable models, we estimated the probability of having a PI trough concentration below the recommended therapeutic range for lopinavir (<1000 ng/mL or <4000 ng/mL) and outside the recommended range for atazanavir (<150 ng/mL or >850 ng/mL) for various scenarios (‘hypothetical patients’).
Results
Lopinavir
Lopinavir concentrations were assessed in 439 patients fulfilling the inclusion criteria (Table 1). The median time from last intake was 12 h [interquartile range (IQR): 12–13 h]. The current prescribing information for lopinavir recommends a dose increase when co-administered with NNRTIs,14 which was reflected in our patient group; the higher dose of 533 mg twice daily was used in 86 (72%) of patients receiving an NNRTI compared with 51 (16%) of patients not on an NNRTI. Rifabutin was co-administered in 15 (3%) patients, of whom 13 received 400 mg of lopinavir twice daily. The rifabutin regimen was 150 mg three times a week in nine patients, 150 mg once daily in two patients and was not known in four patients.
Table 1.
Patient characteristics
| Lopinavir group (n = 439) | Atazanavir group (n = 313) | |
|---|---|---|
| Age (years) | 40.5 (35.2–45.7) | 41.8 (37.0–46.9) |
| Gender | ||
| female | 95 (22%) | 54 (17%) |
| male | 344 (78%) | 259 (83%) |
| Ethnicity | ||
| black | 141 (32%) | 75 (24%) |
| white | 298 (68%) | 238 (76%) |
| Weight (kg) | 71.8 (64.5–80.5) | 72.7 (65.0–82.8) |
| Time from last PIa intake (h) | 12 (12–13) | 19 (13–24) |
| Time on PIa (weeks) | 39 (12–99) | 16 (4–36) |
| Time on current regimen (weeks) | 20 (7–49) | 11 (3–28) |
| Dosing schedule | ||
| once daily 400 mg | 0 | 25 (8%) |
| once daily 300 mg + ritonavir | 0 | 187 (60%) |
| once daily 400 mg + ritonavir | 0 | 101 (32%) |
| twice daily 400 mg + ritonavir | 302 (69%) | 0 |
| twice daily 533 mg + ritonavir | 137 (31%) | 0 |
| PIa plasma concentration (ng/mL) | 5358 (3116–8133) | 919 (534–1968) |
| Number of previous PIs before start of PIa | 2 (0–3) | 2 (0–3) |
| PI-naive before start of PIa | 142 (32%) | 89 (28%) |
| Concomitant antiretroviral drugsb | ||
| NRTIs | ||
| zidovudine | 88 (20%) | 40 (13%) |
| lamivudine | 228 (52%) | 114 (36%) |
| stavudine | 40 (9%) | 15 (5%) |
| tenofovir | 212 (48%) | 208 (66%) |
| didanosine | 123 (28%) | 100 (32%) |
| abacavir | 90 (21%) | 91 (29%) |
| emtricitabine | 11 (3%) | 40 (13%) |
| NNRTIs | ||
| nevirapine | 51 (12%) | 23 (7%) |
| efavirenz | 74 (17%) | 30 (10%) |
| PIs | ||
| lopinavir | 439 (100%) | 17 (5%) |
| atazanavir | 7 (2%) | 313 (100%) |
| saquinavir | 67 (15%) | 22 (7%) |
| fosamprenavir | 26 (6%) | 2 (1%) |
| Concomitant TB drug | ||
| no | 424 (97%) | 312 (99.7%) |
| rifabutin | 15 (3%) | 1 (0.3%) |
| rifampicin | 0 | 0 |
PI, protease inhibitor, NRTIs, nucleoside reverse transcriptase inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; TB, tuberculosis.
Values are number (%) or median (IQR).
aLopinavir in the lopinavir group; atazanavir in the atazanavir group.
bOnly drugs given in at least 5% of patients in one of the two groups.
Factors potentially influencing lopinavir serum concentration are listed in Table 2. Because of strong inter-relationships between various patient characteristics, results from the multivariable models are more interpretable than the unadjusted values from the univariable models. Results from multivariable models without and with consideration of concomitant antiviral drugs were very similar (Table 2; multivariable 1 and multivariable 2). Including all potential predictors, lopinavir plasma concentration was significantly influenced by weight (11% lower per additional 10 kg, P < 0.001), lopinavir dose (25% higher in 533 mg regimen, P = 0.024) and concomitant use of rifabutin (116% higher, P < 0.001). The strong effect of rifabutin was also present when patients on 150 mg of rifabutin once daily were excluded (+83%; P = 0.009), and the effect of rifabutin was neither statistically different in patients with a low weight compared with patients with a high weight nor associated with raised ALT (not shown). There was no demonstrable effect of NNRTIs (including no statistical interaction with lopinavir dose, i.e. patients who received NNRTIs and were on the standard lopinavir dose did not have a lopinavir concentration different from that of patients on NNRTIs and on the increased lopinavir dose) or any of the other antiretroviral drugs. Fractional polynomial models supported a linear effect of weight, with no evidence of a threshold effect.
Table 2.
Factors influencing lopinavir plasma concentration
| Change in drug concentration (% change in ng/mL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| univariable |
multivariable 1 |
multivariable 2 |
|||||||
| Parameter | effect | 95% CI | P | effect | 95% CI | P | effect | 95% CI | P |
| Age (per 10 years) | 2.8 | −6.1 to 12.5 | 0.55 | 2.0 | −6.8 to 11.6 | 0.67 | 3.4 | −5.7 to 13.3 | 0.48 |
| Gender (female versus male) | 17.0 | −3.0 to 41.2 | 0.10 | 15.9 | −8.1 to 46.1 | 0.21 | 19.0 | −6.4 to 51.2 | 0.16 |
| Ethnicity (black versus white) | 0.8 | −14.6 to 19.0 | 0.93 | −10.7 | −26.8 to 9.1 | 0.27 | −12.2 | −28.6 to 8.0 | 0.22 |
| Weight (per 10 kg) | −13.2 | −18.3 to −7.8 | <0.001 | −10.7 | −16.1 to −5.0 | <0.001 | −10.5 | −15.9 to −4.7 | 0.001 |
| Time on LPV (per 4 weeks) | 0.1 | −0.4 to 0.6 | 0.74 | −0.1 | −0.6 to 0.5 | 0.85 | −0.1 | −0.7 to 0.5 | 0.76 |
| Time on current regimen (per 4 weeks) | 0.2 | −0.6 to 0.9 | 0.62 | 0.4 | −0.5 to 1.3 | 0.38 | 0.5 | −0.4 to 1.5 | 0.26 |
| LPV dose (533 versus 400 mg/r) | 17.0 | −0.9 to 38.3 | 0.06 | 22.2 | 4.2 to 43.4 | 0.014 | 24.8 | 2.9 to 51.3 | 0.024 |
| On rifabutin (yes versus no) | 119.3 | 44.1 to 233.8 | <0.001 | 108.6 | 37.2 to 217.2 | 0.001 | 116.0 | 41.0 to 231.1 | <0.001 |
| On zidovudine (yes versus no) | 9.8 | −9.5 to 33.2 | 0.34 | 6.1 | −15.2 to 32.8 | 0.60 | |||
| On lamivudine (yes versus no) | 5.2 | −9.9 to 22.8 | 0.52 | 13.4 | −4.7 to 35.0 | 0.16 | |||
| On stavudine (yes versus no) | −8.2 | −29.9 to 20.2 | 0.53 | 0.8 | −23.5 to 32.7 | 0.96 | |||
| On tenofovir DF (yes versus no) | −4.8 | −18.5 to 11.1 | 0.53 | 8.3 | −8.7 to 28.3 | 0.36 | |||
| On didanosine (yes versus no) | 3.8 | −12.7 to 23.3 | 0.67 | 7.9 | −10.0 to 29.4 | 0.41 | |||
| On abacavir (yes versus no) | −6.6 | −22.9 to 13.2 | 0.49 | −11.0 | −26.3 to 7.5 | 0.22 | |||
| On efavirenz (yes versus no) | −2.3 | −20.5 to 20.2 | 0.83 | −10.5 | −28.5 to 11.9 | 0.33 | |||
| On nevirapine (yes versus no) | 22.7 | −3.6 to 56.1 | 0.10 | 8.0 | −17.0 to 40.5 | 0.57 | |||
| On saquinavir (yes versus no) | 2.3 | −17.5 to 26.9 | 0.84 | 13.8 | −8.9 to 42.2 | 0.25 | |||
| On fosamprenavir (yes versus no) | 7.0 | −22.9 to 48.6 | 0.68 | 19.2 | −14.9 to 66.8 | 0.31 | |||
LPV, lopinavir; /r, ritonavir boosted; DF, disoproxil fumarate.
Multivariable model 1: concomitant antiretroviral drugs not considered.
Multivariable model 2: considering concomitant antiretroviral drugs.
Both multivariable models are adjusted for hours since last lopinavir intake.
All results with P < 0.05 are shown in bold.
Neither in univariable nor in multivariable models did we find an association between liver variables (hepatitis B surface antigen, hepatitis C antibody, raised ALT) and lopinavir concentration (data not shown).
The probability of having a lopinavir trough concentration <4000 ng/mL increased with higher body weight and was 30% at 50 kg but 45% at 80 kg. Of interest, the probability of having a lopinavir plasma concentration <1000 ng/mL, i.e. below the recommended therapeutic range, was very small (1%–4%) across all groups.
Atazanavir
Atazanavir concentrations were assessed in 313 patients fulfilling the inclusion criteria (Table 1). Atazanavir once-daily regimens were ritonavir-boosted 300 mg in 187 (60%) patients and ritonavir-boosted 400 mg in 101 (32%) patients; the remainder received unboosted 400 mg. Of the patients on concomitant tenofovir, 61% were treated with boosted 300 mg, 37% with boosted 400 mg and 2% with unboosted 400 mg. Of the 30 patients on concomitant efavirenz, 23% and 77% were treated with boosted 300 or 400 mg, respectively; 20 patients received both efavirenz and tenofovir. Compared with lopinavir, the distribution of time from last intake was wider for atazanavir [median (IQR): 19 (13–24) h].
In multivariable analyses including concomitant antiviral drugs, atazanavir plasma concentration was significantly associated with regimen (40% higher for 400 mg/r and 67% lower for 400 mg, each compared with 300 mg/r; overall P < 0.001) and co-administration of efavirenz (32% lower, P = 0.016) (Table 3). The negative influence of efavirenz persisted when analysing only patients on 400/100 mg atazanavir/ritonavir. There was a trend for lower atazanavir plasma concentration with increasing weight (6% lower per additional 10 kg, P = 0.06), whereas there was no significant association with nevirapine (P = 0.37) or with tenofovir (P = 0.54).
Table 3.
Factors influencing atazanavir plasma concentration
| Change in drug concentration (% change in ng/mL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| univariable |
multivariable 1 |
multivariable 2 |
|||||||
| Parameter | effect | 95% CI | P | effect | 95% CI | P | effect | 95% CI | P |
| Age (per 10 years) | −6.0 | −18.2 to 7.9 | 0.38 | −2.6 | −13.1 to 9.2 | 0.65 | 0.1 | −11.2 to 12.5 | 0.99 |
| Gender (female versus male) | −6.1 | −29.8 to 25.6 | 0.67 | 0.5 | −26.5 to 37.5 | 0.97 | −3.3 | −29.9 to 33.2 | 0.84 |
| Ethnicity (black versus white) | −1.8 | −24.1 to 27.1 | 0.89 | −12.2 | −33.4 to 15.6 | 0.35 | −12.4 | −34.1 to 16.5 | 0.36 |
| Weight (per 10 kg) | −5.6 | −13.0 to 2.4 | 0.16 | −6.3 | −12.1 to −0.1 | 0.047 | −6.3 | −12.4 to 0.3 | 0.06 |
| Time on ATV (per 4 weeks) | 0.6 | −1.1 to 2.5 | 0.48 | 0.2 | −2.5 to 3.0 | 0.89 | 0.1 | −2.6 to 2.9 | 0.94 |
| Time on current regimen (per 4 weeks) | 1.1 | −0.9 to 3.2 | 0.28 | 0.6 | −2.5 to 3.8 | 0.70 | 0.7 | −2.5 to 4.0 | 0.68 |
| ATV regimen (versus 300 mg/r)a | |||||||||
| 400 mg/r | 35.2 | 8.8 to 67.9 | 0.007 | 29.3 | 6.4 to 57.1 | 0.010 | 40.3 | 13.7 to 73.1 | 0.002 |
| 400 mg | −75.0 | −82.8 to −63.7 | <0.001 | −69.4 | −78.0 to −57.5 | <0.001 | −66.8 | −76.7 to −52.7 | <0.001 |
| On zidovudine (yes versus no) | −0.7 | −28.6 to 38.0 | 0.97 | 19.8 | −9.8 to 59.1 | 0.21 | |||
| On lamivudine (yes versus no) | −26.2 | −41.1 to −7.5 | 0.009 | −17.4 | −33.5 to 2.7 | 0.09 | |||
| On stavudine (yes versus no) | −0.7 | −40.7 to 66.2 | 0.98 | 17.0 | −22.5 to 76.7 | 0.45 | |||
| On tenofovir DF (yes versus no) | 40.1 | 11.3 to 76.3 | 0.004 | 7.1 | −14.0 to 33.4 | 0.54 | |||
| On didanosine (yes versus no) | −4.1 | −24.3 to 21.4 | 0.73 | −4.0 | −22.1 to 18.3 | 0.70 | |||
| On abacavir (yes versus no) | −13.8 | −32.3 to 9.8 | 0.23 | −5.7 | −23.6 to 16.4 | 0.59 | |||
| On efavirenz (yes versus no) | −8.3 | −36.9 to 33.2 | 0.65 | −32.3 | −50.6 to −7.1 | 0.016 | |||
| On nevirapine (yes versus no) | −19.9 | −47.4 to 22.0 | 0.30 | −15.0 | −40.4 to 21.5 | 0.37 | |||
| On emtricitabine (yes versus no) | 48.7 | 7.3 to 106.1 | 0.017 | −0.4 | −27.1 to 36.1 | 0.98 | |||
| On saquinavir (yes versus no) | −2.9 | −36.8 to 49.4 | 0.89 | 6.6 | −26.7 to 55.0 | 0.74 | |||
| On lopinavir (yes versus no) | −15.1 | −47.8 to 37.9 | 0.51 | −11.3 | −40.3 to 31.8 | 0.55 | |||
ATV, atazanavir; /r, ritonavir boosted; DF, disoproxil fumarate.
Multivariable model 1: concomitant antiretroviral drugs not considered.
Multivariable model 2: considering concomitant antiretroviral drugs.
Both multivariable models are adjusted for hours since last atazanavir intake.
All results with P < 0.05 are shown in bold.
aOverall P value for all three models <0.001.
Neither in univariable nor in multivariable models did we find an association between liver variables (hepatitis B surface antigen, hepatitis C antibody, raised ALT) and atazanavir concentration (data not shown).
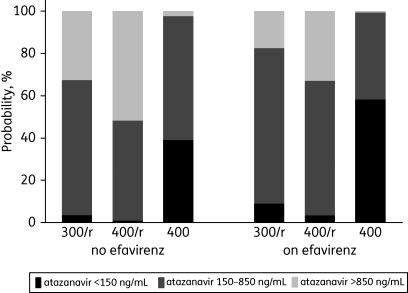
The probability of having an atazanavir trough concentration below, within or above the recommended therapeutic range is shown in Figure 1, which illustrates the influence of dose and concomitant efavirenz. Of interest, the probability of having a trough concentration within the recommended therapeutic range (150–850 ng/mL) on boosted 400 mg atazanavir plus efavirenz was the same as on boosted 300 mg atazanavir without efavirenz (64% in both scenarios), and the probability of having a trough concentration <150 ng/mL was only relevant on unboosted 400 mg atazanavir.
Figure 1.
Probabilities of having a predicted atazanavir trough concentration below (<150 ng/mL), within (150–850 ng/mL) or above (>850 ng/mL) the recommended therapeutic range for various scenarios. Numbers were derived from a multivariable regression model including atazanavir regimen, concomitant efavirenz and time post-drug intake (24 h). 300/r, 300/100 mg atazanavir/ritonavir; 400/r, 400/100 mg atazanavir/ritonavir; 400, 400 mg unboosted atazanavir (all once daily).
Discussion
In this study we observed an effect of body weight on exposure to lopinavir (and a borderline effect on atazanavir), as well as important interactions between lopinavir and rifabutin (previously unreported) and between atazanavir and efavirenz.
Drug interactions of rifabutin and ritonavir-boosted PIs such as lopinavir are complex because rifabutin and lopinavir are both inducers and substrates of CYP3A, whereas ritonavir inhibits CYP3A. Pharmacokinetic studies have shown that boosted PIs greatly increase rifabutin exposure, and current guidelines recommend that the dosage of rifabutin should be reduced by ∼75% of the usual dose (i.e. to a maximum dose of 150 mg every other day or three times per week) and that extra vigilance is warranted.14,25,26 In contrast, the impact of rifabutin on the concentration of boosted PIs has been less well studied, and in a recent review on rifabutin drug interactions no associations were described for lopinavir.27 Most of the data available to date are from the manufacturer's prescribing information. For lopinavir, it is reported that co-administration of rifabutin (150 mg once daily for 10 days) and lopinavir/ritonavir (400/100 mg for 20 days) to 14 subjects significantly increased the AUC of lopinavir by 17%; increases were also observed for Cmax (8%) and Cmin (20%), even though not statistically significant.14 A potential to increase PI exposure is also suggested by the prescribing information for some other PIs such as darunavir or fosamprenavir. Some pharmacokinetic data on the combination rifabutin and lopinavir/ritonavir are available from two reports on a small number of HIV/TB-co-infected patients, which do not suggest a clear effect of rifabutin on lopinavir exposure; however, both reports focused mainly on rifabutin parameters.28,29 To our knowledge, our study is the first population-based report describing an effect of rifabutin on lopinavir, and most of the patients took rifabutin as currently recommended (150 mg three times a week). Somewhat surprisingly, we found an even stronger interaction than described before, and patients on rifabutin had a lopinavir concentration more than twice as high as patients not on this drug. Of note, this association was present in univariable and multivariable analyses, i.e. it did not seem to be affected by demographic factors (including body weight) or other antiretroviral drugs, even though we cannot rule out an effect of other co-administered drugs (potential inhibitors of cytochrome P450 enzymes) such as isoniazid, although the impact on lopinavir has not been studied. Therefore, even if our patients are a selected group of all patients on the combination lopinavir/rifabutin, our finding would support the current recommendation of a close monitoring in these patients. It is possible that there is an interaction between rifabutin and lopinavir at the level of hepatic influx since there are reports that lopinavir is a substrate for OATP1B130 and that rifamycins are inhibitors of both OATP1B1 and OATP1A2.31
Similar to lopinavir and atazanavir, NNRTIs are metabolized by CYP3A enzymes, potentially leading to drug interactions when co-administered. In several studies it has been described that concomitant therapy with efavirenz or nevirapine results in decreased exposure to lopinavir.4,7,32,33 As a consequence, current prescribing information for lopinavir recommends a dose increase when co-administered with NNRTIs, which was reflected by the treatment regimens in our patient group. However, in our study there was no significant association between concomitant NNRTI and lopinavir concentrations, whereas, in contrast, we found decreased atazanavir concentrations with concomitant efavirenz. Somewhat conflicting are results from other studies in HIV-infected populations. No association between NNRTIs and atazanavir exposure was seen in two population pharmacokinetic studies,5,34 while decreased atazanavir plasma concentrations were observed in another study in patients on 300/100 mg and in those on 400/100 mg atazanavir/ritonavir with a concomitant NNRTI (results were not reported separately for efavirenz and nevirapine).35 Differences in patient characteristics or co-medication might have contributed to these heterogeneous results. Data from the manufacturer's Summary of Product Characteristics suggest that atazanavir trough concentrations remain low even at 400/100 mg atazanavir/ritonavir, and the manufacturer has recently recommended to consider, with close clinical monitoring, the use of 400 mg of atazanavir plus 200 mg of ritonavir if co-administration with efavirenz is required.36 The effect of 200 mg of ritonavir, however, is unknown in this context. Our data (Figure 1) suggest that the atazanavir–efavirenz interaction may be overcome by dose increment. Of note, we did not see an independent effect of nevirapine on atazanavir exposure.
Conflicting results have been found when examining the effect of concomitant tenofovir on atazanavir exposure. In some studies, atazanavir exposure was decreased when given with tenofovir, in healthy volunteers with unboosted 400 mg atazanavir9 and in HIV-infected patients with boosted 300 mg atazanavir.10 However, the mechanisms for this possible interaction are unclear, and no influence of tenofovir was found in other, observational studies in HIV-infected patients.8,11,12 In our study, including a much larger number of patients on concomitant tenofovir, we also did not see an effect of tenofovir on atazanavir exposure. Of note, in the univariable analysis individuals on tenofovir had a 40% higher atazanavir concentration, but since these patients were proportionally more often on a boosted regimen the association disappeared when adjusting for the atazanavir regimen.
In addition, we found an association between increasing weight and decreasing lopinavir concentrations, and a trend in the same direction also for atazanavir. Similar associations have been found before for both drugs.6,37 In combination with other factors this might lead to subtherapeutic lopinavir concentrations in patients who are overweight.
We found a higher lopinavir plasma concentration in patients taking 533/133 mg lopinavir/ritonavir compared with those on the standard dose of 400/100 mg. This higher dose was recommended for consideration when certain other drugs were administered concomitantly, e.g. NNRTIs. However, our analysis is based on the old capsule formulation of 133/33 mg lopinavir/ritonavir and since 2006 this has been phased out and replaced by new tablet formulations (100/25 mg and 200/50 mg tablets), which have less pharmacokinetic variability and a lower food effect.38 Whereas this change left the standard dose untouched, the recommended higher dose is now 500/125 mg lopinavir/ritonavir,14 which has been shown to result in a similar pharmacokinetic exposure when given with 600 mg of efavirenz compared with the standard dose of lopinavir/ritonavir without efavirenz.39
Our analysis is limited in so far as it is based on an observational TDM data set, rather than on a controlled study, with well-known methodological limitations.40 For example, observational data sets are by their very nature selective because drug monitoring is not universally applied to patients receiving ART but often for specific indications only. Conventional drug interaction studies are usually performed during drug development, mostly in healthy volunteers.41 Whilst of obvious advantages, these studies are only able to target suspected drug interactions and, as a result, clinically significant drug interactions that are unanticipated may remain missed. In addition, exposure to HIV drugs may be influenced by gender, age, body weight, liver function or pharmacogenetic variability, which are not captured in formal pharmacokinetic studies that are usually of small sample size.13,14 In contrast, observational population studies or TDM registries if sufficiently large are a valuable resource since they contain ‘real world’ data incorporating significant numbers of individuals from diverse groups. Therefore, we argue that observational data sets yield complementary information to formal pharmacokinetic interaction studies, and, while they cannot entirely rule out an interaction with certainty, they can detect ‘signals’ that include previously unsuspected interactions for confirmation in prospective pharmacokinetic studies.
In summary, the linkage of our TDM Registry with a well-characterized clinical cohort has made it possible to evaluate important drug interactions. Our analysis confirms that concomitant efavirenz decreases atazanavir concentrations, and we found a surprisingly strong impact of concomitant rifabutin on lopinavir that requires further study.
Funding
This work was funded by a grant from the UK Department of Health (New and Emerging Applications of Technology). The National Institute of Health Research (NIHR–Department of Health) and the Northwest Development Agency (NWDA) provided infrastructural and project support. The UK CHIC Study has been funded by research grants from the UK Medical Research Council (G0000199 and G0600337).
Transparency declarations
D. B., D. D., C. S., A. W., J. Ainsworth, B. G., C. L., M. F., C. O., J. Anderson, M. J., P E. and S. K. have received research funding, travel reimbursement, honoraria, speaker fees or consultancy fees from either or both Bristol-Myers Squibb and Abbott or have been part of their advisory boards. W. S., R. G., D. P., T. H., L. B. and S. G.: none to declare.
Therapeutic Drug Monitoring in the UK is supported by GlaxoSmithKline, Abbott, Roche and Merck.
Disclaimer
The views expressed in this manuscript are those of the researchers and not necessarily those of the MRC.
Acknowledgements
Participating Centres: Barts and The London NHS Trust, London (Chloe Orkin, Kevin Jones and Rachel Thomas); Brighton and Sussex University Hospitals NHS Trust, Brighton (Martin Fisher, Nicky Perry, Anthony Pullin, Duncan Churchill and Wendy Harris); Chelsea and Westminster NHS Trust, London (Brian Gazzard, Steve Bulbeck, Sundhiya Mandalia and Jemima Clarke); Health Protection Agency–Centre for Infections (HPA), London (Valerie Delpech); Homerton University Hospital NHS Trust, London (Jane Anderson and Selina Gann); King's College Hospital, London (Philippa Easterbrook, Yasar Khan, Fatimah Karim, Eghosa Bazuaye and Stephen Duffell); Medical Research Council Clinical Trials Unit (MRC CTU), London (Abdel Babiker, David Dunn, Kholoud Porter and Stephen Sheehan); Mortimer Market Centre, Royal Free and University College Medical School (RFUCMS), London (Richard Gilson, Julie Dodds, Shuk-Li Man and Ian Williams); North Middlesex University Hospital NHS Trust, London (Achim Schwenk); Royal Free NHS Trust and RFUCMS, London (Margaret Johnson, Mike Youle, Fiona Lampe, Colette Smith, Helen Grabowska, Clinton Chaloner, Dewi Ismajani Puradiredja, Loveleen Bansi, Teresa Hill, Andrew Phillips and Caroline Sabin); St Mary's Hospital, London (John Walsh, Jonathan Weber, Christian Kemble and Mark Carder); The Lothian University Hospitals NHS Trust, Edinburgh (Clifford Leen and Alan Wilson).
We also acknowledge the contribution of Dr John Tjia in bioanalysis.
UK CHIC Steering Committee: Jonathan Ainsworth, Jane Anderson, Abdel Babiker, David Dunn, Philippa Easterbrook, Martin Fisher, Brian Gazzard (Chair), Richard Gilson, Mark Gompels, Teresa Hill, Margaret Johnson, Clifford Leen, Chloe Orkin, Andrew Phillips, Deenan Pillay, Kholoud Porter, Caroline Sabin, Tariq Sadiq, Achim Schwenk, John Walsh and Valerie Delpech.
References
- 1.Gazzard BG. British HIV Association Guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. 2008;9:563–608. doi: 10.1111/j.1468-1293.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 2.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 3.Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–55. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 4.Crommentuyn KM, Kappelhoff BS, Mulder JW, et al. Population pharmacokinetics of lopinavir in combination with ritonavir in HIV-1-infected patients. Br J Clin Pharmacol. 2005;60:378–89. doi: 10.1111/j.1365-2125.2005.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo S, Buclin T, Cavassini M, et al. Population pharmacokinetics of atazanavir in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2006;50:3801–8. doi: 10.1128/AAC.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Leur MR, Burger DM, la Porte CJ, et al. A retrospective TDM database analysis of interpatient variability in the pharmacokinetics of lopinavir in HIV-infected adults. Ther Drug Monit. 2006;28:650–3. doi: 10.1097/01.ftd.0000245681.12092.d6. [DOI] [PubMed] [Google Scholar]
- 7.Solas C, Poizot-Martin I, Drogoul MP, et al. Therapeutic drug monitoring of lopinavir/ritonavir given alone or with a non-nucleoside reverse transcriptase inhibitor. Br J Clin Pharmacol. 2004;57:436–40. doi: 10.1046/j.1365-2125.2003.02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winston A, Bloch M, Carr A, et al. Atazanavir trough plasma concentration monitoring in a cohort of HIV-1-positive individuals receiving highly active antiretroviral therapy. J Antimicrob Chemother. 2005;56:380–7. doi: 10.1093/jac/dki235. [DOI] [PubMed] [Google Scholar]
- 9.Kaul S, Bassi K, Damle B, et al. Pharmacokinetic evaluation of the combination of atazanavir (ATV), enteric coated didanosine (ddI-EC), and tenofovir disoproxil fumarate (TDF) for a once-daily antiretroviral regimen. Abstracts of the Forty-third Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, USA. Washington, DC, USA: American Society for Microbiology; 2003. Abstract A-1616. [Google Scholar]
- 10.Taburet AM, Piketty C, Chazallon C, et al. Interactions between atazanavir-ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2004;48:2091–6. doi: 10.1128/AAC.48.6.2091-2096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Hentig N, Dauer B, Haberl A, et al. Tenofovir comedication does not impair the steady-state pharmacokinetics of ritonavir-boosted atazanavir in HIV-1-infected adults. Eur J Clin Pharmacol. 2007;63:935–40. doi: 10.1007/s00228-007-0344-y. [DOI] [PubMed] [Google Scholar]
- 12.Pellegrin I, Breilh D, Ragnaud JM, et al. Virological responses to atazanavir-ritonavir-based regimens: resistance-substitutions score and pharmacokinetic parameters (Reyaphar study) Antivir Ther. 2006;11:421–9. [PubMed] [Google Scholar]
- 13.Reyataz. Prescribing Information. Princeton, NJ, USA: Bristol-Myers Squibb Company; 2008. [Google Scholar]
- 14.Kaletra. Prescribing Information. North Chicago, IL, USA: Abbott Laboratories; 2008. [Google Scholar]
- 15.The UK Collaborative HIV Cohort Steering Committee CHIC. The creation of a large UK-based multicentre cohort of HIV-infected individuals: the UK Collaborative HIV Cohort (UK CHIC) Study. HIV Med. 2004;5:115–24. doi: 10.1111/j.1468-1293.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson L, Robinson L, Tjia J, et al. Simultaneous determination of HIV protease inhibitors amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir and saquinavir in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;829:82–90. doi: 10.1016/j.jchromb.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Carlin JB, Li N, Greenwood P, et al. Tools for analyzing multiple imputed datasets. The Stata Journal. 2003;3:226–44. [Google Scholar]
- 18.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–74. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 19.la Porte CJL, Back DJ, Blaschke T, et al. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev Antivir Ther. 2006;3:4–14. [Google Scholar]
- 20.Ray JE, Marriott D, Bloch MT, et al. Therapeutic drug monitoring of atazanavir: surveillance of pharmacotherapy in the clinic. Br J Clin Pharmacol. 2005;60:291–9. doi: 10.1111/j.1365-2125.2005.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleijsen RM, van de Ende ME, Kroon FP, et al. Therapeutic drug monitoring of the HIV protease inhibitor atazanavir in clinical practice. J Antimicrob Chemother. 2007;60:897–900. doi: 10.1093/jac/dkm298. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Novoa S, Martin-Carbonero L, Barreiro P, et al. Genetic factors influencing atazanavir plasma concentrations and the risk of severe hyperbilirubinemia. AIDS. 2007;21:41–6. doi: 10.1097/QAD.0b013e328011d7c1. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez de Requena D, Bonora S, Canta F, et al. Atazanavir Ctrough is associated with efficacy and safety: definition of therapeutic range. Abstracts of the Twelfth Conference on Retroviruses and Opportunistic Infections; Boston, USA. Alexandria, VA, USA: Foundation for Retrovirology and Human Health; 2005. Abstract 645. [Google Scholar]
- 24.Breilh D, Pellegrin I, Rouzes A, et al. Virological, intracellular and plasma pharmacological parameters predicting response to lopinavir/ritonavir (KALEPHAR study) AIDS. 2004;18:1305–10. doi: 10.1097/00002030-200406180-00009. [DOI] [PubMed] [Google Scholar]
- 25.Pozniak AL, Miller RF, Lipman MC, et al. BHIVA treatment guidelines for tuberculosis (TB)/HIV infection 2005. HIV Med. 2005;6(Suppl 2):62–83. doi: 10.1111/j.1468-1293.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 26.US Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf. (10 July 2009, date last accessed) [Google Scholar]
- 27.Baciewicz AM, Chrisman CR, Finch CK, et al. Update on rifampin and rifabutin drug interactions. Am J Med Sci. 2008;335:126–36. doi: 10.1097/MAJ.0b013e31814a586a. [DOI] [PubMed] [Google Scholar]
- 28.Bonora S, Boffito M, D'Avolio A, et al. Pharmacokinetics (PKS) of rifabutin (RIF) coadministered with lopinavir/ritonavir (LPV/r) in HIV patients affected by tuberculosis (TB). Antivir Ther; Abstracts of the Second IAS Conference on HIV Pathogenesis and Treatment; 2003; Paris, France. 2003. pp. S427–8. Abstract 863. [Google Scholar]
- 29.Khachi H, O'Connell R, Ladenheim D, et al. Pharmacokinetic interactions between rifabutin and lopinavir/ritonavir in HIV-infected patients with mycobacterial co-infection. J Antimicrob Chemother. 2009;64:871–3. doi: 10.1093/jac/dkp263. [DOI] [PubMed] [Google Scholar]
- 30.Shallcross VL, Kwan WS, Hartkoorn R, et al. Lopinavir is a substrate for SLCO1A2 but 516A>C and 38T>C polymorphisms do not influence lopinavir plasma concentrations. J Int AIDS Soc; Abstracts of the Ninth International Congress on Drug Therapy in HIV Infection; 2008; Glasgow, UK. 2008. p. P238. Abstract P238. [Google Scholar]
- 31.Niemi M. Role of OATP transporters in the disposition of drugs. Pharmacogenomics. 2007;8:787–802. doi: 10.2217/14622416.8.7.787. [DOI] [PubMed] [Google Scholar]
- 32.Dailly E, Allavena C, Raffi F, et al. Pharmacokinetic evidence for the induction of lopinavir metabolism by efavirenz. Br J Clin Pharmacol. 2005;60:32–4. doi: 10.1111/j.1365-2125.2005.02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu A, Isaacson J, Brun S, et al. Pharmacokinetic-pharmacodynamic analysis of lopinavir-ritonavir in combination with efavirenz and two nucleoside reverse transcriptase inhibitors in extensively pretreated human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2003;47:350–9. doi: 10.1128/AAC.47.1.350-359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solas C, Gagnieu MC, Ravaux I, et al. Population pharmacokinetics of atazanavir in human immunodeficiency virus-infected patients. Ther Drug Monit. 2008;30:670–3. doi: 10.1097/FTD.0b013e3181897bff. [DOI] [PubMed] [Google Scholar]
- 35.Poirier JM, Guiard-Schmid JB, Meynard JL, et al. Critical drug interaction between ritonavir-boosted atazanavir regimen and non-nucleoside reverse transcriptase inhibitors. AIDS. 2006;20:1087–9. doi: 10.1097/01.aids.0000222092.97776.cd. [DOI] [PubMed] [Google Scholar]
- 36.Bristol-Myers Squibb Pharmaceuticals Ltd. Reyataz - Summary of Product Characteristics. http://emc.medicines.org.uk/medicine/14145/SPC/Reyataz+150+mg%2c+200+mg+and+300mg+Hard+Capsules/ (10 July 2009, date last accessed) [Google Scholar]
- 37.Di Giambenedetto S, De Luca A, Villani P, et al. Atazanavir and lopinavir with ritonavir alone or in combination: analysis of pharmacokinetic interaction and predictors of drug exposure. HIV Med. 2008;9:239–45. doi: 10.1111/j.1468-1293.2008.00555.x. [DOI] [PubMed] [Google Scholar]
- 38.Klein CE, Chiu YL, Awni W, et al. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft-gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. J Acquir Immune Defic Syndr. 2007;44:401–10. doi: 10.1097/QAI.0b013e31803133c5. [DOI] [PubMed] [Google Scholar]
- 39.Ng J, Klein C, Xiong J, et al. Lopinavir/ritonavir 500/125 mg twice-daily+efavirenz approximate the pharmacokinetic exposure of LPV/r 400/100 mg twice-daily administered alone in healthy adult subjects. Abstracts of the Fifteenth Conference on Retroviruses and Opportunistic Infections; Boston, USA. Alexandria, VA, USA: Foundation for Retrovirology and Human Health; 2008. Abstract 765. [Google Scholar]
- 40.Stohr W, Back D, Dunn D, et al. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir Ther. 2008;13:675–85. [PubMed] [Google Scholar]
- 41.FDA. In Vivo Drug Metabolism/Drug Interaction Studies—Study Design, Data Analysis, and Recommendations for Dosing and Labeling. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072119.pdf. (10 July 2009, date last accessed) [Google Scholar]